
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 19 August 2021
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.693693
 Shuixian Zhang1†
Shuixian Zhang1† Fengchun Zhao1†
Fengchun Zhao1† Tengyuan Zhou1†
Tengyuan Zhou1† Dan Liu1
Dan Liu1 Xiaohong Yao2
Xiaohong Yao2 Wenjuan Fu2
Wenjuan Fu2 Zhi Liu1
Zhi Liu1 Chuan Lan1
Chuan Lan1 Zhaopan Lai1
Zhaopan Lai1 Chen Liu3
Chen Liu3 Haitao Li3
Haitao Li3 Yuhong Li1
Yuhong Li1 Shengli Hu1
Shengli Hu1 Yi Yin1
Yi Yin1 Liang Tan1
Liang Tan1 Wenyan Li1
Wenyan Li1 Fei Li1
Fei Li1 Rong Hu1*
Rong Hu1* Hua Feng1*
Hua Feng1*Both subventricular zone (SVZ) contact and isocitrate dehydrogenase 1 (IDH1) mutation have been reported to be related to the outcome of glioma, respectively. However, far too little attention has been paid to the role of tumor edge-SVZ distance in the outcome of glioma. We aim to assess the value of tumor-SVZ distance, as well as combined tumor-SVZ distance and IDH status, in predicting the outcome of gliomas (WHO grade II–IV). Here, the MR images and clinical data from 146 patients were included in the current study. The relationship between survival and the tumor-SVZ distance as well as survival and combination of tumor-SVZ distance and IDH status were determined via univariate and multivariate analyses. In univariate analysis of tumor-SVZ distance, the patients were divided into three types (SVZ involvement, tumor-SVZ distance from 0 to 10 mm, and tumor-SVZ distance >10 mm). The results showed that the OS (p = 0.02) and PFS (p = 0.002) for the patients had a positive correlation with the tumor-SVZ distance. In addition, simple linear correlation found a significant relationship between the two parameters (OS and PFS) and tumor-SVZ distance in patients with non-SVZ-contacting glioma. Combination analysis of the tumor-SVZ distance and IDH status showed that IDH1 mutation and SVZ non-involvement enable favorable outcomes, whereas IDH1 wild type with SVZ involvement indicates a significantly worse prognosis in all patients. Moreover, in patients with non-SVZ-contacting glioma, IDH1 mutation concurrent with tumor-SVZ distance >10 mm has better OS and PFS. IDH1 wild type and tumor-SVZ distance from 0 to 10 mm suggest poorer OS and PFS. Multivariate analysis showed WHO grade IV, SVZ involvement, tumor-SVZ distance from 0 to 10 mm, IDH1 mutation, gross total resection, and chemotherapy serve as independent predictors of OS. WHO grade IV, SVZ involvement, tumor-SVZ distance from 0 to 10 mm, IDH1 mutation, and chemotherapy serve as independent predictors of PFS of patients with glioma. In conclusion, tumor-SVZ distance and IDH1 mutation status are the determinants affecting patient outcome.
Glioma is the most common type of tumor in the brain, representing 75% of primary brain tumors in adults (1). Glioblastoma (GBM), as the most common aggressive cancer of the central nervous system, represents 45% of gliomas (2). GBM presents with the poorest prognosis and frequent relapses and is resistant to conventional therapies, including chemotherapy and radiation (3), with a 5-year survival of around 5% (2). Despite therapeutic strategies that have been improved, there is no substantial improvement in the overall survival of glioma (especially GBM) over the past decade, partially owing to the not-fully-understood mechanisms of glioma initiation and progression.
It has been proposed that glioma is initiated and propagated from a subpopulation of cancer cells with self-renewal ability, proliferative capacity, and multilineage potency, which are termed as glioma stem cells (GSCs) (4, 5). Converging evidence over the last decade has demonstrated that GSCs may arise from neural stem cells (NSCs) residing in the adult subventricular zone (SVZ) (6). This notion has been supported by the direct genetic evidence that astrocyte-like NSCs in the SVZ harbor the driver mutations of human glioma, which could lead to glioma development (6–11). These findings established a clinical link between NSCs in the SVZ and the initiation and progression of the glioma, and possible therapeutic interventions to improve outcomes of glioma.
Despite the critical roles of SVZ in the tumorigenesis and progression of glioma, the association of SVZ with clinical characteristics of glioma patients remains incompletely clarified. Recent studies have demonstrated that patients with glioma involving the SVZ are more likely to present multifocal lesions on MR images when initially diagnosed (12) and are associated with higher risk of recurrence and shorter overall survival (OS) (13–17). In addition, a shorter distance from the tumor centroid to the SVZ (≤30 mm) was correlated with unfavorable clinical outcome in patients with SVZ contact (17). However, many gliomas located in the parenchyma without SVZ contact are close to the SVZ. There is a lack of evidence with respect to the clinical correlation of the shortest distance from tumor edge to SVZ with prognostic consequence of patients with glioma.
While isocitrate dehydrogenase 1 (IDH1) mutations are present in 70% of lower-grade gliomas and secondary glioblastomas (18–20), and predict better patient survival, whether combined IDH1 mutation status and anatomical tumor-SVZ distance would facilitate more accurate prediction of prognosis has not yet been investigated. In the present study, we aim to assess the relationship of combined IDH1 mutation status and anatomical tumor-SVZ distance to clinical features of patients with glioma.
We performed a retrospective investigation of patients who were diagnosed with glioma at our institution between 2010 and 2016. One hundred and forty-six patients who met the following criteria were enrolled: histopathological confirmation of glioma (WHO classification II–IV), underwent radiation and/or chemotherapy post surgical resection without prior craniotomy or stereotactic biopsy. All patients underwent preoperative and postoperative enhanced MRI scan and genetic detection of IDH1 mutation status. The shortest distance between the tumor edge and lateral ventricles was determined according to the preoperative MR (T2) images.13 Based on the distance=0 (SVZ involvement) and distance=10 mm (the median distance of all tumors that did not contact the ventricle) (16), patients were categorized into three groups according to the following criteria: type I (SVZ involvement), type II (tumor-SVZ distance from 0 to 10 mm), and type III (tumor-SVZ distance >10 mm). The extent of surgical resection was defined by comparing the preoperative and postoperative MR images. The gross total resection (GTR) was achieved by complete resection of the contrast-enhancing tumor on T1-weighted sequences. Otherwise, < GTR was defined as any resection that failed to achieve GTR (21). The tumor size and edema volume were determined by preoperative MR images (T2/fluid attenuation inversion recovery, T2 weighted image). Other clinical variables such as age, sex, and preoperative KPS score were obtained from medical records. All manipulations were performed under the approval of the Research Ethics Committee of the First Hospital Affiliated to Army Medical University (Southwest Hospital), and written informed consent was obtained from all patients.
