
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 04 June 2021
Sec. Hematologic Malignancies
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.694594
This article is part of the Research Topic Metabolic Rewiring in Leukemias View all 8 articles
 Fei Han1,2,3†
Fei Han1,2,3† Huanhuan Zhao1,2,3†
Huanhuan Zhao1,2,3† Jun Lu1,2,3
Jun Lu1,2,3 Weina Yun1,2,3
Weina Yun1,2,3 Lingling Yang1,2,3
Lingling Yang1,2,3 Yude Lou1,2,3
Yude Lou1,2,3 Dan Su1,2,3
Dan Su1,2,3 Xin Chen1,2,3
Xin Chen1,2,3 Shixuan Zhang1,2,3
Shixuan Zhang1,2,3 Hanwei Jin1,2,3
Hanwei Jin1,2,3 Xiang Li1,2,3
Xiang Li1,2,3 Jie Sun2,4
Jie Sun2,4 He Huang2,3,4
He Huang2,3,4 Qishan Wang5
Qishan Wang5 Xi Jiang1,2,3*
Xi Jiang1,2,3*Dysregulation of ketone metabolism has been reported in various types of cancer. In order to find out its role in acute myeloid leukemia (AML) pathogenesis, we first analyzed the expression levels of 10 key genes involved in ketone metabolism in AML blasts and CD34+ hematopoietic stem cells (HSCs) from healthy donors. We found that the expression level of BDH1 was significantly lower in AML than in normal HSCs. The downregulation of BDH1 gene expression in AML cell lines as compared with normal HSCs was further confirmed with real-time RT-PCR. Analysis of TCGA and other database revealed that the downregulation of BDH1 was associated with worse prognosis in AML patients. In addition, we showed that overexpression of BDH1 inhibited the viability and proliferation of AML cells. In contrast, BDH1 knock-down promoted AML cell growth. Collectively, our results suggest the previously unappreciated anti-tumor role of BDH1 in AML, and low BDH1 expression predicts poor survival.
Acute myeloid leukemia (AML), the most common type of acute leukemia in adults, is a group of highly aggressive heterogeneous cancer (1). Despite of intensive traditional chemotherapy, the overall 3-year survival of AML patients was only around 25%. Many subtypes of AML are associated with intermediate to poor prognosis, especially in elder patients (2). Therefore, it is urgently needed to better understand the molecular mechanism of AML, and develop novel therapy targeting key molecules crucial for leukemogenesis.
Energy reprogramming is a hallmark in cancer. Dysregulation of metabolism, including the aerobic glycolysis (3–5), the amino acid (6) and fatty acid metabolism (7, 8), has been found in cancer, including AML. Targeting metabolic pathways provides a potential strategy for cancer therapy (9). Among the metabolic pathways, ketone metabolism, which includes hepatic ketogenesis and extrahepatic ketolysis, was used to be recognized as the byproduct of lipid metabolism. A bunch of regulators, such as the 3-hydroxymethylglutaryl-CoA synthase (HMGCS2), the hydroxymethylglutaryl coenzyme A lyase (HMGCL), the 3-oxoacid CoA transferase 1 (OXCT1), and the phosphatidylcholine-dependent mitochondrial βOHB dehydrogenase (BDH1), etc., are involved in the process. During ketogenesis, the condensation of AcAc-CoA and acetyl-CoA was catalyzed by HMGCS2 to generate hydroxymethylglutaryl (HMG)-CoA. HMGCL cleaves HMG-CoA to release acetyl-CoA and AcAc. AcAc is then converted into βOHB by BDH1 in a NAD+/NADH- coupled near-equilibrium reaction. During ketolysis, extrahepatic mitochondrial BDH1 catalyzes the conversion between βOHB and AcAc, and OXCT1 mediates the conversion of AcAc to AcAc-CoA. A reversible AcAc-CoA thiolase reaction catalyzed by any of the four mitochondrial thiolases ACAT1, ACAT2, HADHA, HADHB yields acetyl-CoA, which thereafter enters the TCA cycle for ATP production (10).
Several clinical and preclinical studies suggest the anti-tumor effect of ketone diet (KD) in solid tumors (11), such as glioma (12) and pancreatic cancer (13). It is also shown that KD can improve the response of PI3K inhibitor BKM120 in MLL-AF9 AML mouse model (14). However, the effects of KD in cancer patients still remain quite controversial (15). Up till now, knowledge about the ketone metabolism in AML is very limited. Therefore, it is necessary to identify the role and molecular mechanism of ketone body metabolism in AML.
In this study, we show the downregulation of key genes involved in ketone body metabolism in AML blasts as compared with normal hematopoietic stem cells (HSCs), and identify the association between BDH1 expression and AML prognosis. The anti-tumor role of BDH1 was then further confirmed in AML cells. Together, we show the anti-tumor effects of BDH1 in AML, which indicate the therapeutic potential of targeting BDH1 to cure AML.
The CDS of BDH1, encoding the human BDH1 gene, was amplified through PCR using primers 5′-TATAGCTAGCATGCTGGCCACCCG-3′ and 5′-TATAACCGGTTCAGCGGATGTAGATCAT-3′, and then cloned into pLJM1-EGFP lentiviral vector (Addgene, 19319). The CDS of mouse Bdh1 gene was amplified through PCR using primers 5′-TATACTCGAGATGCTAGCTGCC-3′ and 5′- TATAGAATTCTCAGTGTATGTAGATCTTGT-3’, and then ligated into a retroviral vector, namely MSCV-PIG (Addgene, 105594).
THP1 cells were maintained in RPMI 1640 supplemented with 10% FBS, and 1% penicillin-streptomycin. Mouse leukemic cells were kept in RPMI 1640 supplemented with 10 ng/mL interleukin 3 (IL-3), 10 ng/mL IL-6, 100 ng/mL SCF, 10% FBS and 1% penicillin-streptomycin. Human HSCs were maintained in IMDM supplemented with 20% FBS, 1% penicillin-streptomycin, 10 ng/mL IL-3, 10 ng/mL IL-6, 10 ng/mL Flt3 ligand, 10 ng/mL TPO, and 100 ng/mL SCF.
pMD2.G, psPAX2, pLJM1-EGFP plasmids were purchased from Addgene (Cambridge, MA). 0.7 μg pMD2.G, 0.7 μg psPAX2 and 1.5 μg pLJM1-EGFP constructs, i.e. pLJM1-BDH1 or pLJM1-EGFP control were co-transfected into HEK-293T cells in a 60 mm cell culture dish with Lipofectamine 2000 reagent (ThermoFisher, 11668). Lentiviral particles were harvested 48 and 72 hours after transfection. The lentivirus particles were directly added into leukemic cells and two rounds of ‘spinoculation’ (16–18) were performed to allow the infection of viruses.
