
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol., 01 June 2021
Sec. Thoracic Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.684098
This article is part of the Research TopicImmunological Evolution from Precancerous Lesion to Advanced Lung Cancer: Immunologic Surveillance, Immunologic Balance and Immunologic EscapeView all 5 articles
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment paradigm for lung cancer in recent years. These strategies consist of neutralizing antibodies against negative regulators of immune function, most notably cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and PD-1 ligand 1 (PD-L1), thereby impeding the ability of tumor cells to escape immune surveillance. Though ICIs have proven a significant advance in lung cancer therapy, overall survival rates remain low, and lung cancer continues to be the leading cause of cancer-related death in the United States. It is therefore imperative to better understand the barriers to the efficacy of ICIs, particularly additional mechanisms of immunosuppression within the lung cancer microenvironment. Recent evidence suggests that regulatory T-lymphocytes (Tregs) serve as a central mediator of immune function in lung cancer, suppressing sterilizing immunity and contributing to the clinical failure of ICIs. Here, we provide a comprehensive summary of the roles of Tregs in lung cancer pathobiology and therapy, as well as the potential means through which these immunosuppressive mechanisms can be overcome.
Non-small cell lung cancer (NSCLC) is the most common form of lung cancer, representing roughly 85% of all new lung cancer diagnoses (1). Most patients are diagnosed with advanced disease, stemming from inadequate screening and the late onset of symptoms (1). The treatment for NSCLC is highly varied and can include a combination of surgery, chemotherapy, radiation, targeted therapy, and most recently, immunotherapy (2). Lung cancer immunotherapy is backboned by immune checkpoint inhibitors (ICIs), which have demonstrated significant antitumor activity in most solid tumors (3–9). ICI-based immunotherapy consists of neutralizing antibodies against negative regulators of immune function, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and PD-1 ligand 1 (PD-L1), thereby limiting the ability of tumor cells to escape the cytotoxic immune program (10). However, despite the advent of immunotherapy, NSCLC carries a combined 5-year survival rate of only 25% (11). Similarly, ICI-based immunotherapy is approved as a first-line treatment for small cell lung cancer (SCLC) (12). However, patients with SCLC display even poorer outcomes, with an overall 5-year survival rate of 7% (11). Hence, there are still several remaining obstacles limiting the therapeutic efficacy of ICIs in lung cancer that must be overcome in order to further enhance drug responses and improve patient outcomes.
Recent evidence suggests that several resident immune cell subsets within the NSCLC tumor microenvironment (TME) may contribute to immune evasion, thereby blunting the effects of ICI-based immunotherapy. This includes regulatory T-lymphocytes (Tregs), a specialized T-cell subpopulation that acts to suppress sterilizing immune responses, thus promoting self-tolerance (13). While Tregs have central roles in maintaining normal airway tolerance (14), Tregs are abundant in NSCLC and predict for an increased risk of disease recurrence in early-stage disease (15). Similarly, increased tumor-infiltrating Tregs are associated with poor overall survival in SCLC (16). Here, we discuss the mechanistic contributions of Tregs to lung cancer pathobiology, as well the means through which Tregs limit therapeutic responses to ICI-based immunotherapy regimens and the means through which this can be overcome.
Tregs are a subset of CD4+ T-cells, typically defined by the expression of the transcription factor Forkhead box protein P3 (FoxP3) (17). In contrast to other CD4+ T-cells that enhance local immune function, Tregs maintain immune homeostasis and self-tolerance by suppressing the activity of other immune cell subsets, thereby restraining autoimmune responses in the periphery (18–21). Treg-mediated immune suppression occurs through a variety of mechanisms. Under physiologic conditions, an activated antigen-presenting cell (APC) will display a processed antigen peptide on its surface via an MHC molecule. This antigen/MHC complex will then associate with the T-cell receptor (TCR) of a nearby T-cell. This interaction, when combined with an additional co-stimulatory signal mediated in part by association of the APC’s B7 and T-cell’s CD28, lead to the activation of the T-cell, which then can clonally expand and/or exert its enhanced effector function (Figure 1A) (22).
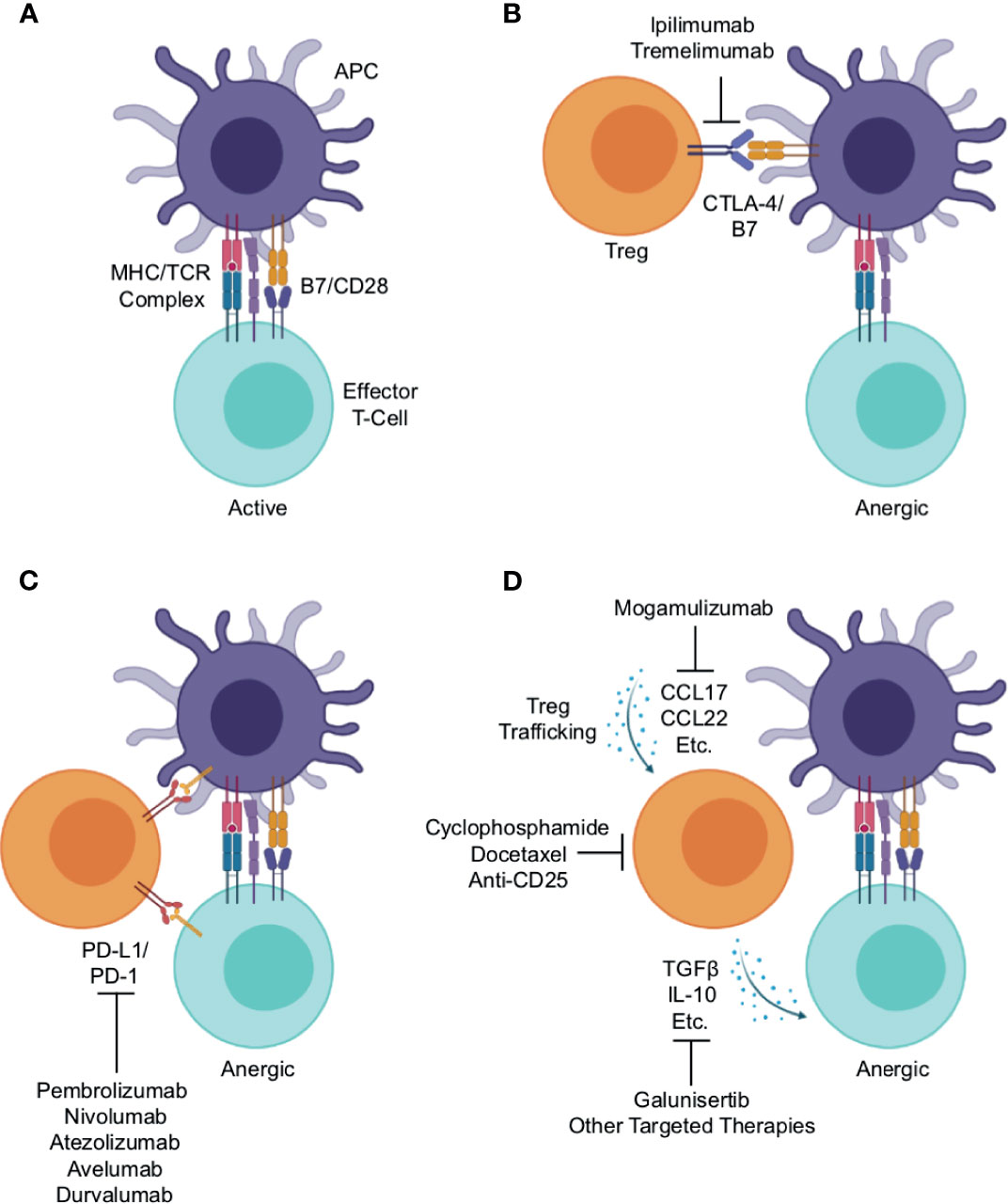
Figure 1 Mechanisms of Treg-mediated immune evasion within the lung cancer immune microenvironment and strategies for therapeutic intervention. (A) Under physiologic conditions, an activated antigen-presenting cell (APC) will associate with an effector T-cell, presenting antigen peptide on an MHC molecule. This will associate with the T-cell receptor, which combined with additional stimuli such as co-stimulation mediated in part by association of the APC’s B7 and T-cell’s CD28, lead to T-cell activation and enhanced effector function. Regulatory T-cells (Tregs) suppress effector T-cell activation through a variety of mechanisms. (B) One such mechanism is the association of Treg’s cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) with the APC’s B7, outcompeting the effector T-cell for the co-stimulation signal thereby leading to anergy. This serves as the rationale for the use of anti-CTLA-4 antibodies such as ipilimumab and tremelimumab, which function to block this interaction, thereby enhancing anti-tumor immune responses. (C) Both APCs and effector T-cells can express programmed cell death protein 1 (PD-1) on their surface. Treg expressed PD-1 ligand 1 (PD-L1) can associate with PD-1, leading to reduced co-stimulation by the APC or functional inactivation of the effector T-cell. This association is interrupted by antibodies against PD-1 (pembrolizumab and nivolumab) or PD-L1 (e.g., atezolizumab, avelumab, and durvalumab), and this strategy is now considered the cornerstone of lung cancer therapy. (D) Tregs traffic into tumor tissues largely by following gradients of CCR4 ligands such as CCL17 and CCL22. There, they produce a variety of suppressive cytokines, namely interleukin 10 (IL-10) and transforming growth factor β (TGFβ). These both can limit effector T-cell responses, and facilitate tumor escape from immune surveillance. This also provides several potential opportunities for therapeutic intervention including: anti-CCR4 antibodies such as mogamulizumab (KW-0761) to block Treg trafficking, anti-CD25 antibodies or chemotherapy agents, cyclophosphamide and docetaxel, to deplete Tregs, or anti-TGFβ agents such as galunisertib or other targeted therapies to block the immune suppressive actions of Treg-derived cytokines within the lung tumor microenvironment.