All patients underwent the same MRI protocol. MR images were acquired using conventional spin echo sequences on a 3.0-Tesla Siemens MR Magnetron System (Siemens AG, Erlangen, Germany) in axial plane, sagittal section, and coronal section. Patients received intravenous contrast (gadolinium diethylenetriamine pentaacetate) at a dose of 0.1 mmol/kg body weight. The MRI slice thickness was 5 mm.
For imaging analysis, tumors lesions and the lateral ventricle were segmented manually (Figure 1B) by two experienced neurosurgeons (more than 10 years of work experience) using INFINITT PACS software and 3D slicer (Surgical Planning Laboratory, version 4.10.2, Harvard University, Boston, MA, USA), and further reviewed by two independent neuroradiologists; they were blinded to outcomes for subsequent analysis. In both software packages, tumor segmentation was performed by delineating the abnormal signal in T2/fluid attenuation inversion recovery (T2/FLAIR) images (Figure 1A) (22). Surfaces representing the SVZ were created manually based on the description given by Vescovi et al. (23) and the rendering of the lateral ventricles given in the Hammersmith atlas (24). The volume of gross tumors (VG) and the volume of gross whole (VGW) are calculated by 3D slicer (Supplementary Figure 1); the workflow for this software has previously been described by others (25). The tumor-SVZ distance was defined as the shortest distance between the edge of tumor and the ependyma of the ventricles (13) and calculated by INFINITT PACS software (Figure 1B).
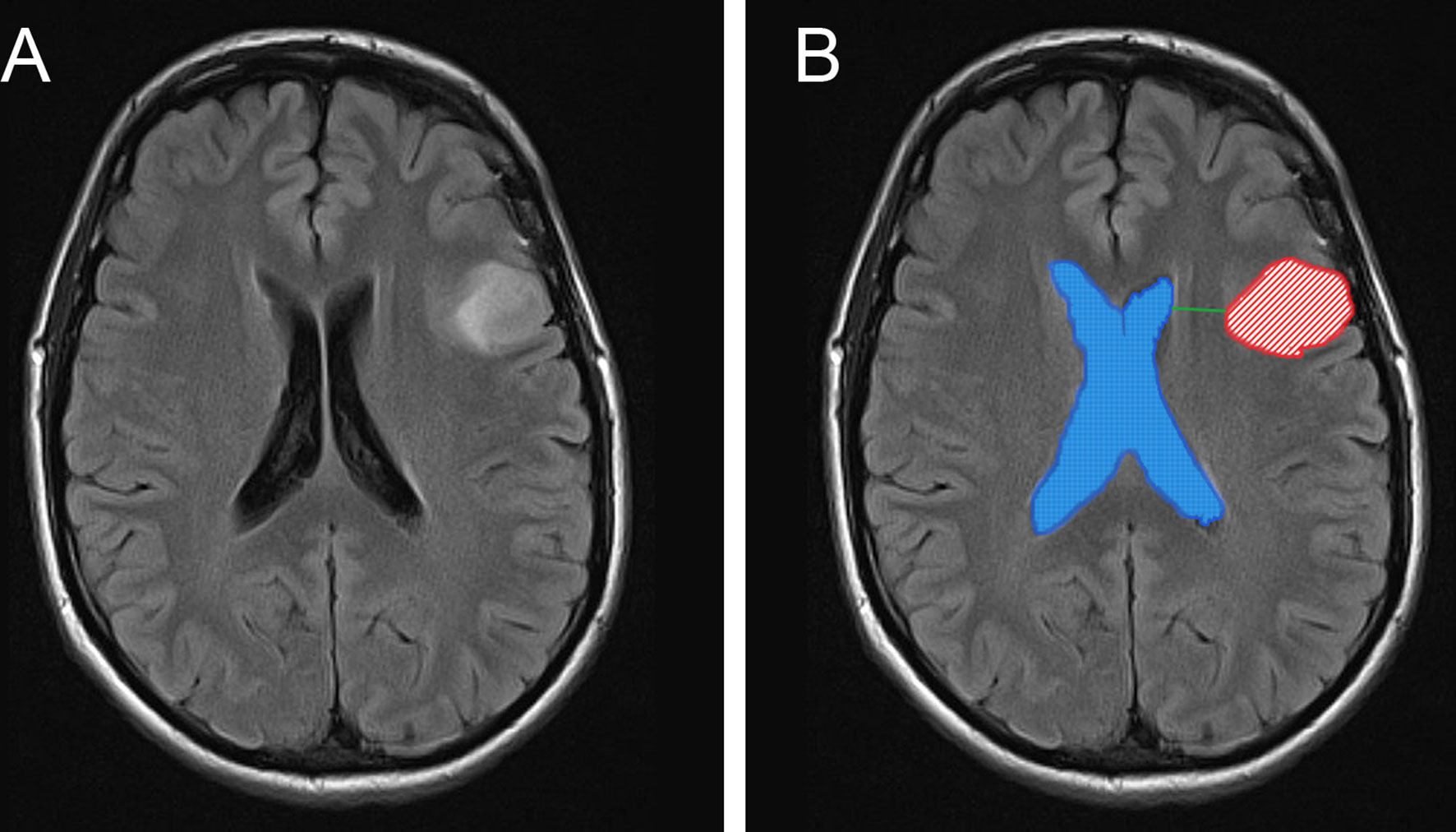
Figure 1 MR images depicts the anatomical position between tumors and the SVZ, calculation methods of VG and VGW. Representative MR image of tumor without SVZ involvement (A). The tumor lesions and ventricles are manually segmented and shown as red and blue masks. The shortest distance between the tumor edge and the lateral ventricles was showed as green line (B).
The clinical records of the included patients were gathered. OS was measured from the date of initial surgery to the date of death or date of last contact if alive. Progression-free survival (PFS) was calculated from the date of the primary surgical resection to disease progression or the last recorded date of follow-up without progression. All patients with disease progression were determined based on MR images, and disease progression was defined as a new or progressive increase within the initial surgical resection site and/or in a remote location, presenting symptoms or postoperative neurological dysfunction by a neurosurgeon and a radiologist blinded to outcomes.
