- 1Musculoskeletal Tumor Center, Peking University People’s Hospital, Beijing, China
- 2Orthopedic Oncology, Xijing Hospital Air Force Medical University of PLA (The Fourth Military Medical University), Xi’an, China
- 3Medical Oncology, Shanghai Sixth People’s Hospital, Shanghai, China
- 4Orthopedic Oncology, Qilu Hospital of Shandong University, Jinan, China
- 5Musculoskeletal Tumor Center, The First Affiliated Hospital of Fujian Medical University, Fuzhou, China
- 6Orthopedic Oncology, Shanghai Changzheng Hospital, Shanghai, China
- 7Orthopedic Oncology, Jinan Military General Hospital, Jinan, China
- 8Orthopedic Oncology, Ruijin Hospital Affiliated to Shanghai Jiaotong University, Shanghai, China
- 9Orthopedic Oncology, Shanghai General Hospital, Shanghai, China
- 10Orthopedic Oncology and Medical Oncology, Wuhan Union Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China
- 11Medical Oncology, The First Affiliated Hospital of Jilin University, Changchun, China
- 12Orthopedic Oncology, General Hospital of Eastern Theater Command, Nanjing, China
- 13Orthopedic Oncology, Huaxi Hospital West China School of Medicine/West China Hospital of Sichuan University (WCSM/WCH), Chengdu, China
- 14Musculoskeletal Tumor Center, Liaoning Cancer Hospital & Institute, Shenyang, China
Four prospective trials have reported apatinib-related efficacy in osteosarcoma, with a high response rate of 43.2%. Currently, Adverse Events (AEs) have increasingly gained attention, as treatment with multiple tyrosine kinase inhibitors (TKIs) is potentially lifelong. For this reason, a consensus meeting of the Chinese Sarcoma Study Group (CSSG), which is a multidisciplinary panel composed of pediatric, medical and surgical oncologists specializing in sarcoma, nurse specialists, oncological senior pharmacists and gastroenterologists, was held to develop comprehensive guidelines on AEs emerging due to apatinib treatment to better assist in the prevention, management, and understanding of AE development. We summarized all AEs that arose in ≥10% of the participants as well as rare AEs that required extra caution to prevent that were observed in these four published prospective trials and arranged these AEs into 14 disorder systems according to CTCAE 5.0. In this review, we discuss strategies for the management of AEs in patients with advanced osteosarcoma, with the aim of maximizing treatment benefits and minimizing the need for apatinib treatment discontinuation. We also focus on providing recommendations for the prophylaxis and treatment of advanced osteosarcoma using apatinib to achieve optimal outcomes.
Introduction
Osteosarcoma has a five-year overall survival of 71% (95% CI: 68%−73%) based on the European and American Osteosarcoma Study (EURAMOS-1) trial (1–3). However, treatment options for chemotherapy refractory cases remain limited, and prognosis is dismal. Tyrosine kinase inhibitors (TKIs) targeting angiogenesis, such as vascular endothelial growth factor receptors (VEGFRs), have been verified to be helpful in prolonging the progression-free survival (PFS) of advanced osteosarcoma patients who progressed on chemotherapy (4, 5). Apatinib, a specific TKI that mainly targets VEGFR-2, has been investigated in patients with advanced osteosarcoma (6). The combination of apatinib with camrelizumab, a high-affinity, humanized,IgG4-κ PD-1 monoclonal antibody (7), has resulted in the prolongation of PFS and two durable responses (8).
However, more than half of our enrolled patients had a dose reduction or temporal interruption due to toxicity (6, 7, 9, 10). In real-world practice, at least a quarter of patients change their TKIs at least once during their lifetime because of drug intolerance (11–13). Although the tumor burden can be significantly reduced by apatinib, the quality of life (QoL) of patients was not improved, which seems to place apatinib in the same situations as sorafenib, sunitinib, regorafenib, and pazopanib (14–17). The need for the continuous daily use of apatinib until secondary resistance is hampered by the associated long-term adverse events (AEs) and the resulting decrease in QoL. The attention given by the Chinese Sarcoma Study Group (CSSG) to AEs has grown in the past few years, but our understanding remains poor. Furthermore, we noticed that publications on the prevention and management of apatinib-related AEs were limited (18, 19). Thus, the CSSG working party asked authors LX, JX and WG to convene a group of physicians or medical workers who had previously published and/or taken part in apatinib-related trials or frequently used this drug in the clinic and reported their findings at local meetings. These people were asked to retrospectively investigate all valuable methods in relieving the toxicities of apatinib and to provide medical advice on when apatinib is the appropriate treatment regimen and when it should be avoided. This publication provides a consensus on the use of apatinib based on discussions by email, as well as discussions at a meeting held in Dalian, China on August 30, 2019. Here, we review the tolerability profiles of apatinib during the treatment of advanced osteosarcoma, mainly focusing on AE management strategies.