This provides several avenues through which Tregs can suppress the activation of a neighboring T-cell. For instance, Tregs constitutively express cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) on their surface, which binds to the B7 on the APC surface, outcompeting CD28 expressed on a nearby T-cell. In the absence of a B7/CD28 interaction, the effector T-cell will remain refractory from full activation (Figure 1B) (23). This serves as the rationale for using anti-CTLA-4 antibodies such as ipilimumab and tremelimumab that block this interaction as a means of cancer immunotherapy, aiming to enhance anti-tumor immune responses (Figure 1B) (24). Similarly, both APCs and T-cells can express programmed cell death protein 1 (PD-1) on their surface. Treg expressed PD-1 ligand 1 (PD-L1) can associate with PD-1, leading to reduced co-stimulation by the APC or direct inactivation of an effector T-cell (Figure 1C) (23). This, in part, is the rationale for using blocking anti-PD-1 antibodies (e.g., pembrolizumab and nivolumab) or anti-PD-L1 antibodies (e.g., atezolizumab, avelumab, and durvalumab) as cancer therapy (Figure 1C) (25).
Finally, Tregs traffic to tissues predominantly by following gradients of CCR4 ligands such as CCL17 and CCL22 (26). At the tumor site, Tregs produce a variety of suppressive cytokines such as 10 (IL-10) and transforming growth factor β (TGFβ), which can bind receptors on other T-cells or other nearby immune cells and prevent their full activation (Figure 1D). This also provides several potential opportunities for therapeutic intervention discussed in detail in a subsequent section of this article. In brief, these include the use of anti-CCR4 antibodies such as mogamulizumab (KW-0761) to block Treg trafficking (26), anti-CD25 antibodies (27) or chemotherapy to deplete Tregs (28, 29), anti-TGFβ agents such as galunisertib (30), or other targeted therapies to block the immune suppressive actions of Treg-derived cytokines (Figure 1D).
Tregs can be induced early in thymocyte development during T-cell selection in the thymus (31) or generated in the periphery through the conversion of naïve CD4+ T-cells to Tregs (32). In the thymus, the primary factor guiding Treg differentiation appears to be the specificity of the T cell receptor (TCR). In brief, there is a range of TCR self-reactivity that is permissive for Treg differentiation, provided the appropriate co-stimulatory and cytokine signals are present in subsequent stages of development to induce FoxP3 expression (31, 33). These thymus-derived Tregs serve important roles in maintaining central tolerance, and deficiency of FoxP3 is associated with severe autoimmune disease, colitis, and allergies in mice and humans alike (34–37).
Contrastingly, the extrathymic genesis of peripheral Tregs involves the conversion of naïve Tregs in the periphery, and is primarily dictated by the local cytokine milieu, though still dependent on FoxP3 expression following antigen exposure (32). Though peripheral Tregs represent a small percentage of total Tregs under physiologic conditions, peripheral Tregs are strongly represented in the gastrointestinal tract and placenta, and maintain immune tolerance toward commensal bacteria, ingested antigens, allergens, and the fetus during pregnancy (37). Peripheral Tregs have been suggested to be the main Treg subset represented in most cancers, and are thought to be converted within the tumor microenvironment (38), though this warrants continued exploration. To date, most studies have focused on the contributions of transforming growth factor β (TGFβ) signaling in peripheral Treg conversion. TGFβ has been shown to induce FoxP3 expression in both human and murine T-cells, and TGFβ-induced Tregs are central to preventing house dust mite-induced allergic lung pathogenesis in a murine model of asthma (39–41). Similarly, blockade of TGFβ signaling has been shown to reduce FoxP3 expression in ex vivo T-cell cultures (42), as well as disrupt Treg-mediated tolerance to inhaled antigen in vivo (43).
These and other studies suggest that Tregs play essential roles in maintaining normal airway tolerance. For instance, a seminal study demonstrated that animals with a selective deficit in extrathymically generated Tregs spontaneously develop pronounced T-helper 2 (Th2)-associated pathologies at mucosal sites in the lungs, specifically in the form of allergic inflammation and asthma (44). These and several other studies have substantiated Tregs as a central mediator of immune responses to inhaled antigens, limiting the activation of pathogenic immune cells and preventing tissue-damaging inflammatory responses (14, 45). Importantly, these studies also suggest that Treg-dependent maintenance of airway tolerance requires continuous exposure to airborne antigens, as antigen withdrawal results in a decreased number of Tregs, enhancing susceptibility to pathologic Th2-dependent response against respiratory antigens (46, 47).
In addition to maintaining airway tolerance, Tregs are also an established component of the lung cancer microenvironment, functioning to inhibit autologous T-cell proliferation and impede local immune responses (48). This has led to the long-standing hypothesis that Tregs have driving roles in lung cancer pathogenesis, by inhibiting the action of auto-reactive T-cells and allowing for the continued escape from immune surveillance. As the relative abundance of Tregs has been shown to predict for poor clinical outcomes in most cancer types (49), several studies have explored Tregs as a potential prognostic biomarker in lung cancer (Table 1). A recent pan-cancer meta-analysis determined that lung cancer patients with higher tumor densities of FoxP3+ Tregs had significantly poorer disease-free survival rates (49). A lung cancer-specific meta-analysis similarly found that, in a combined cohort of 1,303 NSCLC patients across 11 studies, an increase in tumor-infiltrating FoxP3+ Tregs was associated with poor overall survival. Interestingly, the authors also found that the Treg infiltrate was strongly associated with smoking status, though they did not find a relationship between Tregs and other clinicopathological features (50). Similarly, a large study conducting gene expression analysis of 196 NSCLC and 137 normal samples found that immune-infiltrating Treg-related genes were strongly associated with poor overall survival (55).