Data were summarized as categorical variables, and differences between groups were tested using the Chi-square test. All collected data were statistically analyzed in SPSS version 23.1 (IBM Corp, Armonk, NY, USA). OS and PFS were plotted using the Kaplan-Meier method, and log-rank test was used to compare Kaplan-Meier plots. For patients without SVZ involvement, simple linear regression was used to determine the strength of association between the tumor-SVZ distance and the OS, PFS as indicated by Pearson’s correlation coefficients (GraphPad Prism 7, La Jolla, CA, USA). A p < 0.05 was considered to be statistically significant, and two-sided statistical tests were performed. Multivariate proportional hazards regression (Cox) analysis was used to analyze an association of independent factors with survival and recurrence. We performed both univariate and multivariate analyses. Factors with a probability value (p) < 0.05 on univariate analyses were subjected to multivariate Cox regression analyses.
One hundred and forty-six cases who met the inclusion criteria were retrospectively reviewed. Detailed clinical information is summarized in Table 1. Of the enrolled patients, 72 cases were found to be SVZ involved, which was grouped as type I. For type II (tumor-SVZ distance from 0 to 10 mm), 36 patients were selected. Thirty-eight cases were grouped as type III (tumor-SVZ distance >10 mm). In all patients, tumors and edemas were bigger in patients with SVZ-positive tumor than in patients with SVZ-negative tumor. A lower rate of GTR and low-grade gliomas (WHO II) was observed in patients with SVZ involvement (20.8 and 40.3%) than in patients with SVZ non-involvement (50 and 61.8%). The comparison in all patients indicates a lower IDH1-mutation ratio in patients with SVZ involvement (41.7%) than in patients with SVZ non-involvement (61.8%). Among the 146 patients included, 63 recurrent cases were observed, and 54 patients had died. The median follow-up time was 1,576 days at the time of last update during follow-up.
Based on the tumor-SVZ distance stratification, the OS (p = 0.02) and PFS (p = 0.002) for the patients had a positive correlation with the tumor-SVZ distance. The patients with SVZ involvement had the worst OS and PFS. In striking contrast, tumor-SVZ distance >10 mm enabled favorable outcomes (Figures 2A, B). Simultaneously we investigated the relationship between the clinical outcomes (PFS and OS) and tumor-SVZ distance in low-grade gliomas (II) and high-grade gliomas (III-IV). Our results showed that tumor-SVZ distance >10 mm was better than tumor-SVZ distance from 0 to 10 mm in respect to OS in two groups, and tumor-SVZ distance >10 mm was associated with better PFS in low-grade gliomas (Supplementary Figure 2). Then, we divided the non-SVZ-contacting glioma into two groups: IDH1-wild glioma and IDH1-mut glioma. Simple linear correlation analysis was applied to investigate the relation between the two parameters (OS and PFS) and tumor-SVZ distance. The results revealed the positive correlation between the tumor-SVZ distance and two parameters (OS and PFS) except PFS of patients with IDH1-wild glioma (Figures 2C–F).
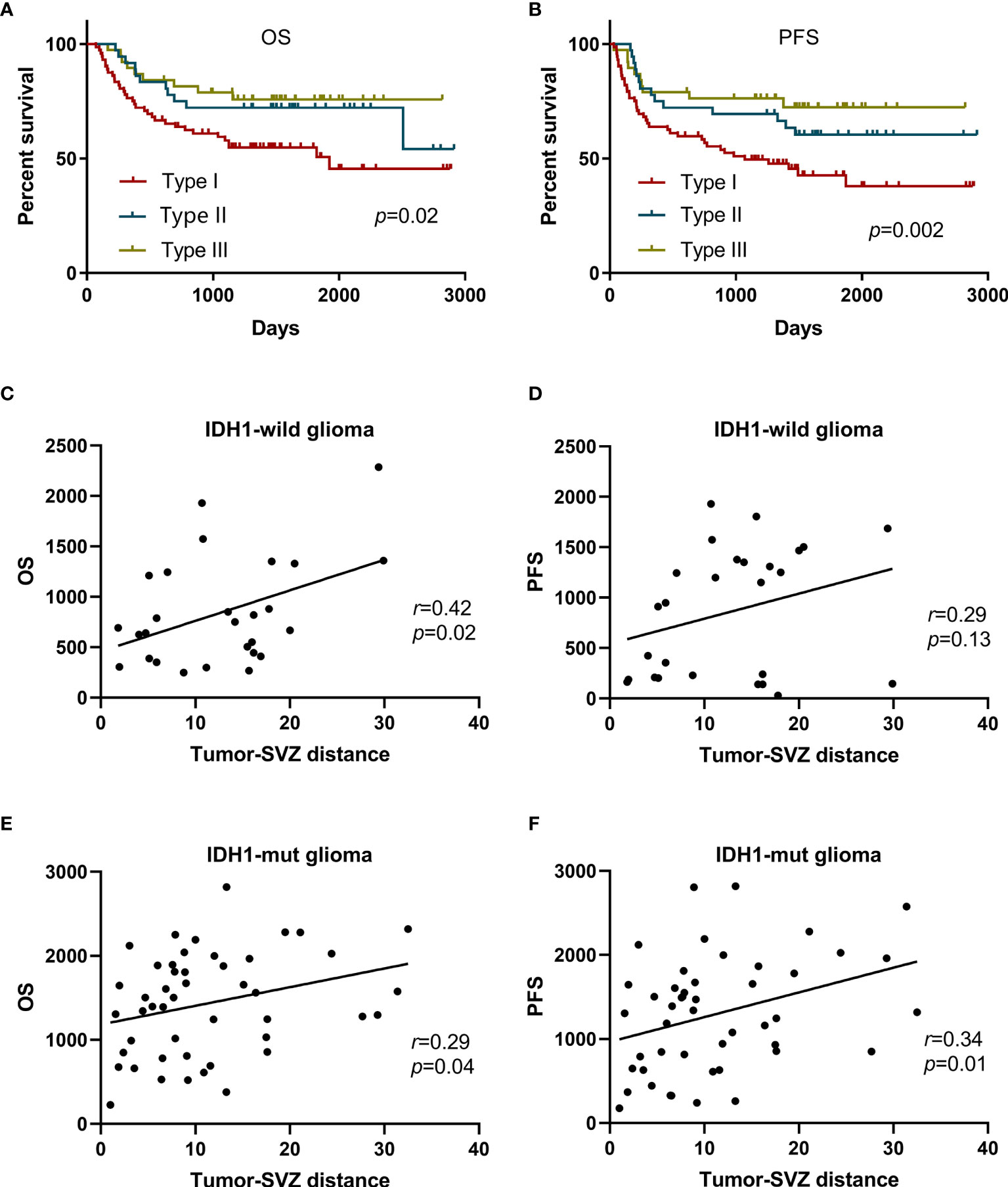
Figure 2 Kaplan-Meier curve analysis of all patients and simple linear correlation analysis of patients with non-SVZ-contacting glioma. Kaplan-Meier curves plotting of patient OS (A) and PFS (B) stratified by the tumor-SVZ distance. Simple linear correlation between the OS and tumor-SVZ distance in IDH1-wild glioma (C) and IDH1-mut glioma (E). Simple linear correlation between the PFS and tumor-SVZ distance in IDH1-wild glioma (D) and IDH1-mut glioma (F). Type I means SVZ involvement, type II means tumor-SVZ distance from 0 to 10 mm, type III means tumor-SVZ distance >10 mm.
