- 1School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 2College of Medical Technology, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3Department of Laboratory Medicine, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
Multiple myeloma (MM) is a hematologic malignancy characterized by aberrant expansion of monoclonal plasma cells with high mortality and severe complications due to the lack of early diagnosis and timely treatment. Circulating miRNAs have shown potential in the diagnosis of MM with inconsistent results, which remains to be fully assessed. Here we updated a meta-analysis with relative studies and essays published in English before Jan 31, 2021. After steps of screening, 32 studies from 11 articles that included a total of 627 MM patients and 314 healthy controls were collected. All data were analyzed by REVMAN 5.3 and Stata MP 16, and the quality of included literatures was estimated by Diagnostic Accuracy Study 2 (QUADAS-2). The pooled area under the curve (AUC) shown in summary receiver operating characteristic (SROC) analyses of circulating miRNAs was 0.87 (95%CI, 0.81–0.89), and the sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were 0.79, 0.86, 5, 0.27, 22, respectively. Meta-regression and subgroup analysis exhibited that “miRNA cluster”, patient “detailed stage or Ig isotype” accounted for a considerable proportion of heterogeneity, revealing the importance of study design and patient inclusion in diagnostic trials; thus standardized recommendations were proposed for further studies. In addition, the performance of the circulating miRNAs included in MM prognosis and treatment response prediction was summarized, indicating that they could serve as valuable biomarkers, which would expand their clinical application greatly.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=234297, PROSPERO, identifier (CRD42021234297).
Introduction
Multiple myeloma (MM), the second most common hematological malignancy (1), develops from monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM), through the malignant transformation of long-lived plasma cells deriving from memory B cells and plasma-blasts (2). Normal interactions between plasma cells and their environment (the bone remodeling chambers) enable the stability of normal plasma cell genotypes and phenotypes, which may be disrupted by multiple factors at a precursor stage of MM (MGUS, pre-MGUS state) leading to MM tumorigenesis (3). The risk of progression for MGUS is about 1% yearly and 10% for SMM in the first 5 years (4). Early treatment is of benefit, inhibiting disease progression but with non-negligible side effects (5), and a novel biomarker with high accuracy for early diagnosis is imperative.
As a member of non-coding RNAs, miRNAs are small RNA molecules that function as negative regulators of gene expression. The decrease or increase of specific miRNAs in MM is associated with the dysregulation of the target gene expression, reflecting their impact on tumor-suppression or promotion (6, 7). Circulating miRNAs have been explored as valuable tools for various tumor diagnoses and prognoses by several studies (8). Similarly, the diversification of circulating miRNA expression in MM has been investigated, indicating that miRNAs could serve as potential diagnostic biomarkers (9, 10). However, not every miRNA was eligible, and there was significant heterogeneity among the findings. We updated a meta-analysis to address whether the circulating miRNAs could be promising biomarkers for early detection of MM with the latest evidence and try to resolve the problems that contributed to heterogeneity in previous studies.
Materials and Methods
This systematic review and meta-analysis was conducted following the guidance of the PRISMA 2020 Statement: an Updated Guideline for Reporting Systematic Reviews (11), and registered on PROSPERO prior to the start.
Literature Search
Multiple databases (Cochrane Library, PubMed, Embase, Web of Science, SinoMed, CQVIP database, Wan Fang database, China National Knowledge Infrastructure (CNKI), and Clinical Trials.gov) were systematically searched for related studies and essays published in English up to Jan 31, 2021. Subject headings and all the free words of ‘multiple myeloma’, ‘microRNAs’, ‘sensitivity’, ‘specificity’, ‘predictive value’, ‘accuracy’, ‘diagnostic’, and ‘AUC’ were applied for the regular advanced search. To minimize search omissions, we searched ScienceDirect and ResearchGate and pored over the reference list of the articles cited in this review to look for potential studies.
Study Selection
Studies that met the following criteria were included: 1) all the MM patients were diagnosed according to standard diagnostic criterion; 2) control subjects were analyzed concurrently; 3) miRNA measured by qRT-PCR and the process clearly described; 4) same outcome: sensitivity, specificity, AUC; 5) specimens are limited to serum or plasma; and 6) sample size was given. The exclusion criteria were as follows: 1) different control groups; 2) reviews and meta-analysis; 3) abstracts, editorials, conference papers, and letters without valid data; 4) repeated articles; 5) not conducted on humans; 6) sample size was insufficient; 7) non-English literature. To avoid any selection bias, two independent authors decided whether to include or exclude a study when they reach a consensus; otherwise, a third author reviewed the article again and resolved the disagreement.
Quality Assessment of Literature and Data Extraction
The quality of the selected studies was assessed according to QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies-2) on RevMan (version 5.3, London, UK, RRID: SCR_003581) by two authors and illustrated in a graph. Also, basic information from eligible articles/supplementary materials (first authors, publication year, specimen, sample size, miRNAs, expression mode, sensitivity, specificity, AUC, patient information including Ig isotype, stage, study design, gender structure, and age range) was extracted by two authors independently using a standardized form and then reviewed by a third author in detail.
Statistical Analysis
The data of true positives (TPs), false positives (FPs), true negatives (TNs), and false negatives (FNs) of 32 miRNAs extracted from 11 individual studies were analyzed in REVMAN 5.3 and STATA (version MP16, Texas, USA, RRID : SCR_012763). The sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under the curve (AUC) were pooled with the random effects meta-analysis model. Total diagnostic accuracy was assessed by summary receiver operating characteristic (SROC) curve with the sensitivity and specificity data of each qualified study. To evaluate the existence of heterogeneity, I2 over 50% and/or P-value of Q-test under 0.05 were set as statistically significant. Meta-regression was executed with univariable model and multivariate model respectively. Subgroup analyses were employed to dissociate the heterogeneity among the studies using the random-effects inverse-variance model with DerSimonian–Laird estimate of tau². All P-values less than 0.05 were considered statistically significant.
Results
Literature Selection, Quality Assessment, and Study Characteristics
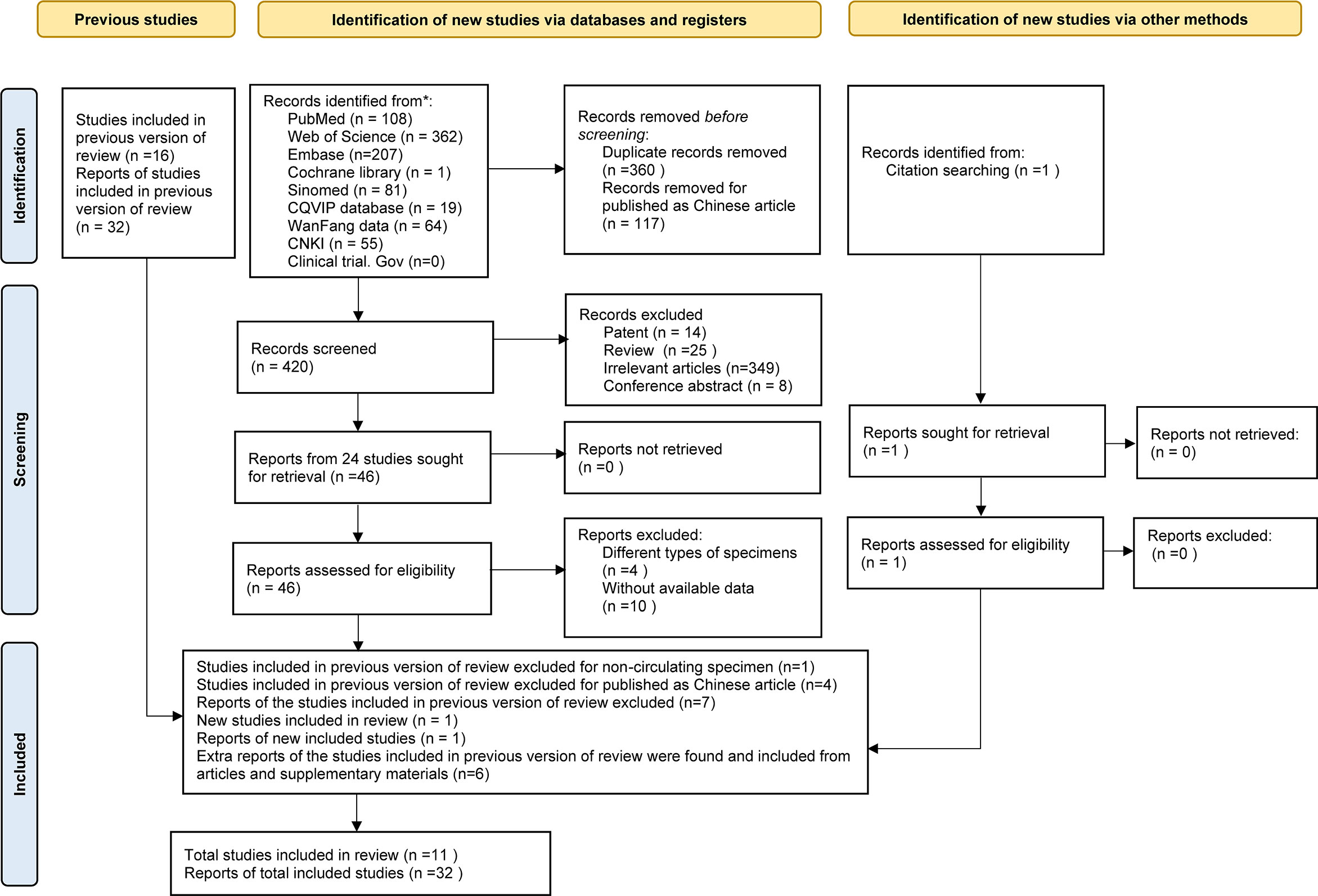
As the study search and selection process show in Figure 1, a total of 898 articles were initially obtained; 897 studies were identified from Cochrane Library, PubMed, Embase, Web of Science, SinoMed, CQVIP database, Wan Fang Data, China National Knowledge Infrastructure (CNKI), and Clinical Trials.gov, and 1 was acquired from reference lists of articles cited in this paper. All of the articles were imported into Zotero. After carefully screening for the title. abstract, and full-text, 117 Chinese articles, 360 duplicates, 14 patent, 25 reviews, 8 conference abstracts, 349 with irrelevant themes, 5 with different types of specimen, and 10 without available data were excluded. Finally, 32 miRNAs from 11 articles were included for this meta-analysis.
