
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 14 June 2021
Sec. Hematologic Malignancies
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.691064
This article is part of the Research Topic Molecular and Immunological Advances in Hematological Malignancies: Volume II View all 10 articles
 Kunming Qi1,2,3†
Kunming Qi1,2,3† Zhiling Yan1,2,3†
Zhiling Yan1,2,3† Hai Cheng1,2,3†
Hai Cheng1,2,3† Wei Chen1,2,3
Wei Chen1,2,3 Ying Wang1,2,3
Ying Wang1,2,3 Xue Wang1,2,3
Xue Wang1,2,3 Jiang Cao1,2,3
Jiang Cao1,2,3 Huanxin Zhang1,2,3
Huanxin Zhang1,2,3 Wei Sang1,2,3
Wei Sang1,2,3 Feng Zhu1,2,3
Feng Zhu1,2,3 Haiying Sun1,2,3
Haiying Sun1,2,3 Depeng Li1,2,3
Depeng Li1,2,3 Qingyun Wu1,2,3
Qingyun Wu1,2,3 Jianlin Qiao1,2,3
Jianlin Qiao1,2,3 Chunling Fu1,2,3
Chunling Fu1,2,3 Lingyu Zeng1,2,3
Lingyu Zeng1,2,3 Zhenyu Li1,2,3*
Zhenyu Li1,2,3* Junnian Zheng4*
Junnian Zheng4* Kailin Xu1,2,3*
Kailin Xu1,2,3*Introduction: Chimeric antigen receptor T (CAR-T) cells are effective in treating hematological malignancies. However, in patients receiving CAR-T therapy, data characterizing cardiac disorders are limited.
Methods: 126 patients with hematologic malignancies receiving CAR-T cell therapy were analyzed to determine the impact of CAR-T therapy on occurrence of cardiac disorders, including heart failure, arrhythmias, myocardial infarction, which were defined by the Common Terminology Criteria for Adverse Events (CTCAE). Parameters related to cardiac disorders were detected including myocardial enzyme, NT-proBNP and ejection fraction (EF). Cardiovascular (CV) events included decompensated heart failure (HF), clinically significant arrhythmias and CV death.
Results: The median age of patients was 56 years (6 to 72 years). 58% patients were male, 62% had multiple myeloma, 20% had lymphoma and 18% had ALL. 33 (26%) patients had cardiac disorders, most of which were grade 1-2. 13 patients (10%) were observed with cardiac disorders grade 3-5, which comprised 5(4%) patients with new-onset HF, 2 (2%) patients with new-onset arrhythmias, 4 (3%) patients with the acute coronary syndrome, 1(1%) patient with myocardial infarction and 1(1%) patient with left ventricular systolic dysfunction. There were 9 CV events (7%) including 6 decompensated heart failure, 1 clinically significant arrhythmias and 2 CV deaths. Among the 33 patients with cardiac disorders, the patients with cardiac disorders CTCAE grade 3-5 had higher grade CRS (grade ≥ 3) than those with cardiac disorders CTCAE grade ≤ 2 (P <0.001). More patients with cardiac disorders CTCAE grade 3-5 were observed in the cohort who did not receive corticosteroids and/or tocilizumab therapy timely comparing with those who received corticosteroids and/or tocilizumab therapy timely (P =0.0004).
Conclusions: Cardiac disorders CAR-T cell therapy were common and associated with occurrence of CRS. However, most cases were mild. For patients with CRS grade 3-5, timely administration of corticosteroids and/or tocilizumab can effectively prevent the occurrence and progression of cardiac disorders.
Chimeric antigen receptor (CAR)-T cell therapy has sparked a wave of optimism in relapsed/refractory hematologic malignancies. CAR-T cells targeting new tumor antigens would emerge in the near future. Some side effects were observed after CAR-T cell therapies with cytokine releasing syndrome (CRS) as the most common one, presenting as fever, hypotension, hypoxia, and capillary leakage. Neurotoxicity and coagulation dysfunction have also been reported (1–4). These side effects were intensively studied, however, other side effects such as cardiac disorders might be underestimated. Case report has shown some cases of cardiac disorders (5). Thus, a comprehensive study is needed to evaluate occurrence of cardiac disorders after CAR-T cell therapy.
Here, we performed a comprehensive analysis of the incidence, dynamic changes and outcomes of cardiac disorders defined by CTCAE in 126 patients with relapsed/refractory (R/R) hematologic malignancies after receiving CAR-T cell therapy. The correlation between cardiac disorders and CRS was also analyzed.
The study cohort was derived from the patients receiving CAR-T at Affiliated Hospital of Xuzhou Medical University between January 1, 2019, and November 20, 2020; 126 patients were included. (China) (Clinical Trials: NCT02782351, NCT03207178, ChiCTR-OIC-17011272). All patients were followed-up until a fixed calendar date (i.e., January 31, 2021). Clinical events were extracted by detailed chart review. For the patients who died during the follow-up, the last follow-up date was the date of death. This was a retrospective study conducted according to the Declaration of Helsinki’s principles with approval by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University. Informed consents were obtained from all patients.
The humanized single-chain variable fragment (scFv) sequence specific for CD19 was derived from clone FMC63 as previously described (6) and the anti-CD20, anti-CD22 and anti-BCMA scFv was derived from a murine anti-human CD20, CD22 and BCMA monoclonal antibody. The scFv sequence for CD19, CD20 and BCMA were inserted in tandem with the human CD8 transmembrane, CD8 hinge, 4-1BB costimulatory domain, CD3z intracellular regions, and T2A-EGFRt sequence. The scFv sequence for CD22 was inserted in tandem with the human CD8 transmembrane, CD8 hinge, CD28 costimulatory domain, CD3z intracellular regions, and T2A-EGFRt sequence. CARs targeting CD19, CD20, CD22 and BCMA were synthesized and subcloned into lentivirus expression vector Lenti-EF1a-puro and stably expressed in CD3-positive T cells after transfection of lentiviral vector.