AEs During Apatinib-Related Therapy
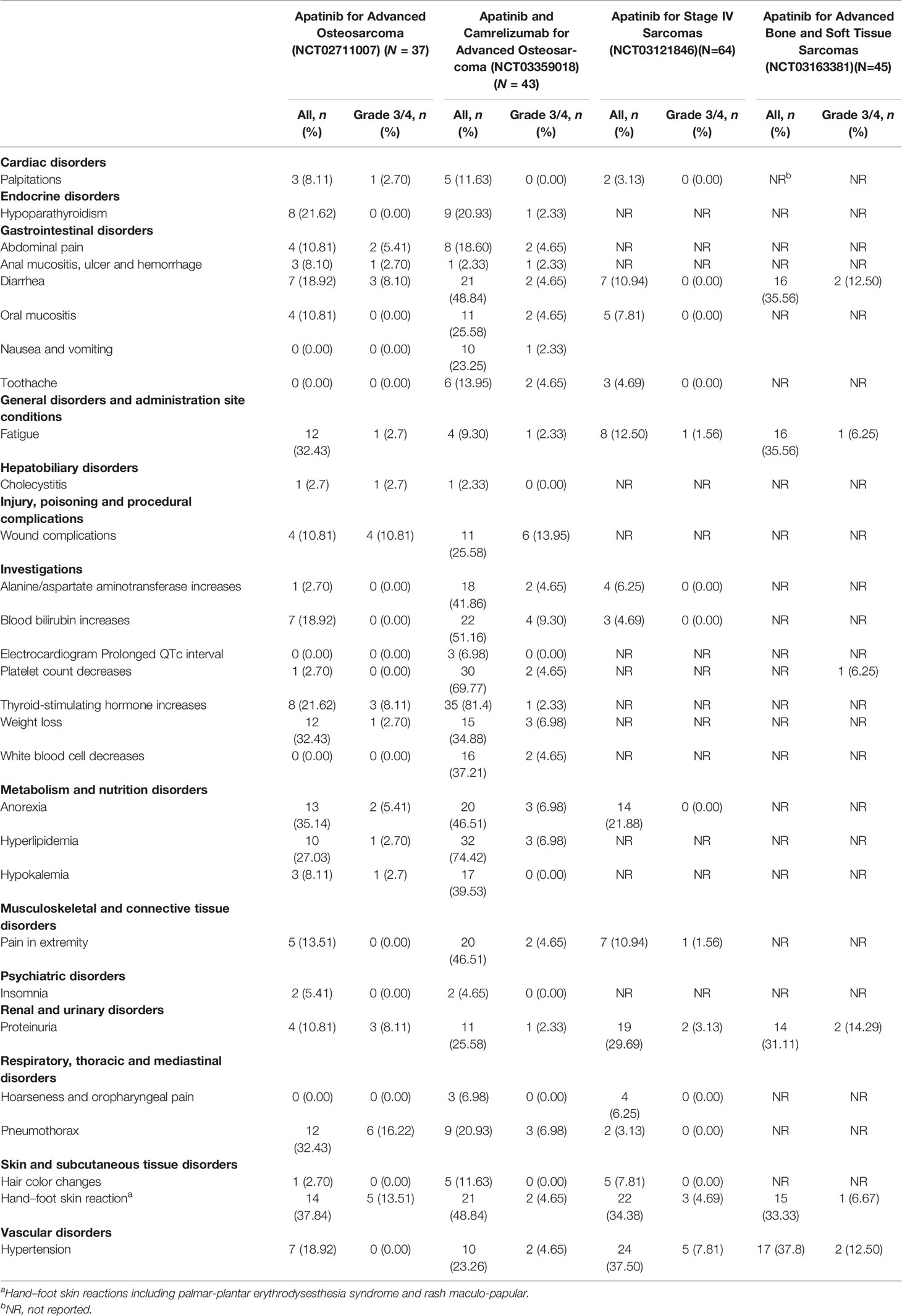
The Peking University People’s Hospital Sarcoma Group (PKUPH-sarcoma) study is a single-arm, nonblind, phase-two study that evaluated the efficacy and safety of apatinib in patients with ≥16 years of advanced osteosarcoma progressing on chemotherapy and is registered at ClinicalTrials.gov (NCT02711007) (6). Another single-arm phase II trial (NCT03121846) was designed to assess the biological activity of apatinib in relation to its efficacy and safety for bone sarcomas and soft tissue sarcomas and was conducted at Tianjin Medical University Cancer Institute & Hospital (9). The third study came from the Affiliated Cancer Hospital of Zheng Zhou University, Henan Cancer Hospital and was an open-label, nonrandomized, single-center study of 45 patients with bone and soft tissue sarcomas conducted from May 2017 to July 2018 (NCT03163381) (10). Apatinib plus camrelizumab (SHR-1210) for unresectable high-grade osteosarcoma (APFAO) is the fourth independent study investigating the administration of apatinib mesylate and camrelizumab for treating patients with inoperable locally advanced or metastatic osteosarcoma who progressed after chemotherapy (NCT03359018) (7). In general, drug-related AEs were limited to grade one or two. The frequency of apatinib administration (the total dose over time) was 40.5%-60% of the planned dose (6, 7, 9, 10). The most common grade 3 or 4 AEs are listed in Table 1.
Tumor growth in relation to angiogenesis that is effectively targeted may be the cause of mechanism-induced toxicity but is also linked with efficacy (20, 21). Some TKI-associated AEs were reported to be correlated with better patient prognosis. In our single-agent apatinib trial for osteosarcoma, loss of appetite was also significantly related to longer PFS based on a Cox regression model (HR, 0.35; p = 0.01) (6). For the clinical benefit rate (CBR, CR+PR+SD for more than 6 months according to RECIST 1.1), patients who had pneumothorax and hypothyroidism showed prolonged PFS compared with those who did not (p = 0.07 and 0.00, respectively) (6). These AEs that arise early in the treatment course, which are reversible and alleviated by supportive care, would predict better outcomes. However, if they deteriorated to persistent dose reductions, drug efficacy might be compromised afterwards.
Manifestation and Management of AEs
Over the last decade, a wealth of strategies have been developed for the management of TKI-associated AEs in other solid tumors. Most of these strategies can be used in the management of AEs associated with apatinib (18, 19). Before using this drug, it is important to determine whether the study participants have a medical history of hypertension, cerebrovascular disease, thrombosis and hemorrhage, heart disease, or even history of AEs using other antiangiogenic TKIs because this would help prevent possible severe AEs later. In addition, monitoring some laboratory parameters at baseline and during treatment (e.g., routine blood tests, hepatorenal functions, urine protein, and thyroid function) and other systems (e.g., cardiovascular, cutaneous, mucosal, and neurological systems) may also prevent the development of severe AEs.
Table 2 shows the recommended dose reductions of apatinib during treatment. Comprehensive recognition in the early stage of treatment is crucial for optimal prophylaxis in the case of inevitably compromised treatment continuity. Most of the study protocols have used the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) for classifying the severity of AEs, and there are similarities in management strategies across different tumors. It is worth noting that the NCI-CTCAE criteria are not always comprehensive enough to describe the diversity of AEs, but as common criteria used by scholars, physicians, and investigators all over the world, we also choose its definitions and systems to describe and classify these AEs. In clinical practice, recommendations should be flexible and individualized and must take into account many variables, such as disease phase, patients’ general conditions, and local medical facilities for handling severe emergencies.

Table 2 General algorithm for dose interruptions and reductions according to the severity of apatinib-associated adverse events.
Hand-Foot Skin Reactions (HFSRs)
HFSRs (also known as palmar-plantar erythrodysesthesia) generally affect the palms and feet. When these become more severe, they are described as rash acneiform, which occurred in 37.8%–48.8% apatinib-treated patients. Typical manifestations are localized thick hyperkeratotic lesions that are surrounded by erythematous regions, which are always painful (22). Thus, although HFSRs are described differently in CTCAE, we usually incorporate these two definitions together. Occasionally, symptoms may also occur in other areas, such as the knees and elbows. HFSRs of any grade were the most common AEs associated with antiangiogenic TKIs in advanced sarcoma studies, occurring in 18% of patients in the pazopanib for metastatic soft-tissue sarcoma (PALETTE) trial (23) and 51% of patients treated with regorafenib for advanced osteosarcoma (5). It was also one of the most common AEs in sorafenib (24), lenvatinib (25) and cabozantinib (26) phase 3 advanced hepatocellular carcinoma studies (occurring in 21%, 27%, and 46% of the patients, respectively). Grade 3 HFSR, which can substantially impair patients’ ability to perform daily tasks and overall QoL, occurred in 3%–17% of patients in these studies (24–26). These lesions usually occur within the first 45−80 days of apatinib administration (6) and can negatively affect their QoL.
