
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 31 May 2021
Sec. Genitourinary Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.683190
This article is part of the Research Topic Post-Operative Radiation Treatment for Prostate Cancer View all 8 articles
 Young Dong Yu1
Young Dong Yu1 Young Hwii Ko2
Young Hwii Ko2 Jong Wook Kim3
Jong Wook Kim3 Seung Il Jung4
Seung Il Jung4 Seok Ho Kang5
Seok Ho Kang5 Jinsung Park6
Jinsung Park6 Ho Kyung Seo7
Ho Kyung Seo7 Hyung Joon Kim8
Hyung Joon Kim8 Byong Chang Jeong9
Byong Chang Jeong9 Tae-Hwan Kim10
Tae-Hwan Kim10 Se Young Choi11
Se Young Choi11 Jong Kil Nam12
Jong Kil Nam12 Ja Yoon Ku13
Ja Yoon Ku13 Kwan Joong Joo14
Kwan Joong Joo14 Won Sik Jang15
Won Sik Jang15 Young Eun Yoon16
Young Eun Yoon16 Seok Joong Yun17
Seok Joong Yun17 Sung-Hoo Hong18
Sung-Hoo Hong18 Jong Jin Oh19,20*
Jong Jin Oh19,20*Aim: This study evaluated the prognosis and survival predictors for bladder urachal carcinoma (UC), based on large scale multicenter cohort with long term follow-up database.
Methods: A total 203 patients with bladder UC treated at 19 hospitals were enrolled. Clinical parameters on carcinoma presentation, diagnosis, and therapeutic methods were reviewed for the primary cancer and for all subsequent recurrences. The stage of UC was stratified by Mayo and Sheldon pathological staging system. Oncological outcomes and the possible clinicopathological parameters associated with survival outcomes were investigated.
Results: The mean age of the patients was 54.2 years. Among the total of 203 patients, stages I, II, III, and IV (Mayo stage) were 48 (23.8%), 108 (53.5%), 23 (11.4%), and 23 (11.4%), respectively. Gross hematuria and bladder irritation symptoms were the two most common initial symptoms. The mean follow-up period was 65 months, and 5-year overall survival rates (OS), cancer-specific survival rates (CSS), and recurrence-free survival rates (RFS) were 88.3, 83.1, and 63.9%, respectively. For the patients with Mayo stage ≥III, OS, CSS, and RFS were significantly decreased to 38.0, 35.2, and 28.4%, respectively. The higher pathological stage (Mayo stage ≥III, Sheldon stage ≥IIIc), positive surgical margin (PSM), and positive lymphovascular invasion (PLM) were independent predictors of shorter OS, CSS, and RFS.
Conclusion: The pathological stage, PSM, and PLM were significantly associated with the survival of UC patients, emphasizing an importance of the complete surgical resection of tumor lesion.
During the fetal development, the urachus obliterates and subsequently forms the median umbilical ligament, a 5–10 cm long fibromuscular canal, which extraperitoneally connects between umbilicus and the roof of bladder in the midline. Incomplete closure of the urachus produces urachal remnants such as urachal cyst, or urachal fistula (1–3). Urachal carcinoma (UC) is relatively rare urologic malignancy, which most frequently occurs within the urachal remnant located at the junction between umbilical ligament and dome of the urinary bladder, whereas it can be even found in any location along the midline of the bladder (4). Regarding the incidence of disease entity, recent retrospective study based on the National Cancer Registry in Ireland presented that UC accounts for 0.3% of overall bladder cancer incidence (5). In addition, previous studies have presented that adenocarcinomas, including signet-ring cell carcinoma or mucinous adenocarcinoma, accounts for almost 90% of histological subtypes of UC (6). UC commonly lacks early clinical symptoms and this results in relatively late diagnosis of the disease whereas the carcinoma is already progressed to an advanced stage such as systemic metastasis at the time of beginning therapeutic intervention (7). The treatment methods of UC are different for localized or metastatic disease, but the current main therapy for localized UC is surgery including partial resection or radical resection (8). Many previous studies have reported that UC cases treated with surgical resection had a median survival of 48 months, and no definite difference in survival rates were observed according to different surgical modalities including partial and radical cystectomy (9). The oncological benefits of bilateral pelvic lymphadenectomy in UC is still highly debatable (6, 10). Moreover, regarding metastatic UC, there is no current standard therapy due to the resistant nature of UC to both chemotherapy and radiation therapy (11). Due to the low prevalence of UC, previous literatures regarding clinical characteristics of UC had a relatively small case series with retrospective nature. Furthermore, studies regarding the Asian population with urachal cancer have been rarely reported (4, 11, 12). Thus, the present study is a retrospective multi-center based research, which primarily investigated the clinical characteristics and oncological outcomes of UC patients treated at overall 19 large scale medical institutions in South Korea. Secondarily, we tried to clarify the clinical and therapeutic factors influencing the oncological outcomes of UC cases.