Interestingly, peripheral blood Tregs increase in a stage-dependent manner in NSCLC, and have also been suggested to have potential utility as a prognostic biomarker (59). One of the earliest studies evaluating Tregs and lung cancer prognosis included 64 stage I NSCLC patients. Using excisional biopsies, the authors found that 81% of samples had detectable tumor-infiltrating lymphocytes, with 51% positive for FoxP3+ Tregs. They then determined that patients with a higher proportion of tumor Tregs relative to total tumor-infiltrating lymphocytes had a significantly higher risk of recurrence and worse clinical outcomes (15). Similarly, a recent study evaluated peripheral blood samples from 156 chemotherapy-naive patients with stage III or IV NSCLC. Similar to studies exploring tumor-infiltrating Tregs, the authors observed a significant relationship between peripheral Tregs and clinical outcomes. This study also stratified Tregs into three additional subsets based on surface marker expression: naïve, effector, and terminal effector. Naive Tregs were defined as being CD4+CD25highCD127−/lowCD152-FoxP3lowCD45RO−, and represent an early stage of Treg differentiation with reduced sensitivity to apoptotic stimuli (60). Effector Tregs were those that were CD4+CD25highCD127lowCD152+FoxP3+CD45RO+, and represent a transient stage of Treg differentiation that rapidly divide prior to their disappearance (61). Terminal effector Tregs were defined as CD4+CD25highCD127−CD152+FoxP3+CD45RO+, and are considered the most immune-suppressive Treg subtype (62, 63).
Using this approach, they determined that the increased presence of terminal effector Tregs predicts for improved overall and progression-free survival, whereas an increase in either naïve or effector Tregs was associated with worse survival (56). Similarly, the increased presence of peripheral blood Tregs has also been suggested to predict for clinical responses to radiotherapy. In a group of 70 NSCLC patients undergoing radiation, increased Tregs independently predicted for poor progression-free survival (57). This may be particularly noteworthy given the frequent cooperation between radiation and immunotherapy in several cancers (64), and warrants continued exploration.
Tregs have also been shown to have potential utility in predicting for poor survival in NSCLC patients undergoing definitive surgery. In a retrospective analysis of 100 patients who had undergone a complete resection for NSCLC, the authors evaluated the prognostic utility of the combination of epithelial Cyclooxygenase-2 (COX-2) expression and tumor-infiltrating Tregs. In this group, patients with high COX-2 expression had significantly worse recurrence-free survival, accompanied by a relative increase in Treg infiltration. They found that only lymph node involvement was an independent predictor of recurrence-free survival in a multivariate analysis. However, in node-negative NSCLC, they determined that FoxP3+ tumor-infiltrating Tregs was an independent predictor of shorter recurrence-free survival (51).
A similar study of 196 NSCLC patients found improved overall survival for patients with increased tumor-infiltrating CD8+ T-cells, but poorer overall survival for patients with increased tumor-infiltrating FoxP3+ Tregs (53). While these studies have relied on FoxP3+ Tregs, others have included additional parameters, including FoxP3 expression in tumor cells. For example, a study of 87 excisional biopsies from operable NSCLC patients found that FoxP3+ tumor cells were found in 31% of lung cancer specimens, with no significant relationship to tumor-infiltrating Tregs. Further, increased tumor-infiltrating Tregs were associated with poor overall and relapse-free survival, and though tumor expression of FoxP3 was not an independent predictor of outcomes, when FoxP3+cancer cells were present, the relationship between tumor Treg infiltration and worse prognosis was attenuated. Conversely, patients without FoxP3- tumor cells and high Tregs had worse outcomes than all other groups (52).
In addition to tumor cell expression of FoxP3, there are other markers that may also influence the prognostic utility of Tregs. One such example is the transcription factor Helios, which has been suggested as a means of differentiating between thymus-derived and peripherally induced Tregs (65). Helios-expressing Tregs are generated during thymic selection, whereas Helios-non-expressing Tregs represent those induced in the periphery. These two Treg subsets are functionally distinct, and have largely non-overlapping TCR reservoirs (66). In a cohort of 64 NSCLC patients, 45 of whom had undergone surgery and 19 that had received only chemotherapy, Helios was expressed in 47.5 ± 13.3% in peripheral blood and 18.1 ± 13.4% of tumor-infiltrating Tregs. Patients with reduced Helios expression in tumor-infiltrating Tregs had significantly poorer survival, suggesting that peripherally induced Tregs are a more significant driver of immune evasion in the lung TME (58). As no other study to date has attempted to differentiate between thymus-derived and peripherally induced Tregs in lung cancer, this warrants continued exploration.
Additionally, the co-inhibitory signal B7-H3 may also influence the relationship between Tregs and prognosis. In a group of 110 NSCLC specimens, FoxP3 expression in tumor-infiltrating T-cells was associated with male gender, regional lymph node involvement, advanced clinical stage, and poor overall survival. B7-H3 expression was strongly associated with tumor-infiltrating FoxP3+ Tregs, and patients with the highest expression of B7-H3+FoxP3+ Tregs had the poorest survival of all groups (54).
Though several studies have affirmed the utility of Tregs as a predictor of clinical outcomes in NSCLC, Tregs are less established as a prognostic marker in small cell lung cancers (SCLC). There is emerging evidence supporting a similar role for Tregs in SCLC, namely that several SCLC tumor cell lines can induce de novo differentiation of Tregs from naïve peripheral blood lymphocytes in an IL-15 dependent mechanism. The same study also evaluated SCLC tumor biopsies, and found that an increase in tumor-infiltrating Tregs was associated with poor overall survival, similar to previous studies in NSCLC (16).
Given the advent of ICIs in lung cancer treatment, there is considerable interest in identifying clinically useful biomarkers to predict for therapeutic responses. While PD-L1 expression is perhaps the most widely used predictive biomarker, emerging data suggests that several other factors may be as or possibly more informative than established biomarkers, e.g., PD-L1 status. For instance, several studies have now indicated that the presence of Tregs may also have utility as a predictor of responses to ICI-based immunotherapy (Table 2). For example, a recent study evaluated patients with NSCLC, gastric cancer, and malignant melanoma that were treated with the anti-PD-1 antibodies nivolumab or pembrolizumab, or the anti-PD-L1 antibody atezolizumab. The authors found that non-responsive patients typically displayed increased PD-1 on Tregs. The ratio of tumor-infiltrating PD-1+CD8+ T-cells relative to Tregs was a superior predictor of therapeutic responses than all other predictors, including PD-L1 expression and tumor mutational burden. The authors therefore concluded that PD-1+ Tregs might have utility as a predictive biomarker, and warrant consideration as a therapeutic target to augment the clinical efficacy of ICIs in lung cancer (67).
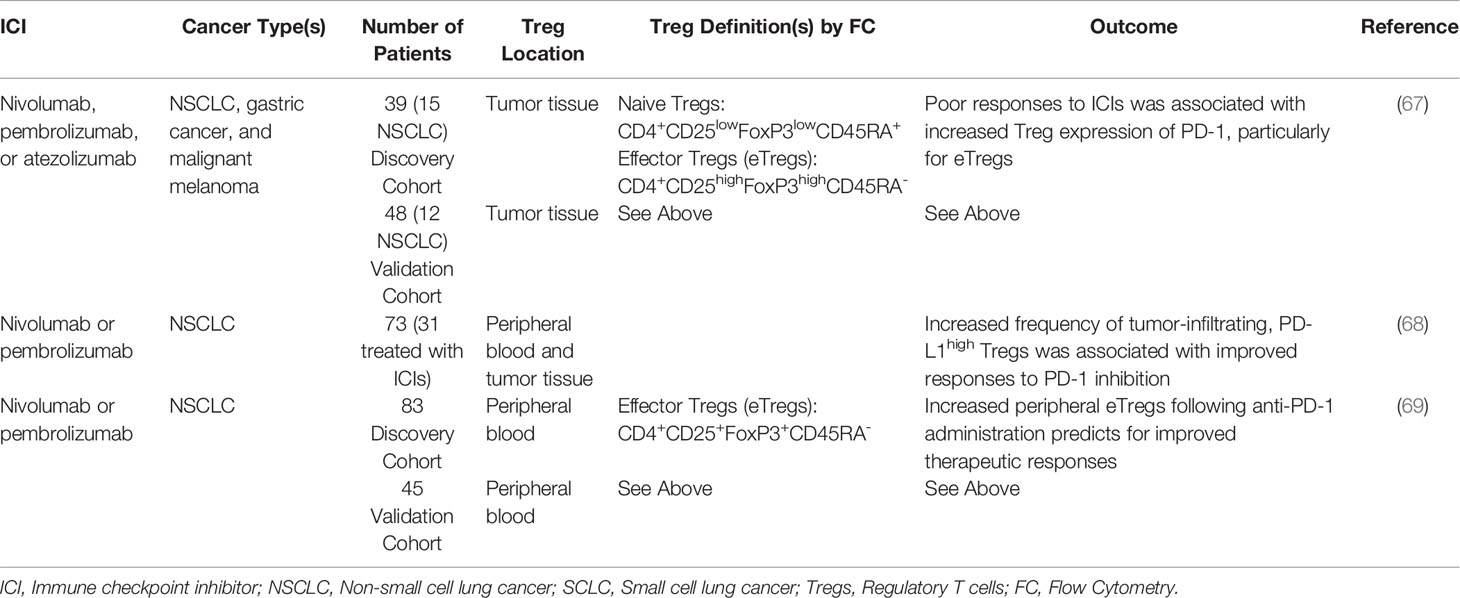
Table 2 Studies exploring regulatory T cells as a predictor of responses to immune checkpoint inhibition.