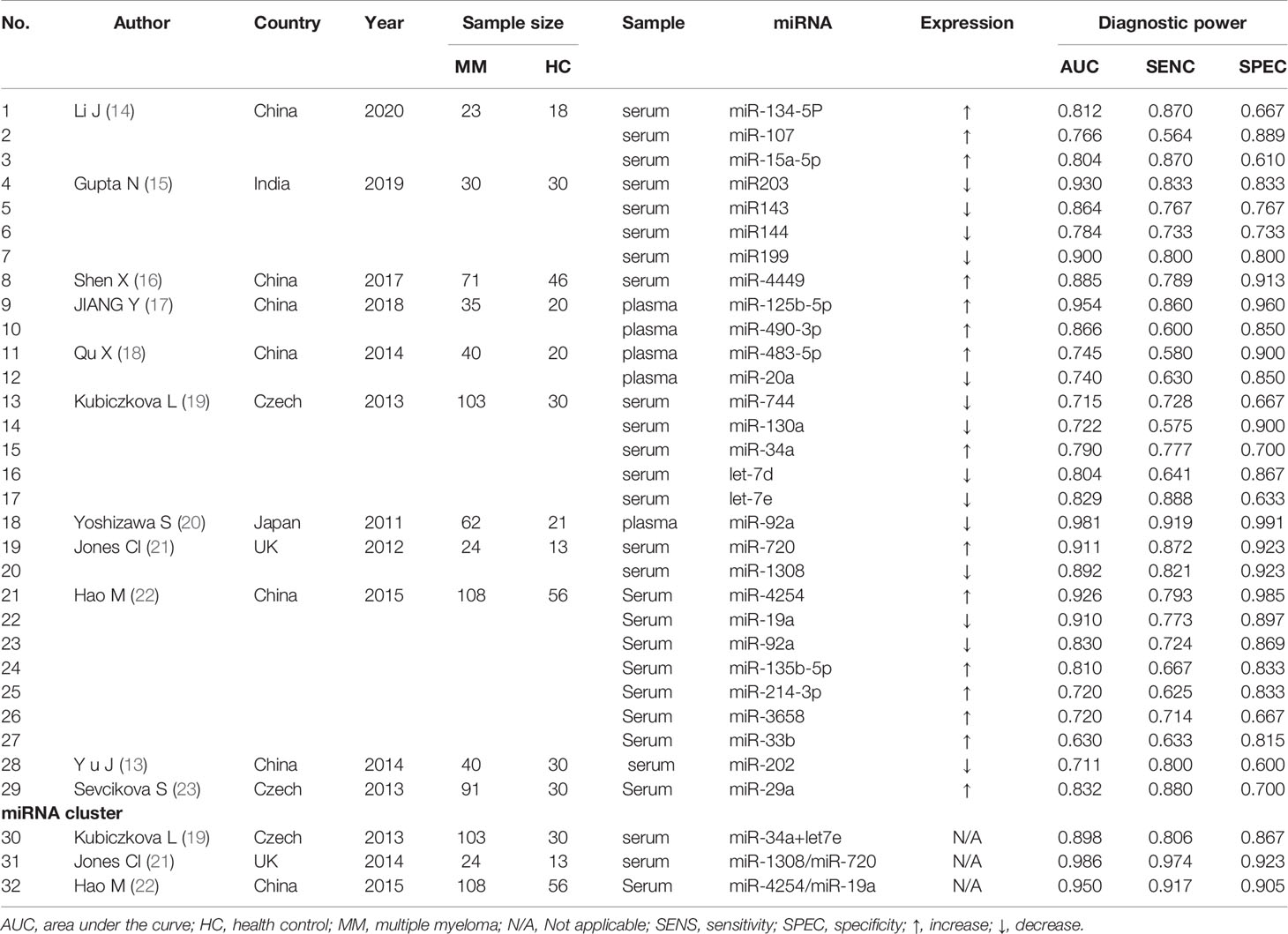
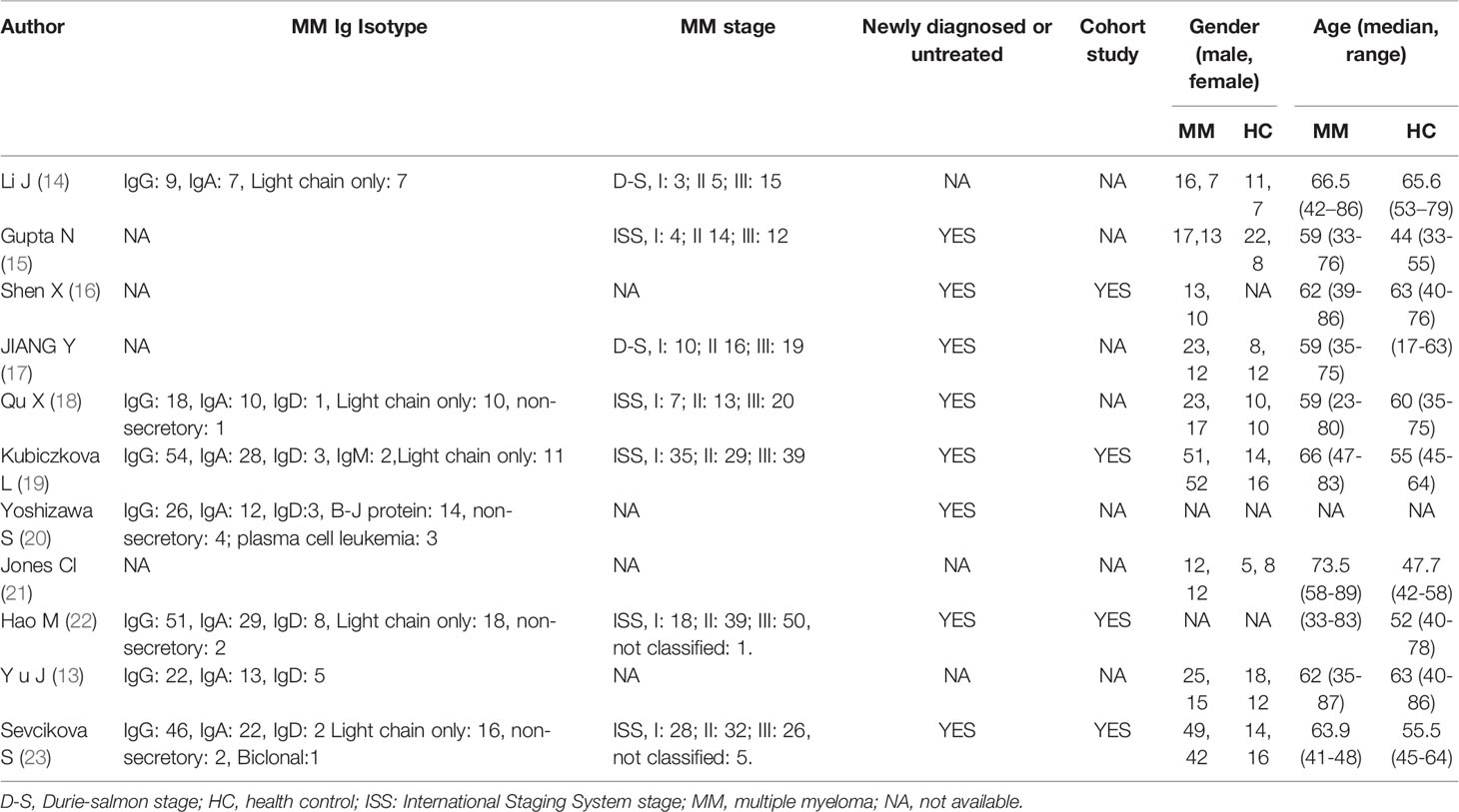
In comparison to the previous version of review (10), one report of a study for non-circulating specimen (12) and seven reports of four articles published in Chinese were excluded; and one report of a new study was included (13). The main information of circulating miRNA reports extracted from the qualified studies is displayed in Table 1. Among these, six extra reports of the studies included in the previous version of review were found from the articles and supplementary materials (14, 18, 21, 22). Characteristics of MM patients and healthy controls are available in Table 2. All the eligible articles were published before Jan 31, 2021 containing 627 MM patients and 314 healthy controls. The expression levels of miRNAs in serum (n = 27) or plasma (n = 5) were detected by qRT-PCR. Of these 32 studies, three studies evaluated miRNA clusters, whereas the others evaluated individual miRNA.
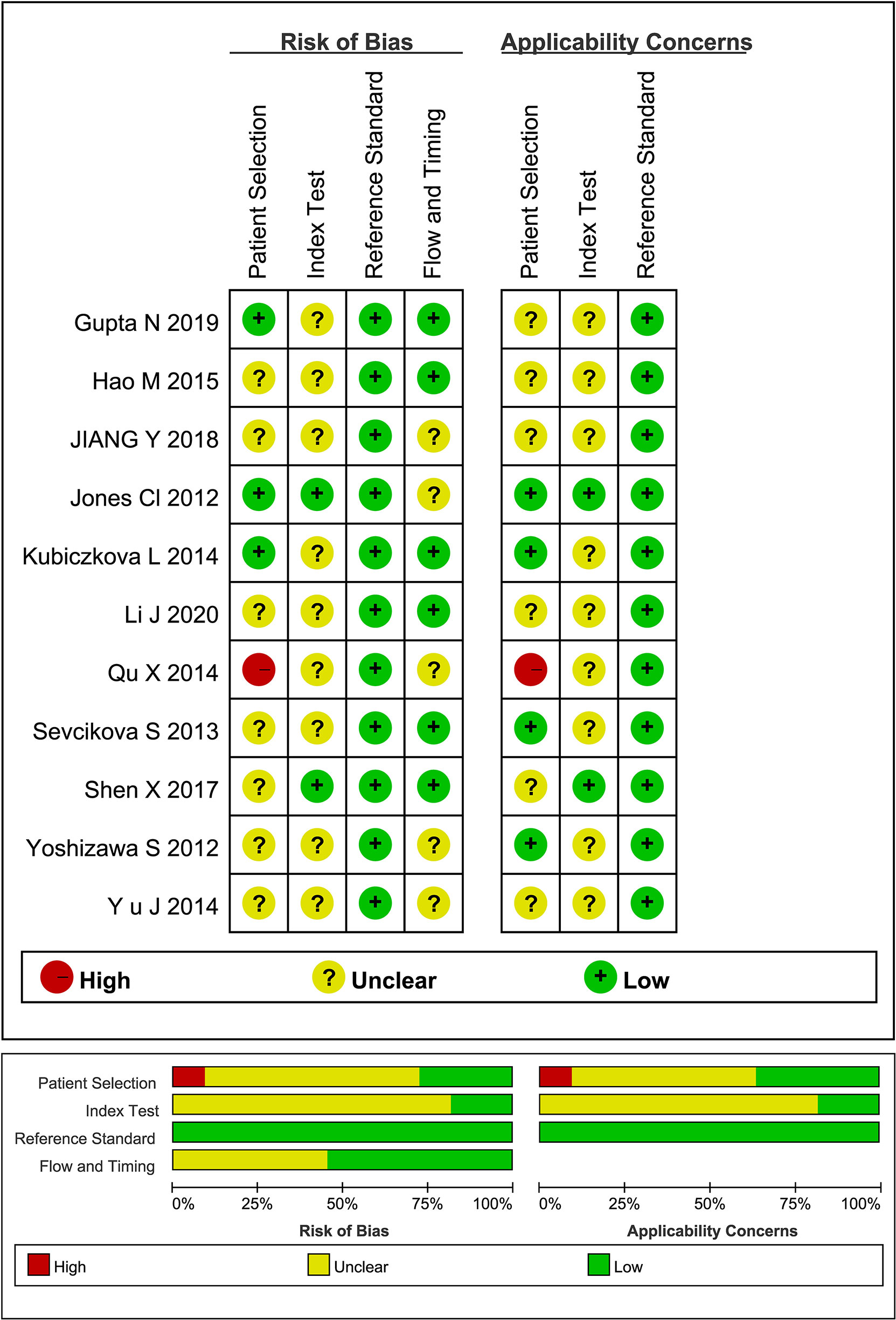
Quality assessment of qualified literatures by QUADAS-2 tool reveal that the overall quality of the studies included was acceptable, but with a few unignorable flaws and uncertainties (Figure 2). Some of the literatures did not clarify the study design, the patient information (stage/Ig isotype/prior to any treatment), and whether there was an appropriate time interval between the reference standard and miRNA detection. Besides, the absence of suspected cases in some of the literatures may lead to bias in the evaluation of diagnostic power.
