- 1Health Management Center, The First Affiliated Hospital, University of South China, Hengyang, China
- 2Department of Spine Surgery, The Second Xiangya Hospital, Central South University, Changsha, China
- 3Department of Radiation Oncology, Indiana University School of Medicine, IU Simon Comprehensive Cancer Center, Indianapolis, IN, United States
- 4Department of Spine Surgery, The First Affiliated Hospital, University of South China, Hengyang, China
Background: Currently, the clinicopathological and prognostic characteristics of dedifferentiated chordoma (DC) and poorly differentiated chordoma (PDC) remain poorly understood. In this study, we sought to characterize clinicopathological parameters in a large PDC/DC cohort and determine their correlations with progression-free survival (PFS) and overall survival (OS) of patients. We also attempted to compare clinical features between PDC/DC and conventional chordoma (CC).
Methods: Literature searches (from inception to June 01, 2020) using Medline, Embase, Google Scholar and Wanfang databases were conducted to identify eligible studies according to predefined criteria. The local database at our center was also retrospectively reviewed to include CC patients for comparative analysis.
Results: Fifty-eight studies from the literature and 90 CC patients from our local institute were identified; in total, 54 PDC patients and 96 DC patients were analyzed. Overall, PDC or DC had distinct characteristics from CC, while PDC and DC shared similar clinical features. Adjuvant radiotherapy and chemotherapy were associated with both PFS and OS in PDC patients in the univariate and/or multivariate analyses. In the DC cohort, tumor resection type, adjuvant chemotherapy and tumor dedifferentiation components significantly affected PFS, whereas none of them were predictive of outcome in the multivariate analysis. By analyzing OS, we found that surgery, resection type and the time to dedifferentiation predicted the survival of DC patients; however, only surgery remained significant after adjusting for other covariables.
Conclusions: These data may offer useful information to better understand the clinical characteristics of PDC/DC and may be helpful in improving the outcome prediction of patients.
Introduction
Chordoma is a rare and locally invasive malignant mesenchymal tumor that is considered to originate from embryonic residual notochordal tissues (1, 2). Chordoma accounts for approximately 1-4% of all bone tumors (3) and has a population-based incidence rate of 0.8/1000000 (4). The peak age for chordoma onset ranges from 60 to 70 years (5–8). Chordomas most commonly involve the sacrococcygeal or skull base (9), and cases occurring outside the axial skeleton have also been reported (10). Chordoma is unresponsive to traditional radiotherapy and chemotherapy; therefore, surgery constitutes the main treatment of choice. Due to its infiltrative nature and proximity to key neurovascular structures, complete resection of chordoma lesions may be challenging (11, 12). Therefore, the risk of tumor recurrence after surgery is high, and 40-60% of patients may even have distant metastasis during the course of disease, exerting a significant adverse effect on the quality of life and survival of patients (1, 13).
Histologically, chordomas can be divided into three subtypes: classical, chondroid and dedifferentiated types (14). Specifically, conventional chordoma (CC) is microscopically characterized by the presence of physaliferous cells in the mucous matrix, while chondroid chordoma contains both chordoma and chondroma components. By contrast, dedifferentiated chordoma (DC) is determined when sarcomatous elements emerge within CC tissues (14). Recently, a new chordoma subtype called poorly differentiated chordoma (PDC) has been proposed based on its clinicopathological and/or genetic features (6). Unlike other subtypes, PDC has no physaliferous cells or rich myxoid matrix on microscopy, although obvious atypia and active mitosis are commonly observed. In addition, it has also been suggested that Brachyury expression is positive, while the expression of SMARCB1/INI-1 is commonly absent in PDC tissues (6, 14, 15).
Currently, reports on the clinical characteristics of and prognostic factors for CC have been widely documented in the literature (16–18). Furthermore, studies have also shown that the prognosis of chondroid chordoma is better than that of CC (19). However, due to the rarity, studies addressing PDC/DC are limited in the literature, with most of them being single case reports or small sample case series. Therefore, little is known about the detailed clinicopathological features of PDC/DC, and no summary of the complete prognostic factors in these two chordoma subtypes has been reported. Previous studies have shown that compared with CC, PDC/DC are highly aggressive with worse patient survival (20–22). Considering the dismal prognosis of PDC/DC, systematically summarizing their prognostic factors may be helpful in stratifying patients and guiding therapeutic optimization, thereby improving patient survival. In this study, we sought to characterize the clinicopathological data of a large PDC/DC cohort and explore factors affecting the progression-free survival (PFS) and overall survival (OS) of patients. We also attempted to analyze the differences in clinicopathological and prognostic features between PDC/DC and CC.
Methods and Materials
Literature Review
Electronic searches of MEDLINE, Embase, Google Scholar and Wanfang databases were performed from inception to June 1, 2020, to identify eligible studies. The keywords used for searching were (“chordoma” or “chordomas”) and (“poorly differentiated” or “anaplastic” or “dedifferentiated” or “dedifferentiation” or “sarcomatous” or “sarcomatoid” or “transformation” or “malignant fibrous histiocytoma”). No other restrictions were put on the above searching terms in order to obtain comprehensive outcomes and avoid omissions. Bibliographies of the included studies were also manually checked to find any additional eligible records. The detailed search strategy is depicted in Figure 1. We included studies that assessed PDC/DC cases originally occurring in the spine or the skull base areas. Tumor diagnosis was confirmed by pathological and immuno- histochemical findings. Studies were restricted to English or Chinese reports involving human beings. The study exclusion criteria were as follows: no clinical information recorded for patients, duplicate publications, unconfirmed diagnosis of PDC/DC, and failure to provide survival data.
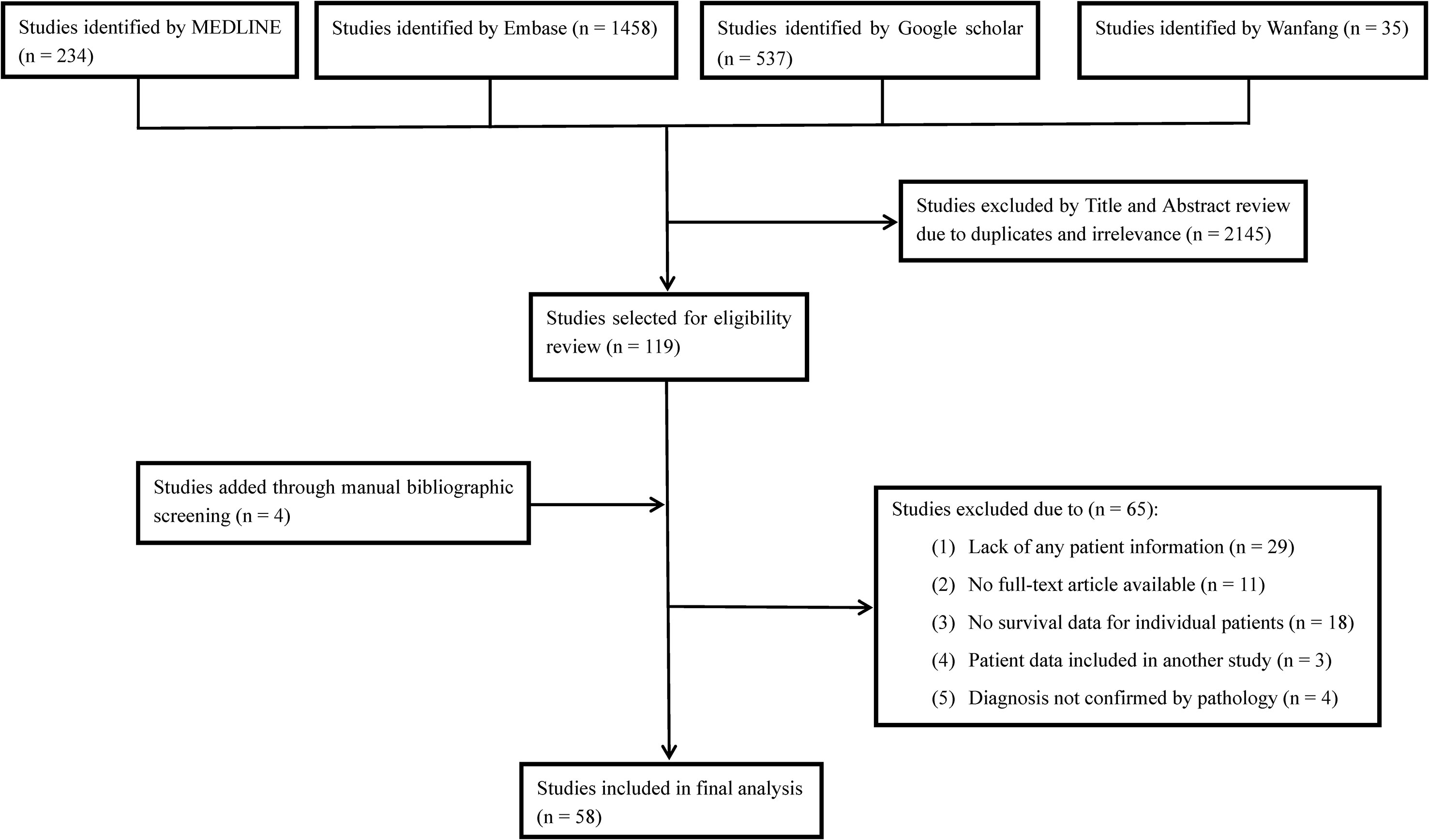
Figure 1 Flow diagram of literature search showing studies identified, included and excluded at each stage.