Furthermore, we performed comparison, taking into account tumor localization to the SVZ, IDH1 status, and WHO grade, respectively. In our cohort, patients with SVZ involvement were found to be associated with worse OS (p = 0.006) and PFS (p = 0.002) compared with those without SVZ contact (Supplementary Figures 3A, B), similar to previous studies (14, 17). IDH-mut glioma had better OS (p < 0.001) and PFS (p < 0.001) compared with patients without IDH1 mutation (Supplementary Figures 3C, D), which was in agreement with studies reported elsewhere (26, 27). The prognosis of patients with high WHO grade was worse than the patients with low WHO grade (Supplementary Figures 3E, F).
Considering the long-lasting debate of ventricular entry and possible dissemination, we analyzed the prognosis of low-grade patients (WHO grade II) according to the ventricular entry during surgical resections. Among the 76 low-grade gliomas (WHO II), there occurred 36 ventricular entries (VEs) during resection and 40 did not occur. There were 3 (8.3%) distant recurrences and 7 (19.4%) local recurrences in patients with ventricular entry. Whereas in patients with non-ventricular entry, no distant recurrence was observed and 8 (20%) patients had local recurrence. There was no statistically significant difference in local or distant recurrence by analysis of Chi-square test (p = 0.20). In addition, Kaplan-Meier analysis was used to perform survival analysis, and the differences were not statistically significant (Figures 3A, B).
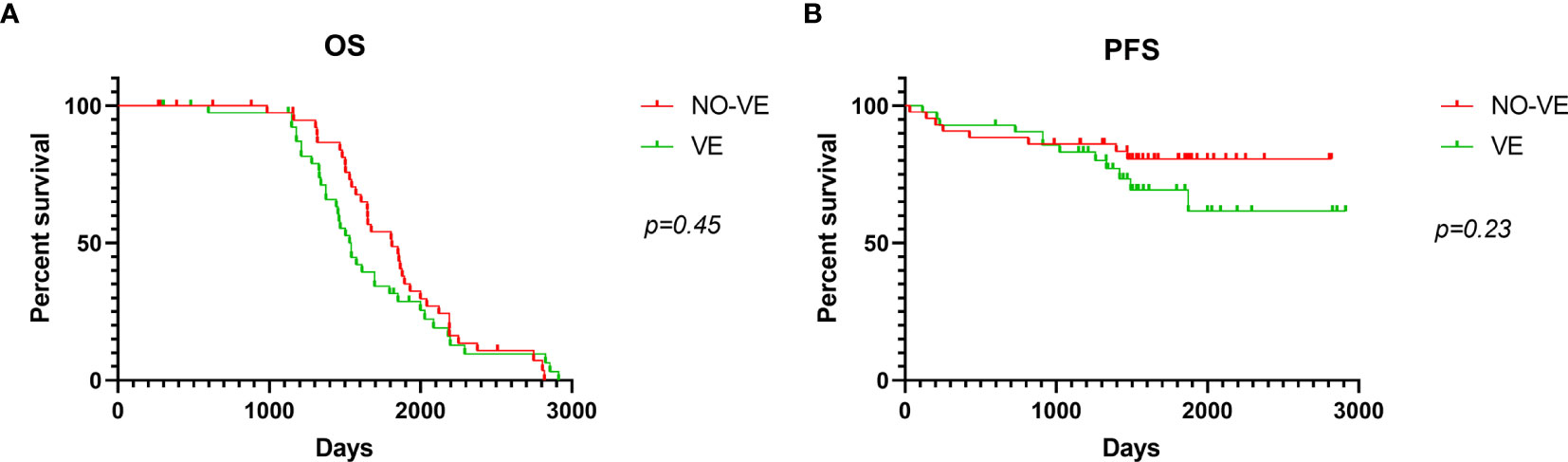
Figure 3 Kaplan-Meier curve analysis according to ventricular entry. OS (A) and PFS (B) analysis using Kaplan-Meier curves in patients with or without ventricular entry. VE means ventricular entry.
Given that SVZ contact and IDH1 mutation are potent factors predicting patient survival time and tumor relapse, it is unknown whether the prognostic performance would be improved with combined use of SVZ contact and IDH1 mutation. In patients with SVZ-positive tumors, no significant difference was found in PFS and OS between IDH1 mutation and IDH wild type. Among patients with SVZ-negative glioma, there was a positive association of IDH1 mutation with OS (p = 0.007) and PFS (p = 0.007) by contrast to wild-type IDH1. Importantly, both IDH1 mutation and SVZ-negative tumors enabled favorable patient outcomes and longer time to relapse, whereas IDH1-wild glioma with SVZ-positive displayed a significantly worse prognosis and shorter time to relapse (Figures 4A, B). These observations highlight the prognostic significance of combined detection of IDH1 status and SVZ involvement for patients with glioma.