Diagnostic Performance Evaluation
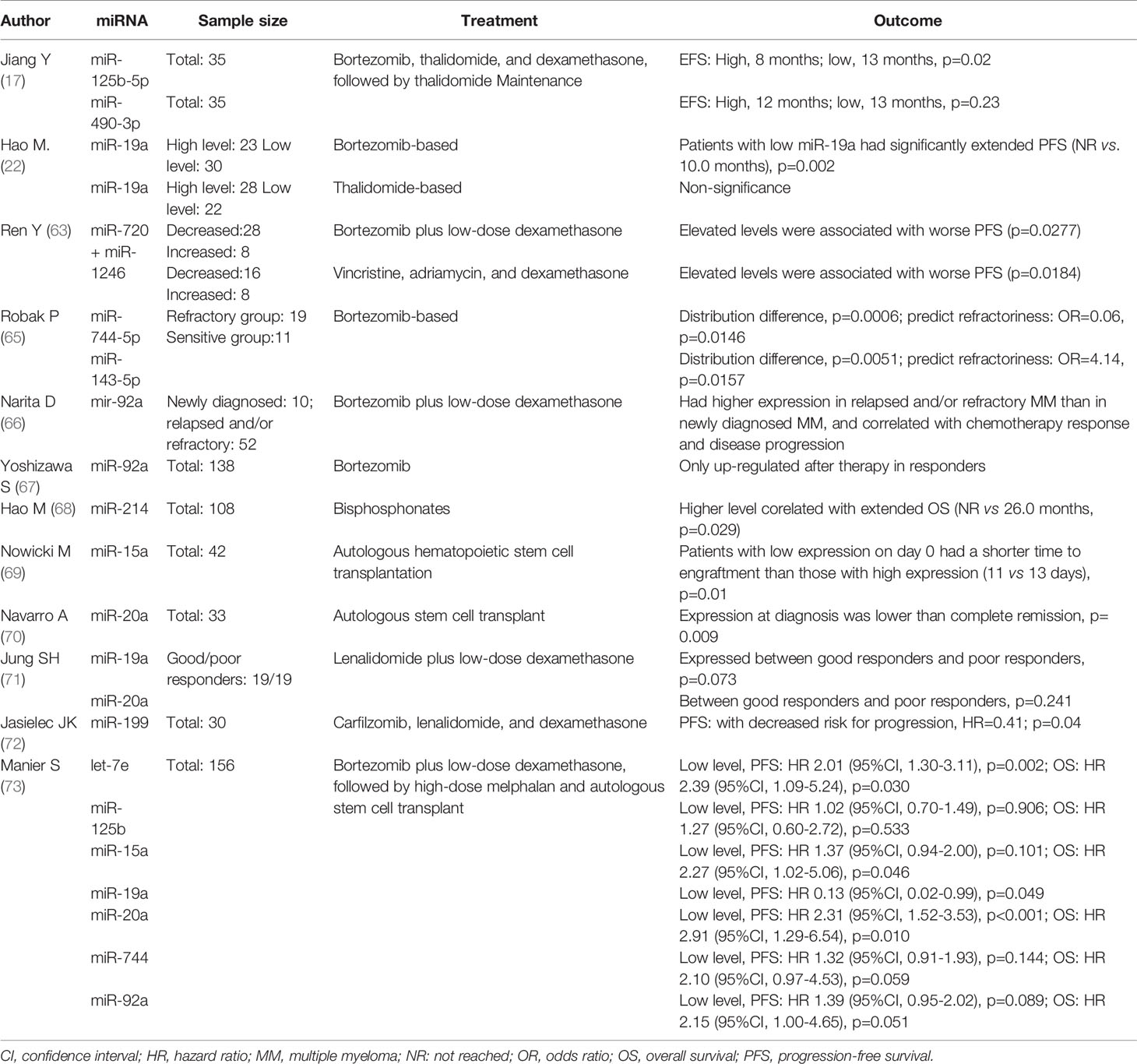
All the data were included to calculate the combined diagnostic accuracy of miRNAs by STATA MP 16 software. As Forest plots present, the sensitivity and specificity of miRNAs in various studies were significantly heterogeneous (sensitivity: I2 = 76.86%, p < 0.01; specificity: I2 = 62.94%, p < 0.01). By recombining the data with random effect models, the pooled sensitivity and specificity were 0.79 (95%CI, 0.73–0.83) and 0.86 (95%CI, 0.81–0.89) respectively (Figure 3A). Spearman correlation analysis was performed on the Logit conversion values of sensitivity and false-positive rate to identify the source of heterogeneity, and no threshold effect was suggested (coefficient = −0.0817, p = 0.657). Based on the SROC curve, the pooled AUC of miRNAs in MM diagnosis was 0.87 (95%CI, 0.81–0.89), PLR 5 (95%CI, 4–6), NLR 0.27 (95%CI, 0.23–0.33) (Figure 3B). The pooled DOR was 22 (95%CI, 14–35) using a random effect model (Figure 3C).
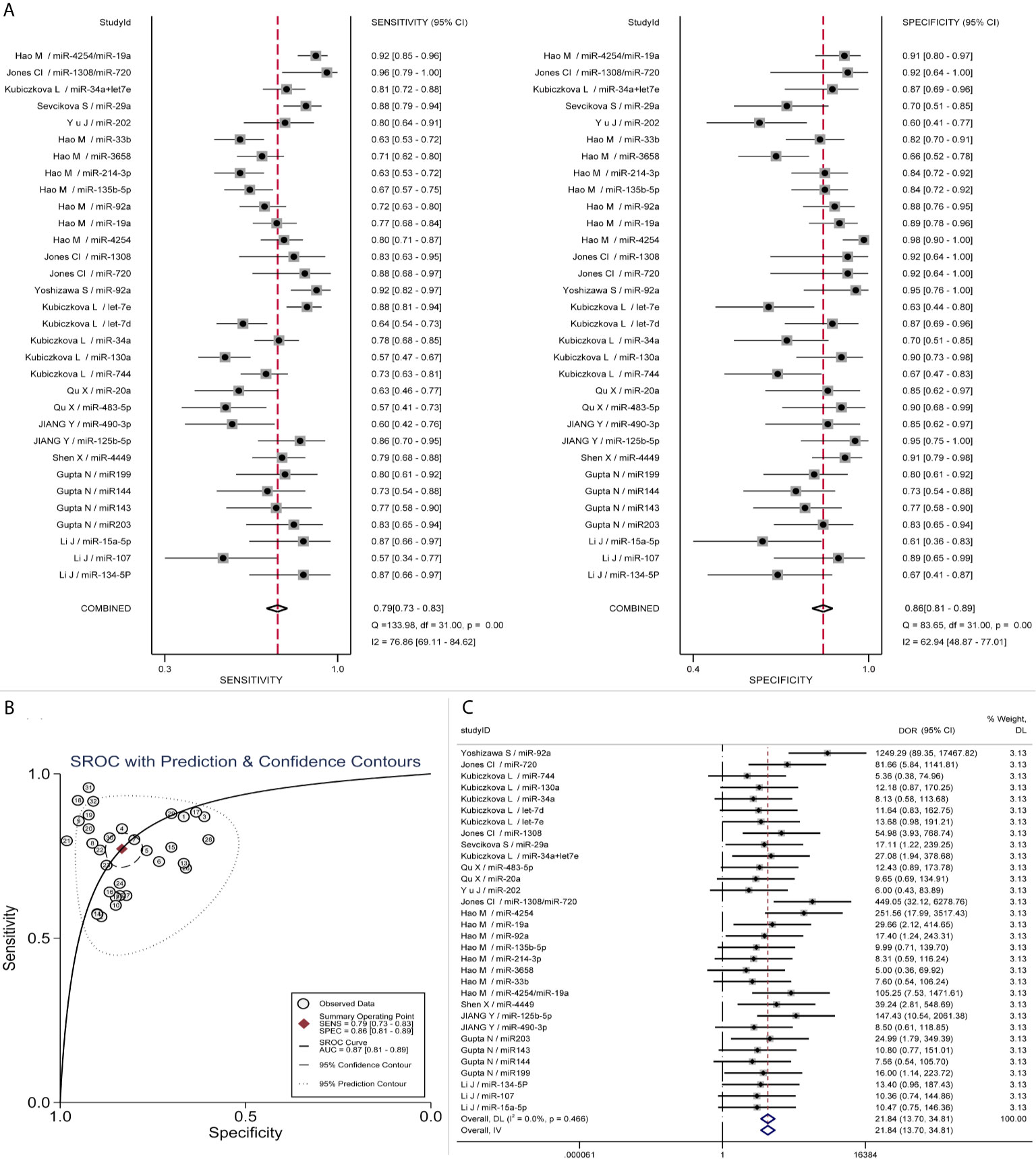
Figure 3 Pooled diagnostic parameters of all microRNA studies. (A) Forest plot of Sensitivity and Specificity; (B) SROC curve; (C) Forest plot of DOR.
Summary LRP and LRN for Index Test are depicted in Figure 4A, demonstrating that a small number of miRNAs (No. 9/18/19/20/21/31/32) contribute to the confirmation or exclusion of MM, whereas the others appear to be under-achieving. The pre-test and post-test probabilities were evaluated by Fagan’s nomogram, presenting that when the prior probability was 20%, post-test probabilities were 54 and 6% for positive and negative circulating miRNAs in MM patients, respectively (Figure 4B).
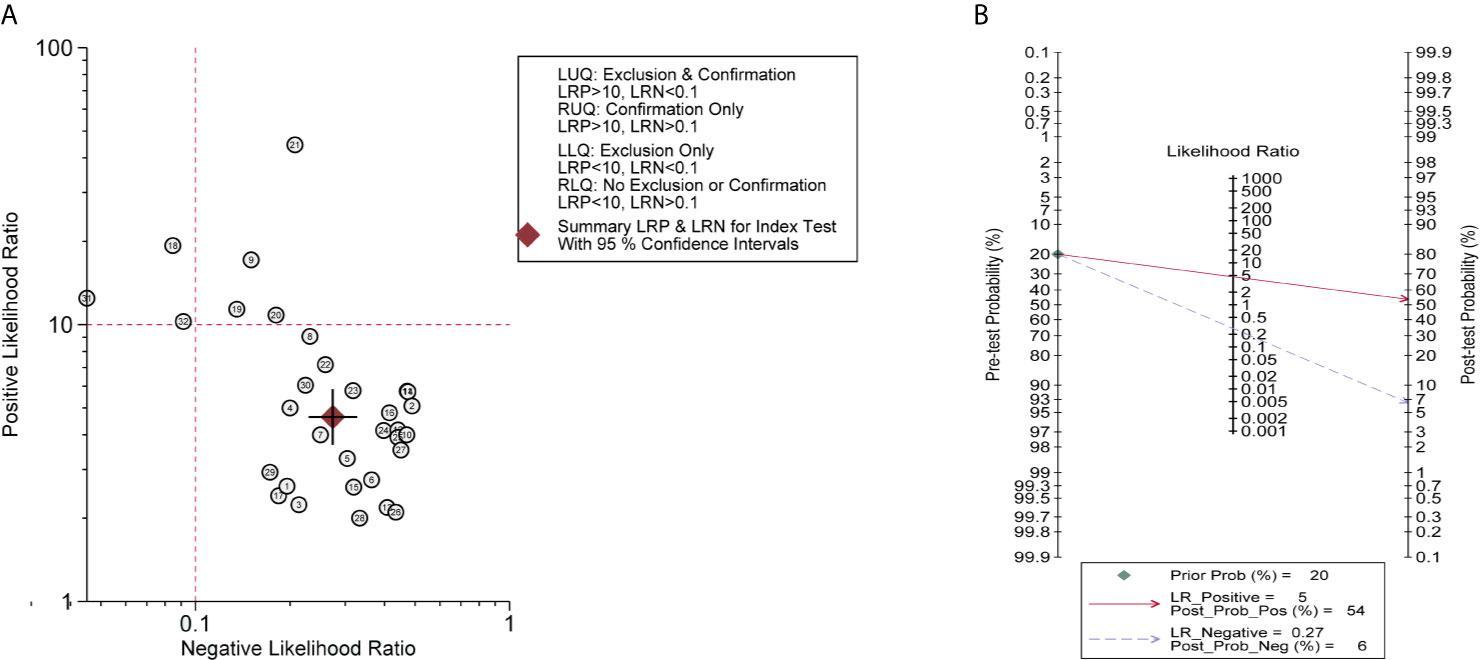
Figure 4 Clinical utility of circulating miRNAs. (A) Summary LRP & LRN for Index Test showed that a few miRNAs (No. 9/18/19/20/21/31/32) had relatively good clinical diagnostic value. LLQ, left lower quadrant; LRN, likelihood ratio negative; LRP, likelihood ratio positive; LUQ, left upper quadrant; RLQ, right lower quadrant; RUQ, right upper quadrant; (B) Fagan nomogram of Pre-test probability and post-test probability.
