
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 30 August 2021
Sec. Gastrointestinal Cancers
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.691380
 Yuan Ding1,2,3,4,5,6†
Yuan Ding1,2,3,4,5,6† Xin Han1,2,3,4,5,6†
Xin Han1,2,3,4,5,6† Zhongquan Sun1,2,3,4,5,6
Zhongquan Sun1,2,3,4,5,6 Jinlong Tang7
Jinlong Tang7 Yingsheng Wu1,2,3,4,5,6*
Yingsheng Wu1,2,3,4,5,6* Weilin Wang1,2,3,4,5,6*
Weilin Wang1,2,3,4,5,6*Intrahepatic cholangiocarcinoma (CCA), always diagnosed at an advanced stage in recent years, is of high aggression and poor prognosis. There is no standard treatment beyond first-line chemotherapy and no molecular-targeted agents or immune checkpoint inhibitors approved for advanced intrahepatic CCA. Hence, we firstly report an original therapeutic strategy for a 60-year-old patient diagnosed with intrahepatic CCA categorized as Stage IIIB (T3N1M0) by the American Joint Committee on Cancer staging system. After histopathological examination and next-generation sequencing, the patient was treated with four courses of novel systemic sequential therapy (intravenous gemcitabine 1,000 mg/m2 and cisplatin 25 mg/m2 on days 1 and 8; oral lenvatinib 8 mg/day from days 1 to 21; intravenous tislelizumab 200 mg on day 15). Then, the patient achieved partial response and was operated on right hemihepatectomy, cholecystectomy, and abdominal lymph node dissection. Without any perioperative complications, the patient was discharged from our hospital in perfect condition. Thereafter, the patient continued to use this new regimen 1 month after surgery for adjuvant therapy and was confirmed without recurrence when we followed up. In a word, we found an effective therapeutic regimen for preoperative advanced intrahepatic CCA conversion therapy, which may become a new approach in cancer treatment in the future.
Cholangiocarcinoma (CCA) is a heterogeneous group of cancers arising from the epithelial cells of intrahepatic and extrahepatic bile ducts (1). According to anatomic locations, intrahepatic cholangiocarcinoma (ICC) is one of the three CCAs, and the incidence of ICC has steadily risen in recent decades (2, 3). Radical resection (R0) that involves formal hepatectomy and portal lymphadenectomy is the best method among ICC patients for long-term survival (4). Unfortunately, because of highly aggressive malignancy, most of the patients are diagnosed at an advanced stage and even lose the chance to undergo surgery (2, 3, 5).
As more effective and novel chemotherapy, targeted therapies, and immunotherapy become available, multiple treatments can be chosen for the patients with advanced ICC (6). For instance, a double chemotherapy regimen using gemcitabine and cisplatin (CisGem) is supported by several recommendations (7, 8). Targeted agents, such as pemigatinib, show great efficacy in ICC therapy (9). In addition, on account of high genetic aberrations, most patients are sensitive to immunotherapy, taking pembrolizumab, for example (10). However, how to choose the most suitable therapy regimen is difficult, and most of them have not been approved at present.
Recently, whole-genome and transcriptome sequencing revealed the diversity of CCAs, offering a clearer understanding of carcinogenesis, classification, and treatment strategy (11). With the requirement of personalized therapies, multidrug combinations may also be the trend of novel treatments. Therefore, we describe a case that CisGem, lenvatinib, and tislelizumab were used to treat a patient for preoperative conversion therapy after genomic profiling in our hospital.
A 60-year-old male who was diagnosed with liver tumor by upper abdominal contrast-enhanced computed tomography (CT) in a local hospital presented to our department for further diagnosis and treatment on May 21, 2020, complaining of dull pain in the upper right abdomen without any symptoms of diarrhea, hematochezia, nausea, or vomiting for some weeks. Further inquiry revealed a history of hypertension, coronary heart disease, coronary stenting, and smoking, without any other history related to ICC such as primary sclerosing cholangitis and schistosomiasis. No other aberrations were noted, and physical examination was normal. Routine blood counts, coagulation function, and liver and renal function demonstrated normal levels except for alanine aminotransferase (ALT) (52 U/L) and aspartate aminotransferase (AST) (43 U/L). No clear elevation of tumor biomarkers including carcinoembryonic antigen (CEA), alpha fetoprotein (AFP), and carbohydrate antigen 125 (CA 125) was observed, while carbohydrate antigen 19-9 (CA 19-9) was a little higher (42.1 ng/ml). Hepatitis virus markers were all negative. Further examinations were performed after admission of the patient to our department. Acoustic contrast of the viscera showed parenchymal hypoechoic masses at liver segments IV and V, with blood flow signal by color Doppler flow imaging (CDFI) (Figure 1). Then, contrast-enhanced CT and magnetic resonance imaging (MRI) revealed an irregular-morphology mass (49 mm * 39.6 mm) in segments V–VIII of the liver; the hepatic portal and retroperitoneal lymph nodes were enlarged—the larger one (30.4 mm * 23.1 mm) lying between the inferior vena cava and the abdominal aorta (Figures 2, 3). Based on the above information, the patient was diagnosed as having ICC clearly, which was classified as stage IIIB (T3N1M0) according to the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging system.