A similar study evaluated peripheral blood samples and tumor specimens from 73 NSCLC patients, 31 of whom had received either pembrolizumab or nivolumab. Using this approach, the authors identified a population of high-PD-L1+, CD25+ Tregs (PD-L1hi Tregs) that was preferentially expressed within tumor tissues and correlated with PD-1 expression in tumor-infiltrating CD8+ T-cells. Patients with an increased frequency of tumor-infiltrating PD-L1hi Tregs showed high PD-1/PD-L1 pathway dependence, and improved CD8+ T-cell responses following PD-1 inhibition. This corresponded to improved clinical outcomes compared to patients with a low frequency of PD-L1hi Tregs (68).
A recent study has also explored peripheral Tregs as a potential biomarker for responses to ICIs. One such study evaluated peripheral blood samples from 83 NSCLC patients before and after ICI-based immunotherapy. In this group, patients with a high frequency of Tregs one week after anti-PD-1 administration had significantly improved response rates, as well as longer progression-free and overall survival, though the number of peripheral Tregs prior to therapy had no predictive value. Similar results were observed regarding serum levels of TGFβ, which was also associated with improved clinical outcomes. These results were affirmed in a second cohort of 45 patients, suggesting that increased peripheral Tregs or elevated levels of TGFβ can also predict for clinical outcomes (69).
As discussed, given the many roles of Tregs in blunting the effector function of CD8+ T-cells, Tregs have long been suggested as a barrier to the efficacy of ICIs and a potential target for therapy (70). This approach has shown encouraging preclinical efficacy, particularly combined with other treatment modalities such as radiation (71–73). Several strategies to deplete or modulate the activity of Tregs have been introduced (Figure 1). Though it has been suggested that the efficacy seen with CTLA-4 inhibitors may be mediated in part through the depletion of Tregs, recent evidence suggests that CTLA-4 inhibitors do not significantly change or deplete Tregs within the tumor microenvironment (74). However, the effects of select chemotherapy agents on Tregs are well documented, particularly regarding cyclophosphamide (75).
The tumoricidal effects of cyclophosphamide appear primarily dependent on the immune system, as a single injection of high-dose cyclophosphamide increased the survival of immunocompetent mice, though this was not observed in immune-deficient mice (76). Accordingly, Tregs are highly sensitive to cyclophosphamide, particularly when compared to CTLs and helper T cells, which was presumed to be due to a DNA repair defect (77). This has been shown to be a central mediator of cyclophosphamide-induced type-1 diabetes in mice, which was prevented by the allogenic transfer of Tregs (78). Likewise, in vitro studies demonstrate that low-dose cyclophosphamide has been reported to induce Treg apoptosis, restrain Treg proliferation, and limit their immunosuppressive functions (79).
Thus, cyclophosphamide has been suggested as a potential means of targeting Tregs in cancer, particularly in light of observations that cyclophosphamide-mediated depletion of Tregs allows immunotherapy to be curative in a rat model of implanted PROb colon cancer cells (80). Similar results have been observed in a variety of mouse models. Cyclophosphamide reduced regulatory T-cells, enhancing the efficacy of non-myeloablative allogeneic stem cell transplantation through increased activation of autoreactive T-cells and IFN-γ production (81). In a mouse model of cutaneous melanoma, cyclophosphamide has also been demonstrated to increase the frequency of tumor-infiltrating, IFN-γ producing T-cells, as well as decrease the relative abundance of Tregs (82). In colon cancer patients, low-dose cyclophosphamide depleted tumor-associated Tregs, enhanced anti-tumor immune responses, and extended overall survival (29). Though encouraging, the clinical utility for cyclophosphamide as an adjuvant to immunotherapy is still emerging in lung cancer.
Other chemotherapy agents have also been suggested to selectively target Tregs. For example, docetaxel has been shown to selectively reduce Tregs in vitro, and patients who received four cycles of docetaxel-based chemotherapy showed fewer peripheral Tregs than at baseline (28). The multidrug regimens FOLFOX (5-FU, leucovorin, and oxaliplatin) and FOLFIRI (5-FU, leucovorin, and irinotecan) also significantly reduced peripheral blood Tregs in patients with metastatic colorectal cancer (83). Hence, the mechanistic intersection between chemotherapy-mediated Treg depletion and lung cancer immunotherapy warrants continued exploration (29).
Other therapeutic strategies more directly targeting Tregs are also emerging. For example, the high-affinity IL-2 receptor CD25 (also known as IL-2 receptor alpha) is strongly expressed on Tregs, and neutralizing antibodies against CD25 have been suggested as a means of Treg depletion. This approach has been highly effective in reducing Tregs in preclinical models, enhancing CD8-mediated anti-tumor immune function (27, 84). Though anti-CD25 has shown limited efficacy against established tumors, select studies have evaluated the combination of anti-CD25-mediated Treg depletion and ICI-based immunotherapy. One such study assessed the combination of anti-CD25 and anti-PD-1 in mouse models of tumorigenesis and found that this approach promoted complete tumor rejection, substantiating CD25 as a therapeutic target for combination approaches in immuno-oncology (85). Anti-CD25 has also been used in combination with near-infrared photoimmunotherapy (NIR-PIT), a method of treating cancer using the activation of an antibody-photoabsorber conjugate activated by NIR light (86, 87). Anti-CD25 guided NIR-PIT led to the selective depletion of Tregs, as well as robust CD8+ T-cell and natural killer cell activation in models where anti-CD25 alone was ineffective at depleting Tregs (87).
While these and other related findings support anti-CD25 as a potentially useful means to deplete Tregs in cancer therapy (88–90), other approaches are also showing early promise. The chemokine receptor CCR4 is expressed on 90% of Tregs and has been linked to ICI-resistance. ICIs have been suggested to upregulate CCR4 ligands, e.g., CCL17 and CCL22, thereby increasing Treg trafficking into tumors. This was ameliorated through the addition of a pharmacological inhibitor of CCR4, substantiating CCR4 as a potential means of targeting Tregs for cancer therapy (91). CCR4-inhibition has also been suggested as a means of depleting Tregs for cancer immunotherapy, augmenting cytotoxic T-cell responses (92). This approach has been explored in clinical trials, where the anti-CCR4 antibody mogamulizumab has been shown to deplete Tregs and potentially enhance cytotoxicity in adult T-cell leukemia/lymphoma (26). However, though mogamulizumab successfully depleted Tregs in lung cancer patients (93), a recent multi-cancer trial determined that the combination of mogamulizumab and durvalumab or tremelimumab does not result in improved efficacy for patients with advanced solid tumors (94). Hence, the potential for mogamulizumab as an adjuvant to ICIs in lung cancer is unclear at this time and requires further investigation.