Peripheral blood mononuclear cells were obtained from patients by leukapheresis as previously described (3). Most patients received lymphodepletion chemotherapy with the FC regimens included both fludarabine (750 mg/m2, day-5) and cyclophosphamide (30 mg/m2/d, days -5~2). On day 0, patients with MM received CD19 CAR-T cell and BCMA CAR-T cell infusion at the median dose 2 × 106 cells/kg (1.4 - 4 × 106 cells/kg), patients with B-NHL received CD19 CAR-T cell and CD22 CAR-T cell infusion at the median dose 2 × 106 cells/kg (0.8-6 × 106 cells/kg) and patients with ALL received a single dose of CD19 CAR-T cell infusion at the median dose 1 × 106 cells/kg (0.8-2 × 106 cells/kg) respectively.
Peripheral blood was collected before lymphodepletion, on day -3 or day -1, and at approximately 1~3, 4~6, 7~10, 11~13, 14~16, 17~20, 21-24, 25~30, 31-40, 41-50 days after CAR-T cell infusion for analysis of complete blood counts, hepatic function, renal function, hs-cTnT, NT-proBNP, cytokine including IL-6, Ferritin, CRP, IL-8, IFN-γ and IL-10. The morphological characteristics of bone marrow were evaluated on day 0, 14 and 28 respectively. Minimal residual disease was detected by flow cytometry. If the patient did not die, the CAR-T cells were followed up for at least 90 days.
Cardiac disorders was defined according to Common Terminology Criteria for Adverse Events (CTCAE; version 4.03) (7). According to the hs-cTnT assay, the lower limit of detection is 3 ng/L for the 99th percentile is 14 ng/L according to the manufacturer. The diagnosis of myocardial infarction required a rise and/or fall of hs-cTnT with at least one value above the 99th percentile upper reference limit, with the symptoms of ischaemia or development electrocardiogram (8). NT-proBNP elevation of patients ≤ 50 years old, patients > 50 years old and patients with baseline glomerular filtration rate (GFR) < 60ml/min were defined > 450 pg/ml, > 900 pg/ml and >1200 pg/ml respectively. A reduction in left ventricular ejection fraction (LVEF) was defined as a reducing at least ten percentage points, less than 50%. Cardiovascular events included decompensated HF, clinically significant arrhythmias and CV death (9). CRS was graded according to consensus criteria proposed by Lee et al. (10). All results were reviewed and confirmed by the research team without considering other variables. The onset of CRS was defined as the first appearance of a fever after CAR-T therapy, excluding other factors for fever. The time to corticosteroid or tocilizumab administration was defined as the time from the onset of CRS to the administration of corticosteroid or tocilizumab. This article reports cardiac disorders presenting within 50 days after the first CAR-T cell infusion.
Descriptive statistics (median/interquartile range [IQR], count, and percentage) are reported for key variables. Continuous data were compared using unpaired Student’s t-tests and categorical data were compared using the chi-square or the Fisher exact test. The factors associated with cardiac disorders were explored using ordinal regression with cardiac disorders CTCAE frequency as the dependent variable and baseline glomerular filtration rate, sex, age, diagnosis, underlying disease (including diabetes, hypertension, etc) and CRS as independent variables. The Wilcoxon test was adopted for continuous variables between 2 groups, and the Kruskal-Wallis test was for multiple groups. The Spearman correlation test was used for correlation analysis. Statistical significance was defined using a 2-tailed p-value < 0.05. Statistical analyses were performed using IBM SPSS for Windows 25.0 software (SPSS, Inc., Chicago, IL).
Baseline demographics and clinical characteristics of the 126 patients with R/R hematologic malignancies receiving lymphodepletion chemotherapy and CAR-T cell therapy, are shown in Table 1. In the entire cohort, the median age was 56 years (range, 6 to 72 years), including 73(58%) males and 53(42%) females. The most of patients were multiple myeloma (MM) (n = 78, 62%), followed by non-Hodgkin’s lymphoma (NHL) (n = 25, 20%) and acute lymphoblastic leukemia (ALL) (n=23, 18%). In the MM cohort, 13 patients (86%) had low baseline glomerular filtration rate (< 60ml/min) and 44(57%) patients bone marrow plasma cells were ≥10%. In the NHL cohort, 21 patients (84%) were Ann Arbor stage III~IV. The percentage of bone marrow blasts was ≥20% in 13 (57%) patients with ALL. Before the CAR-T cell therapy, 120 (95%) patients received lymphodepletion chemotherapy of FC regimen, and 6 (5%) received non-FC regimen, including 2 with fludarabine alone, 1 with cyclophosphamide alone and 3 without regimen (Table 1).
Within 50 days of CAR-T cell infusion, cardiac disorders of any grade were more frequent in female patients (P = 0.012), the patients with low baseline glomerular filtration rate (< 60ml/min) (P < 0.0001) and the patients with CRS (P < 0.0001) by univariate analyses (Table 1). The patient’s age, diagnosis, ECOG, the number of plasma cells in bone marrow of MM patients, Charlson Comorbidity Index and hypertension were not associated with cardiac disorders by univariate analyses. The baseline of hs-cTnT, NT-proBNP, Ann Arbor stage of NHL, ALL patients with a high tumor burden (blasts ≥20% in bone marrow), lymphodepletion regimens and underlying diseases (including diabetes, coronary artery disease, chronic heart failure and atrial fibrillation) were no statistical significance. Multivariable analysis showed that after CAR-T cell therapy, the patients with low baseline glomerular filtration rate (< 60ml/min) and CRS were associated with an increased risk of cardiac disorders (P = 0.031, P < 0.0001 perspective) (Table 1).
Of 126 patients treated with lymphodepletion chemotherapy and CAR-T cell infusion, 33(26%) patients (25 MM patients, 3 NHL patients, 5 ALL patients) had cardiac disorders as defined by the Common Terminology Criteria for Adverse Events (CTCAE). There were 10 patients (8%) with CTCAE grade 1, 10 patients (8%) with CTCAE grade 2, 8 patients (6%) with CTCAE grade 3, 2 patients (2%) with CTCAE grade 4 and 3 patients (2%) with CTCAE grade 5. In MM cohort, cardiac disorders CTCAE 1, CTCAE 2, CTCAE 3, CTCAE 4 and CTCAE 5 were 9, 7, 6, 2 and 1 patients. In NHL cohort, cardiac disorders CTCAE 1, CTCAE 2, CTCAE 3, CTCAE 4 and CTCAE 5 were 1, 1, 1, 0 and 0 patients respectively. In ALL cohort, cardiac disorders CTCAE 1, CTCAE 2, CTCAE 3, CTCAE 4 and CTCAE 5 were 0, 2, 1, 1, 1 patients respectively (Figure 1).