Proactive measures should be taken before skin lesion development, including oral administration of vitamin B tablets, frequent use of creams and moisturizers from the start of drug therapy, and avoidance of pressure and friction (i.e., plantar pads and wearing clothes with adequate room for the hands and feet) (27). Routine skin examination before treatment initiation and callus softening and removal are necessary before patients develop more severe conditions. Sun exposure and unprotected cold exposure should be avoided, and more aggressive strategies may include applying keratolytic creams that contain urea or salicylic acid (6%) on minor hyperkeratotic areas and locally spraying a solution of recombinant human basic endothelial or fibroblast growth factor or vitamin B on skin areas showing minor wear out (28).
In cases of HFSR developing into grade ≥2, consultation with a dermatologist is recommended. Nonsteroidal anti-inflammatory drugs (NSAIDs) (29) are suggested under some circumstances. The key strategies for HFSR management are to maintain or restore patient comfort to avoid reducing their QoL and influencing their daily activities and to maintain their treatment with apatinib for as long as possible. Dose reductions or treatment interruptions may be warranted in some scenarios, which usually leads to symptom alleviation within 2–7 days (6, 7, 13). Moisturizers containing 20%–40% urea, salicylic acid, ammonium lactate, or alpha hydroxyl acid may be used to soften and exfoliate hyperkeratotic and callus areas. Patients should consider using topical treatments, such as cortisone and 0.05% clobetasol, as well as antibiotic ointment to prevent infections (30). Sometimes local anesthetics are recommended for severe pain. In our experience, some Chinese herbal preparations for external use are clinically helpful, and these contain Portulacaria, Geranium wilfordii Maxim, Rhizoma, Flos carthami, and Cortex phellodendri, all of which have been formulated into medical products in some clinics in Beijing (6, 13).
Stomatitis and Mucositis
Theoretically, stomatitis and mucositis are similar to HFSRs in that they are caused by capillary vasoconstriction due to antiangiogenic TKIs; they had an incidence of 18.9%–27.9% in apatinib-treated patients. However, to date, studies on treatments for VEGFR-2 inhibitor-induced mucosal toxicity are limited, and no randomized controlled trial data are currently available (31, 32). Furthermore, most published information specifically focuses on oral mucosal inflammation (stomatitis), ignoring inflammation of the nasal, anal, vaginal, or even whole alimentary tract mucosae. Information on the management of mucosal toxicity is usually available through document retrievals. Good oral hygiene is highly encouraged, including frequent teeth brushing, flossing and tongue cleaning with a soft brush (every 2–3 h for mild stomatitis; every 1–2 h for more severe symptoms) (33). Soft, nonirritating foods that are easy to chew and swallow are advised, and spicy, acidic, and salty foods should be avoided; however, this suggestion might exacerbate anorexia in individuals from some provinces in China, especially in Sichuan, Hubei and Hunan, where local people enjoy spicy and salty foods. Commercial mouthwashes containing alcohol should be avoided (27). Taking compound vitamin B tablets is also encouraged. Cleaning the rectal area with mild soap and water and using disposable wipes and a moisture-barrier ointment are recommended to prevent anal mucosal ulcers.
Because stomatitis/mucositis can become more severe than grade 2, it might be necessary to stop apatinib or reduce the dose. Topical anesthetics, mucosal coating agents and/or benzydamine HCl may be administered locally for pain alleviation (34–36). In cases of infections, topical or systemic antimicrobials may also be administered. Patients are suggested to go to specialist clinics for advice. For stomatitis/mucositis reaching grade 3, treatment with apatinib should be discontinued, and the patient is usually admitted to the hospital for supportive care (28). Cortisol paste should be suggested, and the dose of oral antibiotics should be increased. In addition, locally spraying a solution of recombinant human basic fibroblast growth factor that has been manufactured as an externally applied agent or vitamin B on the surfaces of ulceration may be considered. Some Chinese herbal preparations may also be used to promote ulcer wound healing (6). For anal ulcers, a Chinese patent medicine named Mayinglong hemorrhoid ointment, which contains traditional Chinese medicine anti-inflammatory ingredients and the pain reliever borneol, is extremely effective in improving patients’ QoL.
Diarrhea
Although diarrhea is the most common AE experienced by patients receiving aa-TKIs, the underlying mechanism is not totally understood, although some experts believe that TKI-associated diarrhea is due to excessive chloride secretion (37). In the METEOR clinical trial, 63% of patients receiving cabozantinib experienced grade 1–2 diarrhea, and 11% experienced grade 3 diarrhea, of which the median onset of diarrhea was 4.9 weeks. This AE was the most common reason for cabozantinib dose reduction (16%) (38). Diarrhea was also the most common AE associated with sorafenib and apatinib, occurring in 26% and 19% of patients with advanced osteosarcoma, respectively (4, 6).
Generally, recommendations for TKI-associated diarrhea management are similar across tumor types. To decrease the risk of diarrhea, patients should be encouraged to maintain liquid volume, eat frequent small meals, and avoid lactose-containing food, high-fat products and alcohol (22). Using a stool diary to help identify foods that may trigger digestive problems may be useful for these patients. Patients are recommended to eat bananas, rice, potatoes, apple sauce, toast, and probiotics to help digestion. It is feasible and recommended for patients to refer to a dietician for evidence-based information to reduce the risk of diarrhea (27, 33). However, physicians must always be aware that dietary restrictions might have a negative impact on weight loss and hypokalemia in populations whose body weight maintenance can already be difficult.
In cases of diarrhea that cannot be managed by dietary control, loperamide (4 mg then 2 mg every 4 h, or even 2 mg every 2 h depending on personalized situations) can be prescribed (39). For patients experiencing diarrhea frequently, loperamide could also be orally taken preemptively 30 min before TKI treatment. It is suggested that patients drink enough liquids that contain water and electrolytes, and in appropriate situations, patients can even take atropine-diphenoxylate, codeine, or tincture of opium (40) for palliation, which have been suggested in the literature; however, in clinical practice, these are rarely used. Moreover, pancreatic enzyme supplements, such as azintamide and Aspergillus oryzae trypsin, may be considered to reduce diarrhea and improve digestion (29). It is usually suggested to take 1-2 pills three times after each meal every day as conventional medical care. At the same time, any concurrent gastrointestinal infections should be treated appropriately. If diarrhea continues to grade ≥ 3/4, then an absolute diet with parenteral nutrition and intravenous electrolytes and fluid supply is advised, and somatostatin analogs such as octreotide are commonly prescribed (21, 27, 33). Patients should be hospitalized or referred to a gastroenterologist, particularly when severe cramping, nausea and vomiting, fever, or even dehydration occurs (19).
