
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 03 June 2021
Sec. Hematologic Malignancies
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.685786
This article is part of the Research Topic Insights in Hematologic Malignancies: 2021 View all 28 articles
 Iole Cordone1*
Iole Cordone1* Serena Masi1
Serena Masi1 Diana Giannarelli1
Diana Giannarelli1 Alessia Pasquale1
Alessia Pasquale1 Laura Conti1
Laura Conti1 Stefano Telera2
Stefano Telera2 Andrea Pace2
Andrea Pace2 Elena Papa2
Elena Papa2 Mirella Marino1
Mirella Marino1 Paolo de Fabritiis3
Paolo de Fabritiis3 Andrea Mengarelli2
Andrea Mengarelli2Cerebrospinal fluid (CSF) flow cytometry has a crucial role in the diagnosis of leptomeningeal disease in onco-hematology. This report describes the flow cytometry characterization of 138 CSF samples from patients affected by non-Hodgkin lymphoma, negative for disease infiltration. The aim was to focus on the CSF non-neoplastic population, to compare the cellular composition of the CSF with paired peripheral blood samples and to document the feasibility of flow cytometry in hypocellular samples. Despite the extremely low cell count (1 cell/µl, range 1.0–35) the study was successfully conducted in 95% of the samples. T lymphocytes were the most abundant subset in CSF (77%; range 20–100%) with a predominance of CD4-positive over CD8-positive T cells (CD4/CD8 ratio = 2) together with a minority of monocytes (15%; range 0–70%). No B cells were identified in 90% of samples. Of relevance, a normal, non-clonal B-cell population was documented in 5/7 (71%) patients with primary central nervous system lymphoma at diagnosis (p<0.0001), suggesting a possible involvement of blood-brain barrier cell permeability in the pathogenesis of cerebral B-cell lymphomas. The highly significant differences between CSF and paired peripheral blood lymphoid phenotype (p<0.0001) confirms the existence of an active mechanism of lymphoid migration through the meninges.
Neoplastic meningitis is a dramatic complication in cancer patients and the diagnosis of leptomeningeal metastasis represents one of the greatest challenges in neuro-oncology. Cerebrospinal fluid (CSF) analysis has a key role in routine clinical practice however conventional cytology, the gold standard for cell type identification, has considerable limitations regarding sensitivity and specificity, with a reported false-negative rate of up to 40% (1, 2).
In recent years, several studies have demonstrated that CSF flow cytometry is superior to conventional cytology for detection of CNS involvement in non-Hodgkin lymphomas, acute leukemia and multiple myeloma (3–11). Thereafter, flow cytometry is recognized among the basic elements for the diagnosis of leptomeningeal metastasis in hematologic cancers (12, 13), although the low cell count of CSF samples, combined with the rapidly declining leukocyte viability, makes CSF flow cytometry challenging (14). More recently, flow cytometry application and efficiency in diagnosis of solid tumors leptomeningeal metastasis is gaining more evidence (15–18).
However, cancer cells represent only a proportion, often a minority, of the CSF population in neoplastic meningitis. A significant presence of lymphocytes has been documented, together with floating malignant cells, in CSF samples from patients with non-Hodgkin lymphomas and breast cancer leptomeningeal metastasis (18, 19); an active mechanism of reactive CD8 T-lymphocyte migration has been observed in primary central nervous system lymphomas (PCNSL) of B-cell type (20, 21). These findings suggest an active role of the central nervous system (CNS) lymphatic system in both lymphoid and tumor cells migration into and out of the meninges.
Focusing on non-neoplastic populations, we report here the immunophenotype of the CSF leukocytes of patients affected by non-Hodgkin lymphomas without leptomeningeal involvement. The aim was to document the feasibility of flow cytometry in normal, thereafter, extremely hypocellular samples, to document the immunophenotype of CSF non-neoplastic population in non-Hodgkin lymphoma, to compare the cellular composition of the CSF with paired peripheral blood samples. Moreover, a possible correlation between the CSF lymphocyte subpopulations and diagnosis was evaluated.
From March 2010 to December 2015 a cohort of 138 samples with non-Hodgkin lymphoma who underwent diagnostic lumbar puncture according to the routine clinical practice entered the study (22). All PCNSL cases diagnosed until December 2019 were also included. Lymphomas were classified according to the World Health Organization (WHO) classification (23).
All CSF samples were analyzed by cytology and flow cytometry and had no evidence of infiltration. Patients with a positive diagnostic lumbar puncture due to CSF infiltration by pathological cells were excluded from this analysis. The Central Ethical Committee IRCCS Lazio, Section I.F.O. approved this retrospective study. Protocol n° 0009524, July 27th 2020.
CSF was collected in a tube without any transport medium or anticoagulant and processed within 1 to 3 h to minimize cell loss. To avoid peripheral blood contamination, the first 0.2 to 0.4 ml (four to eight drops) of CSF were discarded before sample collection. A standard cell count was performed using the Turk reagent and a Nageotte chamber. CSF was spun at 1,400 rpm for 5 min, the supernatant fluid was discarded and the cell pellet was suspended in 500 µl of phosphate buffered saline (PBS): 100 µl of cell suspension was used for cytomorphology and 100 µl/tube for the flow cytometric study.
Cytospins were prepared using a Shandon CytoSpin cytocentrifuge. Morphological examination was performed by expert cytopathologists using May–Grünwald–Giemsa staining. All cases were morphologically negative for CNS localization.
