
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 12 August 2021
Sec. Cancer Epidemiology and Prevention
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.684917
This article is part of the Research Topic Disparities in Cancer Prevention and Epidemiology View all 15 articles
Background: Cancer has become the leading cause of mortality in Singapore and among other Asian populations worldwide. Despite the presence of National Cancer Screening programmes in Singapore, less than half of the population has had timely screening according to guidelines. The underlying factors of poor cancer screening rates and health outcomes among Asian ethnic groups remain poorly understood. We therefore examined cancer screening participation rates and screening behavior in a multi-ethnic Singapore population.
Methods: We collected data from 7,125 respondents of the 2015–2016 Singapore Community Health Study. Factors associated with cervical, breast, and colorectal cancer screening were evaluated using modified Poisson regression. Adjusted prevalence ratios were computed with 95% confidence intervals after adjusting for confounders.
Results: The mean age of the respondents was 57.7 ± 10.9 years; 58.9% were female and were predominately Chinese (73.0%), followed by Malay (14.2%), and Indian (10.9%). Less than half of the respondents in the recommended age groups had undergone cancer screening (cervical, 43%; breast, 35.1%; colorectal, 27.3%). Malay respondents were significantly less likely to screen as recommended for cervical (aPR = 0.75, CI = 0.65–0.86, p < 0.001), breast (aPR = 0.83, CI = 0.68–0.99, p = 0.045), and colorectal cancer (aPR = 0.55, CI = 0.44–0.68, p < 0.001), as compared to Chinese respondents. Respondents who had obtained lower secondary level education were 42% more likely to screen for cervical cancer (aPR = 1.42, CI = 1.23–1.64, p < 0.001), and 22% more likely to screen for breast cancer (aPR = 1.22, CI = 1.02–1.46, p = 0.032), compared to those with primary level education and below. Respondents with a household income ≥S$10,000/month were 71% more likely to screen for breast cancer (aPR = 1.71, CI = 1.37–2.13, p < 0.001), as compared with <$2,000/month.
Conclusions: Ethnicity and socio-economic status were significantly associated with lower uptake of cancer screening tests in Singapore. To improve the screening uptake among disadvantaged groups, a multi-faceted approach is needed that addresses the barriers to screening such as the adequacy of subsidy schemes and ethnic differences.
GLOBOCAN estimated 18.1 million new cases and 9.6 million cancer deaths worldwide in 2018 (1). Approximately half of the global burden of cancer was attributed to Asia in part due to 60% of the global population residing there and is projected to continue increasing as life expectancy improves (1). Cancer is the leading cause of mortality among both native and immigrant Asians irrespective of their country of residence (2–6). Those residing in Western countries where they are the ethnic minority are more likely to present with advanced stages of cancer and to have lower cancer screening rates in comparison to non-Hispanic whites (2–6). A study in Canada demonstrated breast cancer screening disparities among immigrant women by world region of origin and found that South Asian women, which included Indians, had the lowest screened as recommended rate at 48.5%. East Asian and Pacific women, which included Chinese, had a screened as recommended rate of 61.1% (7). In another study in the United States, regression models showed that foreign-born women from Southeast Asia, which included Singaporean Chinese, Indian and Malays, were more likely to be unscreened for cervical cancer (13.7%) compared to US-born women (7.6%) (8). Studies conducted in Western countries are often too underpowered to distinguish different Asian ethnic sub-groups (9, 10). Singapore is an opportune country to explore cancer screening behaviors among Asian ethnic sub-groups due to the nation’s large population of East Asians (Chinese), South Asians (Indians), and South East Asians (Malays).
In Singapore, cancer was the leading cause of death with 29.1% of total deaths in 2017 (11, 12). The Singapore Cancer Registry data showed that colorectal cancer (17.2%) had replaced lung cancer (14.8%) to become the most common cancer in men (13). Breast cancer (29.1%) and colorectal cancer (13.4%) remained the most common cancers in women (13). National Cancer Screening programmes have been launched to reduce morbidity and mortality in breast, cervical, and colorectal cancers. Through the Health Promotion Board (HPB), Singapore became the first Asian country to launch a population-wide national breast cancer screening programme in 2002 for females aged 50–69 years (14), which was shortly followed by the launch of a national cervical cancer screening programme in 2004 for females aged 25–69 years (15). From 2003, Singapore Cancer Society has been involved in large-scale opportunistic colorectal cancer screening. In 2011, HPB launched a national screening programme for colorectal cancer for individuals aged 50 and above (16). Although public awareness of screening and accessibility increased, the National Health Survey 2010 data showed that timely screening remained low with less than half of the population having had timely screening according to guidelines (17). Therefore, it is necessary to evaluate the progress of cancer screening.
This study aims to examine cervical, breast, and colorectal cancer screening behaviors in Singapore and identify how socio-demographic factors such as ethnicity and socio-economic status are associated with cancer screening rates. We will also examine the extent of the knowledge–behavior gap in cancer screening behavior. In doing so, we aim to better understand the determinants of cancer screening behaviors in the population of Singapore to improve screening programmes for the under-screened groups.
Data used in this cross-sectional study was derived from the Singapore Community Health Study (CHS), a population health survey that was conducted in Queenstown and Bukit Panjang (18, 19) between April 2015 and August 2016. The surveyed districts were catchment areas for the National University Health System and resembled the age, gender, and ethnic distribution of the national population census (20). All Singaporean citizens and permanent residents aged 40 and above were eligible for participation in CHS. A total of 7,125 residents in this age group were interviewed (Bukit Panjang—4,906; Queenstown—2,219).
Recruitment in CHS occurred through community club events and advertisements (banners/posters) in residential blocks. All household members were eligible to participate in the study, which was voluntary and self-selected. Households also received invitation letters at least two weeks before being visited by a trained interviewer. A group of field work team members were required to pass an assessment after undergoing a minimum of three days of training by qualified staff from the University on consent-taking and administering the questionnaire before they were allowed to interview participants. A response rate could not be ascertained due to the multi-modal recruitment process.
Interviewer-administered standardized questionnaires were conducted in the preferred language and location of the participant (own home or at the nearby Residents’ Committee centre). A translator was arranged if required. Informed consent was taken from all participants.
The questionnaire explored socio-demographics (age, gender, ethnicity), socio-economic indicators (education level, household income, housing type), living arrangement (alone or with others), lifestyle practices including smoking and alcohol consumption, medical history (previous cancer diagnosis or any family history of any cancer) and cancer screening practices. Education level was categorized as primary [passing the Primary School Leaving Examination (PSLE)], lower secondary (years 1–3), secondary (passing the Singapore-Cambridge General Certificate of Education (GCE) Normal or Ordinary Level Examination), junior college (passing the GCE-Advanced Level Examination), polytechnic/arts institution (obtaining a diploma), and university (obtaining a degree, masters or PhD). For cervical cancer screening, the questions were: “Do you know what a Pap smear is?”; “Have you ever had a Pap smear test?”; “How long ago did you have your last smear done?”. For breast cancer screening the questions were: “Do you know what a mammogram is?”; “Have you ever had a mammogram?”, “How long ago did you have your last mammography done?”. Finally, for colorectal cancer screening the questions were: “Have you ever had a blood stool test to determine whether the stool contains blood?”; “How long ago did you have your last blood stool test done?’; “Have you ever had either sigmoidoscopy or colonoscopy, an examination in which a tube is inserted in the rectum to view the colon for signs of cancer or other health problems?”; “How long ago did you have your last sigmoidoscopy or colonoscopy done?”.