Sensitivity Analysis and Heterogeneity Exploration
Then, a sensitivity analysis was performed to evaluate the stability of the combined DOR. After subtracting the No. 18 study with the maximal deviation, the DOR value changed from 22 to 19, presenting that there was no significant change in the results (Figure 5A). Similarly, other studies, excluding one, showed at the time consistent combinatorial DOR without significant fluctuations. However, the fixed-effect model had a greater influence on the pooled DOR than the random-effect model (DOR, 17 vs. 22), indicating that an improper data analysis method may have a great impact on the pooled results. Among these miRNAs, miR-4254 was the most promising diagnostic biomarker with 0.80 sensitivity (95%CI, 0.71–0.87), 0.98 specificity (95%CI, 0.90–1.0) and 252 DOR (95%CI, 18–3517). Bivariate boxplot revealed that some of the studies with relatively higher AUC (No.18/21/31) showed stronger heterogeneity (Figure 5B). Meanwhile, publication bias was not found by Deek’s funnel plot asymmetry test (P = 0.73) (Figure 5C).
Figure 5 Sensitivity analysis and Heterogeneity exploration. (A) Sensitivity Analysis showed that the combination results were stable; (B) Bivariate Boxplot revealed that No.18/21/31 studies presented strong heterogeneity; (C) Deek’s Funnel Plot Asymmetry Test found no publication bias.
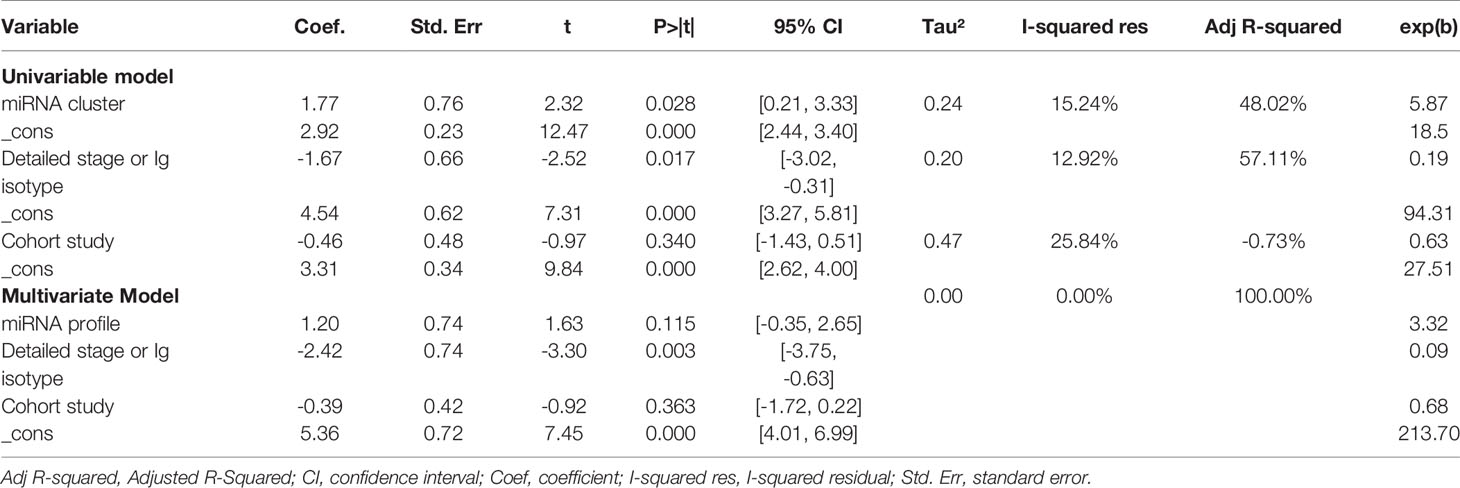
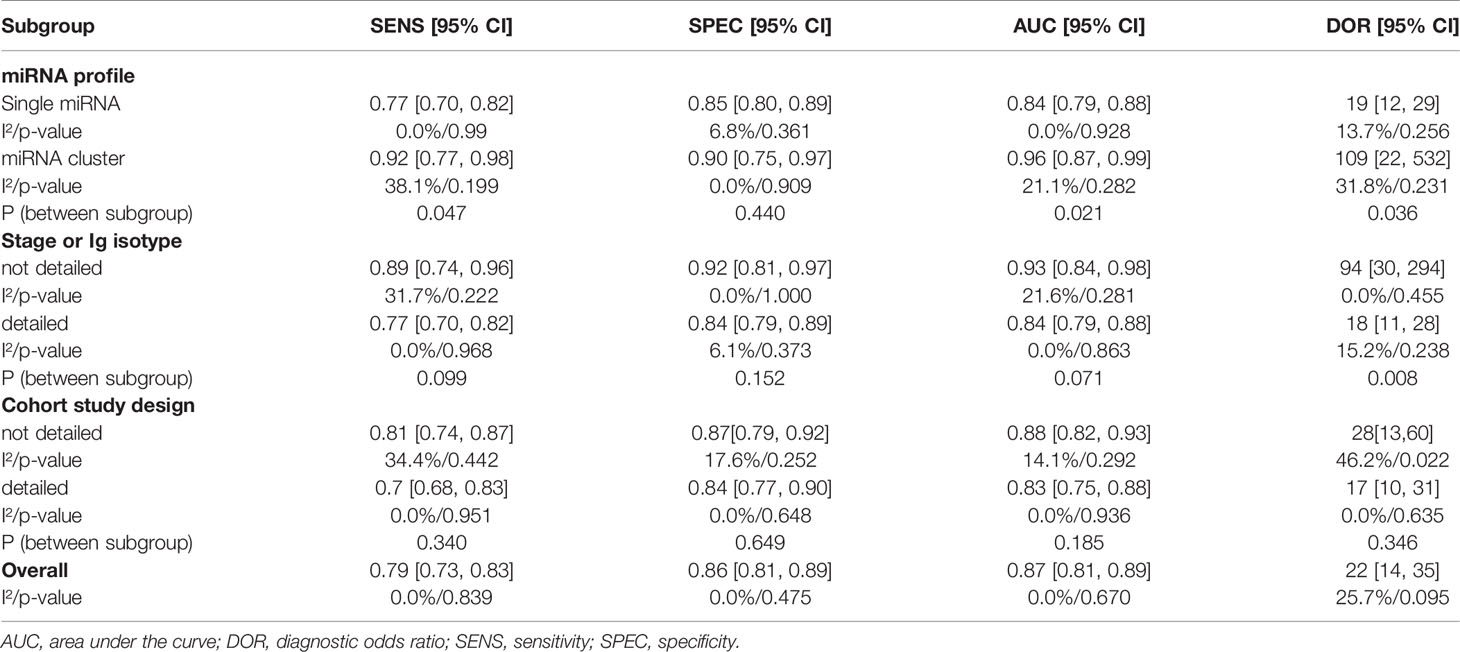
Further heterogeneity analysis by meta-regression according to “miRNA cluster”, “ detailed stage or Ig isotype”, “newly diagnosed or untreated”, “determined cohort study design”, “sample size>30”, “serum or plasma” and “ethnicity” were conducted with univariable model, demonstrating that “miRNA cluster”, “detailed stage or Ig isotype” and “determined cohort study design” reached statistical significance. Subsequently, these three variables were analyzed by meta-regression with multivariate model, and there were significant differences between the “miRNA cluster” subgroups and the “detailed stage or Ig isotype” subgroups (Table 3). Then subgroup analyses based on sensitivity, specificity, AUC, and DOR were performed using the random-effects inverse-variance model with DerSimonian–Laird estimate of tau² to investigate the specific heterogeneity existing within and between subgroups, and the results are displayed in Table 4. The diagnostic parameters of miRNA clusters were better than the single miRNA subgroup, whereas the parameters of the detailed patient information subgroup and determined cohort study design subgroup were inferior than their contrasts. The DOR-based subgroup analysis results are shown in Figure 6.
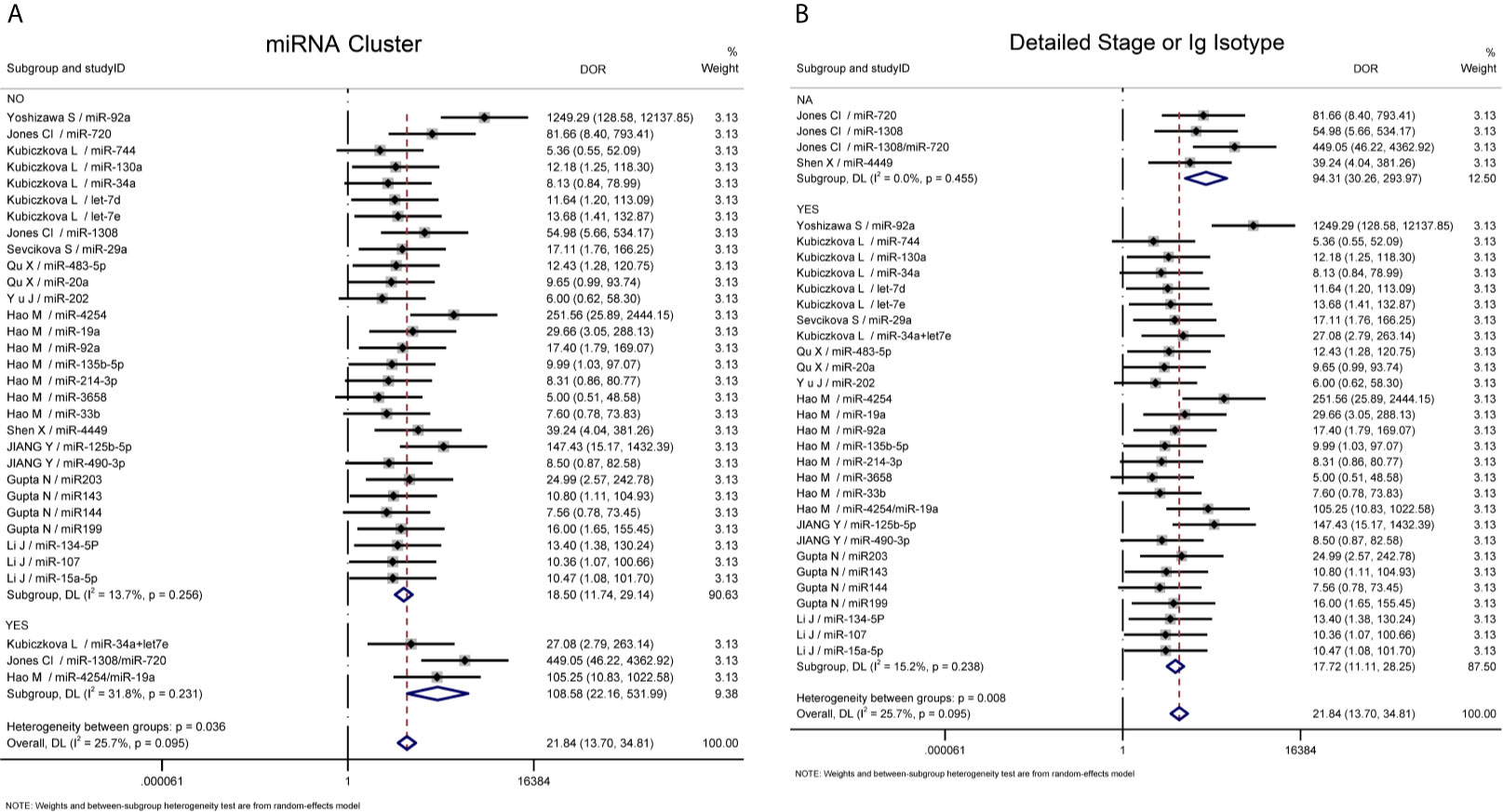
Figure 6 Subgroup analysis. (A) Subgroup analysis based on DOR sorted by “miRNA cluster”; (B) Subgroup analysis based on DOR sorted by “detailed stage or Ig isotype”. NA, not available.