The medical records of patients with UC who were treated in 19 large scale institutions in South Korea between 1994 and 2020 were retrospectively reviewed. Before the data collection, research approvals were obtained from the institutional ethics committee of each hospital involved in the current study. Clinical parameters and pathological outcomes of the cohort such as initial cystoscopy findings, computed tomography (CT) findings, laboratory blood test results including squamous cell carcinoma-related antigen (SCC), carbohydrate antigen 19-9 (CA19-9), and carcinoembryonic antigen (CEA) levels, initial symptoms, treatment modalities, tumor stage, pathologic types of carcinoma, and immunohistochemistry markers were analyzed. Regarding immunohistochemistry markers, surgical tissue specimen analyses for overexpression of antigen KI-67, p53, epidermal growth factor receptor (EGFR), as well as cytokeratin-7/-20 (CK-7/CK-20), and KRAS overexpression were performed as potential tumor markers.
During the follow-up period, all patients underwent routine blood and urine tests, cystoscopy, and abdomino-pelvis CT at each outpatient clinic visit. Visiting interval was different at each hospital, but it was between 2 and 3 months. If there was any evidence of suspected recurrence in cystoscopy, urine cytology or abdomino-pelvis CT scans, further imaging studies including brain magnetic resonance imaging (MRI), bone scintigraphy, and positron emission tomography (PET) scans were additionally undertaken. The recurrence-free survival (RFS), cancer-specific survival (CSS), and overall survival (OS) were evaluated for the study cohort. OS was evaluated from the date of initial diagnosis to the date of death from any cause or the date of last follow-up visit. RFS was defined as the time from surgery to the date of first recurrence confirmed by radiological tests or follow-up biopsy. CSS was calculated from the date of initial diagnosis to the date of death by UC.
Mean and proportion were used to present categorical data. Multivariate Cox proportional hazards regression analyses were performed to evaluate prognostic and independent factors of survival rates. OS, CSS, and RFS were calculated using the Kaplan–Meier survival analyses with log-rank tests. All statistical analyses were undertaken by using the SPSS package version 24.0 (SPSS Inc., Chicago, IL, USA), and p-values less than 0.05 were considered significant.
The baseline characteristics of the cohort are presented in Table 1. Overall, 203 patients (male: 125 patients, female: 78 patients) treated for UC from 19 different institutions are included in the current study, while the mean follow-up period was 65 months. The mean age was 54.2 years, and most of the patients (202 patients, 99.5%) showed Eastern Cooperative Oncology Group (ECOG) performance status ≤ grade 1. 78 patients (38.4%/mean smoking amount 7.1 pack–years) had smoking history, whereas 33 patients were current smoker. 71.4% of the cohort (145 patients) showed gross hematuria as the cancer-related initial symptom, and suprapubic symptom was the second most common initial symptom (19 patients, 9.4%). Among the cancer related symptoms, omphalitis or mucosuria was not present with any patient, whereas seven patients (3.4%) were asymptomatic. Solid mass (109 patients, 53.7%) and cystic mass (23 patients, 11.3%) were the most common CT findings, whereas only four patients (2.0%) showed cystic mass with calcification in the initial CT scans. According to the initial blood test results, 94.6 and 89.7% patient accompanied elevated CA19-9 and CEA levels, respectively. Regarding therapeutic modalities, 136 patients (67.0%) were treated with surgery alone, whereas 66 patients (32.5%) underwent adjuvant therapy after surgery. Among the modalities of adjuvant therapy, chemotherapy, radiotherapy, and chemotherapy with radiotherapy were performed to 64 patients (97.0%), one patient (1.5%), and one patient (1.5%), respectively. Only one patient received radiotherapy for neoadjuvant therapy before surgical treatment. Regarding mass excision method, 82.8 and 11.3% of the patients underwent partial cystectomy and radical cystectomy, respectively. The other 5.9% patients had initial transurethral resection of bladder (TUR-B) and subsequent partial cystectomy. En-bloc resection of the umbilicus and the median umbilical ligament was performed to 12 patients (5.9%). For adjuvant chemotherapy, cisplatin/5-fluorouracil regimen (22 patients/34.4%) and gemcitabine/cisplatin regimen (14 patients/21.9%) were the most commonly used chemotherapy regimen, whereas the other regimens were applied with similar frequencies (1.5–9.4%).