Anorexia and Weight Loss
Anorexia occurred in 35% of patients treated with apatinib and in 31% of patients treated with regorafenib in trials for advanced osteosarcoma (5, 6). Weight loss was experienced by 11%–35% of these patients. Severe hypokalemia usually follows anorexia and weight loss. Although both AEs are common and closely related, patients can also experience weight loss as a result of diarrhea, with or without anorexia, which is more subjective according to the patient’s complaints.
In our experience, appetite and weight should be monitored in each treatment cycle. Consuming nutritionally endowed foods with high calorific value as well as high-protein foods and snacking throughout the day (on foods such as eggs, poultry, fish, honey, cheese, and gelatin) is encouraged; foods that could cause gastrointestinal events should be limited or avoided (22, 30, 40). Consulting an endocrinologist in some circumstances is necessary because of the presence of underlying conditions, including hypothyroidism and low testosterone in men (22, 30). However, similar to that in patients presenting with fatigue, the administration of corticoids to stimulate appetite is also controversial. For patients who experience anorexia, stimulants such as megestrol acetate might be suggested, but they are rarely administered in China because of the restrictions on physicians’ prescriptions (41).
Blood Bilirubin Increases
Few reports have described increases in blood bilirubin with aa-TKI treatment (42). However, in two trials, nearly 20% of the patients showed an increase in bilirubin levels after the long-term use of apatinib, and chronic cholestatic cholecystitis might develop, causing severe abdominal pain and drug interruption. Thus, for those with increased bilirubin, various initial work-ups to exclude infections or other etiologies should be performed. Ursodeoxycholic acid could be considered in individuals with cholestatic drug-induced liver injury (DILI) (43). When DILI occurs, it is recommended to detect variables reflecting hepatic uptake, conjugation, or excretion as well as biliary obstruction and/or hemolysis. Isolated elevation of the conjugated fraction does not truly signify DILI. When these factors are associated with an increase in alanine aminotransferase (ALT) (Hy’s law) (44), we assume DILI might occur. Corticosteroids are frequently administered to define DILI (such as that associated with liver dysfunction) (44). When acute liver failure occurs, a hepatologist should be consulted for the need for artificial liver and liver transplantation.
Wound Complications
Approximately 11% of apatinib-treated osteosarcoma patients may develop wound problems (6). Currently, there are no evidence-based guidelines on perioperative management and drug suspension time in patients treated with TKIs due to the lack of formal clinical data. Impaired wound healing is a potential problem following treatment with TKIs, such as axitinib, cabozantinib, pazopanib, ponatinib, regorafenib, sorafenib, sunitinib, and vandetanib (45). In terms of frequency, there are no data available because no formal studies have been conducted to assess the potential impact of any TKI on wound healing. There is an urgent demand for unified retrospective studies to analyze existing data from various clinical trials investigating wound complications following all kinds of operations in patients receiving TKIs. We summarized all the half-lives of aa-TKIs and the recommendations for drug interruption intervals for selective surgeries (Table 3) according to the drug labels, which in our opinion was not reliable in real-life scenarios due to tumor progression after long drug interruption time intervals. It is suggested from the package insert that apatinib should be stopped at least 30 days prior to scheduled surgery, which is usually not executable due to “tumor rebound” after therapy discontinuation for such a long period of time. However, the appropriate time for stopping apatinib treatment before surgery should be established with caution based on the location and complexity of elective surgery, and there are diverse opinions from different medical institutions in China. Collaborations between surgeons and oncologists on debridement and dressing changes in patients are encouraged.
Hypertension
Hypertension is a frequent AE associated with VEGF pathway inhibition, with any-grade hypertension occurring in 9%–30% of the population and grade ≥3 hypertension occurring in 0%–24% of advanced osteosarcoma patients (4–6). In apatinib-treated children and young adults, the incidence of hypertension was 18.9%–23.3% (4–6). Some studies have also suggested that hypertension may be utilized as a marker for favorable clinical outcomes (46). However, in most adolescents and young adults, hypertension is not as severe as that observed in adult patients treated with other aa-TKIs (6).
Blood pressure should be monitored and under control before initiating TKI treatment and should be regularly under surveillance for the first few months of treatment; blood pressure monitoring could be conducted during clinic visits. However, in our opinion, if appropriate, it is preferable for patients to self-monitor their blood pressure at home multiple times a day (47). If blood pressure is stable, then the monitoring frequency may be subsequently reduced. To manage hypertension, current guidelines recommend initial antihypertensive treatment with angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and beta blockers (22, 27–29, 41, 46). Sometimes, ACEIs might cause severe cough in adults, and thus, these agents may be substituted with ARBs, as these agents could also reduce proteinuria. In addition, some studies have shown that the combination of apatinib and ACEIs may have a synergistic effect in controlling tumor growth (48). Calcium channel blockers (CCBs) may also be administered (33, 49); however, caution should be taken in selecting the specific agent to use. Diltiazem and verapamil are nondihydropyridine CCBs that inhibit CYP3A4, and similar agents should be avoided with apatinib due to potential drug–drug interactions (50). For difficult-to-control hypertension, we recommend three standard antihypertensive agents, although ACEIs and ARBs should not be combined. Caution should be taken when using thiazide diuretics owing to the risk of diarrhea, electrolyte loss, and QT prolongation (51). The BP goal for patients older than 60 years could be 150/90 mmHg, and for younger patients, it should be 140/90 mmHg. When antihypertensives are not effective, TKI dose reductions or interruptions may be necessary (28, 29, 51). In persistent cases, TKI discontinuation should be considered (3, 6, 15).
Proteinuria
Proteinuria is usually difficult to notice, especially in patients who do not have any obvious complaints, and it is also hard to address. In the two trials, proteinuria usually occurred 158 (95% CI: 12–186) days after the initiation of apatinib treatment in 11% of the cohort (6, 7). It is recommended to regularly monitor proteinuria monthly, if possible. In China, some Chinese patent medicines containing musk mallow and Paecilomyces hepiali Chen may be considered to prevent patients with proteinuria of less than grade 2+ from developing a more severe grade (6, 12, 13). Twenty-four-hour urine protein quantitation is advised when patients’ routine urine analysis indicates proteinuria. ACEIs or ARBs may also be considered to reduce proteinuria (22, 30). Long-term proteinuria might cause renal failure and uncontrollable hypoproteinemia (52).