Retrovirus vectors were co-transfected with pCL-Eco packaging vector into HEK293T cells using Lipofectamine 2000 reagent (ThermoFisher, 11668) to produce retrovirus. BM cells were harvested from 6-week-old B6.SJL (CD45.2) mice after five days of 5-fluorouracil (5-FU) treatment, and primitive hematopoietic progenitor cells were enriched with Mouse Lineage Cell Depletion Kit (Miltenyi Biotec Inc., 130-090-858). An aliquot of enriched hematopoietic progenitor cells was added to retroviral supernatant together with polybrene in cell culture plates, which were centrifuged at 2,000 g for 3 hours at 30°C [i.e., ‘spinoculation’ (16–18)] and then the medium was replaced with fresh medium and incubated for 20 hours at 37°C. Next day, the same procedure was repeated once. Cultures were incubated at 37°C in a humidified atmosphere of 5% CO2 in air.
10,000 cells were seeded into each well of a 96-well plate, and allowed to grow for 4-5 days to build a growth curve. For cell viability assay, cells were seeded into 96-well plates in triplicates and MTT (Sigma, M2128) was used to assess cell proliferation and viability following the manufacturer’s instructions. Briefly, cells were seeded at a density of 5000-10000 cells/100 μL. Dye solution was added at indicated time points and incubated at 37°C for 3-4 hours before adding of solubilization/stop solution to stop the reaction. The absorbance at 540 nm was read at the end point using SynergyMx M5.
For quantitative real-time PCR (qRT-PCR) analysis, the cDNAs were amplified using HiScript® II Q RT SuperMix (Vazyme, R223) for qRT-PCR. Relative gene expression levels were detected through qRT-PCR using the ChamQ™ SYBR® qPCR Master Mix (Vazyme, Q311) and LightCycler® 480 System. Using GAPDH as an internal reference, the ΔΔCT method was used to quantify the relative expression of target genes. The primer sequences were as follows: GAPDH-Forward, 5’-AATCCCATCACCATCTTCCAG-3’; GAPDH-Reverse, 5’-AAATGAGCCCCAGCCTTC-3’; BDH1-Forward, 5’-GGCAGAAGTGAACCTTTGGG-3’; BDH1-Reverse, 5’-GCAGTCCGAGAAAGCCTCTAC-3’; Gapdh-Forward, 5’-AGGTCGGTGTGAACGGATTTG-3’; Gapdh-Reverse, 5’-TGTAGACCATGTAGTTGAGGTCA-3’; Bdh1-Forward, 5’-TTCCCCTTCTCCGAAGAGC-3’; Bdh1-Reverse, 5’ -CCCAGAGGGTGCATCTCATAG-3’.
The RNA-seq databases (TCGA-LAML, n=151) and patient information were obtained from National Cancer Institute GDC Data Portal. The RNA-seq and patient clinical data in BEATAML database were downloaded from cBioPortal online tool (https://www.cbioportal.org). The Agilent microarray data sets (GSE9476, GSE6891, GSE24006, GSE65409) were obtained from Gene Expression Omnibus (GEO) database.
The Agilent microarray probes IDs for GSE9476, GSE6891, GSE24006 and GSE65409 were annotated using the platform GPL96 [(HG-U133A) Affymetrix Human Genome U133A Array], GPL570 [(HG-U133_Plus_2) Affymetrix Human Genome U133 Plus 2.0 Array] and GPL10881 [Affymetrix Human Genome U133 Plus 2.0 Array (CDF: HGU133Plus2_Hs_REFSEQ_12.1.0)], GPL6947 (Illumina HumanHT-12 V3.0 expression beadchip), respectively. When multiple probes mapped to the same gene, the mean of the signal intensities was used.
To investigate the association of gene expression with survival time in the TCGA-LAML and GSE6891 database, the median expression level was employed to divide the patients into expression-high and expression-low group. Kaplan-Meier plot and log-rank (Mantel-Cox) test were used to compare overall survival months between two groups.
Unsupervised hierarchical clustering of 8 ketone metabolism genes was conducted using the Pheatmap function in R package pheatmap. cDNA microarray expression data were subjected as a numeric matrix to clustering analysis, using ‘complete linkage’ as clustering method, using ‘Euclidean Distance’ as distance method.
Statistical analysis was performed by using GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA). The independent Student’s t-test was used to analyze the results and data are expressed as the means ± SEM. Kaplan-Meier plots were analyzed for survival analysis. The Mann-Whitney test was used for non-normally distributed data and was analyzed by IBM SPSS Statistics 21 software for Windows (SPSS Inc., Chicago, IL, USA).
To explore the expression pattern of genes participating in ketogenesis and ketolysis in AML, a total of 10 genes from “Synthesis and degradation of ketone bodies” (hsa00072) of Kyoto Encyclopedia of Genes and Genomes (KEGG) were selected for the following analysis (Table 1). The 10 genes were: ACAT1, ACAT2, BDH1, BDH2, HMGCS1, HMGCS2, HMGCL, HMGCLL1, OXCT1, OXCT2. We analyzed the cDNA microarray data of 26 cases of AML blasts and 18 cases of normal HSCs from GEO database (GSE9476), and found information of 9 of the above 10 genes (except HMGCLL1, which was not included in the database) from the database. Results show that expression levels of 8 of the 9 genes, i.e., ACAT1, ACAT2, BDH1, BDH2, HADHB, HMGCL, HMGCS1, and OXCT1, were significantly downregulated in AML (Figure 1A). We also analyzed the transcriptional levels of the above 8 genes using mRNA expression data downloaded from GSE24006 database, including 7 AML blasts and 7 normal HSCs, and GSE65409 database including 30 AML blasts and 8 normal HSCs. The significant downregulation of 3 of the 8 genes, i.e., BDH1, HADHB and OXCT1, in AML, was further confirmed as compared to the normal HSCs (Figures 1B and S1A, B). This suggests the dysregulation in expression of ketone body metabolism associated genes in AML.