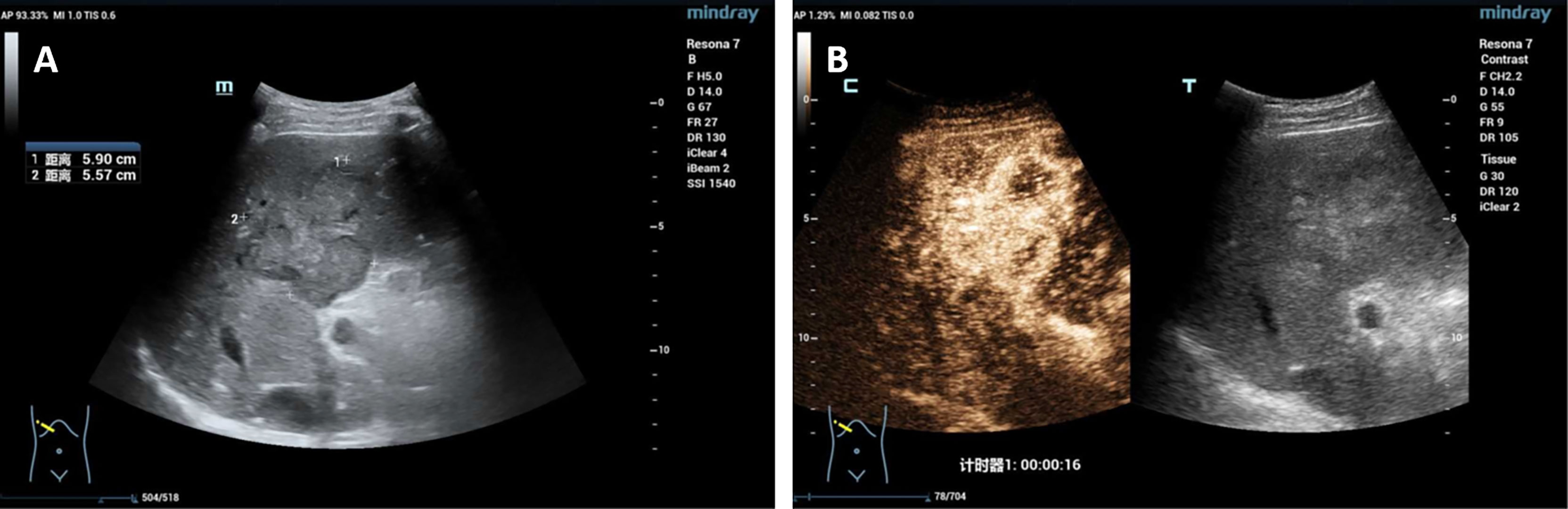
Figure 1 (A) Acoustic contrast of the viscera showed parenchymal hypoechoic masses; (B) the arterial phase showed uneven and high enhancement.
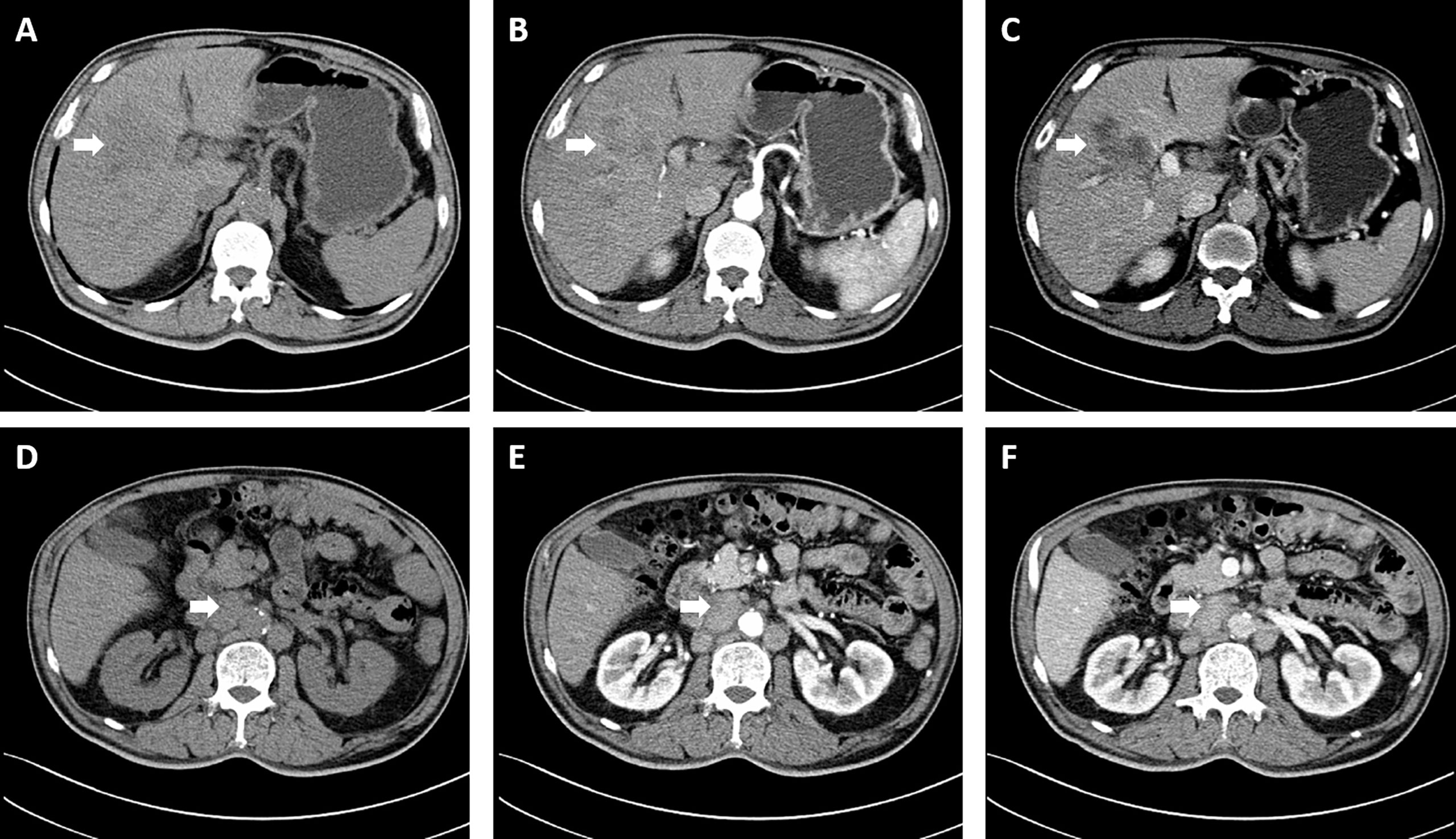
Figure 2 Computed tomography revealed an irregular morphology mass (49 mm * 39.6 mm) in the liver; the hepatic portal and retroperitoneal lymph nodes were enlarged. (A, D) Plain scan; (B, E) Arterial phase; (C, F) Portal venous phase. The white arrowheads direct the lesion or enlarged lymph nodes.