CSF samples were processed and stained using a 6-color monoclonal antibodies panel, 5 µl of each, and the “Duo-lyse” program of the Becton Dickinson Bioscience (BDB) Lyse-Wash-Assistant according to the following combinations: tube 1) CD3Fitc (BD Biosciences Cat# 345763, RRID : AB_2811220), CD56Pe (BD Biosciences Cat# 345812, RRID : AB_2629216), CD45PerCP-Cy5.5 (BD Biosciences Cat# 332784, RRID : AB_2868632), CD4PE-Cy7 (BD Biosciences Cat# 348809, RRID : AB_2783789), CD19APC (BD Biosciences Cat# 345791, RRID : AB_2868817) and CD8APC-Cy7 (BD Biosciences Cat# 348813, RRID : AB_2868857); tube 2) CD5Fitc (BD Biosciences Cat# 345781, RRID : AB_2868807), CD10Pe (BD Biosciences Cat# 332776, RRID : AB_2868625), CD45PerCP-Cy5.5, CD2PE-Cy7 (BD Biosciences Cat# 335821, RRID : AB_2868684), CD79bAPC (BD Biosciences Cat# 335834, RRID : AB_2868695) and CD20APC-Cy7 (BD Biosciences Cat# 335829, RRID : AB_2868690); tube 3) anti-Lambda Fitc (BD Biosciences Cat# 347247, RRID : AB_2868845, anti-Kappa Pe (BD Biosciences Cat# 347246, RRID : AB_2868844), CD45PerCP-Cy5.5, CD34PE-Cy7 (BD Biosciences Cat# 348811, RRID : AB_2868855), CD22APC (BD Biosciences Cat# 333145, RRID : AB_2868646) and CD14APC-Cy7 (BD Biosciences Cat# 333951, RRID : AB_2868679). All antibodies were from BDB. Prior to sample acquisition, a flow cell cleaning with FACS flow (for 1 to 2 min run) was performed to avoid any event carry over. The whole volume of sample was acquired and analyzed using the FACSCanto II 2L flow cytometer and the FACSDiva software Version 6.1.3 (BDB). Single-stained cellular controls, BD FACS™ 7-color setup beads and BD FACSDiva CS&T IVD Beads have been used to adjust detector voltage, to set fluorescence compensation and to monitor instrument performance.
Data are presented as the percentage of positive cells evaluated on the CD45-positive population. Lymphocytes were identified by CD45-strong/side scatter (SSC)-low. The CD4 and CD8 subsets were evaluated as a percentage of CD3-positive T lymphocytes. Monocytes were identified using the CD4-weak or CD14 staining. Neutrophils as CD45/SSC-high. Surface immunoglobulins (Ig) kappa and lambda light chain expression was evaluated on CD22-positive B cells. In agreement with the recommendations for the analysis of rare events, a cluster of 10 events was considered to define a positive result and identify a leukocyte subpopulation (10). Disease infiltration of the CSF was excluded, being negative for the lymphoma-associated phenotype identified by histopathology. We defined as CSF negative all sample negative by cytology and flow cytometry.
The peripheral blood lymphocyte characterization, using the CD3 CD56 CD45 CD4 CD19 and CD8 combination (tube n°1), was conducted in 104 paired cases.
Qualitative items were reported as absolute and percentage counts, while quantitative variables were summarized using mean and standard deviation, median and range. The difference in distribution between CSF and PB lymphoid subpopulations was assessed by Wilcoxon rank-sum test. Association between variables was evaluated with the Spearman’s ρ coefficient. The test was two-sided with a p-value of <0.05 indicating a statistically significant difference. All statistical analyses were performed using SPSS (version 21.0).
A cohort of 138 samples from 127 non-Hodgkin lymphoma patients, all negative for CNS disease involvement, entered the study (Table 1). Eighty-three patients (65%) were male and median age was 60 years (range 18–84). The lumbar puncture for analysis was performed at diagnosis (n=108), at follow up (n=11), at relapse (n=12), or with progressive disease (n=7).

Table 1 Diagnostic distribution of 127 non-Hodgkin lymphoma patients who underwent diagnostic lumbar puncture according to the routine clinical practice and were negative for leptomeningeal involvement.
The study focuses on 107 cases; 24 cases (17%) were not included in analysis due to peripheral blood contamination of the CSF documented by the identification of red blood cells in the cytospin assessed morphologically as well as a population of CD45/SSC high (46%; range 30–89%) positive neutrophils. In seven cases the flow cytometry analysis was not evaluable due to the absence of clustered events.
A median volume of 4.0 ml (range 2.0–12.0) of CSF was available for flow cytometry analysis. The CSF cell count was extremely low (1 cell/µl, range 1.0–35); in 9 cases (8%) the CSF cell count was higher than the normal reference value of 4 cell/µl, with a median value of 22 cells/µl (5.0–35). Despite the low absolute cell number, flow cytometry characterization was successfully conducted in 95% of cases (131/138).
Gating on the CD45-positive population in combination with the side scatter, a median of 384 (range 49–23649; mean 1518 ± 3772) events were acquired and analyzed. A positive correlation was found between the volume (ml) of CSF and the number of events analyzed by flow cytometry (Δ 0.36; p<0.001) (Figure 1).

Figure 1 Positive correlation between the ml of cerebrospinal fluid and the number of cells (events) analyzed by flow cytometry (p 0.36; P < 0.001).
The CSF population was represented by lymphocytes (77%; range 20–100%) together with a minor population of monocytes (CD4-weak or CD14-positive 15%; range 0–70%).
The CSF lymphoid population was represented by CD2 CD3 CD5-positive T cells (94%; range 62–100%) with a prevalence of CD4-positive lymphocytes (CD4/CD8 ratio = 2). A minority of CD56-positive cells were also documented (5%; range 0–47%). No B cells (< 10 clustered events) were identified in 90% of cases (Table 2).