The histopathological results of the cohort are shown in Table 2. More than half of the patients (156 patients/77.3%) were classified as Mayo stage ≤II, and 174 patients (85.7%) were assigned as Sheldon stage ≤IIIB. Mean number of positive regional lymph node invasion at the time of surgery was 0.3 lymph nodes per patient. Positive surgical margin PSM) was confirmed in 17 patients (8.4%), and 48 patients (23.6%) accompanied positive lymphovascular invasion (PLM). Positive urine cytology with malignant cells was observed in only seven patients (3.4%). 21 patients (10.3%) had positive distant metastasis at the time of diagnosis, whereas peritoneum and lung were the two most common metastatic sites. More than half (58.1%) of the patients had tumor size ≥4 cm. Regarding pathologic types of tumor, adenocarcinoma (89.7%) was the most frequent type. The other pathological types include urothelial carcinoma (7.9%), undifferentiated carcinoma (2.0%), and small cell carcinoma (0.5%). For histologic sub-classification, mucinous feature (55.9%) and enteric feature (23.3%) were the two most commonly observed histologic sub-types. Immunohistochemical results presented that elevated expression of EGFR, and p53 was confirmed in 90.1% of the patients, whereas KRAS mutation and increased CK-20 expression were detected in 56.2 and 64.0% of the study population.
Among the study cohort, 82 patients (40.4%) had recurrence of urachal cancer after initial treatment (Table 2). For salvage treatment to the recurred UC patients, concurrent chemotherapy with radiotherapy (42 patients, 51.2%) was the most commonly used therapeutic modality (Table 2). Mean survival of OS, CSS, and RFS were 46.8, 44.7, and 39.6 months, respectively (Table 2, Figure 1). For OS, 2-, 5-, and 10-year survival rates were 98.0, 88.3, and 69.5%, respectively (Table 2, Figure 1A). 2-, 5- and 10-year CSS rates were 95.9, 83.1, and 68.4% (Table 2, Figure 1B), and RFS rates were 91.5, 63.9, and 59.8%, respectively (Table 2, Figure 1C). 5-year OS, CSS, and RFS for the patients with Mayo stage ≥III were 38.0, 35.2, and 28.4%, which indicated significantly poorer survival outcomes associated with advanced stage UC (Table 2, Figure 2). The results for survival predictors evaluated by multivariate Cox proportional hazards regression analyses are presented in Table 3. PSM and PLM were the independent predictors for shorter OS, CSS, and RFS (Table 3). Mayo stage ≥III and Sheldon stage ≥IIIC were also significantly associated with shorter survival outcomes including OS, CSS, and RFS (Table 3). Among pathologic types of UC, small cell cancer was an independent predictor for shorter OS and CSS (Table 3). However, body mass index (BMI) was associated with longer OS and CSS (OS: p = 0.037. CSS: p = 0.021) (Table 3). Regarding surgical methods, radical cystectomy was not associated with superior survival outcomes compared with partial cystectomy (Table 3).
