- 1Department of Orthopedic Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Orthopedics Research Institute of Zhejiang University, Hangzhou, China
- 3Key Laboratory of Motor System Disease Research and Precision Therapy of Zhejiang Province, Hangzhou, China
- 4Department of Orthopedics, Ningbo Municipal Hospital of TCM, Ningbo, China
Purpose: Bone metastasis from endometrial cancer (EC) is rare and poorly described. The purpose of the present study was to investigate the correlation between the clinically accessible factors and survival time among EC patients with bone metastasis.
Patients and Methods: We retrospectively identified and reviewed EC patients with bone metastasis from 2010 to 2016, based on the Surveillance, Epidemiology and End Results (SEER) database. Univariable and multivariable Cox regressions were applied to evaluate the effects of clinical variables on survival. Kaplan–Meier plots were used to visually demonstrate the correlation between independent risk factors and survival.
Results: Clinical data of 584 EC patients with bone metastasis from the SEER database were analyzed. EC patients with bone metastasis experienced extremely poor survival, with 1-year overall survival (OS) and cancer-specific survival (CSS) rates 33.8 and 35.8%, respectively. Variables associated with OS and CSS in the univariable analysis included race, tumor grade, tumor subtype, tumor size, lung, liver and brain metastases, surgery, radiotherapy, and chemotherapy. In the multivariable analysis, tumor grade, tumor subtype, liver and brain metastases, local surgery, and systemic chemotherapy remained independent risk factors for OS and CSS. However, local radiotherapy was an independent predictor of OS, not CSS.
Conclusions: We identified several factors affect the survival of EC patients with bone metastasis, which is useful for clinicians to assess patients’ outcomes. Our study supports surgery and radiotherapy of primary EC, and systemic chemotherapy for prolonging survival among EC patients with bone metastasis, which lays a solid foundation for defining optimal treatment strategy in this specific cohort.
Introduction
As the most common malignancy of female endometrium, endometrial cancer (EC) incidence rate has been rapidly rising in recent years (1, 2). With the rapid development of imaging techniques and treatment of EC, the incidence of bone metastasis is also increasing. Compared with other tumors, bone metastasis is relatively uncommon in EC, with an incidence 0.6% (3). Among patients in stage IV EC, bone metastasis occurred in 6.8% of patients (4). Distant organ metastasis usually predicts a poor prognosis (5). Although 5-year overall survival (OS) of EC was estimated around 80% in patients without metastatic disease (1, 2), metastatic EC patients experienced extremely poor prognosis with 5-year OS less than 20% (3, 4). Common treatment methods for EC include surgery, radiotherapy, as well as systemic therapies. However, the standard management of EC with bone metastasis is unknown.
Some studies reported risk factors associated with survival among EC, including tumor stage, histological type, adjuvant chemotherapy, etc. (6, 7). However, EC patients with bone metastasis constitute a heterogeneous cohort, survival predictors and appropriate treatments of them remain unknown. Additionally, the Surveillance, Epidemiology and End Results (SEER) database used in the study, is widely used in rare tumor entities. This study was aimed to reveal the clinicopathologic features and assess the risk factors associated with prognosis of this rare population.
Material And Methods
Study Population
In this retrospective study, we included patients diagnosed as EC with bone metastasis between 2010 and 2016. Clinical data regarding EC with bone metastasis were collected from the SEER database, including basic clinicopathological information and treatment methods. As a large population-based database, the SEER represents nearly 30% of the US population and provides a free tool for clinical study of malignant tumors, which covers 20 geographic areas in the USA (8).
We used two keywords Primary site C54.1-Endometrium and SEER Combined Mets at DX-bone (2010+) to retrieve EC cases with bone metastasis in the SEER database. In addition, we only included cases with pathological diagnosis. According to the International Classification of Diseases for Oncology, Third Edition (ICD-O-3), we categorized EC with bone metastasis into three histological subtypes: endometrioid subtype (8380), non-endometrioid subtype (8000, 8010, 8013, 8020, 8041, 8045– 8046, 8050, 8070, 8140, 8246, 8255, 8260, 8310, 8323, 8441, 8460–8461, 8480, 8560, 8570, 8574), and sarcoma subtype (8800, 8805, 8890,8900, 8930–8931,8950,8980). Surgery or radiotherapy in the current research refers to the primary EC (9).