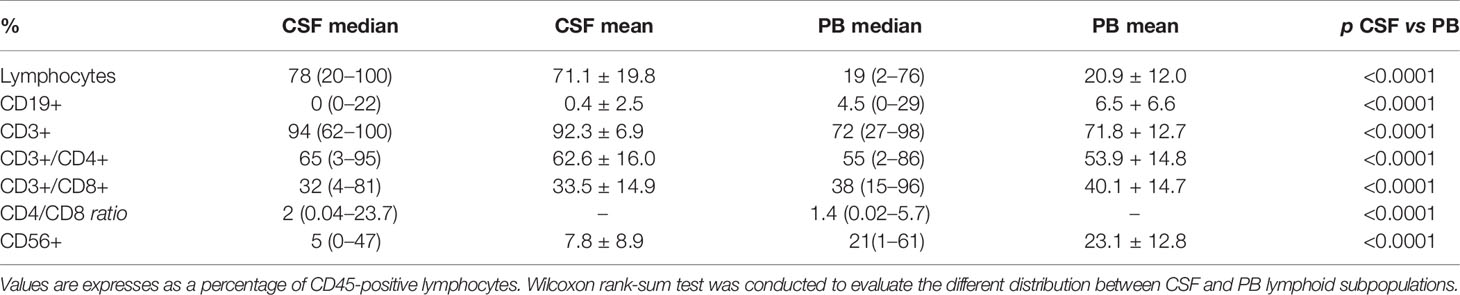
Table 2 Cerebrospinal fluid (CSF) and corresponding peripheral blood (PB) lymphoid immunophenotype comparison in 104 non-Hodgkin lymphoma patients negative for leptomeningeal involvement.
In 11 patients a subpopulation of CD19 CD20 CD22 CD79b-positive B lymphocytes (4%; range 1–22%), with a normal/balanced Ig kappa/lambda ratio evaluated on the CD22-positive population, was identified: 5 PCNSL, 5 diffuse large B-cell lymphoma (DLBCL), 1 follicular lymphoma (Table 3) (Figures 2A–C). All 11 patients were at diagnosis. Non-clonal B lymphocytes were documented in 45.5% (5/11) of PCNSL, in 5.5% (5/90) DLBCL and 1/5 FL cases. Seven PCNSL were at diagnosis and 4 in disease progression; normal B cells were present in 5/7 (71%) PCNSL at diagnosis, identifying a significant correlation between a non-clonal B-cell subpopulation in the CSF and a diagnosis of cerebral B-cell lymphoma (p<0.0001).

Table 3 Analysis of the cerebrospinal fluid (CSF) and corresponding peripheral blood (PB) lymphocytes of 11/107 non-Hodgkin lymphoma patients, negative for leptomeningeal involvement, where a subpopulation of B cells has been identified at diagnosis by flow cytometry of the CSF sample.
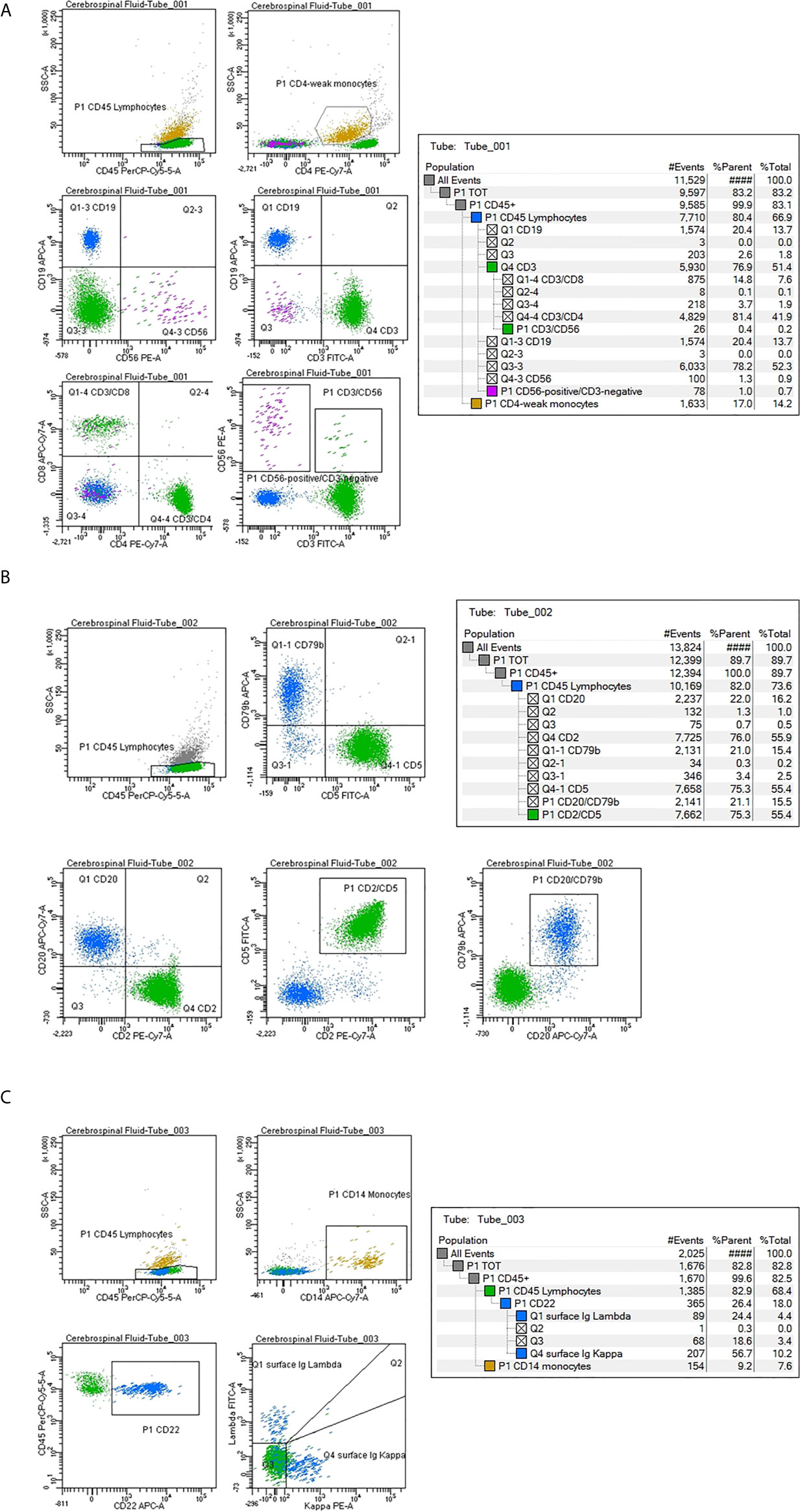
Figure 2 Cerebrospinal fluid (CSF) flow cytometry characterization in a case of diffuse large B-cell lymphoma negative for disease infiltration. Cell count: 24 cells/µl. The CSF lymphocyte immunophenotype is reported as percentage of positive cells within the lymphoid population, identified as CD45-strong/low SSC. (A) Tube number 1: Green color has been utilized to mark CD3 CD4 CD8-positive T lymphocytes; purple for CD56-positive cells, blue for CD19-positive B lymphocytes and dark yellow for CD4-weak monocytes. (B) Tube number 2: Green color has been utilized to mark CD2 CD5-positive T lymphocytes; blue for CD79b CD20-positive B cells. (C) Tube number 3: Blue color has been utilized to mark CD22-positive B lymphocytes. The Ig light chain expression shows a normal kappa/lambda ratio. Dark yellow marks CD14-positive monocytes.