Pneumothorax
The development of cavities for pulmonary nodules is evidence of a treatment response to TKIs and is also a risk factor for pneumothorax (53). As pneumothorax is a potentially fatal complication due to apatinib-based treatment, its risk and treatment interruption hazard should be balanced with discretion. In prospective trials of aa-TKIs for advanced osteosarcoma, pneumothorax developed in 3%, 0%, and 32% of patients who received sorafenib (4), regorafenib (5), and apatinib, respectively (6). In a retrospective report evaluating the combination of sorafenib, bevacizumab, and low-dose cyclophosphamide in children’s solid tumors, an unexpectedly high incidence of pneumothorax was observed, with an incidence of 25% (11/44) (54). It is hypothesized that the use of VEGF blockers induces pulmonary nodule necrosis secondary to vascular constrictions, which causes subsequent necrosis and results in pathologic pneumothorax (55).
There is no prophylactic treatment for pneumothorax. The risk factors for spontaneous pneumothorax are related to pulmonary metastasis, the majority of which are located at the pleural or peripheral zone of the lung. Although some patients who have pneumothorax during treatment are asymptomatic, most of them have chief complaints of chest pain and dyspnea (54). Appropriate measures for pneumothorax include observation without intervention, observation with oxygen inhalation, needle aspiration, insertion of thoracostomy tube to exhaust gas, or even invasive thoracoscopy or thoracotomy (56). Although treatment options usually depend on patients’ clinical symptoms and the severity of the pneumothorax, the management of pneumothorax, especially in those with osteosarcoma, is challenging, and to date, there have been no established guidelines for dealing with pneumothorax induced by TKI usage. Furthermore, establishing a consolidated and publicly accepted approach is difficult, as the data in osteosarcoma are from small-sample studies. This AE usually requires interruption of apatinib but causes disease progression. Thus, we recommend the use of a pigtail catheter or chest tube to evacuate pneumothorax and then the use of chemical or mechanical pleurodesis. Pleurodesis is an efficient method for the treatment of recurrent pneumothorax, as extensive studies on various agents, such as talc, silver nitrate, bleomycin, tetracycline, mitoxantrone, mepacrine, Corynebacterium parvum, and povidone, have shown that these adhere to the visceral and parietal pleura (57–59). From our experience, it is recommended to use Staphylococcus aureus byproducts for pleurodesis. Usually, after three to five injections of a combination solution of S. aureus byproducts and local anesthetics, pleurodesis is performed without interruption of apatinib; however, during this procedure, patients usually experience fever and chest pain, which should be managed with symptomatic treatment.
Nausea and Vomiting
In the phase 3 clinical trials discussed here, the nausea rates were 24% for sorafenib, 17% for regorafenib, 20% for lenvatinib, 23.3% for apatinib and 31% for cabozantinib (24–26, 60). Vomiting occurred in 15%, 13%, 16%, and 26% of the patients in these trials. In each study, ≤2% of patients experienced grade ≥ 3 nausea or vomiting (26).
It has been suggested that avoiding chocolate, caffeine, alcohol, and nicotine may be helpful (Table 4) (29). Antiemetics, such as metoclopramide and levosulpiride, may alleviate symptoms. 5-HT3 antagonists have been recommended for only NK1 receptor antagonists (e.g., dexamethasone) to avoid CYP3A4 modulation. Ondansetron and granisetron should be used with caution owing to potential interactions with apatinib, causing QTc prolongation (50, 61). In addition, we recommend a healthy lifestyle, dietary modifications and prophylactic use of proton-pump inhibitors to avoid nausea and vomiting.
Myelosuppression
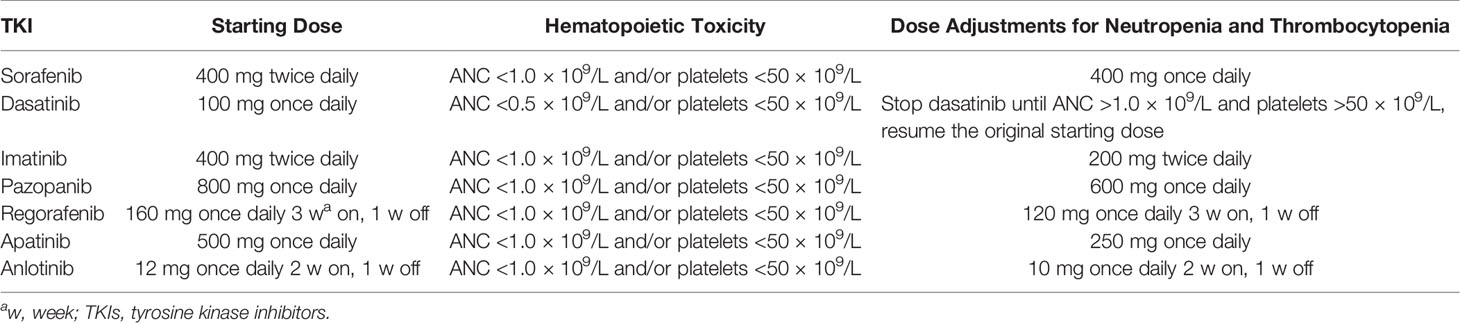
Myelosuppression developing during apatinib treatment of osteosarcoma is rare. The hematological toxicity of TKIs is usually mild, persistent, dose-dependent, and reversible upon cessation or dose reduction; TKIs casually affect leukocytes, erythrocytes and thrombocytes to varying degrees (62). We compared the hematopoietic toxicities of different TKIs with dose adjustments for neutropenia and thrombocytopenia (Table 5).
Conclusions
Patients who receive apatinib-based therapy should be educated on the commonly seen AE manifestations and prophylactic and symptomatic measures and should routinely arrange follow-ups to obtain timely and appropriate supportive care and be monitored for rare, asymptomatic events. Otherwise, these AEs may immensely influence patients’ QoL and increase the risk of dose reduction or drug discontinuation, which in turn may compromise clinical outcomes. In addition, the interventions being described should be better assessed in prospective trials to evaluate whether these interventions may improve symptoms and to evaluate the dose delivered to patients.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7223462/. The datasets are available from the corresponding author upon reasonable request. We promised to cover patients’ data confidentially. But patients’ data will be made disguisedly available, including data dictionaries, for approved data sharing requests. Individual data will be shared that underlie the results reported in this Article, after de-identification and normalization of information (text, tables, figures, and appendices). Proposals should be directed to eGllLmx1QGhvdG1haWwuY29t. To gain access, data requestors will need to sign a data access agreement.