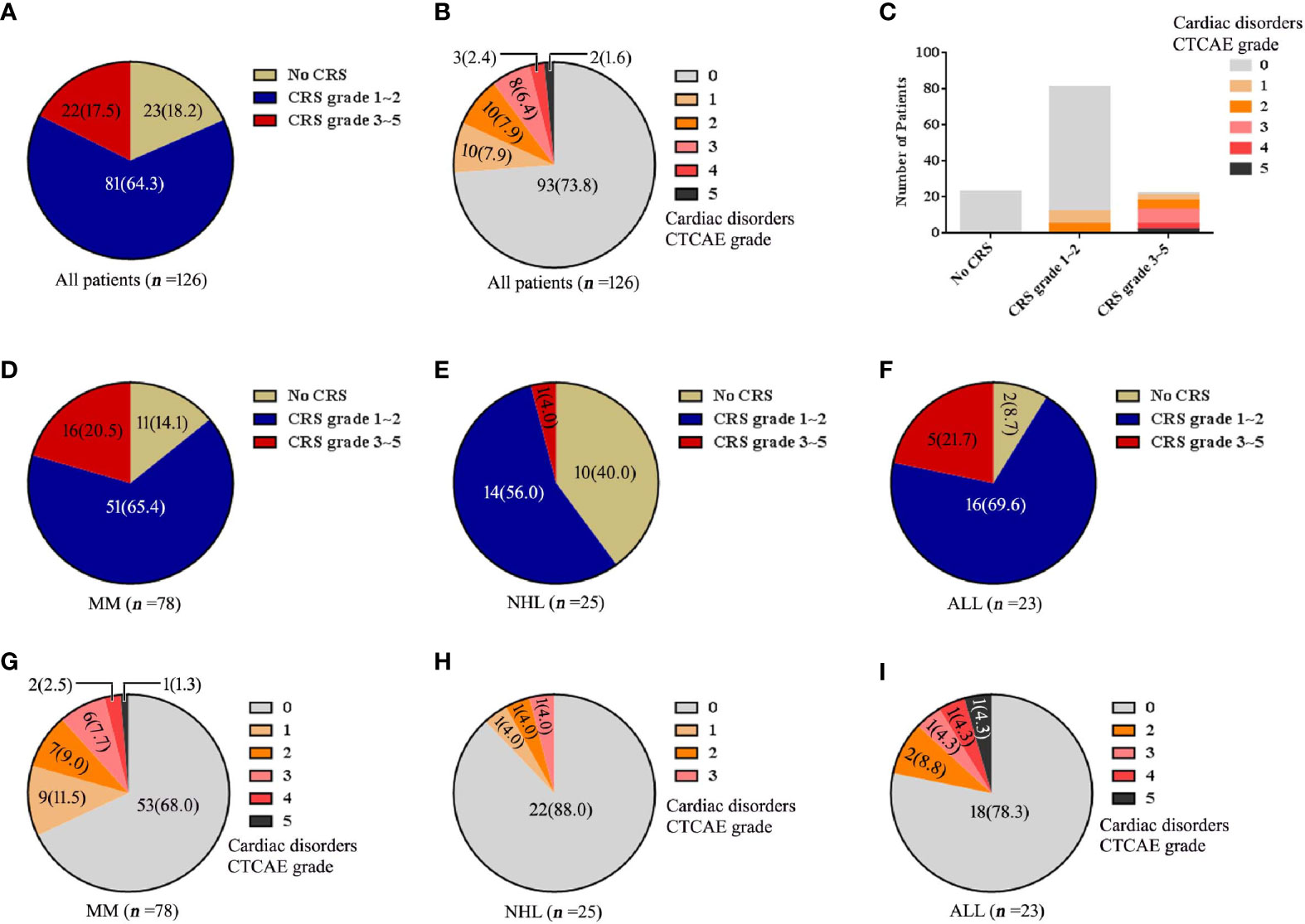
Figure 1 The changes of CRS, cardiac disorders CTCAE before and after CAR-T cell infusion. (A) Numbers of patients with each CRS. (B) Numbers of patients with each cardiac disorders grade. (C) Numbers of patients with each grade of cardiac disorders and CRS. The numbers of patients with each CRS grade are shown for each disease of (D) MM, (E) NHL and (F) ALL. Cardiac disorders CTCAE for each disease of (G) MM, (H) NHL and (I) ALL. Percentage is shown in parentheses.
CRS was found in all 33 patients (26%) with cardiac disorders. Among 20 (16%) patients with cardiac disorders CTCAE grade 1-2, there were 12 patients with CRS grade 1-2 and 8 patients with CRS grade 3-5. Among 13 (10%) patients with cardiac disorders CTCAE grade 3-5, all patients had severe CRS (grade 3-5) (Figure 1).
Of 13 patients (10%) with cardiac disorders grade 3-5, 5(4%) patients had new-onset HF, 2 (2%) patients had new-onset arrhythmias, 4 (3%) patients had the acute coronary syndrome, 1(1%) patient had myocardial infarction and 1(1%) patient had left ventricular systolic dysfunction. There were 9 CV events (7%) with a median onset time of 5 days (IQR: 3 to 9 days) among all cardiac disorder patients. The CV events included 6 decompensated heart failures, 1 clinically significant arrhythmias and 2 CV deaths (Table 2).
The patients who developed CTCAE grade ≥3 cardiac disorders had more severe CRS (p < 0.001, Tables 1, 3). The occurrence time of CRS grade 3-5 was earlier than that of cardiac disorders CTCAE grade 1-2 and 3-5. The median onset time of CRS grade 3-5 and cardiac disorders CTCAE grade 3-5 was 3 days (IQR: 1 to 7 days) and 8 days (IQR: 4 to 9 days) (P = 0.0054) (Table 4). Earlier onset of CRS after CAR-T therapy was associated with a higher risk of subsequent developing severe cardiac disorders. The severity of cardiac disorders was related to the higher peak concentrations of hs-cTnT, NT-proBNP, ferritin, C-reactive protein (CRP) and multiple cytokines, including IL-6, IL-8, IFN-γ and IL-10 (Figure 2).