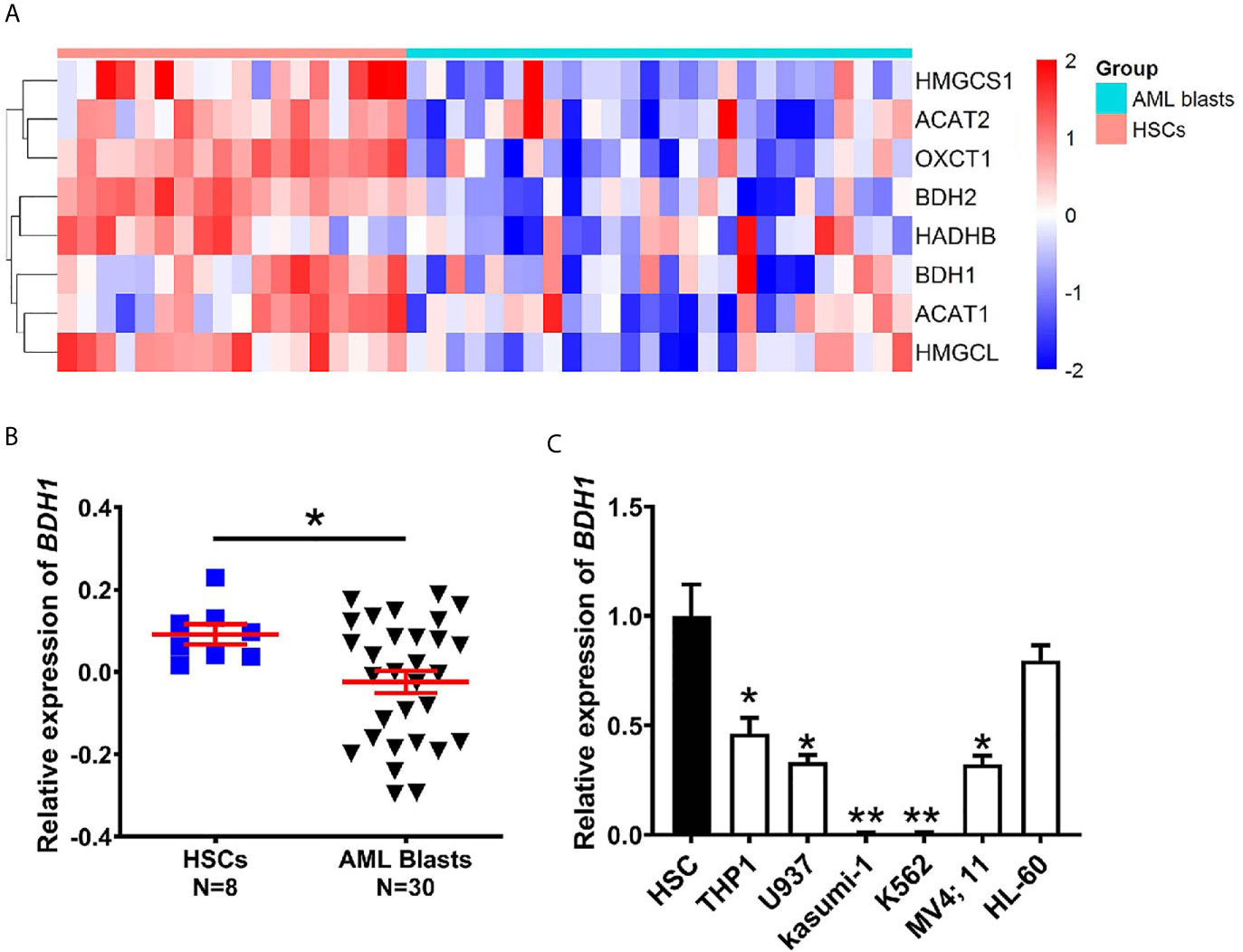
Figure 1 The expression of BDH1 is downregulated in AML as compared with normal HSCs. (A) A heatmap of the hierarchical clustering of 8 key genes involved in ketone body metabolism. Input data are the mRNA expression values of AML blasts over normal HSCs. Upregulated genes are designated with red color, while blue color indicates downregulated genes. (B) The mRNA expression analysis of BDH1 in 30 cases of AML blasts and 8 cases of normal HSCs in GSE65409 database. The expression values in GSE65409 were log2-transformed and mean centered. The P values were detected by t-test. (C) The expression level of BDH1 was determined by qRT-PCR in AML cell lines and normal HSCs, normalized to GAPDH. *p < 0.05, **p < 0.01.
To further verify the findings, we performed qRT-PCR, and tested the expression levels of BDH1, HADHB and OXCT1 in several AML cell lines, i.e., THP1/t(9;11), U937/t(10;11), Kasumi-1/t(8;21), MV4;11/t(4;11), HL-60/del(16), K562/t(9;22) (CML) and normal HSCs. The expression level of BDH1 was significantly lower in AML cells than in normal HSCs (Figure 1C), in consistence with results of the above data analysis (Figures 1A, B). Given the fact that BDH1 was the most broadly and consistently suppressed gene among the 3 genes investigated in AML, we focused on BDH1 in the following study.
In order to find out the potential relationship between BDH1 gene expression and AML prognosis, we first compared the expression patterns of BDH1 in different subtypes of AML. Results show that the expression level of BDH1 was significantly lower in patients with poor cytogenetics risk than in those with favorable or intermediate cytogenetics risk (Figures 2A, B). The above pattern was further confirmed in another analysis according to the ELN2017 risk classification (19) (Figure S2A). More, AML-M3, which is classified as acute promyelocytic leukemia (APL) and has a favorable prognosis (20), was the FAB subtype that had significantly up-regulated BDH1 expression (Figures 2C, D and S2B). Increased age is often associated with increased risk of chemoresistance and death in AML patients (21). We found that BDH1 expression was remarkably lower in patients over 65-years old (Figure S2C). CEBPA biallelic mutations were shown to be associated with a better prognosis in AML (22). Here we found that the BDH1 expression level in patients with CEBPA biallelic mutations was significantly higher than in the those with no CEBPA mutations (Figures S2D, E). The above results indicate the association between BDH1 expression and AML clinicopathological characteristics.
