
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 17 August 2021
Sec. Head and Neck Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.692390
 Xinwen Zhang1†
Xinwen Zhang1† Yong Fu1†
Yong Fu1† Zhuang Ding1
Zhuang Ding1 Nisha Zhu1
Nisha Zhu1 Mengxiang Zhao1
Mengxiang Zhao1 Yuxian Song1
Yuxian Song1 Xiaofeng Huang2
Xiaofeng Huang2 Sheng Chen2
Sheng Chen2 Yan Yang2
Yan Yang2 Caihong Zhang3
Caihong Zhang3 Qingang Hu1*
Qingang Hu1* Yanhong Ni1*
Yanhong Ni1* Liang Ding1*
Liang Ding1*Background: Reelin, an extracellular glycoprotein, is expressed on neuronal cells and participates in neuronal migration during brain development. Recently, Reelin also has a vital role in carcinogenesis. However, its role in oral squamous cell carcinoma (OSCC) remains to be explored. The purpose of this study was to explore the roles of Reelin in OSCC.
Methods: The expression of Reelin in cancer-associated fibroblasts (ReelinCAF) and tumor cells (ReelinTC) was analyzed by the Gene Expression Omnibus (GEO) database. Immunohistochemistry (IHC) was used to detect the spatial pattern of Reelin in 75 OSCCs. The diagnostic and prognostic values of Reelin were evaluated and also verified by The Cancer Genome Atlas (TCGA) database. Primary CAFs from 13 OSCC patients were isolated to confirm Reelin expression. Thirty-nine OSCC peripheral blood samples were used to analyze the change of immunocytes based on Reelin levels by flow cytometry. The relationship between Reelin and tumor immune microenvironment in head and neck squamous cell carcinoma (HNSCC) tissues was determined by TISIDB and the Tumor Immune Estimation Resource (TIMER) database.
Results: In breast cancer, pancreatic cancer and rectal cancer, Reelin in CAFs was significantly upregulated compared with Reelin in TCs. The IHC results in OSCC also showed that Reelin levels were higher in CAFs. Upregulated ReelinTC was related to a decreased pN stage and distant metastasis. Strikingly, patients with enhanced ReelinCAF had a high risk of lymph node metastasis, poor worst pattern of invasion (WPOI), and distant metastasis, but showed comparable Ki-67 level in all OSCC patients, resulting in shorter overall survival (OS) and disease-specific survival (DSS). Unexpectedly, Reelin in tumor-infiltrating lymphocytes (ReelinTIL) was correlated with postoperative relapse. Patients with high ReelinTIL, but not ReelinTC and ReelinCAF, had poor cytotoxicity of CD8+ T cells and higher ratio of CD4/CD8 in peripheral blood. However, Reelin was positively associated with tissue-resident B cells and NK cells in the tumor microenvironment.
Conclusion: Reelin has a versatile function in distinct cell types during the development of OSCC via governing tumor cell and stroma microenvironment.
Head and neck squamous cell carcinoma (HNSCC) was the sixth most common cancer worldwide in 2018. Oral squamous cell carcinoma (OSCC) is the most common malignant tumor of the head and neck with a high mortality rate (1–3). Therefore, the potential predictors of OSCC are awaited to identify for guiding diagnosis and treatment. Reelin is a secreted extracellular matrix glycoprotein that is formed with 3,461 amino acids. It is secreted by Cajal-Retzius cells in the marginal zone and encoded by the RELN gene, which is located on chromosome 7q22. The molecular weight of Reelin is about 400 kDa (4–6). To date, a majority of Reelin-related studies have focused on neurological diseases, such as schizophrenia, autism, and Alzheimer’s disease because Reelin helps modulate the processes of neuronal migration and positioning during brain development (7–10). Importantly, Reelin is expressed in many extraneuronal tissues, including plasma, blood cells, liver, and intestine. However, the roles of peripheral Reelin remain unknown, especially in oral tissues with a large number of nerves (4).
Notably, alterations in Reelin expression are observed in cancers of nonneuronal origin (11). Specifically, the expression of Reelin is reduced in hepatocellular carcinoma, gastric carcinoma, colorectal cancer, glioblastoma, breast cancer, and pancreatic cancers (12–17). In contrast, upregulation of Reelin is observed in retinoblastoma, esophageal carcinoma, high-grade prostate cancer, multiple myeloma, and non-Hodgkin lymphoma (18–22). The alterations in Reelin expression are related to the tumor metastasis and invasion (23, 24). For example, in a human esophageal carcinoma cell line, RELN was related to TGF-β, which was related to metastasis, and knockdown of RELN increased the rate of cell migration (25). However, the role of Reelin in OSCC is far from known.
Although the studies related to Reelin mainly focuses on brain development and tumor progression, some studies have proved that Reelin is also linked to immune function. For example, some studies pointed out that interleukin (IL)-1β mRNA was highly expressed in mice with RELN mutation by stimulating with lipopolysaccharide (26, 27). Decreased Reelin was associated with the alterations in serotonin transporter (SERT) clustering in blood lymphocytes, which may be essential in some cardiovascular or immune system alterations (28). Thus, correlation between Reelin and immune balance in OSCC remains elucidated.
Therefore, in the present study, we focused on the expression pattern of Reelin in OSCC, including tumor cells (ReelinTC), cancer-associated fibroblasts (ReelinCAF), and tumor-infiltrating lymphocytes (ReelinTIL). Furthermore, we evaluated the correlation between Reelin and clinicopathological characteristics, its prognostic significance, and immune cell infiltrations.
All methods used for this study were approved by the Ethics Committee of Nanjing Stomatology Hospital [2019NL-009(KS)]. The study was carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the patients. Seventy-five OSCC patients were recruited in this study. All the 75 cases included 14 cases of cheek cancer, 30 cases of tongue cancer, 16 cases of gingival cancer, and 15 cases of other types of oral cancer. The inclusion and exclusion criteria of patients were the same as those of our previous studies (29). Specifically, the patients must have primary OSCC without anti-cancer therapies including chemotherapy and/or radiotherapy before surgery, and no distant metastasis prior to surgery, and these patients underwent curative resection between 2013 and 2016 at Nanjing Stomatology Hospital. Lactating individuals, pregnant women, and patients who were diagnosed with other malignant diseases or certain autoimmune diseases were excluded. These patients with primary tumor were diagnosed by hematoxylin and eosin staining by two experienced pathologists. All 75 patients were followed-up until April 2020. Paraffin-embedded OSCC tissue slices were obtained from the pathology department and used for IHC study. Thirty-nine blood samples from OSCC patients were obtained for flow cytometry assay before any related treatments.
The protocol of IHC of formalin-fixed paraffin-embedded sections and scoring details of IHC was performed as previously described (29, 30). Specifically, IHC was performed on 3-µm formalin-fixed paraffin-embedded sections using anti-Reelin (monoclonal, 1:100 dilution; MAB5366; Abcam) and anti-Ki-67 (monoclonal, 1:200 dilution; ab16667; Abcam). Slides were deparaffinized with xylene and rehydrated in an ethanol series. Antigen retrieval was performed with 10 mmol/L citric acid (pH 6.0) in a pressure cooker. Then, endogenous peroxidase activity was blocked with a 3% hydrogen peroxide solution. After washing in phosphate-buffered saline (PBS; pH 7.4) three times, slides were incubated with primary antibodies against Reelin (MAB5366; Abcam) at 4°C overnight. Polink-2 plus HRP Detection Kit was used as the secondary antibody at 37°C for 40 min. Finally, slides were developed in diaminobenzidine (DAB). The primary antibody was replaced by PBS as negative control. The IHC staining results of Reelin and Ki-67 were independently and double blindly evaluated by two senior pathologists who did not know the patients’ data, and the average values were calculated for further analysis. IHC staining was scored according to the percentage of positive cells and staining intensity. The percentage of stained cells was defined as 0 = 0–5%; 1 = 6%–25%; 2 = 26%–50%; 3 = 51%–75%; and 4 = 75–100%. Staining intensity was defined as follows: 0 = negative staining; 1 = weak staining; 2 = moderate staining; and 3 = strong staining (29, 30). The IHC score was calculated by multiplying the grade of the staining intensity by that of the staining percentage. High and low expressions of Reelin were defined by the median of IHC scores.
