
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 08 September 2021
Sec. Cancer Genetics
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.682917
This article is part of the Research TopicAltered Epigenetic Modification Mediated Dysregulated Transcriptional Networks in Drug ResistanceView all 5 articles
Background: Apigenin (APG), a natural flavonoid, can affect the development of a variety of tumors, but its role in ovarian cancer remains unclear. There has been an increasing amount of evidence supporting the vital role played by mast cells and the bioactive mediators they release, as components of the tumor microenvironment, in the progression of ovarian cancer (OC); however, the mechanism warrants further exploration.
Methods and Results: In this study, a combination of transcriptomics analysis and application of TCGA database was performed, and we found that the expression of genes related to mast cell degranulation in ovarian cancer tissues changed remarkably. We then explored whether histamine, a major constituent of mast cell degranulation, could affect the development of ovarian cancer through immunohistochemistry analysis and cell proliferation assays. The results showed that a certain concentration of histamine promoted the proliferation of ovarian cancer cells by upregulating the expression of estrogen receptor α (ERα)/estrogen receptor β (ERβ). Additionally, we found that the inhibition of ERα or the activation of ERβ could inhibit the proliferation of ovarian cancer cells induced by histamine through real-time PCR and western blot assays. Finally, we demonstrated the attenuation effect imparted by apigenin in histamine-mediated ovarian cancer via the PI3K/AKT/mTOR signaling pathway.
Conclusion: Our research revealed that apigenin decelerated ovarian cancer development by downregulating ER-mediated PI3K/AKT/mTOR expression, thus providing evidence of its applicability as a potentially effective therapeutic agent for ovarian cancer treatment.
As one of the most commonly reported cancers among women, ovarian cancer (OC) ranks first in mortality among gynecological tumors, and is characterized by a high recurrence, thus establishing itself as a grave risk to the lives and health of women worldwide (1). There seems to be a multifactorial contribution to this disease, such as psychology, environment, the expression of oncogenes, and the inhibition of tumor suppressor genes; however, its pathogenesis remains unknown (2). Currently, there are many clinical treatment approaches available for ovarian cancer. Among them, the most widely used approach is tumor cytoreductive surgery combined with platinum therapy or paclitaxel administration (3). Although surgery and chemotherapy using platinum-based treatments promote better survival in patients with advanced or metastatic ovarian cancer, most patients continue to present with deterioration of their case; therefore, there is an urgent need to explore and develop new therapeutic targets for this debilitating disease.
As components of the tumor microenvironment, mast cells (MCs) play a complex role in promoting angiogenesis, tissue remodeling, and in regulating the host immune response by enabling the secretion of proteases to trigger the activation of receptors, prostaglandins, histamine (HA), and other mediators, thereby promoting tumor growth (4). Among them, histamine, a low-molecular-weight biogenic amine, is the main bioactive substance released upon the degranulation of MCs. Its expression and synthesis have been detected in various cancer cells and malignant tumors (5). The action of histamine is realized after its binding to different receptors (6); the binding induces different responses pertaining to cell proliferation through different signaling pathways. Studies have shown that the histamine content of breast tumor tissue is positively correlated with the number of MCs, which indicates that MCs are the source of histamine in tumor tissues. The increased activity of L-histidine decarboxylase, an enzyme that catalyzes the production of histamine, was observed in certain tumors, including colon cancer, ovarian cancer, endometrial cancer, and melanoma, via immunohistochemistry and RT-PCR, and a high level of histamine release was observed via radioimmunoassays (7–9). Similarly, a considerable number of mast cells were observed in colorectal cancer tissues, and cancer cells near histamine-producing cells were observed to possess a high proliferation ability, and such aspects were found to negatively impact the overall prognosis of patients (10). Therefore, the impact of histamine on the development and prognosis of ovarian cancer should be further explored.
Apigenin (APG) is a natural plant flavonoid compound that is widely present in celery, chamomile, olive, thyme, and other plants (11). In the 1960s, apigenin first attracted the attention of the scientific community because of its ability to inhibit the release of histamine from basophils (12). Studies have shown that in addition to its antioxidant and anti-inflammatory effects (13), APG can inhibit cell proliferation in pancreatic, colorectal, lung, and other tumors, characterized by a reduction in the expression of Hypoxia Inducible Factor-1 (HIF-1), Vascular Endothelial Growth Factor (VEGF), and their receptors in the tumor environment (14).
In this study, we conducted a bioinformatics analysis based on RNA-Seq and TCGA database and in vitro studies to investigate the role of histamine and apigenin in OC, and found that APG attenuated the excessive proliferation of HA-induced OC cell lines. Our results showed that APG mitigated the HA-induced proliferation of OC cells by regulating the ER-mediated PI3K/AKT/mTOR pathway, which might provide insights into the development of potential treatment strategies for ovarian cancer.
The ovarian cancer tissues used in this study were collected from hospitals in Beijing that extended cooperation with our research team. The experiment complied with the relevant regulations and was approved by Human Research Ethics Committee. Six fresh tissues were analyzed using RNA-Seq technology; the tissues were derived from patients who underwent radical ovarian cancer surgery in the hospital from January 2015 to December 2016, including those derived from three cases of healthy ovary tissue and three cases of serous ovarian tumors. The information of patients are given in the Supplementary Table 1. All tissues were stored at -80°C before being subjected to further analyses.
Total RNA extraction from frozen tissues was conducted using the TRNzol reagent (TransGen Biotech, Beijing, China). After the performance of quality control, at least 3 µg RNA with RNA integrity number (RIN) greater than 8.0 was used for database construction. A 100-bp paired-end RNA sequencing experiment was performed using the HiSeq2500® system (Illumina, USA). The database construction and sequencing services were provided by Novogene (Beijing, China).
Data retrieved from TCGA were used for the integrated analysis in this study. RNA-Seq data derived from patients with OC (n=308) in TCGA and normal samples (n=88) in the GTEx database were obtained from UCSC Xena (https://xenabrowser.net/datapages/). Raw data were downloaded and normalized using a robust multi-array average (RAM) method using the “affy” package in R software.
The RNA sequencing reads were aligned to the reference sequence information provided by the Ensembl project using TopHat based on the Bowtie algorithm (15). Using the limma package, the differentially expressed genes were determined, and the genes with P < 0.05, and |log2(fold change)| > 1 were selected as the significantly differentially expressed genes (DEGs). To better understand the function of the selected DEGs, we used the clusterprofiler package of R to perform GO function enrichment analysis and KEGG pathway enrichment analysis. Statistical significance was set at P<0.05.
Human ovarian cancer cell lines OVCAR-3 and Anglne were provided by the BeNa Culture Collection and Procell Life Science & Technology Co., Ltd, respectively, which were sourced from the American Type Culture Collection. The OVCAR-3 and Anglne cells were routinely cultured in dulbecco’s modified eagle medium (DMEM) containing 10% FBS and 1% penicillin/streptomycin/amphotericin B and were maintained in a humidified incubator at 37°C with 5% CO2. The cells were subjected to treatment with different concentrations of histamine (1, 5, 10, 50, and 100 ng/mL) and apigenin (0.1, 0.5, 1, 5, and 10 μM) for 48 h. Additionally, 1 μM AZD9496, PHTPP, PPT, and DPN were added to the treatment group and incubation was observed for 48 h.