According to the screening guidelines of Singapore (21), the frequency of cervical and breast cancer screening was considered done as recommended if women aged 25–69 years reported having a Pap smear every 3 years, and if women aged 50–69 years reported having a mammogram every 2 years, respectively. Colorectal cancer screening was done as recommended if fecal occult blood test (FOBT) was done annually or sigmoidoscopy/colonoscopy was done once every 10 years for individuals aged ≥50 years.
Ethics approval was obtained from the National Health Group Domain Specific Review Board (2015-00095) as well as the National University of Singapore IRB (S-19-340).
Baseline characteristics were reported as categorical variables and tabulated using proportions for the descriptive analysis. For estimating prevalence ratios in cross-sectional studies, Zou’s method using multivariate modified Poisson regression with robust sandwich variance was chosen as the most viable statistical option as described in Lee’s Practical Guide for Multivariate Analysis of Dichotomous Outcomes (22). This method was utilized to estimate the adjusted prevalence ratios (aPRs) and 95% confidence intervals (CIs) using R packages lmtest v0.9-3.7 and sandwich v2.5-1. Variables identified as determinants of screening behaviors in previous studies (23–28) that proved to be significant in the univariate analysis for the respective cancer groups (e.g. age, ethnicity, education, household income, housing type, living arrangement, past history of any cancer, family history of any cancer, and frequent smoking) were used to adjust for potential confounding. The analysis was also stratified by family history. A P-value ≤0.05 was used to determine statistical significance. The knowledge–behavior gap was calculated as the difference in proportions between those that reported having knowledge of the screening test and those that ever did the screening test or screened as recommended. All analysis was performed using R version 3.6.2.
Respondents of the survey (N = 7,125) were mostly aged 40–69 years (85%) with a mean age of 57.7 ± 10.9 years and ethnically Chinese (73%) with a slight majority of females (58.9%) (Table 1). The age, gender, and ethnic distribution of our survey sample resembled the population census during the same time period (Supplemental Figures 1–3).
A majority of the screening-eligible female respondents reported having knowledge of Pap smear (80.0%) and mammography (93.6%). At least three quarters had ever been screened (cervical, 77.2%; breast, 75.2%); whereas, less than half had undergone screening as recommended (cervical, 43.0%; breast, 35.1%) (Table 2).
Nearly half of the eligible respondents (49.0%) had ever been screened for colorectal cancer, but only 27.3% had screened within the recommended time period. More respondents had ever had FOBT (42.9%) compared to colonoscopy or sigmoidoscopy (22.1%). Among female respondents aged 50–69 years, only 10.7% had screened for all three cancers (cervical, breast, colorectal) within the recommended time period.
In the multivariate analysis, Malay and Indian ethnicity and higher level of education were significantly associated with reporting having knowledge of the Pap smear test (Table 3).
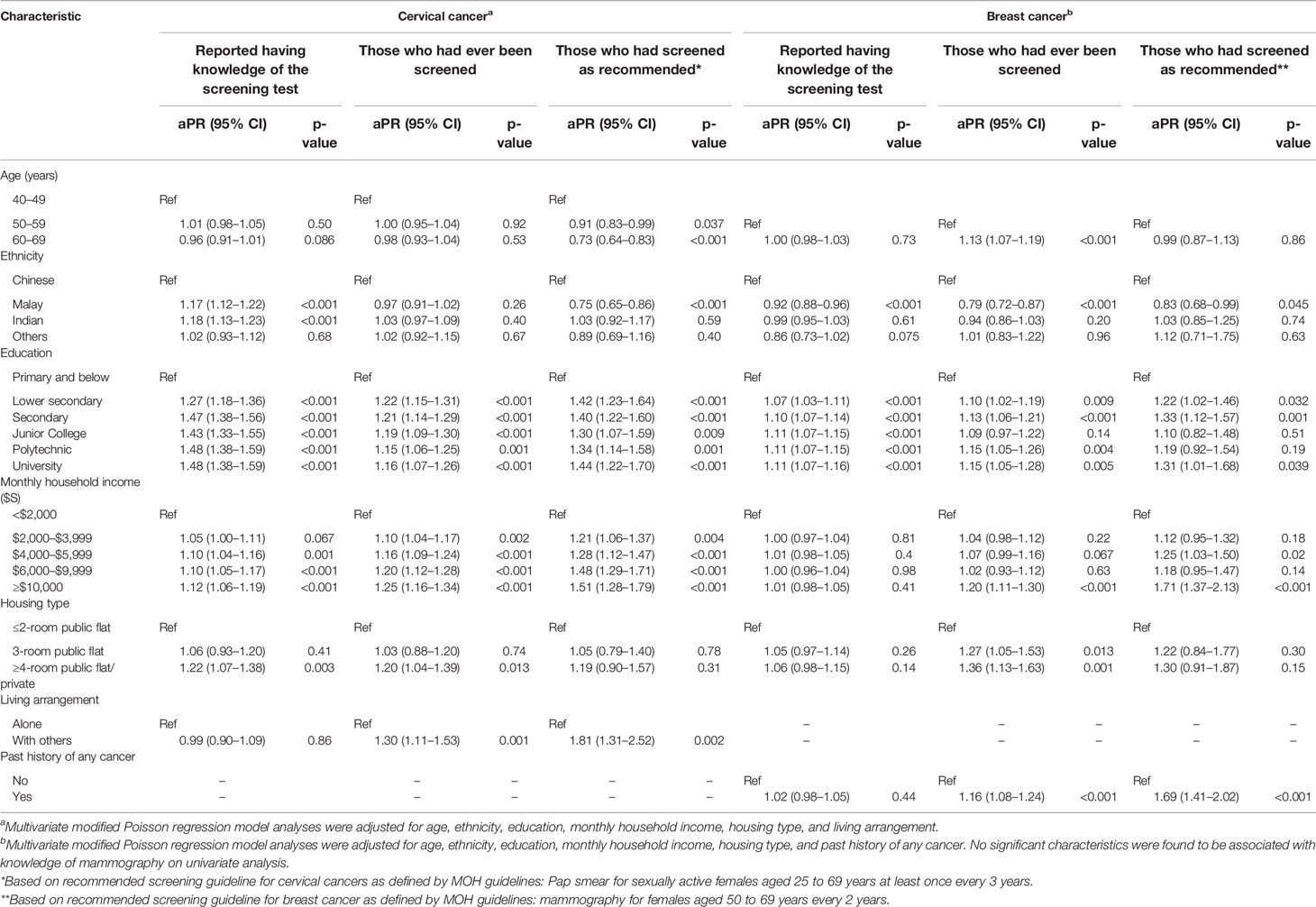
Table 3 Adjusted prevalence ratio (aPR) estimates for characteristics associated with knowledge of and participation in cervical and breast cancer screening.
Individuals of Malay (aPR = 1.17, CI = 1.12–1.22, p < 0.001) and Indian (aPR = 1.18, CI = 1.13–1.23, p < 0.001) ethnicity were more likely to report knowledge of Pap smear testing as compared with ethnic Chinese. In contrast, Malay women were less likely than Chinese women to report having knowledge of mammography (aPR = 0.92, CI = 0.88–0.96, p < 0.001) (Table 3).
All levels of education higher than primary school and below were significantly associated with self-reported knowledge of the screening tests even for those with only lower secondary school education. Compared with having attained at most primary school education, the prevalence of self-reported knowledge regarding Pap smear was already 47% higher at secondary school level education (aPR = 1.47, CI = 1.38–1.56, p < 0.001). Household income and housing type showed weaker associations with self-reported Pap smear knowledge.