CAFs used in this study were isolated from fresh 13 OSCC tumor tissues immediately after surgical resection. Details of this protocol were the same as previously described (29).
Immunofluorescence performed on primary CAFs was carried out as previously described (29): primary antibodies, α-SMA (1:200, Abcam, England), FSP-1 (1:200, Abcam, England), and PDGFR-β (1:200, Abcam, England); secondary antibodies, Alexa Fluor 647-conjugated (1:100, Abcam, England), Alexa Fluor 555-conjugated (1:200, Abcam, England), and Alexa Fluor 488-conjugated (1:400, Abcam, England).
Thirteen cultured primary CAFs were classified as WPOI = 1–3 or WPOI = 4–5. The details of extracting total mRNA and conducting quantitative PCR (q-PCR) were performed as previously described (29). Comparative 2−ΔCt or 2−ΔΔCt method was used to quantify the relative mRNA expression as indicated.
For the cell subtypes of peripheral blood mononuclear cell (PBMC) analysis, details of flow cytometry were the same as previously described (31).
The differential expression of Reelin between CAFs and TCs was performed by Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) database analysis in specific tumors. The Cancer Genome Atlas (TCGA, https://cancergenome.nih.gov/abouttcga/overview) database was used to further validate survival implication of Reelin. Tumor Immune Estimation Resource (TIMER, https://cistrome.shinyapps.io/timer/) was used to learn the association between Reelin mRNA expression and the expression of particular immune infiltrating cell subset markers according to the TIMER’s online instructions. An integrated repository portal for tumor–immune system interactions (TISIDB, http://cis.hku.hk/TISIDB/index.php) was utilized to examine Reelin mRNA and immune system interactions in 28 types of TILs across human cancers.
SPSS 25.0 software and GraphPad Prism 8.0 were used for statistical analyses and figure process. The results of the experiments were presented as mean ± SEM. Chi-square test, Pearson’s chi-square test, or Fisher’s exact test was used to compare clinicopathological parameters of the patients. Kolmogorov-Smirnov tests were used to learn the relationship between Reelin and Ki-67. The Mann–Whitney U test was used to compare the two groups (e.g., Reelin in CAFs with relapse, with relapse versus without relapse). Survival analysis including overall survival (OS), disease-specific survival (DSS), and progression-free survival (PFS) were evaluated by Kaplan–Meier survival test. The Cox regression model was used to evaluate the correlations between different prognostic factors in a multivariate analysis. The immune infiltration level was compared with the t test. All the analyses were two-sided test and differences were considered statistically significant with p < 0.05.
Based on the GEO database, we found that Reelin was significantly upregulated in CAFs compared with TCs (p = 0.0063, p = 0.0021, and p = 0.0033; Figures 1A–C). Thus, to validate Reelin expression in OSCC, IHC staining was performed to assess the expression of Reelin in different cell types including TCs, FLCs, and TILs. Consistently with the database, the IHC score of ReelinFLC was significantly higher than that of ReelinTC in OSCC (p < 0.0001 Figure 1E). In addition, representative IHC staining is shown in Figure 1D.
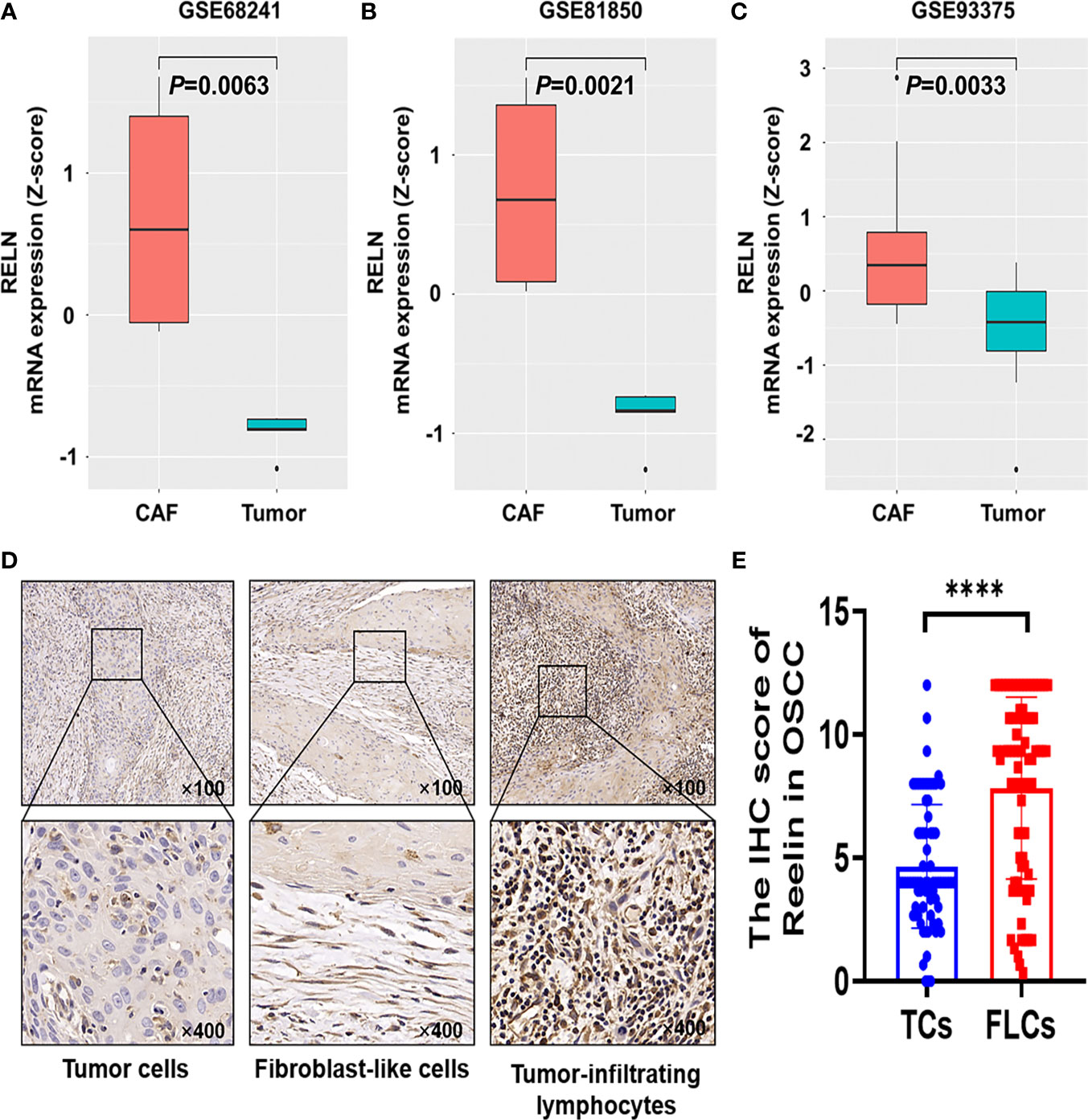
Figure 1 Reelin was upregulated in CAFs compared with TCs derived from OSCC and other several cancers. Enhanced level of RELNCAF compared with RELNTC in breast cancer (A), pancreatic cancer (B), and rectal carcinoma (C) using the GEO database. Representative immunohistochemistry staining of Reelin in OSCC, according to TCs, FLCs and TILs (D). The IHC score of ReelinTC and ReelinFLC in OSCC (E). **** denoted that differences were considered statistically significant with p < 0.0001.