Circulating miRNAs in MM, MGUS, and SMM
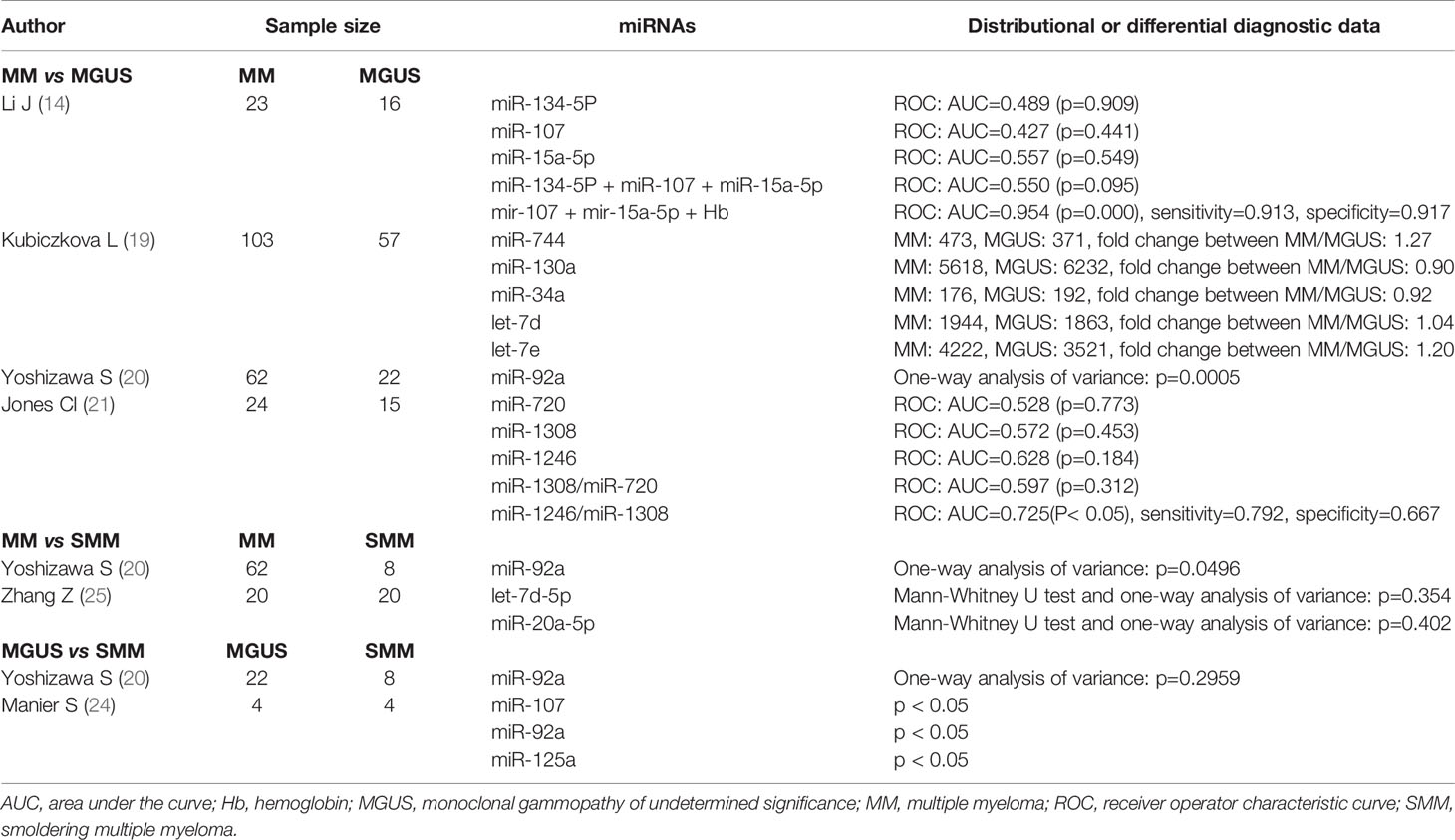
In order to investigate the ability of the included circulating miRNAs to discriminate MM from MGUS/SMM, we summarized their distributional and differential diagnostic data among those disease states. Between the MM and MGUS groups, the levels of miR-20, miR-15a, and miR-92a were significantly different. In ROC analysis, the individual miRNAs did not exhibit substantial discriminative performance, which would be improved by the combination of miRNAs and other clinical parameters. The combination of miR-107, miR-15a-5p, and hemoglobin gained the best differential performance with AUC = 0.954 (14), and the combination of miR-1246 and miR-1308 ranked second with AUC = 0.725 (21). For MM and SMM, the expression level of miR-92a was significantly different, but the differential diagnostic value remains to be verified (20). For MGUS and SMM, Manier et al. found significant differences in the expression levels of miR-107, miR-92a, and miR-125a in circulating exosomes (24); however, the included serum or plasma miRNAs have not shown any differential diagnostic value (Table 5).
Discussion
Although survival of MM patients have been improved with the rapid advances of therapeutic strategy and supportive care, myriad patients still suffer from relapsed/refractory MM, entailing a reliable biomarker for early diagnosis. The remarkable impact of miRNA on protein expression is emerging with the discovery that about one-third of human encoding genes are regulated by miRNAs (26). Pioneering studies have found a mass of specific miRNAs carried by circulating microparticles significantly distinct from their maternal cells, depicting an interesting transfer pathway for the gene-regulating function of miRNAs from microparticles releasing cells to the target cells (27).
MicroRNAs transfer between MM plasma cells and bone marrow microenvironment, enabling the development and metastasis of malignancy through messenger RNA destruction or translation inhibition (28, 29). Circulating microRNAs have been applied in hematological diseases as diagnostic biomarkers due to their reliable stability and non-invasive properties (30). In recent years, the important role of circulating miRNAs in MM diagnosis has received considerable attention; however, the results vary and leave questions open (10). We updated this meta-analysis to figure out whether the circulating miRNAs could be promising means for early detection of MM with the latest evidence.
The diagnostic performance of miRNAs differed in this meta-analysis; among the top three individual miRNAs, miR-4254 was the highest [DOR = 252, 95% CI = (18,3517)] followed by miR-125b-5p [DOR = 147, 95% CI = (11,2061)], then miR-720 [DOR = 82, 95% CI= (6,1142)], whereas miR-3658 was the lowest [DOR = 5, 95% CI = (0.36,70)]. The miRNA cluster exhibited better diagnostic performance in subgroup analysis, with the combined parameters (sensitivity, 0.92; specificity, 0.90; AUC, 0.96; DOR 109) being far beyond individual miRNAs (sensitivity, 0.77; specificity, 0.85; AUC, 0.84; DOR 19).
The pooled sensitivity, specificity, AUC, PLR, NLR, and DOR were 0.79, 0.86, 0.87, 5, 0.27, and 22, respectively, consistent with the previous meta-analysis (10). Summary LRP and LRN for Index Test suggest a small number of studies have relatively high value for diagnosis conformation and/or exclusion. The Fagan nomogram of post-test probability also indicated that circulating miRNAs were of relatively good diagnostic value but still had room for improvement. Researchers found that the tests based on serum or plasma did not show significant differences, nor between ethnic groups; and our meta-analysis confirmed it.
However, the quality assessment of literatures presents a few ignorable flaws and uncertainties, which are somewhat inconsistent with the results of the previous meta-analysis (10), possibly because of the different versions of evaluation tools or the strictness in our assessment. We found that some of the literatures did not specify the study design, patient information (stage/Ig isotype/treatment information), or the time interval between the reference standard and miRNA detection. Besides, the absence of suspected cases in some of the studies may lead to bias in evaluation.
The combined diagnostic power did not fluctuate significantly in sensitivity analysis. The DOR value only changed from 22 to 19 even when the study with the greatest deviation was eliminated. To dissociate the sources of heterogeneity, a publication bias assessment was performed and no publication bias was found (p = 0.73). Inevitably, the exclusion of non-English literatures may also lead to selection bias. However, significant differences were found in the “miRNA cluster” and “detailed stage or Ig isotype” subgroups through meta-regression with univariable model and multivariate model (p < 0.05), but not in the “cohort study” and “newly diagnosed and untreated” subgroups, which may also be influenced by the limited number of studies included in each subgroup. Besides, no significant differences were found in the “specimen” and “ethnicity” subgroups, consistent with previous studies. These results reveal that the study design and the enrolment of patients and healthy controls may have an impact on the diagnostic value of the index to be assessed, and a standardized recommendation is imperative.
At present, the efficacy of early treatment determined by the free light chain ratio for the precursor-stage of MM (MGUS or SMM) to improve longevity and health-related quality of life is still unclear (31, 32), and distinguishing symptomatic multiple myeloma from those conditions is of great importance. In our summary, not much distributional or differential diagnostic evidence of circulating miRNAs in MM, MGUS and SMM were found. Although miRNA expression levels differed significantly, no individual miRNA exhibited excellent differential diagnostic ability, indicating that this area needs to be further explored with more meticulous design for subject enrolment. Moreover, a variety of diseases such as inflammation (33), cardiovascular diseases (34), or other non-cancerous illnesses (35) may also alter miRNA profile and level, which should be taken into account by researchers when setting up control groups.