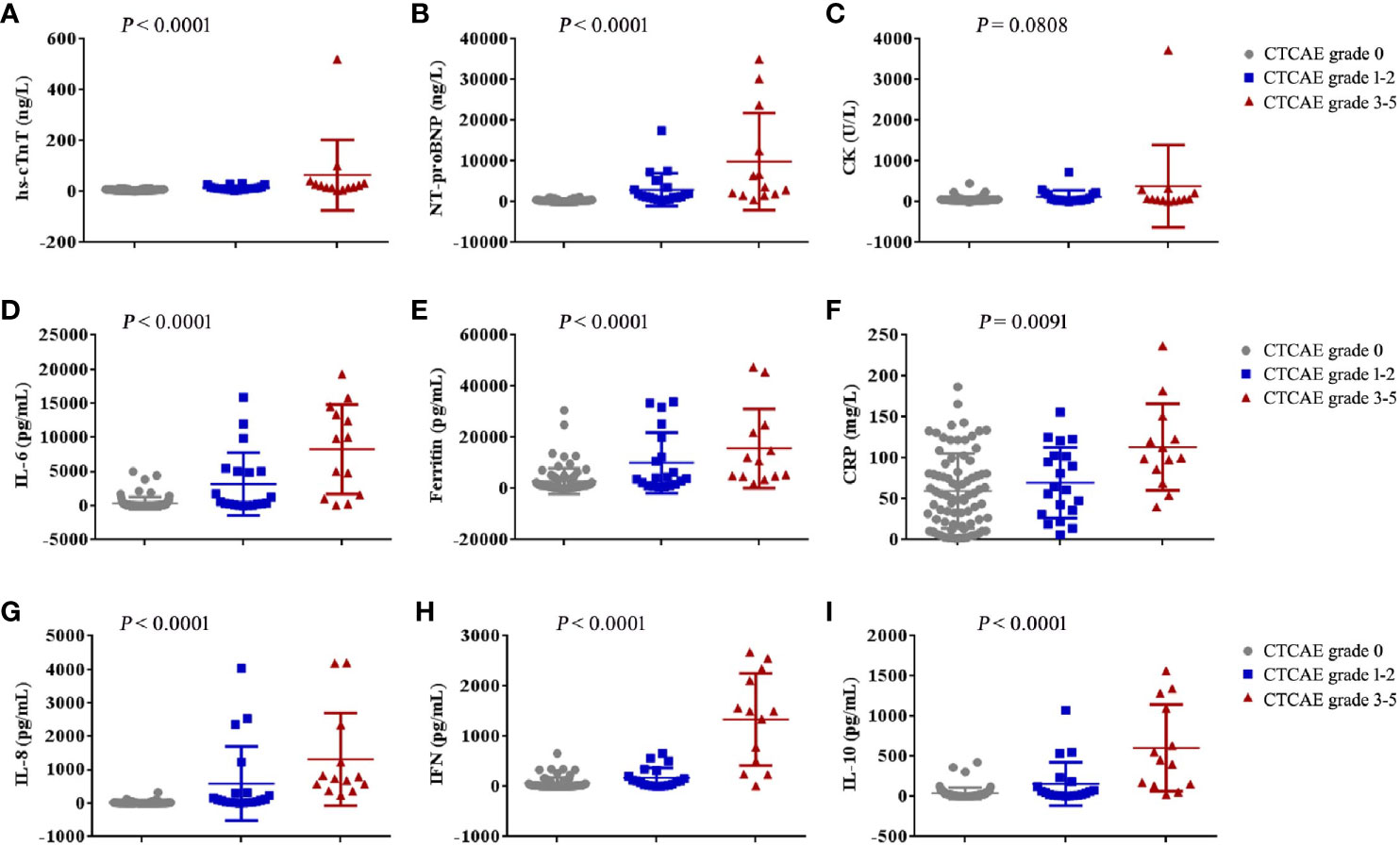
Figure 2 The maximum values of myocardial enzyme parameters, NT-proBNP and cytokines in patients with cardiac disorders CTCAE grade 0, 1-2 and 3-5 after CAR-T cell infusion. The maximum hs-cTnT (A), maximum NT-proBNP (B), maximum CK (C), maximum IL-6 (D), maximum Ferritin (E), maximum CRP (F), maximum IL-8 (G), maximum IFN-γ (H) and maximum IL-10 (I) in the first 30 days after CAR-T cell infusion are shown in patients who had cardiac disorders CTCAE grade 0 (n = 80), cardiac disorders CTCAE grade 1-2 (n = 20) and cardiac disorders CTCAE grade 3-5 (n =13). Each point represents data from a single patient. The median and IQR are shown. Two-sided P values were determined using the Kruskal-Wallis test.
After further analysis of the correlation between the levels of hs-cTnT, NT-proBNP and cytokines in patients with the grade 3-5 cardiac disorders CTCAE (n =13), we found that the values of hs-cTnT were positively correlated with the levels of serum IL-6, Ferritin, IFN-γ (Figures 3A, B, E). The values of NT-proBNP were positively correlated with the levels of serum IL-6, Ferritin, IFN-γ and IL-10 (Figures 3G, H, K, L).