After confirming the contrary expression pattern of ReelinTC and ReelinFLC in OSCC, we further evaluated the correlation between Reelin and various clinicopathological variables according to different cell types in Table 1. The results demonstrated that patients with low-expressed ReelinTC (p = 0.041, Figure 2A) but high-expressed ReelinFLC (p = 0.007, Figure 2B) had a high risk of lymph node metastasis. Also, more ReelinFLC was closely linked to poor WPOI (p = 0.034, Figure 2C). In addition, we continued to study the role of Reelin in primary CAFs from OSCC patients. CAFs were successfully isolated and cultured from fresh OSCC tissues with reliable phenotypic identification using immunofluorescence (Figure 2D). We divided CAFs into WPOI (1–3) and WPOI (4–5) groups. Consistently with the IHC results, high-expressed Reelin in primary CAFs had a poor WPOI using qPCR analysis (p = 0.0016, Figure 2E). However, there were no differences between Reelin and sex, age, smoking habit, T stage, or differentiation (all p>0.05). Ki-67 IHC staining also showed that there was no difference between low Reelin levels and high Reelin levels in TCs (p = 0.1232, Figure 3A), CAFs (p = 0.6257 Figure 3B), and TILs (p = 0.4325, Figure 3C).
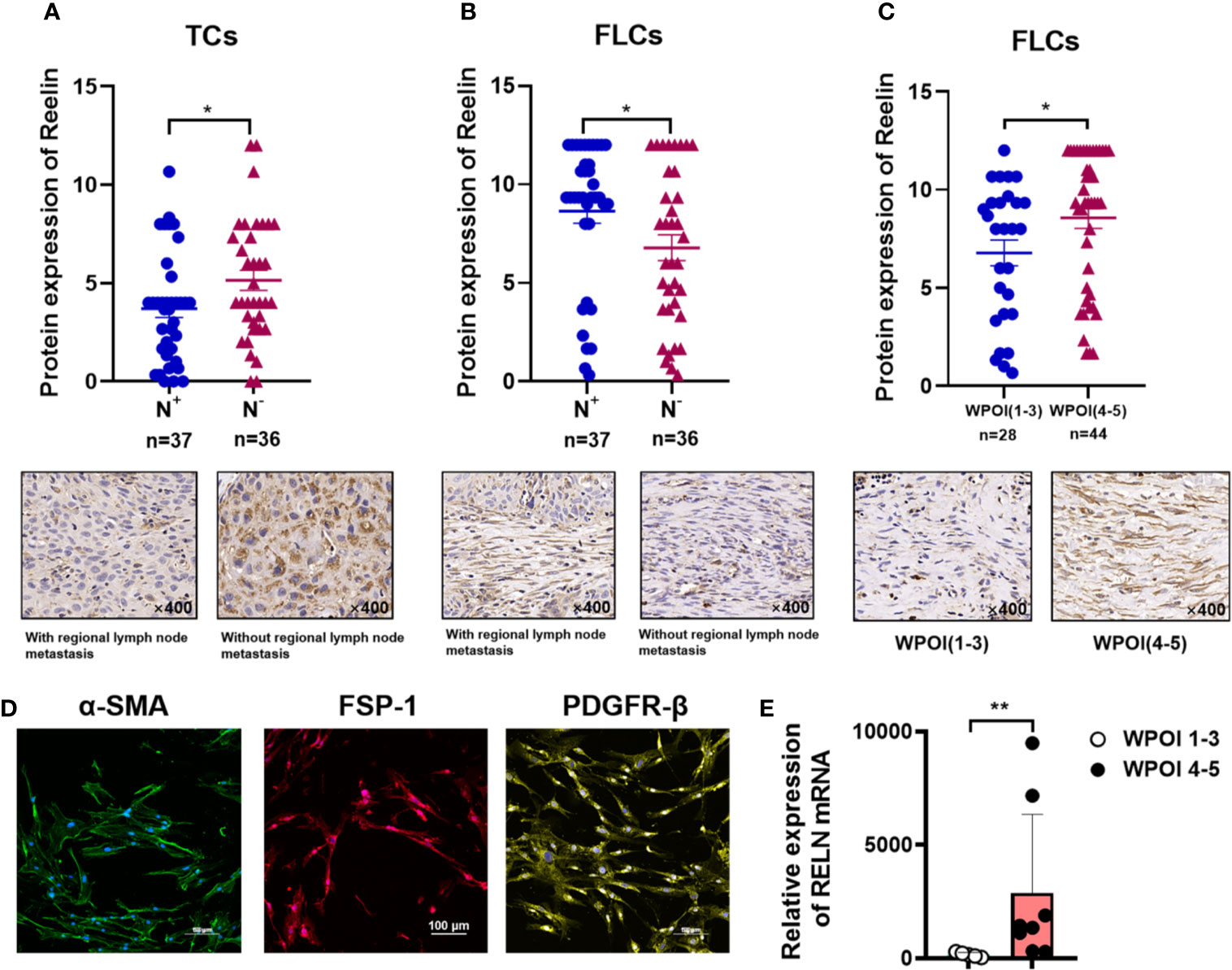
Figure 2 Reelin expression with regional lymph node metastasis in TCs (A), FLCs (B), and WPOI in FLCs (C). Immunofluorescence of isolated primary fibroblasts showed that CAFs express α-SMA, FSP-1, and PDGFR-β (D). Quantitative analysis of RELN mRNA between WPOI (1-3) and WPOI (4-5) revealed significant difference between two groups (E). * and ** denote that differences were considered statistically significant with p < 0.05 and p < 0.01, respectively.