Two investigators independently selected eligible studies according to predefined criteria and extracted data from each included study. These data included the following: patient information (age, sex, duration of symptoms), tumor features [including location, size, dedifferentiation type (de novo or secondary), treatment history before tumor dedifferentiation 9such as surgery or radiotherapy), dedifferentiation components and time to dedifferentiation], immunohistochemical results of proteins expression (including Brachyury, pancytokeratin [CK], S-100, vimentin and epithelial membrane antigen [EMA]), treatment (including surgery or not, the type of resection, adjuvant radiotherapy and chemotherapy), endpoint events (tumor progression and death) and time to critical events. The type of resection was determined as wide (such as gross total or en bloc resection with negative margins) or not wide (including intralesional or marginal resection) according to a previously described method (11). The main data of interest were PFS and OS. PFS was defined as the time interval from the date of diagnosis to the earliest date of disease progression (local and distant) or death from all causes if no progression was observed (16). Similarly, OS was defined as the interval from the date of diagnosis to death related to all causes or to the date of last follow-up if the patient was alive or lost to follow-up. For subsequent statistical analysis, immunohistochemical results were simply classified into two subgroups according to a published method (10): the high expression group (defined when the protein was diffusely or strongly positive in tumor tissues) and the low expression group (determined when the protein was weakly or focally positive and negative in tumor tissues). For patients with DC, the time to dedifferentiation was defined as the period from initial diagnosis of CC to the first appearance of dedifferentiated sarcomatous component in tumor tissues by pathological examinations.
Local Cohort
A retrospective review of our pathological database was conducted to identify patients at our center. A total of 90 patients with CC who underwent radical surgery at our institute from June 2002 to November 2018 were included for a comparative analysis with PDC or DC cohort. However, no PDC or DC cases were identified in our institute. Seventy-five of the CC patients had been previously described in our study (10). All patients received regular clinical and radiological examinations after surgery, and the follow-up information was updated in April 2019. The tumor diagnosis, pathological classification and resection type were evaluated by two experienced neuropathologists based on the findings in HE-staining sections of intraoperatively resected specimens. Expression levels of proteins in 75 patients were directly obtained from our published study (10), and the data of 15 newly added patients were retrieved by immunohistochemical analysis. To ensure comparability, the same antibodies (Supplemental Digital Content 1), dilution ratio and staining procedures were used during the experiment. Protein expression data were separated into two groups (low and high expression) according to the criteria described above. Patient demographics, treatment history, clinical outcomes and tumor features were obtained from medical records. Observations were censored when a patient was progression-free (PFS analysis) or alive (OS analysis).
Statistical Analysis
All statistical analyses were performed using R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria) and the “surv_cutpoint” function in the “survminer” package was used to determine cut-off values for patient age (in PDC cohort), as well as duration of symptoms and time to tumor dedifferentiation (both in DC data) in survival analysis with OS as the outcome parameter (Supplemental Digital Content 2) (23). Specifically, the threshold value was defined as the point with the minimum P value in the log-rank test, which was subsequently corrected to avoid overstating the significance (24). For analysis, the data of patient age in other two cohorts (≤ 50 yr or > 50 yr) and tumor size in all cohorts (≤ 5 cm or > 5 cm), as well as duration of symptoms in CC cohort (≤ 36.5 mo or > 36.5 mo) were separated into two groups as previously suggested (10). Continuous data are expressed as the mean ± standard deviation and were analyzed by Student’s t-test or one-way ANOVA. Categorical data are summarized as the frequency or composition ratio and were analyzed by chi-square test. Univariate survival analysis was performed by Kaplan-Meier curves using the log-rank test to compare survival probabilities between groups. Multivariate survival analysis was conducted with a Cox proportional hazards model to identify independent predictive factors of PFS and OS. This analysis included variables that were statistically significant in our univariate analysis as well as the prognostic factors reported in the literature (14, 21). All tests were two-sided, and a P < 0.05 was considered statistically significant.
Results
Patient and Tumor Features of the PDC, DC, and CC Cohorts
A total of 58 studies (Supplemental Digital Content 3) were finally included, resulting in 150 PDC/DC cases for analysis, including 54 PDC cases and 96 DC cases. For comparative analysis, 90 CC cases were also included in the study. The patient characteristics are detailed in Table 1. Most of the included studies did not offer complete clinicopathological data for patients.
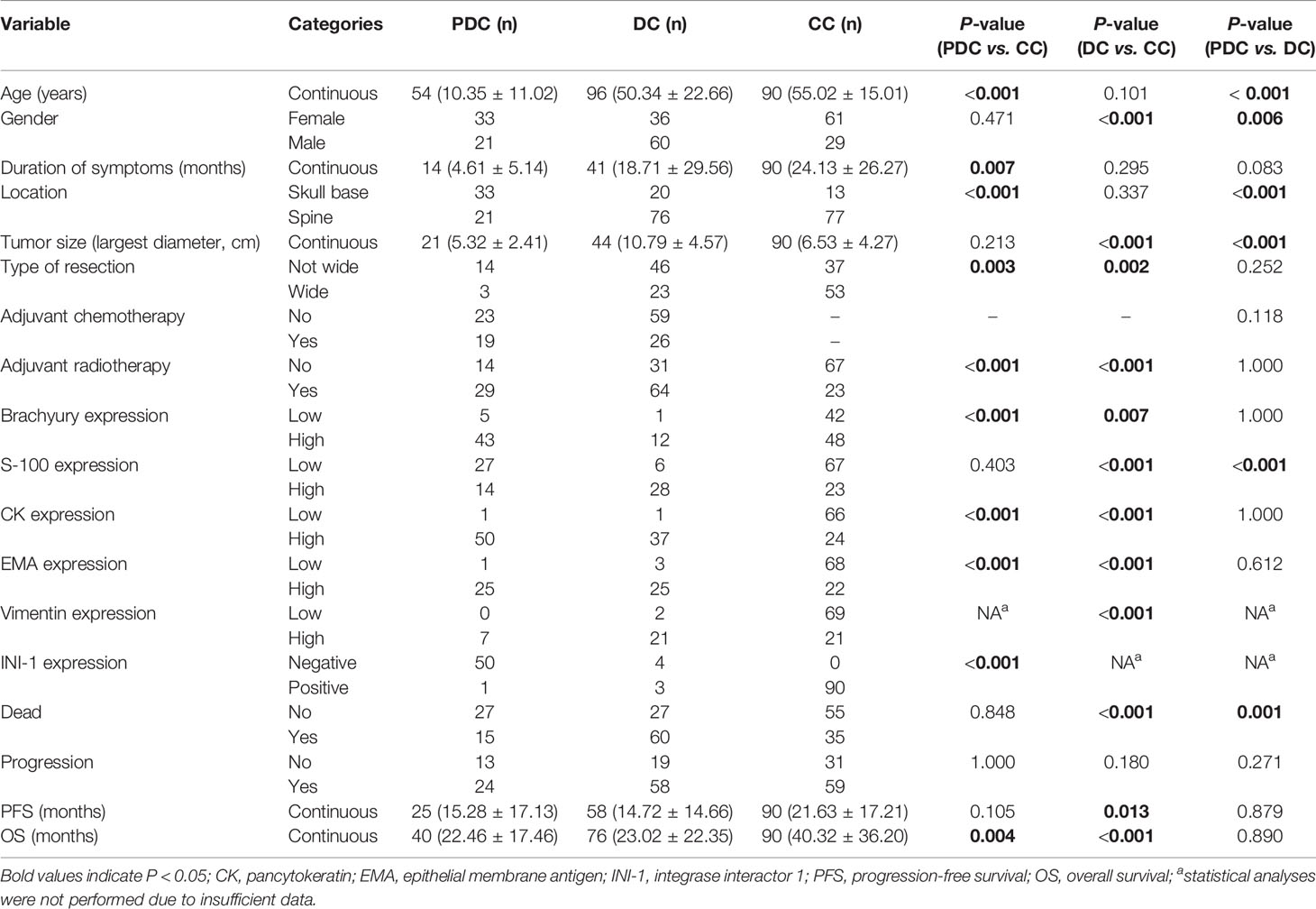
Table 1 Comparison of baseline characteristics among patients with classic chordoma (CC, n = 90), dedifferentiated chordoma (DC, n = 96) and poorly differentiated chordoma (PDC, n = 54).