Tissue specimens were embedded in paraffin and cut into 4 μm-thick sections. After performing high-temperature retrieval at 98°C with antigen, tumor sections were incubated with the respective antibodies and biotin-conjugated secondary anti-mouse IgGs. The tissues were stained with diaminobenzidine and visualized using the electron microscopy protocol proposed by Soslow (16).
Cell proliferation was measured using the CCK-8 kit (Beyotime, Beijing, China). Briefly, 2×104 OVCAR-3 and Anglne cells suspended in 100 μL culture medium were inoculated into 96-well culture plates and cultured in a humidified incubator at 37°C with 5% CO2 for 12 h to enable cell adherence to the plate. Then, different doses of histamine (100, 50, 10, 5, and 1 ng/mL) were added to the treatment wells. After culturing under the same conditions for 48 h,10 μL of the CCK-8 reagent was added. After incubation for 1 h, the absorbance was measured at 450 nm using a microplate reader. The cell proliferation rate was calculated as follows:
Total mRNA isolation from OVCAR-3 cells was performed using Trizol Up reagent (TransGen Biotech) and samples were reverse-transcribed using a cDNA reverse transcription kit (Tiangen Biotech) according to the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) analyses of histamine receptors and ERs were performed using the TransStart® Green qPCR SuperMix (Bio-Rad Laboratories, California, USA). The primer pairs used for the amplification of the target genes are presented in Supplementary Table 2.
Briefly, the cells subjected to treatments were homogenized in lysis buffer and centrifuged at 14,000 × g for 20 min to collect the supernatants. Protein concentrations were quantified using the Bradford protein assay kit (Beyotime). Cell proteins were separated on a 12% polyacrylamide gel and transferred onto polyvinylidene fluoride membranes. Then, the membranes were blocked with freshly prepared 5% skimmed milk powder in TBS/Tween-20 buffer for 2 h, following which they were washed and incubated overnight at 4°C with the specific primary antibodies. Thereafter, horseradish peroxidase-conjugated secondary antibodies were incubated with the membranes for 2 h. Proteins were visualized using chemiluminescence reagents, and the signals were detected using a gel documentation system.
All data in the experiment were analyzed using the SPSS 13.0 and GraphPad Prism 5 software. The experimental data are expressed as mean ± SD from three independent biological replicates. Changes observed in the experiment were assessed for statistical significance using one-way analysis of variance (ANOVA). Values were considered significant at P<0.05.
To investigate the influence of mast cell degranulation on ovarian cancer, the differentially expressed genes between normal ovary tissues and ovarian cancer tissue were analyzed according to the count values of all detected genes (Figure 1A). After quality evaluation, the sequencing results were observed to satisfy the requirements, as shown in Supplementary Figure 1 and Supplementary Table 3. A total of 1,068 differentially expressed genes (DEGs) were identified at a cutoff of |log2(fold change)| > 1, Padj<0.05, and included 524 upregulated genes and 544 downregulated genes (Figure 1B). To increase the credibility of the results, we downloaded OC transcriptome data from TCGA database and selected 2,018 genes with a significance level of P <0.05, and |log2(fold change)| > 1 at intersection with our sequencing results (Figure 1C). Supplementary Table 4 depicts the top 20 differentially expressed intersection genes. To further analyze the biological functions and signaling pathways of the 2,018 DEGs, GO and KEGG enrichment analyses were performed. GO analysis mainly concentrated on the extracellular matrix, collagen-containing extracellular matrix, extracellular structure organization, extracellular matrix organization, and cell-cell junction, among others. KEGG pathway analysis indicated the presence of PI3K-Akt signaling pathway, MAPK signaling pathway, human papillomavirus infection, among others (Figures 1D, E).
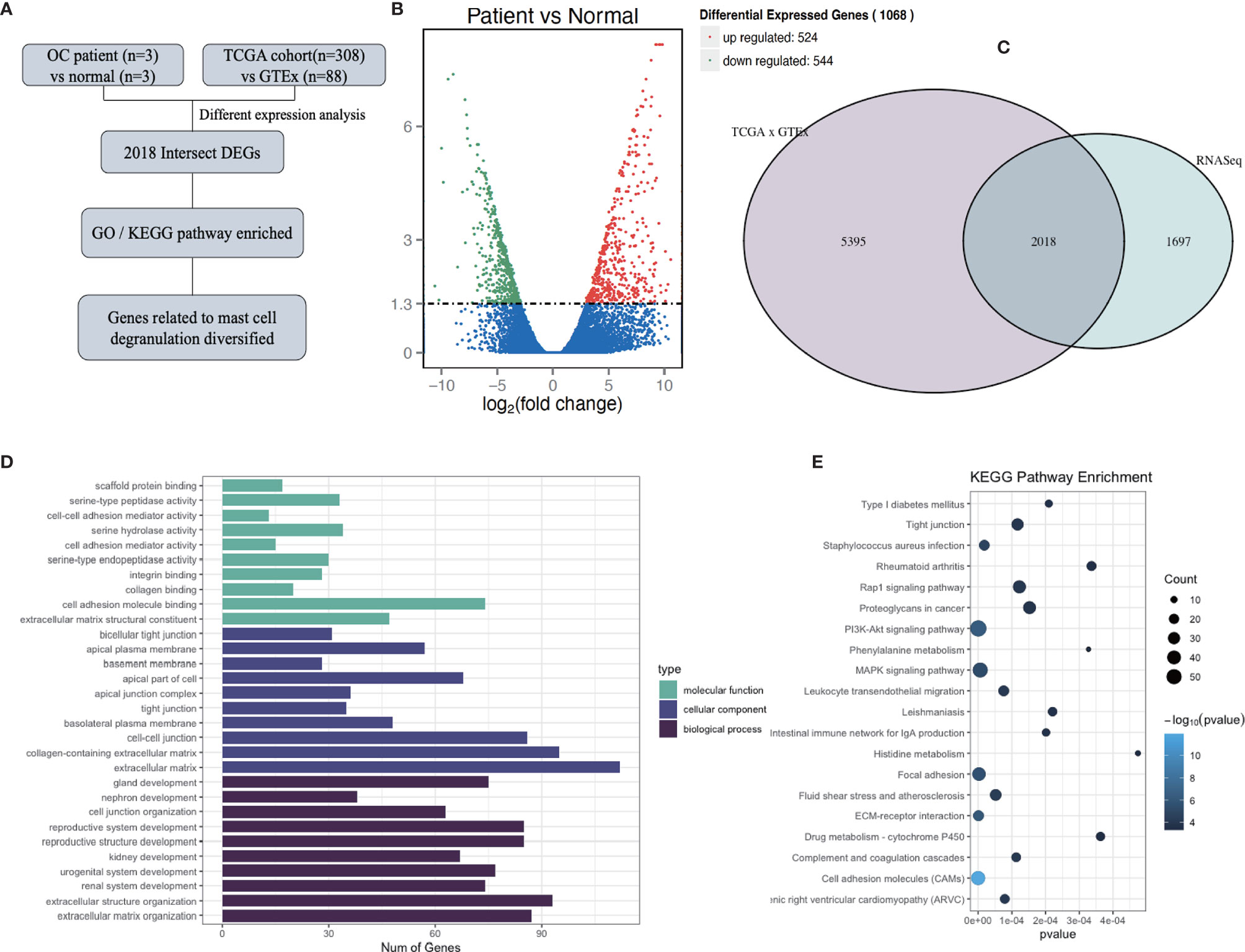
Figure 1 Determination of genes differently expressed between normal ovarian tissue and ovarian cancer tissue. (A) Flow diagram of current bioinformatics research: a combination of RNA-Seq analysis and TCGA database. (B) Volcano plot of all genes differentially expressed between normal ovarian tissue (n = 3) and ovarian cancer tissue (n = 3), at a significance thresholds of |log2(Fold Change)| > 1, Padj<0.05. (C) Venn diagram of the results of the analyses of RNA-Seq and differently expressed genes (DEGs) in TCGA and GTEx. (D, E) GO (D) and KEGG (E) enrichment pathway analysis involving intersection DEGs.