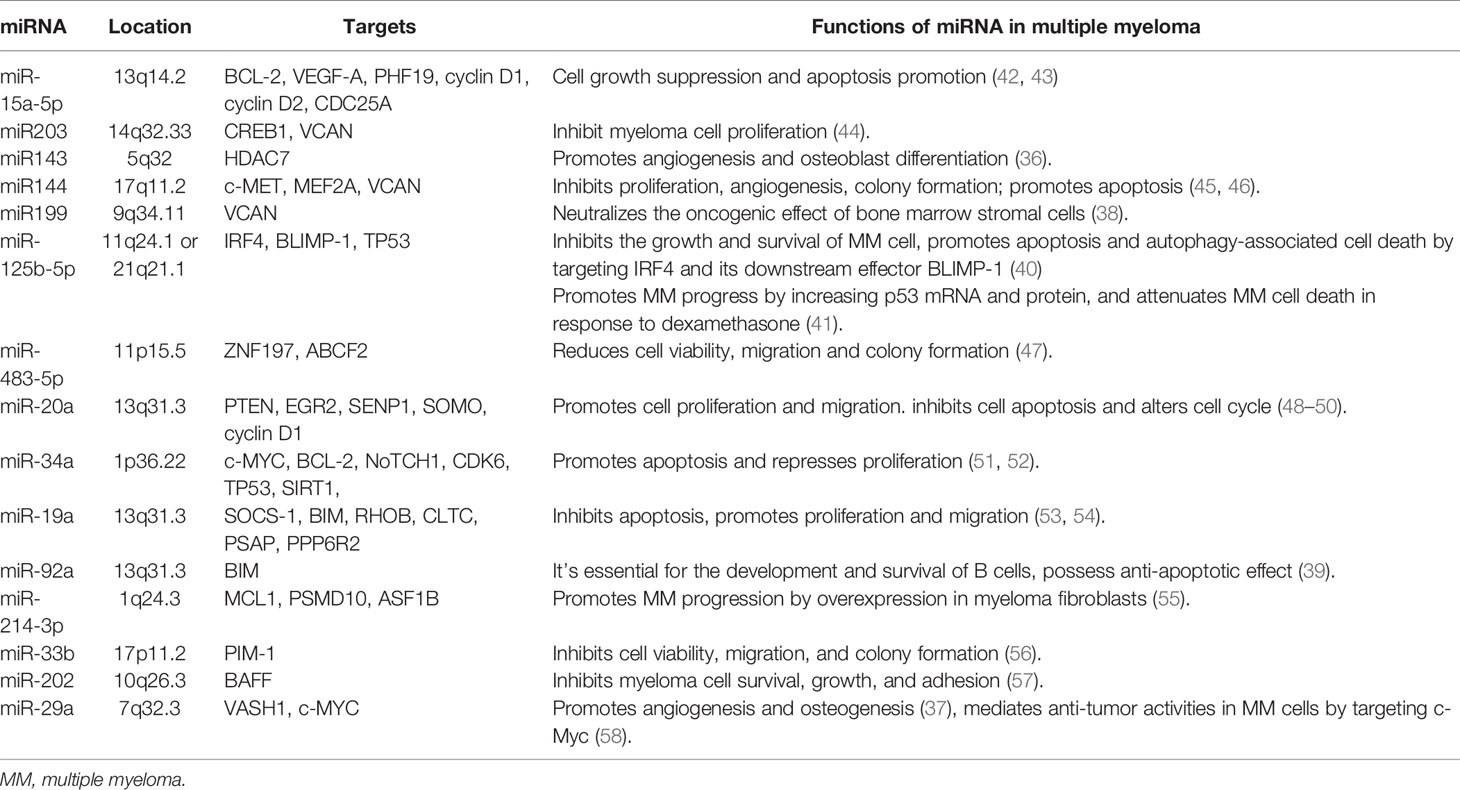
The past decade has seen remarkable achievements in the understanding of miRNAs in MM, including their various targets, effects, and dysregulation modes in disease development and progression. Some miRNAs, such as miR-20a, miR-19a, miR-92a, and miR-214-3p act as oncomiR playing important roles in anti-apoptosis, proliferation, migration, and invasion. Other miRNAs, including miR-15a-5p, miR203, miR144, miR199, miR-483-5p, miR-34a, miR-33b, miR-202, and miR-29a act as tumor suppressors. Also, some miRNAs are involved in the development of bone marrow microenvironment in MM; for example, miR143 and miR-29a promote angiogenesis and osteoblast differentiation (36, 37), miR199 neutralizes the oncogenic effect of bone marrow stromal cells (38), and miR-92a is essential for B cell development (39). These results provide valuable resources for the investigation of the etiology and treatment methods for MM. Interestingly, the role of miR-125b-5p in MM is still controversial since it has the ability to inhibit the growth and survival of MM cell and promote apoptosis and autophagy-associated cell death by targeting IRF4 and its downstream effector BLIMP-1 (40), but miR-125b-5p may generate counterproductive effects through different target genes or signaling pathways; it promotes MM progress by increasing p53 mRNA and protein and attenuating MM cell death in response to dexamethasone (41). Further exploration of miRNAs may enrich our perspective. More details are listed in Table 6.
Due to the important role of miRNAs in the tumorigenesis and progression of MM, circulating miRNAs have also shown their prognostic ability in MM, expanding their clinical application for identifying high-risk MM patients. As shown in Table 7, several studies have demonstrated that patients with different levels of miR-483-5p, miR-744, let-7e, miR-19a, miR-92a, miR-33b, miR-214, or miR-20a appeared with quite different survival rates.
More interestingly, the expression levels of miR-19a, miR-744-5p, miR-143-5p, and miR-92a, had significantly different outcomes in bortezomib-based treatment (22, 65–67). Patients with higher serum levels of miR-214 had extended overall survival upon bisphosphonate-based therapy (68). In patients with autologous stem-cell transplantation, the lower level of miR-15a on day 0 was associated with the shorter time to engraftment, and miR-20a decreased at complete remission (69, 70). These results demonstrate that the expression patterns of circulating microRNAs are valuable markers for predicting treatment response. See Table 8 for details.
Strength and Limitations
The main strengths of this meta-analysis are the follows: 1) this is the first time that “cohort study design”, patient “stage or Ig isotype” and “newly diagnosed or untreated” information of MM patients were included in the subgroup analysis, summarizing the possible influencing factors of the current results; 2) it uses extensive but rigorous search strategies to optimize the quality of included literature; 3) it is stricter in assessing the quality of the included literature, providing a more detailed summary of included patients and healthy donors; 4) it structures a comprehensive review of the current understanding of circulating miRNAs in multiple myeloma, including the value of diagnosis, differential diagnosis, prognosis, and therapy-guiding. The major limitations are the following: 1) due to the insufficiency of miRNA studies included, the pooled diagnostic power of miRNA may be of some deviation, and the results of the subgroup analysis may also be biased to some extent; 2) some miRNAs are also dysregulated in other hematologic diseases; for example, miR-143, miR-144, and miR-199 are also under-expressed in child acute lymphoblastic leukemia, suggesting that circulating miRNAs may not be independent diagnostic biomarkers of MM, but can only be used as auxiliary and discriminatory diagnostic biomarkers (74).
Recommendations
For diagnostic markers, randomized controlled trials are hard to achieve due to the limitations on patient choice and the invasiveness of the gold standard test; many studies have adopted case–control study designs. However, the reproducibility of miRNA-based studies in the diagnosis of MM is conducive to the feasibility of clinical application. Optimizing the flow of miRNA diagnostic tests according to the following suggestions would enable researchers to conduct systematic reviews and/or meta-analyses and draw more practical conclusions.
Patient Selection
“newly diagnosed or untreated patient” should be regarded as one of the criteria for the inclusion of diagnostic test, for circulating miRNA profile and level may change due to treatment. Pathological stage or IG isotype information of MM patients should be provided in detail, since the progression and Ig isotype of MM may affect circulating miRNA expression.
Study Design
Case–control study should not be perceived as a valid design for investigating the diagnostic value of miRNAs in MM patients. A cohort study may be a more applicable design initially, owing to the limited source of patients. Randomized controlled trials could be taken into consideration after the diagnostic power has been qualified.
Control Groups
To attenuate selection bias and avoid overestimation of diagnostic value, researchers should consider setting up control groups for suspected cases, individuals with MM precursor state, and patients with other conditions (such as inflammation, cardiovascular disease, or other non-cancerous conditions) separately, rather than just healthy controls. It was reported that about 28.6% of MM patients were diagnosed at the age of 65–74 years, and about 3.5% were under 44 (75). Besides, the incidence of MM is more prevalent in black race than in white, and higher in males than in females (76). Hence, the composition of age, ethnicity and gender of the control group should be consistent with that of the experimental group.
Sample Size
A sufficient sample size should be rigorously calculated in advance and can be achieved through collaboration between specialized institutions.
Specimen and Storage
Based on the long-term storage stability, circulating miRNAs have reliable performance as biomarkers. Both serum and plasma samples can be used to detect circulating miRNAs since there is no significant difference between serum or plasma-based tests. The researchers found that preservation at −20°C barely influenced the total amount of miRNAs for at least 2–4 years, with only slight changes in the concentration of individual miRNAs; in addition, storage at −80°C is even better (77).
Reference Standard
There are two widely used diagnostic criteria for patients with multiple myeloma and MUGS, one from the National Comprehensive Cancer Network (NCCN) and the other from the International Myeloma Working Group (IMWG) (78, 79). To avoid ambiguity and bias, researchers should clearly define the reference criteria.
Experimental Flow and Result Interpretation
Strict concealment measures should be observed throughout the study, including grouping, detection, and interpretation of test results. In addition the instruments, reagents, operating procedures, and the cutoff value of the test should be determined before the validation process and be detailed in the paper to avoid subjective bias and to ensure reproducibility. Except for the data on sensitivity, specificity and AUC, the direct presentation of true positive (TP), false positive (FP), true negative (TN), and false negative (FN) data would be of great benefit for future systematic evaluation.
Conclusion
Through the unremitting efforts of researchers, miRNAs have been confirmed to be implicated in many pathophysiological processes of MM; however, the exact regulation mechanism remains to be fully elucidated. Much attention has been given to the diagnostic value of circulating miRNAs in MM over the past decade. This meta-analysis reveals that miR-4254 has the best potential to be a biomarker for MM diagnosis, and miRNA cluster might be a good choice to optimize the utilization. Successfully unraveling the diagnostic value of circulating miRNAs in MM will depend on multicenter large-scale studies with rigorous process design and a broad enrolment in patient and control groups.
Data Availability Statement
The original data presented in the study were derived from the included article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
JZ designed the study, assessed the quality of the manuscript. YX and PX were responsible for study searching, data extraction and meta-analysis implementation. LZ reviewed the results of each step and resolved any differences in the evaluation opinions of other authors. YX, PX, and LZ co-drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project was supported by Youth Foundation of National Natural Science Foundation of China (81802075/H2003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We sincerely appreciate the efforts of researchers and volunteers in investigating the application of miRNAs in the diagnosis of multiple myeloma.