PDC Cohort
In the PDC cohort, 39 patients (72.2%) underwent surgery, and of them, 3 (5.6%) had wide resection, 14 (25.9%) had nonwide resection, and the remaining 22 had an unknown type of surgical resection. Nineteen patients received chemotherapy (13 were treated with unknown chemotherapy regimens; four, with tazemetostat; one, with doxorubicin, vincristine and ifosfamide; and one, with tazemetostat, nivolumab and ipilumimab), while 23 patients did not receive chemotherapy. Twenty-nine patients received adjuvant radiotherapy (12 were treated with unknown radiotherapy types; 5, with pencil beam scanning; 3, with conventional photon radiotherapy; 2, with 3D conformal radiation therapy; and 7, with combinatorial adjuvant radiotherapy), and 14 patients did not. The median PFS was 17.0 months, and the 1-year, 3-year, and 5-year PFS rates were 56%, 27%, and 14%, respectively. The median OS was 52.0 months, and the 1-, 3- and 5-year OS rates were 80%, 51%, and 34%, respectively.
DC Cohort
In the DC cohort, 29 patients had treatment history before tumor dedifferentiation (9 patients underwent tumor surgical resection, 8 received adjuvant radiotherapy, and 12 had both tumor resection and radiotherapy), and 67 patients did not. The average time to tumor dedifferentiation was 5.74 ± 6.53 years. Fifty-eight DC cases had dedifferentiation component data available. Of them, 18 tumors had fibrous sarcomatous components (including 11 with malignant fibrous histiocytomas, 5 with fibrosarcomas and 2 with both types), and 40 harbored nonfibrous sarcomatous elements (including 4 with osteosarcomas, 1 with rhabdomyoblastoma, 2 with rhabdomyosarcomas and 33 with other sarcomas). Twenty-three patients (24.0%) underwent wide resection, and 46 patients (47.9%) had nonwide resection. Twenty-six patients (27.1%) received chemotherapy (6 were treated with unknown chemotherapy drugs; two, with doxorubicin; one, with etoposide; and seventeen, with combinatorial adjuvant chemotherapy), and 59 patients did not. Sixty-four patients (66.7%) received adjuvant radiotherapy (including 20 treated with unknown radiotherapy approaches; 26, with traditional photon radiotherapy; 9, with proton beam radiation; 5, with external beam radiotherapy; 3, with carbon-ion radiotherapy; and one, with intensity-modulated radiotherapy), while 31 patients did not receive radiotherapy. The median PFS was 11.7 months, and the 1-, 3- and 5-year PFS rates were 48%, 17%, and 7%, respectively. The median OS was 26.0 months, and the OS rates at 1 year, 3 years and 5 years were 59%, 43% and 19%, respectively.
CC Cohort
Clinicopathological data of 90 CC patients have been partially communicated by our group (10, 23). No patients received chemotherapy. All patients underwent surgery. Of them, 53 (58.9%) underwent wide resection, and 37 (41.1%) had nonwide resection. Twenty-three patients (25.6%) received adjuvant radiotherapy (including 13 treated with traditional photon radiotherapy and 10 treated with intensity-modulated radiotherapy), and 67 patients (74.4%) did not. The median PFS was 22.0 months, and the 1-, 3- and 5-year PFS rates were 73%, 20% and 14%, respectively. The median OS was 73.0 months, and the OS rates at 3 years, 5 years and 10 years were 60%, 51% and 39%, respectively.
Comparison of Clinicopathological Characteristics Among Patients With Different Chordoma Subtypes
The comparative results of clinicopathological features between PDC, DC and CC patients are shown in Table 1. Overall, we found significantly different clinicopathological characteristics between the PDC or DC and CC groups, while PDC and DC displayed overlap in their clinical data. Of note, however, as no CC patients received adjuvant chemotherapy, we therefore did not analyze chemotherapy data in this group. In addition, our analysis revealed that the PDC and DC cohorts had more patients undergoing adjuvant radiotherapy than the CC group. However, this outcome might be biased by the fact that the CC cohort included only small number of patients with skull base chordoma who were more likely to be treated with radiotherapy after surgery.
Immunohistochemical results showed that protein biomarkers were differentially expressed among the three chordoma subtypes (Table 1). Specifically, INI-1 protein was negatively expressed in most PDC tissues, while all CC cases had positive INI-1 expression (Supplemental Digital Content 4), suggesting that INI-1 is a reliable maker for PDC diagnosis. However, due to the lack of enough INI-1 expression information in DC, we therefore did not assess these data in this group. This was also the case for tumoral vimentin expression in the PDC cohort.
Regarding clinical outcomes, we found that the PFS of DC patients was significantly shorter than that of CC patients. In addition, a significantly worse OS was observed in the PDC and DC groups than in the CC cohort. Similarly, Kaplan-Meier curves by the log-rank test also revealed significant differences in terms of PFS and OS among the three chordoma subtypes (Figure 2). Further subgroup analysis revealed that there was a remarkable difference in PFS between the DC and CC groups (median PFS: 11.7 vs 22.0 mo, P = 0.012). Similarly, a significant difference was also seen in the OS between the DC and CC groups (median OS: 26.0 vs 73.0 mo, P < 0.001), but there was only a borderline significant survival difference between the PDC and DC groups (median OS: 52.0 vs 26.0 mo, P = 0.062).
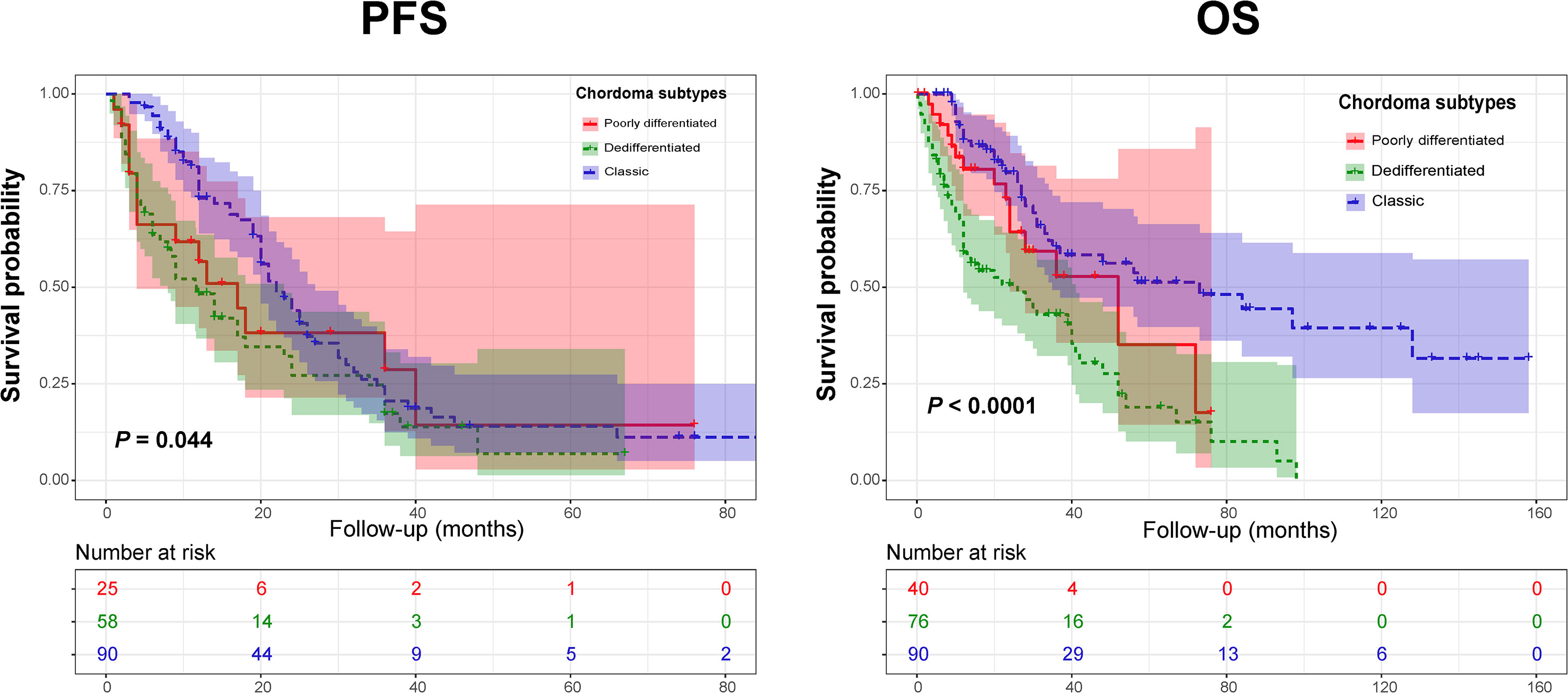
Figure 2 Kaplan-Meier curves of progression-free survival (Left) and overall survival (Right) of chordoma patients stratified by tumor pathology subtypes.