Education level and household income were significantly associated with ever having a Pap smear test (Table 3). In addition, women living with others (aPR = 1.30, CI = 1.11–1.53, p = 0.001) were 30% more likely to ever have a Pap smear compared with those living alone. Older age, higher education level, high household income, and having a more expensive housing type were significantly associated with ever having a mammogram, whereas Malay ethnicity was associated with a lower likelihood of ever having a mammogram (Table 3).
Among those who reported no knowledge of the screening tests (N = 711 for Pap smear; N = 161 for mammogram), 44.7% underwent screening with Pap smear (n = 318) and 26.1% with mammogram (n = 42). For Pap smear, respondents of Malay (aPR = 0.45, CI = 0.27–0.75, p = 0.002) and Indian (aPR = 0.36, CI = 0.16–0.82, p = 0.015) ethnicity were less likely to report this behavior compared to Chinese (Supplemental Table 1). The sub-group analysis was not reported for mammogram due to the small sample size.
Participants of Malay ethnicity (aPR = 0.75, CI = 0.65–0.86, p < 0.001) and those aged 60–69 years (aPR = 0.73, CI = 0.64–0.83, p < 0.001) were significantly less likely to undergo Pap smear screening as recommended at least once every three years (Table 3). Socio-economic factors directly associated with screening as recommended were higher education level and higher household income. Respondents living with others (aPR = 1.81, CI = 1.31–2.52, p = 0.002) were 81% more likely to screen as recommended compared to those living alone. Similar to cervical cancer screening, Malay ethnicity (aPR = 0.83, CI = 0.68–0.99, p = 0.045) was observed to be less likely to screen for breast cancer as recommended compared to Chinese. Higher education and higher household income were also significantly associated with mammogram screening as recommended at least once every two years (Table 3). A higher proportion of respondents reported desirable cancer screening behavior among those who had any family history of any cancer in comparison with those without any family history (Supplementary Table 4).
Older age (60–79 years), higher education level, higher household income, past history of any cancer, and family history of any cancer were significantly associated with having ever screened for colorectal cancer by FOBT and/or scope (colonoscopy/sigmoidoscopy) (Table 4). Malay and Indian respondents as well as those who smoked daily were significantly less likely to be ever screened. The same variables that were significantly associated with having ever been screened by FOBT, colonoscopy, or sigmoidoscopy were also significantly associated with screening as recommended (Table 4).
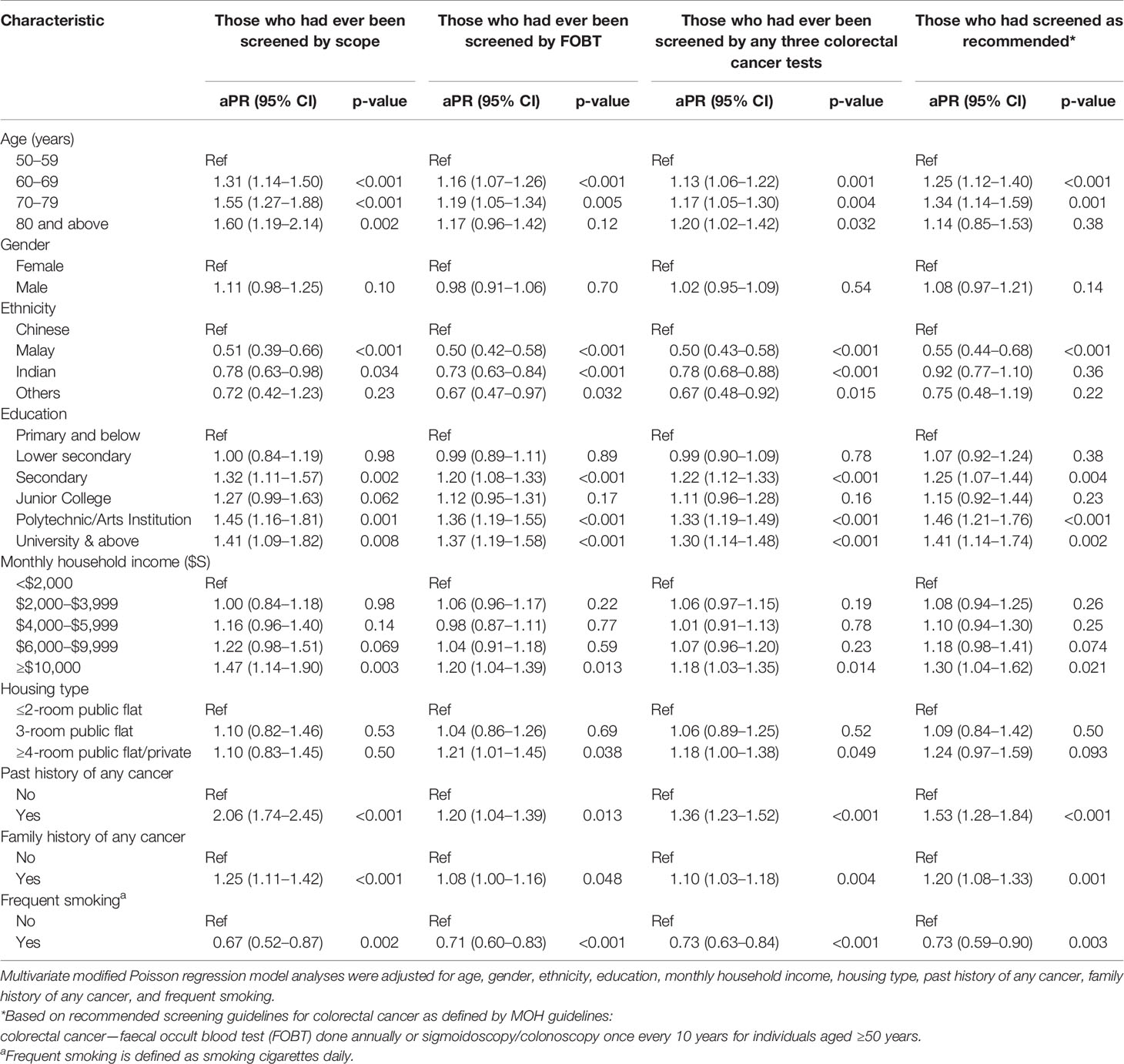
Table 4 Adjusted prevalence ratio (aPR) estimates for characteristics associated with participation in colorectal cancer screening.
A key difference was that among the ethnic groups, only Malay ethnicity (aPR = 0.55, CI = 0.44–0.68, p < 0.001), and not Indian ethnicity, remained significantly associated with a lower likelihood of screening as recommended.
We examined determinants of screening as recommended for all three cancers among eligible women aged 50–69. Higher level of education and higher household income were significantly associated with having screened as recommended for all three cancers, whereas Malay ethnicity (aPR = 0.53, CI = 0.33–0.84, p = 0.008) was significantly associated with a lower likelihood as compared with Chinese ethnicity (Supplemental Table 2).
The gap between the percentage that reported knowledge of Pap smear and were ever screened with Pap smear was 2.8% (Table 2). For mammography, the gap was higher at 18.4%. Our multivariate analysis indicated the Malay ethnicity was in general less likely to exhibit cancer screening behavior compared with ethnic Chinese. The knowledge–behavior gap among the ethnicities was calculated using the difference in proportions between those that reported having knowledge of the screening test and those that ever did the screening test or screened as recommended. For ever having done the screening test, Malays had the largest knowledge–behavior gap with 13.1% for Pap smear and 26.5% for mammography (Figure 1).