The peripheral blood lymphocyte subset was evaluated in 104 cases (97%) and compared to the corresponding CSF lymphoid subpopulations. The absolute number of lymphocytes was 1100 cell/µl (range 70–3300), the median percentage was 19% (range 2–76). The analysis documented a population of CD3-positive cells (72%, range 27–98) with a CD4/CD8 ratio of 1.4, CD56-positive (21%; range 1–61) and CD19-positive (5%; range 0–29) lymphocytes. A different distribution of CD3 CD56 and CD19 lymphoid subpopulations was observed between CSF and corresponding peripheral blood samples (p<0.0001) (Table 2) (Figure 3).
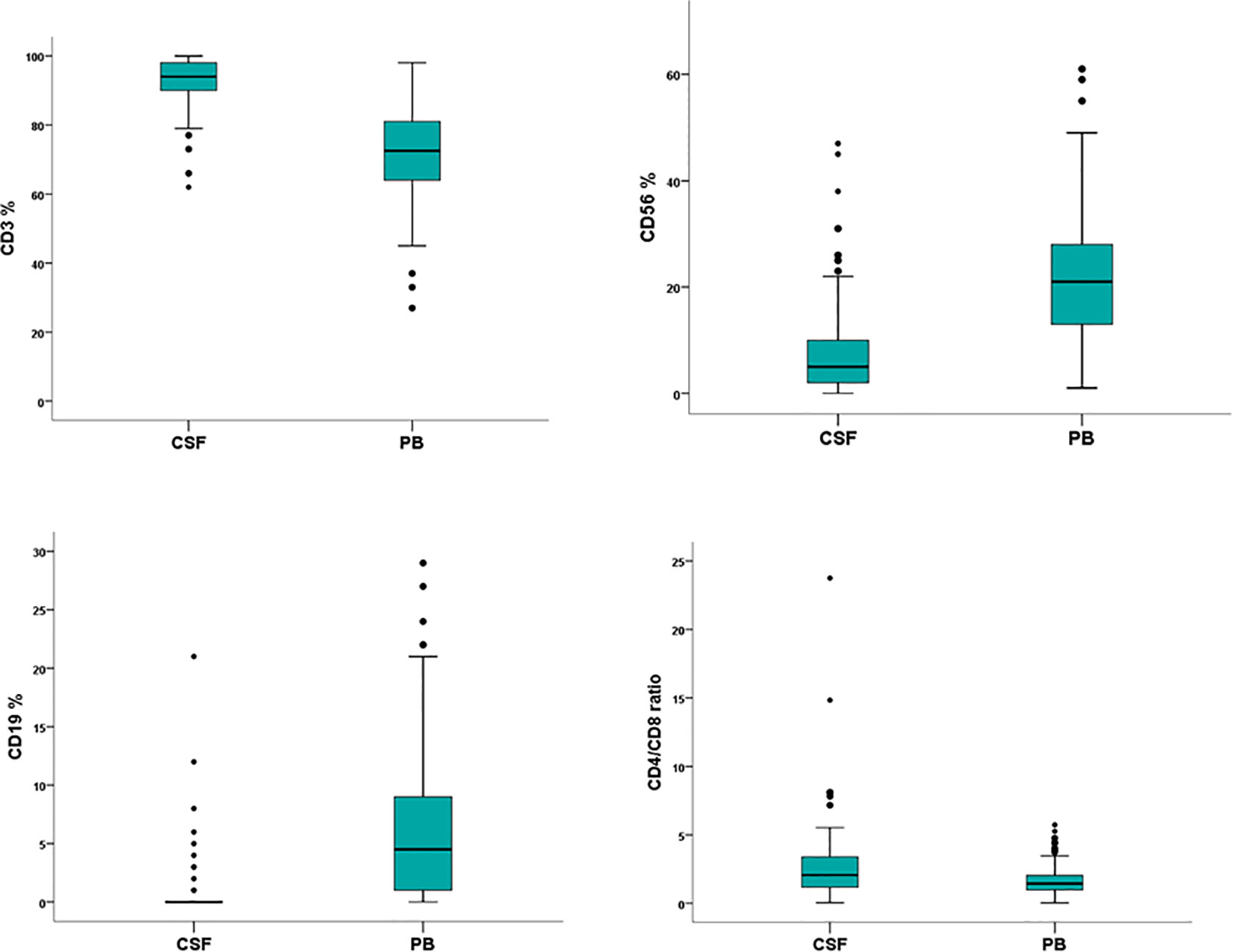
Figure 3 Flow cytometry characterization of cerebrospinal fluid (CSF) and peripheral blood (PB) lymphocytes in 107 non-Hodgkin lymphoma patients negative for leptomeningeal involvement. Wilcoxon rank-sum test documents a significant different distribution between CSF and PB lymphoid subpopulations.