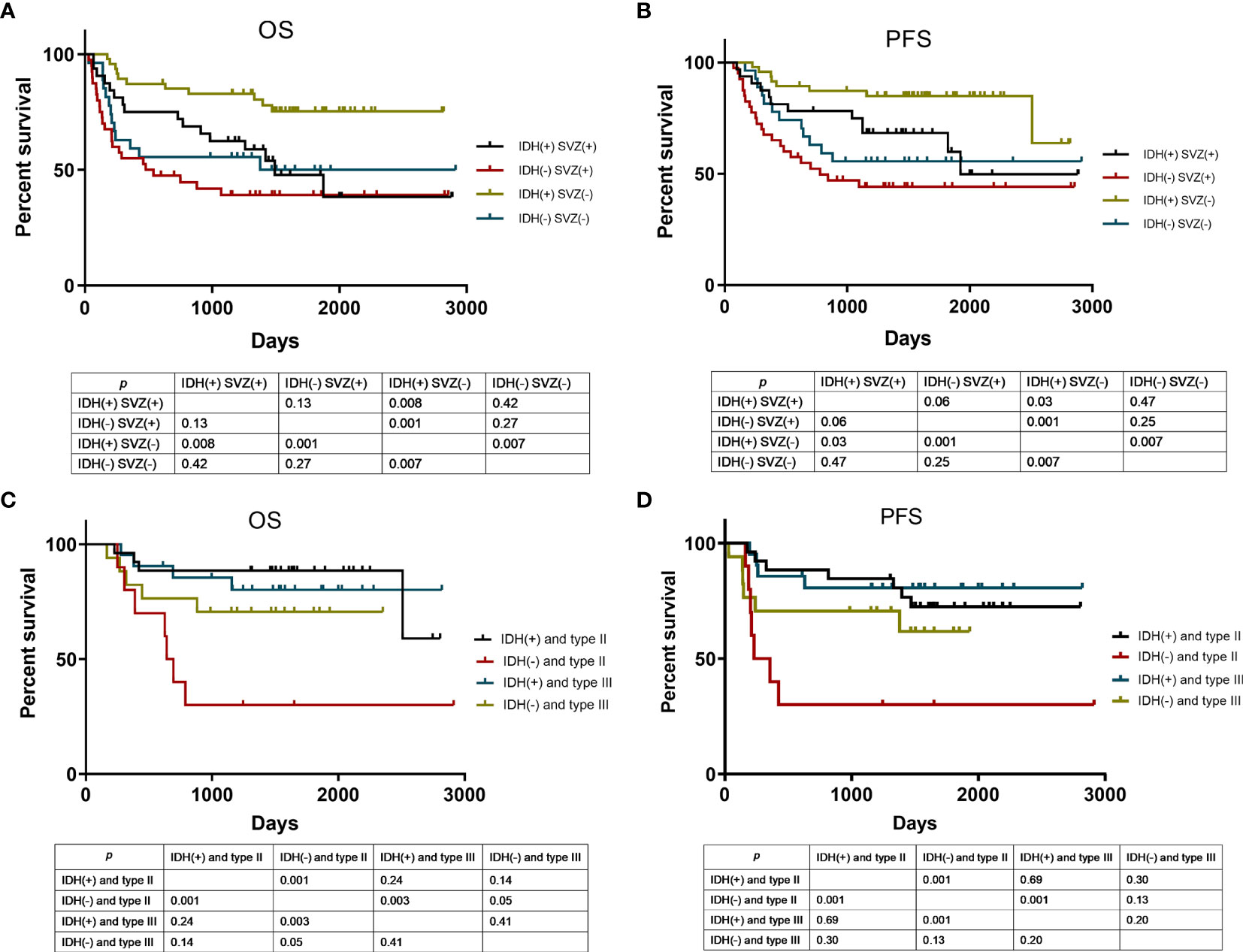
Figure 4 Kaplan-Meier curve analysis according to various risk categories. OS (A) and PFS (B) analysis using Kaplan-Meier curves in patients stratified by SVZ involvement and IDH1 mutation status. Of the patients without SVZ involvement, they were stratified by tumor-SVZ distance and IDH1 mutation status for OS (C) and PFS (D) analysis using Kaplan-Meier curves. SVZ (+) means SVZ involvement, SVZ (−) means SVZ non-involvement. IDH (+) means IDH1 mutation, IDH (−) means IDH1 wild type. Type II means tumor-SVZ distance from 0 to 10 mm; type III means tumor-SVZ distance >10 mm.
As revealed above, in patients without SVZ involvement, the tumor-SVZ distance impacts the outcome of patients with glioma, among which patients with tumor-SVZ distance >10 mm have better prognosis than those with tumor-SVZ distance from 0 to 10 mm. To estimate the prognostic performance of combined tumor-SVZ distance and IDH1 status in patients without SVZ involvement, we made an additional comparison. As shown in Figures 4C, D, tumor-SVZ distance showed no obvious interaction with the OS and PFS of patients with IDH1 mutations. But in patients with IDH1 wild type, the OS (p = 0.05) of patients with tumor-SVZ distance >10 mm was significantly better than in patients with tumor-SVZ distance from 0 to 10 mm. Therefore, patients bearing wild-type IDH1 and tumor-SVZ distance from 0 to 10 mm displayed poorer OS and PFS. Whereas patients with IDH1 mutation concurrent with tumor-SVZ distance >10 mm exhibited particularly satisfactory prognosis and longer time to relapse.
The contribution of individual variables was evaluated by both univariate and multivariate analyses. Univariate analysis identifies unfavorable factors for the outcome of patients with glioma including age ≥40 years, KPS <80, WHO grades (III–IV) and SVZ involvement, and tumor-SVZ distance from 0 to 10 mm. GTR, IDH1 mutation, and chemotherapy were found to predict good prognosis of patients. A detailed summary is shown in Table 2. Similarly, multivariate analysis showed that WHO grade IV (p = 0.001), SVZ involvement (p = 0.01), tumor-SVZ distance from 0 to 10 mm (p = 0.03), GTR (p = 0.05), chemotherapy (p = 0.003), and IDH1 mutation (p = 0.01) served as independent predictors for patient OS. In addition, WHO grade IV (p = 0.001), SVZ involvement (p = 0.002), tumor-SVZ distance from 0 to 10 mm (p = 0.02), chemotherapy (p = 0.02), and IDH1 mutation (p = 0.03) served as independent predictors for patient PFS (Table 3).
Our study demonstrates the relationship between the shortest distance from tumor edge to SVZ and the prognosis of patients with glioma. Although SVZ contact has been identified as a prognostic factor for outcome of patients with glioma, it remains undetermined whether and what distance from tumor to SVZ determine the prognosis of patients with glioma. We show that the clinical outcomes of patients with SVZ involvement were worse than patients with SVZ non-involvement regardless of IDH1 status. In patients with wild-type IDH1, the clinical outcomes of patients with tumor-SVZ distance from 0 to 10 mm were worse than those with tumor-SVZ distance >10 mm. Therefore, these data suggest that combining the distance from the tumor edge to the subventricular zone and IDH mutation provides a more accurate predictor for the prognosis of patients with Glioma.
