- 1Department of Urology, Center for Urologic Cancer, Research Institute and Hospital of National Cancer Center, Goyang, South Korea
- 2National Cancer Control Institute, National Cancer Center, Goyang, South Korea
We retrospectively analyzed therapeutic strategies and risk factors for overall survival (OS) in disease recurrence following curative nephrectomy for localized renal cell carcinoma (loRCC) using the Korean National Cancer Registry Database. We selected 1295 recurrent loRCC patients who underwent either partial or radical nephrectomy from 2007–2013. Patients were excluded for age <19 years, secondary RCC, multiple primary tumors, other SEER stages except for a localized or regional stage, postoperative recurrence within 3-month, and non-nephrectomized cases. Four therapeutic groups were statistically analyzed for OS and risk factors: surgery (OP, 12.0%), other systemic therapy (OST, 59.5%), radiotherapy (RT, 2.8%), and targeted therapy (TT, 25.8%). The overall mortality rate for recurrent loRCC was 32.5%, including 82.4% for RCC-related deaths. The baseline comparison among groups showed statistical differences for the diagnostic age of cancer and the SEER stage (p<0.05). Multivariate analysis of OS showed significance for the TT (hazard ratio [HR]: 6.27), OST (HR: 7.05), and RT (HR: 7.47) groups compared with the OP group, along with significance for the sex, SEER stage, and the time from nephrectomy to treatment for disease recurrence (p<0.05). The median OS curve showed a significantly better OS in the OP group (54.9 months) compared with the TT, OST, and RT groups (41.7, 42.9, and 38.0 months, respectively; p<0.001). In conclusion, the surgery-treated group had the best OS among the different therapeutic strategies for recurrent loRCC after nephrectomy, and the importance of the time from nephrectomy to secondary treatment was a significant prognostic factor.
Introduction
Renal cell carcinoma (RCC) is a radio- and chemo-resistant tumor for which surgical removal of primary kidney tumor via radical or partial nephrectomy in localized RCC (loRCC) is the standard curative strategy (1, 2). Once the RCC becomes either advanced or metastatic, the 5-year overall survival (OS) rates significantly decrease from 85–95% to <30% for loRCC (3). Among the surgically treated loRCC cases, about 33–50% of patients experience disease recurrence within two years resulting in a survival rate of <30% (1, 4–7), with rarely later recurrence after postoperative 5-year (6, 8–10). Therefore, it is important to identify any predisposing characteristics of disease recurrence after nephrectomy and detect them as early as possible to initiate an earlier therapeutic strategy with adequate periodic imaging methods to improve the survival prognosis.
The recurrent disease includes distant metastasis and local disease recurrence. Several therapeutic modalities are available for its treatment, dependent on tumor-related risk factors, the intrinsic tumor pathology, and the extent and location of the recurrence; this results in diverse and unpredictable therapeutic outcomes (5, 6). In addition to there being multiple therapeutic priorities, deciding the most appropriate and best therapeutic options for disease recurrence is also complicated by its rarity, unpredictability, tumor heterogeneity, and the lack of large-scale, randomized studies that support any management type (1, 6–8). Since the disease recurrence can be a local recurrence at the operative area, distant metastasis, or synchronous local recurrence and distant metastasis, various and complex therapeutic strategies exist in combination with local and systemic therapies.
Due to the differences, heterotrophic heterogenicity, and complex appearances of local recurrence from distant metastasis, it is difficult to determine the most appropriate treatment and to generalize recommendations from randomized trials for improving survival prognoses and treatment outcomes (4–6, 8). This creates uncertainty in real-world clinics for choosing the best treatment for recurrent loRCC following a nephrectomy based on information from the national insurance coverage system, clinicians’ experiences, and a few multicentric, retrospective, and prospective studies (1, 4–8).
This study retrospectively evaluated, for the first time in Korea, the therapy methods and patterns of recurrent loRCC after either radical or partial nephrectomy using a population-based National Cancer database and the National Insurance Claim databases in Korea to analyze overall survival (OS) and related risk factors.
Materials and Methods
Ethics Statement
This study was approved by the Institutional Review Board (IRB) of the National Cancer Center and Cancer Research Institute in Korea (IRB no. NCC2015-0217). The analytical methodology for using the Korean National Policy System database has been previously described (10). A detailed cohort was selected for cancer incidence and mortality data from 2006–2013 from the Korean National Health Insurance System of Statistics and the Korea National Cancer Incidence Database of Korean Central Cancer Registry.
Patients’ Criteria
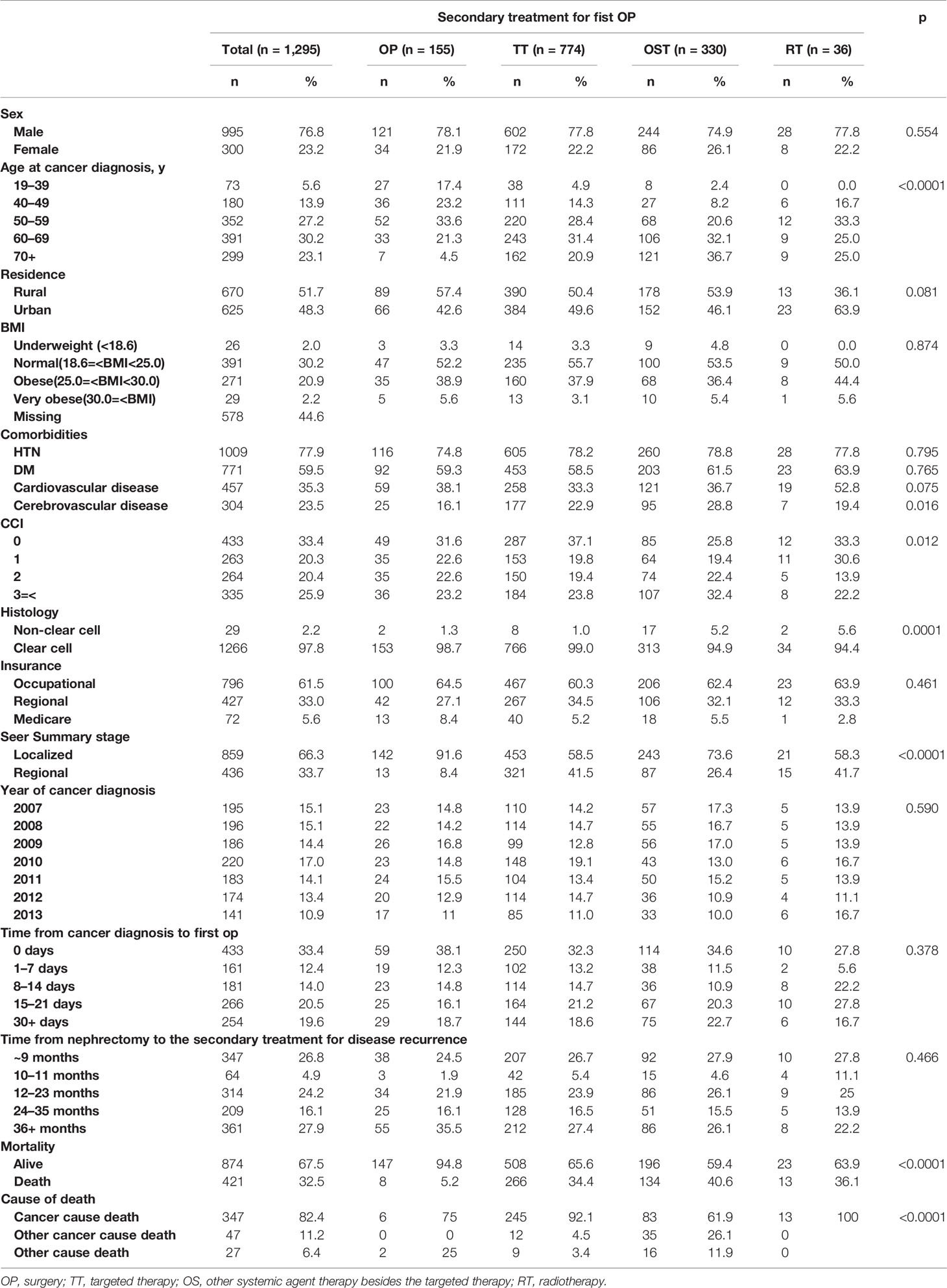
An ICD-10 code of C64 for RCC from the Korean National Insurance Policy Database was used to extract data for patients with localized RCC following either partial or radical nephrectomy who had postoperative secondary treatment from 2007–2013. LoRCC was defined as a localized or regional stage according to the SEER staging system. Patients were excluded for age <19 years, secondary RCC, multiple primary tumors, other SEER stages except for localized and regional, postoperative therapies within 3 months after nephrectomy, and non-nephrectomized cases. There were 1295 (4.6%) nephrectomy cases selected out of 25,792 cases Figure 1.