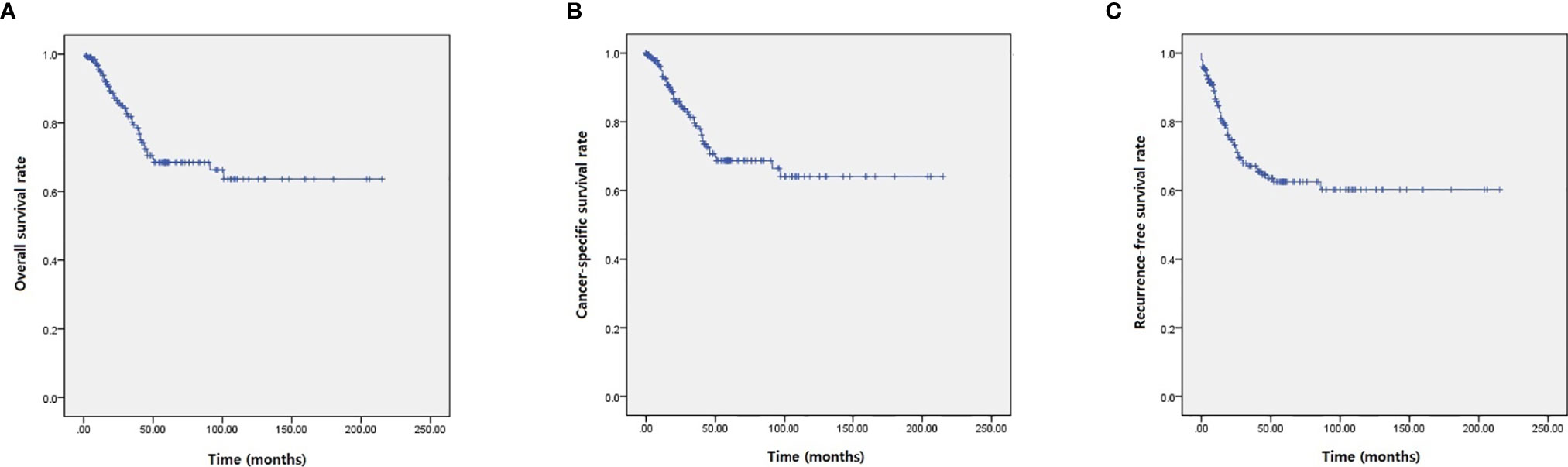
Figure 1 Kaplan–Meier analyses presenting survival outcomes of UC patients. (A) OS. (B) CSS. (C) RFS.
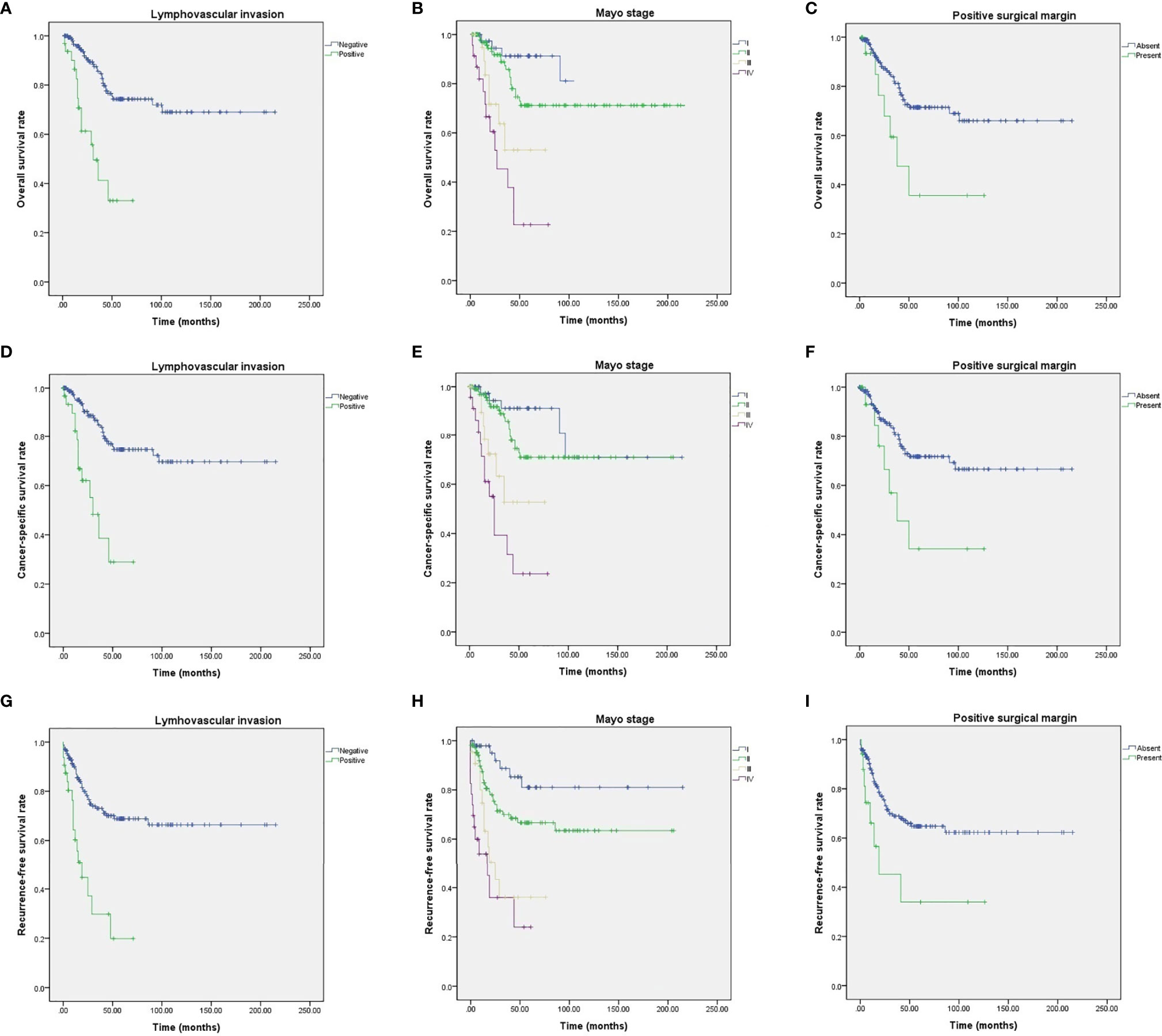
Figure 2 Kaplan–Meier analyses of UC patients with different survival outcome predictors. (A) OS for PLM. (B) OS for Mayo stages. (C) OS for PSM. (D) CSS for PLM. (E) CSS for Mayo stages. (F) CSS for PSM. (G) RFS for PLM. (H) RFS for Mayo stages. (I) RFS for PSM.
The current study retrospectively reviewed 203 patients treated for confirmed UC, and this study described their several unique and original clinicopathological findings. Moreover, this study also evaluated the clinicopathological predictors of oncological outcomes for UC. The mean age of our study cohort was 54.2 years, and 61.6% of the patients were male. These values are similar to the result of the recent SEER database analysis (age range 46–71 years/60% males) (13). Previous studies including Molina et al. have suggested strong association between Tobacco exposure and UC (14), and our study results also showed that more than half of the cohort (125 patients, 61.6%) were current or ex-smoker. As previous literatures demonstrated (2, 15), gross hematuria was the most common initial symptom. Solid mass lesion was the most commonly observed initial CT findings, and it provided a general impression of UC such as the size and location, which were consistent with previous reports (1, 16). Our study data presented that elevations in some tumor markers including CEA, and CA19-9 were accompanied in majority of the patients. Some previous researches including Siefker-Radtke et al. (17) showed similar results, and these tumor marker analyses strongly suggested diagnostic value of CEA and CA19-9 in UC although the disease-specificity for diagnosis might be relatively low. In addition, CEA or CA19-9 showed no association with patients’ survival in this study.