Accumulating evidence indicates that SVZ and IDH1 mutation are the leading determinants for prognostic prediction of glioma. Studies over the last decade have established the critical role of SVZ in glioma development and progression. It is now considered that neural stem cells residing in the SVZ undergo neoplastic transformation and result in tumorigenesis, progression, and resistance to standard treatment (5, 11). Mutations in IDH1 occur in the vast majority of low-grade gliomas (WHO I–II) and secondary high-grade gliomas. Growing data indicate that these mutations drive gliomagenesis and are of prognostic importance for gliomas. However, little is known about whether combined IDH1 mutation and SVZ involvement are clinically relevant and provide improved prognostic performance for patients with glioma. Moreover, while its clinical role and significance in SVZ-positive patients were extensively studied, the functional consequence of tumor-SVZ distance in SVZ-negative patients has not been investigated. In this study, we performed volumetric analysis using MR images to reveal the anatomical relationship between the tumor progression and the distance from tumor edge to SVZ. SVZ involvement in our cohort is correlated with larger tumor and edema volumes, leading to lower rate of GTR. IDH1 mutation, which was found to disrupt SVZ and drive tumor progression (28), was detected in 53.2% of glioma population here, similar to prior studies (29, 30). We revealed the anatomical relationship between the tumor progression and the SVZ correlates with patient prognosis. However, further research is needed to conclusively determine the underlying mechanisms that have led to our results. Consistently, SVZ contact predicts patient outcomes described here and elsewhere regardless of IDH1 status. The observation that SVZ involvement was associated with shorter OS and PFS by univariate analysis is generally in line with multiple studies previously described (13, 14, 31, 32). Our findings here highlight the therapeutic implications of targeting SVZ for glioma intervention. Indeed, it has been shown that radiotherapy co-targeted to the SVZ improves PFS of patients compared to radiotherapy targeting the tumor alone (33). Additionally, increasing the mean dose of radiotherapy to the ipsilateral SVZ was associated with significantly improved OS (32, 34).
In addition, glioma, no matter the SVZ involvement or not, is thought to arise from SVZ where NSCs reside (5, 35–38). This means that non-SVZ-contacting glioma located at the parenchyma of the brain still has some correlation with SVZ, implying that the tumor-SVZ distance might affect the outcome of glioma. We evaluated the association between tumor-SVZ distance and the PFS and OS. Dramatically, our data suggest that tumor-SVZ distance was significantly associated with the PFS and OS of patients with glioma. Moreover, our data revealed the positive correlation between the two parameters (OS and PFS) and tumor-SVZ distance in patients with non-SVZ-contacting gliomas (Figures 2C–F). In multivariate analysis, SVZ involvement and tumor-SVZ distance from 0 to 10 mm serve as independent predictors for patients (Table 3). Thus, the present study supports that the tumor-SVZ distance correlates with the PFS and OS of glioma. This finding suggests that tumor-SVZ distance could be used as a new routine variable that can be used as a marker to predict the prognosis of patients, especially in patients without IDH mutation. The prognosis of tumors close to the SVZ may be worse than that of patients with tumors distant from the SVZ.
Despite the prognostic significance of SVZ involvement and IDH1 mutation status alone, evidence is lacking concerning their combined use. Mutations of the IDH1 are considered as an important event that occurs at an early stage during gliomagenesis (39) and was included in the (WHO) classification of the central nervous system published in 2016 (40). IDH1 mutations were thought to control the balance between glioma stem cell property and cell differentiation (41–45). A meta-analysis of 55 observational studies revealed glioma patients harboring IDH1 mutations have improved OS and PFS, especially for patients with WHO grade III and grades II–III (27, 46). We demonstrated in the current study that patients of IDH1 mutation concurrent with tumor-SVZ distance >10 mm have the best clinical outcome compared with patients bearing wild-type IDH1 and tumor-SVZ distance <10 mm, which exhibit the poorest survival time and shorter tumor relapse. It is interesting to clarify whether IDH1 mutation and tumor-SVZ distance >10 mm would serve as specific biomarkers that are predictive of effective response to current therapy for gliomas.
Our study also has limitations. This is a retrospective study, and its conclusion needs to be validated in future studies. Some established prognostic biomarkers, such as 1p/19q codeletion and MGMT promoter methylation status, are missing. Moreover, only the mutation status of IDH1 but not IDH2 was assessed here, although the IDH1 mutations were predominantly found (27). Additionally, current MR technology can hardly delineate the pathological boundary of a tumor lesion. A previous study pointed out that abnormality on T2WI represents a variable admixture of vasogenic edema and non-enhancing tumor, whereas abnormality on contrast-enhanced T1WI may correlate more closely with the site of origin of the glioma (13). Therefore, the edges of tumor are guided by standard MRI: T2WI for non-enhancing tumor in our study, which is consistent with the guideline of clinical treatment (47, 48). At the same time, to reduce inaccuracies, all tumor boundaries were manually defined by two experienced neurosurgeons and then reevaluated by two independent senior neuroradiologists. A prospective study designed for considering multiple variables might provide more accurate prognostic prediction for glioma.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Research Ethics Committee of the First Hospital Affiliated to Army Medical University (Southwest Hospital). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