After the intersection DEGs were screened and analyzed, the DEGs associated with mast cells were filtered and were observed to play a role in the growth, migration, and degranulation of mast cells, as listed in Table 1. The increased expression of vesicle-associated membrane protein-8 (VAMP-8), a fusion protein of the soluble N-ethyl maleimide sensitive factor (NSF) adhesion protein receptor (SNARE) family, garnered our attention, which regulates exocytosis (17). Meanwhile, as a member of the glysin superfamily, the protein CapG is reportedly involved in the remodeling of muscle fibers and in the regulation of endocytosis by binding with Ca2+ and by adding caps to the F-actin extension terminal (18). These findings suggest that ovarian cancer may be related to the degranulation of MCs.
It is not clear whether histamine, the main active constituent released during the degranulation of MCs, plays a role in the development of ovarian cancer. Therefore, to determine the relationship between histamine release and the development of ovarian cancer, we analyzed histamine levels in normal ovary tissues and ovarian cancer tissues via immunohistochemical staining. The results indicate that compared with normal ovary tissue, tumors in ovarian cancer tissues are poorly differentiated, with more cell nuclei and conspicuous interstitial infiltration. Histamine levels in cancer tissues were significantly higher than those in normal tissues (*P<0.05) (Figures 2A–C). These results suggest that high levels of histamine release in tumor tissues may be related to the formation or development of tumors.
Next, we used the human ovarian cancer cell line OVCAR-3 for further experiments. OVCAR-3 cells were subjected to treatment with 100, 50, 10, 5, and 1 ng/mL histamine for 48 h, with no treatment considered as a control, the proliferation rate of which was recorded as 100%. As shown in Figure 2D, the proliferation of OVCAR-3 ovarian cancer cells was significantly increased when subjected to co-culture with 50 ng/mL histamine (*P < 0.05), similar to the phenomenon observed in Anglne cells (Figure 2E). Taken together, these results indicate that histamine at a certain concentration accelerates the proliferation of tumor cells.
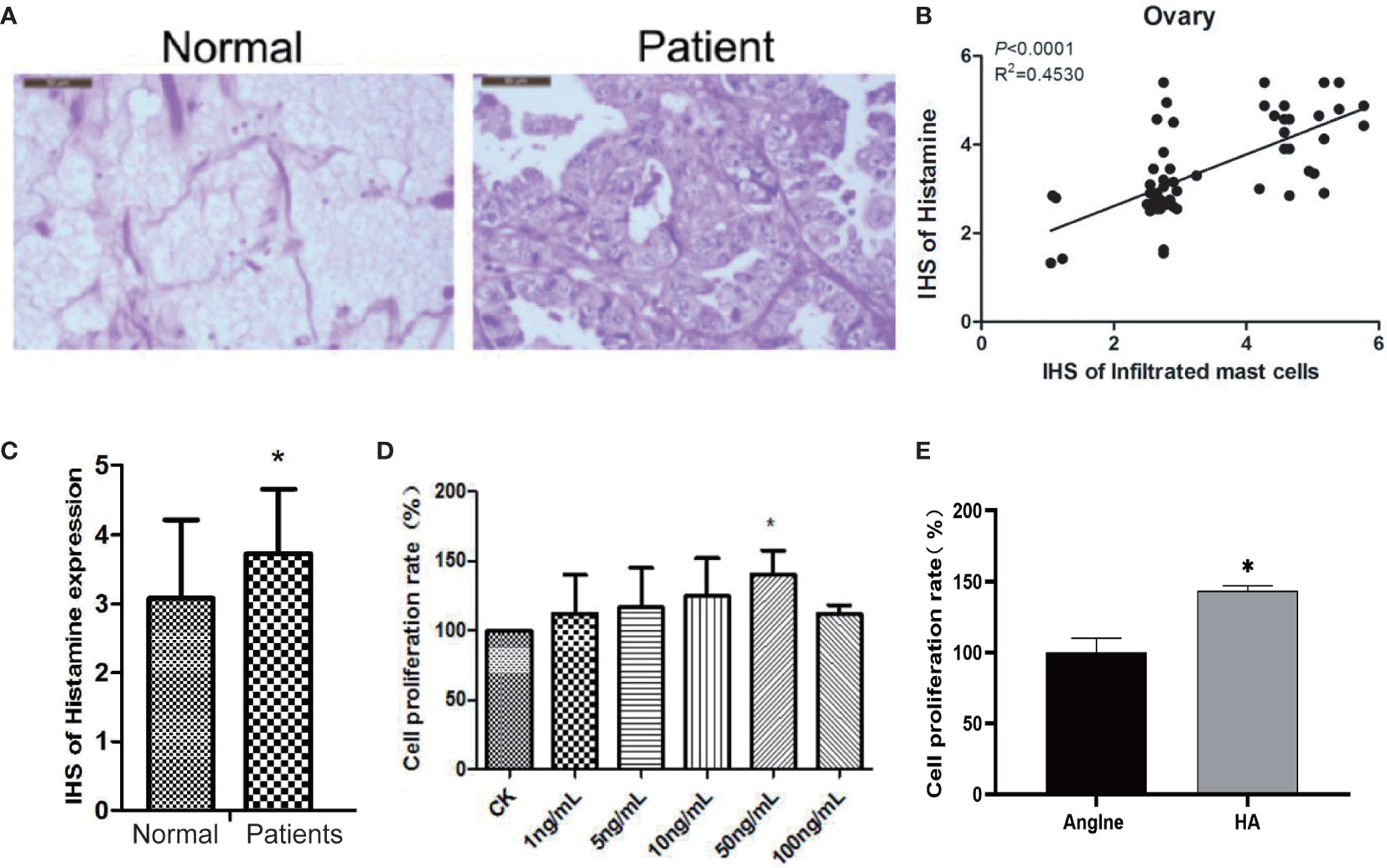
Figure 2 Histamine released by mast cell degranulation stimulates the proliferation of ovarian cancer cells. (A) HE staining of normal ovarian tissue (left) and ovarian cancer tissue (right). (B) Correlation between mast cell infiltration and histamine release in ovarian cancer tissue. (C) Histamine release levels in normal ovarian and ovarian cancer tissues, *P < 0.05 (compared to normal tissues). (D) OVCAR-3 cell proliferation after treatment with different concentrations of histamine. (E) Anglne cell proliferation rate after treatment with histamine at a concentration of 50 ng/mL. *P < 0.05 (compared to untreated Anglne cell group).