Leptomeningeal metastasis represents one of the greatest challenges in neuro-oncology and CSF is one of the most promising diagnostic tissues utilized in routine clinical practice (13, 24). In addition to cytology, the diagnostic use of flow cytometry is strongly recommended for CSF samples of patients clinically suspected of neoplastic meningitis (12). This report describes the flow cytometry characterization of 138 CSF samples of non-Hodgkin lymphoma patients who underwent diagnostic lumbar puncture according to the routine clinical practice and who were negative for disease infiltration. Knowledge of normal values is essential for diagnostic and research interpretation and, to the best of our knowledge, this is the first, single-institution, report on the flow cytometry characterization of CSF non-neoplastic leukocytes in a large cohort of onco-hematology patients.
The normal cell count of the CSF in adults is up to 4 cells/μL and CSF cell count is a diagnostic criterion for leptomeningeal infiltration. The median cellularity in this cohort of patients was extremely low (1 cell/µl, range 1.0–35), but nevertheless flow cytometry was successfully conducted in 95% of cases, confirming its role of a highly sensitive and specific technique for detection of rare cell sub-populations, even in samples with as few as 1 leukocyte per µl of sample. Of note, in 8% of samples (n=9) the CSF cell count was significantly higher than the normal reference value (median 22 cell/µl). In these cases, unequivocal identification of the cell population was mandatory to exclude false positive interpretation: therefore, in addition to cytology, flow cytometry becomes essential. Moreover, the role of an increased number of lymphocytes in the CSF of non-Hodgkin lymphomas patients deserves to be investigated.
High sensitivity has been reported utilizing a volume of 2.0 ml of CSF for flow cytometry characterization (6). The present study was conducted on a median volume of 4 ml of CSF (≤2 ml being withdrawn in six cases only) with a median of 384 (mean 1518 ± 3772) events analyzed. This number is appreciably higher than the minimum number of events, 10 dots, required for minimal disease identification in CSF flow cytometry (6, 25), confirming the feasibility of comprehensive leukocyte characterization in low volume/low count samples. In this series only 7 cases were not evaluable due to the lack of clustered events. However, cancer cells represent only a proportion, often a minority, of the CSF population in neoplastic meningitis (18, 19, 26). Since there is a positive correlation between the volume of CSF (ml) and the number of events available for flow cytometric analysis (Δ 0.36; p < 0.001) and taking into account the potentially extremely low cell count of the CSF, as well as some cell loss related to the staining technique, we recommend the withdrawal of not less than 4 ml of sample to ensure an adequate number of events for a reliable identification of minimally represented sub-populations. Moreover, peripheral blood contamination of the CSF, due to difficulty in the execution of the lumbar puncture, can occur. False positive results represent the major pitfall in all cases with peripheral blood infiltration by leukemic/lymphoma cells and blood contamination of the CSF (author statement manuscript in preparation) (12). Thereafter, discarding the first drops of sample must represent the reference standard in all CSF samples collected for flow cytometry analysis. Moreover, the different distribution of lymphocyte subsets between CSF and peripheral blood can potentially represent a new, useful tool to discriminate between primary and peripheral blood derived populations, particularly at diagnosis or in neutropenic patients.
Studies conducted on normal CSF observed that the vast majority of leucocytes is represented by central memory T lymphocytes with significantly higher percentage compared to blood. The proportion of CD56-positive cells is low while B cells are almost absent (<1%) (27–30). This indicates a selective recruitment of memory T cells into normal CSF. Our study confirms, in a large cohort of samples, that CSF is a tissue rich in CD2 CD3 CD5-positive T lymphocytes with a predominance of CD4-positive over CD8-positive T cells (CD4/CD8 ratio = 2), together with a minority of CD56-positive cells in patients with B-cell non-Hodgkin lymphomas negative for leptomeningeal involvement. The presence of normal T cells in the CSF sample represents not only a strong, reliable internal quality control of the technique but also documents a selective recruitment of T-cell into CSF. The CNS is an immunological sanctuary with restricted access and a unique microenvironment however scientific evidences have recently documented that CNS is no longer an immune-privileged site, but rather a virtual secondary lymphoid organ (31, 32). The tumor inflammatory response is involved in both cancer growth inhibitions as well as in cancer invasiveness (33–37). A relevant proportion of infiltrating T lymphocytes and monocytes beside cancer cells has been documented in patient with breast cancer neoplastic meningitis, with a significant difference in the lymphoid immunophenotype between CSF and peripheral blood (18). Likewise, a sub-population of T cells has been identified in CSF samples positive for B non-Hodgkin lymphoma infiltration (7, 19). Moreover, an active mechanism of reactive CD8 T-lymphocyte migration through the blood-brain barrier has been consistently shown in PCNSL (20, 21). In this study, the ratio between CD4/CD8-positive T cells was shifted significantly in favor of CD4-positive T cells in CSF compared to corresponding peripheral blood (ratio = 2 versus ratio=1.4 respectively; p < 0.0001). This different distribution documents that the brain barrier actively selects a sub-population of T lymphocytes, supporting the involvement of the meningeal lymphatic network in lymphoid cell migration into the meninges as a potential alternative route to the cardiovascular system. This finding documents the existence of an active mechanism of lymphocyte localization and provides a promising rationale for the investigation of cellular immunotherapy in brain diseases.