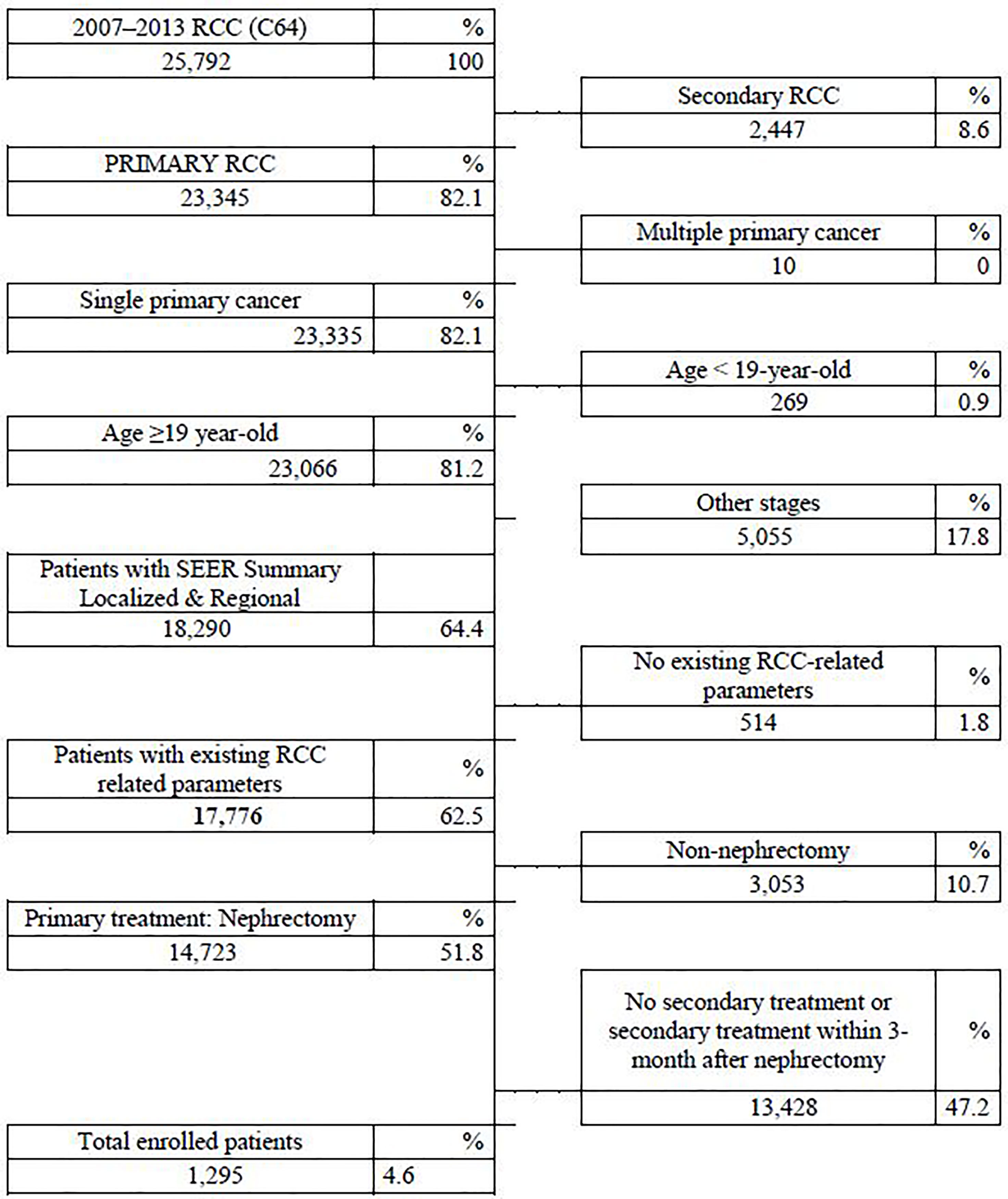
Figure 1 Flowchart of patient selection according to inclusion and exclusion criteria in the National Insurance Policy Database.
Group Definitions According to the Secondary Therapies
The therapeutic treatments 3-months after nephrectomy for the 1295 patients were grouped as OP for surgery (n=155, 12.0%), OST for other systemic therapy (n=334, 25.8%), RT for radiation therapy (n=36, 2.8%), and TT for targeted therapy (n=770, 59.5%) according to the first method of secondary treatment after nephrectomy. For example, the patient was enrolled into the OP group if the recurrent loRCC received surgery and adjuvant or neoadjuvant chemotherapy, radiation therapy, or targeted therapy. However, when the patient received only TT as the first therapy, the patient was enrolled into the TT group. When the patient received OST first and then either TT or RT, the patient was enrolled in the OST group. This means the RT group was composed of patients that received RT only. We determined the postoperative 3-month time index to exclude the obscured synchronous metastatic RCC at nephrectomy and include the post-nephrectomized recurrent LoRCC.
Statistical Analysis
Student’s t-test, the chi-square test, and Fisher’s exact test were used to compare the baseline characteristics between groups, including age at RCC diagnosis, sex, residential location, insurance type, SEER stage, year of RCC diagnosis, time from cancer diagnosis to the first nephrectomy, time from nephrectomy to the secondary treatment for disease recurrence, secondary treatment type, and mortality/survival (Table 1). The OS of the four therapeutic groups were compared. Multivariate analysis was performed for the risk factors of OS, and Kaplan-Meier analysis with the log-rank test were performed; a p-value <0.05 was considered significant. From the starting date of nephrectomy, hazard ratios (HR) with 95% confidence interval (CI) values from Cox proportional hazard models for mortality were analyzed to investigate the effects of secondary treatments. Age at RCC diagnosis, sex, residential location, insurance type, SEER stage, year of RCC diagnosis, time from cancer diagnosis to the first nephrectomy, and time from nephrectomy to the secondary treatment for disease recurrence were used to adjust the Cox proportional model. Two-sided p-values <0.05 were considered statistically significant. All statistical analyses were performed using SAS (release 9.4, SAS Institute Inc., North Carolina, USA).