Statistical Analyses
We completed all statistical analyses by IBM SPSS Statistics 22. We defined cancer-specific survival (CSS) as the interval from diagnosis till death due to EC (10, 11). We used the univariable Cox regression model to screen for statistically significant indicators associated with OS and CSS. Then, we performed multivariable Cox regression model to confirm independent predictors of OS and CSS. Kaplan–Meier plots were used to visually demonstrate the correlation between independent risk factors and survival. Hazard ratios (HRs) and 95% confidence interval (CI) were used to show the impact of variables on survival during Cox regression analyses. Analysis variables with bilateral p less than 0.05 were considered statistically significant.
Results
Clinicopathological Characteristics
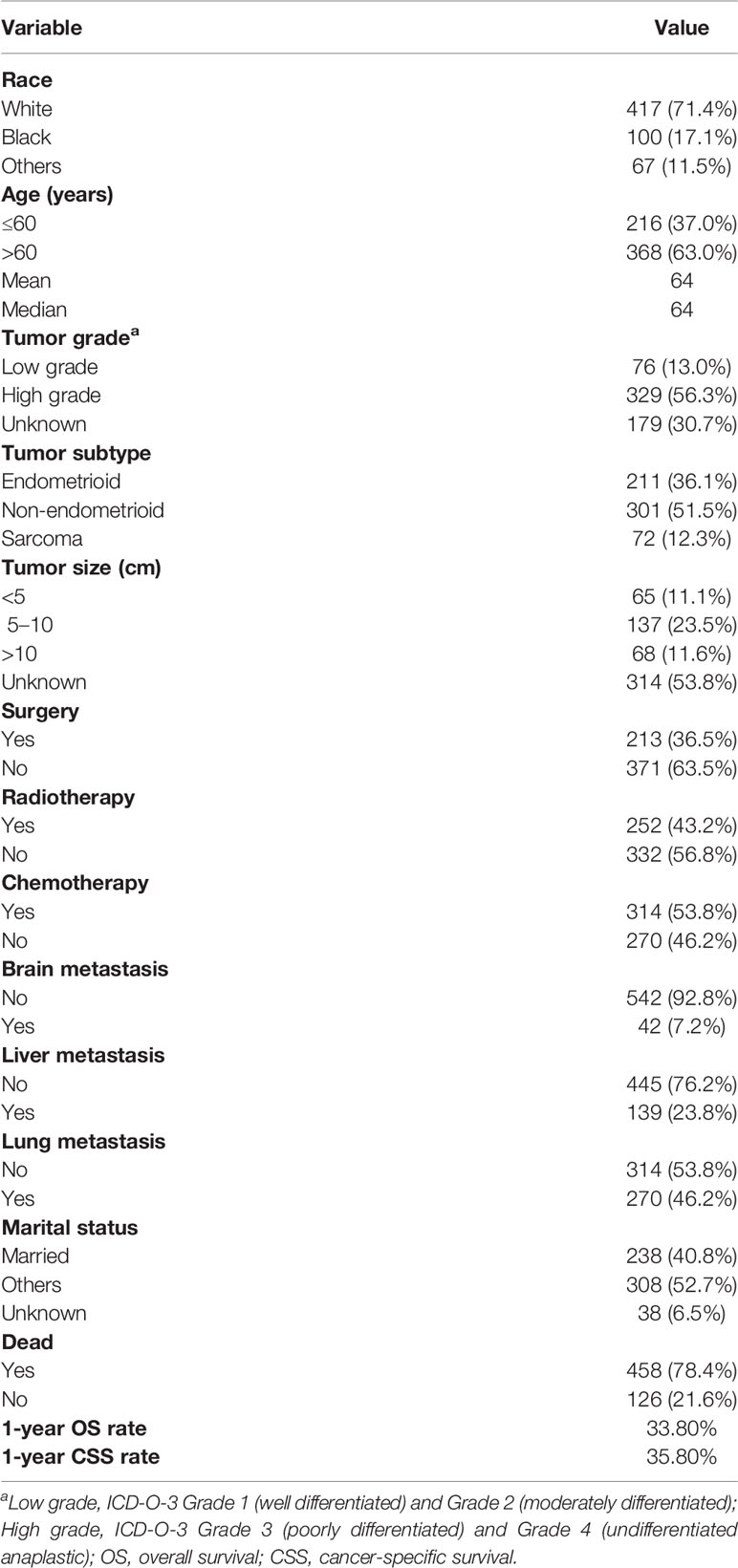
Of the 584 EC patients with bone metastasis identified, more than two-thirds (71.4%) of patients were white. Average and median age are both 64 years old. Two hundred sixteen (37.0%) patients were aged less than 60 years old and 368 (63.0%) patients were aged over 60 years old. Tumor grade was defined as low grade (n = 76, 13.0%), high grade (n = 329, 56.3%), and unknown grade (n = 179, 30.7%). Histological subtype distribution was endometrioid 36.1%, non-endometrioid 51.5%, and sarcoma 12.3%. Sixty-five (11.1%), 137 (23.5%), and 68 (11.6%) of the patients had tumor size <5 cm, 5–10 cm, and >10 cm, respectively. Lung metastasis accounted for 46.2%, liver metastasis accounted for 23.8%, and brain metastasis accounted for 7.2%. About two-fifths (40.8%) of the patients were married. Surgery was performed for 213 (36.5%) patients, radiotherapy was performed for 252 (43.2%), and chemotherapy was performed for 314 (53.8%). One-year OS and CSS rate for all patients was 33.8 and 35.8%, respectively (Table 1).