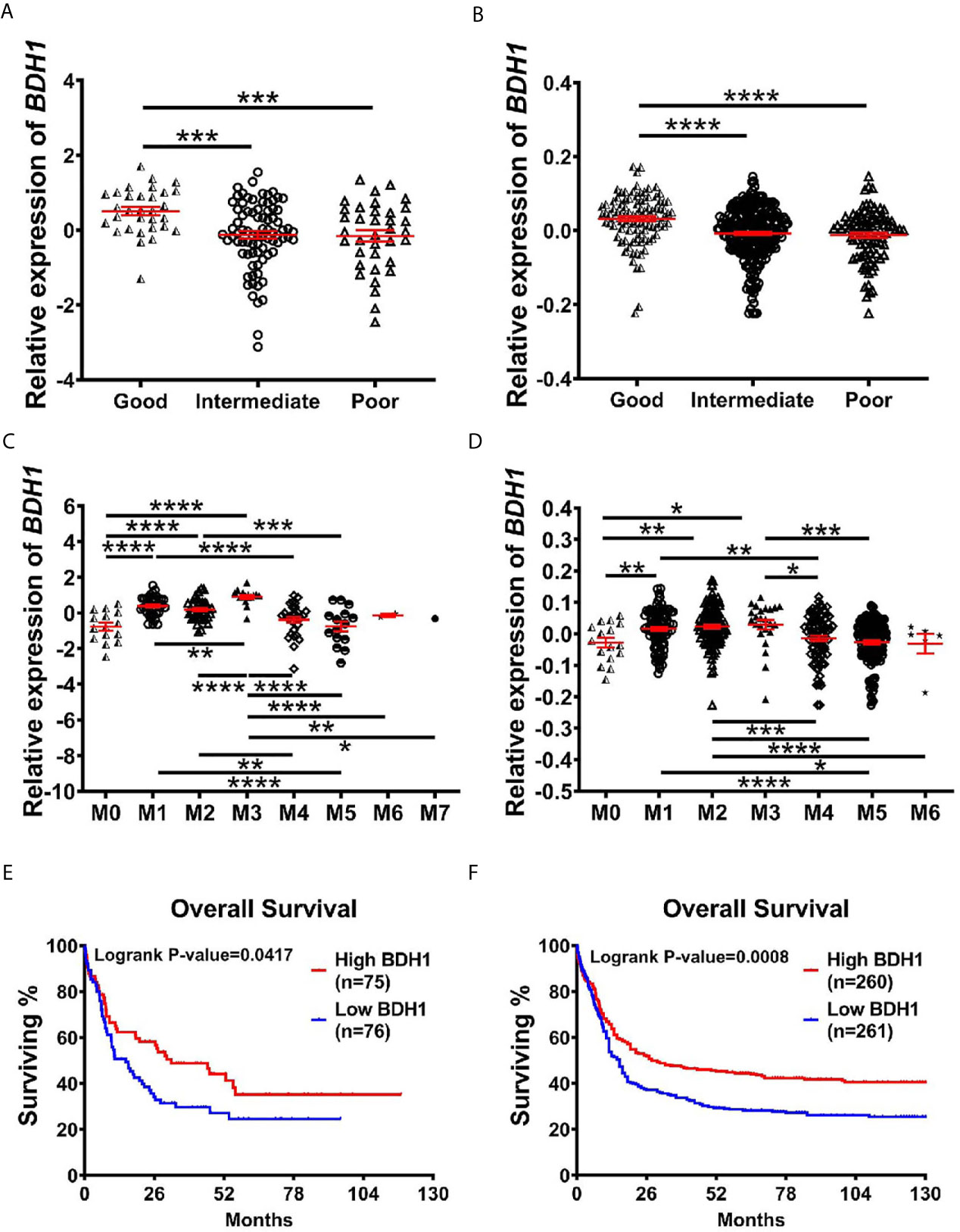
Figure 2 The lower expression of BDH1 predicts the worse prognosis for AML patients. (A, B) Shown are comparisons of BDH1 gene expression levels among AML patients of different cytogenetic-risk classifications in TCGA-LAML database (A) and GSE6891 database (B), and comparison of BDH1 gene expression among AML patients of different FAB subtypes in TCGA-LAML database (C) and GSE6891 database (D). The expression values were log2-transformed and mean centered. The P values were detected by t-test. (E, F) Kaplan-Meier survival curves of BDH1 in TCGA-LAML (left) (E) and GSE6891 (right) (F) database. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Further, we investigated the potentially prognostic significance of BDH1 expression levels in AML. Kaplan-Meier analysis of TCGA-LAML database consisting of 151 AML cases including 63 normal karyotypes, 10 inv(16), 7 t(8;21), 14 t(15;17), 7 t(11q23) and 50 other karyotypes showed that the lower expression of BDH1 correlated with the worse overall survival (OS, log-rank test, p=0.0417) in AML patients (Figure 2E). A similar pattern was then confirmed in the GSE6891 database consisting of 521 AML cases including 187 normal karyotypes, 33 inv(16), 35 t(8;21), 21 t(15;17), 10 t(11q23) and 235 other karyotypes (OS, log-rank test, p=0.0008) (Figure 2F). These results suggest BDH1 predicts favorable outcome in AML.
A variety of genes, such as HOXA9, MEIS1 and TP53, have been shown to be critically involved in AML pathogenesis (23–26). The upregulation of HOXA9 was reported in more than 50% of AML and is highly associated with poor prognosis (23). MEIS1, a critical regulator of leukemia stem cells (LSCs), is often found overexpressed in Hox-gene-driven leukemia (24). The combinational overexpression of Hoxa9 and Meis1 leads to a massive acceleration of leukemia development (25). As a tumor suppressor gene, TP53 inactivation by gene deletion or mutation potently promotes AML (26). Here we investigated the correlation between expression levels of BDH1 and HOXA9, MEIS1, or TP53 genes in AML in TCGA-LAML and BEATAML database. Results show that the expression of BDH1 positively correlated with TP53 (r=0.2602, p=0.0013 in TCGA, r=0.4786, p<0.0001 in BEATAML) (Figures 3A, B), while negatively correlated with HOXA9 (r=-0.3216, p<0.0001 in TCGA; r=-0.1041, p=0.0298 in BEATAML) (Figures 3C, D) and MEIS1 (r=-0.3935, p<0.0001 in TCGA; r=-0.1152, p=0.0154 in BEATAML) (Figures 3E, F). The above results suggest the potential correlation between BDH1 and other oncogenes and/or tumor suppressors in AML.