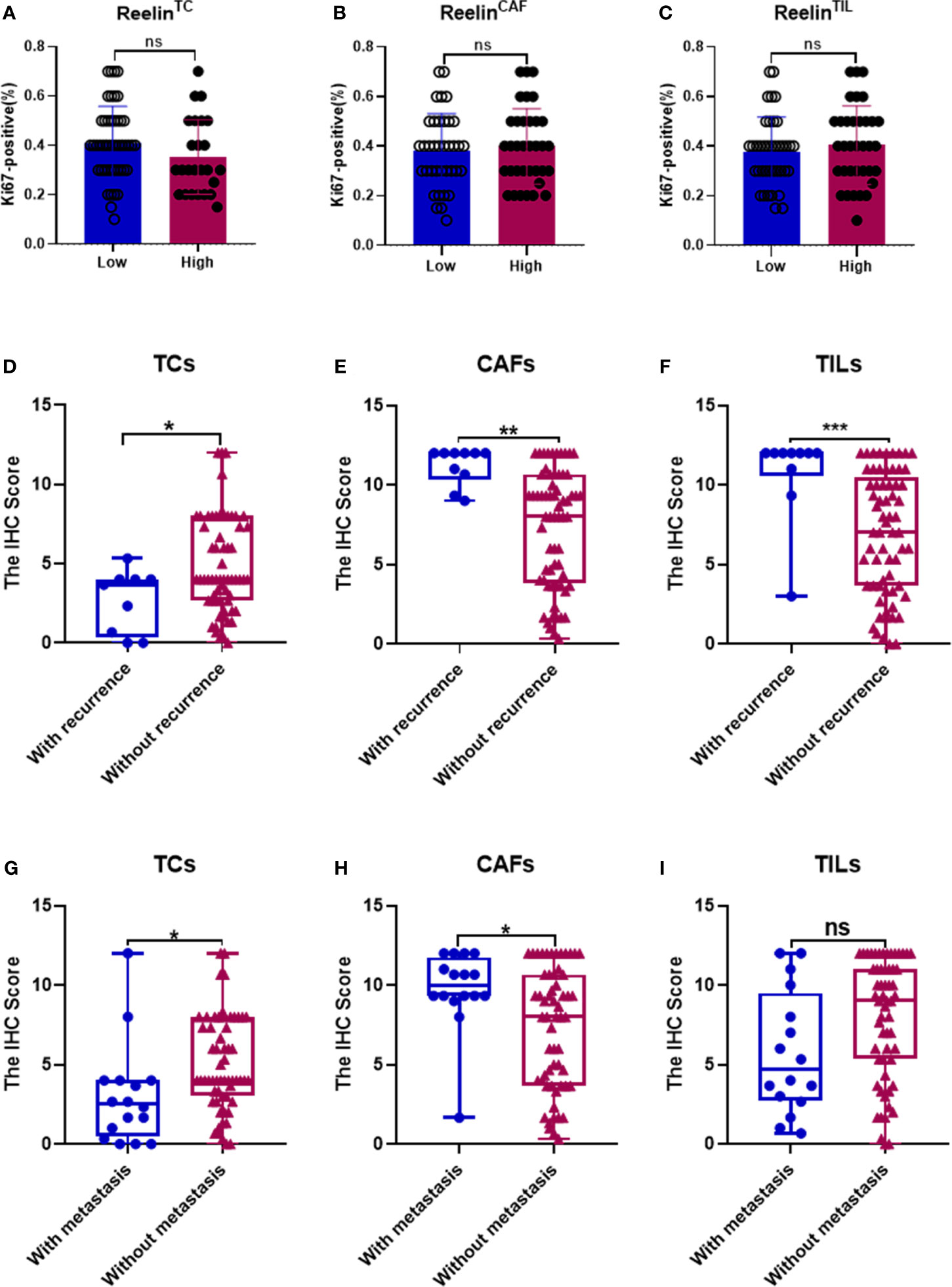
Figure 3 Correlation between Ki-67 in TCs and Reelin in TCs, CAFs, and TILs (A–C). Correlation between Reelin and postoperative recurrence as well as distant metastasis, according to TCs (D, G), CAFs (E, H), and TILs (F, I). *, ** and *** denote that differences were considered statistically significant with p < 0.05, p < 0.01 and p < 0.001 respectively. ns represented no statistical differences.
Considering that decreased ReelinTC, but increased ReelinCAF was correlated with poor clinicopathological characteristics, we further analyzed the association between Reelin and postoperative relapse as well as distant metastasis according to distinct cell types. The results indicated that patients with postoperative relapse had a low expression of ReelinTC (p = 0.0465, Figure 3D), but a high expression of Reelin CAF (p = 0.0015, Figure 3E). Moreover, patients with low-expressed ReelinTC (p = 0.0221, Figure 3G) but high-expressed ReelinCAF (p = 0.0165 Figure 3H) were prone to postoperative metastasis. Strikingly, patients with high ReelinTIL also had a high risk of postoperative relapse (p = 0.0007, Figure 3F). Reelin in TILs didn't have relationship with metastasis (P = 0.0565, Figure 3F). Considering that the role of Reelin in TCs was contrary to that in CAFs in cancer metastasis and relapse, we further evaluated its prognostic value in OS and DSS. Kaplan–Meier curve analysis showed that patients with lower-expressed ReelinTC but higher-expressed ReelinCAF had a shorter OS (p = 0.0391, Figure 4A; p<0.0001, Figure 4B) and DSS (p = 0.0028, Figure 4D; p = 0.0005, Figure 4E). Reelin in TILs wasn’t linked with OS and DSS (P = 0.7794, Figure 4C; P = 0.5798, Figure 4F). Consistent with our results, the TCGA database (TCGA.HNSC.sampleMap/HiSeqV2) also showed that patients with high-expressed ReelinTC had a longer DSS (p = 0.0128, Figure 4G) and PFS (p = 0.0173, Figure 4H).
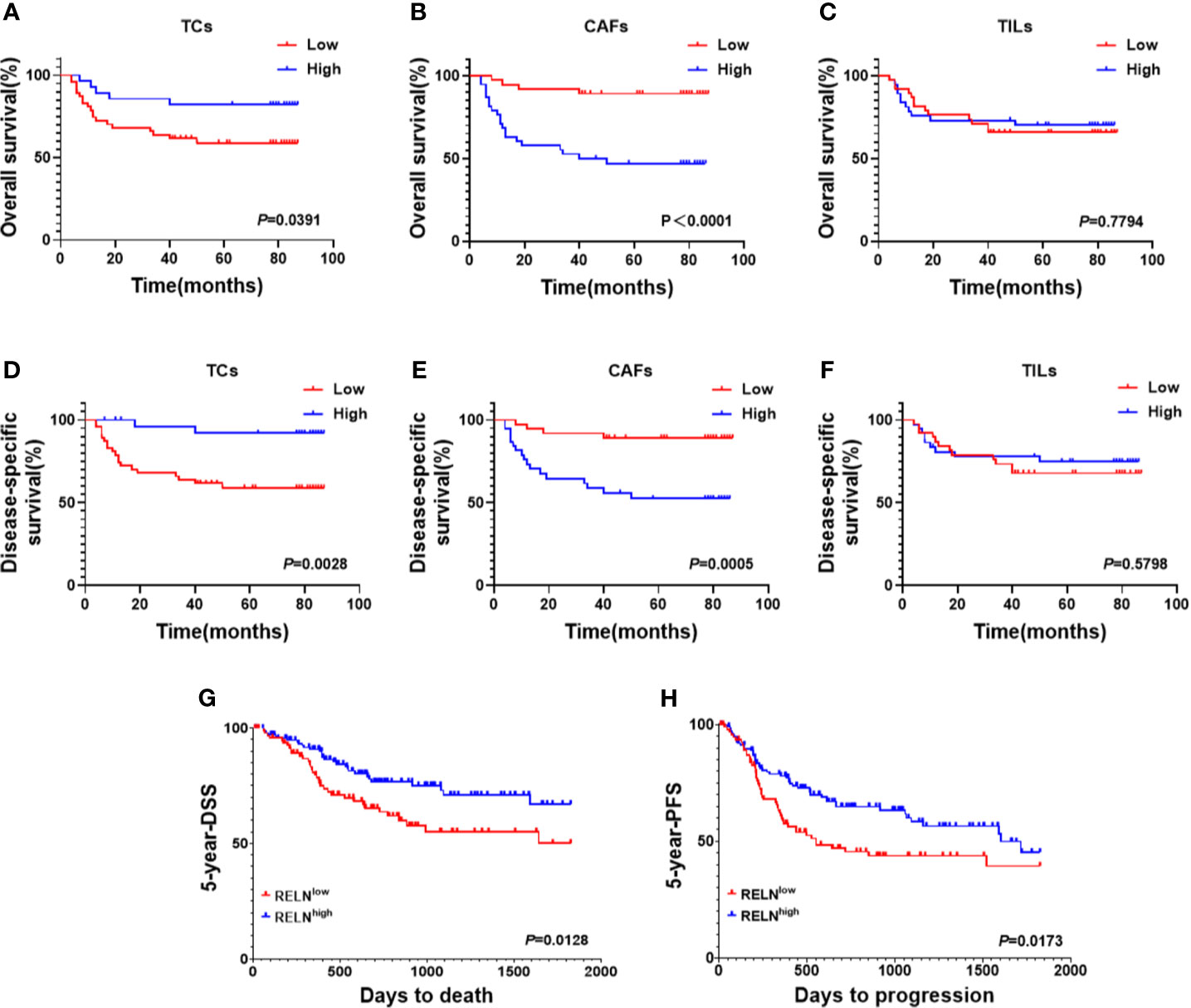
Figure 4 Kaplan–Meier survival curves of OS (A–C) and DSS (D–F) for OSCC patients according to expression level of Reelin in TCs, CAFs, and TILs. Based on the TCGA database (TCGA.HNSC.sampleMap/HiSeqV2), Kaplan–Meier survival curves of DSS (G) and PFS (H) for OSCC patients according to expression level of Reelin.