Univariable Cox Regression Analysis
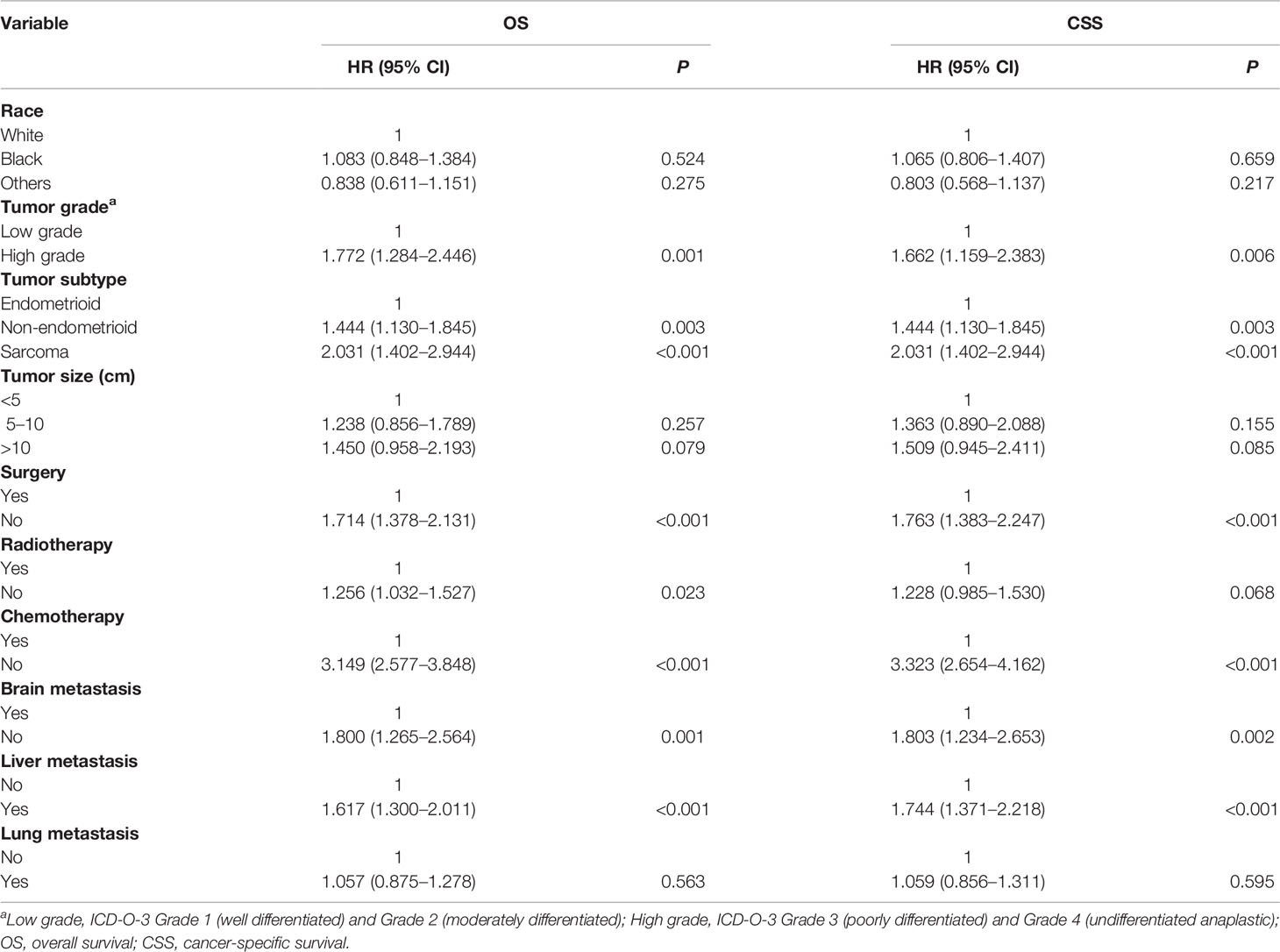
On univariable analysis, black race, high tumor grade, non-endometrioid, and sarcoma subtype, tumor size >10 cm, the presence of lung, liver and brain metastases, no surgery, no radiotherapy, and no chemotherapy were significant predictors for decreased OS and CSS (Table 2). There was no difference in OS and CSS by age and marital status (Table 2). The Kaplan–Meier curve plots displayed that patients with endometrioid subtype had the best survival, followed by non-endometrioid and sarcoma subtypes (Figure 1, p < 0.05). Moreover, surgery, radiotherapy, and chemotherapy had a significant survival benefit for patients (Figure 2, p < 0.05).