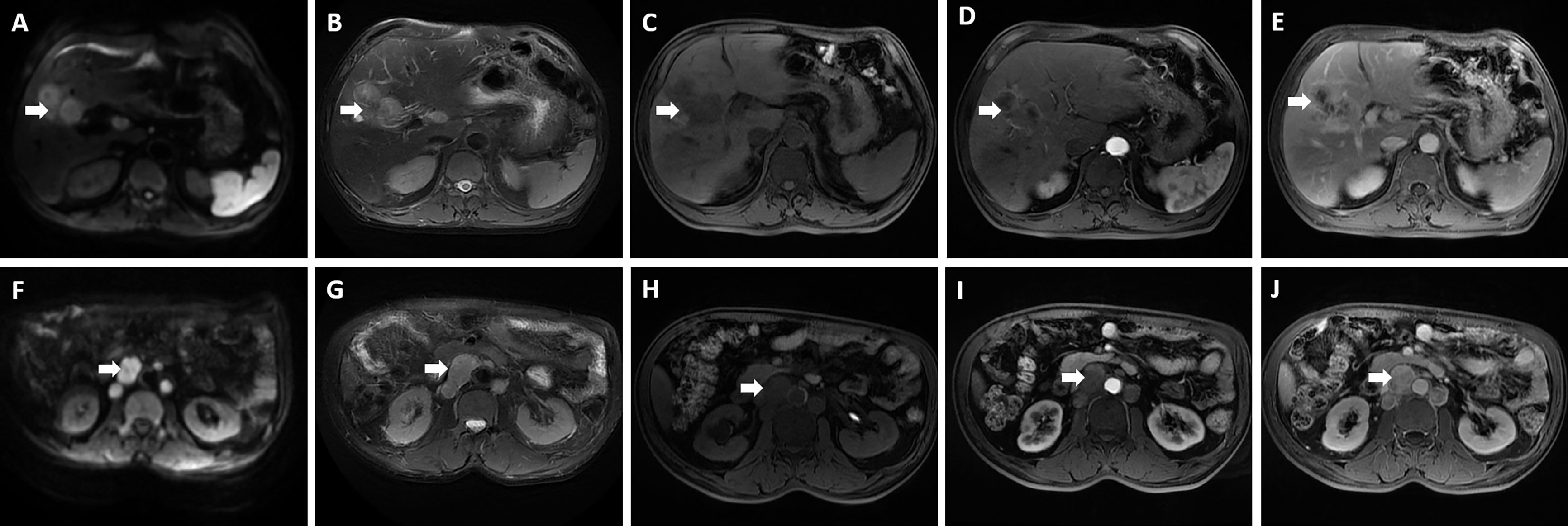
Figure 3 Magnetic resonance imaging showed an irregular morphology mass, with low signal in T1-weighted imaging, high signal in T2-weighted imaging, and limited diffusion in diffusion-weighted imaging (DWI); enlarged hepatic portal and retroperitoneal lymph nodes were also revealed—the larger one was 30.4 mm * 23.1 mm in size. (A, F) DWI; (B, G) T2-weighted imaging; (C, H) T1-weighted imaging; (D, I) The arterial phase; (E, J) The delayed phase. The white arrowheads direct the lesion or enlarged lymph nodes.
To further evaluate, we did liver biopsy and pathological examination. The results revealed a poorly differentiated adenocarcinoma (Figure 4). Immunohistochemical staining results were as follows: arginase-1 (-), Muc-1 (+), CK7 (+), CK20 (+), CDX2 (+), SATB2 (-), P53 (-), Ki-67 (60% +). In addition, next-generation sequencing (NGS) was performed at the same time; it showed the following: somatic mutation of gene: BRCA2 p.G2270, mutation frequency: 35.50%, and TP53 p.V73fs, mutation frequency: 50.00%. FGFR2 gene fusion was detected as well. The tumor mutational burden (TMB) was also determined to be 51.37 mut/Mb. Moreover, 48 unstable loci were revealed, and microsatellite instability (MSI) score was 0.5926. All of the above suggested that immunotherapy and targeted therapy might be effective against the patient’s tumor.
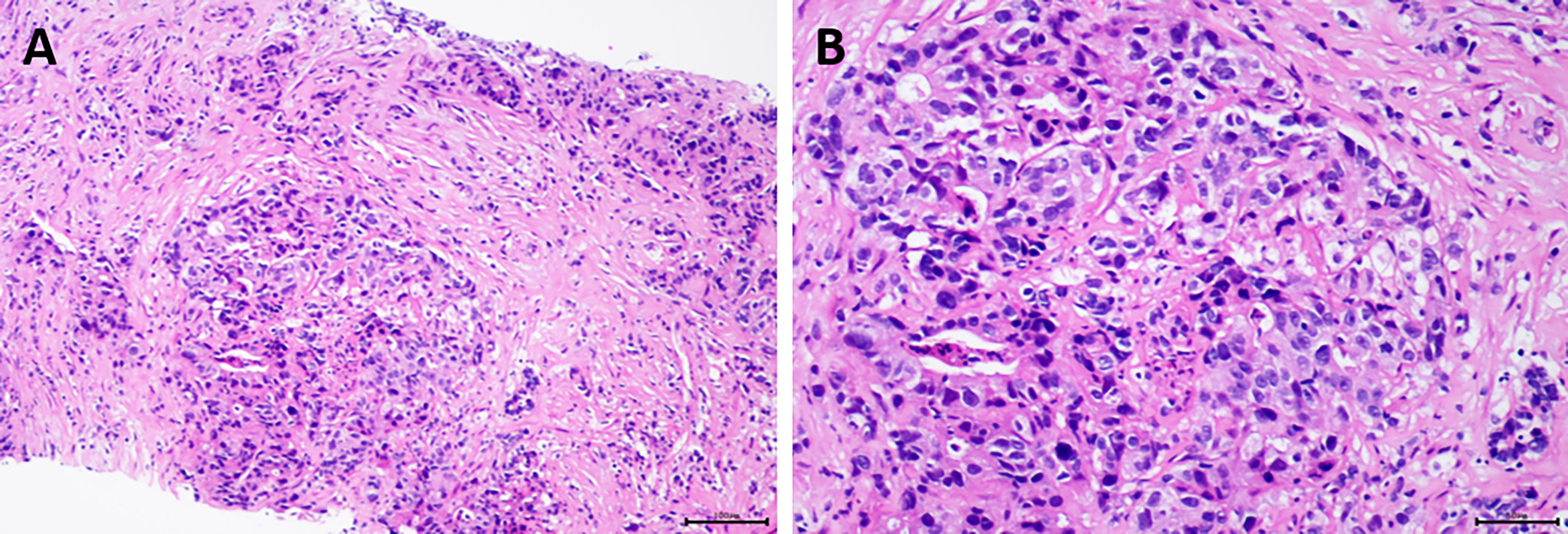
Figure 4 Pathological examination of the patient revealed poorly differentiated adenocarcinoma. (A) Hematoxylin and eosin (H&E), original magnification ×100; (B) H&E, original magnification ×200.