Univariate and multivariate analyses suggested that ReelinCAF was a valuable prognostic factor among the clinicopathologic variables examined, including gender, age, smoking, T stage, N stage, differentiation, and WPOI. Note that patients with high-expressed ReelinCAF had a worse OS (p = 0.004, Table 2) and DSS (p = 0.009, Table 2). These data clearly showed that ReelinCAF level may further refine the prognostic criteria so as to find OSCC patients who may need extra therapy.
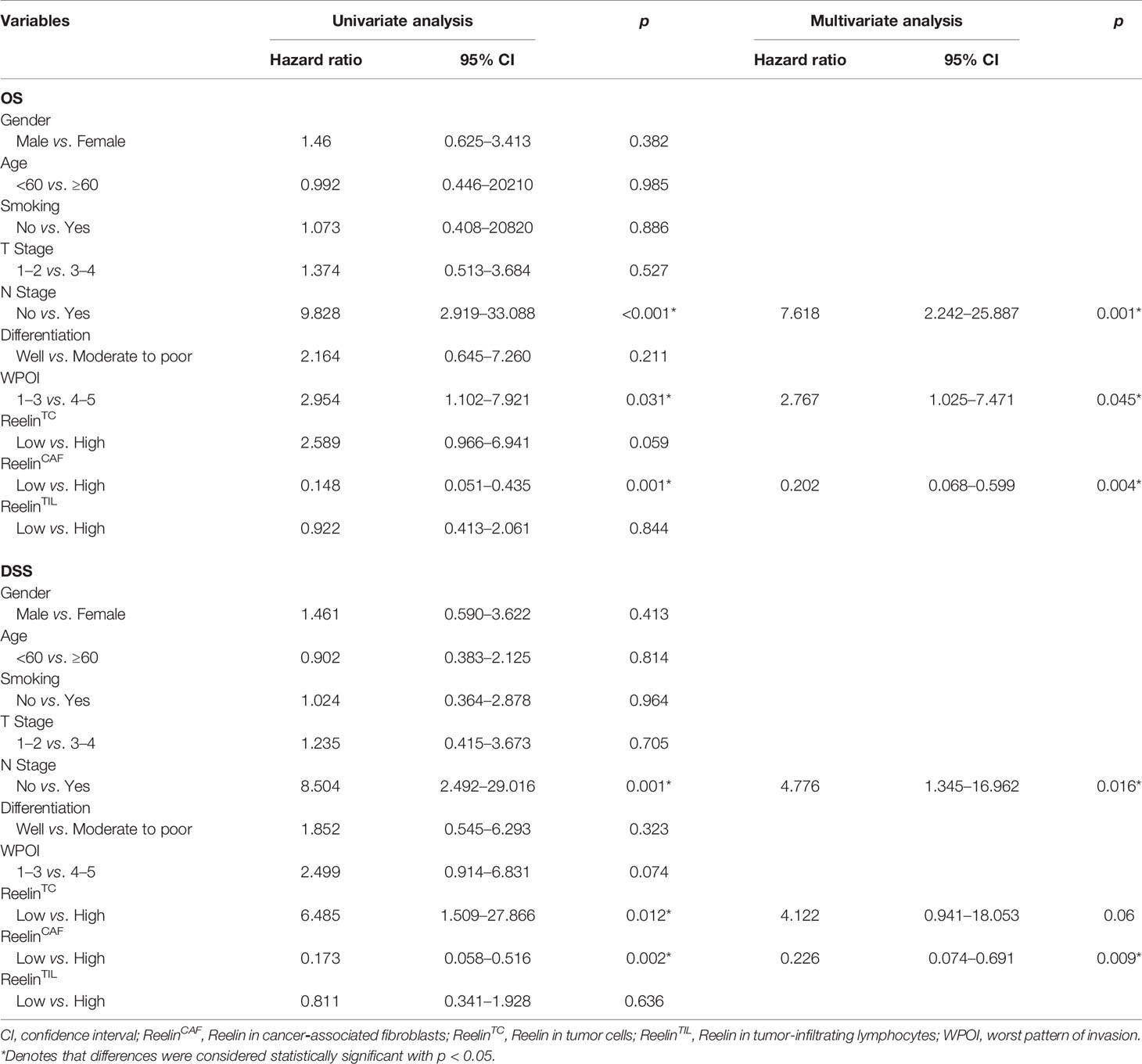
Table 2 Cox-regression analyses of overall survival (OS) and disease-specific survival (DSS) in OSCC patients.
Several previous studies have pointed out that Reelin also played a vital role in immune function. Our data showed that patients with high-expressed ReelinTIL had a high risk of postoperative relapse. We further analyzed the correlation between Reelin and lymphocyte subsets from PBMCs of OSCC using flow cytometry. Lymphocytes were gated as shown in Figure 5A. The results demonstrated that patients with high-expressed ReelinTIL, not ReelinTC or ReelinCAF, had a low ratio of CD3+CD8+ T cells (p = 0.0085, Figure 5B) and a high ratio of CD3+CD4+ T cells (p = 0.0118, Figure 5B). A study reported that elevated CD4/CD8 ratio was related to poor survival in head and neck squamous cancer (32). Therefore, we evaluated the CD4/CD8 ratio between low and high expression of ReelinTIL. The result showed that more ReelinTIL had a higher ratio of CD4/CD8 (p = 0.0018, Figure 5C). Besides, our data showed no relationship between Reelin and B cells as well as NK cells from peripheral blood.