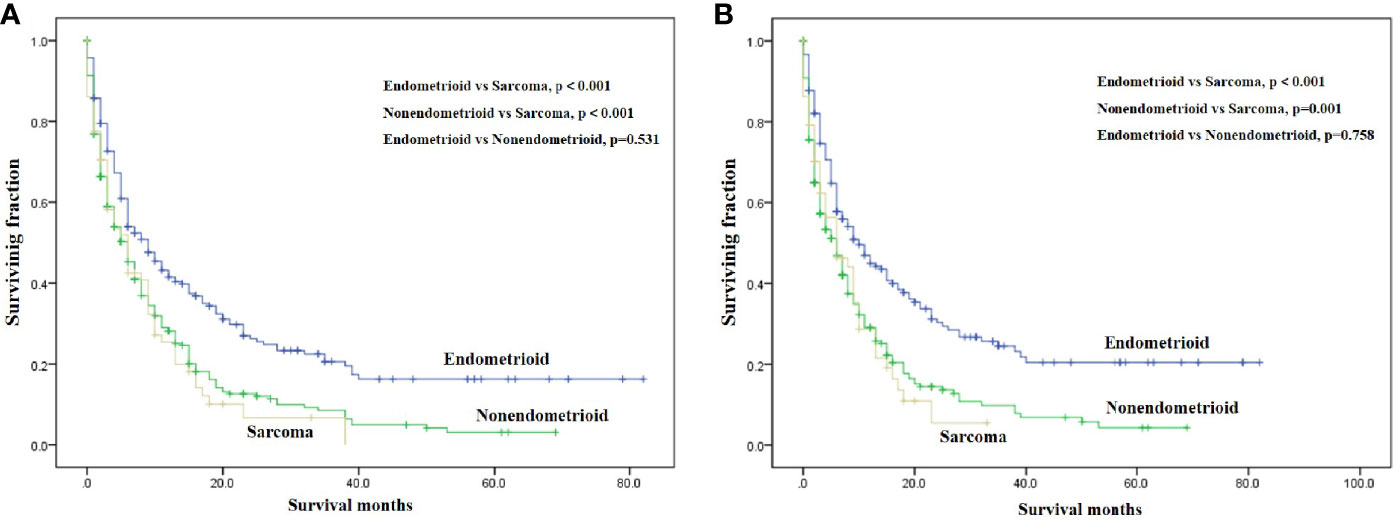
Figure 1 Kaplan-Meier method estimated OS (A) and CSS (B) in endometrial cancer bone metastasis stratified by tumor subtype. (OS, overall survival; CSS, cancer-specific survival).