Later, multidisciplinary treatment (MDT) was conducted to discuss the appropriate therapy. After MDT, a new therapeutic scheme, uniting CisGem, lenvatinib, and tislelizumab, was fully concerned. Hence, the patient was treated with four cycles of systemic sequential therapy (Table 1) without any obvious complications. Soon afterward, the measurement of target lesion was detected again by liver MRI on September 3, 2020. The lesion in the liver segments V–VIII was reduced, which was about 15 mm in diameter with clear boundary. Additionally, multiple lymph nodes metastasized in the hepatic portal and retroperitoneum, but they were obviously decreased compared with prior treatment (Figure 5). As expected, the patient achieved partial response (PR) successfully according to the standard RECIST 1.1 criteria. The CA 19-9 levels were reduced to normal range, in company with normal routine blood counts, coagulation function, other tumor markers, and liver and kidney function after one cycle of therapy. All these blood test results remained normal until four cycles of systemic sequential therapy finished. Subsequently, the patient underwent right hemihepatectomy, cholecystectomy, and abdominal lymph node dissection with enhanced recovery after surgery (ERAS) pathway in the Department of Hepatobiliary and Pancreatic Surgery. The postoperative pathological examination showed the tumor bed with necrotic fibrous tissue proliferation, chronic inflammatory cell infiltration, cholesterol crystallization, and hemosiderin deposition; was 4.5 cm * 2.5 cm in size, and low-grade intraepithelial neoplasia was seen in the surrounding bile duct; no clear tumor residue was found, and the Evans grade is IV; and the four hepatic portal lymph nodes dissected were negative, except one of Group 12 (Figure 6). Moreover, the postoperative immunohistochemical staining results suggested the following: CK (AE1/AE3) (+), CK7 (+), CK8 (+), CK18 (+), CK19 (+), MUc-1 (+), KI-67 index of 80%, arginase-1 (-) (Figure 7). Without any complications, the patient was discharged from the hospital in good condition.
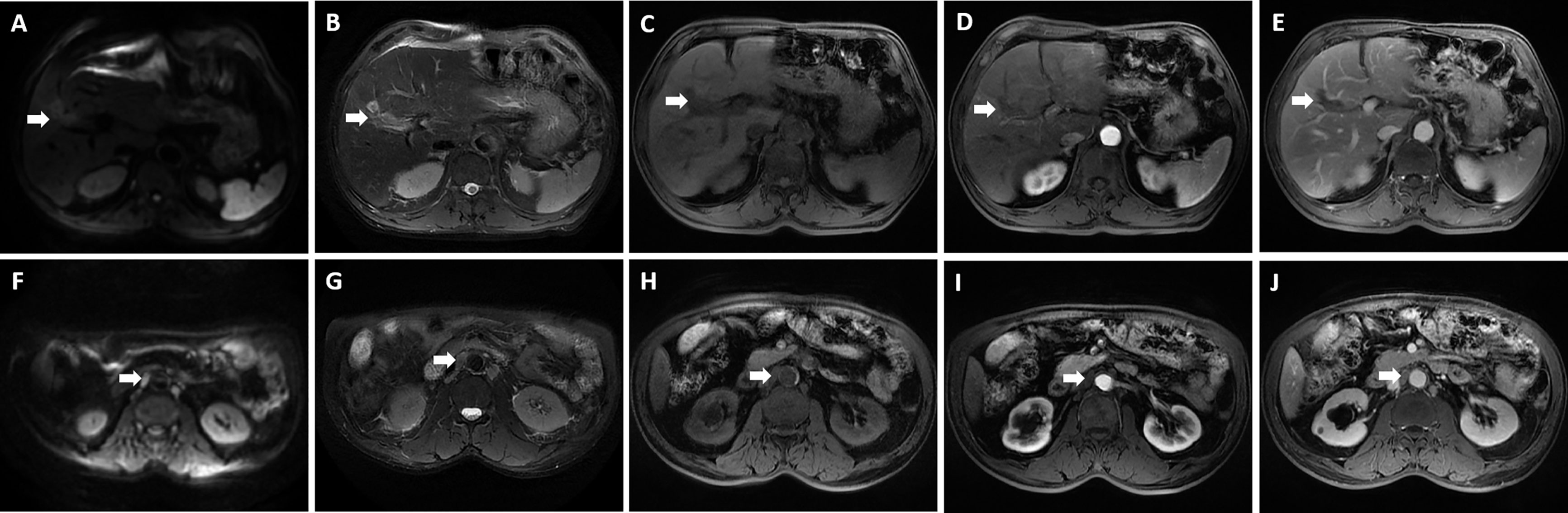
Figure 5 Preoperative magnetic resonance imaging was performed for the patient. The reduced lesion with clear boundary has low signal in T1-weighted imaging, high signal in T2-weighted imaging, and limited diffusion in diffusion-weighted imaging (DWI); enlarged hepatic portal and retroperitoneal lymph nodes were also revealed—the larger one was about 10 mm in diameter. (A, F) DWI; (B, G) T2-weighted imaging; (C, H) T1-weighted imaging; (D, I) The arterial phase; (E, J) The delayed phase. The white arrowheads direct the lesion or enlarged lymph nodes.