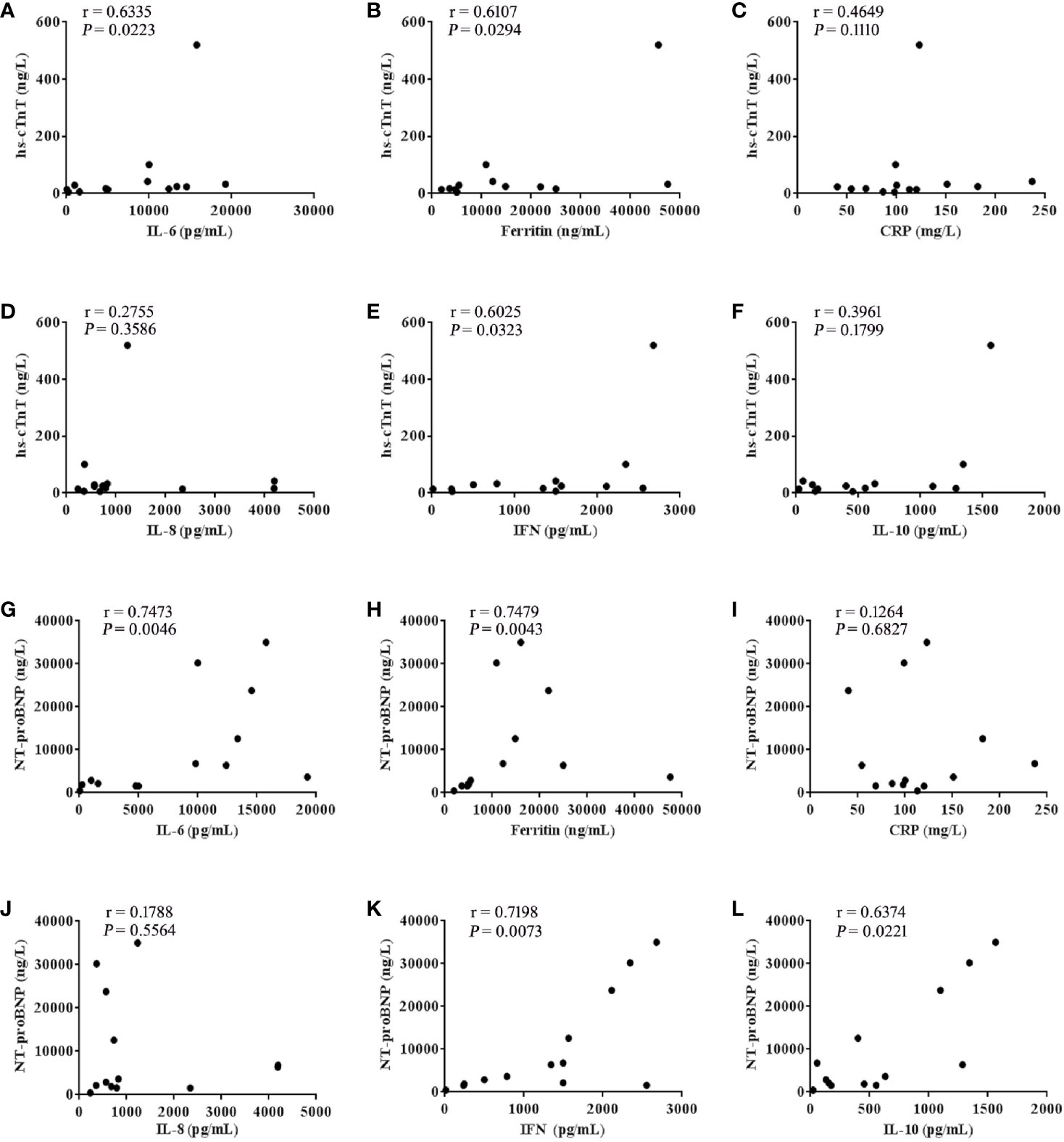
Figure 3 hs-cTnT and NT-proBNP were closely correlated to cytokine levels in patients with CTCAE grade 3-5 (n = 13). The correlation of hs-cTnT concentration with serum (A) IL-6, (B) Ferritin, (C) CRP, (D) IL-8, (E) IFN-γ and (F) IL-10. The correlation of NT-proBNP concentration with serum (G) IL-6, (H) Ferritin, (I) CRP, (J) IL-8, (K) IFN-γ and (L) IL-10. Each point represents data from a single patient; r values were determined using the spearman correlation test.
In 10 patients with cardiac disorders CTCAE grade 1, which were asymptomatic or mild, no intervention was needed. In 10 patients with CTCAE grade 2, 3 patients with HF were given torasemide and the other patients were only given symptomatic treatment. In cardiac disorders CTCAE grade≥3 cohort, all patients required an intervention that included the administration of diuretic, amiodarone, cedilanid and/or IV metoprolol and/or vasopressors and oxygen supplementation. Some patients were typically transferred to the intensive care unit for further management. After treatment, 2 (2%) patients died, among which one patient died of malignant arrhythmia and another died of myocardial infarction. However, 31 of 33 patients (94%) recovered. The arrhythmia and HF disappeared quickly after treatment. NT proBNP and hs TnT returned to normal baseline levels within one month after treatment.
Tables 5 and 6 shows the treatments in patients with CRS (n = 103) and cardiac disorders. In cohort 1, patients were given corticosteroids and/or tocilizumab therapy within 24 hours after the onset of CRS and in cohort 2 patients were not given corticosteroids and/or tocilizumab therapy within 24 hours or received corticosteroids and/or tocilizumab therapy more than 24 hours after the onset of CRS. Among the 51 patients with CRS grade 1 and 30 patients with CRS grade 2, there was no difference in the incidence of cardiac disorders between cohort 1 and cohort 2 (P = 0.1336, 0.3742). While among the 22 patients with grade 3-5, 2 of 8 patients (25%) in cohort 1 developed cardiac disorders, which was lower than 11 of 14 patients (79%) in cohort 2 (P = 0.0260). In 33 patients with cardiac disorders, compared with 11 of 15 patients (73%) developed cardiac disorders (CTCAE Grade 3-5) in cohort 2, there were fewer patients in cohort 1, with only 2 of 18 patients (11%) progressed to cardiac disorders (CTCAE grade 3-5) (P = 0.0004) (Tables 5, 6).
CRS is one of the most common side effects in CAR-T cell therapy (12–14). Other toxicities including neurotoxicity and coagulation dysfunction were also reported (2–4). However, there have been very few reports of cardiac disorders after CAR-T. Cardiac disorders had different manifestations, of which the most common was HF (12%), followed by acute coronary syndrome (7%) and arrhythmia (6%), and the least was myocardial infarction (1%). The diagnosis of CAR-T cell-related cardiac disorders required the appearance of new symptoms or signs of HF, arrhythmia or myocardial infarction. Although most cardiac disorders are mild and temporary, some patients developed severe cardiac disorders, which may be life-threatening in the form of malignant arrhythmias, myocardial infarction, and decompensated HF. Early diagnosis and treatment of CAR-T-related cardiac disorders is an important step to reduce the mortality after CAR-T cell therapy. Serial testing of myocardial enzymes, especially hs-cTnT and NT-proBNP, is very necessary for early detection of cardiac disorders. Alvi RM (9) reported that an elevated troponin occurred in 29 of 53 tested patients (54%) and was associated with an increased risk for a CV event. In our study, 9 and 12 of 13 patients with cardiac disorders (CTCAE grade 3-5) had elevated hs-cTnT and NT-proBNP, with the median peak value 23.60 ng/L (IQR: 14.00 to 37.52 ng/L) and 3657 pg/ml (IQR: 1724 to 18186 pg/ml) respectively, suggesting that continuous testing of hs-cTnT and NT-proBNP may be of use for identifying high-risk patients with cardiac disorders after CAR-T. Shalabi H also found out troponin and proBNP may help to earlier identify those patients at highest risk of severe cardiac systolic dysfunction (15).