Additional strategies are targeting Treg-derived cytokines, namely TGFβ. TGFβ is a potent and pleiotropic cytokine with several, often contradictory, roles in tumorigenesis (95). TGFβ has been shown to contribute to immune evasion in cancer (96) and attenuate therapeutic responses to PD-L1 inhibition by contributing to T-cell exclusion (97). Accordingly, the combination of TGFβ signal inhibition and ICIs are showing promise in several solid tumors (30, 98–100). Early results for bintrafusp alfa (a bifunctional fusion protein of the extracellular domain of the type 2 TGFβ receptor fused to an anti-PD-L1 antibody) showed promising efficacy and manageable toxicity in NSCLC patients previously treated with platinum-based immunotherapy (101). This has been supported by additional studies, with durable immune responses observed in many NSCLC patients after two-years, particularly those with high PD-L1 expression (102).
The immunosuppressive effects of Tregs in lung cancer are well documented with several recent studies affirming the prognostic relevance of circulating and tumor-infiltrating Tregs alike. Emerging evidence also supports the longstanding hypothesis that Tregs have a driving role in the clinical failure of ICI-based immunotherapy. Hence, Tregs and their associated cell processes may represent a promising therapeutic target in lung cancer, particularly in the setting of ICIs. However, despite the promise of targeting Tregs in lung cancer, there are several important distinctions that must be made prior to advancing such strategies in the clinic. As highlighted in this review, Tregs are a highly heterogeneous T-cell subset with several subcategories including thymus-derived, peripherally generated (extrathymic), naïve, effector, terminal effector, and others. Though many studies support Tregs as a potential prognostic biomarker and/or predictor of treatment failure, very few such studies account for this variance, with most using the generalized term “Tregs”. Additionally, the criteria used to define these Tregs are highly varied, both regarding methodology and the surrogate markers used. Hence, the true utility of Tregs as a predictive biomarker remains unclear, and warrants continued exploration using standardized methodology, as well as particular attention to the many unique and functionally distinct Treg subsets.
Finally, it is also important to note that targeting Tregs requires extreme caution. For instance, in a murine model of pancreatic cancer, genetic ablation of Tregs led to extensive remodeling of the tumor microenvironment and rapidly accelerated tumor formation (103). Hence, the combination of Treg-targeted therapy and ICIs warrants careful study prior to advancing to clinical practice. Additionally, Tregs (particularly those developed during thymic selection) have a central role in suppressing autoreactive T-cells and restraining tissue inflammation. Therefore, it remains a distinct possibility that broadly targeting Tregs in tandem with ICI-based immunotherapy may also lead to severe autoimmune-mediated adverse effects. Hence, this too warrants additional study, as do whether unique Treg subsets can be safely targeted for therapy (e.g., peripherally-induced Tregs) while still offering a potential clinical benefit to lung cancer patients, as this may be a useful means of maximizing efficacy while also limiting toxicity.
ICIs are the cornerstone of lung cancer therapy. Though a significant advance, overall survival rates remain poor. Hence, there is a clear need to identify new strategies to further improve the efficacy of ICI-based immunotherapy. Tregs represent a highly suppressive T-cell subset that is abundant within the lung cancer TME. Accordingly, Tregs have many roles in maintaining peripheral tolerance and impeding anti-tumor immunity in lung cancer, likely contributing to the clinical failure of ICIs. While select strategies to deplete Tregs or neutralize their immunosuppressive effects are showing early promise, these are most effective when combined with other treatment modalities. Hence, further study is required to determine the most appropriate combinations of Treg-targeted therapies and ICI-based immunotherapy in order to maximize efficacy and minimize off-target toxicity.
DP drafted the manuscript and assembled figures. LC, NM, and HM edited the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by NIH F30CA236031 to DP and Veterans Affairs LPOP Award I50CU000179 to HM and NM.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Gridelli C, Rossi A, Carbone DP, Guarize J, Karachaliou N, Mok T, et al. Non-Small-Cell Lung Cancer. Nat Rev Dis Primers (2015) 1:15009. doi: 10.1038/nrdp.2015.9
2. Ettinger DS, Wood DE, Aggarwal C, Aisner DL, Akerley W, Bauman JR, et al. Nccn Guidelines Insights: non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw (2019) 17:1464–72. doi: 10.6004/jnccn.2019.0059
3. Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival With Ipilimumab in Patients With Metastatic Melanoma. N Engl J Med (2010) 363:711–23. doi: 10.1056/NEJMoa1003466
4. Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab Plus Dacarbazine for Previously Untreated Metastatic Melanoma. N Engl J Med (2011) 364:2517–26. doi: 10.1056/NEJMoa1104621
5. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab Versus Docetaxel in Advanced Nonsquamous non-Small-Cell Lung Cancer. N Engl J Med (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
6. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the Treatment of non-Small-Cell Lung Cancer. N Engl J Med (2015) 372:2018–28. doi: 10.1056/NEJMoa1501824
7. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med (2015) 373:23–34. doi: 10.1056/NEJMoa1504030
8. Gibney GT, Weiner LM, Atkins MB. Predictive Biomarkers for Checkpoint Inhibitor-Based Immunotherapy. Lancet Oncol (2016) 17:e542–e51. doi: 10.1016/S1470-2045(16)30406-5
9. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune Checkpoint Inhibitors: Recent Progress and Potential Biomarkers. Exp Mol Med (2018) 50:1–11. doi: 10.1038/s12276-018-0191-1
10. Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discovery (2018) 8:1069–86. doi: 10.1158/2159-8290.CD-18-0367
11. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin (2020) 70:7–30. doi: 10.3322/caac.21590
12. Pavan A, Attili I, Pasello G, Guarneri V, Conte PF, Bonanno L. Immunotherapy in Small-Cell Lung Cancer: From Molecular Promises to Clinical Challenges. J Immunother Cancer (2019) 7:205. doi: 10.1186/s40425-019-0690-1
13. Kondelkova K, Vokurkova D, Krejsek J, Borska L, Fiala Z, Ctirad A. Regulatory T Cells (TREG) and Their Roles in Immune System With Respect to Immunopathological Disorders. Acta Med (Hradec Kralove) (2010) 53:73–7. doi: 10.14712/18059694.2016.63
14. Duan W, Croft M. Control of Regulatory T Cells and Airway Tolerance by Lung Macrophages and Dendritic Cells. Ann Am Thorac Soc (2014) 11 Suppl 5:S306–13. doi: 10.1513/AnnalsATS.201401-028AW
15. Petersen RP, Campa MJ, Sperlazza J, Conlon D, Joshi MB, Harpole DH Jr., et al. Tumor Infiltrating Foxp3+ Regulatory T-Cells are Associated With Recurrence in Pathologic Stage I NSCLC Patients. Cancer (2006) 107:2866–72. doi: 10.1002/cncr.22282
16. Wang W, Hodkinson P, McLaren F, MacKinnon A, Wallace W, Howie S, et al. Small Cell Lung Cancer Tumour Cells Induce Regulatory T Lymphocytes, and Patient Survival Correlates Negatively With FOXP3+ Cells in Tumour Infiltrate. Int J Cancer (2012) 131:E928–37. doi: 10.1002/ijc.27613