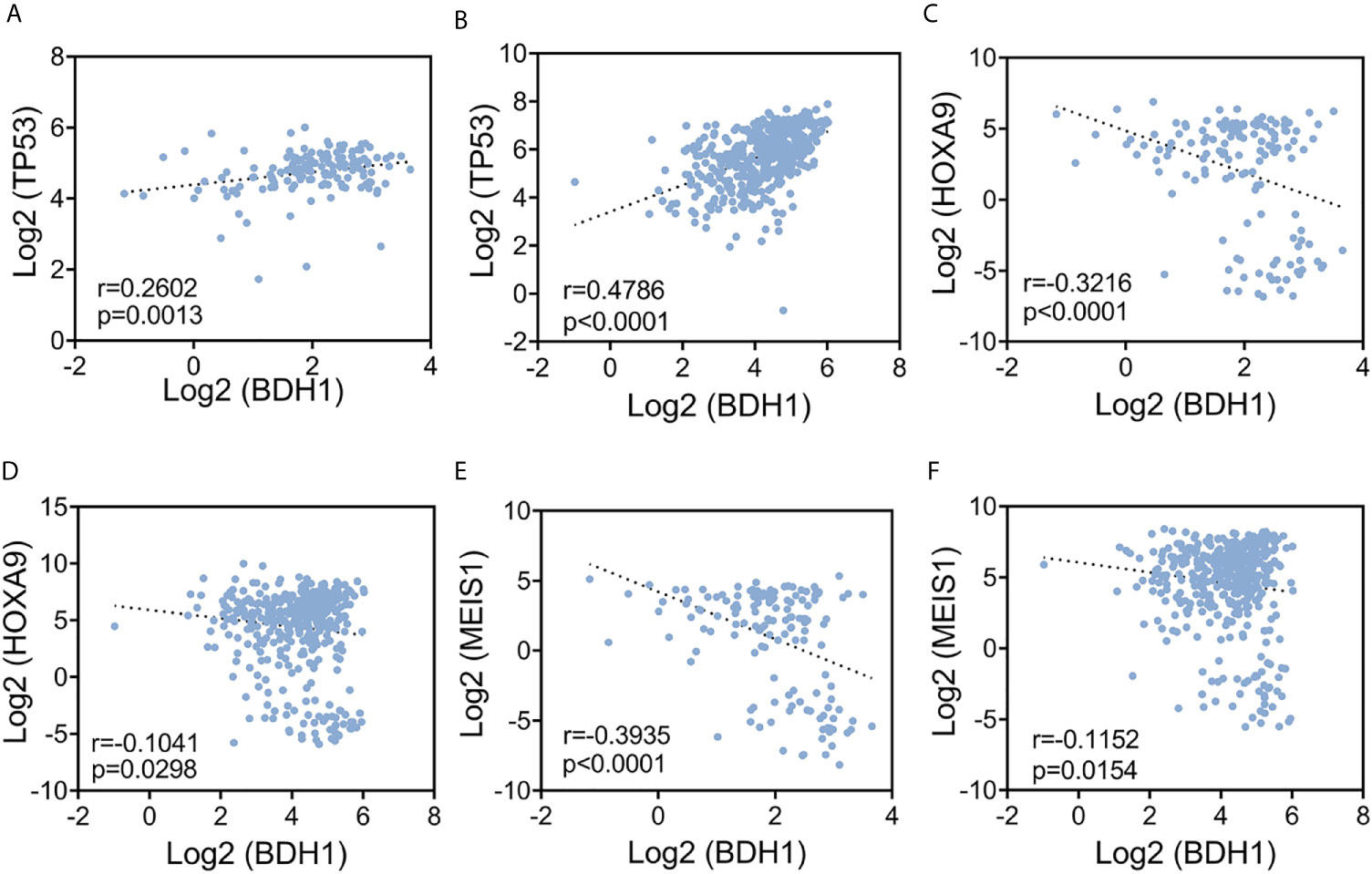
Figure 3 The expression level of BDH1 is significantly correlated with that of HOXA9, MEIS1, and TP53 in AML. Results of the pair-wise inter-correlation analysis of gene expression levels of BDH1 and TP53, HOXA9, MEIS1 based on TCGA-LAML database (A, C, E) and BEATAML database (B, D, F) are shown.
To assess the function of BDH1 gene in AML, we first cloned the Bdh1 CDS into MSCV-PIG retroviral vector, and then co-infected mouse bone marrow (BM) progenitor cells with MSCVneo-MLL-AF9 (MA9) and MSCV-PIG-Bdh1 (Bdh1) or MSCV-PIG (Ctrl) retroviruses. The expression of exotic Bdh1 was confirmed at mRNA level with real-time RT-PCR assays (Figure 4A). We found that cell viability of MA9 BM cells overexpressing Bdh1 was significantly lower than control group (Figure 4B). Bdh1 overexpression remarkably suppressed cell proliferation (Figure 4C). In order to interfere BDH1 expression in human AML cell line, human BDH1 was cloned into PLJM1-EGFP vector, and PLJM1-EGFP was used as the negative control. Then THP1/t(9;11) cells were infected with PLJM1-EGFP (Control) or PLJM1-BDH1 (BDH1) lentivirus. The expression of exotic BDH1 was confirmed at mRNA level with real-time RT-PCR assays (Figure 4D). Forced expression of BDH1 significantly inhibited cell proliferation in THP1 cells (Figure 4E). We then knocked down BDH1 expression by use of shRNAs. In contrast, the speed of proliferation of THP1 cells with BDH1 knockdown was significantly higher than that of the control cells (Figures 4F, G). Taken together, our data verified that BDH1 exhibited an anti-tumor effect in MA9 AML cells.
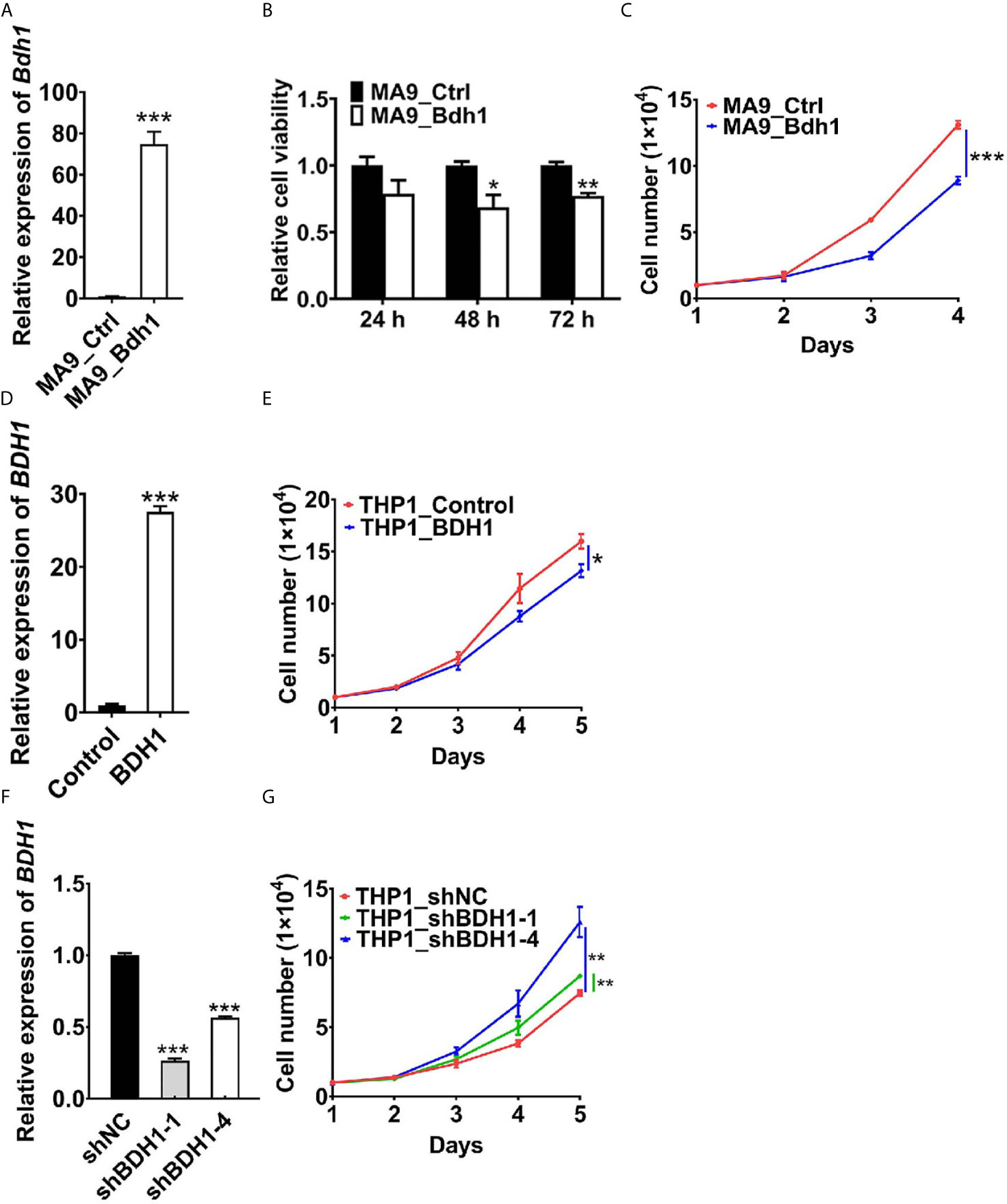
Figure 4 BDH1 suppresses the growth of MLL-AF9 AML cells. (A) The expression level of Bdh1 in mouse BM cells infected with MSCVneo-MLL-AF9 (MA9), MSCV-PIG-Bdh1 (Bdh1) or MSCV-PIG (Ctrl) retrovirus was verified through qRT-PCR after positive screening for 5 days. (B) Bdh1 overexpression repressed cell viability of MLL-AF9-transduced mouse BM cells. Mouse BM cells were infected with MSCVneo-MLL-AF9 (MA9), MSCV-PIG-Bdh1 (Bdh1) or MSCV-PIG (Ctrl) retrovirus. MTT assays were conducted 24, 48, 72 hours after seeding. (C) Cell number of each of the above groups was counted once per day until 4 days post-seeding. (D) The gene expression level of BDH1 in THP1 cells infected with PLJM1-GFP (Control), PLJM1-BDH1 (BDH1) lentivirus was verified through qRT-PCR after drug screening for 7 days. (E) BDH1 overexpression inhibited cell growth of THP1 cells. Cell number of each of the above groups was counted once per day until 5 days post-seeding. (F) The expression level of BDH1 in THP1 cells infected with PLKO.1-TRC (shNC), PLKO.1-BDH1-shRNA-1 (shBDH1-1) and PLKO.1-BDH1-shRNA-4 (shBDH1-4) lentivirus was determined by qRT-PCR after positive screening for 7 days. (G) BDH1 knock-down promoted the growth of THP1 cells. Cell number of each of the above groups was counted once per day until 5 days post-seeding. *p < 0.05, **p < 0.01, ***p < 0.001.