Results
Baseline Characteristics
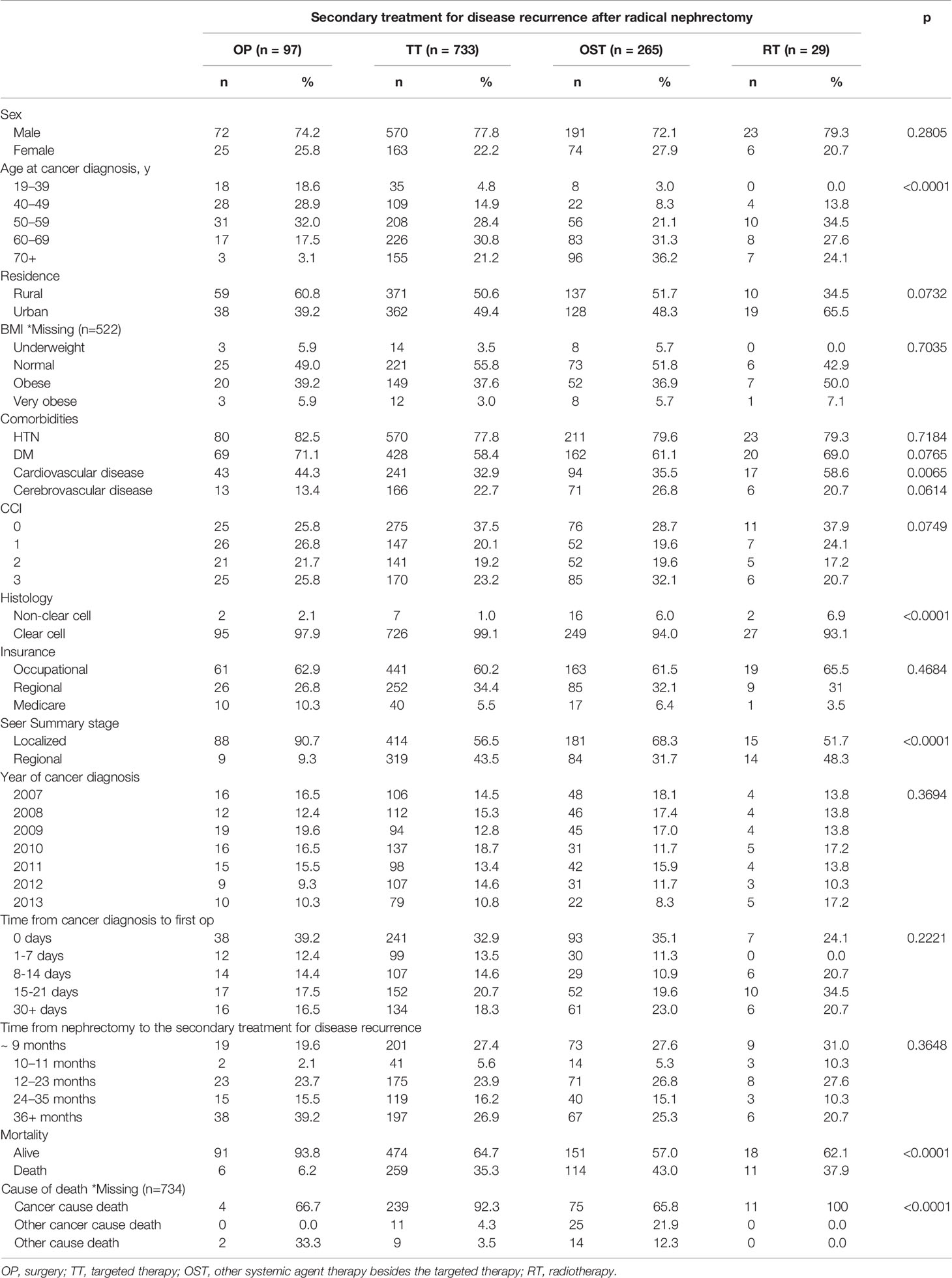
Among 1124 radically nephrectomized patients and 171 partially nephrectomized patients, 421 (32.5%) deaths were observed during the study period, including 17.6% of non-RCC-specific deaths (Table 1). The median OS of each group was 54.9, 41.7, 42.9, and 38.0 months for the OP, TT, OST, and RT groups, respectively (Figure 2). The median periods for each treatment group from nephrectomy to the secondary treatment after postoperative disease progression were 32.7, 26.0, 25.2, and 24.1 months for the OP, TT, OST, and RT groups, respectively.
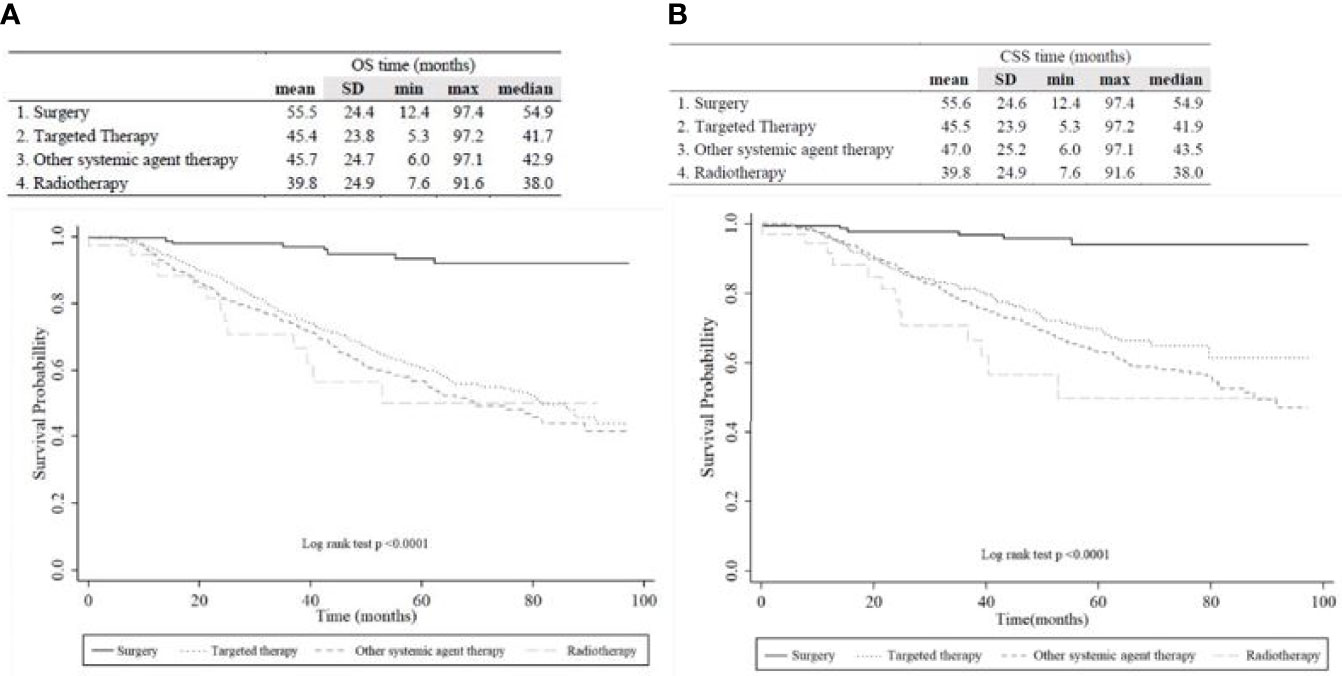
Figure 2 Kaplan-Meier plot of (A) overall survival and (B) cancer-specific survival among overall patients according to the therapeutic group.