Activation of estrogen-related signaling pathways is an important condition for the development of gynecological tumors since estrogen performs biological functions by binding to its receptors (18). There are two subtypes of estrogen receptors (ERs), namely ERα and ERβ. Different ERs play distinct roles in various tumor types, stages, and development phases (19–21). Bioinformatics analysis showed that the estrogen receptor gene was differentially expressed in ovarian cancer tissues (Table 2). Therefore, we speculated that HA contributed to the development of ovarian cancer tumors by regulating the expression of ERs. We measured the expression levels of ERα and ERβ in normal ovary and ovarian cancer tissues and observed that the expression level of ERα in ovarian cancer tissues was significantly increased, while the level of ERβ was decreased (Figure 3A).
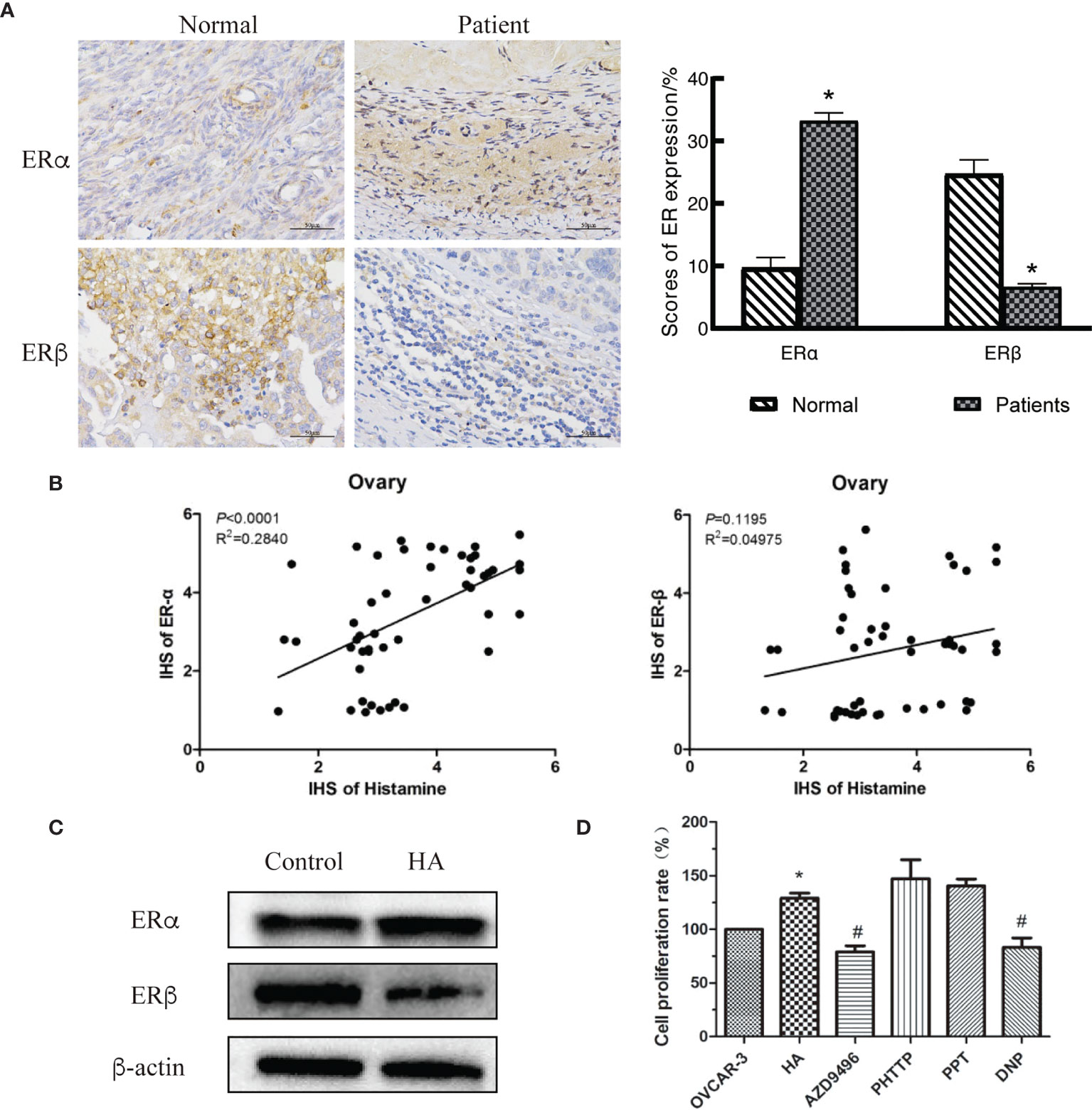
Figure 3 Histamine induces the development of ovarian cancer by disordering expression of estrogen receptors (ERs). (A) Immunohistochemical detection of ovarian tissues, *P < 0.05 (compared to normal tissues). (B) Correlation analysis between histamine and ERα (left)、ERβ (right) in ovarian cancer tissues. (C) Western blot analysis of the expression of ERα and ERβ in histamine-treated OVCAR-3 cells. (D) Effect of Inhibiting or activating of estrogen receptors on the proliferation of OVCAR-3 cells. AZD9496, PHTTP, PPT, DNP are respectively ERα inhibitors, ERβ inhibitors, ERα agonists, ERβ agonists; *P <0.05 (Compared to untreated OVCAR-3 cell group); #P < 0.05 (compared with the HA-treated group).
We also investigated the relationship between histamine, ERα, and ERβ in human tissues. In ovarian tissues, HA was significantly and positively correlated with the expression of ERα (P<0.0001), but was not correlated with ERβ expression (P=0.1195) (Figure 3B). The poor correlation between HA and ERβ may be limited by the small number of samples in this study. Western blotting was performed to examine the expression of ERs in HA-treated cells. Results showed that the expression of ERα was upregulated, while the expression of ERβ was downregulated in HA-treated cells (Figure 3C). To further verify whether histamine played a tumor-promoting effect through estrogen receptors, AZD9496 (an ERα inhibitor), PHPPT (an ERβ inhibitor), PPT (an ERα agonist), and DNP (an ERβ agonist) were used to subject OVCAR-3 cells to the desired treatment (Figure 3D). As depicted, treatment with AZD9496 and DNP, but not PHPPT or PPT, significantly reversed the histamine-induced increase in ovarian cancer cell proliferation (#P<0.05). This finding supports and adds credibility to the hypothesis that histamine may accelerate the development of ovarian cancer through the differential expression of ERα and ERβ.