Author Contributions
WG, LX, and JXu made substantial contributions to the conception and design of the study. LX and JXu drafted the article. All authors contributed to the article and approved the submitted version.
Funding
Financial support was provided by the National Natural Science Foundation of China (grant no. 81572633).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the patients, investigators, and data-monitoring communities. We thank colleagues from the Chinese Medical Doctor Association (CMDA) and the Chinese Sarcoma Study Group (CSSG) for providing medical materials; We also thank our sponsor, Jiangsu Hengrui Medicine Co., Ltd., for trial support. We thank Springer Nature Author Services for their linguistic assistance during the preparation of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.696865/full#supplementary-material
Supplementary Table 1 | PRISMA flow diagram.
References
1. Smeland S, Bielack SS, Whelan J, Bernstein M, Hogendoorn P, Krailo MD, et al. Survival and Prognosis With Osteosarcoma: Outcomes in More Than 2000 Patients in the EURAMOS-1 (European and American Osteosarcoma Study) Cohort. Eur J Cancer (2019) 109:36–50. doi: 10.1016/j.ejca.2018.11.027
2. Marina NM, Smeland S, Bielack SS, Bernstein M, Jovic G, Krailo MD, et al. Comparison of MAPIE Versus MAP in Patients With a Poor Response to Preoperative Chemotherapy for Newly Diagnosed High-Grade Osteosarcoma (EURAMOS-1): An Open-Label, International, Randomised Controlled Trial. Lancet Oncol (2016) 17(10):1396–408. doi: 10.1016/S1470-2045(16)30214-5
3. Bielack SS, Smeland S, Whelan JS, Marina N, Jovic G, Hook JM, et al. Methotrexate, Doxorubicin, and Cisplatin (MAP) Plus Maintenance Pegylated Interferon Alfa-2b Versus MAP Alone in Patients With Resectable High-Grade Osteosarcoma and Good Histologic Response to Preoperative MAP: First Results of the EURAMOS-1 Good Response Randomized Controlled Trial. J Clin Oncol (2015) 33(20):2279–87. doi: 10.1200/JCO.2014.60.0734
4. Grignani G, Palmerini E, Dileo P, Asaftei SD, D’Ambrosio L, Pignochino Y, et al. A Phase II Trial of Sorafenib in Relapsed and Unresectable High-Grade Osteosarcoma After Failure of Standard Multimodal Therapy: An Italian Sarcoma Group Study. Ann Oncol (2012) 23(2):508–16. doi: 10.1093/annonc/mdr151
5. Duffaud F, Mir O, Boudou-Rouquette P, Piperno-Neumann S, Penel N, Bompas E, et al. Efficacy and Safety of Regorafenib in Adult Patients With Metastatic Osteosarcoma: A Non-Comparative, Randomised, Double-Blind, Placebo-Controlled, Phase 2 Study. Lancet Oncol (2019) 20(1):120–33. doi: 10.1016/S1470-2045(18)30742-3
6. Xie L, Xu J, Sun X, Tang X, Yan T, Yang R, et al. Apatinib for Advanced Osteosarcoma After Failure of Standard Multimodal Therapy: An Open Label Phase II Clinical Trial. Oncologist (2019) 24(7):e542–50. doi: 10.1634/theoncologist.2018-0542
7. Xie L, Xu J, Sun X, Guo W, Gu J, Liu K, et al. Apatinib Plus Camrelizumab (Anti-PD1 Therapy, SHR-1210) for Advanced Osteosarcoma (APFAO) Progressing After Chemotherapy: A Single-Arm, Open-Label, Phase 2 Trial. J Immunother Cancer (2020) 8(1):e000798. doi: 10.1136/jitc-2020-000798
8. Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in Advanced Soft-Tissue Sarcoma and Bone Sarcoma (SARC028): A Multicentre, Two-Cohort, Single-Arm, Open-Label, Phase 2 Trial. Lancet Oncol (2017) 18(11):1493–501. doi: 10.1016/S1470-2045(17)30624-1
9. Liao Z, Li F, Zhang C, Zhu L, Shi Y, Zhao G, et al. Phase II Trial of VEGFR2 Inhibitor Apatinib for Metastatic Sarcoma: Focus on Efficacy and Safety. Exp Mol Med (2019) 51(3):24. doi: 10.1038/s12276-019-0221-7
10. Yao W, Wu F, Cai Q, Wang J. Efficacy and Safety of Apatinib in Advanced Sarcoma. Anticancer Drugs (2019) 30(7):749–56. doi: 10.1097/cad.0000000000000778
11. Gou M, Si H, Zhang Y, Qian N, Wang Z, Shi W, et al. Efficacy and Safety of Apatinib in Patients With Previously Treated Metastatic Colorectal Cancer: A Real-World Retrospective Study. Sci Rep (2018) 8(1):4602. doi: 10.1038/s41598-018-22302-z
12. Zhang Y, Han C, Li J, Zhang L, Wang L, Ye S, et al. Efficacy and Safety for Apatinib Treatment in Advanced Gastric Cancer: A Real World Study. Sci Rep (2017) 7(1):13208. doi: 10.1038/s41598-017-13192-8
13. Xie L, Guo W, Wang Y, Yan T, Ji T, Xu J. Apatinib for Advanced Sarcoma: Results From Multiple Institutions’ Off-Label Use in China. BMC Cancer (2018) 18(1):396. doi: 10.1186/s12885-018-4303-z
14. Spinzi G, Terreni N. Health-Related Quality of Life and Sorafenib. Hepatology (2010) 52(4):1523. doi: 10.1002/hep.23756
15. Pan X, Huang H, Huang Y, Liu B, Cui X, Gan S, et al. Sunitinib Dosing Schedule 2/1 Improves Tolerability, Efficacy, and Health-Related Quality of Life in Chinese Patients With Metastatic Renal Cell Carcinoma. Urol Oncol (2015) 33(6):268.e9–e15. doi: 10.1016/j.urolonc.2015.03.008