The currently accepted surgical treatment of UC throughout previous studies is partial cystectomy with complete resection of the tumor and en-bloc resection of the median umbilical ligament and the umbilicus is also recommened (1, 17, 18). However, this standard surgical modality and regional pelvic lymph node dissection (PLND) approach still carry some contraversies (18). In this study, most of the patients underwent partial cystectomy (82.8%), whereas 12 of them received TUR-B prior to partial cystectomy. Due to the retrospective multi-center based nature of this study, diversity of surgical modalities was inevitable. Nevertheless, umbilectomy with umbilical ligament resection and PLND were performed to only 5.9 and 23.2% of the patients, respectively. We reckon this deflection of surgical method might be due to the surgeon’s reluctance of extensive surgical dissection when the therapeutic effect of the method is not fully confirmed. Although PLND was not associated with survival of UC cohort in this study, we still believe the importance of PLND should not be diminished at any time. Since preoperative radiologic exams cannot detect all lymph invasions, pelvic exploring should be performed even if no lymph node invasion was suspected in preoperative imaging. In addition, further evaluations with larger size cohort would be helpful to define the surgical extent of PLND. Most of the previous studies about chemotherapy regimen for urachal adenocarcinoma have analyzed combination chemotherapy. As urachal adenocarcinoma is pathologically similar to colorectal adenocarcinoma, mFOLFOX-6 (leucovorin, fluorouracil, oxaliplatin) regimens have shown effective therapeutic outcomes in previous studies (17). Early analysis on the combination regimen of 5-fluorouracil, leucovorin, gemcitabine, and cisplatin also showed promising oncological outcomes with 30–40% of radiographic therapeutic response rates, but long-term outcomes need to be further evaluated (19). In addition, previous literatures have shown that the most effective chemotherapy regimen might be the combination of 5-fluorouracil and cisplatin, which seems to produce better outcomes in terms of response rate compared with other cisplatin-based regeimens (19, 20). Our study results showed that 12 different chemotherapy regimens were used, and even the most commonly used regimen (cisplatin with 5-fluorouracil) were applied to only 22 patient. Thus, it was relatively difficult to evaluate the optimal chemotherapy regimen with superior outcomes in the current therapy.