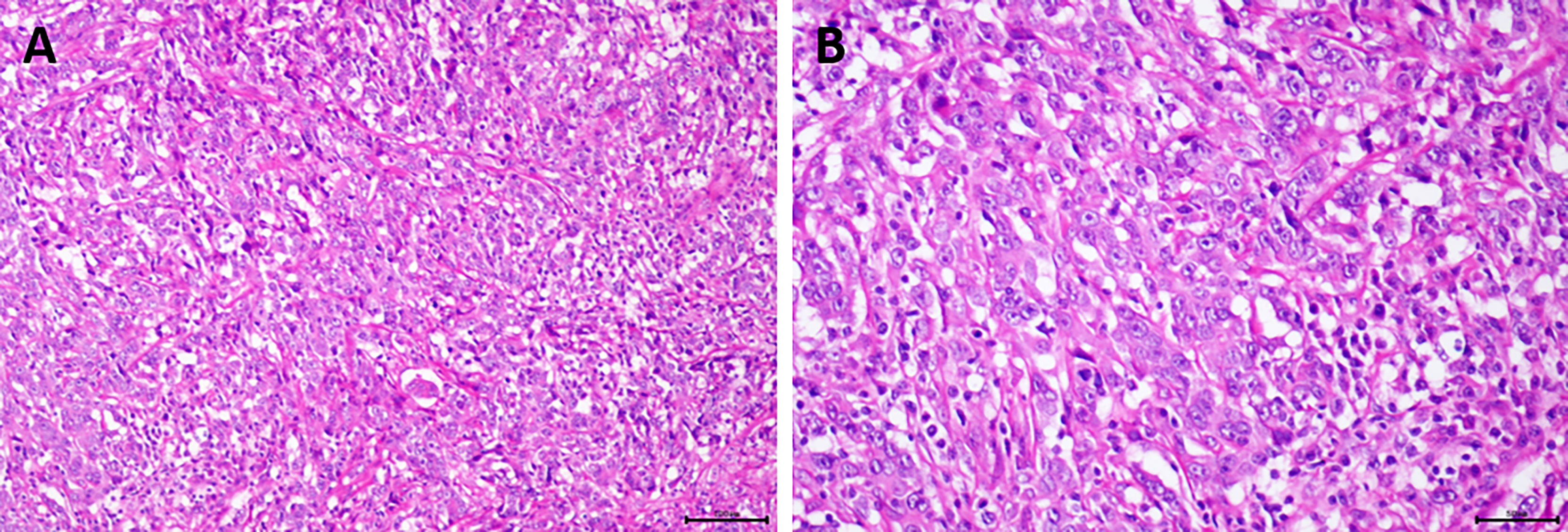
Figure 6 The postoperative pathological examination of the patient showed the tumor bed contained necrotic fibrous tissue proliferation, chronic inflammatory cell infiltration, cholesterol crystallization, and hemosiderin deposition, and low-grade intraepithelial neoplasia was seen in the surrounding bile duct. (A) Hematoxylin and eosin (H&E), original magnification ×100; (B) H&E, original magnification ×200.

Figure 7 The postoperative immunohistochemical staining results, original magnification ×200. (A) CK (AE1/AE3); (B) CK7; (C) CK8; (D) CK18; (E) CK19; (F) MUc-1; (G) KI-67; (H) arginase-1.
One month after surgery, MRI examination was performed again; it exhibited the following: postoperative changes of liver and gallbladder, a little exudation and effusion in the operative area (Figure 8). Furthermore, the patient used the new therapeutic regimen again for adjuvant therapy on October 24, 2020. The tumor has disappeared without recurrence after four cycles of adjuvant therapy, fortunately (Figure 9). Thereafter, the patient kept on using this original therapeutic regimen for further treatment. Up to now, the patient recovers very well without any severe side effects and recurrence during follow-up, which is more than 10 months after the operation.
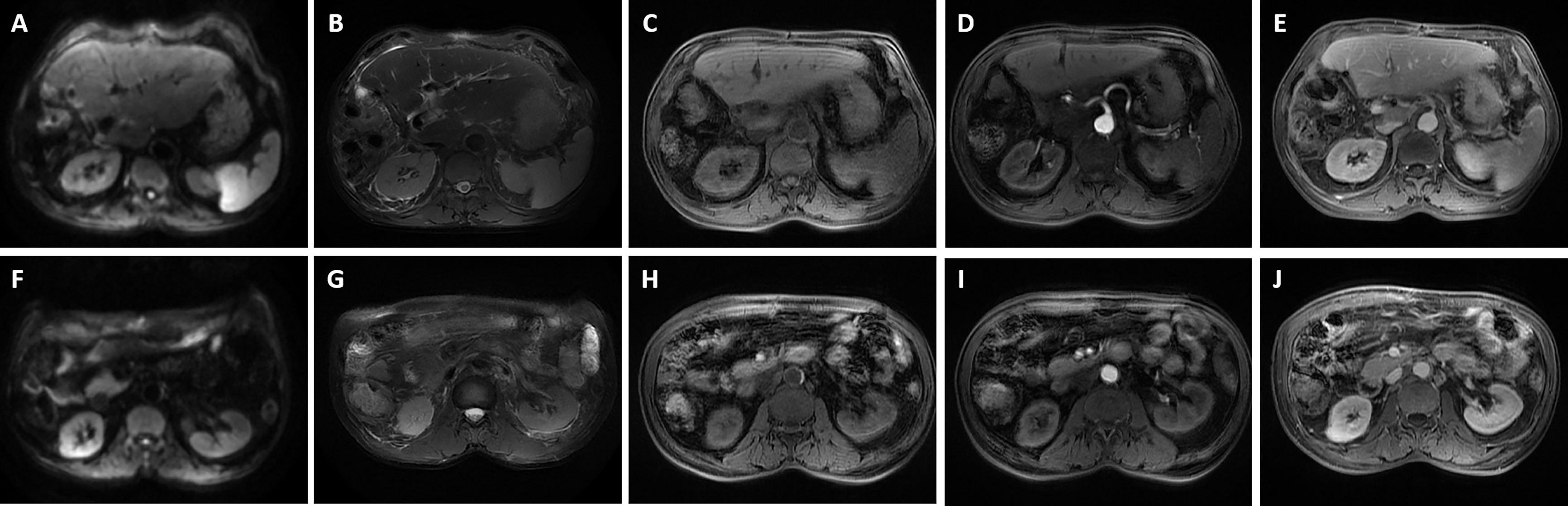
Figure 8 Magnetic resonance imaging was performed for the patient 1 month after surgery. It revealed postoperative changes of liver and gallbladder, a little exudation and effusion in the operative area. (A, F) Diffusion-weighted imaging (DWI); (B, G) T2-weighted imaging; (C, H) T1-weighted imaging; (D, I) The arterial phase; (E, J) The delayed phase.