16. Martin AJ, Gibbs E, Sjoquist K, Pavlakis N, Simes J, Price T, et al. Health-Related Quality of Life Associated With Regorafenib Treatment in Refractory Advanced Gastric Adenocarcinoma. Gastric Cancer (2018) 21(3):473–80. doi: 10.1007/s10120-017-0754-1
17. Coens C, van der Graaf WT, Blay JY, Chawla SP, Judson I, Sanfilippo R, et al. Health-Related Quality-of-Life Results From PALETTE: A Randomized, Double-Blind, Phase 3 Trial of Pazopanib Versus Placebo in Patients With Soft Tissue Sarcoma Whose Disease has Progressed During or After Prior Chemotherapy-a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Global Network Study (EORTC 62072). Cancer (2015) 121(17):2933–41. doi: 10.1002/cncr.29426
18. Liu X, Qin S, Wang Z, Xu J, Xiong J, Bai Y, et al. Early Presence of Anti-Angiogenesis-Related Adverse Events as a Potential Biomarker of Antitumor Efficacy in Metastatic Gastric Cancer Patients Treated With Apatinib: A Cohort Study. J Hematol Oncol (2017) 10(1):153. doi: 10.1186/s13045-017-0521-0
19. Liu X, Qin S, Wang Z, Xu J, Xiong J, Bai Y, et al. Correction to: Early Presence of Anti-Angiogenesis-Related Adverse Events as a Potential Biomarker of Antitumor Efficacy in Metastatic Gastric Cancer Patients Treated With Apatinib: A Cohort Study. J Hematol Oncol (2018) 11(1):5. doi: 10.1186/s13045-017-0545-5
20. Kalmanti L, Saussele S, Lauseker M, Muller MC, Dietz CT, Heinrich L, et al. Safety and Efficacy of Imatinib in CML Over a Period of 10 Years: Data From the Randomized CML-Study IV. Leukemia (2015) 29(5):1123–32. doi: 10.1038/leu.2015.36
21. Liu S, Kurzrock R. Understanding Toxicities of Targeted Agents: Implications for Anti-Tumor Activity and Management. Semin Oncol (2015) 42(6):863–75. doi: 10.1053/j.seminoncol.2015.09.032
22. Rimassa L, Danesi R, Pressiani T, Merle P. Management of Adverse Events Associated With Tyrosine Kinase Inhibitors: Improving Outcomes for Patients With Hepatocellular Carcinoma. Cancer Treat Rev (2019) 77:20–8. doi: 10.1016/j.ctrv.2019.05.004
23. van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for Metastatic Soft-Tissue Sarcoma (PALETTE): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet (2012) 379(9829):1879–86. doi: 10.1016/S0140-6736(12)60651-5
24. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in Advanced Hepatocellular Carcinoma. N Engl J Med (2008) 359(4):378–90. doi: 10.1056/NEJMoa0708857
25. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib Versus Sorafenib in First-Line Treatment of Patients With Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet (2018) 391(10126):1163–73. doi: 10.1016/S0140-6736(18)30207-1
26. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in Patients With Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med (2018) 379(1):54–63. doi: 10.1056/NEJMoa1717002
27. Hofheinz RD, Segaert S, Safont MJ, Demonty G, Prenen H. Management of Adverse Events During Treatment of Gastrointestinal Cancers With Epidermal Growth Factor Inhibitors. Crit Rev Oncol Hematol (2017) 114:102–13. doi: 10.1016/j.critrevonc.2017.03.032
28. Califano R, Tariq N, Compton S, Fitzgerald DA, Harwood CA, Lal R, et al. Expert Consensus on the Management of Adverse Events From EGFR Tyrosine Kinase Inhibitors in the UK. Drugs (2015) 75(12):1335–48. doi: 10.1007/s40265-015-0434-6
29. Gerendash BS, Creel PA. Practical Management of Adverse Events Associated With Cabozantinib Treatment in Patients With Renal-Cell Carcinoma. Onco Targets Ther (2017) 10:5053–64. doi: 10.2147/OTT.S145295
30. Sastre J, Argiles G, Benavides M, Feliu J, Garcia-Alfonso P, Garcia-Carbonero R, et al. Clinical Management of Regorafenib in the Treatment of Patients With Advanced Colorectal Cancer. Clin Transl Oncol (2014) 16(11):942–53. doi: 10.1007/s12094-014-1212-8
31. Lacouture ME, Ciccolini K, Kloos RT, Agulnik M. Overview and Management of Dermatologic Events Associated With Targeted Therapies for Medullary Thyroid Cancer. Thyroid (2014) 24(9):1329–40. doi: 10.1089/thy.2013.0700
32. Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, et al. MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy. Cancer (2014) 120(10):1453–61. doi: 10.1002/cncr.28592
33. Khan G, Moss RA, Braiteh F, Saltzman M. Proactive Strategies for Regorafenib in Metastatic Colorectal Cancer: Implications for Optimal Patient Management. Cancer Manag Res (2014) 6:93–103. doi: 10.2147/CMAR.S52217
34. Monjazeb S, Wilson J. Epidermal Growth Factor Receptor Inhibitors: Cutaneous Side Effects and Their Management. Skin Ther Lett (2017) 22(5):5–7.
35. Stulhofer Buzina D, Martinac I, Ledic Drvar D, Ceovic R, Bilic I, Marinovic B. The Most Common Cutaneous Side Effects of Epidermal Growth Factor Receptor Inhibitors and Their Management. Acta Dermatovenerol Croat (2015) 23(4):282–8.