Although some previous studies tried to evaluate the immunohistochemical characteristics of UC, no UC specific immunohistochemical analysis based biomarkers have been found. Previous studies, which performed immunohistochemical analyses for UC, suggested several biomarkers including Ki67 and p53 (21, 22). Ki67 is expressed in proliferating cells and highly elevated expression of Ki67 is observed in UC (15, 21). An accumulation of p53 protein indicates mutations in tumor suppressor gene TP53, and strong positivity of p53 accumulation has been described in previous studies (22). Our study results showed increase of Ki57 expression and p53 positivity, but immunoreactivity of both p53 and Ki67 were not associated with survival outcomes of the patients.
According to a study that analyzed the UC cohort from MD Anderson Cancer Center, bone, lung, and liver were the three most common metastatic sites (10). Our study results showed that lung, liver, and skin were the three most metastatic sites of metastasis when peritoneum and abdominal wall are excluded from the analysis. These study results emphasize the importance of regular evaluation of lung and liver for monitoring the progression and recurrence of UC.
Another noticeable finding of this study is the clinical significance of PSM and PLM on survival of UC patients. Many previous studies including Ashley et al. (23) presented PSM has a strong negative impact on survival of UC cohort. Our study results also showed that PSM is significantly associated with shorter OS, CSS, and RFS. In addition, PLM was also independent predictor of OS, CSS, and RFS in our study. To our knowledge, the current study is the first research presenting the influence of PLM on survival of UC cohort.
The mean values of OS, CSS, and RFS in this study are similar to previous studies (24). However, 5- and 10-year survival rates are relatively higher than previous studies (10, 14, 25). We believe these better long-term survival rates are mainly due to small cohort size and relatively longer follow-up period. In the current study, 25.1% of the patients, who had excellent oncological outcomes such as no UC recurrence, underwent surgery more than 10 years ago. Thus, longer follow-up period of these patients might have exaggerated survival rates of the entire study cohort. Moreover, overall 203 patients are included in this study, which implies the cohort size was not big enough to minimize the selection bias affecting patients’ long-term survival analysis.
Although no confirmative staging system for UC has been validated, Sheldon and Mayo staging systems are the two most commonly used stages (23, 25, 26). The current study results showed that higher tumor stages (Mayo stage ≥III and Sheldon stage ≥IIIC) were strongly associated with poor survival outcomes of UC patients, and these results coincide with the previous study results (20, 25). As higher tumor stages of UC significantly increased negative prognostic predictive ability of the Mayo and Sheldon staging systems, early and active therapeutic intervention might need to be emphasized for the patients having UC with progressed stages.
There are some potential limitations in this study. First, due to the retrospective multi-center based nature of the study, standardization of therapeutic modalities was not performed. This limited evaluation of therapeutic methods on survival outcomes. Second, because of the rareness of UC, relatively small sample size diminished statistical power of the study results including immunohistochemical biomarkers. Thus, further research studies with larger cohort size need to be undertaken to confirm the study results.
Despite the limitations, to the best of our knowledge, this study is the first multi-institutional study with the largest sample size, which evaluated therapeutic outcomes and potential predictors of UC patients in Asia.
Immunohistochemical biomarkers including Ki67 and p53 are markedly increased in UC patients. The strong association of PSM, PLM with survival outcomes of UC patients emphasizes an importance of the complete surgical resection of tumor lesion. Higher Mayo and Sheldon stages were significantly associated with long-term survival. Due to the relatively small cohort size, the universal predictors of oncological outcomes in UC patients were not confirmed.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Seoul National University Bundang Hospital IRB. The patients/participants provided their written informed consent to participate in this study.