Univariate and Multivariate Analyses of Prognostic Factors in PDC and DC Patients
PDC Cohort
In the PDC cohort, univariate Kaplan-Meier analysis showed that adjuvant radiotherapy and chemotherapy were closely related to a good PFS (Figures 3A, B and Table 2). Similarly, our analysis found that adjuvant radiotherapy was positively associated with OS (Figure 3C and Table 2). In addition, our results also revealed a marginally significant effect of adjuvant chemotherapy on patients’ OS (χ2 = 3.221, P = 0.073) (Table 2). Multivariate Cox regression analysis showed that adjuvant radiotherapy and chemotherapy could independently predict a good PFS (Table 3), while only radiotherapy was an independent predictor of OS (Table 3).
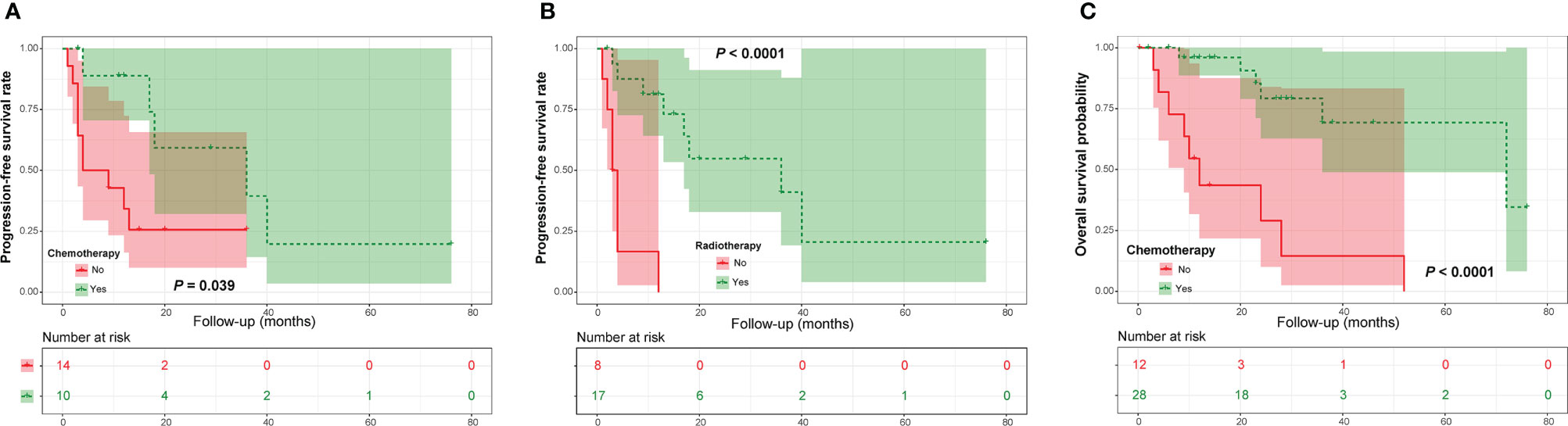
Figure 3 Kaplan-Meier curves of progression-free survival of patients with poorly differentiated chordoma stratified by adjuvant chemotherapy (A) and radiotherapy (B), as well as overall survival of patients stratified by adjuvant chemotherapy (C).
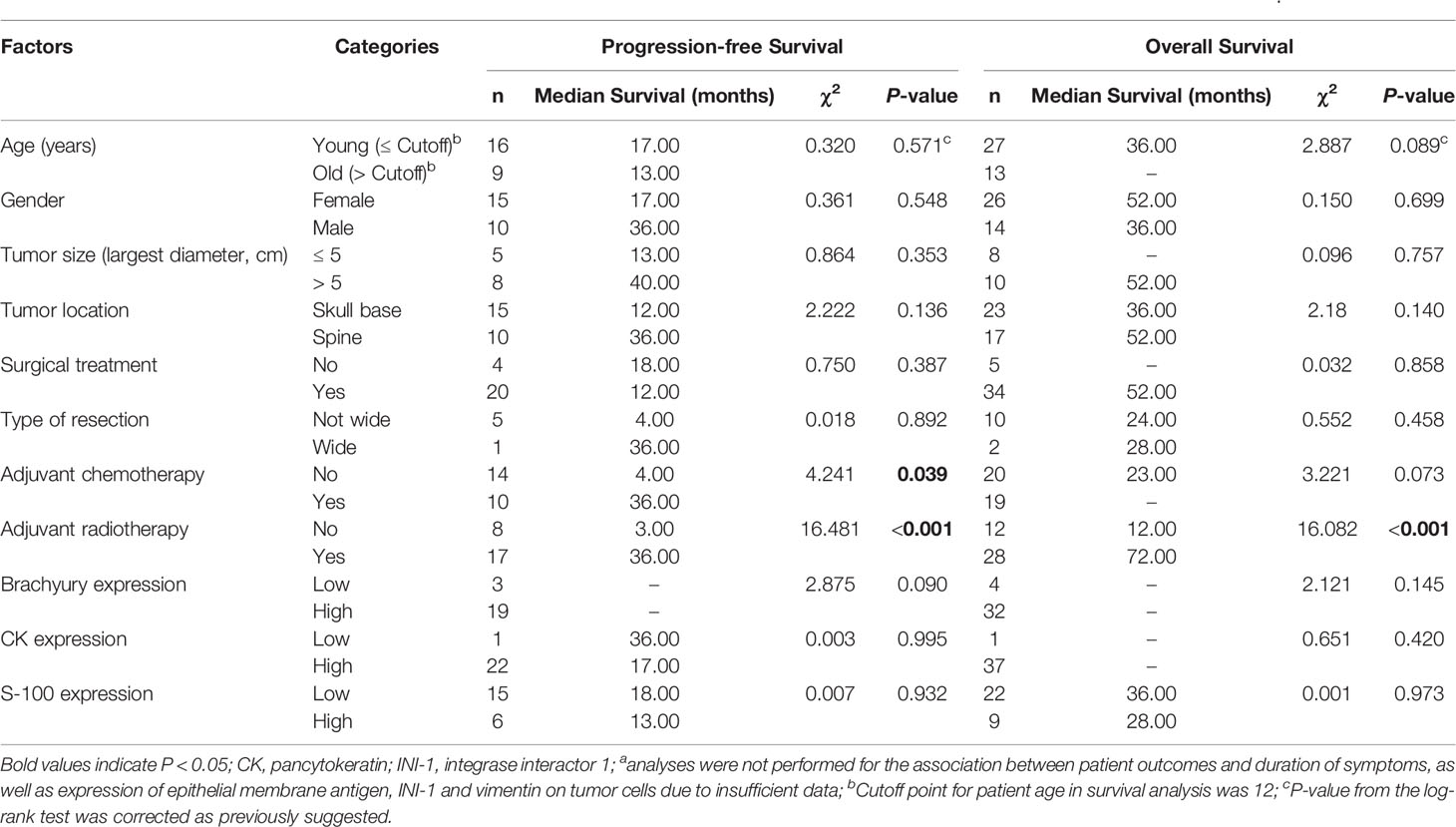
Table 2 Univariate analyses of prognostic factors for progression-free survival and overall survival in patients with poorly differentiated chordomaa.

Table 3 Multivariate Cox proportional hazard analyses of prognostic factors for progression-free survival and overall survival in patients with poorly differentiated chordoma.
DC Cohort
In the DC group, we found that a nonfibrous dedifferentiated component and wide tumor resection were associated with a good PFS (Figures 4A, B and Table 4). However, in contrast to PDC, adjuvant chemotherapy negatively influenced the PFS of DC patients (Figure 4C and Table 4). By analyzing OS, we found that the time to tumor dedifferentiation, surgery and resection type were correlated with OS (Figures 4D–F and Table 4). Multivariate Cox analysis showed that only wide resection of tumors seemed to have a significant correlation with patients’ PFS (P = 0.098, Table 5). Similarly, this analysis only disclosed that surgical treatment was an independent predictor of OS (Table 5).