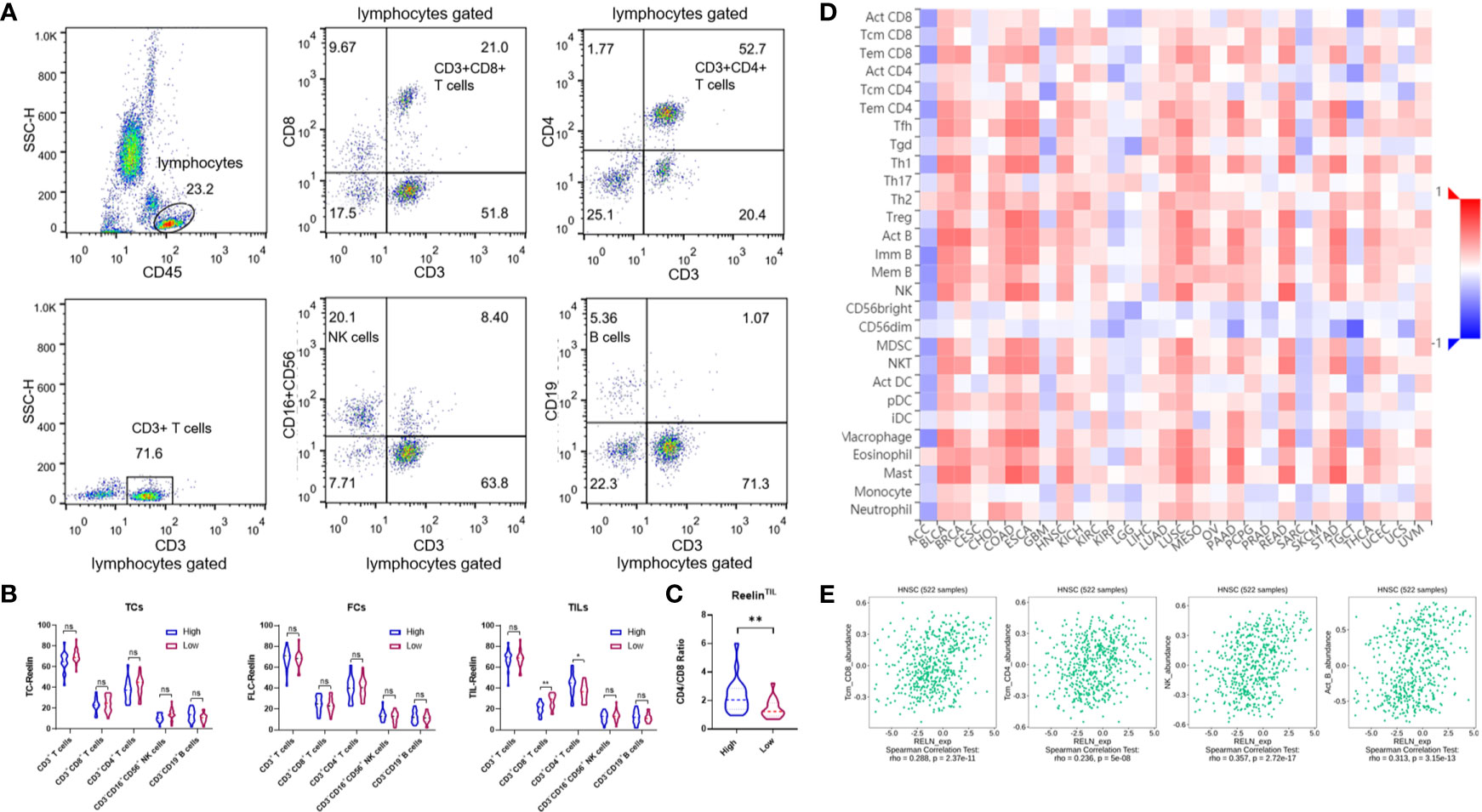
Figure 5 Flow cytometry analysis of lymphocytes, gated based on characteristic light-scatter properties, and single lymphocytes were gated based on forward scatter height vs. forward scatter area (FSC-A). The numbers in the quadrants or over line indicate the percentage of cells (A). Correlation of Reelin expression with immune infiltration level in OSCC before surgery, according to TCs, CAFs, and TILs (B). Correlation of CD4/CD8 ratio with Reelin in TILs (C). Relations between expression of RELN and 28 types of TILs across human heterogeneous cancers (D). RELN significantly correlated with CD8+ T cells, CD4+ T cells, NK cells, and B cells in HNSCC (E). * and ** denote that differences were considered statistically significant with p < 0.05 and p < 0.01, respectively. ns represented no statistical differences.
We furthermore evaluated the relationship between RELN and tumor immune microenvironment. We found correlations of RELN with 28 types of TILs across human heterogeneous cancers according to the TISIDB database (Figure 5D). In HNSCC, RELN positively correlated with abundance of central memory CD8 T cells (Tcm_CD8 T cells; rho = 0.288, p < 0.001, Figure 5E), central memory CD4 T cells (Tcm_CD4 T cells; rho = 0.236, p < 0.001, Figure 5E), natural killer cells (NK cells; rho = 0.357, p < 0.001, Figure 5E), and activated B cells (B cells; rho = 0.313, p < 0.001, Figure 5E). Meanwhile, the Spearman’s correlation test was further used to examine the correlation among RELN and immune cell infiltration of HNSCC according to the TIMER database (Table 3). Elevated RELN was significantly associated with T-cell, B-cell, monocyte, macrophage, and dendritic cell infiltration (all p < 0.05), leading to a multitype immune cell infiltration in the tissue microenvironment.
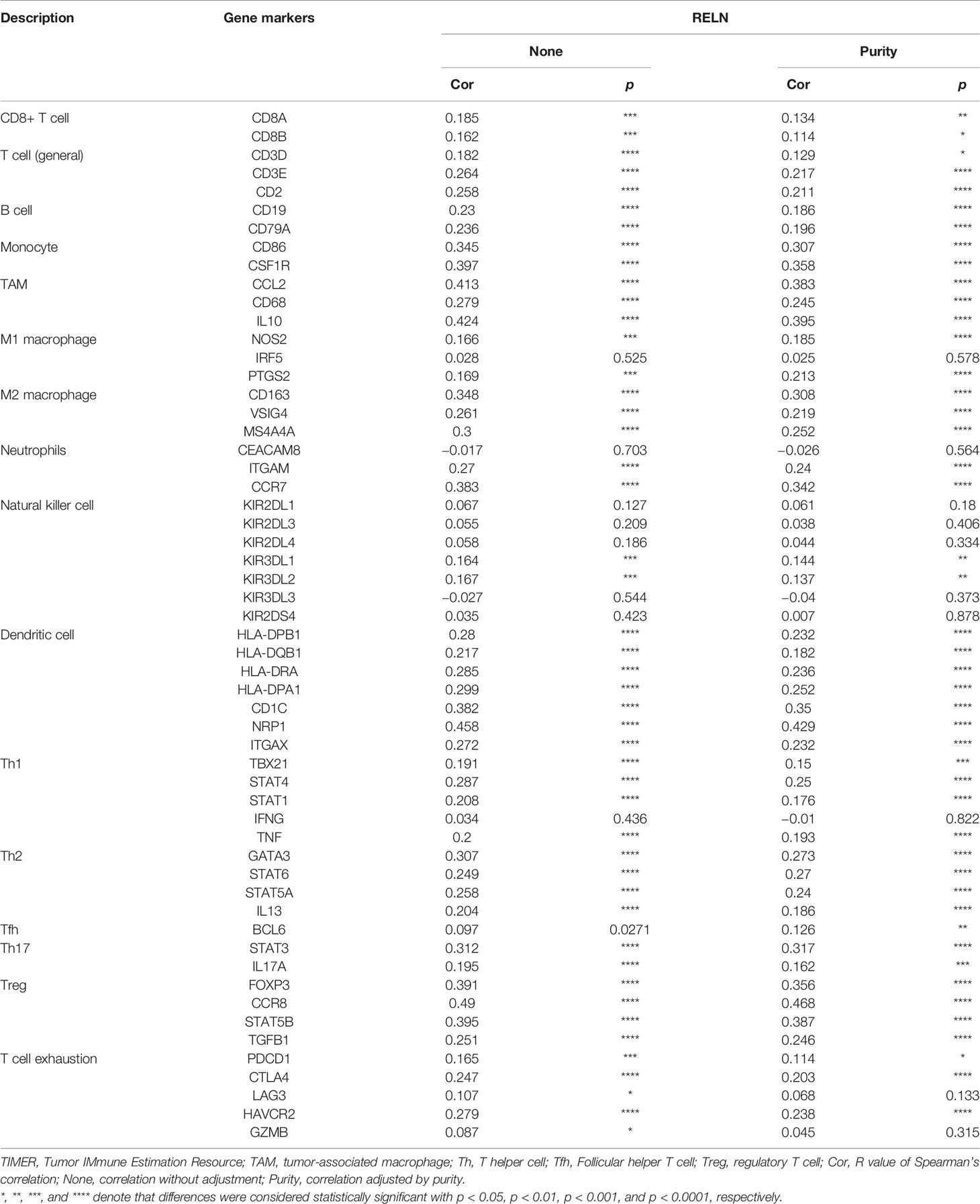
Table 3 Correlation analysis between RELN and immune cell infiltrations in HNSCC samples using TIMER.
Reelin has a great impact on carcinogenesis and tumor progression. However, the expression of Reelin in different cancers was different. For example, Reelin is decreased in hepatocellular carcinoma, but increased in esophageal carcinoma (13, 22). Based on the GEO database, we found that Reelin was significantly upregulated in CAFs compared with TCs in breast cancer, pancreatic cancer, and rectal carcinoma. However, the expression of Reelin in OSCC had not yet been investigated. In this study, we examined the expression pattern of Reelin in OSCC for the first time. The result also demonstrated that Reelin levels were higher in CAFs compared with TCs in OSCC. Besides, TIL-derived Reelin was expressed distinctively according to different OSCC patients. Therefore, we concluded that Reelin might have a versatile function in different cell types during the development of OSCC via governing tumor cell and stroma microenvironment.