The comparison of baseline characteristics between the four treatment groups showed that the cancer-diagnostic age, the presence of cerebrovascular disease, the CCI, the histology, the SEER stage, and the mortality and survival rates were significantly different (p<0.05, Table 1). The comparison of baseline characteristics between the radical and partially nephrectomized groups showed that only the SEER stage and mortality/survival rates were significantly different (p<0.001, Supplementary Table 1). Among the radically nephrectomized group (n=1124, Table 2), the comparison of baseline characteristics among the four different therapeutic groups showed results similar to those in Table 1: significant differences were observed in the cancer-diagnostic age, the presence of cardiovascular disease, the histology, the SEER stage, and the mortality and survival rates (p<0.05).
Multivariate Results for Survival Outcomes
In the multivariate analysis of OS, the obese BMI (HR: 0.47, 95%CI: 0.23–0.96), regional SEER stage (HR: 1.78, 95%CI: 1.29–2.47), a postoperative after-two-year from nephrectomy to secondary treatment for disease recurrence (HR: <1.0, years post-nephrectomy), and the secondary treatment types (HR: 7.62 for TT and HR 7.68 for OST) were significant risk factors (p<0.05, Table 3). In adjusted multivariate analysis, only 1124 radically nephrectomized RCC patients had predictive OS risk factors similar to 1295 overall patients (p<0.05, Supplementary Table 2).
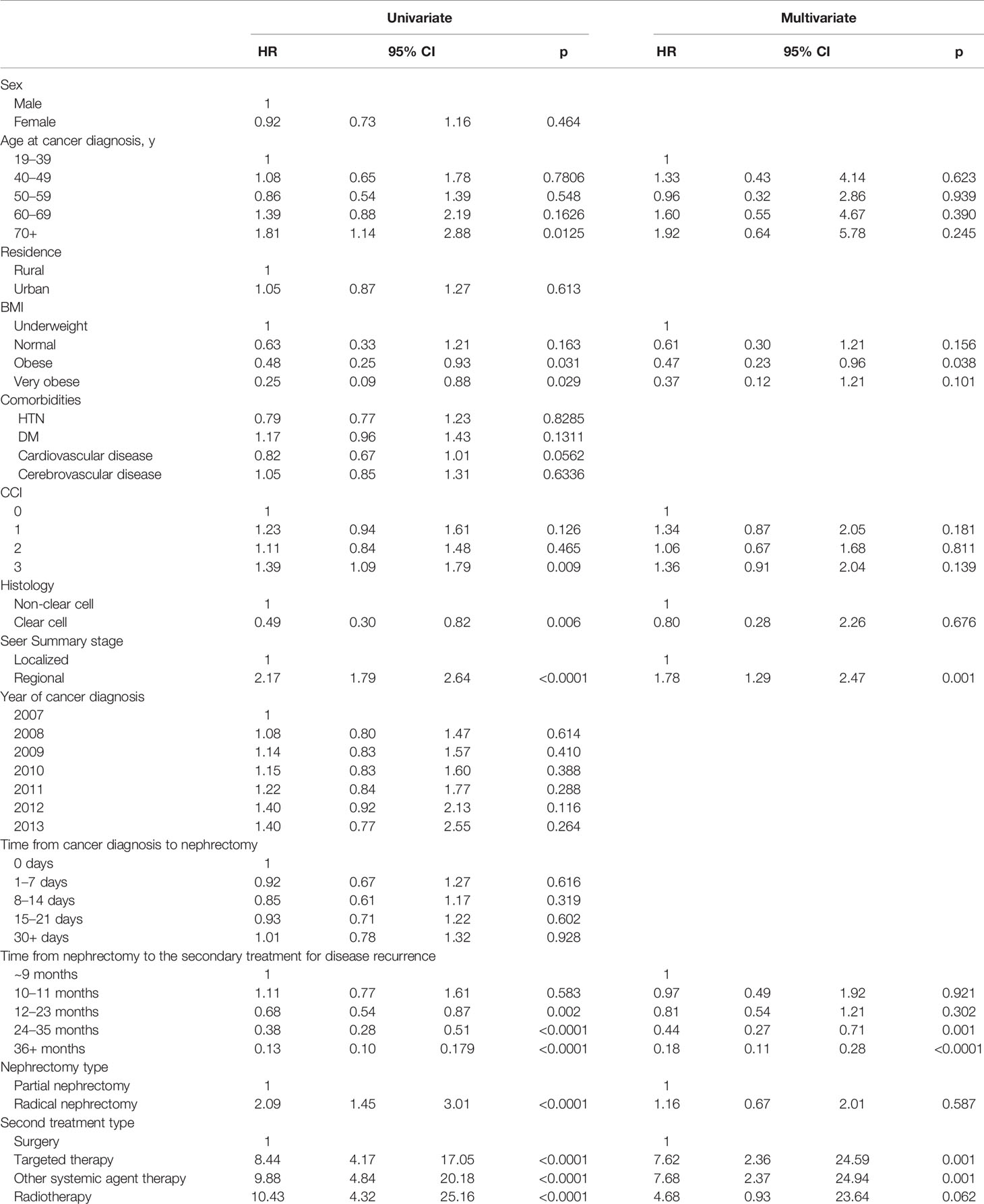
Table 3 Multivariate analysis of overall survival in RCC patients with postoperative disease progression after nephrectomy.
In the multivariate analysis of CSS, the regional SEER stage (HR: 1.83, 95%CI: 1.28–2.62), a postoperative after-two-year from nephrectomy to secondary treatment for disease recurrence (HR: <1.0, years post-nephrectomy), and the secondary treatment types (HR: 9.86 for TT, HR 7.77 for OST, and HR 8.01 for RT) were significant risk factors (p<0.05, Table 4).