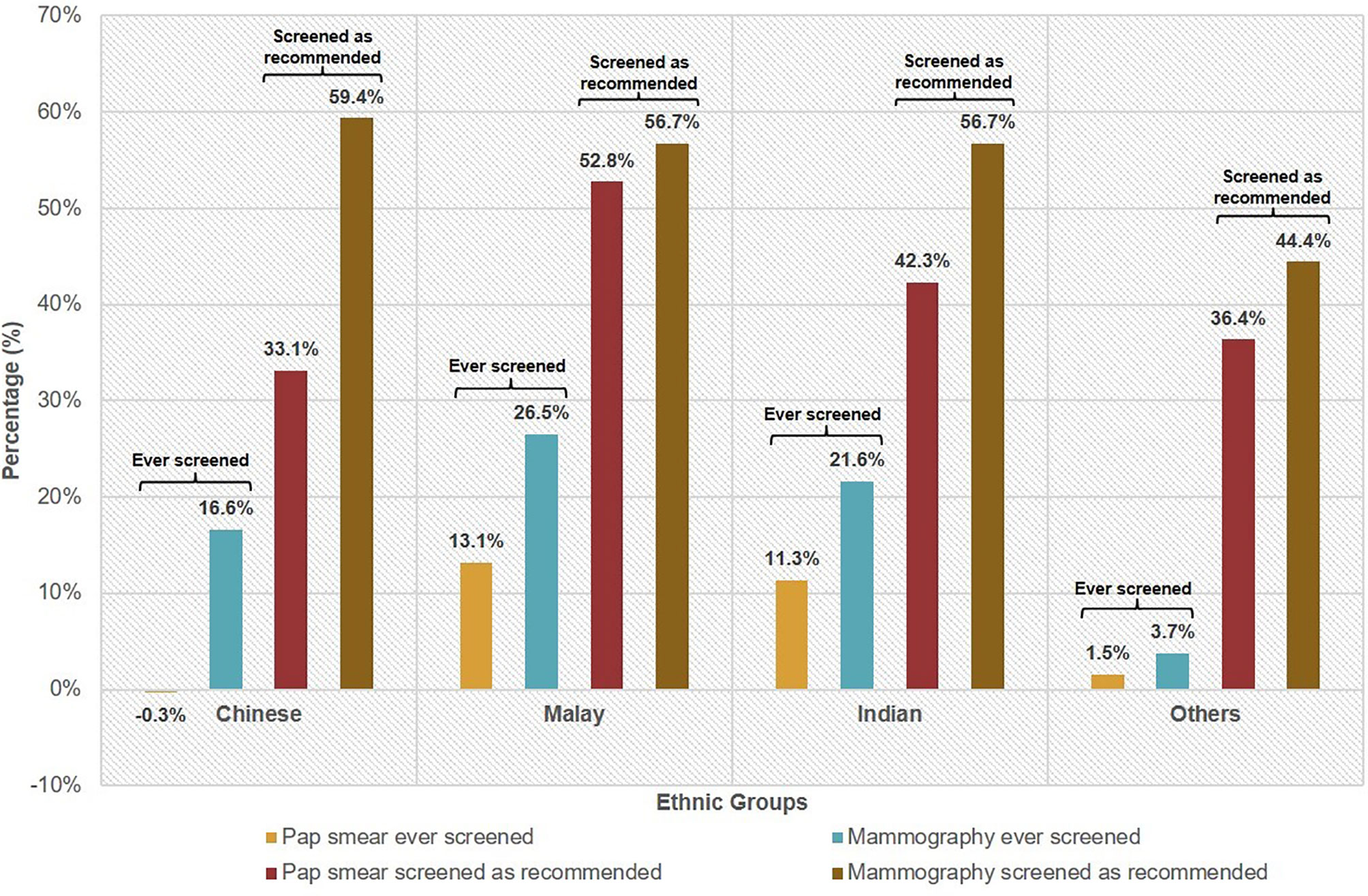
Figure 1 Knowledge–behaviour gap╤ of female cancer screening* by ethnicity. ╤Knowledge–behaviour gap is defined as the difference in proportions between those that reported having knowledge of the screening test and those that ever did the screening test or screened as recommended. *Based on recommended screening guidelines for selected cancers as defined by MOH guidelines: cervical cancer—Pap smear for sexually active females aged 25 to 69 years at least once every 3 years; breast cancer—mammography for females aged 50 to 69 years every 2 years.
Likewise, Malays exhibited the largest knowledge–behavior gap at 52.8% for having screened with Pap smear as recommended. For having screened with mammography as recommended, the gaps were similarly high across the three ethnicities—Chinese (59.4%), Malay (56.7%), Indian (56.7%).
Although screening recommendation guidelines vary slightly between countries, our screened as recommended participation rates fell behind other high-income East Asian countries such as Taiwan in 2016 (cervical, 72.1%; breast, 39.3%; colorectal, 40.7%) (29), and South Korea in 2014 (cervical, 66.1%; breast, 66.0%; colorectal, 29.1%) (30). We also performed poorer compared to Western countries such as the United States in 2015 (cervical, 81%; breast, 71.6%; colorectal, 62.9%) (31) and the United Kingdom in 2017/18 (cervical, 71.4%; breast, 71.1%; colorectal, 57.7%) (32).
Compared to the cancer screening participation rates measured in the 2004 and 2010 national health surveys (17, 33), our screened as recommended participation rates did not indicate significant improvements (Supplemental Table 3). For example, the proportion of women who had gone for mammogram as recommended was 35.1% in our study, down from 39.6% in 2010. The proportion of Singapore residents who underwent colorectal screening as recommended was 27.3% in our study, up from 20.2% in 2010. Although the health promotion efforts over the years may have resulted in only modest changes in the screened as recommended participation rates, it is reassuring to observe that between 2004 and 2016, the ever screened rates have seen an upward trend (cervical, 70.1 vs 77.1%; breast, 54.2 vs 75.2%) in tandem with a downward trend in the size of the knowledge–behavior gap (cervical, 10.7 vs 2.8%; breast, 25.7 vs 18.4%). Improvements were also seen in colorectal cancer screening participation rates between 2004 and 2016 in ever screened with FOBT (17.3 vs 42.9%) and ever screened with sigmoidoscopy/colonoscopy (11.2 vs 22.1%).
Our results demonstrate that screening knowledge and behaviors differ substantially by socio-economic status and ethnicity in Singapore. Higher educational level and household income were found to be significantly associated with screening as recommended for cervical, breast, and colorectal cancers. Malay ethnicity was associated with a lower likelihood of screening as recommended as compared with Chinese ethnicity. Cancer screening disparities associated with socio-economic status and ethnicity were reported in previous studies in Singapore (25–27, 34–38), as well as internationally (39, 40). However, limitations to the existing local literature include small sample sizes of the Malay and Indian ethnic minorities with oversampling of the Chinese ethnic majority, assessment of a single cancer screening modality, and age of the data. These limit the ability to generalize findings to the population and develop targeted population health interventions. Our study attempts to better estimate the true population effect sizes through our large representative sample size of 5,203 Chinese, 1,014 Malay, and 777 Indian respondents in the community setting.
Over the years, the Singapore Ministry of Health has endeavored to address the need to improve cancer screening participation rates, which culminated in the 2017 launch of the Enhanced Screen for Life Programme by the Health Promotion Board. This enabled eligible Singaporeans to screen for cervical, breast, and colorectal cancer from as low as $0–$5 per screening visit (41). Although affordability is an important consideration to address the socio-economic disparities, the continued low participation rates suggest there are additional barriers to address. A survey conducted at four polyclinics in Singapore reported that the most commonly cited reasons for not attending breast cancer screening programmes were lack of any breast problems, lack of time, and fear of pain (37). Another local mixed-methods study on barriers to breast and cervical cancer screening reported that fear of unnecessary treatments, ineffective treatments for early stage cancer, and low test sensitivity for early stage cancer were barriers to screening (28).