Previous studies have shown that apigenin inhibits the development of a variety of gynecological tumors (22, 23); however, whether it can inhibit ovarian cancer induced by histamine remains unclear. Therefore, we first investigated whether apigenin inhibited the proliferation of OVCAR-3 cells after histamine treatment. Apigenin at concentrations of 0.1, 0.5, 1, 5, and 10 μM was added to the co-culture of histamine (50 ng/mL) and ovarian cancer cells. The results revealed that OVCAR-3 cell proliferation was inhibited by the introduction of apigenin at different concentrations in a dose-dependent manner. Apigenin at concentrations of 1, 5, and 10 μM remarkably suppressed the proliferation of ovarian cancer cells compared with the histamine-treated group (#P<0.05) (Figure 4A, Supplementary Figure 2), which proved that after histamine treatment, apigenin inhibited the proliferation of tumor cells. Additionally, 1 μM APG was selected for addition for subsequent experiments, which was the lowest concentration where apigenin demonstrated remarkable efficacy.
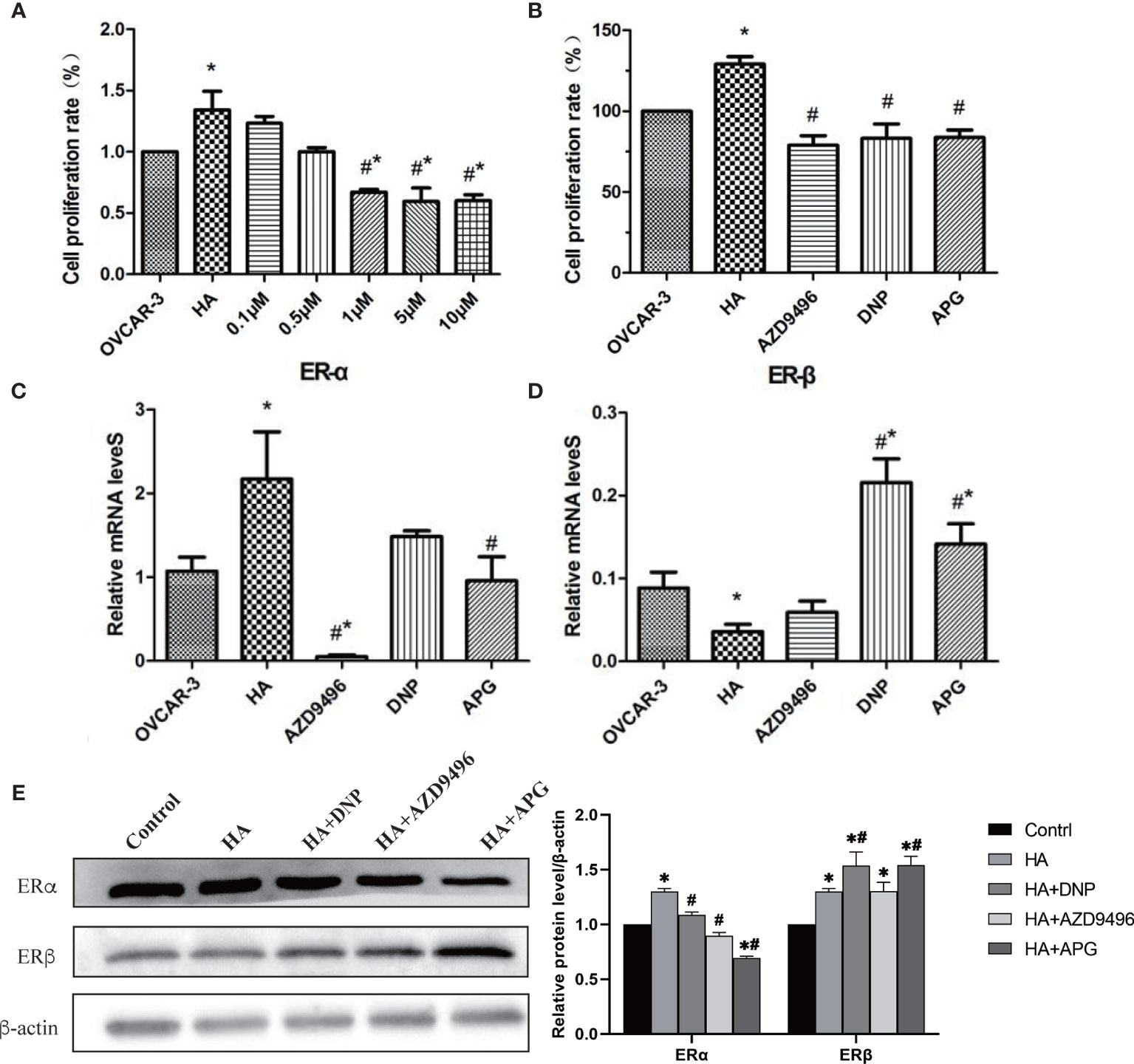
Figure 4 Apigenin inhibits the tumor-promoting effect of histamine by down-regulating ERα/ERβ. (A, B) The effect of different concentrations of apigenin, AZD9496, and DNP on the proliferation of OVCAR-3 cells. The concentration of AZD9496 and DNP was 1 µM. (C, D) The mRNA expression of ERα (C) and ERβ (D). (E) The protein expression of ERα and ERβ by western blot. *P < 0.05 (Compared to untreated OVCAR-3 cell group); #P < 0.05 (compared with the HA-treated group).
Previous studies have shown that histamine promotes the proliferation of ovarian cancer cells by regulating the expression of ERs, and this can be inhibited by using the ERα inhibitor AZD9496 and the ERβ agonist DNP. As a natural flavonoid, apigenin affects the proliferation of ovarian cancer cells by targeting the estrogen receptor. We then compared the antitumor activity of apigenin by conducting treatment of ovarian cancer cells with AZD9496 and DNP. Our results indicated that apigenin effectively inhibited the histamine-induced proliferation of OVCAR-3 cells, similar to the effect shown by AZD9496 and DNP treatment (Figure 4B).
We also determined whether apigenin suppressed histamine-induced tumor cell proliferation by affecting the expression of the histamine H1 (HRH1) or H3 receptor (HRH3). Results showed that the mRNA levels of HRH1 and HRH3 were not significantly changed by apigenin treatment, indicating that apigenin did not inhibit the histamine-induced increase in tumor cell proliferation by affecting the expression of HRH1 and HRH3 (Supplementary Figure 3). We then investigated whether apigenin affected the mRNA and protein expression levels of ERα and ERβ in OVCAR-3 cells (Figures 4C–E). The results showed that after HA treatment, the expression level of ERα increased significantly and that of ERβ decreased (P<0.05). However, apigenin treatment reversed this change and resulted in the establishment of almost normal levels, both at the transcriptional and translational levels.