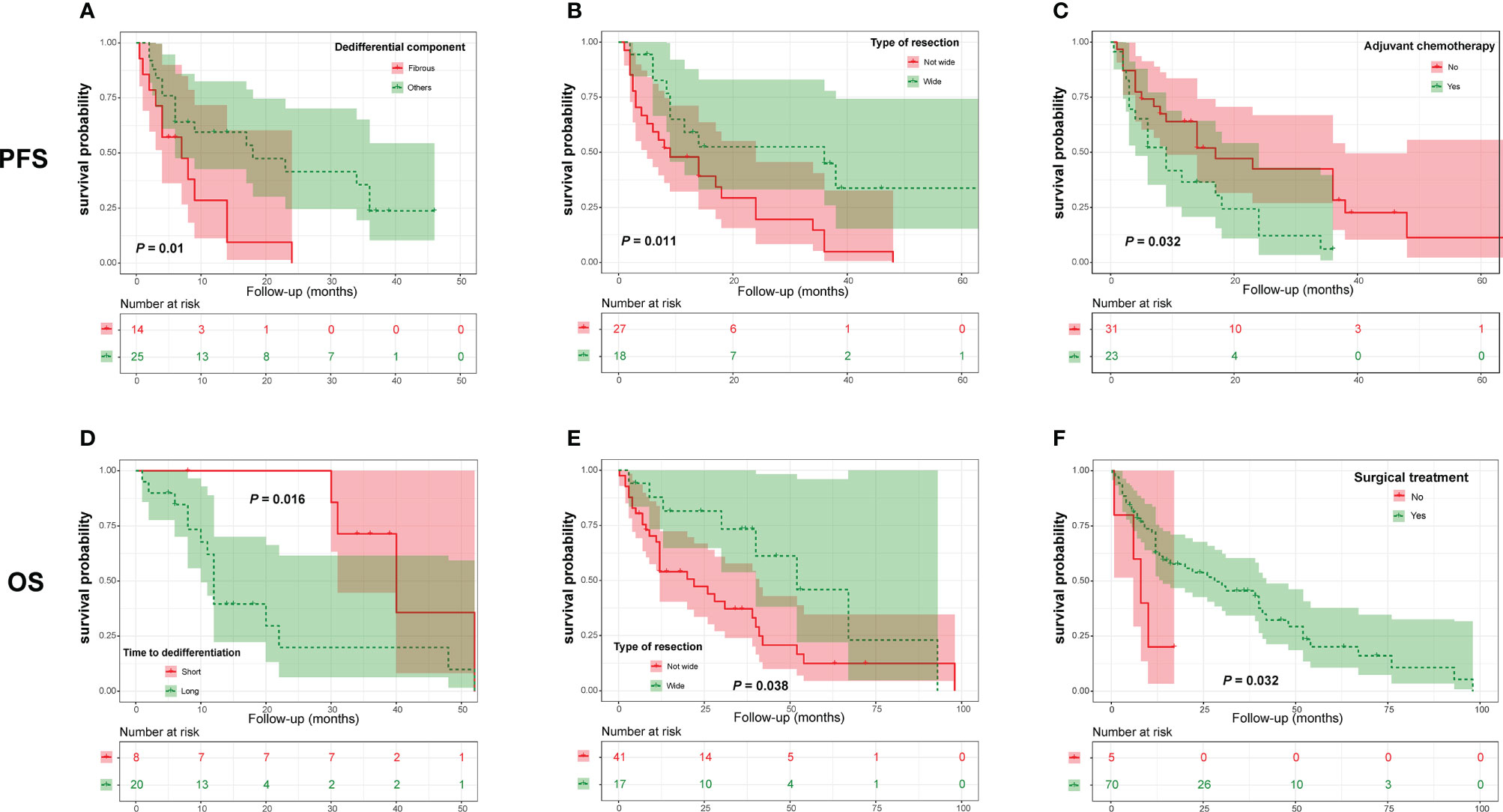
Figure 4 Kaplan-Meier curves of progression-free survival of patients with dedifferentiated chordoma stratified by dedifferentiation component (A), resection type (B) and adjuvant chemotherapy (C), as well as overall survival of patients stratified by time to tumor dedifferentiation (D), type of resection (E) and surgery (F).
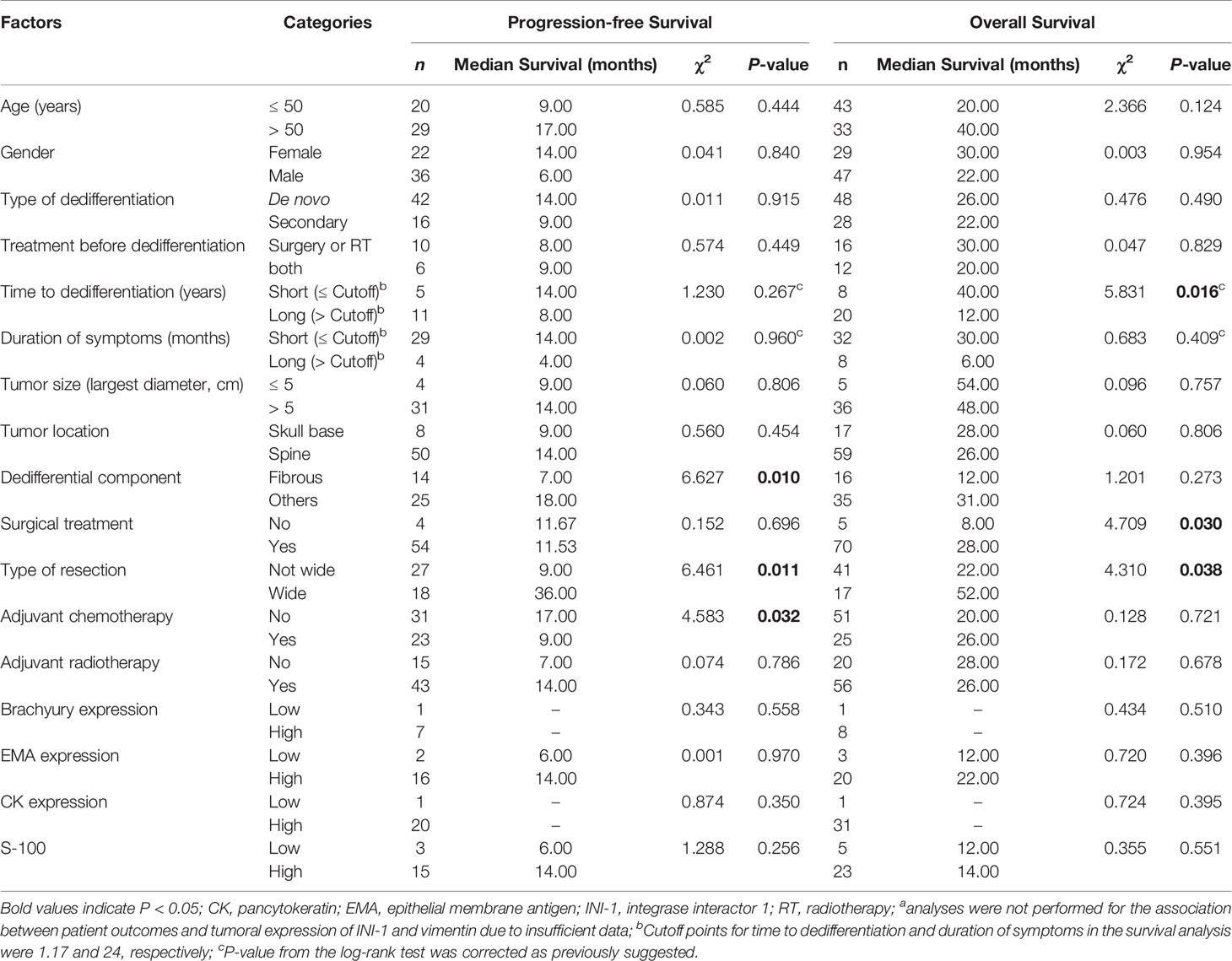
Table 4 Univariate analyses of prognostic factors for progression-free survival and overall survival in patients with dedifferentiated chordomaa.