YDY: conceived of the presented idea, analysis and interpretation of data, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, writing—initial draft, critical revision of the article for important intellectual content, statistical analysis, and administrative, technical, and administrative support. JO: study concept and design, acquisition of data, analysis and interpretation of data, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, editing—initial draft, critical revision of the article for important intellectual content, statistical analysis, administrative, technical, and material support, and study supervision. YK, JWK, SJ, SK, JP, HS, HK, BJ, T-HK, SC, JN, JYK, KJ, WJ, YEY, SY, and S-HH: study concept and design, acquisition of data, and administrative, technical, and material support. All authors contributed to the article and approved the submitted version.
This research was neither funded nor supported by any institution or organization. The authors wish to acknowledge all participating investigators.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Bruins HM, Visser O, Ploeg M, Hulsbergen-van de Kaa CA, Kiemeney LA, Witjes JA. The Clinical Epidemiology of Urachal Carcinoma: Results of a Large, Population Based Study. J Urol (2012) 188(4):1102–7. doi: 10.1016/j.juro.2012.06.020
2. Upadhyay V, Kukkady A. Urachal Remnants: An Enigma. Eur J Pediatr Surg (2003) 13:372–6. doi: 10.1055/s-2003-44725
3. Mennitto A, Vernieri C, Procopio G. Urachal Carcinoma: Towards a Precision Medicine. Transl Cancer Res (2016) 5(Suppl 7):S1307. doi: 10.21037/tcr.2016.12.28
4. Paner GP, McKenney JK, Barkan GA, Yao JL, Frankel WL, Sebo T, et al. Immunohistochemical Analysis in Amorphologic Spectrum of Urachal Epithelial Neoplasms: Diagnostic Implications and Pitfalls. Am J Surg Pathol (2011) 35:787–98. doi: 10.1097/PAS.0b013e3182189c11
5. Collins DC, Velazquez-Kennedy K, Deady S, Brady AP, Sweeney P, Power DG. National Incidence, Management and Survival of Urachal Carcinoma. Rare Tumors (2016) 8:6257. doi: 10.4081/rt.2016.6257
6. Siefker-Radtke A. Urachal Adenocarcinoma: A Clinician’s Guide for Treatment. Semin Oncol (2012) 39:619–24. doi: 10.1053/j.seminoncol.2012.08.011
7. Besarani D, Purdie CA, Townell NH. Recurrent Urachal Adenocarcinoma. J Clin Pathol (2003) 56(11):882. doi: 10.1136/jcp.56.11.882
8. Aggarwal A, Agarwal S, Pandey S, Sankhwar S. Urachal Adenocarcinoma. BMJ Case Rep (2018) 12:bcr2018226207. doi: 10.1136/bcr-2018-226207
9. Milhoua PM, Knoll A, Bleustein CB, Ghavamian R. Laparoscopic Partial Cystectomy for Treatment of Adenocarcinoma of the Urachus. Urology (2006) 67(423):423.e15–17. doi: 10.1016/j.urology.2005.08.044
10. Wright JL, Porter MP, Li CI, Lange PH, Lin DW. Differences in Survival Among Patients With Urachal and Nonurachal Adenocarcinomas of the Bladder. Cancer (2006) 107(4):721–8. doi: 10.1002/cncr.22059
11. Gopalan A, Sharp DS, Fine SW, Tickoo SK, Herr HW, Reuter VE, et al. Urachal Carcinoma: A Clinicopathologic Analysis of 24 Cases With Outcome Correlation. Am J Surg Pathol (2009) 33:659–68. doi: 10.1097/PAS.0b013e31819aa4ae
12. Hayashi T, Yuasa T, Uehara S, Inoue Y, Yamamoto S, Masuda H, et al. Clinical Outcome of Urachal Cancer in Japanese Patients. Int J Clin Oncol (2016) 21:133–8. doi: 10.1007/s10147-015-0866-8
13. Mylonas KS, O'Malley P, Ziogas IA, El-Kabab L, Nasioudis D. Malignant Urachal Neoplasms: A Population-Based Study and Systematic Review of Literature. Urol Oncol-Semin Ori (2017) 35:33. doi: 10.1016/j.urolonc.2016.07.021