The proportion of those reporting having a family history of cancer was similar across cervical, breast, and colorectal cancer screening respondents; however, the association between a positive family history of cancer and cancer screening was only found to be significant among colorectal cancer screening respondents. While other studies have also reported this association among Asian women (26, 42), local screening rates particularly among the higher risk first degree relatives of colorectal cancer patients continue to be low (43, 44). Barriers include poor understanding of the screening guidelines, lack of health promotion messaging by healthcare professionals, fear of the test and the diagnosis, scheduling difficulties, feeling invulnerable since young and asymptomatic, unawareness of genetic risk, and the high cost of colonoscopy (43–45). Risk perception should be emphasized in health promotion messaging among Asian ethnicities as perceived susceptibility to breast, cervical, and colorectal cancers was found to be the lowest among Asian women as compared with White, African American, and Latino women (42).
Observing past studies in tandem with our current study, there is a repetitive trend of Malay ethnicity being less likely to participate in cancer screening when compared to the Chinese ethnic majority and their Indian counterparts (17, 26, 27, 33, 46, 47). For female cancer screening, this may be partly explained by the knowledge–behavior gap demonstrated in our study. This gap may be linked to cultural beliefs among Asian women, which should be appropriately understood in order to craft effective policies and health promotion messages. Previous studies have reported cancer screening barriers related to social stigma, personal modesty, fatalistic attitudes, beliefs that breast cancer is a Western women-affliction, beliefs that mammograms cause cancer, and a preference to be unaware of a fatal disease diagnosis to postpone accompanying fears (28, 34, 37, 48–52). However, these findings are limited to predominantly Chinese respondents. In the neighboring country Malaysia with a high proportion of ethnic Malays, their National Health & Morbidity Survey in 2006 showed that only 7.9% of eligible women had underwent mammography as recommended, and only 12.8% had underwent Pap smear as recommended in 2011 (53). Malaysian studies have reported that Malay women are apprehensive about doing Pap smears especially if they are single or unmarried as it indicates sexual activity. A woman’s partner or family members also hold great influence over decisions to screen due to strong family ties. Lack of knowledge among partners and male family members as well as perceived inaccessibility to a female health-care provider are commonly reported barriers (54–56). Similarly, the presence of male technicians/radiographers was found to be a barrier to mammogram screening (57).
The difference in the knowledge–behavior gap between ethnicities alludes to potential health literacy issues related to language barriers in Pap smear testing. Limited English proficiency and low health literacy among Asian women have been identified as barriers to cancer screening in several international studies where English is the predominant language (58–63). We also observed the phenomenon of Chinese women proceeding with Pap smear testing, despite not being fully aware of the purpose of the test. This may be linked to high trust among Chinese women towards their primary physician, which was reported by a study among Redhill residents in Singapore who were predominantly Chinese. Over half of the respondents rated trust towards primary care doctors and the medical profession as high or very high (64), which has been supported by other studies that reported high regard towards general practitioners in the Asian context (65, 66).
In our study, the knowledge–behavior gap was higher for mammography (18.4%) than for Pap smear (2.8%). Previous studies have suggested logistical and operational issues as reasons for the difference between uptake of Pap smear versus mammograms (34, 51, 52). The widespread availability of Pap smear tests as a bedside procedure in general practice clinics has made it readily accessible in contrast to the limited availability of mammography. In addition, most patients are able to state preferences or choose female doctors to perform the Pap smears; however, there is no freedom of choice for radiographer doing the mammograms. Having a male radiographer has been shown to be a barrier to screening in both Western and Asian cultures (67–70).
Strategies to further narrow the knowledge–behavior gap should include developing tailored cancer screening promotion campaigns for the Malay ethnic group, which can be done in close consultation with employers, religious, and community authorities to ensure the messages stay culturally relevant (71–77). To further incentivize cancer screening behavior, we must inculcate a culture of cancer screening through community screening initiatives so that they are seen as a form of social event (71, 78, 79). Targeted and frequent mass media campaigns have been shown to be effective in increasing awareness and compliance for cancer screening (71, 80, 81) as well as being frequently exposed to reminders with cues to action (23, 24, 71, 82, 83). Addressing polyclinic proximity and screening appointment logistics may contribute to improving mammography uptake (51). Further studies will need to be done on Malay-specific barriers and facilitators for screening across the three screening modalities as our analysis showed that only 10.7% screened as recommended for all three, and Malays had a higher propensity to not be screened. Existing studies in Singapore had predominantly Chinese respondents and focused on specific screening modalities (23–28). Further studies comparing cancer screening knowledge and behavior between Malays residing in Singapore versus Malays residing in Malaysia would help to elicit environmental and cultural influences.
Strengths of this study include a large sample population that resembles the overall age, ethnic, and gender distribution of the Singapore population (Supplemental Figures S1–S3) (84). Self-selection bias was minimized through the use of a door-to-door recruitment strategy. Misclassification due to interviewer bias, social desirability bias, or recall bias was reduced through the use of a standardized questionnaire consisting of closed-ended, easy to understand questions, simple response options, and trained interviewers that followed the designed question and answer format strictly. However, there are a few limitations to our study. As our survey questions were modelled after the National Health Survey to allow for comparisons, the questions did not differentiate whether the tests were done for screening or diagnostic purposes. In addition, the questions did not differentiate if the participant was screening regularly as recommended or had coincidentally last screened in the recommended time period. As a result, the reported screened as recommended participation rates may be an overestimation of the true value. We were unable to corroborate the self-reported cancer screening data with objective data from medical databases. Another limitation was the inclusion criteria due to the interest of regional health system in targeting interventions on those aged 40 and above in their catchment area, which meant the cervical cancer screening age group from 25 to 39 years was unrepresented. Due to this targeted population, all household members who met the inclusion criteria were included in the Community Health Study; however, data on the proportion of households with more than one member who participated in the study were not available, and statistical analysis adjusting for such potential clustering effects was not performed.
Cancer screening knowledge and behaviors differ substantially between Asian ethnic sub-groups even within the confines of the island state of Singapore. Asian ethnicity represents a heterogeneous group with different religious and cultural traditions, and our results suggest that it is important to distinguish different ethnic sub-groups in future studies of screening behavior. Ethnic Malays are therefore, a key target population for further research and interventions to narrow the knowledge–behavior gap. Design of targeted cancer screening programmes and health promotion messaging by healthcare providers should include sensitivity to ethnic differences as well as female-specific cancer screening facilitators and barriers, which will help to further increase the uptake of cancer screening. The population-based cancer screening programmes are essential to Singapore’s preventive health strategy. The availability of subsidized rates has allowed more members of the population to access cancer screening, but the overall cancer screening rates still remain low. Socio-economic factors such as educational and income level remain important aspects that policy makers and healthcare organizations should address to improve cancer screening.
The datasets used in this article are available from the corresponding authors on reasonable request.
The studies involving human participants were reviewed and approved by National Health Group Domain Specific Review Board (2015-00095) and the National University of Singapore IRB (S-19-340). The patients/participants provided their written informed consent to participate in this study.
TC participated in the design of this study, performed the statistical analysis, interpreted the data, and drafted the manuscript. LT participated in the design of the Community Health Study, coordination, and data collection. RD is the principal investigator of the Community Health Study and participated in the manuscript revision of this study. WJS participated in the design of this study, the statistical analysis, interpretation of the data, and the manuscript revision. All authors contributed to the article and approved the submitted version.