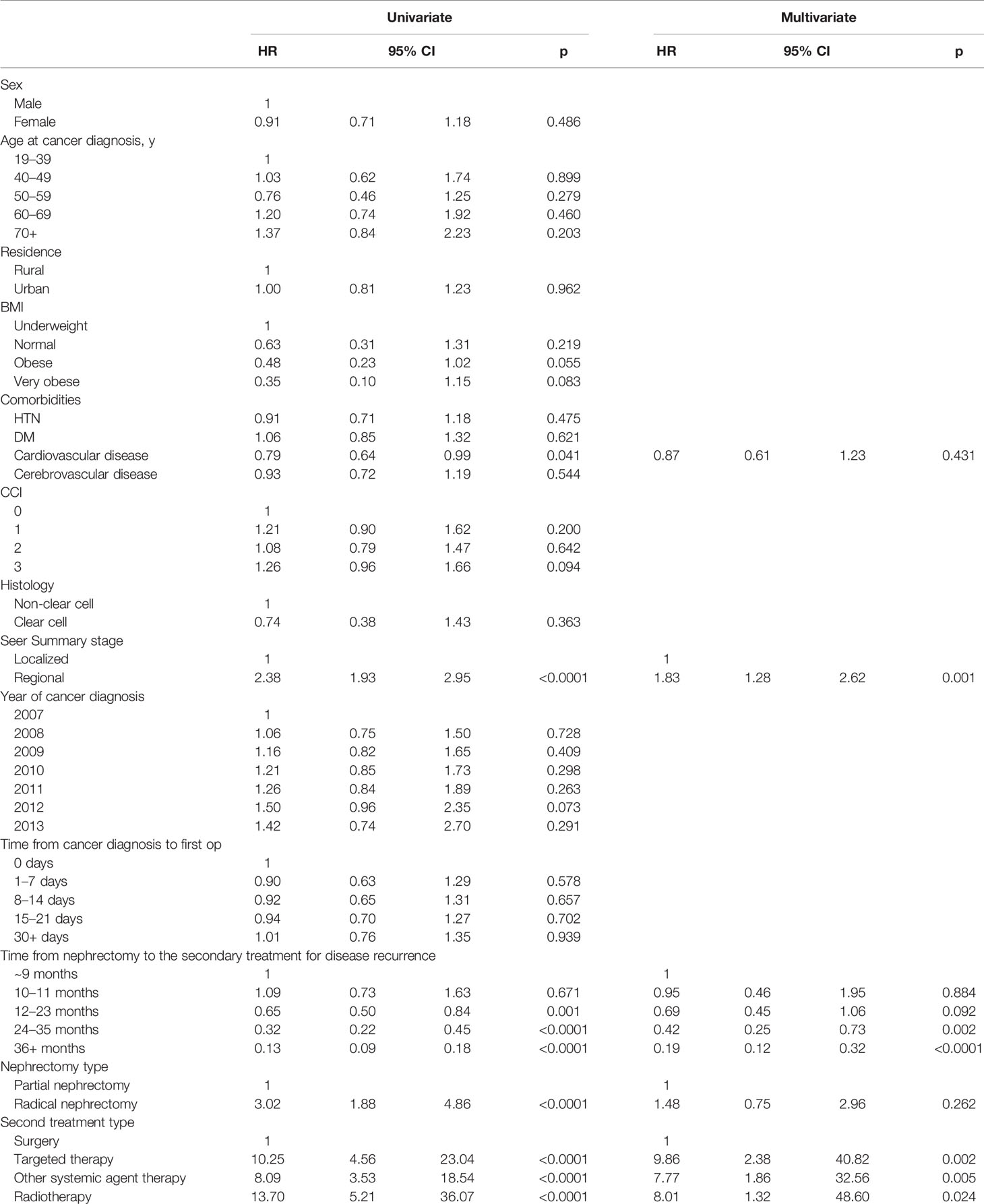
Table 4 Multivariate analysis of RCC specific survival in RCC patients with postoperative disease progression after nephrectomy.
Propensity Score Matching Results
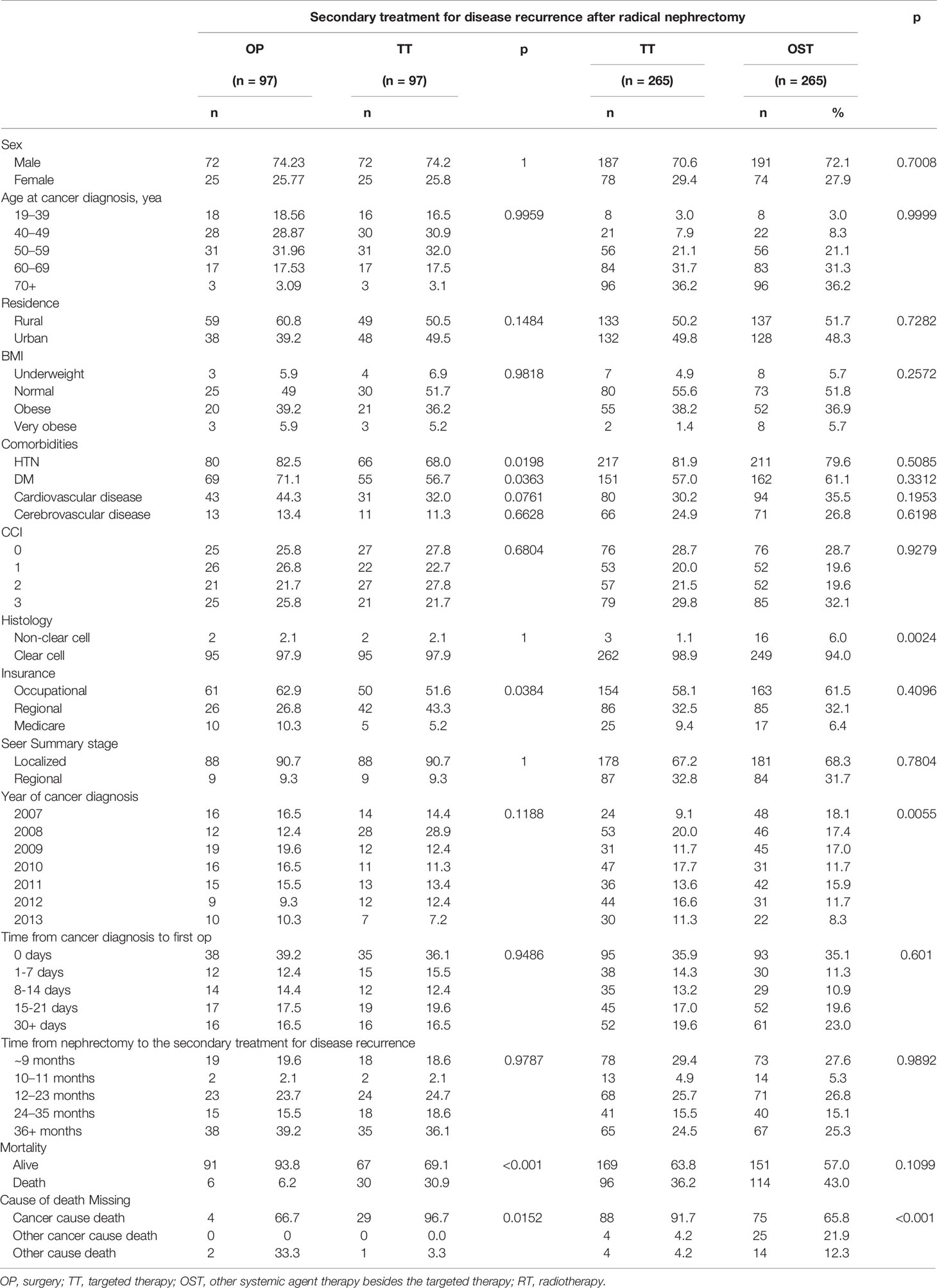
The three different treatment groups were compared after 1:1 Propensity-score matching by adjusting the gender, age, CCI, and SEER staging. In the comparison between the OP and TT groups, the presence of comorbid diseases such as hypertension and diabetes, mortality and cause of death were significantly different (p<0.05, Table 5). In comparing TT and OST groups, histology, year of cancer diagnosis, and cause of death were significantly different (p<0.05).