Some study had demonstrate that ≈10% of patients develop cardiomyopathy in the context of high-grade CRS after CAR-T cell therapy (16). In our study, baseline factors, including patients with CRS and low baseline GFR levels were associated with an increased risk of subsequent cardiac disorders by multivariable analysis. According to univariate and multivariable analysis, patients with baseline GFR < 60ml/min were more likely to have cardiac disorders than that with baseline GFR ≥ 60ml/min. Lefebvre B also found that baseline creatinine and CRS grade 3 or 4 were independently associated with major adverse cardiovascular events (17). However, after CAR-T treatment, if the treatment were effective, many patients’ renal function would be improved with creatinine and glomerular filtration rate (GFR) returning to normal, which is consistent with our previous published paper (18). Alvi RM showed that troponin elevation was associated with CRS, but not with CAR-T type, cancer type, therapies before CAR-T, race or age (9). The Baseline of NT-proBNP was higher in patients with renal dysfunction, which was prone to heart failure (19). In our study, the baseline of hs-cTnT and NT-proBNP were no statistical significance.
There was a graded relationship between CRS and cardiac disorders. After CAR-T treatment, fever is the most common sign of CRS (2). In our study, 100% of patients with CAR-T-related cardiac disorders had CRS. Patients who developed cardiac disorders CTCAE grade ≥3 were more frequently observed in the severe CRS (grade ≥3) cohort (Tables 1, 3) and no severe CRS (grade ≥3) was found in patients without cardiac disorders, which indicated that there was a close and graded relationship between the development of CRS and cardiac disorders. We found the occurrence time of CRS grade 3-5 was earlier than that of cardiac disorders grade 3-5, and the median onset time of CRS grade 3-5 and cardiac disorders CTCAE grade 3-5 was 3 days (IQR: 1 to 7 days) and 8 days (IQR: 4 to 9 days) (Table 4), suggesting that CRS may cause cardiac disorders. Some studies have found that CRS after CAR-T therapy was related to hepatic dysfunction, coagulation abnormality and neurotoxicity (20–22). Our study indicated that CRS was closely related to cardiac disorders, or cardiac disorders was a part of CRS.
Endothelial dysfunction and hypercoagulability are considered to be the main factors of CAR-T toxicity. Indeed, the biomarkers of endothelial cell activation, such as angiopoietin 2, the angiopoietin-2 to angiopetin-1 ratio and von Willebrand Factor (VWF) were higher in patients with neurotoxicity (grade ≥4) (2). Similarly, it should be noted that endothelial dysfunction is considered an early event in the pathophysiology of cardiovascular disease (23). In our study, after CAR-T therapy, cytokines such as IL-6, IFN-γ which can cause endothelial injury, were significantly increased in patients with severe cardiac disorders. We also observed that patients with earlier peak concentrations of IL-6, IFN-γ had a higher grade of cardiac disorders, suggesting that the rising rate of serum cytokine concentration as well as the peak concentration, may be determinants of the severity of cardiac disorders. IL-6, Ferritin and CRP are the most frequently detected cytokines in CRS (24, 25). After further analysis of the correlation between the levels of myocardial enzyme parameters and cytokines in patients with cardiac disorders (grade ≥3), we found the values of both hs-cTnT and NT-proBNP were positively correlated with the levels of serum IL-6, Ferritin and IFN-γ (Figure 3). Like IL-6, IFN-γ is a proinflammatory cytokine involved in the biological process of inducing macrophages to secrete tumor necrosis factor (TNF-α) and stimulating macrophages to release reactive oxygen species (26, 27). Thus, we speculate that besides targeting IL-6, targeting IFN-γ may be a novel strategy for managing CRS or cardiac disorders that are caused by CAR-T, although this theory requires further investigation.
Among the 103 patients with CRS administered different treatments, we found that there was no difference in the incidence of cardiac disorders of patients with CRS grade 1-2 between cohort 1 and cohort 2. However, patients with CRS grade 3-5 in cohort 2 were more likely to develop cardiac disorders than those in cohort 1. In other words, when severe CRS (grade 3-5) occurs, prompt treatment (given corticosteroids and/or tocilizumab therapy within 24 hours) could reduce the incidence of cardiac disorders (Tables 5, 6).
Tocilizumab, an antagonistic IL-6R mAb, effectively ameliorates fever and hypotension in most patients developed severe CRS after CAR-T cell (28). Our study found that in addition to IL-6, other cytokines such as IFN-γ, CRP, ferritin, IL-8 and IL-10 were significantly increased in the occurrence of cardiac disorders. Corticosteroids may be a double-edged sword in the management of immunomodulation and inflammatory response. Its pharmacological mechanisms involve reducing inflammatory cytokines production, leukocyte infiltration and phagocytosis at the onset of inflammation (29, 30). Besides, the corticosteroid can interfere with the interaction between proinflammatory factors (31, 32). Although corticosteroids may cause injury to the infused CAR-T cell and reduce the curative effect, previous studies and our results show that corticosteroids are still very important for the treatment of cytokine storm-related diseases as hemophagocytic syndrome and CRS (33–35). Our study suggested that timely administration of corticosteroids and/or tocilizumab at the early stage of CRS grade 3-5 may relieve syndromes and delay cardiac disorders progression.