The publication of this report was funded by the National University of Singapore Start-Up and the Ministry of Education Tier 1 grants. The Community Health Study was supported by grants from the Ministry of Health, Singapore, National University of Singapore and National University Health System, Singapore. The funding bodies had no role in the interpretation of the data and the formulation of this report.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank Dr. Asano Miho, Associate Professor Wong Mee Lian, Dr. Lim Wei-Yen, and Ms. Mao Yinan for their valuable comments and assistance.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.684917/full#supplementary-material
Supplementary Table 1 | Adjusted prevalence ratio (aPR) estimates for characteristics associated with those tested without knowledge of Pap smear. Multivariate modified Poisson regression model analyses were adjusted for age, ethnicity, education, monthly household income, housing type, and living arrangement. *Based on recommended screening guideline for cervical cancers as defined by MOH guidelines: Pap smear for sexually active females aged 25 to 69 years at least once every 3 years.
Supplementary Table 2 | Adjusted prevalence ratio (aPR) estimates for characteristics associated with screening as recommended for all three cancers. Multivariate modified Poisson regression model analyses were adjusted for age, ethnicity, education, monthly household income, housing type, living arrangement, past history of any cancer, family history of any cancer, and frequent smoking. *Based on recommended screening guidelines for selected cancers as defined by MOH guidelines: cervical cancer—Pap smear for sexually active females aged 25 to 69 years at least once every 3 years; breast cancer—mammography for females aged 50 to 69 years every 2 years; colorectal cancer–faecal occult blood test (FOBT) done annually or sigmoidoscopy/colonoscopy once every 10 years for individuals aged ≥50 years. aFrequent smoking is defined as smoking cigarettes daily.
Supplementary Table 3 | Cancer screening participation rates in Singapore. NHS National Health Survey; CHS, Community Health Survey. Unless otherwise stated, the screening questions involved age groups 25–69 for cervical, 50–69 for breast, and 50 and above for colorectal. ¢CHS 2016 age groups were 40–69 for cervical screening questions. ╤The difference in proportion between knowledge of the cancer screening test and ever screened with the test.
Supplementary Table 4 | Cancer screening knowledge and participation rates, stratified by family history.
Supplementary Figure 1 | Age distribution in the survey sample in comparison to Singapore’s population. **Based on Singapore SingStat 2016 Population Data ages 40 and above.
Supplementary Figure 2 | Gender distribution in the survey sample in comparison to Singapore’s population. **Based on Singapore SingStat 2016 Population Data ages 40 and above.
Supplementary Figure 3 | Ethnic distribution in the survey sample in comparison to Singapore’s population. **Based on Singapore SingStat 2016 Population Data ages 40 and above.
aPR, adjusted prevalence ratio; CI, confidence interval; HPB, Health Promotion Board; FOBT, faecal occult blood test; NUS, National University of Singapore; SSHSPH, Saw Swee Hock School of Public Health.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Thompson CA, Gomez SL, Hastings KG, Kapphahn K, Yu P, Shariff-Marco S, et al. The Burden of Cancer in Asian Americans: A Report of National Mortality Trends by Asian Ethnicity. Cancer Epidemiol Biomarkers Prev (2016) 25:1371–82. doi: 10.1158/1055-9965.EPI-16-0167
3. Sentell T, Braun KL, Davis J, Davis T. Health Literacy and Meeting Breast and Cervical Cancer Screening Guidelines Among Asians and Whites in California. Springerplus (2015) 4:432. doi: 10.1186/s40064-015-1225-y
4. Szczepura A, Price C, Gumber A. Breast and Bowel Cancer Screening Uptake Patterns Over 15 Years for UK South Asian Ethnic Minority Populations, Corrected for Differences in Socio-Demographic Characteristics. BMC Public Health (2008) 8:346. doi: 10.1186/1471-2458-8-346
5. Lee HY, Ju E, Vang PD, Lundquist M. Breast and Cervical Cancer Screening Disparity Among Asian American Women: Does Race/Ethnicity Matter [Corrected]? J Womens Health (Larchmt) (2010) 19:1877–84. doi: 10.1089/jwh.2009.1783
6. Lofters AK, Hwang SW, Moineddin R, Glazier RH. Cervical Cancer Screening Among Urban Immigrants by Region of Origin: A Population-Based Cohort Study. Prev Med (2010) 51:509–16. doi: 10.1016/j.ypmed.2010.09.014
7. Vahabi M, Lofters A, Kumar M, Glazier RH. Breast Cancer Screening Disparities Among Immigrant Women by World Region of Origin: A Population-Based Study in Ontario, Canada. Cancer Med (2016) 5:1670–86. doi: 10.1002/cam4.700
8. Endeshaw M, Clarke T, Senkomago V, Saraiya M. Cervical Cancer Screening Among Women by Birthplace and Percent of Lifetime Living in the United States. J Low Genit Tract Dis (2018) 22:280–7. doi: 10.1097/LGT.0000000000000422
9. Thompson CA, Gomez SL, Chan A, Chan JK, McClellan SR, Chung S, et al. Patient and Provider Characteristics Associated With Colorectal, Breast, and Cervical Cancer Screening Among Asian Americans. Cancer Epidemiol Biomarkers Prev (2014) 23:2208–17. doi: 10.1158/1055-9965.EPI-14-0487
10. Torre LA, Sauer AM, Chen MS, Kagawa-Singer M, Jemal A, Siegel RL. Cancer Statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: Converging Incidence in Males and Females. CA Cancer J Clin (2016) 66:182–202. doi: 10.3322/caac.21335
11. Trinh-Shevrin C, Sacks R, Ahn J, Yi SS. Opportunities and Challenges in Precision Medicine: Improving Cancer Prevention and Treatment for Asian Americans. J Racial Ethn Health Disparities (2018) 5:1–6. doi: 10.1007/s40615-016-0334-9
12. Principal Causes of Death. Singapore: Ministry of Health (2018). Available at: https://www.moh.gov.sg/resources-statistics/singapore-health-facts/principal-causes-of-death.
13. Lee HP, Ling A, Foo LL, Kuo SM, Lee E, Lim GK, et al. Singapore Cancer Registry Annual Registry Report. Singapore: Health Promotion Board (2015).
14. Wang SC. The Singapore National Breast Screening Programme: Principles and Implementation. Ann Acad Med Singapore (2003) 32:466–76.
15. Yeoh KG, Chew L, Wang SC. Cancer Screening in Singapore, With Particular Reference to Breast, Cervical and Colorectal Cancer Screening. J Med Screen (2006) 13 Suppl 1:S14–9.
16. Tan WS, Tang CL, Koo WH. Opportunistic Screening for Colorectal Neoplasia in Singapore Using Faecal Immunochemical Occult Blood Test. Singapore Med J (2013) 54:220–3. doi: 10.11622/smedj.2013077
18. van Dam RM, Merchant RA, Tan Wl, Foo jm, Yap KS. Community Health @ Queenstown: A Report on the Baseline Survey. Singapore: National University of Singapore p. 1–41.
19. van Dam RM, Merchant RA, Tan WL, Foo JM, Yao J. Community Health @ Bukit Panjang: A Report on the Baseline Survey. Singapore: National University of Singapore p. 1–45.
20. Singapore Residents by Planning Area Subzone, Age Group, Sex and Type of Dwelling, June 2011-2019. Singapore: Department of Statistics Singapore. Available at: https://www.singstat.gov.sg/-/media/files/find_data/population/statistical_tables/singapore-residents-by-planning-areasubzone-age-group-sex-and-type-of-dwelling-june-20112019.zip.
22. Lee J, Tan CS, Chia KS. A Practical Guide for Multivariate Analysis of Dichotomous Outcomes. Ann Acad Med Singapore (2009) 38:714–9.