Despite of dysregulation of the aerobic glycolysis (3–5), the amino acid (6) and fatty acid metabolism (7, 8), etc., the role and regulatory mechanism of ketone metabolism in AML still remain obscure. In this study, through analysis of several AML databases, we first showed the downregulation of BDH1, a key regulator of ketone body metabolism in AML blasts, as compared with normal HSCs. Then we proved that BDH1 expression positively correlated with AML prognosis. Through in vitro studies in human and mouse AML cells, we further confirmed the anti-tumor effect of BDH1 in AML. Our findings not only reveal the previously unappreciated anti-tumor function of BDH1 in AML, but also indicate the potential of targeting BDH1 to cure AML.
Abnormal metabolism such as enhanced glycolysis, nutrient addiction, lipid metabolism dysregulation, has been shown to be involved in AML pathogenesis (27). Therefore, targeting metabolic pathways holds great potential in treating AML. For example, the Internal tandem duplication (ITD) mutations in Fms-like tyrosine kinase 3 gene (FLT3-ITD) are known to be associated with the upregulation of the glycolytic gate keeper enzyme hexokinase 2 (HK-2) and increased glycolytic activity in AML. Glycolytic inhibitors such as 3-Bromopyruvate propyl ester (3-BrOP) and 2-deoxyglucose (2-DG) exhibited cytotoxicity in murine and human leukemia cells carrying FLT3/ITD mutation (28). Lise et al. found that knockdown of SLC1A5, the transporter for glutamine uptake, could induce apoptosis in MOLM-14 AML cells and inhibit tumor formation in the mouse AML xenotransplantation model (6). More, Yoko et al. showed that inhibition of fatty acid oxidation (FAO) by Etomoxir (FAO inhibitor) induced apoptosis in U937 AML cells co-cultured with BM adipocytes (29). And it was recently reported that BRQ (dihydroorotate dehydrogenase (DHODH) inhibitor) treatment could sensitize chemoresistant AML cells in AML mice (30). However, knowledge of some metabolic pathways, such as the ketone metabolism, in cancer, and especially in AML, still remains limited. Therefore, it is crucial to further explore into various metabolic pathways in AML, and develop novel targeted therapy based on a better understanding of the molecular mechanisms of each metabolic pathway.
Among these metabolisms, ketones are known as an important alternative energy source during metabolic stress. The KD is regarded as a promising adjuvant and a patient-specific multifactorial cancer therapy. As a key regulator in both ketogenesis and ketolysis, BDH1 has been shown to determine the cell fate of adipocytes (31, 32). However, the role of BDH1 in cancer still remains unclear. The expression levels of BDH1 vary among different cancer cells. Low expression levels of ketolytic enzymes, including BDH1, have been found in tumors such as pancreatic cancer and gliomas (12, 33, 34). BDH1 expression level is closely associated with the amount of ketone body in cancer cell lines (35). βOHB treatment was shown to be able to partly rescue the growth inhibition effect of low glucose in Hela cells, which has relatively high BDH1 expression, but not in PNAC-1 cells with low BDH1 level (33). The role of BDH1 and ketone body in cancer and cancer therapy still remains quite controversial. Here we show the anti-tumor role of BDH1 in AML, which enlightens the possibility of restoration of BDH1 expression as an AML therapy. It is highly possible that KD would benefit AML patients especially those with abnormal BDH1 expression and dysregulated ketone metabolism.