These data are obtained retrospectively. As with all retrospective studies, there may be missing data at some points after CAR-T infusion. For example, some patients with CRS grade 1-2 had mild symptoms, and sometimes may miss cytokine assay. Meanwhile, because the hs-cTnT and NT-proBNP assay in our study was often based on clinical suspicion of cardiac injury (not a protocol driven study as expected), with no symptoms of heart failure, acute coronary syndrome, some patients were not given hs-cTnT or NT-proBNP assay and color doppler echocardiography examination. Therefore, the value of hs-cTnT or NT-proBNP at some time points were missed. In addition, some patients undergo cardiac MRI or ECT examinations at designated locations after developing severe cardiac disorders. This part of the data was also unavailable and without a cardiac biopsy, the exact mechanism of cardiac disorders cannot be determined.
In summary, cardiac disorders were common events after CAR-T cell therapy closelyassociated with CRS. However, the most cardiac disorders were mild. Patients who developed cardiac disorders CTCAE grade ≥3 had more severe CRS (≥3 grade). The values of hs-cTnT were positively correlated with the levels of serum IL-6, Ferritin, IFN-γ. The values of NT-proBNP were positively correlated with the levels of serum IL-6, Ferritin, IFN-γ and IL-10. The onset time of CRS and administration time of corticosteroids/tocilizumab were associated with severity of CRS and cardiac disorders.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Ethics Committee of the Xuzhou Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
KQ, ZY, and HC designed the research, analyzed the data and drafted the paper. WC, YW, XW, JC, HZ, WS, FZ, HS, and DL were mainly responsible for data collection and analysis. QW, JQ, CF, and LZ were primarily responsible for statistical analysis. KX, ZL, and JZ contributed to study design and revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (81871263, 81930005, 82070127), Natural Science Foundation of Jiangsu Province (BK2020022348), and the Postgraduate Research & Practice Innovation Program of Jiangsu (KYCX19-2229).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.691064/full#supplementary-material
1. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated With Immune Effector Cells. Biol Blood Marrow Transplant (2019) 25:625–38. doi: 10.1016/j.bbmt.2018.12.758
2. Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity After Adoptive Immunotherapy With CD19 CAR-T Cells. Cancer Discov (2017) 7:1404–19. doi: 10.1158/2159-8290.CD-17-0698
3. Wang Y, Qi K, Cheng H, Cao J, Shi M, Qiao J, et al. Coagulation Disorders After Chimeric Antigen Receptor T Cell Therapy: Analysis of 100 Patients With Relapsed and Refractory Hematologic Malignancies. Biol Blood Marrow Transplant (2020) 26:865–75. doi: 10.1016/j.bbmt.2019.11.027
4. Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric Antigen Receptor T-Cell Therapy - Assessment and Management of Toxicities. Nat Rev Clin Oncol (2018) 15:47–62. doi: 10.1038/nrclinonc.2017.148
5. Brudno JN, Kochenderfer JN. Toxicities of Chimeric Antigen Receptor T Cells: Recognition and Management. Blood (2016) 30:3321–30:127. doi: 10.1182/blood-2016-04-703751
6. Cao J, Wang G, Cheng H, Chen W, Qi K, Sang W, et al. Potent Anti-Leukemia Activities of Humanized CD19-Targeted Chimeric Antigen Receptor T (CAR-T) Cells in Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia. Am J Hematol (2018) 93:851–8. doi: 10.1002/ajh.25108
7. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 [National Institutes of Health Web site]. (2010). Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
8. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third Universal Definition of Myocardial Infarction. Eur Heart J (2012) 33:2551–67. doi: 10.1093/eurheartj/ehs184
9. Alvi RM, Frigault MJ, Fradley MG, Jain MD, Mahmood SS, Awadalla M, et al. Cardiovascular Events Among Adults Treated With Chimeric Antigen Receptor T-Cells (CAR-T). J Am Coll Cardiol (2019) 24:3099–108:74. doi: 10.1016/j.jacc.2019.10.038
10. Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current Concepts in the Diagnosis and Management of Cytokine Release Syndrome. Blood (2014) 10:188–95:124. doi: 10.1182/blood-2014-05-552729
11. Charlson M, Pompei P, Ales KL, MacKenzie CR. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J Chronic Dis (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
12. Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR-T Cell-Induced Cytokine Release Syndrome Is Mediated by Macrophages and Abated by IL-1 Blockade. Nat Med (2018) 24:731–8. doi: 10.1038/s41591-018-0041-7
13. Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, et al. Intent to Treat Leukemia Remission by CD19CAR T Cells of Defined Formulation and Dose in Children and Young Adults. Blood (2017) 129:3322–31. doi: 10.1182/blood-2017-02-769208
14. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N Engl J Med (2014) 371:1507–17. doi: 10.1056/NEJMoa1407222
15. Shalabi H, Sachdev V, Kulshreshtha A, Cohen JW, Yates B, Rosing DR, et al. Impact of Cytokine Release Syndrome on Cardiac Function Following CD19 CAR-T Cell Therapy in Children and Young Adults With Hematological Malignancies. J Immunother Cancer (2020) 8:e001159. doi: 10.1136/jitc-2020-001159
16. Ganatra S, Redd R, Hayek SS, Parikh R, Azam T, Yanik GA, et al. Chimeric Antigen Receptor T-Cell Therapy-Associated Cardiomyopathy in Patients With Refractory or Relapsed Non-Hodgkin Lymphoma. Circulation (2020) 142:1687–90. doi: 10.1161/CIRCULATIONAHA.120.048100