References
1. Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV, et al. Multiple Myeloma. Nat Rev Dis Primers (2017) 3:17046. doi: 10.1038/nrdp.2017.46
2. Kyle R, Larson D, Therneau T, Dispenzieri A, Kumar S, Cerhan J, et al. Long-Term Follow-Up of Monoclonal Gammopathy of Undetermined Significance. N Engl J Med (2018) 378:241–9. doi: 10.1056/NEJMoa1709974
3. Capp JP, Bataille R. Multiple Myeloma Exemplifies a Model of Cancer Based on Tissue Disruption as the Initiator Event. Front Oncol (2018) 8:355(6). doi: 10.3389/fonc.2018.00355
4. Kyle R, Durie B, Rajkumar S, Landgren O, Blade J, Merlini G, et al. Monoclonal Gammopathy of Undetermined Significance (MGUS) and Smoldering (Asymptomatic) Multiple Myeloma: IMWG Consensus Perspectives Risk Factors for Progression and Guidelines for Monitoring and Management. Leukemia (2010) 24(6):1121–7. doi: 10.1038/leu.2010.60
5. He Y, Wheatley K, Glasmacher A, Ross H, Djulbegovic B. Early Versus Deferred Treatment for Early Stage Multiple Myeloma. Cochrane Database Syst Rev (2003) 2003(1):CD004023. doi: 10.1002/14651858.CD004023
6. Handa H, Murakami Y, Ishihara R, Kimura-Masuda K, Masuda Y. The Role and Function of microRNA in the Pathogenesis of Multiple Myeloma. Cancers (2019) 11(11):1738. doi: 10.3390/cancers11111738
7. Che F, Chen J, Wan C, Huang X. MicroRNA-27 Inhibits Autophagy and Promotes Proliferation of Multiple Myeloma Cells by Targeting the NEDD4/Notch1 Axis. Front Oncol (2020) 10:571914. doi: 10.3389/fonc.2020.571914
8. D’Angelo B, Benedetti E, Cimini A, Giordano A. MicroRNAs: A Puzzling Tool in Cancer Diagnostics and Therapy. Anticancer Res (2016) 36(11):5571–75. doi: 10.21873/anticanres.11142
9. Zhang L, Cao D, Tang L, Sun C, Hu Y. A Panel of Circulating miRNAs as Diagnostic Biomarkers for Screening Multiple Myeloma: A Systematic Review and Meta-Analysis. Int J Lab Hematol (2016) 38(6):589–99. doi: 10.1111/ijlh.12560
10. Gao S-S, Wang Y-J, Zhang G-X, Zhang W-T. Potential Diagnostic Value of Circulating miRNA for Multiple Myeloma: A Meta-Analysis. J Bone Oncol (2020) 25:100327. doi: 10.1016/j.jbo.2020.100327
11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
12. Li F, Xu Y, Deng S, Li Z, Zou D, Yi S, et al. MicroRNA-15a/16-1 Cluster Located at Chromosome 13q14 is Down-Regulated But Displays Different Expression Pattern and Prognostic Significance in Multiple Myeloma. Oncotarget (2015) 6(35):38270–82. doi: 10.18632/oncotarget.5681
13. Yu J, Qiu X, Shen X, Shi W, Wu X, Gu G, et al. miR-202 Expression Concentration and its Clinical Significance in the Serum of Multiple Myeloma Patients. Ann Clin Biochem (2014) 51(Pt 5):543–9. doi: 10.1177/0004563213501155
14. Li J, Zhang M, Wang C. Circulating miRNAs as Diagnostic Biomarkers for Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance. J Clin Lab Anal (2020) 34(6):e23233. doi: 10.1002/jcla.23233
15. Gupta N, Kumar R, Seth T, Garg B, Sati HC, Sharma A. Clinical Significance of Circulatory microRNA-203 in Serum as Novel Potential Diagnostic Marker for Multiple Myeloma. J Cancer Res Clin Oncol (2019) 145(6):1601–11. doi: 10.1007/s00432-019-02896-1
16. Shen X, Ye Y, Qi J, Shi W, Wu X, Ni H, et al. Identification of a Novel microRNA, miR-4449, as a Potential Blood Based Marker in Multiple Myeloma. Clin Chem Lab Med (2017) 55(5):748–54. doi: 10.1515/cclm-2015-1108
17. Jiang Y, Luan Y, Chang H, Chen G. The Diagnostic and Prognostic Value of Plasma microRNA-125b-5p in Patients With Multiple Myeloma. Oncol Lett (2018) 16(3):4001–7. doi: 10.3892/ol.2018.9128
18. Qu X, Zhao M, Wu S, Yu W, Xu J, Xu J, et al. Circulating microRNA 483-5p as a Novel Biomarker for Diagnosis Survival Prediction in Multiple Myeloma. Med Oncol Northwood Lond Engl (2014) 31(10):219. doi: 10.1007/s12032-014-0219-x
19. Kubiczkova L, Kryukov F, Slaby O, Dementyeva E, Jarkovsky J, Nekvindova J, et al. Circulating Serum microRNAs as Novel Diagnostic and Prognostic Biomarkers for Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance. Haematologica (2014) 99(3):511–18. doi: 10.3324/haematol.2013.093500
20. Yoshizawa S, Ohyashiki JH, Ohyashiki M, Umezu T, Suzuki K, Inagaki A, et al. Downregulated Plasma miR-92a Levels Have Clinical Impact on Multiple Myeloma and Related Disorders. Blood Cancer J (2012) 2(1):e53. doi: 10.1038/bcj.2011.51
21. Jones CI, Zabolotskaya MV, King AJ, Stewart HJS, Horne GA, Chevassut TJ, et al. Identification of Circulating microRNAs as Diagnostic Biomarkers for Use in Multiple Myeloma. Br J Cancer (2012) 107(12):1987–96. doi: 10.1038/bjc.2012.525
22. Hao M, Zang M, Wendlandt E, Xu Y, An G, Gong D, et al. Low Serum miR-19a Expression as a Novel Poor Prognostic Indicator in Multiple Myeloma. Int J Cancer (2015) 136(8):1835–44. doi: 10.1002/ijc.29199
23. Sevcikova S, Kubiczkova L, Sedlarikova L, Slaby O, Hajek R. Serum miR-29a as a Marker of Multiple Myeloma. Leuk Lymphoma (2013) 54:189–91. doi: 10.3109/10428194.2012.704030
24. Manier S, Boswell E, Sacco A, Maiso P, Banwait R, Aljawai Y, et al. Comparative miRNA Expression Profiling of Circulating Exosomes From MGUS and Smoldering Multiple Myeloma Patients. Blood (2012) 120:3975. doi: 10.1182/blood.V120.21.3975.3975
25. Zhang ZY, Li YC, Geng CY, Zhou HX, Gao W, Chen WM. Serum Exosomal microRNAs as Novel Biomarkers for Multiple Myeloma. Hematol Oncol (2019) 37(4):409–17. doi: 10.1002/hon.2639
26. Lewis BP, Burge CB, Bartel DP. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates That Thousands of Human Genes are MicroRNA Targets. Cell (2005) 120(1):15–20. doi: 10.1016/j.cell.2004.12.035
27. Diehl P, Fricke A, Sander L, Stamm J, Bassler N, Htun N, et al. Microparticles: Major Transport Vehicles for Distinct microRNAs in Circulation. Cardiovasc Res (2012) 93:633–44. doi: 10.1093/cvr/cvs007
28. Imai Y. Latest Development in Multiple Myeloma. Cancers (2020) 12(9):2544. doi: 10.3390/cancers12092544
29. Soley L, Falank C, Reagan MR. MicroRNA Transfer Between Bone Marrow Adipose and Multiple Myeloma Cells. Curr Osteoporos Rep (2017) 15(3):162–70. doi: 10.1007/s11914-017-0360-5
30. Grasedieck S, Sorrentino A, Langer C, Buske C, Döhner H, Mertens D, et al. Circulating microRNAs in Hematological Diseases: Principles, Challenges, and Perspectives. Blood (2013) 121:4977–84. doi: 10.1182/blood-2013-01-480079
31. Li X, Liu J, Chen M, Gu J, Huang B, Zheng D, et al. Health-Related Quality of Life of Patients With Multiple Myeloma: A Real-World Study in China. Cancer Med (2020) 9(21):7896–913. doi: 10.1002/cam4.3391
32. Goodman AM, Kim MS, Prasad V. Persistent Challenges With Treating Multiple Myeloma Early. Blood (2020) 137(4):456–8. doi: 10.1182/blood.2020009752
33. Vajen T, Mause SF, Koenen RR. Microvesicles From Platelets: Novel Drivers of Vascular Inflammation. Thromb Haemost (2015) 114(2):228–36. doi: 10.1160/TH14-11-0962
34. Guo M, Li R, Yang L, Zhu Q, Han M, Chen Z, et al. Evaluation of Exosomal miRNAs as Potential Diagnostic Biomarkers for Acute Myocardial Infarction Using Next-Generation Sequencing. Ann Transl Med (2021) 9(3):219. doi: 10.21037/atm-20-2337
35. Hulsmans M, Holvoet P. MicroRNA-Containing Microvesicles Regulating Inflammation in Association With Atherosclerotic Disease. Cardiovasc Res (2013) 100(1):7–18. doi: 10.1093/cvr/cvt161
36. Wang R, Zhang H, Ding W, Fan Z, Ji B, Ding C, et al. miR-143 Promotes Angiogenesis and Osteoblast Differentiation by Targeting Hdac7. Cell Death Dis (2020) 11:179. doi: 10.1038/s41419-020-2377-4
37. Lu G, Cheng P, Liu T, Wang Z. BMSC-Derived Exosomal miR-29a Promotes Angiogenesis and Osteogenesis. Front Cell Dev Biol (2020) 8:608521. doi: 10.3389/fcell.2020.608521
38. Gupta N, Kumar R, Seth T, Garg B, Sharma A. Targeting of Stromal Versican by miR-144/199 Inhibits Multiple Myeloma by Downregulating FAK/STAT3 Signalling. RNA Biol (2020) 17(1):98–111. doi: 10.1080/15476286.2019.1669405
39. Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, et al. Targeted Deletion Reveals Essential and Overlapping Functions of the miR-17 Through 92 Family of miRNA Clusters. Cell (2008) 132(5):875–86. doi: 10.1016/j.cell.2008.02.019
40. Morelli E, Leone E, Cantafio M, Di Martino MT, Amodio N, Biamonte L, et al. Selective Targeting of IRF4 by Synthetic microRNA-125b-5p Mimics Induces Anti-Multiple Myeloma Activity. Vitro Vivo Leukemia (2015) 29(11):2173–83. doi: 10.1038/leu.2015.124
41. Murray MY, Rushworth SA, Zaitseva L, Bowles KM, MacEwan DJ. Attenuation of Dexamethasone-Induced Cell Death in Multiple Myeloma is Mediated by miR-125b Expression. Cell Cycle (2013) 12(13):2144–53. doi: 10.4161/cc.25251
42. Hao M, Zhang L, An G, Meng H, Han Y, Xie Z, et al. Bone Marrow Stromal Cells Protect Myeloma Cells From Bortezomib Induced Apoptosis by Suppressing microRNA-15a Expression. Leuk Lymphoma (2011) 52(9):1787–94. doi: 10.3109/10428194.2011.576791
43. Li Z, Liu L, Du C, Yu Z, Yang Y, Xu J, et al. Therapeutic Effects of Oligo-Single-Stranded DNA Mimicking of hsa-miR-15a-5p on Multiple Myeloma. Cancer Gene Ther (2020) 27(12):1–9. doi: 10.1038/s41417-020-0161-3
44. Wong KY, Liang R, So CC, Jin DY, Costello JF, Chim CS. Epigenetic Silencing of MIR203 in Multiple Myeloma. Br J Haematol (2011) 154(5):569–78. doi: 10.1111/j.1365-2141.2011.08782.x
45. Zhao Y, Xie Z, Lin J, Liu P. MiR-144-3p Inhibits Cell Proliferation and Induces Apoptosis in Multiple Myeloma by Targeting C-Met. Am J Transl Res (2017) 9(5):2437–46.