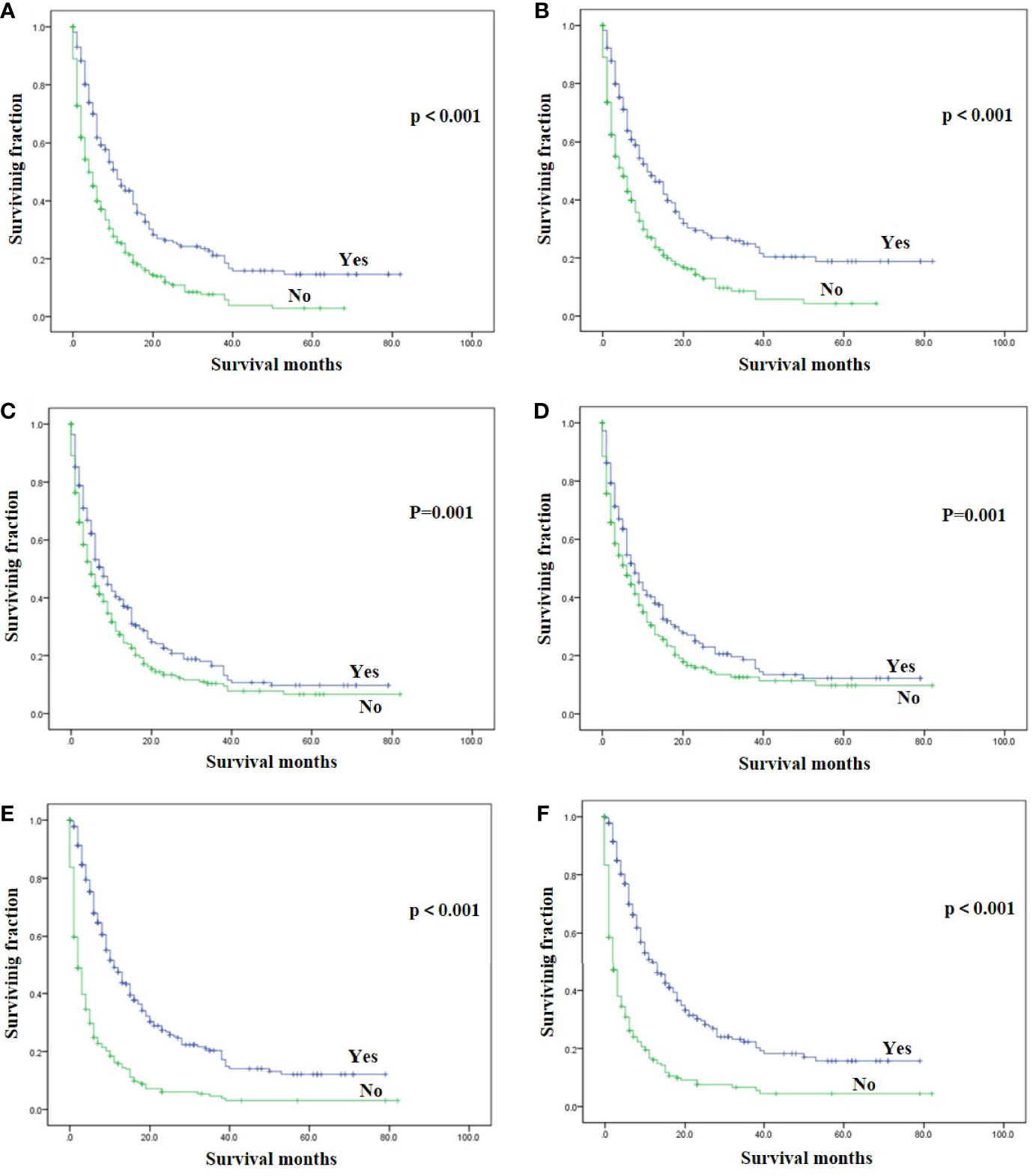
Figure 2 Kaplan-Meier method estimated OS and CSS in endometrial cancer bone metastasis stratified by treatment methods. (A) OS stratified by surgery; (B) CSS stratified by surgery; (C) OS stratified by radiotherapy; (D) CSS stratified by radiotherapy; (E) OS stratified by chemotherapy; (F) CSS stratified by chemotherapy. (OS, overall survival; CSS, cancer-specific survival).
Multivariable Cox Regression Analysis
Using multivariable analysis, high tumor grade, non-endometrioid and sarcoma subtype, the presence of liver and brain metastases, no surgery, and no chemotherapy were significant predictors of worsened OS and CSS (Table 3). Radiotherapy is an independent predictor for OS, not CSS (Table 3). No difference was observed in OS and CSS by race, tumor size, and lung metastasis (Table 3).