Studies have indicated that ERs can establish interactions with PI3K to regulate cancer development (24). In our study, the enrichment of the PI3K/AKT/mTOR signaling pathway played a considerable role in the progression of ovarian cancer (Figure 1E). Therefore, we investigated whether the change in the expression of ERs induced by apigenin could suppress the PI3K/AKT/mTOR pathway. As shown in Figure 5, the overexpression of PI3K, AKT, and mTOR caused by histamine was attenuated after treatment with apigenin. Additionally, the activation of p-PI3K, p-AKT, and p-mTOR was also inhibited, similar to the effect observed with the treatment involving the use of ER modulators DPN and AZD9496. These findings demonstrated that the suppressive effects of apigenin on the proliferation of ovarian cancer were mediated by the PI3K/AKT/mTOR pathway.
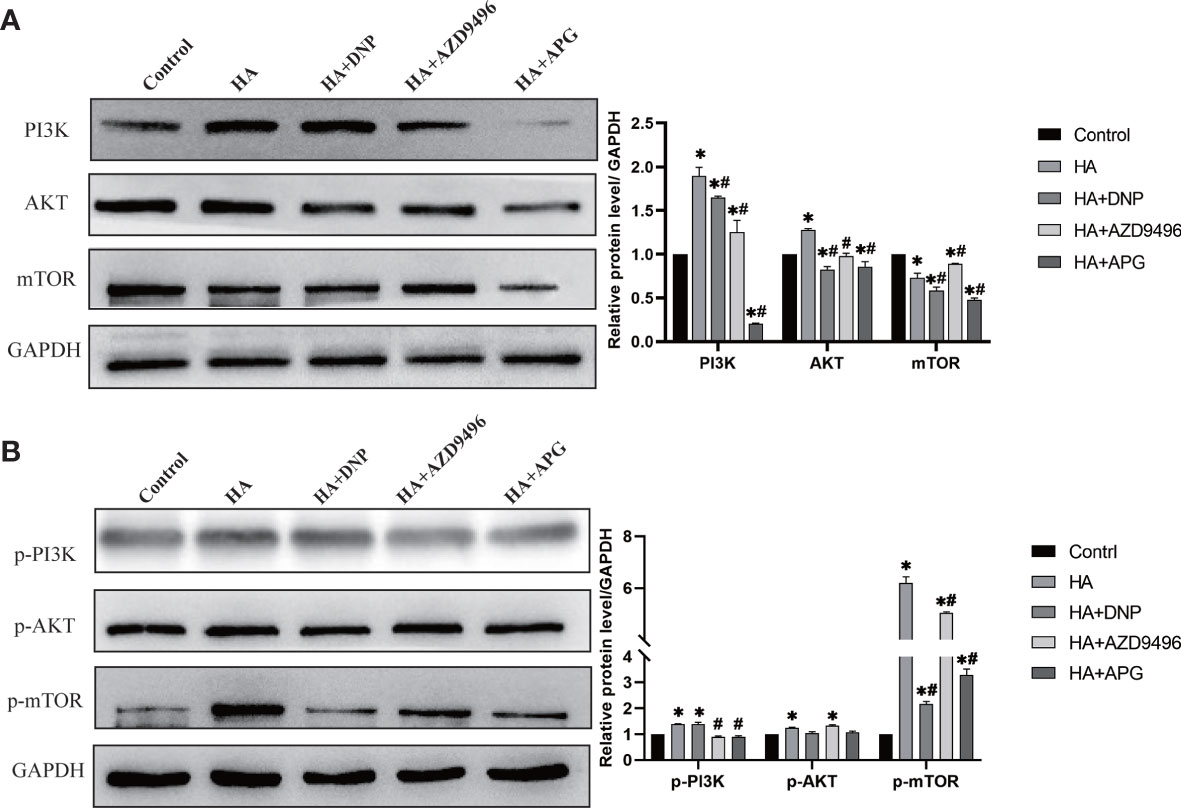
Figure 5 Apigenin inhibits ovarian cancer induced by histamine through attenuating PI3K/AKT/mTOR pathway. (A) Western blot analysis of PI3K, Akt and mTOR expression level of differently-treated OVCAR-3 cells. (B) Western blot analysis of p-PI3K, p-Akt and p-mTOR expression level. *P < 0.05 (Compared to untreated OVCAR-3 cell group); #P < 0.05 (compared with the HA-treated group).
Mast cells are granular cells of the innate immune system that play a role in orchestrating adaptive immune responses. They are well known for their role in allergic reactions following IgE-mediated activation of the high-affinity cell surface-expressed IgE receptor FcεRI (25). MCs participate in many physiological and pathological reactions of the body, mainly through the secretion and release of active mediators and cytokines, also known as degranulation (26). As mast cell degranulation plays a key role in mediating the immune-inflammatory response, it has been used as a target for the treatment of IBD, IBS, and other diseases. Carroll found that the scores of abdominal pain-related behaviors in stress-sensitive Wistar Kyoto rats were decreased after treatment with disodium cromoglycate (DSCG), suggesting that stabilization of the MCs and limitation of their degranulation contributed towards amelioration of visceral hypersensitivity (27). Studies have revealed that MCs also function in the tumor microenvironment (28). Therefore, we screened the differentially expressed genes of MCs between normal ovary tissue and ovarian cancer tissue to assess the potential impact of MC degranulation on the development of ovarian cancer. Spleen tyrosine kinase (Syk) plays an essential role in IgE receptor signaling (FcεRI), which leads to mast cell degranulation. Divalent binding of the tandem SH2 domain (tSH2) of Syk to the intracellular ITAM motif of FcεRI activates the kinase domain of Syk, thereby initiating cell degranulation (29). As a member of the Src family of non-receptor protein tyrosine kinases, Lyn is the first kinase that is activated after FcεRI cross-linking, and its high expression indicates mast cell degranulation (30). Consistent with findings reported in previous studies, variations in the retinoic acid degrading enzyme Cyp26b1 (31), macrophage capping protein CapG (32), calcium-activated potassium channel protein KCNN4 (33), VAMP-8 (17), and leukemia inhibitory factor (LIF) (34) confirmed the accuracy of the RNA-seq results, suggesting that MC degranulation might expedite the progression of ovarian cancer.