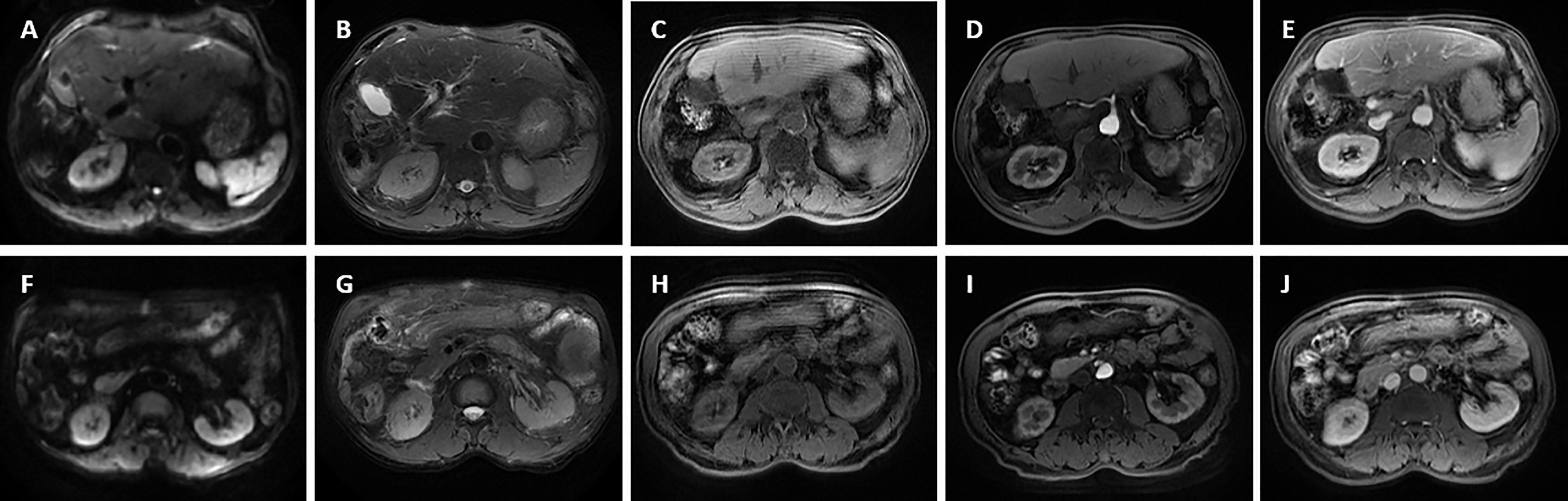
Figure 9 Magnetic resonance imaging was performed for the patient after four cycles of postoperative adjuvant chemotherapy. The tumor has disappeared without recurrence. (A, F) Diffusion-weighted imaging (DWI); (B, G) T2-weighted imaging; (C, H) T1-weighted imaging; (D, I) The arterial phase; (E, J) The delayed phase.
It is the first time that we report a novel therapeutic regimen (uniting CisGem, lenvatinib, and tislelizumab) for preoperative advanced ICC conversion therapy according to pathological examination and NGS. The patient achieved PR after four cycles of treatment. Applying ERAS pathway in perioperation, the patient acquired R0 resection and recovered soon without any severe complications. Then, the patient continued to use the new therapeutic sequential regimen, and the patient recuperated well without recurrence and severe complications at postoperative follow-up.
ICC is relatively common to encounter in clinical practice, accounting for 12% of all primary hepatic carcinomas and approximately 25% of all CCAs (12, 13). Currently, only 30%–40% of patients have an opportunity to undergo surgery because of early metastasis; even after R0 resection, the postoperative recurrence rate in patients is approximately 60%–80% (14, 15). Nevertheless, the CisGem regimen (gemcitabine 1,000 mg/m2 and cisplatin 25 mg/m2 on days 1 and 8, every 21 days) is deemed to be the first-line treatment for advanced and metastatic ICC; the median overall survival (mOS) was 11.7 months and the median progression-free survival (mPFS) was 8.0 months (8). Unfortunately, as mOS is no longer than 1 year and no standard treatment beyond first-line chemotherapy is recommended now, more and more effective therapeutic strategies are to be explored.
In recent years, molecular-targeted therapy, immunotherapy, and multidrug combination therapy have shown interesting and attractive results (16, 17). Molecular-targeted agents, including vascular epidermal growth factor (VEGF) inhibitors, fibroblast growth factor (FGF) inhibitors, and isocitrate dehydrogenase (IDH) inhibitors, have provided new ideas for further treatment in advanced ICC (9, 17, 18). Lenvatinib, confirmed as an inhibitor of VEGF receptors 1–3, FGF receptors 1–4, platelet-derived growth factor (PDGF) receptor α, KIT, and RET, selectively inhibits receptor tyrosine kinases involved in tumor growth and angiogenesis (19, 20). It was successfully investigated in a multicenter phase III clinical trial, revealing that lenvatinib was non-inferior to sorafenib in overall survival in patients with untreated advanced hepatocellular carcinoma (HCC) along with great safety and tolerability (21). Up to now, the multikinase inhibitors sorafenib and lenvatinib are the only approved first-line treatments for advanced HCC by the US Food and Drug Administration (FDA) (22). Due to high expression of the immune checkpoint molecule programmed death-1 (PD-1) and its ligand (PD-L1), immunotherapy, a type of treatment regulating T-lymphocyte activity and enhancing the antitumor immune response, may effectively reduce tumor immune escape and become a promising adjuvant therapy for advanced ICC (23–25). For instance, the KEYNOTE-158 (NCT02628067) trial (n = 104) evaluated treatment with pembrolizumab monotherapy for advanced CCA patients and found that pembrolizumab was highly effective and safe; the mPFS, mOS, and overall response rate (ORR) was 2.0 months, 7.4 months, and 5.8%, respectively (10, 26). Tislelizumab, similar to pembrolizumab, is a PD-1 monoclonal IgG4 antibody of high affinity that is mainly used in hematological cancers and advanced solid tumors, which was conditionally approved after at least second-line chemotherapy in China (27, 28). Moreover, combination of immune checkpoint inhibitors and molecular-targeted agents has promising antitumor activity, taking lenvatinib plus pembrolizumab for example (29, 30).