17. Lefebvre B, Kang Y, Smith AM, Frey NV, Carver JR, Scherrer-Crosbie M. Cardiovascular Effects of CAR T Cell Therapy: A Retrospective Study. JACC CardioOncol (2020) 2:193–203. doi: 10.1016/j.jaccao.2020.04.012
18. Li H, Yin L, Wang Y, Wang X, Shi M, Cao J, et al. Safety and Efficacy of Chimeric Antigen Receptor T-Cell Therapy in Relapsed/Refractory Multiple Myeloma With Renal Impairment. Bone Marrow Transplant (2020) 55:2215–8. doi: 10.1038/s41409-020-0930-5
19. Hogenhuis J, Voors AA, Jaarsma T, Hoes AW, Hillege HL, Kragten JA, et al. Anaemia and Renal Dysfunction are Independently Associated With BNP and NT-proBNP Levels in Patients With Heart Failure. Eur J Heart Fail (2007) 9:787–94. doi: 10.1016/j.ejheart.2007.04.001
20. Locke FL, Neelapu SS, Bartlett NL, Siddiqi T, Chavez JC, Hosing CM, et al. Phase 1 Results of ZUMA-1: A Multicenter Study of KTE-C19 Anti-CD19 CAR T Cell Therapy in Refractory Aggressive Lymphoma. Mol Ther (2017) 25:285–95. doi: 10.1016/j.ymthe.2016.10.020
21. Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E. B Cell Maturation Antigen-Specific CAR T Cells are Clinically Active in Multiple Myeloma. J Clin Invest (2019) 129:2210–21. doi: 10.1172/JCI126397
22. Jiang H, Liu L, Guo T, Wu Y, Ai L, Deng J, et al. Improving the Safety of CAR-T Cell Therapy by Controlling CRS-Related Coagulopathy. Ann Hematol (2019) 98:1721–32. doi: 10.1007/s00277-019-03685-z
23. Gkaliagkousi E, Gavriilaki E, Triantafyllou A, Douma S. Clinical Significance of Endothelial Dysfunction in Essential Hypertension. Curr Hypertens Rep (2015) 17:85. doi: 10.1007/s11906-015-0596-3
24. Nagle SJ, Murphree C, Raess PW, Schachter L, Chen A, Hayes-Lattin B. Prolonged Hematologic Toxicity Following Treatment With Chimeric Antigen Receptor T Cells in Patients With Hematologic. Am J Hematol (2021) 96:455–61. doi: 10.1002/ajh.26113
25. Winkler U, Jensen M, Manzke O, Schulz H, Diehl V, Engert A. Cytokine-Release Syndrome in Patients With B-Cell Chronic Lymphocytic Leukemia and High Lymphocyte Counts After Treatment With an Anti-CD20 Monoclonal Antibody (Rituximab, IDEC-C2B8). Blood (1999) 94:2217–24. doi: 10.1182/blood.V94.7.2217.419k02_2217_2224
26. Damoulis PD, Hauschka PV. Nitric Oxide Acts in Conjunction With Proinflammatory Cytokines to Promote Cell Death in Osteoblasts. J Bone Miner Res (1997) 12:412–22. doi: 10.1359/jbmr.1997.12.3.412
27. Saini NK, Sinha R, Singh P, Sharma M, Pathak R, Rathor N, et al. Mce4A Protein of Mycobacterium Tuberculosis Induces Pro Inflammatory Cytokine Response Leading to Macrophage Apoptosis in a TNF-Alpha Dependent Manner. Microb Pathog (2016) 100:43–50. doi: 10.1016/j.micpath.2016.08.038
28. Gardner RA, Ceppi F, Rivers J, Annesley C, Summers C, Taraseviciute A, et al. Preemptive Mitigation of CD19 CAR T-Cell Cytokine Release Syndrome Without Attenuation of Antileukemic Efficacy. Blood (2019) 134:2149–58. doi: 10.1182/blood.2019001463
29. Urwyler SA, Blum CA, Coslovsky M, Mueller B, Schuetz P, Christ-Crain M. Cytokines and Cortisol - Predictors of Treatment Response to Corticosteroids in Community-Acquired Pneumonia? J Intern Med (2019) 286:75–87. doi: 10.1111/joim.12891
30. Ouisse LH, Remy S, Lafoux A, Larcher T, Tesson L, Chenouard V, et al. Immunophenotype of a Rat Model of Duchenne’s Disease and Demonstration of Improved Muscle Strength After Anti-CD45RC Antibody Treatment. Front Immunol (2019) 10:2131. doi: 10.3389/fimmu.2019.02131
31. Turner MJ, Dauletbaev N, Lands LC, Hanrahan JW. The Phosphodiesterase Inhibitor Ensifentrine Reduces Production of Proinflammatory Mediators in Well Differentiated Bronchial Epithelial Cells by Inhibiting PDE4. J Pharmacol Exp Ther (2020) 375:414–29. doi: 10.1124/jpet.120.000080
32. Löwenberg M, Stahn C, Hommes DW, Buttgereit F. Novel Insights Into Mechanisms of Glucocorticoid Action and the Development of New Glucocorticoid Receptor Ligands. Steroids (2008) 73:1025–9. doi: 10.1016/j.steroids.2007.12.002
33. Rivière S, Galicier L, Coppo P, Marzac C, Aumont C, Lambotte O, et al. Reactive Hemophagocytic Syndrome in Adults: A Retrospective Analysis of 162 Patients. Am J Med (2014) 127:1118–25. doi: 10.1016/j.amjmed.2014.04.034
34. Li F, Yang Y, Jin F, Dehoedt C, Rao J, Zhou Y, et al. Clinical Characteristics and Prognostic Factors of Adult Hemophagocytic Syndrome Patients: A Retrospective Study of Increasing Awareness of a Disease From a Single-Center in China. Orphanet J Rare Dis (2015) 10:20. doi: 10.1186/s13023-015-0224-y
Keywords: CAR-T cell therapy, cardiac disorders, CRS, corticosteroids, tocilizumab
Citation: Qi K, Yan Z, Cheng H, Chen W, Wang Y, Wang X, Cao J, Zhang H, Sang W, Zhu F, Sun H, Li D, Wu Q, Qiao J, Fu C, Zeng L, Li Z, Zheng J and Xu K (2021) An Analysis of Cardiac Disorders Associated With Chimeric Antigen Receptor T Cell Therapy in 126 Patients: A Single-Centre Retrospective Study. Front. Oncol. 11:691064. doi: 10.3389/fonc.2021.691064
Received: 05 April 2021; Accepted: 26 May 2021;
Published: 14 June 2021.
Edited by:
Gurvinder Kaur, All India Institute of Medical Sciences, IndiaReviewed by:
Narendranath Epperla, The Ohio State University, United StatesCopyright © 2021 Qi, Yan, Cheng, Chen, Wang, Wang, Cao, Zhang, Sang, Zhu, Sun, Li, Wu, Qiao, Fu, Zeng, Li, Zheng and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kailin Xu, bGlobWRAMTYzLmNvbQ==; Junnian Zheng, am56aGVuZ0B4em1jLmVkdS5jbg==; Zhenyu Li, bGl6aGVueXVtZEAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.