Histamine, a low-molecular-weight biogenic amine, is the most important substance released upon mast cell degranulation. Histamine exhibits a strong vasodilative effect and increases the permeability of capillary and venule walls, leading to the development of local tissue edema (27). Massive mast cells were observed in colorectal cancer tissues, and cancer cells near histamine-rich regions were found to be highly proliferative, leading to a poorer prognosis (35). Next, we explored whether the tumor-promoting effect mediated by mast cell degranulation was derived from histamine. Immunohistochemical analysis of histamine in normal human and cancerous tissues and the proliferation of OVCAR-3 cells determined the effect of histamine on tumor development. Presently, there have been many reports on the effects of histamine on tumors. Notably, most malignant cell lines and experimental tumors highly express L-histidine decarboxylase (HDC), a type of histamine synthase, and contain high concentrations of endogenous histamine, which can collectively regulate a variety of biological responses related to tumor growth through paracrine or autocrine signaling into the extracellular medium (36), as well as angiogenesis, cell invasion, migration, differentiation, apoptosis, death, and the immune response, indicating that histamine may be considered a key mediator in the development and progression of cancer. Many in vivo studies have reported the use of animal models with homologous or xenogeneic melanoma grafts to prove that both endogenous and exogenous histamine can stimulate tumor growth (37), an observation which is consistent with our findings.
As a hormone-dependent tumor, the development of ovarian cancer is closely related to the levels of estrogen and its receptor. It is generally believed that ERβ is tumor-suppressive, whereas ERα is reportedly tumor-inductive. Our previous studies have found that histamine induces cervical cancer tumor growth by regulating the expression of ERs (38). Therefore, we examined the expression of Erα and ERβ in human ovary tissues and determined the correlation between ERs and OC. The proliferation of ovarian cancer cells is accelerated in the presence of ERα but is inhibited when ERβ levels increase. This was consistent with the findings reported by Chan who stated that ERβ was abnormally expressed in various estrogen-dependent tumors (such as breast cancer and prostate cancer) and resulted in a reduction in cell movement and invasion, leading to increased tumor cell apoptosis (39). Therefore, we report that the different relative expression levels of ER subtypes in individual tumors may produce different effects.
Apigenin, a natural flavonoid, can decelerate the growth of many types of tumors; however, its anti-tumor mechanism remains unclear. Our results confirmed that apigenin could inhibit the proliferation of OVCAR-3 tumor cells induced by histamine, similar to that observed after AZD9496 and DNP treatment. The results of the mRNA expression analysis indicated that apigenin exerted no effect on the expression of histamine receptors, but exhibited a certain regulatory effect on the expression of ERs, the mechanism of which has been illustrated in Figure 6. It has been proven that apigenin possesses estrogen-like properties. Apigenin treatment can increase the number of endometrial glands, stimulate cell growth, and increase the weight of the uterus after establishing interactions with estrogen receptors (42). Apigenin exerts its anti-tumor effects through a variety of mechanisms, including cell growth inhibition, apoptosis induction, cell cycle inhibition, angiogenesis inhibition, cancer proliferation inhibition, and regulation of the expression of oncogenic proteins (43). The PI3K pathway is also closely related to the transcription and expression of ERs. Studies have shown that inhibition of the PI3K pathway in ER-positive breast cancer results in increased ER-dependent transcriptional activity (44).
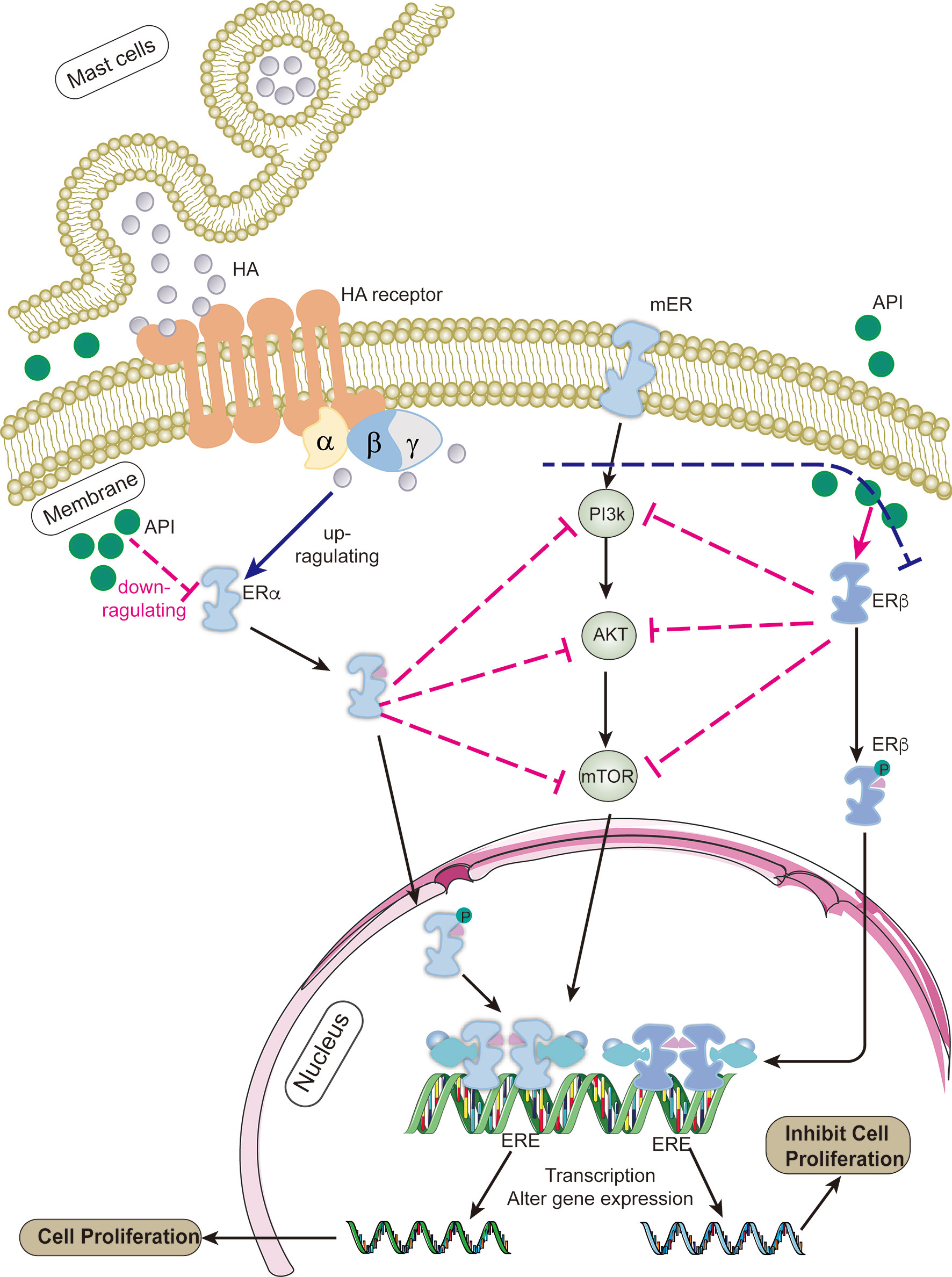
Figure 6 The mechanism of apigenin for inhibiting the proliferation of ovarian cancer cells. After histamine binds to its receptors, it up-regulates the expression of ERα and down-regulates the expression ERβ (blue line). ERs binds to the estrogen response element (ERE) sequence of the target gene promoter after dimerization (38), activating the transcription of downstream target gene (40, 41), such as cell cycle regulators (CCND1, c-Myc) and apoptosis-related proteins (BCL2, BCLXL), etc., which works on promoting cell proliferation and tumor development. Apigenin suppresses PI3K/AKT/mTOR signaling pathway by regulating the expression level of ERα/ERβ (red line), thereby attenuates the development of ovarian cancer induced by histamine.