B cells were by far the smallest subset in the CSF of this cohort of B non-Hodgkin lymphoma patients without CNS involvement. The number of B cells is hardly above detection limit in normal CSF (27–29). By contrast, in patients with paraneoplastic neurological syndrome CSF B cell counts showed significantly elevated numbers compared to normal control, suggesting that B lymphocytes are recruited to CSF in certain pathological conditions (38). In the present study, sporadic/no B cells (<10 clustered events) were identified in 90% of the samples and represented a minority (4%) of the lymphoid population in eleven cases (Table 3). The normal kappa/lambda ratio, evaluated on the CD22-positive population, was crucial for reporting the flow cytometry as negative for infiltration by clonal B cells. A possible correlation between the CSF lymphocyte subpopulations and diagnosis was evaluated. A normal, non-clonal B-cell subpopulation was identified at diagnosis in 71% (5/7) CSF samples of patients with PCNSL (p < 0.0001). In contrast with its low frequency in normal CSF and systemic non-Hodgkin lymphomas, the identification of a subpopulation of B cells in the CSF samples of PCNSL cases raises the question of a possible role of blood-brain barrier cell permeability in the pathogenesis of cerebral B-cell lymphomas. Due to the small number of PCNSL cases evaluated, validation on a larger cohort of patients is warranted to confirm this finding and to investigate the role of the CSF B cells in the pathogenesis and diagnosis of the B-cell lymphomas of the brain. A small clonal B‐cell population has been described in the CSF of patients with B‐cell lymphoproliferative disorders and multiple sclerosis suggesting that this finding is not diagnostic of clinically significant involvement of the CNS by lymphoid malignancy (39, 40). Although a larger prospective study with a long follow‐up is required to validate this finding, the identification of a minority of clonal B cells by flow cytometry at diagnosis deserves a careful clinical and instrumental evaluation and more definitive evidence of CNS lymphoid malignancy before a potentially toxic treatment is given (41).
Finally, after (CD4-positive) T lymphocytes, monocytes represent the second most common leukocyte population of the CSF (15%; range 0–70%). Origin and turnover of this medium-size population, well represented in the CSF, is still largely unexplored. The role of monocytes regarding the CNS cellular immune surveillance and their involvement in onco-hematological meningitis deserves in-depth studies and attention.
The cellular composition of the CSF in non-Hodgkin lymphoma patients negative for leptomeningeal involvement differs profoundly from peripheral blood regarding all major lymphocyte subpopulation. CSF cells are represented by T lymphocytes, in prevalence CD4-positive, and monocytes. B cells are rare and this analysis reveals a possible link with PCNSL. This real-life study confirms the critical role of flow cytometry in routine clinical practice for unequivocal characterization of CSF populations, even in samples with an extremely low cell count. The identification of clusters of normal T cells in the CSF represent a reliable internal quality control of the technique and the significant difference between CSF and paired peripheral blood lymphoid phenotype provides evidence of an independent cerebral lymphatic system. CSF is not an immune-privileged site anymore but a virtual secondary lymphoid organ. An in-depth knowledge of the function and role of the CSF immunological sanctuary is highly needed and has the potential to revolutionize the management of CNS diseases.
The datasets analyzed for this study can be found in the GARRbox (https://gbox.garr.it/garrbox/index.php/s/6BY66PX6n3LaYZP).
The Central Ethical Committee IRCCS Lazio, Section I.F.O. approved this retrospective study. Protocol no 0009524, July 27, 2020. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
IC: designed the study and draft the initial manuscript. SM and APas: flow cytometry studies. IC and SM: analysis, data collection, and quality control. DG: performed the statistical analysis and manuscript revision. MM: histopathological diagnosis and manuscript revision. ST, APac, AM, LC, EP, and PF: patient’s clinical management and critical manuscript revision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
IC thanks Federica Falcioni for management support.
1. Chamberlain MC, Glantz M, Groves MD, Wilson WH. Diagnostic Tools for Neoplastic Meningitis: Detecting Disease, Identifying Patient Risk, and Determining Benefit of Treatment. Semin Oncol (2009) 36(4 Suppl 2):S35–45. doi: 10.1053/j.seminoncol.2009.05.005
2. Taillibert S, Chamberlain MC. Leptomeningeal Metastasis. Handb Clin Neurol (2018) 149:169–204. doi: 10.1016/B978-0-12-811161-1.00013-X
3. Hegde U, Filie A, Little RF, Janik JE, Grant N, Steinberg SM, et al. High Incidence of Occult Leptomeningeal Disease Detected by Flow Cytometry in Newly Diagnosed Aggressive B-Cell Lymphomas At Risk for Central Nervous System Involvement: The Role of Flow Cytometry Versus Cytology. Blood (2005) 105(2):496–502. doi: 10.1182/blood-2004-05-1982