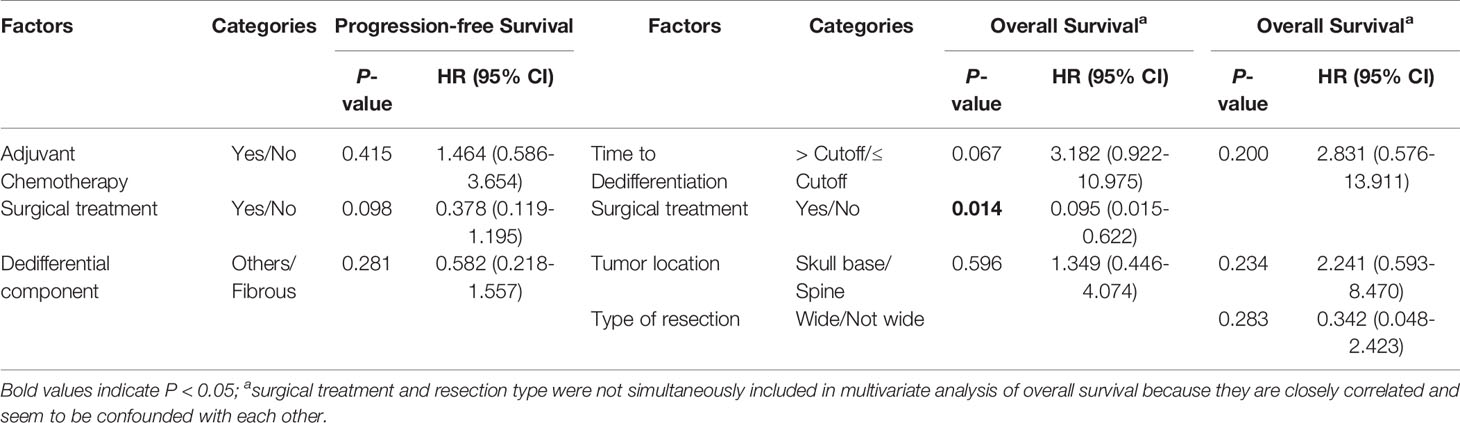
Table 5 Multivariate Cox proportional hazard analyses of prognostic factors for progression-free survival and overall survival in patients with dedifferentiated chordoma.
Univariate and Multivariate Analyses of Prognostic Factors in CC Patients
Prognostic factors in the CC group have been previously reported in our study (10, 23). After updating the sample size in the study, we found that gender, the duration of symptoms, tumor size, the type of resection, radiotherapy and tumoral Brachyury expression were significantly correlated with PFS (Supplemental Digital Contents 5-6). Moreover, this analysis revealed that the duration of symptoms, type of tumor resection and Brachyury expression significantly affected patients’ OS (Supplemental Digital Contents 6-7). Further Cox multivariate analysis showed that gender, the type of tumor resection, adjuvant radiotherapy and Brachyury expression were independent prognostic factors of PFS (Supplemental Digital Content 8), while the duration of symptoms, type of resection and Brachyury expression independently predicted OS (Supplemental Digital Content 8).
Discussion
In this study, we characterized the clinicopathological data and prognostic factors in a large PDC/DC cohort and compared their features with those of a CC cohort. We found that there were significantly distinct clinical features between PDC/DC and CC, while PDC and DC showed similarities except for patient age, gender, tumor location, size and S-100 expression. In the PDC group, adjuvant radiotherapy and chemotherapy were closely associated with patient outcomes. For DC patients, the type of tumor resection, adjuvant chemotherapy and tumor dedifferentiation components significantly affected PFS, while surgery, the type of resection and time to dedifferentiation predicted OS. These data may contribute to a comprehensive understanding of the clinicopathological and prognostic characteristics of PDC/DC and may be helpful in guiding prognostic risk stratification of and therapeutic optimization for patients.
Consistent with previous studies claiming that PDC and DC are aggressive and have a poor prognosis (6, 14, 17, 25, 26), we found that the prognosis of PDC/DC was poorer than that of CC, with DC patients having the shortest survival. These results may suggest different tumorigenesis mechanisms among PDC/DC and CC, thereby leading to different disease phenotypes and clinical outcomes. Given that PDC is more common in children, we speculate that the occurrence of PDC may be attributed to abnormal molecular mechanisms related to growth and development, which deserves further investigation. As DC can occur de novo or after previous treatments, future studies using single-cell sequencing by collecting tissue samples before and after tumor dedifferentiation or matched DC and CC specimens may be helpful in dissecting the precise molecular mechanism of DC development. Such studies are imperative, as they may provide insight into the development of new treatment strategies for DC, considering that the prognosis of this disease is dismal and that the current treatment is highly limited. Furthermore, these therapeutic choices may also be beneficial for PDC patients because PDC and DC shared marked similarity in clinical features as revealed by our data. Noticeably, however, we found no significant survival difference between patients with PDC and CC, in contrast to previous works (14, 20). This phenomenon may be caused by the small number of PDC patients with complete survival data, thus resulting in low statistical power.
Previous studies have confirmed that the type of tumor resection is the main factor affecting the clinical outcomes of chordoma patients (27–29). In contrast, however, we found no significant correlation between the type of resection and patient survival in PDC, which may be due to the small sample size in the survival analysis. It has been shown that PDC (especially for INI-1-negative cases) displays aggressive behavior and tends to have early distant metastasis (20, 30). These conditions may distort evaluation of the true effect of resection type on PDC outcomes. Another major finding of the study was that chemotherapy could improve the survival of PDC patients, similar to previous data (14). One possible reason may be that PDC has poor extracellular matrix; therefore, drugs can easily enter tumor cells to exert their antitumor effects. A recent study provided evidence in support of this conjecture (31). Published data have demonstrated that administration of radiotherapy has benefits in chordoma patients (32–34). Similarly, we found that radiotherapy significantly improved PDC prognosis and could also independently predict both patients’ PFS and OS. These findings together suggest that adjuvant radiotherapy should be performed whenever possible to enhance the survival of PDC patients. Nevertheless, it should be noted that as most of the included studies did not provide detailed information on radiotherapy, we were unable to evaluate the association between specific radiotherapy types and patient outcomes in PDC.
It has been described that dedifferentiated components in DC tissues may include malignant fibrous histiocytoma (35), fibrosarcoma (36), osteosarcoma (37), rhabdomyoblastoma (38) and rhabdomyosarcoma (39). Our study discovered that the presence of a nonfibrous dedifferentiation element had significantly positive prognostic implications in DC, which was in line with previous reports (40). Prior data have suggested that cancer-related fibroblasts can promote cancer development and progression by modulating the integrin signaling pathway (41–43), which has also been linked with tumor progression and metastasis (44). Considering that fibroblasts are the main cell types in DC-containing fibrous sarcomas, whether analogous molecular mechanisms exist in this pathology remains to be fully elucidated. In addition, our analysis found that the time to tumor dedifferentiation was closely related to the OS of DC patients. This is easy to understand because a short period of dedifferentiation may likely hint at a highly invasive tumor phenotype, thereby leading to adverse clinical outcomes. In agreement with published reports (17), we also revealed that wide resection of tumors was correlated with a favorable DC prognosis, with surgical treatment as the only independent predictor. These results highlight the importance of wide resection in the treatment of DC patients, which should be achieved whenever possible to prolong patient survival.
Interestingly, we showed that adjuvant chemotherapy negatively affected the PFS of patients with DC. The exact mechanism underlying this condition is unclear. Prior observations have indicated that chemotherapy can cause shedding of the syndecan-1 proteoglycan to accelerate tumor recurrence and progression (45). This finding may provide an explanatory basis for the poor survival of DC patients treated with chemotherapy. Further transcriptome sequencing studies using DC tissue samples before and after chemotherapy may help uncover the precise molecular mechanisms of how chemotherapy promotes DC progression. However, we failed to identify which chemotherapy drugs could lead to a poor DC prognosis because of insufficient information. Future studies with well-documented chemotherapy data are needed to clarify this issue in order to optimize therapy and improve survival. Additionally, this study found that radiotherapy had no significant effect on DC prognosis, similar to previous observations (27, 46). Studies have suggested that radiotherapy may promote DC progression (27). Considering these findings, we recommend that radiotherapy should be arranged with caution for DC patients, although further confirmation is necessary.
Limitations
Most studies did not provide complete clinicopathological data for PDC/DC patients, which may introduce bias into the results. Despite this, we performed a pooled analysis of individual patient-level data involving DC and PDC subjects from literature to present clinical characteristics of the two chordoma subtypes, which may possibly have implications for clinical decision making of patients. We did this because PDC or DC is a rare entity and collecting sufficient data for analysis in a single institute is difficult. In fact, this method is currently widely used in the literature and is an effective tool for the investigation of rare disease (47). Nevertheless, to reduce the heterogeneities between different studies or between literature data and our local cohort, allow for comparability and then make statistical feasible, we obtained complete and objective patient information (these data cannot have variations among studies, such as age, sex, tumor location, tumor size, etc.), and also simplified criteria for subjective variable grouping in subsequent analysis (divided into low or high and yes or no for most categorical variables). However, additional prospective multicenter cooperative studies with large sample sizes and complete data are still required to confirm our current findings.