23. Seow A, Straughan PT, Ng EH, Lee HP. A Randomized Trial of the Use of Print Material and Personal Contact to Improve Mammography Uptake Among Screening Non-Attenders in Singapore. Ann Acad Med Singapore (1998) 27:838–42.
24. Seow A, Huang J, Straughan PT. Effects of Social Support, Regular Physician and Health-Related Attitudes on Cervical Cancer Screening in an Asian Population. Cancer Causes Control (2000) 11:223–30. doi: 10.1023/A:1008954606992
25. Ong CS, Ooi G, Tan XQ, Lee J, Koh GC, Verkooijen HM. Prevalence of Limited Cancer Knowledge in Singaporeans, Its Determinants and Association With Cancer Screening. Prev Med (2010) 50:304–5. doi: 10.1016/j.ypmed.2010.02.013
26. Wong RK, Wong ML, Chan YH, Feng Z, Wai CT, Yeoh KG. Gender Differences in Predictors of Colorectal Cancer Screening Uptake: A National Cross Sectional Study Based on the Health Belief Model. BMC Public Health (2013) 13:677. doi: 10.1186/1471-2458-13-677
27. Wong HZ, Lim WY, Ma SS, Chua LA, Heng DM. Health Screening Behaviour Among Singaporeans. Ann Acad Med Singapore (2015) 44:326–34.
28. Malhotra C, Bilger M, Liu J, Finkelstein E. Barriers to Breast and Cervical Cancer Screening in Singapore: A Mixed Methods Analysis. Asian Pac J Cancer Prev (2016) 17:3887–95.
32. Report of the Independent Review of Adult Screening Programmes in England. United Kingdom: NHS England (2019).
34. Seow A, Wong ML, Smith WC, Lee HP. Beliefs and Attitudes as Determinants of Cervical Cancer Screening: A Community-Based Study in Singapore. Prev Med (1995) 24:134–41. doi: 10.1006/pmed.1995.1026
35. Wee LE, Koh GC, Toh ZJ. Multi-Disease Health Screening in an Urban Low-Income Setting: A Community-Based Study. Ann Acad Med Singapore (2010) 39:750–7.
36. Sim HL, Seah M, Tan SM. Breast Cancer Knowledge and Screening Practices: A Survey of 1,000 Asian Women. Singapore Med J (2009) 50:132–8.
37. Lim SK, Teo XL, Ng JL, Li FX, Tan SM. A Survey on Singaporean Women’s Knowledge, Perception and Practices of Mammogram Screening. Ann Acad Med Singapore (2015) 44:317–25.
38. Teo CT, Yeo YW, Lee SC. Screening Mammography Behavior and Barriers in Singaporean Asian Women. Am J Health Behav (2013) 37:667–82. doi: 10.5993/AJHB.37.5.11
39. Breen N, Wagener DK, Brown ML, Davis WW, Ballard-Barbash R. Progress in Cancer Screening Over a Decade: Results of Cancer Screening From the 1987, 1992, and 1998 National Health Interview Surveys. J Natl Cancer Inst (2001) 93:1704–13. doi: 10.1093/jnci/93.22.1704
40. James AS, Hall S, Greiner KA, Buckles D, Born WK, Ahluwalia JS. The Impact of Socioeconomic Status on Perceived Barriers to Colorectal Cancer Testing. Am J Health Promot (2008) 23:97–100. doi: 10.4278/ajhp.07041938
41. Enhanced Screen for Life. Singapore: Ministry of Health (2019). Available at: https://www.moh.gov.sg/cost-financing/healthcare-schemes-subsidies/enhanced-screen-for-life.
42. Kim SE, Perez-Stable EJ, Wong S, Gregorich S, Sawaya GF, Walsh JM, et al. Association Between Cancer Risk Perception and Screening Behavior Among Diverse Women. Arch Intern Med (2008) 168:728–34. doi: 10.1001/archinte.168.7.728
43. Tan KK, Lopez V, Wong ML, Koh GC. Uncovering the Barriers to Undergoing Screening Among First Degree Relatives of Colorectal Cancer Patients: A Review of Qualitative Literature. J Gastrointest Oncol (2018) 9:579–88. doi: 10.21037/jgo.2018.03.02
44. Tan KK, Lim TZ, Chan DKH, Chew E, Chow WM, Luo N. Getting the First Degree Relatives to Screen for Colorectal Cancer Is Harder Than It Seems-Patients’ and Their First Degree Relatives’ Perspectives. Int J Colorectal Dis (2017) 32:1065–8. doi: 10.1007/s00384-017-2818-4
45. Yong SK, Ong WS, Koh GC-H, Yeo RMC, Ha TC. Colorectal Cancer Screening: Barriers to the Faecal Occult Blood Test (FOBT) and Colonoscopy in Singapore. Proc Singapore Healthcare (2016) 25:207–14. doi: 10.1177/2010105816643554
48. Huang X, Butow P, Meiser B, Goldstein D. Attitudes and Information Needs of Chinese Migrant Cancer Patients and Their Relatives. Aust New Z J Med (1999) 29:207–13. doi: 10.1111/j.1445-5994.1999.tb00685.x
49. Bottorff JL, Johnson JL, Bhagat R, Grewal S, Balneaves LG, Clarke H, et al. Beliefs Related to Breast Health Practices: The Perceptions of South Asian Women Living in Canada. Soc Sci Med (1998) 47:2075–85. doi: 10.1016/S0277-9536(98)00346-3
50. Kliewer EV, Smith KR. Breast Cancer Mortality Among Immigrants in Australia and Canada. J Natl Cancer Inst (1995) 87:1154–61. doi: 10.1093/jnci/87.15.1154
51. Wee LE, Lim LY, Koh GC-H. Two Sides of the Coin: A Qualitative Study of Patient and Provider Perspectives on Colorectal, Breast and Cervical Cancer Screening in a Low-Income Asian Community. Proc Singapore Healthcare (2016) 25:80–91. doi: 10.1177/2010105815616404
52. Straughan PT, Seow A. Barriers to Mammography Among Chinese Women in Singapore: A Focus Group Approach. Health Educ Res (1995) 10:431–41. doi: 10.1093/her/10.4.431
53. National Strategic Plan for Cancer Control Programme 2016-2020. Malaysia: Ministry of Health Malaysia (2017).
54. Seng LM, Rosman AN, Khan A, Haris NM, Mustapha NAS, Husaini NSM, et al. Awareness of Cervical Cancer Among Women in Malaysia. Int J Health Sci (2018) 12:42–8.
55. Rubini G, Fatini A, Khadijah S, Laxmee H, Noorhasriyantie H, RiniAzmeera K, et al. Barriers and Belief Towards Pap Smear Screening in Sepang, Selangor, Malaysia: Gender Perspective. Int J Educ Res (2018) 6:269–78.
56. Baskaran P, Subramanian P, Rahman RA, Ping WL, Mohd Taib NA, Rosli R. Perceived Susceptibility, and Cervical Cancer Screening Benefits and Barriers in Malaysian Women Visiting Outpatient Clinics. Asian Pac J Cancer Prev (2013) 14:7693–9. doi: 10.7314/APJCP.2013.14.12.7693
57. Mahmud A, Aljunid S. The Uptake of Mammogram Screening in Malaysia and Its Associated Factors: A Systematic Review. Med J Malaysia (2018) 73:202–11.