Here, we highlight the usage of the first-line chemotherapy CisGem, the receptor tyrosine kinase inhibitor lenvatinib in combination with the anti-PD-1 drug tislelizumab for this advanced ICC patient after intense discussion in MDT. Firstly, tumor DNA mismatch repair (MMR) deficiency and high MSI are demonstrated with durability of responses to immune checkpoint inhibitors in some tumor types (31, 32). Similar to MMR and MSI, TMB is another emerging predictive biomarker for immunotherapy (33). In this tumor, deficiency in the MMR pathway was obviously detected, and replication errors with unstable abnormalities in short sequences of nucleotide were accumulated subsequently. Furthermore, the TMB was very high. Secondly, based on a phase II clinical study, lenvatinib was confirmed to have a tolerable safety profile for second-line treatment of CCA (34). Moreover, the cytotoxic cell death activity of chemotherapy would trigger antigen release, enhancing immune stimulation and improving the activity of PD-1/PD-L1-blocking agents (35). Therefore, we finally chose this systemic sequential therapeutic regimen for the ICC patient.
This case demonstrates that with a greater understanding of the molecular pathology and genomics of ICC, the therapy of tumors can gradually enter a more precise phase compared with other regimens. Furthermore, this report is a bold attempt and provides a glimmer of hope for advanced ICC patients, breaking through the bottleneck of traditional therapies. However, the long-term survival of this treatment has not been known. Whether the treatment can truly offer a clinical benefit and be approved needs further exploration with a large-scale randomized controlled trial in the future.
We firstly reported an original systemic sequential therapeutic regimen for preoperative advanced ICC conversion therapy according to pathological examination and genomic profiling, providing an opportunity for radical resection. The patient recovered well with subsequent adjuvant therapy. Additional reliable studies with larger numbers of cases are needed to define certain efficacy and adverse effects for this disease.
(I) Conception and design: WW, YD and XH. (II) Administrative support: WW and YW. (III) Provision of study materials or patients: ZS and JT. (IV) Collection and assembly of data: XH, ZS and JT. (V) Data analysis and interpretation: YD and XH. (VI) Manuscript writing: All authors. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (No. 81773096, 82001673 and 82072650), Key Research and Development Program of Zhejiang Province (No. 2018C03085 and 2021C03121), and the Public Welfare Technology Research Project of Zhejiang Province (No.LGD19C040006).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer QX and the handling editor JC declared a shared affiliation, with no collaboration, with the authors, at the time of the review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CCA, cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma; HCC, hepatocellular carcinoma; R0, radical resection; CisGem, gemcitabine and cisplatin; CT, computed tomography; CEA, carcinoembryonic antigen; AFP, alpha fetoprotein; CA 125, carbohydrate antigen 125; CA 19-9, carbohydrate antigen 19-9; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CDFI, color Doppler flow imaging; MRI, magnetic resonance imaging; AJCC, American Joint Committee on Cancer; UICC, Union for International Cancer Control; TMB, tumor mutational burden; MSI, microsatellite instability; MMR, mismatch repair; NGS, next-generation sequencing; MDT, multidisciplinary treatment; DWI, diffusion-weighted imaging; PR, partial response; ERAS, enhanced recovery after surgery; CR, complete response; mOS, median overall survival; mPFS, median progression-free survival; ORR, overall response rate; VEGF, vascular epidermal growth factor; FGF, fibroblast growth factor; IDH, isocitrate dehydrogenase; PDGF, platelet-derived growth factor; FDA, Food and Drug Administration; PD-1, programmed death-1.
1. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet (2014) 383:2168–79. doi: 10.1016/S0140-6736(13)61903-0
2. Rizvi S, Gores GJ. Pathogenesis, Diagnosis, and Management of Cholangiocarcinoma. Gastroenterology (2013) 145:1215–29. doi: 10.1053/j.gastro.2013.10.013
3. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - Evolving Concepts and Therapeutic Strategies. Nat Rev Clin Oncol (2018) 15:95–111. doi: 10.1038/nrclinonc.2017.157
4. Lang H, Sotiropoulos GC, Sgourakis G, Schmitz KJ, Paul A, Hilgard P, et al. Operations for Intrahepatic Cholangiocarcinoma: Single-Institution Experience of 158 Patients. J Am Coll Surg (2009) 208:218–28. doi: 10.1016/j.jamcollsurg.2008.10.017
5. Sirica AE, Gores GJ, Groopman JD, Selaru FM, Strazzabosco M, Wei Wang X, et al. Intrahepatic Cholangiocarcinoma: Continuing Challenges and Translational Advances. Hepatology (2019) 69:1803–15. doi: 10.1002/hep.30289
6. Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discovery (2017) 7:943–62. doi: 10.1158/2159-8290.CD-17-0245
7. Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D. Biliary Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2016) 27:v28–37. doi: 10.1093/annonc/mdw324
8. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin Plus Gemcitabine Versus Gemcitabine for Biliary Tract Cancer. N Engl J Med (2010) 362:1273–81. doi: 10.1056/NEJMoa0908721
9. Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for Previously Treated, Locally Advanced or Metastatic Cholangiocarcinoma: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol (2020) 21:671–84. doi: 10.1016/S1470-2045(20)30109-1
10. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of Tumour Mutational Burden With Outcomes in Patients With Advanced Solid Tumours Treated With Pembrolizumab: Prospective Biomarker Analysis of the Multicohort, Open-Label, Phase 2 KEYNOTE-158 Study. Lancet Oncol (2020) 21:1353–65. doi: 10.1016/S1470-2045(20)30445-9