Discussion
Although metastatic EC patients experienced poor prognosis, few researches explored risk factors of survival and effective treatments for them. We first performed the largest study for EC patients with bone metastasis to reveal their clinical characteristics, prognosis, and risk factors affecting prognosis. Our results validated that tumor grade, tumor subtype, liver and brain metastases were independent predictor associated with the OS and CSS. In terms of treatment methods, surgery, radiotherapy, and chemotherapy were positive independent predictors of OS, while surgery, and chemotherapy were positive independent predictors of CSS. The most important clinical significance of this study is to guide clinicians to better evaluate the survival of EC patients with bone metastasis and provide appropriate treatments.
The prognosis of EC with bone metastasis was extremely poor, with 1-year OS and CSS rate 33.8 and 35.8%, respectively. Therefore, the prognosis of these patients has a great room for improvement. It is very important and necessary to explore the risk factors affecting the prognosis of these patients. Race was not a significant independent risk factor of survival in this study, unlike many previous studies that it had an impact on the prognosis of EC (12, 13). This may be due to the fact that many previous studies have focused on all patients with EC, but this study only focused on EC patients with bone metastasis. Our univariable analysis also showed that age was not correlated with OS or CSS, which was not congruent with many previous studies including metastatic EC (3, 14, 15). Further researches are required to confirm our finding. This study found that tumor grade was an independent prognostic factor for OS and CSS, which was consistent with others (16–18). Our multivariable analysis showed that patients with non-endometrioid and sarcoma subtypes had worse survival than those with endometrioid subtype, consistent with the findings reported by Liu et al. (4). Although tumor size was significant in a univariable analysis, this variable did not remain significant in multivariable analysis. Many previous studies found tumor size was an important independent predictor of EC patients with lower stage (19, 20). Primary tumor size may not play a significant role in prognosis in metastatic patients. Additionally, Caner Çakır et al. (21)reported that tumor size did not have prognostic value in EC patients with stage I or II. In the current study, no association between survival and marital status was found.
Our analysis found that lung was the most common metastatic organ (46.2%) in EC patients with bone metastasis, compared with other metastatic organs. In contrast to previous studies (7, 22), lung metastasis did not remain significant in multivariable analysis. Consistent with previous studies (3, 22), liver or brain metastasis were independent predictors of decreased OS and CSS in the multivariable analysis. Therefore, aggressive management of liver and brain metastases may be helpful in prolonging outcomes in EC patients with bone metastasis.
Traditional medical treatments for EC patients include surgery, radiotherapy, and chemotherapy (12, 23, 24). However, few data factually support their survival benefits in EC patients with bone metastasis. This study emphasized that good outcomes were associated with surgery and radiotherapy of primary tumors, and systemic chemotherapy, which agrees with the previous findings (12, 22). We first found that surgery or radiotherapy of primary tumors were independent predictors associated with increased OS. Although radiotherapy did not remain a significant risk factor in CSS, it can be used for palliative treatment of such patients (6, 25, 26). Multimodality therapy is strongly recommended for EC patients with bone metastasis.
Several limitations should be noted in this study. First, the retrospective nature of this study cannot be ignored. Second, clinical data on other systemic therapies are not available in the SEER database. Third, the types of surgery or radiotherapy are still not available in SEER database. Despite the above disadvantages of the SEER database, this cancer database provides clinicians a very useful tool for clinical cancer research.