In our present work, we found that the transcriptional inactivation of BDH1 significantly correlated with poor prognosis and shorter survival in AML patients. Thus, the gene expression level of BDH1 has a great power in AML diagnosis and prognosis. We further confirmed that BDH1 expression level was negatively correlated with the expression levels of oncogenes, e.g., HOXA9 and MEIS1, but was positively correlated with the tumor suppressor TP53. The regulatory mechanisms of BDH1 on AML leukemogenesis and therapy, as well as on the expression of HOXA9, MEIS1 and TP53 are no doubt worth further investigation.
From the present research, we show the downregulation and the anti-tumor effects of BDH1 in AML. The expression level of BDH1 is positively correlated with AML prognosis. Our study not only provides novel insights into the mechanism of AML pathogenesis and metabolism, but also indicates the potential of targeting BDH1 and ketone metabolism in treating AML.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the institutional ethics review board of the First Affiliated Hospital of Zhejiang University and Zhejiang University School of Medicine. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by the ethics committee of the Laboratory Animal Center of Zhejiang University.
FH and XJ conceived the study and wrote the paper. FH, HZ, JL, WY, LY, YL, DS, XC, SZ, HJ, XL, JS, HH, QW, and XJ performed the experiments and data analysis. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31900426 and 81970144) (to XJ).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Dr. Jianjun Chen of Beckman Research Institute of City of Hope for his kind gift of the MSCVneo-MLL-AF9 plasmid. The authors sincerely appreciate the technical support from the Core Facilities, Zhejiang University School of Medicine, and the support from Key Laboratory of Immunity and Inflammatory Diseases of Zhejiang Province.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.694594/full#supplementary-material
AML, acute myeloid leukemia; AcAc, acetoacetate; βOHB, βhydroxybutyrate; AcAc-CoA, acetoactyl-CoA; HMGCS2, 3-hydroxymethylglutaryl-CoA synthase; HMG-CoA, hydroxymethylglutaryl-CoA; HMGCL, Hydroxymethylglutaryl coenzyme A lyase; KD, ketone diet; IL-3, interleukin 3; 5-FU, 5-fluorouracil; qRT-PCR, quantitative real-time PCR; GEO, Gene Expression Omnibus; KEGG, Kyoto Encyclopedia of Genes and Genomes; HSCs, hematopoietic stem cells; APL, acute promyelocytic leukemia; OS, overall survival; BM, bone marrow; ITD, Internal tandem duplication; FLT3, Fms-like tyrosine kinase 3; HK2, hexokinase 2; 3-BrOP, 3-Bromopyruvate propyl ester; 2-DG, 2-deoxyglucose; FAO, fatty acid oxidation; DHODH, dihydroorotate dehydrogenase.
1. Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med (2015) 373:1136–52. doi: 10.1056/NEJMra1406184
2. Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and Management of Acute Myeloid Leukemia in Adults: Recommendations from an International Expert Panel, on Behalf of the European Leukemianet. Blood (2010) 115:453–74. doi: 10.1182/blood-2009-07-235358
3. Herst PM, Howman RA, Neeson PJ, Berridge MV, Ritchie DS. The Level of Glycolytic Metabolism in Acute Myeloid Leukemia Blasts at Diagnosis Is Prognostic for Clinical Outcome. J Leukoc Biol (2011) 89:51–5. doi: 10.1189/jlb.0710417
4. Ye H, Adane B, Khan N, Alexeev E, Nusbacher N, Minhajuddin M, et al. Subversion of Systemic Glucose Metabolism as a Mechanism to Support the Growth of Leukemia Cells. Cancer Cell (2018) 34:659–73.e656. doi: 10.1016/j.ccell.2018.08.016
5. Chen WL, Wang YY, Zhao A, Xia L, Xie G, Su M, et al. Enhanced Fructose Utilization Mediated by SLC2A5 Is a Unique Metabolic Feature of Acute Myeloid Leukemia with Therapeutic Potential. Cancer Cell (2016) 30:779–91. doi: 10.1016/j.ccell.2016.09.006
6. Willems L, Jacque N, Jacquel A, Neveux N, Maciel TT, Lambert M, et al. Inhibiting Glutamine Uptake Represents an Attractive New Strategy for Treating Acute Myeloid Leukemia. Blood (2013) 122:3521–32. doi: 10.1182/blood-2013-03-493163
7. Tabe Y, Konopleva M, Andreeff M. Fatty Acid Metabolism, Bone Marrow Adipocytes, and AML. Front Oncol (2020) 10:155. doi: 10.3389/fonc.2020.00155
8. Samudio I, Harmancey R, Fiegl M, Kantarjian H, Konopleva M, Korchin B, et al. Pharmacologic Inhibition of Fatty Acid Oxidation Sensitizes Human Leukemia Cells to Apoptosis Induction. J Clin Invest (2010) 120:142–56. doi: 10.1172/jci38942
9. Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab (2016) 23:27–47. doi: 10.1016/j.cmet.2015.12.006
10. Puchalska P, Crawford PA. Multi-Dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab (2017) 25:262–84. doi: 10.1016/j.cmet.2016.12.022
11. Weber DD, Aminzadeh-Gohari S, Tulipan J, Catalano L, Feichtinger RG, Kofler B. Ketogenic Diet in the Treatment of Cancer - Where Do We Stand? Mol Metab (2020) 33:102–21. doi: 10.1016/j.molmet.2019.06.026
12. Vallejo FA, Shah SS, de Cordoba N, Walters WM, Prince J, Khatib Z, et al. The Contribution of Ketone Bodies to Glycolytic Inhibition for the Treatment of Adult and Pediatric Glioblastoma. J Neurooncol (2020) 147:317–26. doi: 10.1007/s11060-020-03431-w
13. Shukla SK, Gebregiworgis T, Purohit V, Chaika NV, Gunda V, Radhakrishnan P, et al. Metabolic Reprogramming Induced by Ketone Bodies Diminishes Pancreatic Cancer Cachexia. Cancer Metab (2014) 2:18. doi: 10.1186/2049-3002-2-18