According to the significant role of Reelin in tumor progression, some studies showed that upregulated Reelin was found to be correlated with proliferation of cancer cells in multiple myeloma (33). However, stimulating Reelin signaling showed that the proliferation of tumor cells in glioblastoma decreased significantly (15). Moreover, in breast cancer, a previous study showed that enhanced Reelin in MDA-MB231 cells suppressed invasiveness of cancer cells in vitro (17). In OSCC, to investigate the association between Reelin and tumor proliferation, we analyzed Ki-67 among high- and low-expressed groups of Reelin. The result showed that there was no statistical relationship between low and high Reelin levels. However, patients with high-expressed ReelinCAF were predicted to have a poor WPOI. Notably, low-expressed ReelinTC, but high-expressed ReelinCAF was related to the lymph node metastasis, which indicated that Reelin has an opposite function in CAFs and TCs during the development of OSCC.
Besides being related to clinicopathological parameters, some previous studies also determined that Reelin is also related to the prognosis of patients. For example, in hepatocellular carcinoma (HCC), relapse was more likely to happen when the expression of Reelin in tumor cells was high (13). In addition, comparing metastatic colorectal diseases with primary tumors, the expression of Reelin increased significantly in metastatic tumors (23). These data indicated that Reelin might play a vital role in local relapse and distant metastasis. In our survival analysis, the result showed that patients with higher-expressed ReelinCAF had a greater risk of relapse and distant metastasis. Instead, patients with higher-expressed ReelinTC had a lower risk of relapse and distant metastasis. Strikingly, patients with high-expressed ReelinTIL also had a high risk of relapse. This finding further suggested that ReelinCAF and ReelinTC play a diverse role in the prognosis of OSCC patients. Also, ReelinTIL might be a potential indicator with an increased risk of OSCC relapse.
Moreover, as a previous study showed, high-expressed Reelin was negatively associated with PFS and OS in multiple myeloma (34). However, low-expressed Reelin significantly correlated with poor clinical outcome in breast cancer (17). Also, in glioblastoma, the expression of Reelin was positively related to survival (15). Just as we predicted, the result showed that higher-expressed ReelinCAF, but lower-expressed ReelinTC is correlated with shorter OS and DSS in this study. Meanwhile, ReelinCAF was an independent prognostic factor of OS and DSS for OSCC patients.
Furthermore, Reelin-related studies had focused on not only the tumor progression but also the immune function. For example, alterations in serotonin transporter (SERT) clustering in blood lymphocytes associated with a decrease in Reelin expression may be operative in some cardiovascular or immune system alterations (28). Our data showed that patients with high-expressed ReelinTIL had a high risk of postoperative relapse. We identified the relationship between the expression of Reelin and lymphocyte subsets from PBMCs of OSCC. The result showed that patients with high ReelinTIL, but not ReelinTC and ReelinCAF had poor cytotoxicity of CD8+ T cells and higher ratio of CD4/CD8 in peripheral blood, which might result in relapse of OSCC patients. However, the database showed that Reelin was positively associated with tissue-resident B cells and NK cells in the tumor microenvironment, suggesting that Reelin regulates immune response depending on the tissue or blood microenvironment.
In conclusion, we identified that increased ReelinCAF had a higher risk for metastasis and relapse. Therefore, ReelinCAF can be used as a potential biomarker in diagnosis and treatment of OSCC patients. Moreover, Reelin could regulate the balance of CD4+/CD8+ T cells in peripheral blood, which indicated that Reelin might affect the prognosis of OSCC patients. However, it is positively correlated with tissue-resident B cells and NK cells, suggesting that Reelin regulates immune response depending on the tissue or blood microenvironment. Altogether, the study mainly explored the prognostic value of Reelin and the role in immune imbalance in OSCC. However, the molecular function and regulation pathway of Reelin in tumorigenesis of OSCC remained unexplored. In addition, the molecular mechanism of Reelin on immunomodulatory effects was still quite unclear. Future research is needed to unravel the molecular function and regulation pathway of Reelin in tumorigenesis of OSCC. Moreover, the molecular mechanism of Reelin on immunomodulatory effects need to be further explored.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
All the patients have signed the written informed consent before the study. And the present study was approved by the Research Ethics Committee of Nanjing Stomatological Hospital.
All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81902754, 81772880, 82002865, and 81702680), the Natural Science Foundation of Jiangsu Province (No. BK20190304), the Fundamental Research Funds for the Central Universities (No. 021014380161), the China Postdoctoral Science Foundation (No. 2019M651789), and the Nanjing Medical Science and Technology Development Foundation, Nanjing Department of Health (Nos. YKK18123 and YKK19091).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Li X, Bu W, Meng L, Liu X, Wang S, Jiang L, et al. CXCL12/CXCR4 Pathway Orchestrates CSC-Like Properties by CAF Recruited Tumor Associated Macrophage in OSCC. Exp Cell Res (2019) 378(2):131–8. doi: 10.1016/j.yexcr.2019.03.013
3. Quan J, Johnson NW, Zhou G, Parsons PG, Boyle GM, Gao J. Potential Molecular Targets for Inhibiting Bone Invasion by Oral Squamous Cell Carcinoma: A Review of Mechanisms. Cancer Metastasis Rev (2012) 31(1-2):209–19. doi: 10.1007/s10555-011-9335-7
4. Bock HH, May P. Canonical and Non-Canonical Reelin Signaling. Front Cell Neurosci (2016) 10:166. doi: 10.3389/fncel.2016.00166
5. D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A Protein Related to Extracellular Matrix Proteins Deleted in the Mouse Mutant Reeler. Nature (1995) 374(6524):719–23. doi: 10.1038/374719a0
6. DeSilva U, D'Arcangelo G, Braden VV, Chen J, Miao GG, Curran T, et al. The Human Reelin Gene: Isolation, Sequencing, and Mapping on Chromosome 7. Genome Res (1997) 7(2):157–64. doi: 10.1101/gr.7.2.157
7. Dulabon L, Olson EC, Taglienti MG, Eisenhuth S, McGrath B, Walsh CA, et al. Reelin Binds Alpha3beta1 Integrin and Inhibits Neuronal Migration. Neuron (2000) 27(1):33–44. doi: 10.1016/s0896-6273(00)00007-6