In the multivariate analysis of CSS with 1:1 propensity score matching cohort between OP and TT groups (Table 6A), the time from nephrectomy to the secondary treatment since 2 years (24-35 months, HR 0.24 CI 0.073-0.784; 36-month< HR 0.124, CI 0.043-0.358) and TT (HR 8.988, CI 3.092-26.126) were significant factors (p<0.05). In the propensity-score matching cohort between TT and OST groups (Table 6B), the CCI group 1 (HR 1.736, CI 1.115-2.703) and CCI group 3 (HR 1.86, CI 1.23-2.812), regional SEER stage (HR 1.617, CI 1.178-2.22), and the time from nephrectomy to the secondary treatment since 2 years (24-35 months, HR 0.261 CI 0.148-0.459; 36-month< HR 0.114, CI 0.066-0.198) were significant factors (p<0.05).
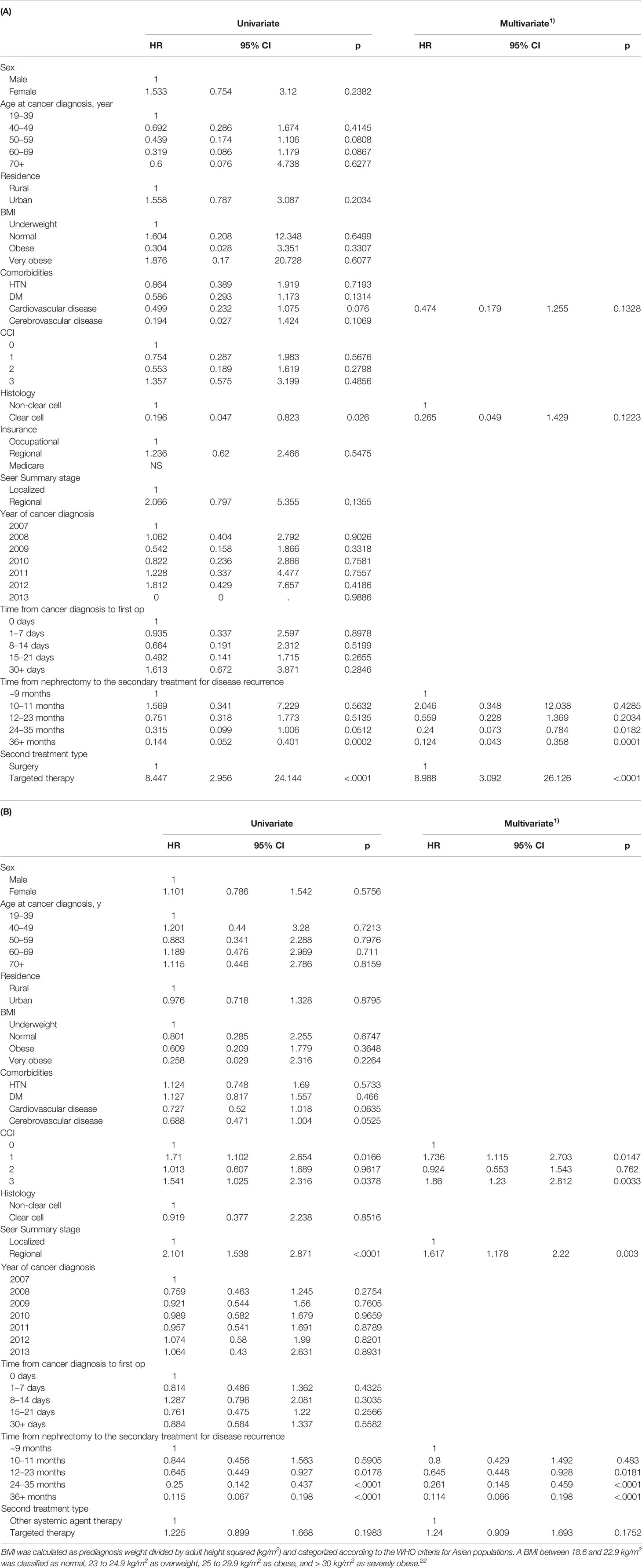
Table 6 Multivariate analysis of cancer specific survival in 1:1 PSM matched RCC patients between (A) OP and TT groups and (B) TT and OST groups with postoperative disease progression after nephrectomy.
Survival Comparisons Between Groups
The Kaplan-Meier OS/CSS curve showed a prominently better OS/CSS in the OP group than in other treatment groups (p<0.05, Figure 2). The median OS/CSS of the OP, TT, OST, and RT groups were 54.9/54.9, 41.7/41.9, 42.9/43.5, and 38.0/38.0 months, respectively (p<0.001, Figure 2). For radically nephrectomized RCC patients, the median OS of the OP, TT, OST, and RT groups were 55.3, 41.6, 40.9, and 27.7 months, respectively (p<0.001, Supplementary Figure 1).
Discussion
This population-based retrospective cancer registry study is the first real-world descriptive and analytic study reporting the Asian therapeutic strategies and predictive risk factors for OS in recurrent loRCC following either radical or partial nephrectomy. Due to its low incidence rate (<5–7% for loRCC) and heterotrophic and heterogenic nature, it is not easy to design randomized clinical trials to predict disease recurrence of nephrectomized loRCC by comparing prognostic outcomes among therapeutic strategies and to generalize standardized therapeutic protocols for recurrent loRCC. Real clinical situations force many clinicians to depend upon their own experience and judgment for treatment decisions in recurrent loRCC, based on retrospective multicentric studies and small-sample case-cohort studies (1, 8, 11–13). This study provides clinical data for informing therapeutic decisions in recurrent loRCC after curative nephrectomy based on real-world outcomes from different therapeutic strategies. Moreover, this study identified that the time from nephrectomy to disease recurrence or secondary treatment for recurrent loRCC is an important prognostic factor for OS.
Various therapeutic treatments have been applied for recurrent loRCC after nephrectomy in combination with local or systemic therapies. Consistent with our findings, the best prognostic outcome was reported for surgical removal of the recurrent tumor to make the patient pathologically and radiologically tumor-free (1, 6, 7, 11, 13); the patients also had better outcomes with younger age, lower isolated tumor burden, and lesions that allowed for successful surgery under general anesthesia such as retroperitoneal lymph node at renal fossa, lung, or bone (11, 13, 14). Having a higher tumor burden with more-disseminated areas, a poorer general condition, and greater underlying disease that require non-surgical treatments for the disease recurrence each resulted in worse OS than that of the OP group.
Furthermore, minimally invasive surgical modalities, including laparoscopy and robot-assisted surgery (1, 6, 7, 15–17), have enabled successful surgery for low recurrent tumor burdens, with longer disease-free states to the next systemic therapy, fewer severe complications, earlier recovery, and allowing for interventive application of adjuvant systemic agent or focal therapy (2, 11, 12, 14, 17–19). Retroperitoneal lymph node dissection, venous thrombectomy, and cytoreductive metastasectomy of distant metastatic organs or perirenal fossa are the best surgical operations that allow better prognostic outcomes in recurrent loRCC (20, 21), in which patients recover earlier, with less-severe complications, and more-enhanced strategic management.
Unexpectedly, the RT group showed the worst OS (HR 7.47; Table 3 and Figure 2). Since RCCs are radioresistant tumors, most recurrent cases received radiation therapy as adjuvant therapy combined with other surgical or systemic agent therapies. However, the RT group comprises cases that received only RT since they were unsuitable for either surgery or systemic therapies due to older age, poorer general condition, greater underlying disease, and higher tumor burdens (Table 2). The radiation therapy in the RT group was performed for palliative rather than the curative purpose and specific recurrent lesions related to high morbidities, such as central nervous system and/or bone lesions. Systemic or external beam radiation therapy targeted at the localized site is reported to have a 5-year OS of 40–73% for recurrent RCC after nephrectomy (18, 19, 21). This might explain why the RT group had the worst OS and a higher rate of non-RCC deaths.