14. Hopkins BD, Pauli C, Du X, Wang DG, Li X, Wu D, et al. Suppression of Insulin Feedback Enhances the Efficacy of PI3K Inhibitors. Nature (2018) 560:499–503. doi: 10.1038/s41586-018-0343-4
15. Erickson N, Boscheri A, Linke B, Huebner J. Systematic Review: Isocaloric Ketogenic Dietary Regimes for Cancer Patients. Med Oncol (2017) 34:72. doi: 10.1007/s12032-017-0930-5
16. Huang H, Jiang X, Li Z, Li Y, Song CX, He C, et al. TET1 Plays an Essential Oncogenic Role in MLL-Rearranged Leukemia. Proc Natl Acad Sci USA (2013) 110:11994–9. doi: 10.1073/pnas.1310656110
17. Li Z, Huang H, Chen P, He M, Li Y, Arnovitz S, et al. miR-196b Directly Targets Both HOXA9/MEIS1 Oncogenes and FAS Tumour Suppressor in MLL-Rearranged Leukaemia. Nat Commun (2012) 3:688. doi: 10.1038/ncomms1681
18. Jiang X, Hu C, Arnovitz S, Bugno J, Yu M, Zuo Z, et al. miR-22 has a Potent Anti-Tumour Role With Therapeutic Potential in Acute Myeloid Leukaemia. Nat Commun (2016) 7:11452. doi: 10.1038/ncomms11452
19. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and Management of AML in Adults: 2017 ELN Recommendations From an International Expert Panel. Blood (2017) 129:424–47. doi: 10.1182/blood-2016-08-733196
20. De Kouchkovsky I, Abdul-Hay M. Acute Myeloid Leukemia: A Comprehensive Review and 2016 Update. Blood Cancer J (2016) 6:e441. doi: 10.1038/bcj.2016.50
21. Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and Acute Myeloid Leukemia. Blood (2006) 107:3481–5. doi: 10.1182/blood-2005-09-3724
22. Dufour A, Schneider F, Metzeler KH, Hoster E, Schneider S, Zellmeier E, et al. Acute Myeloid Leukemia With Biallelic CEBPA Gene Mutations and Normal Karyotype Represents a Distinct Genetic Entity Associated With a Favorable Clinical Outcome. J Clin Oncol (2010) 28:570–7. doi: 10.1200/jco.2008.21.6010
23. Kawagoe H, Humphries RK, Blair A, Sutherland HJ, Hogge DE. Expression of HOX Genes, HOX Cofactors, and MLL in Phenotypically and Functionally Defined Subpopulations of Leukemic and Normal Human Hematopoietic Cells. Leukemia (1999) 13:687–98. doi: 10.1038/sj.leu.2401410
24. Wong P, Iwasaki M, Somervaille TC, So CW, Cleary ML. Meis1 Is an Essential and Rate-Limiting Regulator of MLL Leukemia Stem Cell Potential. Genes Dev (2007) 21:2762–74. doi: 10.1101/gad.1602107
25. Kroon E, Krosl J, Thorsteinsdottir U, Baban S, Buchberg AM, Sauvageau G. Hoxa9 Transforms Primary Bone Marrow Cells Through Specific Collaboration With Meis1a But Not Pbx1b. EMBO J (1998) 17:3714–25. doi: 10.1093/emboj/17.13.3714
26. Welch JS. Patterns of Mutations in TP53 Mutated AML. Best Pract Res Clin Haematol (2018) 31:379–83. doi: 10.1016/j.beha.2018.09.010
27. Kreitz J, Schönfeld C, Seibert M, Stolp V, Alshamleh I, Oellerich T, et al. Metabolic Plasticity of Acute Myeloid Leukemia. Cells (2019) 8:805. doi: 10.3390/cells8080805
28. Ju HQ, Zhan G, Huang A, Sun Y, Wen S, Yang J, et al. ITD Mutation in FLT3 Tyrosine Kinase Promotes Warburg Effect and Renders Therapeutic Sensitivity to Glycolytic Inhibition. Leukemia (2017) 31:2143–50. doi: 10.1038/leu.2017.45
29. Tabe Y, Yamamoto S, Saitoh K, Sekihara K, Monma N, Ikeo K, et al. Bone Marrow Adipocytes Facilitate Fatty Acid Oxidation Activating AMPK and a Transcriptional Network Supporting Survival of Acute Monocytic Leukemia Cells. Cancer Res (2017) 77:1453–64. doi: 10.1158/0008-5472.can-16-1645
30. van Gastel N, Spinelli JB, Sharda A, Schajnovitz A, Baryawno N, Rhee C, et al. Induction of a Timed Metabolic Collapse to Overcome Cancer Chemoresistance. Cell Metab (2020) 32:391–403.e396. doi: 10.1016/j.cmet.2020.07.009
31. Otsuka H, Kimura T, Ago Y, Nakama M, Aoyama Y, Abdelkreem E, et al. Deficiency of 3-Hydroxybutyrate Dehydrogenase (BDH1) in Mice Causes Low Ketone Body Levels and Fatty Liver During Fasting. J Inherit Metab Dis (2020) 43:960–8. doi: 10.1002/jimd.12243
32. Wang W, Ishibashi J, Trefely S, Shao M, Cowan AJ, Sakers A, et al. A PRDM16-Driven Metabolic Signal From Adipocytes Regulates Precursor Cell Fate. Cell Metab (2019) 30:174–89.e175. doi: 10.1016/j.cmet.2019.05.005
33. Zhang J, Jia PP, Liu QL, Cong MH, Gao Y, Shi HP, et al. Low Ketolytic Enzyme Levels in Tumors Predict Ketogenic Diet Responses in Cancer Cell Lines In Vitro and In Vivo. J Lipid Res (2018) 59:625–34. doi: 10.1194/jlr.M082040
34. Chang HT, Olson LK, Schwartz KA. Ketolytic and Glycolytic Enzymatic Expression Profiles in Malignant Gliomas: Implication for Ketogenic Diet Therapy. Nutr Metab (Lond) (2013) 10:47. doi: 10.1186/1743-7075-10-47
Keywords: BDH1, ketone, metabolism, AML - acute myeloid leukemia, tumor suppressor, prognostic factors
Citation: Han F, Zhao H, Lu J, Yun W, Yang L, Lou Y, Su D, Chen X, Zhang S, Jin H, Li X, Sun J, Huang H, Wang Q and Jiang X (2021) Anti-Tumor Effects of BDH1 in Acute Myeloid Leukemia. Front. Oncol. 11:694594. doi: 10.3389/fonc.2021.694594
Received: 13 April 2021; Accepted: 12 May 2021;
Published: 04 June 2021.
Edited by:
Emanuele Ammatuna, University Medical Center Groningen, NetherlandsReviewed by:
Albrecht Reichle, University Medical Center Regensburg, GermanyCopyright © 2021 Han, Zhao, Lu, Yun, Yang, Lou, Su, Chen, Zhang, Jin, Li, Sun, Huang, Wang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xi Jiang, eGppYW5nQHpqdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.