Conclusion
The present study analyzed the clinicopathological characteristics and prognostic factors in a large number of PDC/DC patients and compared their clinical data with that of a CC population. We found that PDC/DC and CC had significantly different clinical features, while PDC and DC shared similarities. In the PDC cohort, adjuvant radiotherapy and chemotherapy were associated with patient survival. In contrast, for DC patients, the presence of a dedifferentiated component, time to tumor dedifferentiation, surgery and radiation therapy significantly affected survival. These data may contribute to a comprehensive understanding of the characteristics of PDC/DC and may be helpful in guiding prognostic risk stratification and therapeutic optimization of patients.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The study protocol was approved by the Institutional Review Board at The First Affiliated Hospital, University of South China, Hunan, P.R. China. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors participated in data acquisition. WH, G-HL, JL, and M-XZ contributed to the conception and design of the study. F-SL, B-WZ, WH, and M-XZ did the data analysis and interpretation. T-LZ, WH, Y-GY, and M-XZ contributed to drafting and revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (82003802 to T-LZ, 81871821 to JL and 82002364 M-XZ), Natural Science Foundation of Hunan Province (2019JJ50542 to T-LZ), Project for Clinical Research of Hunan Provincial Health Commission (20201978 to T-LZ and 20201956 to M-XZ) and China Scholarship Council (CSC201808430085 to T-LZ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dr. Yi Jiang and Dr. Xiao-Ling She from Department of Pathology, The Second Xiangya Hospital, Central South University for pathological analysis of the study. We also thank American Journal Experts for assistance in preparation of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.686565/full#supplementary-material
References
1. Williams BJ, Raper DM, Godbout E, Bourne TD, Prevedello DM, Kassam AB, et al. Diagnosis and Treatment of Chordoma. J Natl Compr Canc Netw (2013) 11(6):726–31. doi: 10.6004/jnccn.2013.0089
2. Whelan JS, Davis LE. Osteosarcoma, Chondrosarcoma, and Chordoma. J Clin Oncol (2018) 36(2):188–93. doi: 10.1200/JCO.2017.75.1743
3. Mavrogenis AF, Angelini A, Panagopoulos GN, Pala E, Calabrò T, Igoumenou VG, et al. Aggressive Chordomas: Clinical Outcome of 13 Patients. Orthopedics (2017) 40(2):e248–54. doi: 10.3928/01477447-20161108-02
4. Smoll NR, Gautschi OP, Radovanovic I, Schaller K, Weber DC. Incidence and Relative Survival of Chordomas: The Standardized Mortality Ratio and the Impact of Chordomas on a Population. Cancer (2013) 119(11):2029–37. doi: 10.1002/cncr.28032
5. McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: Incidence and Survival Patterns in the United States, 1973-1995. Cancer Causes Control (2001) 12(1):1–11. doi: 10.1023/A:1008947301735
6. Mobley BC, McKenney JK, Bangs CD, Callahan K, Yeom KW, Schneppenheim R, et al. Loss of SMARCB1/INI1 Expression in Poorly Differentiated Chordomas. Acta Neuropathol (2010) 120(6):745–53. doi: 10.1007/s00401-010-0767-x
7. Yadav R, Sharma MC, Malgulwar PB, Pathak P, Sigamani E, Suri V, et al. Prognostic Value of MIB-1, P53, Epidermal Growth Factor Receptor, and INI1 in Childhood Chordomas. Neuro Oncol (2014) 16(3):372–81. doi: 10.1093/neuonc/not228
8. Dei Tos AP. Unveiling the Molecular Pathogenesis of Chordoma: A New Paradigm for Molecular Targeting of Rare Cancers. J Pathol (2011) 223(5):565–6. doi: 10.1002/path.2847
9. Stacchiotti S, Sommer J. Chordoma Global Consensus G. Building a Global Consensus Approach to Chordoma: A Position Paper From the Medical and Patient Community. Lancet Oncol (2015) 16(2):e71–83. doi: 10.1016/S1470-2045(14)71190-8
10. Lv GH, Zou MX, Liu FS, Zhang Y, Huang W, Ye A, et al. Clinicopathological and Prognostic Characteristics in Extra-Axial Chordomas: An Integrative Analysis of 86 Cases and Comparison With Axial Chordomas. Neurosurgery (2019) 85(3):E527–42. doi: 10.1093/neuros/nyz073
11. Bettegowda C, Yip S, Lo SL, Fisher CG, Boriani S, Rhines LD, et al. Spinal Column Chordoma: Prognostic Significance of Clinical(Brachyury) Gene SNP Rs2305089 for Local Recurrence and Overall Survival. Neuro Oncol (2017) 19(3):405–13. doi: 10.1093/neuonc/now156
12. Hao S, Song H, Zhang W, Seldomridge A, Jung J, Giles AJ, et al. Protein Phosphatase 2A Inhibition Enhances Radiation Sensitivity and Reduces Tumor Growth in Chordoma. Neuro Oncol (2018) 20(6):799–809. doi: 10.1093/neuonc/nox241
13. Stacchiotti S, Gronchi A, Fossati P, Akiyama T, Alapetite C, Baumann M, et al. Best Practices for the Management of Local-Regional Recurrent Chordoma: A Position Paper by the Chordoma Global Consensus Group. Ann Oncol (2017) 28(6):1230–42. doi: 10.1093/annonc/mdx054
14. Shih AR, Cote GM, Chebib I, Choy E, DeLaney T, Deshpande V, et al. Clinicopathologic Characteristics of Poorly Differentiated Chordoma. Mod Pathol (2018) 31(8):1237–45. doi: 10.1038/s41379-018-0002-1
15. Yeter HG, Kosemehmetoglu K, Soylemezoglu F. Poorly Differentiated Chordoma: Review of 53 Cases. APMIS (2019) 127(9):607–15. doi: 10.1111/apm.12978
16. Tauziede-Espariat A, Bresson D, Polivka M, Bouazza S, Labrousse F, Aronica E, et al. Prognostic and Therapeutic Markers in Chordomas: A Study of 287 Tumors. J Neuropathol Exp Neurol (2016) 75(2):111–20. doi: 10.1093/jnen/nlv010
17. Zhou J, Sun J, Bai HX, Huang X, Zou Y, Tan X, et al. Prognostic Factors in Patients With Spinal Chordoma: An Integrative Analysis of 682 Patients. Neurosurgery (2017) 81(5):812–23. doi: 10.1093/neuros/nyx520
18. Meng T, Yin H, Li B, Li Z, Xu W, Zhou W, et al. Clinical Features and Prognostic Factors of Patients With Chordoma in the Spine: A Retrospective Analysis of 153 Patients in a Single Center. Neuro Oncol (2015) 17(5):725–32. doi: 10.1093/neuonc/nou331
19. Jian BJ, Bloch OG, Yang I, Han SJ, Aranda D, Tihan T, et al. Adjuvant Radiation Therapy and Chondroid Chordoma Subtype are Associated With a Lower Tumor Recurrence Rate of Cranial Chordoma. J Neurooncol (2010) 98(1):101–8. doi: 10.1007/s11060-009-0068-1
20. Hasselblatt M, Thomas C, Hovestadt V, Schrimpf D, Johann P, Bens S, et al. Poorly Differentiated Chordoma With SMARCB1/INI1 Loss: A Distinct Molecular Entity With Dismal Prognosis. Acta Neuropathol (2016) 132(1):149–51. doi: 10.1007/s00401-016-1574-9
21. Hung YP, Diaz-Perez JA, Cote GM, Wejde J, Schwab JH, Nardi V, et al. Dedifferentiated Chordoma: Clinicopathologic and Molecular Characteristics With Integrative Analysis. Am J Surg Pathol (2020) 44(9):1213–23. doi: 10.1097/PAS.0000000000001501
22. Buccoliero AM, Caporalini C, Scagnet M, Baroni G, Moscardi S, Mussa F, et al. A Diagnostic Pitfall: Atypical Teratoid Rhabdoid Tumor Versus Dedifferentiated/Poorly Differentiated Chordoma: Analysis of a Mono-Institutional Series. Appl Immunohistochem Mol Morphol (2019) 27(2):147–54. doi: 10.1097/PAI.0000000000000554