Conclusions
We identified several factors affect the survival of EC patients with bone metastasis, including tumor grade, tumor subtype, liver and brain metastases, surgery, radiotherapy, and chemotherapy. This study is helpful for tailoring treatment regimen for EC patients with bone metastasis. However, further validation of our findings is needed in prospective, multicenter, randomized controlled studies.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Second Affiliated Hospital, Zhejiang University School of Medicine. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
ZW, YR, and ZY conceived and designed the study. HH, ZW, and MZ collected the data. HH, ZW, MZ, FN, and QY performed the statistical analysis. HH and ZW wrote the manuscript. ZW, YR, and ZY revised it. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81872173, 82072959), Natural Science Foundation of Zhejiang Province (LD21H160002), and Medical and Health Science and Technology Plan of Department of Health of Zhejiang Province (WKJ-ZJ-1821).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Brooks RA, Fleming GF, Lastra RR, Lee NK, Moroney JW, Son CH, et al. Current Recommendations and Recent Progress in Endometrial Cancer. CA: Cancer J Clin (2019) 69(4):258–79. doi: 10.3322/caac.21561
2. Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial Cancer. Lancet (London England) (2016) 387(10023):1094–108. doi: 10.1016/s0140-6736(15)00130-0
3. Mao W, Wei S, Yang H, Yu Q, Xu M, Guo J, et al. Clinicopathological Study of Organ Metastasis in Endometrial Cancer. Future Oncol (London England) (2020) 16(10):525–40. doi: 10.2217/fon-2020-0017
4. Liu Y, Chi S, Zhou X, Zhao R, Xiao C, Wang H. Prognostic Value of Distant Metastatic Sites in Stage IV Endometrial Cancer: A SEER Database Study of 2948 Women. Int J Gynaecol Obstetrics: Off Organ Int Fed Gynaecol Obstetrics (2020) 149(1):16–23. doi: 10.1002/ijgo.13084
5. Tanioka M, Katsumata N, Sasajima Y, Ikeda S, Kato T, Onda T, et al. Clinical Characteristics and Outcomes of Women With Stage IV Endometrial Cancer. Med Oncol (Northwood London England) (2010) 27(4):1371–7. doi: 10.1007/s12032-009-9389-3
6. Cowan M, Strauss JB, Barber EL, Matei D. Updates on Adjuvant Chemotherapy and Radiation Therapy for Endometrial Cancer. Curr Opin Obstetrics Gynecol (2019) 31(1):31–7. doi: 10.1097/gco.0000000000000506
7. Seagle BL, Alexander AL, Lantsman T, Shahabi S. Prognosis and Treatment of Positive Peritoneal Cytology In Early Endometrial Cancer: Matched Cohort Analyses From the National Cancer Database. Am J Obstetrics Gynecol (2018) 218(3):329.e1–.e15. doi: 10.1016/j.ajog.2017.11.601
8. Wang Z, Wu B, Zhou Y, Huang X, Pan W, Liu M, et al. Predictors of the Survival of Primary and Secondary Older Osteosarcoma Patients. J Cancer (2019) 10(19):4614–22. doi: 10.7150/jca.32627
9. Wang Z, Cheng Y, Chen S, Shao H, Chen X, Wang Z, et al. Novel Prognostic Nomograms for Female Patients With Breast Cancer and Bone Metastasis at Presentation. Ann Trans Med (2020) 8(5):197. doi: 10.21037/atm.2020.01.37
10. Wang Z, Chen G, Chen X, Huang X, Liu M, Pan W, et al. Predictors of the Survival of Patients With Chondrosarcoma of Bone and Metastatic Disease at Diagnosis. J Cancer (2019) 10(11):2457–63. doi: 10.7150/jca.30388
11. Qian SJ, Wu JQ, Wang Z, Zhang B. Surgery Plus Chemotherapy Improves Survival of Patients With Extremity Soft Tissue Leiomyosarcoma and Metastasis at Presentation. J Cancer (2019) 10(10):2169–75. doi: 10.7150/jca.29874
12. Zhu L, Sun X, Bai W. Nomograms for Predicting Cancer-Specific and Overall Survival Among Patients With Endometrial Carcinoma: A Seer Based Study. Front Oncol (2020) 10:269. doi: 10.3389/fonc.2020.00269
13. Horne ZD, Teterichko SR, Glaser SM, Wegner RE, Hasan S, Crafton SM, et al. Race-Driven Survival Differential in Women Diagnosed With Endometrial Cancers in the USA. Int J Gynecol Cancer: Off J Int Gynecol Cancer Soc (2020) 30(12):1893–901. doi: 10.1136/ijgc-2020-001560