RH and HF designed the experiments. SZ, DL, WF, XY, ZPL, YL, SH, YY, LT, WL, and FL collected the data. SZ, DL, FZ, and TZ evaluated the dataset and performed statistical analyses. CLa, ZL, CLi, and HL reviewed the radiological imaging. SZ, TZ, FZ, and RH drafted the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants WSS-2014-11 from the Science and Technology Foundation of China Academy of Endineering Physics (Intersection of Physics and Biomedicine).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the participants included in our trial for their involvement and enthusiasm.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.693693/full#supplementary-material
1. Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2010-2014. Neuro Oncol (2017) 19(suppl_5):v1–v88. doi: 10.1093/neuonc/nox158
2. Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The Epidemiology of Glioma in Adults: A “State of the Science” Review. Neuro-Oncology (2014) 16(7):896–913. doi: 10.1093/neuonc/nou087
3. Wang Y, Jiang T. Understanding High Grade Glioma: Molecular Mechanism, Therapy and Comprehensive Management. Cancer Lett (2013) 331(2):139–46. doi: 10.1016/j.canlet.2012.12.024
4. Sanai N, Alvarez-Buylla A, Berger MS. Neural Stem Cells and the Origin of Gliomas. N Engl J Med (2005) 353(8):811–22. doi: 10.1056/NEJMra043666
5. Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, et al. Malignant Astrocytomas Originate From Neural Stem/Progenitor Cells in a Somatic Tumor Suppressor Mouse Model. Cancer Cell (2009) 15(1):45–56. doi: 10.1016/j.ccr.2008.12.006
6. Sanai N, Tramontin AD, Quiñones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, et al. Unique Astrocyte Ribbon in Adult Human Brain Contains Neural Stem Cells But Lacks Chain Migration. Nature (2004) 427(6976):740–4. doi: 10.1038/nature02301
7. Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular Zone Astrocytes are Neural Stem Cells in the Adult Mammalian Brain. Cell (1999) 97(6):703–16. doi: 10.1016/s0092-8674(00)80783-7
8. Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in Adult Subventricular Zone. J Neurosc: Off J Soc Neurosci (2002) 22(3):629–34. doi: 10.1523/JNEUROSCI.22-03-00629.2002
9. Quiñones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, et al. Cellular Composition and Cytoarchitecture of the Adult Human Subventricular Zone: A Niche of Neural Stem Cells. J Comp Neurol (2006) 494(3):415–34. doi: 10.1002/cne.20798
10. Gil-Perotin S, Marin-Husstege M, Li J, Soriano-Navarro M, Zindy F, Roussel MF, et al. Loss of P53 Induces Changes in the Behavior of Subventricular Zone Cells: Implication for the Genesis of Glial Tumors. J Neurosc: Off J Soc Neurosci (2006) 26(4):1107–16. doi: 10.1523/jneurosci.3970-05.2006
11. Lee JH, Lee JE, Kahng JY, Kim SH, Park JS, Yoon SJ, et al. Human Glioblastoma Arises From Subventricular Zone Cells With Low-Level Driver Mutations. Nature (2018) 560(7717):243–7. doi: 10.1038/s41586-018-0389-3
12. Lim DA, Cha S, Mayo MC, Chen MH, Keles E, VandenBerg S, et al. Relationship of Glioblastoma Multiforme to Neural Stem Cell Regions Predicts Invasive and Multifocal Tumor Phenotype. Neuro-Oncology (2007) 9(4):424–9. doi: 10.1215/15228517-2007-023
13. Young GS, Macklin EA, Setayesh K, Lawson JD, Wen PY, Norden AD, et al. Longitudinal MRI Evidence for Decreased Survival Among Periventricular Glioblastoma. J Neuro Oncol (2011) 104(1):261–9. doi: 10.1007/s11060-010-0477-1
14. Jafri NF, Clarke JL, Weinberg V, Barani IJ, Cha S. Relationship of Glioblastoma Multiforme to the Subventricular Zone Is Associated With Survival. Neuro-Oncology (2013) 15(1):91–6. doi: 10.1093/neuonc/nos268
15. Chaichana KL, Pendleton C, Chambless L, Camara-Quintana J, Nathan JK, Hassam-Malani L, et al. Multi-Institutional Validation of a Preoperative Scoring System Which Predicts Survival for Patients With Glioblastoma. J Clin Neurosc: Off J Neurosurg Soc Australasia (2013) 20(10):1422–6. doi: 10.1016/j.jocn.2013.02.007
16. Adeberg S, Bostel T, König L, Welzel T, Debus J. Combs SE. A Comparison of Long-Term Survivors and Short-Term Survivors With Glioblastoma, Subventricular Zone Involvement: A Predictive Factor for Survival? Radiat Oncol (2014) 9:95. doi: 10.1186/1748-717x-9-95
17. Liu S, Wang Y, Fan X, Ma J, Ma W, Wang R, et al. Anatomical Involvement of the Subventricular Zone Predicts Poor Survival Outcome in Low-Grade Astrocytomas. PloS One (2016) 11(4):e0154539. doi: 10.1371/journal.pone.0154539
18. Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Sci (New York NY) (2008) 321(5897):1807–12. doi: 10.1126/science.1164382
19. Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 Mutations in Gliomas. New Engl J Med (2009) 360(8):765–73. doi: 10.1056/NEJMoa0808710
20. Ichimura K. Molecular Pathogenesis of IDH Mutations in Gliomas. Brain Tumor Pathol (2012) 29(3):131–9. doi: 10.1007/s10014-012-0090-4
21. Moiraghi A, Prada F, Delaidelli A, Guatta R, May A, Bartoli A, et al. Navigated Intraoperative 2-Dimensional Ultrasound in High-Grade Glioma Surgery: Impact on Extent of Resection and Patient Outcome. Oper Neurosurg (Hagerstown) (2019) 18(4):363–73. doi: 10.1093/ons/opz203
22. Sagberg L, Iversen D, Fyllingen E, Jakola A, Reinertsen I, Solheim O. Brain Atlas for Assessing the Impact of Tumor Location on Perioperative Quality of Life in Patients With High-Grade Glioma: A Prospective Population-Based Cohort Study. NeuroImage Clin (2019) 21:101658. doi: 10.1016/j.nicl.2019.101658
23. Vescovi A, Galli R, Reynolds B. Brain Tumour Stem Cells. Nat Rev Cancer (2006) 6(6):425–36. doi: 10.1038/nrc1889
24. Hammers A, Allom R, Koepp M, Free S, Myers R, Lemieux L, et al. Three-Dimensional Maximum Probability Atlas of the Human Brain, With Particular Reference to the Temporal Lobe. Hum Brain Mapp (2003) 19(4):224–47. doi: 10.1002/hbm.10123