17. Alroqi FJ, Chatila TA. T Regulatory Cell Biology in Health and Disease. Curr Allergy Asthma Rep (2016) 16:27. doi: 10.1007/s11882-016-0606-9
18. Taams LS, Palmer DB, Akbar AN, Robinson DS, Brown Z, Hawrylowicz CM. Regulatory T Cells in Human Disease and Their Potential for Therapeutic Manipulation. Immunology (2006) 118:1–9. doi: 10.1111/j.1365-2567.2006.02348.x
19. Cools N, Ponsaerts P, Van Tendeloo VF, Berneman ZN. Regulatory T Cells and Human Disease. Clin Dev Immunol (2007) 2007:89195. doi: 10.1155/2007/89195
20. Sharabi A, Tsokos MG, Ding Y, Malek TR, Klatzmann D, Tsokos GC. Regulatory T Cells in the Treatment of Disease. Nat Rev Drug Discovery (2018) 17:823–44. doi: 10.1038/nrd.2018.148
21. Corthay A. How do Regulatory T Cells Work? Scand J Immunol (2009) 70:326–36. doi: 10.1111/j.1365-3083.2009.02308.x
22. Smith-Garvin JE, Koretzky GA, Jordan MS. T Cell Activation. Annu Rev Immunol (2009) 27:591–619. doi: 10.1146/annurev.immunol.021908.132706
23. Schmidt A, Oberle N, Krammer PH. Molecular Mechanisms of Treg-Mediated T Cell Suppression. Front Immunol (2012) 3:51. doi: 10.3389/fimmu.2012.00051
24. Wolchok JD, Saenger Y. The Mechanism of Anti-CTLA-4 Activity and the Negative Regulation of T-Cell Activation. Oncologist (2008) 13 Suppl 4:2–9. doi: 10.1634/theoncologist.13-S4-2
25. Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol (2017) 8:561. doi: 10.3389/fphar.2017.00561
26. Ishii T, Ishida T, Utsunomiya A, Inagaki A, Yano H, Komatsu H, et al. Defucosylated Humanized Anti-CCR4 Monoclonal Antibody KW-0761 as a Novel Immunotherapeutic Agent for Adult T-Cell Leukemia/Lymphoma. Clin Cancer Res (2010) 16:1520–31. doi: 10.1158/1078-0432.CCR-09-2697
27. Onda M, Kobayashi K, Pastan I. Depletion of Regulatory T Cells in Tumors With an Anti-CD25 Immunotoxin Induces CD8 T Cell-Mediated Systemic Antitumor Immunity. Proc Natl Acad Sci USA (2019) 116:4575–82. doi: 10.1073/pnas.1820388116
28. Li JY, Duan XF, Wang LP, Xu YJ, Huang L, Zhang TF, et al. Selective Depletion of Regulatory T Cell Subsets by Docetaxel Treatment in Patients With Nonsmall Cell Lung Cancer. J Immunol Res (2014) 2014:286170. doi: 10.1155/2014/286170
29. Scurr M, Pembroke T, Bloom A, Roberts D, Thomson A, Smart K, et al. Low-Dose Cyclophosphamide Induces Antitumor T-Cell Responses, Which Associate With Survival in Metastatic Colorectal Cancer. Clin Cancer Res (2017) 23:6771–80. doi: 10.1158/1078-0432.CCR-17-0895
30. Holmgaard RB, Schaer DA, Li Y, Castaneda SP, Murphy MY, Xu X, et al. Targeting the Tgfbeta Pathway With Galunisertib, a Tgfbetari Small Molecule Inhibitor, Promotes Anti-Tumor Immunity Leading to Durable, Complete Responses, as Monotherapy and in Combination With Checkpoint Blockade. J Immunother Cancer (2018) 6:47. doi: 10.1186/s40425-018-0356-4
31. Hsieh CS, Lee HM, Lio CW. Selection of Regulatory T Cells in the Thymus. Nat Rev Immunol (2012) 12:157–67. doi: 10.1038/nri3155
32. Yadav M, Stephan S, Bluestone JA. Peripherally Induced Tregs - Role in Immune Homeostasis and Autoimmunity. Front Immunol (2013) 4:232. doi: 10.3389/fimmu.2013.00232
33. Kawahata K, Misaki Y, Yamauchi M, Tsunekawa S, Setoguchi K, Miyazaki J, et al. Generation of CD4(+)CD25(+) Regulatory T Cells From Autoreactive T Cells Simultaneously With Their Negative Selection in the Thymus and From Nonautoreactive T Cells by Endogenous TCR Expression. J Immunol (2002) 168:4399–405. doi: 10.4049/jimmunol.168.9.4399
34. Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, et al. JM2, Encoding a Fork Head-Related Protein, is Mutated in X-Linked Autoimmunity-Allergic Disregulation Syndrome. J Clin Invest (2000) 106:R75–81. doi: 10.1172/JCI11679
35. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The Immune Dysregulation, Polyendocrinopathy, Enteropathy, X-Linked Syndrome (IPEX) is Caused by Mutations of FOXP3. Nat Genet (2001) 27:20–1. doi: 10.1038/83713
36. Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-Linked Neonatal Diabetes Mellitus, Enteropathy and Endocrinopathy Syndrome is the Human Equivalent of Mouse Scurfy. Nat Genet (2001) 27:18–20. doi: 10.1038/83707
37. Kanamori M, Nakatsukasa H, Okada M, Lu Q, Yoshimura A. Induced Regulatory T Cells: Their Development, Stability, and Applications. Trends Immunol (2016) 37:803–11. doi: 10.1016/j.it.2016.08.012
38. Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, et al. Tumor Evasion of the Immune System by Converting CD4+CD25- T Cells Into CD4+CD25+ T Regulatory Cells: Role of Tumor-Derived TGF-Beta. J Immunol (2007) 178:2883–92. doi: 10.4049/jimmunol.178.5.2883
39. Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of Peripheral CD4+CD25- Naive T Cells to CD4+CD25+ Regulatory T Cells by TGF-Beta Induction of Transcription Factor Foxp3. J Exp Med (2003) 198:1875–86. doi: 10.1084/jem.20030152
40. Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting Edge: TGF-Beta Induces a Regulatory Phenotype in CD4+CD25- T Cells Through Foxp3 Induction and Down-Regulation of Smad7. J Immunol (2004) 172:5149–53. doi: 10.4049/jimmunol.172.9.5149
41. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal Developmental Pathways for the Generation of Pathogenic Effector TH17 and Regulatory T Cells. Nature (2006) 441:235–8. doi: 10.1038/nature04753
42. Principe DR, DeCant B, Mascarinas E, Wayne EA, Diaz AM, Akagi N, et al. Tgfbeta Signaling in the Pancreatic Tumor Microenvironment Promotes Fibrosis and Immune Evasion to Facilitate Tumorigenesis. Cancer Res (2016) 76:2525–39. doi: 10.1158/0008-5472.CAN-15-1293
43. Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, Ameredes BT, et al. Tolerance Induced by Inhaled Antigen Involves CD4(+) T Cells Expressing Membrane-Bound TGF-Beta and FOXP3. J Clin Invest (2004) 114:28–38. doi: 10.1172/JCI200420509
44. Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, et al. Extrathymically Generated Regulatory T Cells Control Mucosal TH2 Inflammation. Nature (2012) 482:395–9. doi: 10.1038/nature10772
45. Curotto de Lafaille MA, Lafaille JJ, Graca L. Mechanisms of Tolerance and Allergic Sensitization in the Airways and the Lungs. Curr Opin Immunol (2010) 22:616–22. doi: 10.1016/j.coi.2010.08.014
46. Andreev K, Graser A, Maier A, Mousset S, Finotto S. Therapeutical Measures to Control Airway Tolerance in Asthma and Lung Cancer. Front Immunol (2012) 3:216. doi: 10.3389/fimmu.2012.00216
47. Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of Immunological Homeostasis in the Respiratory Tract. Nat Rev Immunol (2008) 8:142–52. doi: 10.1038/nri2236
48. Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, et al. Cutting Edge: Regulatory T Cells From Lung Cancer Patients Directly Inhibit Autologous T Cell Proliferation. J Immunol (2002) 168:4272–6. doi: 10.4049/jimmunol.168.9.4272
49. Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic Value of Tumor-Infiltrating Foxp3+ Regulatory T Cells in Cancers: A Systematic Review and Meta-Analysis. Sci Rep (2015) 5:15179. doi: 10.1038/srep15179
50. Zhao S, Jiang T, Zhang L, Yang H, Liu X, Jia Y, et al. Clinicopathological and Prognostic Significance of Regulatory T Cells in Patients With non-Small Cell Lung Cancer: A Systematic Review With Meta-Analysis. Oncotarget (2016) 7:36065–73. doi: 10.18632/oncotarget.9130
51. Shimizu K, Nakata M, Hirami Y, Yukawa T, Maeda A, Tanemoto K. Tumor-Infiltrating Foxp3+ Regulatory T Cells are Correlated With Cyclooxygenase-2 Expression and are Associated With Recurrence in Resected non-Small Cell Lung Cancer. J Thorac Oncol (2010) 5:585–90. doi: 10.1097/JTO.0b013e3181d60fd7