4. Craig FE, Foon KA. Flow Cytometric Immunophenotyping for Hematologic Neoplasms. Blood (2008) 111(8):3941–67. doi: 10.1182/blood-2007-11-120535
5. Di Noto R, Scalia G, Abate G, Gorrese M, Pascariello C, Raia M, et al. Critical Role of Multidimensional Flow Cytometry in Detecting Occult Leptomeningeal Disease in Newly Diagnosed Aggressive B-Cell Lymphomas. Leukemia Res (2008) 32(8):1196–9. doi: 10.1016/j.leukres.2007.12.016
6. Quijano S, López A, Manuel Sancho J, Panizo C, Debén G, Castilla C. Et Al, Spanish Group for the Study of CNS Disease in NHL (2009). Identification of Leptomeningeal Disease in Aggressive B-Cell Non-Hodgkin’s Lymphoma: Improved Sensitivity of Flow Cytometry. J Clin Oncol Off J Am Soc Clin Oncol (2009) 27(9):1462–9. doi: 10.1200/JCO.2008.17.7089
7. Ahluwalia MS, Wallace PK, Peereboom DM. Flow Cytometry as a Diagnostic Tool in Lymphomatous or Leukemic Meningitis: Ready for Prime Time? Cancer (2012) 118(7):1747–53. doi: 10.1002/cncr.26335
8. Benevolo G, Stacchini A, Spina M, Ferreri AJ, Arras M, Bellio L, et al. Final Results of a Multicenter Trial Addressing Role of CSF Flow Cytometric Analysis in NHL Patients At High Risk for CNS Dissemination. Blood (2012) 120(16):3222–8. doi: 10.1182/blood-2012-04-423095
9. Subirá D, Castañón S, Román A, Aceituno E, Jiménez-Garófano C, Jiménez A, et al. Flow Cytometry and the Study of Central Nervous Disease in Patients With Acute Leukaemia. Br J Haematol (2001) 112(2):381–4. doi: 10.1046/j.1365-2141.2001.02505.x
10. Del Principe MI, De Bellis E, Gurnari C, Buzzati E, Savi A, Consalvo M, et al. Applications and Efficiency of Flow Cytometry for Leukemia Diagnostics. Expert Rev Mol Diag (2019) 19(12):1089–97. doi: 10.1080/14737159.2019.1691918
11. Marchesi F, Masi S, Summa V, Gumenyuk S, Merola R, Orlandi G, et al. Flow Cytometry Characterization in Central Nervous System and Pleural Effusion Multiple Myeloma Infiltration: An Italian National Cancer Institute Experience. Br J Haematol (2016) 172(6):980–2. doi: 10.1111/bjh.13549
12. Chamberlain M, Junck L, Brandsma D, Soffietti R, Rudà R, Raizer J, et al. Leptomeningeal Metastases: A RANO Proposal for Response Criteria. Neuro-oncology (2017) 19(4):484–92. doi: 10.1093/neuonc/now183
13. Boire A, Brandsma D, Brastianos PK, Le Rhun E, Ahluwalia M, Junck L, et al. Liquid Biopsy in Central Nervous System Metastases: A RANO Review and Proposals for Clinical Applications. Neuro-oncology (2019) 21(5):571–84. doi: 10.1093/neuonc/noz012
14. Mlinarić A, Vogrinc Ž, Drenšek Z. Effect of Sample Processing and Time Delay on Cell Count and Chemistry Tests in Cerebrospinal Fluid Collected From Drainage Systems. Biochem Med (2018) 28(3):30705. doi: 10.11613/BM.2018.030705
15. Patel AS, Allen JE, Dicker DT, Peters KL, Sheehan JM, Glantz MJ, et al. Identification and Enumeration of Circulating Tumor Cells in the Cerebrospinal Fluid of Breast Cancer Patients With Central Nervous System Metastases. Oncotarget (2011) 2(10):752–60. doi: 10.18632/oncotarget.336
16. Le Rhun E, Massin F, Tu Q, Bonneterre J, Bittencourt M, Faure GC. Development of a New Method for Identification and Quantification in Cerebrospinal Fluid of Malignant Cells From Breast Carcinoma Leptomeningeal Metastasis. BMC Clin Pathol (2012) 12:21. doi: 10.1186/1472-6890-12-21
17. Stover DG, Parsons HA, Ha G, Freeman SS, Barry WT, Guo H, et al. Association of Cell-Free DNA Tumor Fraction and Somatic Copy Number Alterations With Survival in Metastatic Triple-Negative Breast Cancer. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36(6):543–53. doi: 10.1200/JCO.2017.76.0033
18. Cordone I, Masi S, Summa V, Carosi M, Vidiri A, Fabi A, et al. Overexpression of Syndecan-1, MUC-1, and Putative Stem Cell Markers in Breast Cancer Leptomeningeal Metastasis: A Cerebrospinal Fluid Flow Cytometry Study. Breast Cancer Res: BCR (2017) 19(1):46. doi: 10.1186/s13058-017-0827-4
19. Masi S, Summa V, Pasquale A, Merola R, Ascani R, Antenucci A, et al. Cerebrospinal Fluid Flow Cytometry for Diagnosis and Monitoring of NHL: A Regina Elena National Cancer Institute Experience. Abstract From XV Sies. Haematologica (2018) 103:S117.
20. Cordone I, Masi S, Carosi M, Vidiri A, Marchesi F, Marino M, et al. Brain Stereotactic Biopsy Flow Cytometry for Central Nervous System Lymphoma Characterization: Advantages and Pitfalls. J Exp Clin Cancer Res: CR (2016) 35(1):128. doi: 10.1186/s13046-016-0404-1
21. van der Meulen M, Bromberg J, Lam KH, Dammers R, Langerak AW, Doorduijn JK, et al. Flow Cytometry Shows Added Value in Diagnosing Lymphoma in Brain Biopsies. Cytomet Part B Clin Cytomet (2018) 94(6):928–34. doi: 10.1002/cyto.b.21641
22. Zelenetz AD, Abramson JS, Advani RH, Andreadis B, Byrd JC, Czuczman MS, et al. NCCN Clinical Practice Guidelines in Oncology: Non-Hodgkin's lymphomas. J Natl Compr Canc Netw (2010) 8(3):288–334. doi: 10.6004/jnccn.2010.0021
23. Swerdlow S, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. Who Classification of Tumors of Hematopoietic and Lymphoid Tissues. Lyon: International Agency for Research on Cancer (IARC) (2017).