58. Sentell TL, Tsoh JY, Davis T, Davis J, Braun KL. Low Health Literacy and Cancer Screening Among Chinese Americans in California: A Cross-Sectional Analysis. BMJ Open (2015) 5:e006104. doi: 10.1136/bmjopen-2014-006104
59. Jacobs EA, Karavolos K, Rathouz PJ, Ferris TG, Powell LH. Limited English Proficiency and Breast and Cervical Cancer Screening in a Multiethnic Population. Am J Public Health (2005) 95:1410–6. doi: 10.2105/AJPH.2004.041418
60. Taylor VM, Jackson JC, Tu SP, Yasui Y, Schwartz SM, Kuniyuki A, et al. Cervical Cancer Screening Among Chinese Americans. Cancer Detect Prev (2002) 26:139–45. doi: 10.1016/S0361-090X(02)00037-5
61. Hislop TG, Deschamps M, Teh C, Jackson C, Tu SP, Yasui Y, et al. Facilitators and Barriers to Cervical Cancer Screening Among Chinese Canadian Women. Can J Public Health (2003) 94:68–73. doi: 10.1007/BF03405056
62. Yu ES, Kim KK, Chen EH, Brintnall RA. Breast and Cervical Cancer Screening Among Chinese American Women. Cancer Pract (2001) 9:81–91. doi: 10.1046/j.1523-5394.2001.009002081.x
63. Lu M, Moritz S, Lorenzetti D, Sykes L, Straus S, Quan H. A Systematic Review of Interventions to Increase Breast and Cervical Cancer Screening Uptake Among Asian Women. BMC Public Health (2012) 12:413. doi: 10.1186/1471-2458-12-413
64. Lee YY, Ng CT, Siti Aishah MG, Ngiam JZ, Tai BC, Lim MK, et al. Public Trust in Primary Care Doctors, the Medical Profession and the Healthcare System Among Redhill Residents in Singapore. Ann Acad Med Singapore (2007) 36:655–61.
65. Cheen MH, Kong MC, Zhang RF, Tee FM, Chandran M. Adherence to Osteoporosis Medications Amongst Singaporean Patients. Osteoporos Int (2012) 23:1053–60. doi: 10.1007/s00198-011-1635-9
67. Castle A, Adrian-Harris D, Holloway DG, Race AJ. Continuing Professional Development for Radiographers. Radiography (1997) 3:253–63. doi: 10.1016/S1078-8174(97)90001-8
68. Fitzpatrick P, Winston A, Mooney T. Radiographer Gender and Breast-Screening Uptake. Br J Cancer (2008) 98:1759–61. doi: 10.1038/sj.bjc.6604385
69. Fitzpatrick P, Winston A, Mooney T. Would Male Radiographers Have an Impact on Breast Screening Programme Performance? Breast Cancer Res: BCR (2008) 10:P23–3. doi: 10.1186/bcr2021
70. Aidalina M, Syed Mohamed ASJ. The Uptake of Mammogram Screening in Malaysia and Its Associated Factors: A Systematic Review. Med J Malaysia (2018) 73:202–11.
71. Pasick RJ, Hiatt RA, Paskett ED. Lessons Learned From Community-Based Cancer Screening Intervention Research. Cancer (2004) 101:1146–64. doi: 10.1002/cncr.20508
72. Fox SA, Stein jA, Gonzalez RE, Farrenkopf M, Dellinger A. A Trial to Increase Mammography Utilization Among Los Angeles Hispanic Women. J Health Care Poor Underserved (1998) 9:309–21. doi: 10.1353/hpu.2010.0218
73. Castro FG, Elder J, Coe K, Tafoya-Barraza HM, Moratto S, Campbell N, et al. Mobilizing Churches for Health Promotion in Latino Communities: Companeros En La Salud. J Natl Cancer Inst Monogr (1995), 127–35.
74. Paskett ED, Tatum CM, D'Agostino R Jr, Rushing J, Velez R, Michielutte R, et al. Community-Based Interventions to Improve Breast and Cervical Cancer Screening: Results of the Forsyth County Cancer Screening (FoCaS) Project. Cancer Epidemiol Biomarkers Prev (1999) 8:453–9.
75. Heaney CA, Goetzel RZ. A Review of Health-Related Outcomes of Multi-Component Worksite Health Promotion Programs. Am J Health Promot (1997) 11:290–307. doi: 10.4278/0890-1171-11.4.290
76. Janer G, Sala M, Kogevinas M. Health Promotion Trials at Worksites and Risk Factors for Cancer. Scand J Work Environ Health (2002) 28:141–57. doi: 10.5271/sjweh.658
77. Pelletier KR. A Review and Analysis of the Clinical and Cost-Effectiveness Studies of Comprehensive Health Promotion and Disease Management Programs at the Worksite: Update VIII 2008 to 2010. J Occup Environ Med (2011) 53:1310–31. doi: 10.1097/JOM.0b013e3182337748
78. Agide FD, Sadeghi R, Garmaroudi G, Tigabu BM. A Systematic Review of Health Promotion Interventions to Increase Breast Cancer Screening Uptake: From the Last 12 Years. Eur J Public Health (2018) 28:1149–55. doi: 10.1093/eurpub/ckx231
79. Bonfill X, Marzo M, Pladevall M, Marti J, Emparanza JI. Strategies for Increasing Women Participation in Community Breast Cancer Screening. Cochrane Database Syst Rev (2001) Cd002943. doi: 10.1002/14651858.CD002943
80. Schroy PC 3rd, Glick JT, Robinson PA, Lydotes MA, Evans SR, Emmons KM. Has the Surge in Media Attention Increased Public Awareness About Colorectal Cancer and Screening? J Community Health (2008) 33:1–9. doi: 10.1007/s10900-007-9065-5
81. De Vito C, Angeloni C, De Feo C, Marzuillo E, Lattanzi C, Ricciardi A, et al. A Large Cross-Sectional Survey Investigating the Knowledge of Cervical Cancer Risk Aetiology and the Predictors of the Adherence to Cervical Cancer Screening Related to Mass Media Campaign. BioMed Res Int (2014) 2014:304602. doi: 10.1155/2014/304602
82. Hou SI, Sealy DA, Kabiru CW. Closing the Disparity Gap: Cancer Screening Interventions Among Asians–A Systematic Literature Review. Asian Pac J Cancer Prev (2011) 12:3133–9.
83. Duffy SW, Myles JP, Maroni R, Mohammad A. Rapid Review of Evaluation of Interventions to Improve Participation in Cancer Screening Services. J Med Screen (2017) 24:127–45. doi: 10.1177/0969141316664757
84. M810011 - Singapore Residents By Age Group, Ethnic Group And Sex, End June, Annual. Singapore: Department of Statistics Singapore (2019). Available at: https://www.singstat.gov.sg/find-data/search-by-theme/population/population-and-population-structure/latest-data.
Keywords: behavior, breast cancer, cancer, cervical cancer, colorectal cancer, disparities, knowledge, screening
Citation: Chan TKC, Tan LWL, van Dam RM and Seow WJ (2021) Cancer Screening Knowledge and Behavior in a Multi-Ethnic Asian Population: The Singapore Community Health Study. Front. Oncol. 11:684917. doi: 10.3389/fonc.2021.684917
Received: 24 March 2021; Accepted: 07 July 2021;
Published: 12 August 2021.
Edited by:
Farnam Mohebi, University of California, Berkeley, United StatesReviewed by:
Sophia S. Wang, City of Hope Beckman Research Institute/Comprehensive Cancer Center, United StatesCopyright © 2021 Chan, Tan, van Dam and Seow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tyson Kin-Chung Chan, dHlzb25fY2hhbkBudWhzLmVkdS5zZw==; Wei Jie Seow, ZXBoc3dqQG51cy5lZHUuc2c=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.