14. Molina JR, Quevedo JF, Furth AF, Richardson RL, Zincke H, Burch PA. Predictors of Survival From Urachal Cancer: A Mayo Clinic Study of 49 Cases. Cancer (2007) 110(11):2434–40. doi: 10.1002/cncr.23070
15. Niedworok C, Panitz M, Szarvas T, Reis H, Reis AC, Szendröi A, et al. Urachal Carcinoma of the Bladder: Impact of Clinical and Immunohistochemical Parameters on Prognosis. J Urol (2016) 195(6):1690–6. doi: 10.1016/j.juro.2015.11.067
16. Koster IM, Cleyndert P, Giard RW. Best Cases From the AFIP: Urachal Carcinoma. Radiographics (2009) 29:939–42. doi: 10.1148/rg.293085152
17. Siefker-Radtke A, Gee J, Shen YU, Wen S, Daliani D, Millikan RE, et al. Multimodality Management of Urachal Carcinoma: The M.D. Anderson Cancer Center Experience. J Urol (2003) 169(4):1295–8. doi: 10.1097/01.ju.0000054646.49381.01
18. Yanagihara Y, Tanti N, Miura N, Shirato A, Nishimura K, Fukumoto T, et al. Modified FOLFOX-6 Chemotherapy in Patients With Metastatic Urachal Cancer. Chemotherapy (2013) 59(6):402–6. doi: 10.1159/000362400
19. Claps M, Stellato M, Zattarin E, Mennitto A, Sepe P, Guadalupi V, et al. Current Understanding of Urachal Adenocarcinoma and Management Strategy. Curr Oncol Rep (2020) 22(1):9. doi: 10.1007/s11912-020-0878-z
20. Szarvas T, Módos O, Niedworok C, Reis H, Szendröi A, Szász MA, et al. Clinical. Prognostic, and Therapeutic Aspects of Urachal Carcinoma - A Comprehensive Review With Meta-Analysis of 1,010 Cases. Urol Oncol (2016) 34:388–98. doi: 10.1016/j.urolonc.2016.04.012
21. Kirsch DG, Kastan MB. Tumor-Suppressor p53: Implications for Tumor Development and Prognosis. J Clin Oncol (1998) 16(9):3158–68. doi: 10.1200/JCO.1998.16.9.3158
22. Endl E, Gerdes J. The Ki-67 Protein: Fascinating Forms and an Unknown Function. Exp Cell Res (2000) 257(2):231–7. doi: 10.1006/excr.2000.4888
23. Ashley RA, Inman BA, Sebo TJ, Leibovich BC, Blute ML, Kwon ED, et al. Urachal Carcinoma: Clinicopathologic Features and Long-Term Outcomes of an Aggressive Malignancy. Cancer (2006) 107:712–20. doi: 10.1002/cncr.22060
24. Duan F, Zhai W, Zhang B, Guo S. Urachal Carcinoma: Impact of Recurrence Pattern and Lymphadenectomy on Long-Term Outcomes. Cancer Med (2020) 9(12):4166–74. doi: 10.1002/cam4.3059
25. Pinthus JH, Haddad R, Trachtenberg J, Holowaty E, Bowler J, Herzenberg AM, et al. Population Based Survival Data on Urachal Tumors. J Urol (2006) 175(6):2042–7. doi: 10.1016/S0022-5347(06)00263-1
Keywords: urachal carcinoma, bladder, survival rate, surgical margin, lymphovascular invasion
Citation: Yu YD, Ko YH, Kim JW, Jung SI, Kang SH, Park J, Seo HK, Kim HJ, Jeong BC, Kim T-H, Choi SY, Nam JK, Ku JY, Joo KJ, Jang WS, Yoon YE, Yun SJ, Hong S-H and Oh JJ (2021) The Prognosis and Oncological Predictor of Urachal Carcinoma of the Bladder: A Large Scale Multicenter Cohort Study Analyzed 203 Patients With Long Term Follow-Up. Front. Oncol. 11:683190. doi: 10.3389/fonc.2021.683190
Received: 20 March 2021; Accepted: 04 May 2021;
Published: 31 May 2021.
Edited by:
Fred Saad, University of Montreal Hospital Centre (CRCHUM), CanadaReviewed by:
Alessandro Antonelli, University of Verona, ItalyCopyright © 2021 Yu, Ko, Kim, Jung, Kang, Park, Seo, Kim, Jeong, Kim, Choi, Nam, Ku, Joo, Jang, Yoon, Yun, Hong and Oh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jong Jin Oh, YmVic3V6emFuZ0BuYXZlci5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.