24. Sindeeva OA, Verkhovskii RA, Sarimollaoglu M, Afanaseva GA, Fedonnikov AS, Osintsev EY, et al. New Frontiers in Diagnosis and Therapy of Circulating Tumor Markers in Cerebrospinal Fluid In Vitro and In Vivo. Cells (2019) 8(10):1195. doi: 10.3390/cells8101195
25. Gabelli M, Disarò S, Scarparo P, Francescato S, Zangrando A, Valsecchi MG, et al. Cerebrospinal Fluid Analysis by 8-Color Flow Cytometry in Children With Acute Lymphoblastic Leukemia. Leukemia Lymphoma (2019) 60(11):2825–8. doi: 10.1080/10428194.2019.1602269
26. Sancho JM, Orfao A, Quijano S, García O, Panizo C, Pérez-Ceballos E, et al. Clinical Significance of Occult Cerebrospinal Fluid Involvement Assessed by Flow Cytometry in Non-Hodgkin’s Lymphoma Patients At High Risk of Central Nervous System Disease in the Rituximab Era. Eur J Haematol (2010) 85(4):321–8. doi: 10.1111/j.1600-0609.2010.01478.x
27. Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal Fluid Analysis. Am Family Physician (2003) 68(6):1103–8.
28. Svenningsson A, Andersen O, Edsbagge M, Stemme S. Lymphocyte Phenotype and Subset Distribution in Normal Cerebrospinal Fluid. J Neuroimmunol (1995) 63(1):39–46. doi: 10.1016/0165-5728(95)00126-3
29. de Graaf MT, Smitt PA, Luitwieler RL, van Velzen C, van den Broek PD, Kraan J, et al. Central Memory CD4+ T Cells Dominate the Normal Cerebrospinal Fluid. Cytomet Part B Clin Cytomet (2011) 80(1):43–50. doi: 10.1002/cyto.b.20542
30. Kleine TO. Cellular Immune Surveillance of Central Nervous System Bypasses Blood-Brain Barrier and Blood-Cerebrospinal-Fluid Barrier: Revealed With the New Marburg Cerebrospinal-Fluid Model in Healthy Humans. Cytomet Part A J Int Soc Anal Cytol (2015) 87(3):227–43. doi: 10.1002/cyto.a.22589
31. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and Functional Features of Central Nervous System Lymphatic Vessels. Nature (2015) 523(7560):337–41. doi: 10.1038/nature14432
32. Negi N, Das BK. Cns: Not an Immunoprivilaged Site Anymore But a Virtual Secondary Lymphoid Organ. Int Rev Immunol (2018) 37(1):57–68. doi: 10.1080/08830185.2017.1357719
33. Disis ML. Immune Regulation of Cancer. J Clin Oncol Off J Am Soc Clin Oncol (2010) 28(29):4531–8. doi: 10.1200/JCO.2009.27.2146
34. Chawla A, Alatrash G, Wu Y, Mittendorf EA. Immune Aspects of the Breast Tumor Microenvironment. Breast Cancer Manage (2013) 2(3):231–44. doi: 10.2217/bmt.13.15
35. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-Related Inflammation and Treatment Effectiveness. Lancet Oncol (2014) 15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3
36. Bruno A, Ferlazzo G, Albini A, Noonan DM. A Think Tank of TINK/TANKs: Tumor-Infiltrating/Tumor-Associated Natural Killer Cells in Tumor Progression and Angiogenesis. J Natl Cancer Inst (2014) 106(8):dju200. doi: 10.1093/jnci/dju200
37. Zhou W, Bao S. Reciprocal Supportive Interplay Between Glioblastoma and Tumor-Associated Macrophages. Cancers (2014) 6(2):723–40. doi: 10.3390/cancers6020723
38. de Graaf M, de Beukelaar J, Bergsma J, Kraan J, van den Bent M, Klimek M, et al. B and T Cell Imbalances in CSF of Patients With Hu-antibody Associated PNS. J Neuroimmunol (2008) 195(1-2):164–70. doi: 10.1016/j.jneuroim.2008.01.007
39. Nowakowski GS, Call TG, Morice WG, Kurtin PJ, Cook RJ, Zent CS. Clinical Significance of Monoclonal B Cells in Cerebrospinal Fluid. Cytomet Part B Clin Cytomet (2005) 63(1):23–7. doi: 10.1002/cyto.b.20032
40. Greenfield AL, Dandekar R, Ramesh A, Eggers EL, Wu H, Laurent S, et al. Longitudinally Persistent Cerebrospinal Fluid B Cells can Resist Treatment in Multiple Sclerosis. JCI Insight (2019) 4(6):e126599. doi: 10.1172/jci.insight.126599
Keywords: cerebrospinal fluid, lymphocytes, flow cytometry, NHL, cerebral lymphatic system
Citation: Cordone I, Masi S, Giannarelli D, Pasquale A, Conti L, Telera S, Pace A, Papa E, Marino M, de Fabritiis P and Mengarelli A (2021) Major Differences in Lymphocyte Subpopulations Between Cerebrospinal Fluid and Peripheral Blood in Non-Hodgkin Lymphoma Without Leptomeningeal Involvement: Flow Cytometry Evidence of a Cerebral Lymphatic System. Front. Oncol. 11:685786. doi: 10.3389/fonc.2021.685786
Received: 25 March 2021; Accepted: 22 April 2021;
Published: 03 June 2021.
Edited by:
Alessandro Isidori, AORMN Hospital, ItalyReviewed by:
Roberta Rudà, University Hospital of the City of Health and Science of Turin, ItalyCopyright © 2021 Cordone, Masi, Giannarelli, Pasquale, Conti, Telera, Pace, Papa, Marino, de Fabritiis and Mengarelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iole Cordone, aW9sZS5jb3Jkb25lQGlmby5nb3YuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.