36. Sinclair R. Anticipating and Managing the Cutaneous Side Effects of Epidermal Growth Factor Receptor Inhibitors. Asia Pac J Clin Oncol (2014) 10 Suppl 1:11–7. doi: 10.1111/ajco.12160
37. Yang JC, Reguart N, Barinoff J, Kohler J, Uttenreuther-Fischer M, Stammberger U, et al. Diarrhea Associated With Afatinib: An Oral ErbB Family Blocker. Expert Rev Anticancer Ther (2013) 13(6):729–36. doi: 10.1586/era.13.31
38. Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, et al. Cabozantinib Versus Everolimus in Advanced Renal Cell Carcinoma (METEOR): Final Results From a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2016) 17(7):917–27. doi: 10.1016/S1470-2045(16)30107-3
39. Bolondi L, Craxi A, Trevisani F, Daniele B, Di Costanzo GG, Fagiuoli S, et al. Refining Sorafenib Therapy: Lessons From Clinical Practice. Future Oncol (2015) 11(3):449–65. doi: 10.2217/fon.14.261
40. Markowitz JN, Fancher KM. Cabozantinib: A Multitargeted Oral Tyrosine Kinase Inhibitor. Pharmacotherapy (2018) 38(3):357–69. doi: 10.1002/phar.2076
41. Walko CM, Grande C. Management of Common Adverse Events in Patients Treated With Sorafenib: Nurse and Pharmacist Perspective. Semin Oncol (2014) 41 Suppl 2:S17–28. doi: 10.1053/j.seminoncol.2014.01.002
42. Ghatalia P, Je Y, Mouallem NE, Nguyen PL, Trinh QD, Sonpavde G, et al. Hepatotoxicity With Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitors: A Meta-Analysis of Randomized Clinical Trials. Crit Rev Oncol Hematol (2015) 93(3):257–76. doi: 10.1016/j.critrevonc.2014.11.006
43. Teo YL, Ho HK, Chan A. Formation of Reactive Metabolites and Management of Tyrosine Kinase Inhibitor-Induced Hepatotoxicity: A Literature Review. Expert Opin Drug Metab Toxicol (2015) 11(2):231–42. doi: 10.1517/17425255.2015.983075
44. Andrade RJ, Chalasani N, Bjornsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, et al. Drug-Induced Liver Injury. Nat Rev Dis Primers (2019) 5(1):58. doi: 10.1038/s41572-019-0105-0
45. Shah DR, Dholakia S, Shah RR. Effect of Tyrosine Kinase Inhibitors on Wound Healing and Tissue Repair: Implications for Surgery in Cancer Patients. Drug Saf (2014) 37(3):135–49. doi: 10.1007/s40264-014-0139-x
46. Qi WX, He AN, Shen Z, Yao Y. Incidence and Risk of Hypertension With a Novel Multi-Targeted Kinase Inhibitor Axitinib in Cancer Patients: A Systematic Review and Meta-Analysis. Br J Clin Pharmacol (2013) 76(3):348–57. doi: 10.1111/bcp.12149
47. Qi WX, Lin F, Sun YJ, Tang LN, He AN, Yao Y, et al. Incidence and Risk of Hypertension With Pazopanib in Patients With Cancer: A Meta-Analysis. Cancer Chemother Pharmacol (2013) 71(2):431–9. doi: 10.1007/s00280-012-2025-5
48. Sobczuk P, Szczylik C, Porta C, Czarnecka AM. Renin Angiotensin System Deregulation as Renal Cancer Risk Factor. Oncol Lett (2017) 14(5):5059–68. doi: 10.3892/ol.2017.6826
49. De Wit M, Boers-Doets CB, Saettini A, Vermeersch K, de Juan CR, Ouwerkerk J, et al. Prevention and Management of Adverse Events Related to Regorafenib. Support Care Cancer (2014) 22(3):837–46. doi: 10.1007/s00520-013-2085-z
50. Ding J, Chen X, Gao Z, Dai X, Li L, Xie C, et al. Metabolism and Pharmacokinetics of Novel Selective Vascular Endothelial Growth Factor Receptor-2 Inhibitor Apatinib in Humans. Drug Metab Dispos (2013) 41(6):1195–210. doi: 10.1124/dmd.112.050310
51. Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet Recommendations for the Management of Chronic Myeloid Leukemia: 2013. Blood (2013) 122(6):872–84. doi: 10.1182/blood-2013-05-501569
52. Zhang ZF, Wang T, Liu LH, Guo HQ. Risks of Proteinuria Associated With Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitors in Cancer Patients: A Systematic Review and Meta-Analysis. PloS One (2014) 9(3):e90135. doi: 10.1371/journal.pone.0090135
53. Teuwen LA, Van den Mooter T, Dirix L. Management of Pulmonary Toxicity Associated With Targeted Anticancer Therapies. Expert Opin Drug Metab Toxicol (2015) 11(11):1695–707. doi: 10.1517/17425255.2015.1080687
54. Interiano RB, McCarville MB, Wu J, Davidoff AM, Sandoval J, Navid F. Pneumothorax as a Complication of Combination Antiangiogenic Therapy in Children and Young Adults With Refractory/Recurrent Solid Tumors. J Pediatr Surg (2015) 50(9):1484–9. doi: 10.1016/j.jpedsurg.2015.01.005
55. Hoag JB, Sherman M, Fasihuddin Q, Lund ME. A Comprehensive Review of Spontaneous Pneumothorax Complicating Sarcoma. Chest (2010) 138(3):510–8. doi: 10.1378/chest.09-2292
56. Baumann MH, Strange C, Heffner JE, Light R, Kirby TJ, Klein J, et al. Management of Spontaneous Pneumothorax: An American College of Chest Physicians Delphi Consensus Statement. Chest (2001) 119(2):590–602. doi: 10.1378/chest.119.2.590
57. Wong A, Galiabovitch E, Bhagwat K. Management of Primary Spontaneous Pneumothorax: A Review. ANZ J Surg (2019) 89(4):303–8. doi: 10.1111/ans.14713
58. Kelly AM. Review of Management of Primary Spontaneous Pneumothorax: Is the Best Evidence Clearer 15 Years on? Emerg Med Australas (2007) 19(4):303–8. doi: 10.1111/j.1742-6723.2007.00997.x
59. Devanand A, Koh MS, Ong TH, Low SY, Phua GC, Tan KL, et al. Simple Aspiration Versus Chest-Tube Insertion in the Management of Primary Spontaneous Pneumothorax: A Systematic Review. Respir Med (2004) 98(7):579–90. doi: 10.1016/j.rmed.2004.04.006
60. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for Patients With Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment (RESORCE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet (2017) 389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9
61. Moffett PM, Cartwright L, Grossart EA, O’Keefe D, Kang CS. Intravenous Ondansetron and the QT Interval in Adult Emergency Department Patients: An Observational Study. Acad Emerg Med (2016) 23(1):102–5. doi: 10.1111/acem.12836
Keywords: apatinib, toxicity, prophylaxis, treatment, osteosarcoma
Citation: Xie L, Xu J, Guo W, Wang Z, Yao Y, Li J, Lin J, Xiao J, Yu X, Zhang W, Cai Z, Hua Y, Chen J, Shao Z, Wu D, Wu S, Tu Z and Zhang X (2021) Management of Apatinib-Related Adverse Events in Patients With Advanced Osteosarcoma From Four Prospective Trials: Chinese Sarcoma Study Group Experience. Front. Oncol. 11:696865. doi: 10.3389/fonc.2021.696865
Received: 18 April 2021; Accepted: 29 June 2021;
Published: 22 July 2021.
Edited by:
Jilong Yang, Tianjin Medical University Cancer Institute and Hospital, ChinaReviewed by:
Cristina Meazza, Fondazione Istituto Nazionale dei Tumori, ItalyXiaochun Zhang, The Affiliated Hospital of Qingdao University, China
Yong Chen, Fudan University, China
Copyright © 2021 Xie, Xu, Guo, Wang, Yao, Li, Lin, Xiao, Yu, Zhang, Cai, Hua, Chen, Shao, Wu, Wu, Tu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Guo, Ym9uZXR1bW9yQDE2My5jb20=
 Lu Xie1
Lu Xie1 Wei Guo
Wei Guo Yang Yao
Yang Yao Weibin Zhang
Weibin Zhang