52. Tao H, Mimura Y, Aoe K, Kobayashi S, Yamamoto H, Matsuda E, et al. Prognostic Potential of FOXP3 Expression in non-Small Cell Lung Cancer Cells Combined With Tumor-Infiltrating Regulatory T Cells. Lung Cancer (2012) 75:95–101. doi: 10.1016/j.lungcan.2011.06.002
53. O’Callaghan DS, Rexhepaj E, Gately K, Coate L, Delaney D, O’Donnell DM, et al. Tumour Islet Foxp3+ T-Cell Infiltration Predicts Poor Outcome in Nonsmall Cell Lung Cancer. Eur Respir J (2015) 46:1762–72. doi: 10.1183/13993003.00176-2014
54. Jin Y, Zhang P, Li J, Zhao J, Liu C, Yang F, et al. B7-H3 in Combination With Regulatory T Cell is Associated With Tumor Progression in Primary Human non-Small Cell Lung Cancer. Int J Clin Exp Pathol (2015) 8:13987–95.2015
55. Wang X, Xiao Z, Gong J, Liu Z, Zhang M, Zhang Z. A Prognostic Nomogram for Lung Adenocarcinoma Based on Immune-Infiltrating Treg-Related Genes: From Bench to Bedside. Transl Lung Cancer Res (2021) 10:167–82. doi: 10.21037/tlcr-20-822
56. Kotsakis A, Koinis F, Katsarou A, Gioulbasani M, Aggouraki D, Kentepozidis N, et al. Prognostic Value of Circulating Regulatory T Cell Subsets in Untreated non-Small Cell Lung Cancer Patients. Sci Rep (2016) 6:39247. doi: 10.1038/srep39247
57. Liu C, Wu S, Meng X, Liu G, Chen D, Cong Y, et al. Predictive Value of Peripheral Regulatory T Cells in non-Small Cell Lung Cancer Patients Undergoing Radiotherapy. Oncotarget (2017) 8:43427–38. doi: 10.18632/oncotarget.15238
58. Muto S, Owada Y, Inoue T, Watanabe Y, Yamaura T, Fukuhara M, et al. Clinical Significance of Expanded Foxp3(+) Helios(-) Regulatory T Cells in Patients With non-Small Cell Lung Cancer. Int J Oncol (2015) 47:2082–90. doi: 10.3892/ijo.2015.3196
59. Hu X, Gu Y, Zhao S, Hua S, Jiang Y. Elevated Circulating CD4(+)CD25(-)Foxp3(+) Regulatory T Cells in Patients With Nonsmall Cell Lung Cancer. Cancer Biother Radiopharm (2019) 34:325–33. doi: 10.1089/cbr.2018.2672
60. Fritzsching B, Oberle N, Pauly E, Geffers R, Buer J, Poschl J, et al. Naive Regulatory T Cells: A Novel Subpopulation Defined by Resistance Toward CD95L-Mediated Cell Death. Blood (2006) 108:3371–8. doi: 10.1182/blood-2006-02-005660
61. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional Delineation and Differentiation Dynamics of Human CD4+ T Cells Expressing the Foxp3 Transcription Factor. Immunity (2009) 30:899–911. doi: 10.1016/j.immuni.2009.03.019
62. Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high Regulatory Cells in Human Peripheral Blood. J Immunol (2001) 167:1245–53. doi: 10.4049/jimmunol.167.3.1245
63. Baecher-Allan C, Wolf E, Hafler DA. MHC Class II Expression Identifies Functionally Distinct Human Regulatory T Cells. J Immunol (2006) 176:4622–31. doi: 10.4049/jimmunol.176.8.4622
64. Larson SM, Carrasquillo JA, Cheung NK, Press OW. Radioimmunotherapy of Human Tumours. Nat Rev Cancer (2015) 15:347–60. doi: 10.1038/nrc3925
65. Gottschalk RA, Corse E, Allison JP. Expression of Helios in Peripherally Induced Foxp3+ Regulatory T Cells. J Immunol (2012) 188:976–80. doi: 10.4049/jimmunol.1102964
66. Thornton AM, Lu J, Korty PE, Kim YC, Martens C, Sun PD, et al. Helios(+) and Helios(-) Treg Subpopulations are Phenotypically and Functionally Distinct and Express Dissimilar TCR Repertoires. Eur J Immunol (2019) 49:398–412. doi: 10.1002/eji.201847935
67. Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, et al. The PD-1 Expression Balance Between Effector and Regulatory T Cells Predicts the Clinical Efficacy of PD-1 Blockade Therapies. Nat Immunol (2020) 21:1346–58. doi: 10.1038/s41590-020-0769-3
68. Wu SP, Liao RQ, Tu HY, Wang WJ, Dong ZY, Huang SM, et al. Stromal PD-L1-Positive Regulatory T Cells and PD-1-Positive CD8-Positive T Cells Define the Response of Different Subsets of non-Small Cell Lung Cancer to PD-1/PD-L1 Blockade Immunotherapy. J Thorac Oncol (2018) 13:521–32. doi: 10.1016/j.jtho.2017.11.132
69. Koh J, Hur JY, Lee KY, Kim MS, Heo JY, Ku BM, et al. Regulatory (Foxp3(+)) T Cells and TGF-Beta Predict the Response to Anti-PD-1 Immunotherapy in Patients With non-Small Cell Lung Cancer. Sci Rep (2020) 10:18994. doi: 10.1038/s41598-020-76130-1
70. Whiteside TL. FOXP3+ Treg as a Therapeutic Target for Promoting Anti-Tumor Immunity. Expert Opin Ther Targets (2018) 22:353–63. doi: 10.1080/14728222.2018.1451514
71. Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited Tumor Infiltration by Activated T Effector Cells Restricts the Therapeutic Activity of Regulatory T Cell Depletion Against Established Melanoma. J Exp Med (2008) 205:2125–38. doi: 10.1084/jem.20080099
72. Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. Transient Regulatory T Cell Ablation Deters Oncogene-Driven Breast Cancer and Enhances Radiotherapy. J Exp Med (2013) 210:2435–66. doi: 10.1084/jem.20130762
73. Goding SR, Wilson KA, Xie Y, Harris KM, Baxi A, Akpinarli A, et al. Restoring Immune Function of Tumor-Specific CD4+ T Cells During Recurrence of Melanoma. J Immunol (2013) 190:4899–909. doi: 10.4049/jimmunol.1300271
74. Sharma A, Subudhi SK, Blando J, Scutti J, Vence L, Wargo J, et al. Anti-CTLA-4 Immunotherapy Does Not Deplete FOXP3(+) Regulatory T Cells (Tregs) in Human Cancers. Clin Cancer Res (2019) 25:1233–8. doi: 10.1158/1078-0432.CCR-18-0762
75. Hughes E, Scurr M, Campbell E, Jones E, Godkin A, Gallimore A. T-Cell Modulation by Cyclophosphamide for Tumour Therapy. Immunology (2018) 154:62–8. doi: 10.1111/imm.12913
76. Hong SH, Yoon IH, Kim YH, Yang SH, Park MJ, Nam HY, et al. High-Dose Cyclophosphamide-Mediated Anti-Tumor Effects by the Superior Expansion of CD44(High) Cells After Their Selective Depletion. Immunobiology (2010) 215:182–93. doi: 10.1016/j.imbio.2009.01.010
77. Heylmann D, Bauer M, Becker H, van Gool S, Bacher N, Steinbrink K, et al. Human CD4+CD25+ Regulatory T Cells are Sensitive to Low Dose Cyclophosphamide: Implications for the Immune Response. PloS One (2013) 8:e83384. doi: 10.1371/journal.pone.0083384
78. Brode S, Raine T, Zaccone P, Cooke A. Cyclophosphamide-Induced Type-1 Diabetes in the NOD Mouse is Associated With a Reduction of CD4+CD25+Foxp3+ Regulatory T Cells. J Immunol (2006) 177:6603–12. doi: 10.4049/jimmunol.177.10.6603
79. Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T Regulatory Cell Function Implicated in Enhanced Immune Response by Low-Dose Cyclophosphamide. Blood (2005) 105:2862–8. doi: 10.1182/blood-2004-06-2410
80. Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, et al. CD4+CD25+ Regulatory T Cells Suppress Tumor Immunity But are Sensitive to Cyclophosphamide Which Allows Immunotherapy of Established Tumors to be Curative. Eur J Immunol (2004) 34:336–44. doi: 10.1002/eji.200324181
81. Takeuchi A, Eto M, Yamada H, Tatsugami K, Naito S, Yoshikai Y. A Reduction of Recipient Regulatory T Cells by Cyclophosphamide Contributes to an Anti-Tumor Effect of Nonmyeloablative Allogeneic Stem Cell Transplantation in Mice. Int J Cancer (2012) 130:365–76. doi: 10.1002/ijc.26009
82. Liu P, Jaffar J, Hellstrom I, Hellstrom KE. Administration of Cyclophosphamide Changes the Immune Profile of Tumor-Bearing Mice. J Immunother (2010) 33:53–9. doi: 10.1097/CJI.0b013e3181b56af4
83. Maeda K, Hazama S, Tokuno K, Kan S, Maeda Y, Watanabe Y, et al. Impact of Chemotherapy for Colorectal Cancer on Regulatory T-Cells and Tumor Immunity. Anticancer Res (2011) 31:4569–74.
84. Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor Rejection by In Vivo Administration of Anti-CD25 (Interleukin-2 Receptor Alpha) Monoclonal Antibody. Cancer Res (1999) 59:3128–33.
85. Arce Vargas F, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH, et al. Fc-Optimized Anti-CD25 Depletes Tumor-Infiltrating Regulatory T Cells and Synergizes With PD-1 Blockade to Eradicate Established Tumors. Immunity (2017) 46:577–86. doi: 10.1016/j.immuni.2017.03.013
86. Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer Cell-Selective In Vivo Near Infrared Photoimmunotherapy Targeting Specific Membrane Molecules. Nat Med (2011) 17:1685–91. doi: 10.1038/nm.2554
87. Sato K, Sato N, Xu B, Nakamura Y, Nagaya T, Choyke PL, et al. Spatially Selective Depletion of Tumor-Associated Regulatory T Cells With Near-Infrared Photoimmunotherapy. Sci Transl Med (2016) 8:352ra110. doi: 10.1126/scitranslmed.aaf6843
88. Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25+ Regulatory Cells Uncovers Immune Responses to Shared Murine Tumor Rejection Antigens. Eur J Immunol (2002) 32:3267–75. doi: 10.1002/1521-4141(200211)32:11<3267::AID-IMMU3267>3.0.CO;2-1
89. Oh DS, Kim H, Oh JE, Jung HE, Lee YS, Park JH, et al. Intratumoral Depletion of Regulatory T Cells Using CD25-Targeted Photodynamic Therapy in a Mouse Melanoma Model Induces Antitumoral Immune Responses. Oncotarget (2017) 8:47440–53. doi: 10.18632/oncotarget.17663
90. Zammarchi F, Havenith K, Bertelli F, Vijayakrishnan B, Chivers S, van Berkel PH. CD25-Targeted Antibody-Drug Conjugate Depletes Regulatory T Cells and Eliminates Established Syngeneic Tumors Via Antitumor Immunity. J Immunother Cancer (2020) 8(2):e000860. doi: 10.1136/jitc-2020-000860
91. Marshall LA, Marubayashi S, Jorapur A, Jacobson S, Zibinsky M, Robles O, et al. Tumors Establish Resistance to Immunotherapy by Regulating Treg Recruitment Via CCR4. J Immunother Cancer (2020) 8(2):e000764. doi: 10.1136/jitc-2020-000764
92. Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, et al. Anti-CCR4 Mab Selectively Depletes Effector-Type Foxp3+CD4+ Regulatory T Cells, Evoking Antitumor Immune Responses in Humans. Proc Natl Acad Sci USA (2013) 110:17945–50. doi: 10.1073/pnas.1316796110
93. Kurose K, Ohue Y, Wada H, Iida S, Ishida T, Kojima T, et al. Phase Ia Study of Foxp3+ CD4 Treg Depletion by Infusion of a Humanized Anti-CCR4 Antibody, KW-0761, in Cancer Patients. Clin Cancer Res (2015) 21:4327–36. doi: 10.1158/1078-0432.CCR-15-0357
94. Zamarin D, Hamid O, Nayak-Kapoor A, Sahebjam S, Sznol M, Collaku A, et al. Mogamulizumab in Combination With Durvalumab or Tremelimumab in Patients With Advanced Solid Tumors: A Phase I Study. Clin Cancer Res (2020) 26:4531–41. doi: 10.1158/1078-0432.CCR-20-0328
95. Principe DR, Doll JA, Bauer J, Jung B, Munshi HG, Bartholin L, et al. TGF-Beta: Duality of Function Between Tumor Prevention and Carcinogenesis. J Natl Cancer Inst (2014) 106:(2)djt369. doi: 10.1093/jnci/djt369
96. Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, et al. Tgfbeta Drives Immune Evasion in Genetically Reconstituted Colon Cancer Metastasis. Nature (2018) 554:538–43. doi: 10.1038/nature25492
97. Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. Tgfbeta Attenuates Tumour Response to PD-L1 Blockade by Contributing to Exclusion of T Cells. Nature (2018) 554:544–8. doi: 10.1038/nature25501
98. Chen X, Wang L, Li P, Song M, Qin G, Gao Q, et al. Dual TGF-Beta and PD-1 Blockade Synergistically Enhances MAGE-A3-Specific CD8(+) T Cell Response in Esophageal Squamous Cell Carcinoma. Int J Cancer (2018) 143:2561–74. doi: 10.1002/ijc.31730
99. Principe DR, Park A, Dorman MJ, Kumar S, Viswakarma N, Rubin J, et al. Tgfbeta Blockade Augments PD-1 Inhibition to Promote T-Cell-Mediated Regression of Pancreatic Cancer. Mol Cancer Ther (2019) 18:613–20. doi: 10.1158/1535-7163.MCT-18-0850
100. Principe DR, Narbutis M, Kumar S, Park A, Viswakarma N, Dorman MJ, et al. Long-Term Gemcitabine Treatment Reshapes the Pancreatic Tumor Microenvironment and Sensitizes Murine Carcinoma to Combination Immunotherapy. Cancer Res (2020) 80:3101–15. doi: 10.1158/0008-5472.CAN-19-2959
101. Paz-Ares L, Kim TM, Vicente D, Felip E, Lee DH, Lee KH, et al. Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-Beta and PD-L1, in Second-Line Treatment of Patients With NSCLC: Results From an Expansion Cohort of a Phase 1 Trial. J Thorac Oncol (2020) 15:1210–22. doi: 10.1016/j.jtho.2020.03.003
102. Cho BC, Kim TM, Vicente D, Felip E, Lee DH, Lee KH, et al. Two-Year Follow-Up of Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-β and PD-L1, for Second-Line (2L) Treatment of non-Small Cell Lung Cancer (NSCLC). J Clin Oncol (2020) 38:9558–. doi: 10.1200/JCO.2020.38.15_suppl.9558
Keywords: lung cancer, immunotherapy, regulatory T (Treg) cell, Immune check inhibitor (ICI), tumor immunology
Citation: Principe DR, Chiec L, Mohindra NA and Munshi HG (2021) Regulatory T-Cells as an Emerging Barrier to Immune Checkpoint Inhibition in Lung Cancer. Front. Oncol. 11:684098. doi: 10.3389/fonc.2021.684098
Received: 22 March 2021; Accepted: 17 May 2021;
Published: 01 June 2021.
Edited by:
Chong Sun, German Cancer Research Center (DKFZ), GermanyReviewed by:
Anju Kumari, National Cancer Institute, United StatesCopyright © 2021 Principe, Chiec, Mohindra and Munshi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel R. Principe, cHJpbmNpcGVAaWxsaW5vaXMuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.