The systemic-agent groups in this study, OST (HR 7.05 for OS) and TT (HR 6.27), were composed of inoperable patients, with insignificant differences between them, and a worse OS is expected compared to the OP group. Since the introduction of TT agents in 2005, the survival of RCC has improved markedly by a median 6–18 months, as compared to cytokine usage for advanced or metastatic RCC in the pre-TT era. Treatment with only OST agents indicated only a small portion of specific histological RCC in the TT era (25.79% for OST vs. 59.46% for TT); therefore, it is not easy to compare the OS between the TT and OST groups. Diverse clinical trials using TT agents have been testified and are ongoing as neoadjuvant and adjuvant settings; they failed to provide survival benefits, and adverse events increased in loRCC cohorts after nephrectomy, except for some highly selected high-risk patients (1, 6, 15). However, some positive effects of systemic TT and OST were identified in trials, showing significant downsizing of the tumor burden, decreasing local recurrence or metastasis, and allowing other subsequent treatments (1, 6, 15, 16). Although this study did not include recently introduced immune-checkpoint inhibitors, a combined TT and OST agent might further improve OS without severe adverse events in recurrent loRCC, or in the prevention of disease recurrence, in future neoadjuvant and adjuvant trials (9, 15, 22, 23).
Four significantly favorable predictive factors of OS have been identified: the female sex (HR: 0.72) (24), a regional SEER stage (HR: 1.74), a longer period between nephrectomy and post-nephrectomized secondary treatments after one postoperative year (HR: <1.0 y) (25–27), and the secondary treatment types (HR: >6.0 for TT, OST, and RT) (p<0.05; Table 3 and Supplementary Table 2). Significantly, an interval between nephrectomy and disease recurrence of less than one year is already a proven factor of unfavorable survival prognosis in metastatic and advanced RCC (25–27). The disease recurrence within one year after nephrectomy was affected most by poorer baseline general conditions and poorer pathologic characteristics of the primary loRCC, suggesting that invisible circulating tumors or an inherent dormant metastatic niche might have already existed at the time of nephrectomy despite a free resection margin. Therefore, further studies would be needed to expand the indications for active adjuvant therapies and identify a patient’s baseline general conditions, including immune status and pathologic information on the primary tumor.
Since about half of the disease recurrences occur within 2 years after nephrectomy, and this study showed that one-year postoperative treatment interval was a significant factor for favorable OS (p<0.05, HR: 0.61 for 13–24 months, HR: 0.33 for 25–36 months and HR: 0.12 for > 36 months, Table 3), it is important to discuss the benefit of early secondary treatment for recurrent loRCC between 1–2 years after nephrectomy. Furthermore, since no postoperative follow-up biomarkers, no adequate indicators for surgical timing of metastasectomy or local surgery, and no effective adjuvant therapies have been established in recurrent loRCC (1, 4–7), an intensive conditional postoperative plan and detection protocol should be developed using imaging and blood biomarkers within 1–2 years and afterward (9, 11, 28). For example, in male patients with regional SEER staging, it is important to consider a postoperative follow-up plan within two years using more systemic imaging modalities and more additional prediction biomarkers. The PET-CT and personalized liquid biopsies for blood PD-1 and genetic biomarkers have not been approved as earlier detecting modalities of disease recurrence with a lower tumor burden or isolated tumor recurrence (29, 30). However, such additional modalities within postoperative two years might allow for surgical removal of the targeted recurrent lesion as early as possible, since many clinicians wait for the appearance of recurrent tumor lesion greater than 1 cm confirmed on currently recommended CT imaging modalities (1, 2, 5, 7).
This study had inherent limitations, including its retrospective design and the absence of data for radiological information to estimate the extent of involved organ and site-related tumor burdens, neoadjuvant and adjuvant purposes, the sequential combination of major secondary therapies with the lead-time bias effect, and organ and sites of disease recurrence. However, this is the first study in Korea describing the therapeutic trends of recurrent loRCC after nephrectomy, their survival outcomes, and significant predictive factors. Further prospective, large-scale, longitudinal studies are needed to evaluate disease recurrence for different multi-treatment therapies, including the survival outcomes, intervention measures, and different drug mechanisms.
Conclusion
This population-based retrospective study showed several significant risk factors affecting OS in recurrent loRCC following nephrectomy. Surgery had the highest OS as a secondary therapeutic option for disease recurrence, whereas TT and OST showed insignificant OS differences. The importance of the disease-free period following nephrectomy suggests that an intensive postoperative follow-up protocol should be evaluated for improving the prognostic outcomes of disease recurrence in loRCC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by National Cancer Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
SK: Conceptualization, Data Curation, Investigation, Methodology, Project Administration, Supervision and Writing – Original Draft Preparation. YK, MC, and JS: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Project Administration, Supervision and Writing – Original Draft Preparation. JC: Conceptualization, Data Curation, Investigation, Methodology, Project Administration, Supervision, Funding Acquisition and Writing – Original Draft Preparation. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the National Cancer Center (No. 1610310, 1810021, and 1910171), Republic of Korea. This work was supported by a grant from the Korean Urological Oncology Association in 2019.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.693831/full#supplementary-material
References
1. Kriegmair MC, Bertolo R, Karakiewicz PI, Young Academic Urologists Kidney Cancer Working Group of the European Association of Urology. Systematic Review of the Management of Local Kidney Cancer Relapse. Eur Urol Oncol (2018) 1(6):512–23. doi: 10.1016/j.euo.2018.06.007
2. Levy DA, Slaton JW, Swanson DA, Dinney CP. Stage Specific Guidelines for Surveillance After Radical Nephrectomy for Local Renal Cell Carcinoma. J Urol (1998) 159:1163–7. doi: 10.1016/S0022-5347(01)63541-9
3. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int J Cancer (2019) 144(8):1941–53. doi: 10.1002/ijc.31937
4. Eggener SE, Yossepowitch O, Pettus JA, Snyder ME, Motzer RJ, Russo P. Renal Cell Carcinoma Recurrence After Nephrectomy for Localized Disease: Predicting Survival From Time of Recurrence. J Clin Oncol (2006) 24:3101–6. doi: 10.1200/JCO.2005.04.8280
5. Capogrosso P, Capitanio U, La Croce G, Nini A, Salonia A, Montorsi F, et al. Follow-Up After Treatment for Renal Cell Carcinoma: The Evidence Beyond the Guidelines. Eur Urol Focus (2016) 1:272–81. doi: 10.1016/j.euf.2015.04.001
6. Adamy A, Chong KT, Chade D, Costaras J, Russo G, Kaag MG, et al. Clinical Characteristics and Outcomes of Patients With Recurrence 5 Years After Nephrectomy for Localized Renal Cell Carcinoma. J Urol (2011) 185:433–8. doi: 10.1016/j.juro.2010.09.100
7. Itano NB, Blute ML, Spotts B, Zincke H. Outcome of Isolated Renal Cell Carcinoma Fossa Recurrence After Nephrectomy. J Urol (2000) 164:322–5. doi: 10.1016/S0022-5347(05)67350-8
8. Ha YS, Park YH, Kang SH, Hong SH, Hwang TK, Byun SS, et al. Predictive Factors for Late Recurrence in Patients With Stage T1 Clear Cell Renal Cell Carcinoma: A Multiinstitutional Study. Clin Genitourin Cancer (2013) 11(1):51–5. doi: 10.1016/j.clgc.2012.08.008
9. Takashi M, Hibi H, Ohmura M, Sato K, Sakata T, Ando M. Renal Fossa Recurrence of a Renal Cell Carcinoma 13 Years After Nephrectomy: A Case Report. Int J Urol (1997) 4:508–11. doi: 10.1111/j.1442-2042.1997.tb00294.x
10. Jung KW, Won YJ, Kong HJ, Lee ES. Prediction of Cancer Incidence and Mortality in Korea, 2019. Cancer Res Treat (2019) 51(2):431–37. doi: 10.4143/crt.2019.139
11. Paparel P, Bigot P, Matillon X, Bensalah K, Salomon L, Baumert H, et al. Local Recurrence After Radical Nephrectomy for Kidney Cancer: Management and Prediction of Outcomes: A Multi-Institutional Study. J Surg Oncol (2014) 109:126–31. doi: 10.1002/jso.23473
12. Russell CM, Espiritu PN, Kassouf W, Schwaab T, Buethe DD, Dhilon J, et al. Surgical Outcomes in the Management of Isolated Nodal Recurrences: A Multicenter, International Retrospective Cohort. J Urol (2014) 192:350–6. doi: 10.1016/j.juro.2014.02.010
13. Psutka SP, Heidenreich M, Boorjian SA, Bailey GC, Cheville JC, Stewart-Merrill SB, et al. Renal Fossa Recurrence After Nephrectomy for Renal Cell Carcinoma: Prognostic Features and Oncological Outcomes. BJU Int (2017) 119:116–27. doi: 10.1111/bju.13620
14. Gilbert D, Abaza R. Robotic Excision of Recurrent Renal Cell Carcinomas With Laparoscopic Ultrasound Assistance. Urol (2015) 85:1206–10. doi: 10.1016/j.urology.2015.01.036
15. Martinez Chanza N, Tripathi A, Harshman LC. Adjuvant Therapy Options in Renal Cell Carcinoma: Where do We Stand? Curr Treat Options Oncol (2019) 20(5):44. doi: 10.1007/s11864-019-0639-0
16. Brehmer B, Kauffmann C, Blank C, Heidenreich A, Bex A. Resection of Metastasis and Local Recurrences of Renal Cell Carcinoma After Presurgical Targeted Therapy: Probability of Complete Local Control and Outcome. World J Urol (2016) 34:1061–6. doi: 10.1007/s00345-016-1865-8
17. El Hajj A, Thanigasalam R, Molinié V, Massoud W, Fourati M, Girard F, et al. Feasibility and Oncological Outcomes of Laparoscopic Treatment for Local Relapse of Renal Cell Carcinoma. BJU Int (2013) 112:E307–13. doi: 10.1111/j.1464-410X.2012.11724.x
18. Parker WP, Boorjian SA, Zaid HB, Cheville JC, Leibovich BC, Thompson RH. Surgical Management and Oncologic Outcomes of Recurrent Venous Tumor Thrombus After Prior Nephrectomy for Renal Cell Carcinoma. Eur Urol Focus (2016) 2:625–30. doi: 10.1016/j.euf.2016.05.003
19. Psutka SP, Master VA. Role of Metastasis-Directed Treatment in Kidney Cancer. Cancer (2018) 124(18):3641–55. doi: 10.1002/cncr.31341
20. Paly JJ, Hallemeier CL, Biggs PJ, Niemierko A, Roeder F, Martínez-Monge R, et al. Outcomes in a Multi-Institutional Cohort of Patients Treated With Intraoperative Radiation Therapy for Advanced or Recurrent Renal Cell Carcinoma. Int J Radiat Oncol Biol Phys (2014) 88:618–23. doi: 10.1016/j.ijrobp.2013.11.207
21. Dizman N, Adashek JJ, Hsu J, Bergerot PG, Bergerot CD, Pal SK. Adjuvant Treatment in Renal Cell Carcinoma. Clin Adv Hematol Oncol (2018) 16(8):555–63.
22. Powles T, Albiges L, Staehler M, et al. Updated European Association of Urology Guidelines Recommendations for the Treatment of Firstline Metastatic Clear Cell Renal Cancer. Eur Urol (2018) 73:311–5. doi: 10.1016/j.eururo.2017.11.016
23. Flippot R, Escudier B, Albiges L. Immune Checkpoint Inhibitors: Toward New Paradigms in Renal Cell Carcinoma. Drugs (2018) 78(14):1443–57. doi: 10.1007/s40265-018-0970-y
24. Rampersaud EN, Klatte T, Bass G, Patard JJ, Bensaleh K, Böhm M, et al. The Effect of Gender and Age on Kidney Cancer Survival: Younger Age Is an Independent Prognostic Factor in Women With Renal Cell Carcinoma. Urol Oncol (2014) 32(1):30.e9–13. doi: 10.1016/j.urolonc.2012.10.012
25. Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and Prognostic Stratification of 670 Patients With Advanced Renal Cell Carcinoma. J Clin Oncol (1999) 17(8):2530–40. doi: 10.1200/JCO.1999.17.8.2530
26. Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-Alfa as a Comparative Treatment for Clinical Trials of New Therapies Against Advanced Renal Cell Carcinoma. J Clin Oncol (2002) 20(1):289–96. doi: 10.1200/JCO.2002.20.1.289
27. Shiff B, Breau RH, Patel P, Mallick R, Tanguay S, So A, et al. Impact of Time to Surgery and Surgical Delay on Oncologic Outcomes for Renal Cell Carcinoma. J Urol (2021) 205(1):78–85. doi: 10.1097/JU.0000000000001230
28. Harshman LC, Drake CG, Haas NB, Manola J, Puligandla M, Signoretti S, et al. Transforming the Perioperative Treatment Paradigm in Non-Metastatic RCC—-A Possible Path Forward. Kidney Cancer (2017) 1(1):31–40. doi: 10.3233/KCA-170010
29. Kim EH, Strope SA. Postoperative Surveillance Imaging for Patients Undergoing Nephrectomy for Renal Cell Carcinoma. Urol Oncol (2015) 33(12):499–502. doi: 10.1016/j.urolonc.2015.08.008
Keywords: renal cell carcinoma, nephrectomy, treatment, recurrence, prognosis
Citation: Kim SH, Choi MG, Shin JH, Kim Y-A and Chung J (2021) A Real-World, Population-Based Retrospective Analysis of Therapeutic Survival for Recurrent Localized Renal Cell Carcinoma After Nephrectomy. Front. Oncol. 11:693831. doi: 10.3389/fonc.2021.693831
Received: 12 April 2021; Accepted: 16 August 2021;
Published: 08 September 2021.
Edited by:
Walter J. Storkus, University of Pittsburgh, United StatesReviewed by:
Christoph Würnschimmel, Martini Klinik Prostate Cancer Center, GermanyMelissa A. Reimers, Washington University in St. Louis, United States
Copyright © 2021 Kim, Choi, Shin, Kim and Chung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinsoo Chung, Y2pzNTIyNUBuY2MucmUua3I=; Young-Ae Kim, MTIyNzRAbmNjLnJlLmty
 Sung Han Kim
Sung Han Kim Min Gee Choi2
Min Gee Choi2 Young-Ae Kim
Young-Ae Kim