8. Herz J, Chen Y. Reelin, Lipoprotein Receptors and Synaptic Plasticity. Nat Rev Neurosci (2006) 7(11):850–9. doi: 10.1038/nrn2009
9. Howell BW, Lanier LM, Frank R, Gertler FB, Cooper JA. The Disabled 1 Phosphotyrosine-Binding Domain Binds to the Internalization Signals of Transmembrane Glycoproteins and to Phospholipids. Mol Cell Biol (1999) 19(7):5179–88. doi: 10.1128/mcb.19.7.5179
10. Armstrong NC, Anderson RC, McDermott KW. Reelin: Diverse Roles in Central Nervous System Development, Health and Disease. Int J Biochem Cell Biol (2019) 112:72–5. doi: 10.1016/j.biocel.2019.04.009
11. Khialeeva E, Carpenter EM. Nonneuronal Roles for the Reelin Signaling Pathway. Dev Dyn (2017) 246(4):217–26. doi: 10.1002/dvdy.24462
12. Dohi O, Takada H, Wakabayashi N, Yasui K, Sakakura C, Mitsufuji S, et al. Epigenetic Silencing of RELN in Gastric Cancer. Int J Oncol (2010) 36(1):85–92. doi: 10.3892/ijo_00000478
13. Okamura Y, Nomoto S, Kanda M, Hayashi M, Nishikawa Y, Fujii T, et al. Reduced Expression of Reelin (RELN) Gene Is Associated With High Recurrence Rate of Hepatocellular Carcinoma. Ann Surg Oncol (2011) 18(2):572–9. doi: 10.1245/s10434-010-1273-z
14. Sato N, Fukushima N, Chang R, Matsubayashi H, Goggins M. Differential and Epigenetic Gene Expression Profiling Identifies Frequent Disruption of the RELN Pathway in Pancreatic Cancers. Gastroenterology (2006) 130(2):548–65. doi: 10.1053/j.gastro.2005.11.008
15. Schulze M, Violonchi C, Swoboda S, Welz T, Kerkhoff E, Hoja S, et al. RELN Signaling Modulates Glioblastoma Growth and Substrate-Dependent Migration. Brain Pathol (2018) 28(5):695–709. doi: 10.1111/bpa.12584
16. Serrano-Morales JM, Vázquez-Carretero MD, Peral MJ, Ilundáin AA, García-Miranda P. Reelin-Dab1 Signaling System in Human Colorectal Cancer. Mol Carcinog (2017) 56(2):712–21. doi: 10.1002/mc.22527
17. Stein T, Cosimo E, Yu X, Smith PR, Simon R, Cottrell L, et al. Loss of Reelin Expression in Breast Cancer Is Epigenetically Controlled and Associated With Poor Prognosis. Am J Pathol (2010) 177(5):2323–33. doi: 10.2353/ajpath.2010.100209
18. Dou A, Wang Z, Zhang N, Liu J. Loss of Reelin Suppresses Cell Survival and Mobility in Non-Hodgkin Lymphoma. Oncol Rep (2017) 37(6):3572–80. doi: 10.3892/or.2017.5626
19. Lin L, Wang P, Liu X, Zhao D, Zhang Y, Hao J, et al. Epigenetic Regulation of Reelin Expression in Multiple Myeloma. Hematol Oncol (2017) 35(4):685–92. doi: 10.1002/hon.2311
20. Perrone G, Vincenzi B, Zagami M, Santini D, Panteri R, Flammia G, et al. Reelin Expression in Human Prostate Cancer: A Marker of Tumor Aggressiveness Based on Correlation With Grade. Mod Pathol (2007) 20(3):344–51. doi: 10.1038/modpathol.3800743
21. Seigel GM, Hackam AS, Ganguly A, Mandell LM, Gonzalez-Fernandez F. Human Embryonic and Neuronal Stem Cell Markers in Retinoblastoma. Mol Vis (2007) 13:823–32.
22. Wang Q, Lu J, Yang C, Wang X, Cheng L, Hu G, et al. CASK and Its Target Gene Reelin Were Co-Upregulated in Human Esophageal Carcinoma. Cancer Lett (2002) 179(1):71–7. doi: 10.1016/s0304-3835(01)00846-1
23. Berthier-Vergnes O, El Kharbili M, de la Fouchardière A, Pointecouteau T, Verrando P, Wierinckx A, et al. Gene Expression Profiles of Human Melanoma Cells With Different Invasive Potential Reveal TSPAN8 as a Novel Mediator of Invasion. Br J Cancer (2011) 104(1):155–65. doi: 10.1038/sj.bjc.6605994
24. Vignot S, Lefebvre C, Frampton GM, Meurice G, Yelensky R, Palmer G, et al. Comparative Analysis of Primary Tumour and Matched Metastases in Colorectal Cancer Patients: Evaluation of Concordance Between Genomic and Transcriptional Profiles. Eur J Cancer (2015) 51(7):791–9. doi: 10.1016/j.ejca.2015.02.012
25. Yuan Y, Chen H, Ma G, Cao X, Liu Z. Reelin Is Involved in Transforming Growth Factor-β1-Induced Cell Migration in Esophageal Carcinoma Cells. PloS One (2012) 7(2):e31802. doi: 10.1371/journal.pone.0031802
26. Bakalian A, Kopmels B, Messer A, Fradelizi D, Delhaye-Bouchaud N, Wollman E, et al. Peripheral Macrophage Abnormalities in Mutant Mice With Spinocerebellar Degeneration. Res Immunol (1992) 143(1):129–39. doi: 10.1016/0923-2494(92)80090-8
27. Kopmels B, Wollman EE, Guastavino JM, Delhaye-Bouchaud N, Fradelizi D, Mariani J. Interleukin-1 Hyperproduction by In Vitro Activated Peripheral Macrophages From Cerebellar Mutant Mice. J Neurochem (1990) 55(6):1980–5. doi: 10.1111/j.1471-4159.1990.tb05785.x
28. Rivera-Baltanas T, Romay-Tallon R, Dopeso-Reyes IG, Caruncho HJ. Serotonin Transporter Clustering in Blood Lymphocytes of Reeler Mice. Cardiovasc Psychiatry Neurol (2010) 2010:396282. doi: 10.1155/2010/396282
29. Zhao X, Ding L, Lu Z, Huang X, Jing Y, Yang Y, et al. Diminished CD68(+) Cancer-Associated Fibroblast Subset Induces Regulatory T-Cell (Treg) Infiltration and Predicts Poor Prognosis of Oral Squamous Cell Carcinoma Patients. Am J Pathol (2020) 190(4):886–99. doi: 10.1016/j.ajpath.2019.12.007
30. Ni YH, Ding L, Zhang DY, Hou YY, Huang X, Hu Q. Distinct Expression Patterns of Toll-Like Receptor 7 in Tumour Cells and Fibroblast-Like Cells in Oral Squamous Cell Carcinoma. Histopathology (2015) 67(5):730–9. doi: 10.1111/his.12703
31. Ding L, Zhao X, Zhu N, Zhao M, Hu Q, Ni Y. The Balance of Serum IL-18/IL-37 Levels Is Disrupted During the Development of Oral Squamous Cell Carcinoma. Surg Oncol (2020) 32:99–107. doi: 10.1016/j.suronc.2019.12.001
32. Millrud CR, Månsson Kvarnhammar A, Uddman R, Björnsson S, Riesbeck K, Cardell LO. The Activation Pattern of Blood Leukocytes in Head and Neck Squamous Cell Carcinoma Is Correlated to Survival. PloS One (2012) 7(12):e51120. doi: 10.1371/journal.pone.0051120
33. Qin X, Lin L, Cao L, Zhang X, Song X, Hao J, et al. Extracellular Matrix Protein Reelin Promotes Myeloma Progression by Facilitating Tumor Cell Proliferation and Glycolysis. Sci Rep (2017) 7:45305. doi: 10.1038/srep45305
Keywords: Reelin, oral squamous cell carcinoma, cancer-associated fibroblasts, prognosis, lymphocyte subsets
Citation: Zhang X, Fu Y, Ding Z, Zhu N, Zhao M, Song Y, Huang X, Chen S, Yang Y, Zhang C, Hu Q, Ni Y and Ding L (2021) Functional Heterogeneity of Reelin in the Oral Squamous Cell Carcinoma Microenvironment. Front. Oncol. 11:692390. doi: 10.3389/fonc.2021.692390
Received: 11 April 2021; Accepted: 26 July 2021;
Published: 17 August 2021.
Edited by:
Shankargouda Patil, Jazan University, Saudi ArabiaReviewed by:
A. Thirumal Raj, Sri Venkateswara Dental College, IndiaCopyright © 2021 Zhang, Fu, Ding, Zhu, Zhao, Song, Huang, Chen, Yang, Zhang, Hu, Ni and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanhong Ni, bml5YW5ob25nMTJAMTYzLmNvbQ==; Liang Ding, ODc5MjY5MzM5QHFxLmNvbQ==; Qingang Hu, cWdodUBuanUuZWR1LmNu
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.