25. Skjulsvik A, Bø H, Jakola A, Berntsen E, Bø L, Reinertsen I, et al. Is the Anatomical Distribution of Low-Grade Gliomas Linked to Regions of Gliogenesis? J Neuro Oncol (2020) 147(1):147–57. doi: 10.1007/s11060-020-03409-8
26. Shibahara I, Sonoda Y, Kanamori M, Saito R, Yamashita Y, Kumabe T, et al. IDH1/2 Gene Status Defines the Prognosis and Molecular Profiles in Patients With Grade III Gliomas. Int J Clin Oncol (2012) 17(6):551–61. doi: 10.1007/s10147-011-0323-2
27. Wang XW, Ciccarino P, Rossetto M, Boisselier B, Marie Y, Desestret V, et al. IDH Mutations: Genotype-Phenotype Correlation and Prognostic Impact. BioMed Res Int (2014) 2014:540236. doi: 10.1155/2014/540236
28. Gorovets D, Kannan K, Shen R, Kastenhuber ER, Islamdoust N, Campos C, et al. IDH Mutation and Neuroglial Developmental Features Define Clinically Distinct Subclasses of Lower Grade Diffuse Astrocytic Glioma. Clin Cancer Res: Off J Am Assoc Cancer Res (2012) 18(9):2490–501. doi: 10.1158/1078-0432.CCR-11-2977
29. Bleeker FE, Lamba S, Leenstra S, Troost D, Hulsebos T, Vandertop WP, et al. IDH1 Mutations at Residue P.R132 (IDH1(R132)) Occur Frequently in High-Grade Gliomas But Not in Other Solid Tumors. Hum Mutat (2009) 30(1):7–11. doi: 10.1002/humu.20937
30. Ohgaki H, Kleihues P. Genetic Alterations and Signaling Pathways in the Evolution of Gliomas. Cancer Sci (2009) 100(12):2235–41. doi: 10.1111/j.1349-7006.2009.01308.x
31. Adeberg S, Konig L, Bostel T, Harrabi S, Welzel T, Debus J, et al. Glioblastoma Recurrence Patterns After Radiation Therapy With Regard to the Subventricular Zone. Int J Radiat Oncol Biol Phys (2014) 90(4):886–93. doi: 10.1016/j.ijrobp.2014.07.027
32. Gupta T, Nair V, Paul SN, Kannan S, Moiyadi A, Epari S, et al. Can Irradiation of Potential Cancer Stem-Cell Niche in the Subventricular Zone Influence Survival in Patients With Newly Diagnosed Glioblastoma? J Neurooncol (2012) 109(1):195–203. doi: 10.1007/s11060-012-0887-3
33. Evers P, Lee PP, DeMarco J, Agazaryan N, Sayre JW, Selch M, et al. Irradiation of the Potential Cancer Stem Cell Niches in the Adult Brain Improves Progression-Free Survival of Patients With Malignant Glioma. BMC Cancer (2010) 10:384. doi: 10.1186/1471-2407-10-384
34. Chen L, Guerrero-Cazares H, Ye X, Ford E, McNutt T, Kleinberg L, et al. Increased Subventricular Zone Radiation Dose Correlates With Survival in Glioblastoma Patients After Gross Total Resection. Int J Radiat Oncol Biol Phys (2013) 86(4):616–22. doi: 10.1016/j.ijrobp.2013.02.014
35. Recht L, Jang T, Savarese T, Litofsky NS. Neural Stem Cells and Neuro-Oncology: Quo Vadis? J Cell Biochem (2003) 88(1):11–9. doi: 10.1002/jcb.10208
36. Jackson EL, Alvarez-Buylla A. Characterization of Adult Neural Stem Cells and Their Relation to Brain Tumors. Cells Tissues Organs (2008) 188(null):212–24. doi: 10.1159/000114541
37. Wang Y, Yang J, Zheng H, Tomasek GJ, Zhang P, McKeever PE, et al. Expression of Mutant P53 Proteins Implicates a Lineage Relationship Between Neural Stem Cells and Malignant Astrocytic Glioma in a Murine Model. Cancer Cell (2009) 15(6):514–26. doi: 10.1016/j.ccr.2009.04.001
38. Kappadakunnel M, Eskin A, Dong J, Nelson SF, Mischel PS, Liau LM, et al. Stem Cell Associated Gene Expression in Glioblastoma Multiforme: Relationship to Survival and the Subventricular Zone. J Neurooncol (2010) 96(3):359–67. doi: 10.1007/s11060-009-9983-4
39. Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 Mutations are Early Events in the Development of Astrocytomas and Oligodendrogliomas. Am J Pathol (2009) 174(4):1149–53. doi: 10.2353/ajpath.2009.080958
40. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol (2016) 131(6):803–20. doi: 10.1007/s00401-016-1545-1
41. Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-Associated IDH1 Mutations Produce 2-Hydroxyglutarate. Nature (2010) 465(7300):966. doi: 10.1038/nature09132
42. Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, et al. Oncometabolite 2-Hydroxyglutarate is a Competitive Inhibitor of α-Ketoglutarate-Dependent Dioxygenases. Cancer Cell (2011) 19(1):17–30. doi: 10.1016/j.ccr.2010.12.014
43. Jin G, Reitman ZJ, Spasojevic I, Batinic-Haberle I, Yang J, Schmidt-Kittler O, et al. 2-Hydroxyglutarate Production, But Not Dominant Negative Function, is Conferred by Glioma-Derived NADP-Dependent Isocitrate Dehydrogenase Mutations. PloS One (2011) 6(2):e16812. doi: 10.1371/journal.pone.0016812
44. Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, et al. IDH1 Mutation is Sufficient to Establish the Glioma Hypermethylator Phenotype. Nature (2012) 483(7390):479–83. doi: 10.1038/nature10866
45. Zhang C, Moore LM, Li X, Yung WK, Zhang W. IDH1/2 Mutations Target a Key Hallmark of Cancer by Deregulating Cellular Metabolism in Glioma. Neuro-oncology (2013) 15(9):1114–26. doi: 10.1093/neuonc/not087
46. Xia L, Wu B, Fu Z, Feng F, Qiao E, Li Q, et al. Prognostic Role of IDH Mutations in Gliomas: A Meta-Analysis of 55 Observational Studies. Oncotarget (2015) 6(19):17354–65. doi: 10.18632/oncotarget.4008
47. Niyazi M, Brada M, Chalmers AJ, Combs SE, Erridge SC, Fiorentino A, et al. ESTRO-ACROP Guideline “Target Delineation of Glioblastomas”. Radiother Oncol (2016) 118(1):35–42. doi: 10.1016/j.radonc.2015.12.003
Keywords: glioma, MRI, subventricular zone, isocitrate dehydrogenase 1, prognosis
Citation: Zhang S, Zhao F, Zhou T, Liu D, Yao X, Fu W, Liu Z, Lan C, Lai Z, Liu C, Li H, Li Y, Hu S, Yin Y, Tan L, Li W, Li F, Hu R and Feng H (2021) Combination of the Distance From Tumor Edge to Subventricular Zone and IDH Mutation Predicts Prognosis of Patients With Glioma. Front. Oncol. 11:693693. doi: 10.3389/fonc.2021.693693
Received: 11 April 2021; Accepted: 29 July 2021;
Published: 19 August 2021.
Edited by:
Manabu Kinoshita, Asahikawa Medical University, JapanCopyright © 2021 Zhang, Zhao, Zhou, Liu, Yao, Fu, Liu, Lan, Lai, Liu, Li, Li, Hu, Yin, Tan, Li, Li, Hu and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Hu, aHVjaHJvbmdAdG1tdS5lZHUuY24=; Hua Feng, ZmVuZ2h1YTg4ODhAdmlwLjE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.