23. Zou MX, Pan Y, Huang W, Zhang TL, Escobar D, Wang XB, et al. A Four-Factor Immune Risk Score Signature Predicts the Clinical Outcome of Patients With Spinal Chordoma. Clin Transl Med (2020) 10(1):224–37. doi: 10.1002/ctm2.4
24. Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of Using “Optimal” Cutpoints in the Evaluation of Prognostic Factors. J Natl Cancer Inst (1994) 86(11):829–35. doi: 10.1093/jnci/86.11.829
25. Asioli S, Zoli M, Guaraldi F, Sollini G, Bacci A, Gibertoni D, et al. Peculiar Pathological, Radiological and Clinical Features of Skull-Base De-Differentiated Chordomas. Results From a Referral Centre Case-Series and Literature Review. Histopathology (2020) 76(5):731–9. doi: 10.1111/his.14024
26. Nachwalter RN, Rothrock RJ, Katsoulakis E, Gounder MM, Boland PJ, Bilsky MH, et al. Treatment of Dedifferentiated Chordoma: A Retrospective Study From a Large Volume Cancer Center. J Neurooncol (2019) 144(2):369–76. doi: 10.1007/s11060-019-03239-3
27. Kayani B, Sewell MD, Hanna SA, Saifuddin A, Aston W, Pollock R, et al. Prognostic Factors in the Operative Management of Dedifferentiated Sacral Chordomas. Neurosurgery (2014) 75(3):269–75:discussion 275. doi: 10.1227/NEU.0000000000000423
28. Radaelli S, Fossati P, Stacchiotti S, Akiyama T, Asencio JM, Bandiera S, et al. The Sacral Chordoma Margin. Eur J Surg Oncol (2020) 46(8):1415–22. doi: 10.1016/j.ejso.2020.04.028
29. Yu X, Kou C, Bai W, Yu W, Zhu B, Zhang M, et al. Comparison of Wide Margin and Inadequate Margin for Recurrence in Sacral Chordoma: A Meta-Analysis. Spine (Phila Pa 1976) (2020) 45(12):814–9. doi: 10.1097/BRS.0000000000003386
30. Antonelli M, Raso A, Mascelli S, Gessi M, Nozza P, Coli A, et al. SMARCB1/INI1 Involvement in Pediatric Chordoma: A Mutational and Immunohistochemical Analysis. Am J Surg Pathol (2017) 41(1):56–61. doi: 10.1097/PAS.0000000000000741
31. Zou MX, Zheng BW, Liu FS, Wang XB, Hu JR, Huang W, et al. The Relationship Between Tumor-Stroma Ratio, the Immune Microenvironment, and Survival in Patients With Spinal Chordoma. Neurosurgery (2019) 85(6):E1095–110. doi: 10.1093/neuros/nyz333
32. Iannalfi A, D’Ippolito E, Riva G, Molinelli S, Gandini S, Viselner G, et al. Proton and Carbon Ions Radiotherapy in Skull Base Chordomas: A Prospective Study Based on a Dual Particle and a Patient-Customized Treatment Strategy. Neuro Oncol (2020) 22(9):1348–58. doi: 10.1093/neuonc/noaa067
33. Gatfield ER, Noble DJ, Barnett GC, Early NY, Hoole ACF, Kirkby NF, et al. Tumour Volume and Dose Influence Outcome After Surgery and High-Dose Photon Radiotherapy for Chordoma and Chondrosarcoma of the Skull Base and Spine. Clin Oncol (R Coll Radiol) (2018) 30(4):243–53. doi: 10.1016/j.clon.2018.01.002
34. Jullien-Petrelli AC, Garcia-Sabrido JL, Orue-Echebarria MI, Lozano P, Álvarez A, Serrano J, et al. Role of Intraoperative Radiotherapy in the Treatment of Sacral Chordoma. Spine J (2018) 18(4):632–8. doi: 10.1016/j.spinee.2017.08.255
35. Hruban RH, Traganos F, Reuter VE, Huvos AG. Chordomas With Malignant Spindle Cell Components. A and Immunohistochemical Study With Histogenetic Implications. Am J Pathol (1990) 137(2):435–47.
36. Nanda A, Hirsh LF, Antoiniades K. Malignant Fibrous Histiocytoma in a Recurrent Thoracic Chordoma: Case Report and Literature Review. Neurosurgery (1991) 28(4):588–92. doi: 10.1227/00006123-199104000-00018
37. Kato S, Gasbarrini A, Ghermandi R, Gambarotti M, Bandiera S. Spinal Chordomas Dedifferentiated to Osteosarcoma: A Report of Two Cases and a Literature Review. Eur Spine J (2016) 25 Suppl 1:251–6. doi: 10.1007/s00586-016-4557-6
38. Chan AC, Tsang WY, Chan GP, Lam YL, Chan MK. Dedifferentiated Chordoma With Rhabdomyoblastic Differentiation. Pathology (2007) 39(2):277–80. doi: 10.1080/00313020701230716
39. Bisceglia M, D’Angelo VA, Guglielmi G, Dor DB, Pasquinelli G. Dedifferentiated Chordoma of the Thoracic Spine With Rhabdomyosarcomatous Differentiation. Report of a Case and Review of the Literature. Ann Diagn Pathol (2007) 11(4):262–73. doi: 10.1016/j.anndiagpath.2006.09.002
40. Yasui Y, Abe T, Kurosaki M, Higuchi M, Komiyama Y, Yoshida T, et al. Elastin Fiber Accumulation in Liver Correlates With the Development of Hepatocellular Carcinoma. PLoS One (2016) 11(4):e0154558. doi: 10.1371/journal.pone.0154558
41. Wen S, Hou Y, Fu L, Xi L, Yang D, Zhao M, et al. Cancer-Associated Fibroblast (CAF)-Derived IL32 Promotes Breast Cancer Cell Invasion and Metastasis via Integrin Beta3-p38 MAPK Signalling. Cancer Lett (2019) 442:320–32. doi: 10.1016/j.canlet.2018.10.015
42. Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, et al. A Framework for Advancing Our Understanding of Cancer-Associated Fibroblasts. Nat Rev Cancer (2020) 20(3):174–86. doi: 10.1038/s41568-019-0238-1
43. Zeltz C, Primac I, Erusappan P, Alam J, Noel A, Gullberg D. Cancer-Associated Fibroblasts in Desmoplastic Tumors: Emerging Role of Integrins. Semin Cancer Biol (2020) 62:166–81. doi: 10.1016/j.semcancer.2019.08.004
44. Hamidi H, Ivaska J. Every Step of the Way: Integrins in Cancer Progression and Metastasis. Nat Rev Cancer (2018) 18(9):533–48. doi: 10.1038/s41568-018-0038-z
45. Bandari SK, Purushothaman A, Ramani VC, Brinkley GJ, Chandrashekar DS, Varambally S, et al. Chemotherapy Induces Secretion of Exosomes Loaded With Heparanase That Degrades Extracellular Matrix and Impacts Tumor and Host Cell Behavior. Matrix Biol (2018) 65:104–18. doi: 10.1016/j.matbio.2017.09.001
46. Chen KW, Yang HL, Lu J, Liu JY, Chen XQ. Prognostic Factors of Sacral Chordoma After Surgical Therapy: A Study of 36 Patients. Spinal Cord (2010) 48(2):166–71. doi: 10.1038/sc.2009.95
Keywords: dedifferentiated chordoma, poorly differentiated chordoma, comparative study, clinicopathological characteristics, survival analysis, prognostic factors
Citation: Liu F-S, Zheng B-W, Zhang T-L, Li J, Lv G-H, Yan Y-G, Huang W and Zou M-X (2021) Clinicopathological and Prognostic Characteristics in Dedifferentiated/Poorly Differentiated Chordomas: A Pooled Analysis of Individual Patient Data From 58 Studies and Comparison With Conventional Chordomas. Front. Oncol. 11:686565. doi: 10.3389/fonc.2021.686565
Received: 27 March 2021; Accepted: 27 July 2021;
Published: 13 August 2021.
Edited by:
Ziya Levent Gokaslan, Brown University, United StatesReviewed by:
Tianyi Niu, University of California, Los Angeles, United StatesElias Shaaya, Rhode Island Hospital, United States
Copyright © 2021 Liu, Zheng, Zhang, Li, Lv, Yan, Huang and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Huang, aHVhbmd3ZWk4ODg3QGNzdS5lZHUuY24=
†These authors have contributed equally to this work
‡These authors have contributed equally to this work
 Fu-Sheng Liu1,2‡
Fu-Sheng Liu1,2‡ Jing Li
Jing Li Ming-Xiang Zou
Ming-Xiang Zou