14. Donkers H, Bekkers R, Massuger L, Galaal K. Socioeconomic Deprivation and Survival in Endometrial Cancer: The Effect of BMI. Gynecol Oncol (2020) 156(1):178–84. doi: 10.1016/j.ygyno.2019.10.030
15. AlHilli M, Elson P, Rybicki L, Amarnath S, Yang B, Michener CM, et al. Undifferentiated Endometrial Carcinoma: A National Cancer Database Analysis of Prognostic Factors and Treatment Outcomes. Int J Gynecol Cancer: Off J Int Gynecol Cancer Soc (2019) 29(7):1126–33. doi: 10.1136/ijgc-2019-000465
16. Yilmaz E, Gurocak S, Melekoglu R, Koleli I, Faydali S, Temelli O, et al. The Effect of Prognostic Factors and Adjuvant Radiotherapy on Survival in Patients With High-Grade Early-Stage Endometrial Cancer: A Retrospective Clinical Study. Med Sci Monitor: Int Med J Exp Clin Res (2019) 25:2811–8. doi: 10.12659/msm.913740
17. Matsuo K, Moeini A, Machida H, Scannell CA, Casabar JK, Kakuda M, et al. Tumor Characteristics and Survival Outcome of Endometrial Cancer Arising in Adenomyosis: An Exploratory Analysis. Ann Surg Oncol (2016) 23(3):959–67. doi: 10.1245/s10434-015-4952-y
18. Tanaka K, Kobayashi Y, Sugiyama J, Yamazaki T, Dozono K, Watanabe M, et al. Histologic Grade and Peritoneal Cytology as Prognostic Factors in Type 1 Endometrial Cancer. Int J Clin Oncol (2017) 22(3):533–40. doi: 10.1007/s10147-016-1079-5
19. Chattopadhyay S, Cross P, Nayar A, Galaal K, Naik R. Tumor Size: A Better Independent Predictor of Distant Failure and Death Than Depth of Myometrial Invasion in International Federation of Gynecology and Obstetrics Stage I Endometrioid Endometrial Cancer. Int J Gynecol Cancer: Off J Int Gynecol Cancer Soc (2013) 23(4):690–7. doi: 10.1097/IGC.0b013e31828c85c6
20. Mahdi H, Munkarah AR, Ali-Fehmi R, Woessner J, Shah SN, Moslemi-Kebria M. Tumor Size is an Independent Predictor of Lymph Node Metastasis and Survival in Early Stage Endometrioid Endometrial Cancer. Arch Gynecol Obstetrics (2015) 292(1):183–90. doi: 10.1007/s00404-014-3609-6
21. Çakır C, Kılıç İ, Yüksel D, Karyal YA, Üreyen I, Boyraz G, et al. Does Tumor Size Have Prognostic Value in Patients Undergoing Lymphadenectomy in Endometrioid-Type Endometrial Cancer Confined to the Uterine Corpus? Turkish J Med Sci (2019) 49(5):1403–10. doi: 10.3906/sag-1902-224
22. Li H, Zhang R, Chen C, Wu C, Lin H, Li J, et al. Prognostic Value of Different Metastatic Sites for Patients With FIGO Stage IVB Endometrial Cancer After Surgery: A SEER Database Analysis. J Surg Oncol (2020) 122(5):941–8. doi: 10.1002/jso.26102
23. Lee YC, Lheureux S, Oza AM. Treatment Strategies for Endometrial Cancer: Current Practice and Perspective. Curr Opin Obstetrics Gynecol (2017) 29(1):47–58. doi: 10.1097/gco.0000000000000338
24. Goodman CR, Hatoum S, Seagle BL, Donnelly ED, Barber EL, Shahabi S, et al. Association of Chemotherapy and Radiotherapy Sequence With Overall Survival in Locoregionally Advanced Endometrial Cancer. Gynecol Oncol (2019) 153(1):41–8. doi: 10.1016/j.ygyno.2019.01.007
25. Davidson BA, Moss HA, Arquiette J, Kamal AH. Top Ten Tips Palliative Care Clinicians Should Know When Caring for Patients With Endometrial Cancer. J Palliative Med (2018) 21(6):857–61. doi: 10.1089/jpm.2018.0053
Keywords: endometrial cancer, bone metastasis, clinicopathological characteristics, survival, risk factors
Citation: Hu H, Wang Z, Zhang M, Niu F, Yu Q, Ren Y and Ye Z (2021) Clinicopathological Characteristics and Prognosis in Endometrial Cancer With Bone Metastasis: A SEER-Based Study of 584 Women. Front. Oncol. 11:694718. doi: 10.3389/fonc.2021.694718
Received: 13 April 2021; Accepted: 17 June 2021;
Published: 01 July 2021.
Edited by:
Daniel A. Anaya, Moffitt Cancer Center, United StatesReviewed by:
Hooman Soleymani Majd, Oxford University Hospitals NHS Trust, United KingdomIvana Cataldo, ULSS2 Marca Trevigiana, Italy
Copyright © 2021 Hu, Wang, Zhang, Niu, Yu, Ren and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhan Wang, d2FuZ3poYW5oekB6anUuZWR1LmNu; Ying Ren, MjE4NzAzMEB6anUuZWR1LmNu; Zhaoming Ye, eWV6aGFvbWluZ0B6anUuZWR1LmNu
†These authors have contributed equally to this work
 Hejia Hu1,2,3†
Hejia Hu1,2,3† Zhan Wang
Zhan Wang Zhaoming Ye
Zhaoming Ye