11. Wardell CP, Fujita M, Yamada T, Simbolo M, Fassan M, Karlic R, et al. Genomic Characterization of Biliary Tract Cancers Identifies Driver Genes and Predisposing Mutations. J Hepatol (2018) 68:959–69. doi: 10.1016/j.jhep.2018.01.009
12. El-Diwany R, Pawlik TM, Ejaz A. Intrahepatic Cholangiocarcinoma. Surg Oncol Clin N Am (2019) 28:587–99. doi: 10.1016/j.soc.2019.06.002
13. Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control (2017) 24(3):1073274817729245. doi: 10.1177/1073274817729245
14. Tabrizian P, Jibara G, Hechtman JF, Franssen B, Labow DM, Schwartz ME, et al. Outcomes Following Resection of Intrahepatic Cholangiocarcinoma. HPB (Oxford) (2015) 17:344–51. doi: 10.1111/hpb.12359
15. Park J, Kim MH, Kim KP, Park DH, Moon SH, Song TJ, et al. Natural History and Prognostic Factors of Advanced Cholangiocarcinoma Without Surgery, Chemotherapy, or Radiotherapy: A Large-Scale Observational Study. Gut Liver (2009) 3:298–305. doi: 10.5009/gnl.2009.3.4.298
16. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: The Next Horizon in Mechanisms and Management. Nat Rev Gastroenterol Hepatol (2020) 17:557–88. doi: 10.1038/s41575-020-0310-z
17. Xie C, McGrath NA, Monge Bonilla C, Fu J. Systemic Treatment Options for Advanced Biliary Tract Carcinoma. J Gastroenterol (2020) 55:944–57. doi: 10.1007/s00535-020-01712-9
18. Lowery MA, Burris HA 3rd, Janku F, Shroff RT, Cleary JM, Azad NS, et al. Safety and Activity of Ivosidenib in Patients With IDH1-Mutant Advanced Cholangiocarcinoma: A Phase 1 Study. Lancet Gastroenterol Hepatol (2019) 4:711–20. doi: 10.1016/S2468-1253(19)30189-X
19. Scott LJ. Lenvatinib: First Global Approval. Drugs (2015) 75:553–60. doi: 10.1007/s40265-015-0383-0
20. Fallahi P, Ferrari SM, Galdiero MR, Varricchi G, Elia G, Ragusa F, et al. Molecular Targets of Tyrosine Kinase Inhibitors in Thyroid Cancer. Semin Cancer Biol (2020) S1044-579X(20)30249-2. doi: 10.1016/j.semcancer.2020.11.013
21. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib Versus Sorafenib in First-Line Treatment of Patients With Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 non-Inferiority Trial. Lancet (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
22. Tharehalli U, Svinarenko M, Lechel A. Remodelling and Improvements in Organoid Technology to Study Liver Carcinogenesis in a Dish. Stem Cells Int (2019) 2019:3831213. doi: 10.1155/2019/3831213
23. O'Donnell JS, Teng MWL, Smyth MJ. Cancer Immunoediting and Resistance to T Cell-Based Immunotherapy. Nat Rev Clin Oncol (2019) 16:151–67. doi: 10.1038/s41571-018-0142-8
24. Dai S, Jia R, Zhang X, Fang Q, Huang L. The PD-1/PD-Ls Pathway and Autoimmune Diseases. Cell Immunol (2014) 290:72–9. doi: 10.1016/j.cellimm.2014.05.006
25. Gani F, Nagarajan N, Kim Y, Zhu Q, Luan L, Bhaijjee F, et al. Program Death 1 Immune Checkpoint and Tumor Microenvironment: Implications for Patients With Intrahepatic Cholangiocarcinoma. Ann Surg Oncol (2016) 23:2610–7. doi: 10.1245/s10434-016-5101-y
26. Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A, et al. Efficacy and Safety of Pembrolizumab for the Treatment of Advanced Biliary Cancer: Results From the KEYNOTE-158 and KEYNOTE-028 Studies. Int J Cancer (2020) 147:2190–8. doi: 10.1002/ijc.33013
27. Lee A, Keam SJ. Tislelizumab: First Approval. Drugs (2020) 80:617–24. doi: 10.1007/s40265-020-01286-z
28. Friedlander M, Meniawy T, Markman B, Mileshkin L, Harnett P, Millward M, et al. Pamiparib in Combination With Tislelizumab in Patients With Advanced Solid Tumours: Results From the Dose-Escalation Stage of a Multicentre, Open-Label, Phase 1a/B Trial. Lancet Oncol (2019) 20:1306–15. doi: 10.1016/S1470-2045(19)30396-1
29. Sakurai T, Nishida N, Kudo M. Promising Anticancer Therapy: Combination of Immune Checkpoint Inhibitors and Molecular-Targeted Agents. Hepatobiliary Surg Nutr (2020) 9:777–9. doi: 10.21037/hbsn.2020.03.04
30. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol (2020) 38:2960–70. doi: 10.1200/JCO.20.00808
31. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer Immunology. Mutational Landscape Determines Sensitivity to PD-1 Blockade in non-Small Cell Lung Cancer. Science (2015) 348:124–8. doi: 10.1126/science.aaa1348
32. Baretti M, Le DT. DNA Mismatch Repair in Cancer. Pharmacol Ther (2018) 189:45–62. doi: 10.1016/j.pharmthera.2018.04.004
33. Klempner SJ, Fabrizio D, Bane S, Reinhart M, Peoples T, Ali SM, et al. Tumor Mutational Burden as a Predictive Biomarker for Response to Immune Checkpoint Inhibitors: A Review of Current Evidence. oncologist (2020) 25:e147–e59. doi: 10.1634/theoncologist.2019-0244
34. Ueno M, Ikeda M, Sasaki T, Nagashima F, Mizuno N, Shimizu S, et al. Phase 2 Study of Lenvatinib Monotherapy as Second-Line Treatment in Unresectable Biliary Tract Cancer: Primary Analysis Results. BMC Cancer (2020) 20:1105. doi: 10.1186/s12885-020-07365-4
Keywords: advanced intrahepatic cholangiocarcinoma, systemic sequential therapy, surgery, conversion therapy, next-generation sequencing
Citation: Ding Y, Han X, Sun Z, Tang J, Wu Y and Wang W (2021) Systemic Sequential Therapy of CisGem, Tislelizumab, and Lenvatinib for Advanced Intrahepatic Cholangiocarcinoma Conversion Therapy. Front. Oncol. 11:691380. doi: 10.3389/fonc.2021.691380
Received: 20 April 2021; Accepted: 06 August 2021;
Published: 30 August 2021.
Edited by:
Jiang Chen, Zhejiang University, ChinaReviewed by:
Qiuran Xu, Zhejiang Provincial People’s Hospital, ChinaCopyright © 2021 Ding, Han, Sun, Tang, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weilin Wang, d2FtQHpqdS5lZHUuY24=; Yingsheng Wu, ZHJ3dXlzQGhvdG1haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.