46. Tian F, Wang H, Ma H, Zhong Y, Liao A. mIR−144−3p Inhibits the Proliferation, Migration and Angiogenesis of Multiple Myeloma Cells by Targeting Myocyte Enhancer Factor 2a. Int J Mol Med (2020) 46(3):1155–65. doi: 10.3892/ijmm.2020.4670
47. Bi C, Chung TH, Huang G, Zhou J, Yan J, Ahmann G, et al. Genome-Wide Pharmacologic Unmasking Identifies Tumor Suppressive microRNAs in Multiple Myeloma. Oncotarget (2015) 6(28):26508–18. doi: 10.18632/oncotarget.4769
48. Yuan J, Su Z, Gu W, Shen X, Zhao Q, Shi L, et al. MiR-19b and miR-20a Suppress Apoptosis, Promote Proliferation and Induce Tumorigenicity of Multiple Myeloma Cells by Targeting PTEN. Cancer Biomark (2019) 24(3):1–11. doi: 10.3233/CBM-182182
49. Wang T, Tao W, Zhang L, Li S. Oncogenic Role of microRNA-20a in Human Multiple Myeloma. OncoTargets Ther (2017) 10:4465–74. doi: 10.2147/OTT.S143612
50. Zhang Z, Zhou H, Geng C, Gao W, Chen W. Aberrant Expression of Mir-20a in Serum Exosomes of Multiple Myeloma Lead Abnormal Expression of HIF-1 Signaling Pathway Related Proteins. Blood (2020) 136:43. doi: 10.1182/blood-2020-141182
51. Xiao X, Gu Y, Wang G, Chen S. C-Myc, RMRP, and miR-34a-5p Form a Positive-Feedback Loop to Regulate Cell Proliferation and Apoptosis in Multiple Myeloma. Int J Biol Macromol (2019) 122:526–37. doi: 10.1016/j.ijbiomac.2018.10.207
52. Di Martino MT, Leone E, Amodio N, Foresta U, Lionetti M, Pitari MR, et al. Synthetic miR-34a Mimics as a Novel Therapeutic Agent for Multiple Myeloma: In Vitro and In Vivo Evidence. Clin Cancer Res (2012) 18(22):6260–70. doi: 10.1158/1078-0432.CCR-12-1708
53. Pichiorri F, Suh S-S, Ladetto M, Kuehl M, Palumbo T, Drandi D, et al. MicroRNAs Regulate Critical Genes Associated With Multiple Myeloma Pathogenesis. Proc Natl Acad Sci USA (2008) 105(35):12885–90. doi: 10.1073/pnas.0806202105
54. Lv H, Wu X, Ma G, Sun L, Meng J, Song X, et al. An Integrated Bioinformatical Analysis of Mir−19a Target Genes in Multiple Myeloma. Exp Ther Med (2017) 14(5):4711–20. doi: 10.3892/etm.2017.5173
55. Frassanito MA, Desantis V, Di Marzo L, Craparotta I, Beltrame L, Marchini S, et al. Bone Marrow Fibroblasts Overexpress miR-27b and miR-214 in Step With Multiple Myeloma Progression, Dependent on Tumour Cell-Derived Exosomes. J Pathol (2019) 247(2):241–53. doi: 10.1002/path.5187
56. Tian Z, Zhao J-J, Lin J, Chauhan D, Anderson KC. Investigational Agent MLN9708 Target Tumor Suppressor MicroRNA-33b in Multiple Myeloma Cells. Blood (2011) 118(21):136. doi: 10.1182/blood.V118.21.136.136
57. Shen X, Guo Y, Yu J, Qi J, Shi W, Wu X, et al. miRNA-202 in Bone Marrow Stromal Cells Affects the Growth and Adhesion of Multiple Myeloma Cells by Regulating B Cell-Activating Factor. Clin Exp Med (2016) 16(3):307–16. doi: 10.1007/s10238-015-0355-4
58. Saha M, Chen Y, Abdi J, Chang H. Mir-29a Displays in Vitro and in Vivo Anti-Tumor Activity in Multiple Myeloma. Blood (2014) 124:2090. doi: 10.1182/blood.V124.21.2090.2090
59. Yoshizawa S, Umezu T, Ohyashiki J, Iida S, Ohyashiki K. Lower Plasma Mir-92a Levels Predict Shorter Progression-Free Survival In Newly Diagnosed Symptomatic Multiple Myeloma Patients. Blood (2013) 122(21):1879. doi: 10.1182/blood.V122.21.1879.1879
60. Hao M, Zang M, Yu Q, Fei L, Yang W, Feng X, et al. Serum Mir-4254, Mir-19a and Mir-33b Are Potential Markers For Diagnosis and Prognostic Evaluation In Multiple Myeloma. Blood (2013) 122(21):3222. doi: 10.1182/blood.V122.21.3222.3222
61. Hao M, Zang M, Zhao L, Deng S, Xu Y, Qi F, et al. Serum High Expression of miR-214 and miR-135b as Novel Predictor for Myeloma Bone Disease Development and Prognosis. Oncotarget (2016) 7(15):19589–600. doi: 10.18632/oncotarget.7319
62. Huang J, Yu J, Li J, Liu Y, Zhong R. Circulating microRNA Expression is Associated With Genetic Subtype and Survival of Multiple Myeloma. Med Oncol Northwood Lond Engl (2012) 29(4):2402–8. doi: 10.1007/s12032-012-0210-3
63. Ren Y, Li X, Wang W, He W, Wang J, Wang Y. Expression of Peripheral Blood miRNA-720 and miRNA-1246 Can Be Used as a Predictor for Outcome in Multiple Myeloma Patients. Clin Lymphoma Myeloma Leuk (2017) 17(7):415–23. doi: 10.1016/j.clml.2017.05.010
64. Rocci A, Hofmeister CC, Geyer S, Stiff A, Gambella M, Cascione L, et al. Circulating miRNA Markers Show Promise as New Prognosticators for Multiple Myeloma. Leukemia (2014) 28(9):1922–6. doi: 10.1038/leu.2014.155
65. Robak P, Drozdz I, Jarych D, Mikulski D, Weglowska E, Siemieniuk-Rys M, et al. The Value of Serum MicroRNA Expression Signature in Predicting Refractoriness to Bortezomib-Based Therapy in Multiple Myeloma Patients. Cancers (2020) 12(9):2569. doi: 10.3390/cancers12092569
66. Narita T, Ri M, Kinoshita S, Yoshida T, Totani H, Ashour R, et al. Identification of Circulating Serum microRNAs as Novel Biomarkers Predicting Disease Progression and Sensitivity to Bortezomib Treatment in Multiple Myeloma. Blood (2016) 128(22):4408. doi: 10.1182/blood.V128.22.4408.4408
67. Yoshizawa S, Ohyashiki JH, Ohyashiki M, Umezu T, Suzuki K, Inagaki A, et al. Plasma Mir-92a Levels in Multiple Myeloma Correlate With T-Cell-Derived Mir-92a and Restored in Bortezomib Responder. Blood (2011) 118(21):2871. doi: 10.1182/blood.V118.21.2871.2871
68. Hao M, Zang M, Xu Y, Qin Y, Zhao L, Deng S, et al. Serum High Expression of Micorna-214 Is a Novel Predictor for Myeloma Bone Disease and Poor Prognosis. Blood (2015) 126(23):4186. doi: 10.1182/blood.V126.23.4186.4186
69. Nowicki M, Szemraj J, Wierzbowska A, Misiewicz M, Małachowski R, Pluta A, et al. miRNA-15a, miRNA-16, miRNA-126, miRNA-146a, and miRNA-223 Expressions in Autologous Hematopoietic Stem Cell Transplantation and Their Impact on Engraftment. Eur J Haematol (2018) 100(5):426–35. doi: 10.1111/ejh.13036
70. Navarro A, Díaz T, Tovar N, Pedrosa F, Tejero R, Cibeira MT, et al. A Serum microRNA Signature Associated With Complete Remission and Progression After Autologous Stem-Cell Transplantation in Patients With Multiple Myeloma. Oncotarget (2015) 6(3):1874–83. doi: 10.18632/oncotarget.2761
71. Jung SH, Lee SE, Lee M, Kim SH, Yim SH, Kim TW, et al. Circulating microRNA Expressions can Predict the Outcome of Lenalidomide Plus Low-Dose Dexamethasone Treatment in Patients With Refractory/Relapsed Multiple Myeloma. Haematologica (2017) 102(11):e456–9. doi: 10.3324/haematol.2017.168070
72. Jasielec JK, Chen JL, Rosebeck S, Dytfeld D, Griffith K, Alonge M, et al. Microrna (miRNA) Expression Profiling in Patients With Newly Diagnosed Multiple Myeloma (NDMM) Treated With Carfilzomib, Lenalidomide, and Dexamethasone (CRD). Haematologica (2014) 99(S1):366.
73. Manier S, Liu C-J, Avet-Loiseau H, Park J, Shi J, Campigotto F, et al. Prognostic Role of Circulating Exosomal miRNAs in Multiple Myeloma. Blood (2017) 129:2429–36. doi: 10.1182/blood-2016-09-742296
74. Rashed WM, Hammad AM, Saad AM, Shohdy KS. MicroRNA as a Diagnostic Biomarker in Childhood Acute Lymphoblastic Leukemia; Systematic Review, Meta-Analysis and Recommendations. Crit Rev Oncol Hematol (2019) 136:70–8. doi: 10.1016/j.critrevonc.2019.02.008
75. Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 Patients With Newly Diagnosed Multiple Myeloma. Mayo Clin Proc (2003) 78:21–33. doi: 10.4065/78.1.21
76. Waxman AJ, Mink PJ, Devesa SS, Anderson WF, Weiss BM, Kristinsson SY, et al. Racial Disparities in Incidence and Outcome in Multiple Myeloma: A Population-Based Study. Blood (2010) 116(1):5501–6. doi: 10.1182/blood-2010-07-298760
77. Grasedieck S, Schöler N, Bommer M, Niess JH, Tumani H, Rouhi A, et al. Impact of Serum Storage Conditions on microRNA Stability. Leukemia (2012) 26(11):2414–6. doi: 10.1038/leu.2012.106
78. Kumar SK, Callander NS, Adekola K, Anderson L, Baljevic M, Campagnaro E, et al. Multiple Myeloma, Version 3.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2020) 18(12):1685–717. doi: 10.6004/jnccn.2020.0057
Keywords: biomarker, diagnosis, meta-analysis, microRNAs, multiple myeloma
Citation: Xiang Y, Zhang L, Xiang P and Zhang J (2021) Circulating miRNAs as Auxiliary Diagnostic Biomarkers for Multiple Myeloma: A Systematic Review, Meta-Analysis, and Recommendations. Front. Oncol. 11:698197. doi: 10.3389/fonc.2021.698197
Received: 20 April 2021; Accepted: 22 June 2021;
Published: 08 July 2021.
Edited by:
J. Luis Espinoza, Kanazawa University, JapanReviewed by:
Purvi Purohit, All India Institute of Medical Sciences Jodhpur, IndiaShohei Mizuno, Aichi Medical University, Japan
Copyright © 2021 Xiang, Zhang, Xiang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Zhang, emhhbmdqdWFuODJAMTI2LmNvbQ==
 Yunhui Xiang
Yunhui Xiang Liuyun Zhang
Liuyun Zhang Pinpin Xiang
Pinpin Xiang Juan Zhang
Juan Zhang