In summary, apigenin affects the PI3K/AKT/mTOR signaling pathway by regulating the expression level of ERα/ERβ, thereby decelerating tumor development promoted by histamine. Our study shows that apigenin may be deemed a potential natural compound that can be used to inhibit ovarian cancer progression; however, its specific mechanism should be further explored.
The data presented in the study are deposited in the NCBI Sequence Read Archive, accession number PRJNA757121.
The studies involving human participants were reviewed and approved by China Agricultural University Human Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Conceptualization, QX and YZ. Data curation, ML, QX and YZ. Formal Analysis, ML, GL, QX. and YZ; Methodology, QX, GL and ML. Funding acquisition, HC. Supervision, HC, TH and NS. Writing – original draft, ML, QX and YZ. Writing – review & editing, ML, HC and TH. All authors contributed to the article and approved the submitted version.
This work was supported by Chinese Nutrition Society-Zhongshi Yingke Nutrition Research Fund (CNS-FF2019A21).
Author TH was employed by company Zhongguancun International Medical Inspection and Certification Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.682917/full#supplementary-material.
Supplementary Figure 1 | Determination of DEGs between normal ovarian and ovarian cancer tissues though RNA-Seq analysis. (A) Correlation coefficient diagram between normal ovarian (n = 3) and ovarian cancer tissue samples (n = 3). (B) Principal Component Analysis diagram between ovarian tissue samples; (C, D) GO (C) and KEGG (D) enrichment analysis of DEGs.
Supplementary Figure 2 | The proliferation rate of Anglne cell after HA and APG treatment. *P < 0.05 (Compared to untreated Anglne cell group); #P < 0.05 (compared with the HA-treated group).
Supplementary Figure 3 | The mRNA expression of HRH1 (A) and HRH3 (B) after HA, AZD9496 DNP and APG treatment. *P < 0.05 (Compared to untreated OVCAR-3 cell group).
ERα, Estrogen receptor α; ERβ, Estrogen receptor β; MCs, Mast cells; APG, Apigenin; HIF-1, Hypoxia Inducible Factor -1; VEGF, Vascular Endothelial Growth Factor; RIN, RNA Integrity Number; PCA, Principal Component Analysis; VAMP-8, Vesicle associated membrane protein-8; NSF, N-ethyl maleimide sensitive factor; SNARE, Soluble N-ethyl maleimide sensitive factor adhesion protein receptor; LIF, Leukemia Inhibitory Factor; HDC, L-histidine decarboxylase; HRH1, Histamine H1 receptor; HRH3, Histamine H3 receptor.
1. Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, et al. MicroRNA Signatures in Human Ovarian Cancer. Cancer Res (2007) 67:8699–707. doi: 10.1158/0008-5472.CAN-07-1936
2. Ma Y, Wang W, Idowu MO, Oh U, Wang XY, Temkin SM, et al. Ovarian Cancer Relies on Glucose Transporter 1 to Fuel Glycolysis and Growth: Anti-Tumor Activity of BAY-876. Cancers (Basel) (2019) 11:33. doi: 10.3390/cancers11010033
3. Lheureux S, Ledermann JA, Runswick S, Hodgson DR, Timms K, Lanchbury JS, et al. Genomic Characterization of Long-Term Responders to Olaparib. J Clin Oncol (2015) 33:5566–6. doi: 10.1200/jco.2015.33.15_suppl.5566
4. Ch’ng S, Wallis RA, Yuan L, Davis PF, Tan ST. Mast Cells and Cutaneous Malignancies. Mod Pathol (2006) 19:149–59. doi: 10.1038/modpathol.3800474
5. Kennedy L, Hodges K, Meng F, Alpini G, Francis H. Histamine and Histamine Receptor Regulation of Gastrointestinal Cancers. Transl Gastrointest Cancer (2012) 1:215–27.
6. Kubecova M, Kolostova K, Pinterova D, Kacprzak G, Bobek V. Cimetidine: An Anticancer Drug? Eur J Pharm Sci (2011) 42:439–44. doi: 10.1016/j.ejps.2011.02.004
7. Adams WJ, Lawson JA, Morris DL. Cimetidine Inhibits In Vivo Growth of Human Colon Cancer and Reverses Histamine Stimulated In Vitro and In Vivo Growth. Gut (1994) 35:1632–6. doi: 10.1136/gut.35.11.1632
8. Chanda R, Ganguly AK. Diamine-Oxidase Activity and Tissue Di- and Poly-Amine Contents of Human Ovarian, Cervical and Endometrial Carcinoma. Cancer Lett (1995) 89:23–8. doi: 10.1016/0304-3835(95)90153-1
9. Hegyesi H, Somlai B, Varga VL, Toth G, Kovacs P, Molnar EL, et al. Suppression of Melanoma Cell Proliferation by Histidine Decarboxylase Specific Antisense Oligonucleotides. J Invest Dermatol (2001) 117:151–3. doi: 10.1046/j.0022-202X.2001.01406.x
10. Michael Moriarty C, Stucky JL, Hamburger KW, Patil KD, Foley JF, Koefoot RR. Blood Histamine and Solid Malignant Tumors. J Cancer Res Clin Oncol (1988) 114:588–92. doi: 10.1007/BF00398182
11. Liu R, Ji P, Liu B, Qiao H, Wang X, Zhou L, et al. Apigenin Enhances the Cisplatin Cytotoxic Effect Through P53-Modulated Apoptosis. Oncol Lett (2017) 13:1024–30. doi: 10.3892/ol.2016.5495
12. Tong X, Pelling J. Targeting the PI3K/Akt/mTOR Axis by Apigenin for Cancer Prevention. Anticancer Agents Med Chem (2013) 13:971–8. doi: 10.2174/18715206113139990119
13. Salmani J, Zhang XP, Jacob JA, Chen BA. Apigenin’s Anticancer Properties and Molecular Mechanisms of Action: Recent Advances and Future Prospectives. Chin J Nat Med (2017) 15:321–9. doi: 10.1016/S1875-5364(17)30052-3
14. Shi MD, Shiao CK, Lee YC, Shih YW. Apigenin, a Dietary Flavonoid, Inhibits Proliferation of Human Bladder Cancer T-24 Cells via Blocking Cell Cycle Progression and Inducing Apoptosis. Cancer Cell Int (2015) 15:1–12. doi: 10.1186/s12935-015-0186-0
15. Jia W, Qiu K, He M, Song P, Zhou Q, Zhou F, et al. SOAPfuse: An Algorithm for Identifying Fusion Transcripts From Paired-End RNA-Seq Data. Genome Biol (2013) 14:R12. doi: 10.1186/gb-2013-14-2-r12
16. Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Nasir Khan K, Masferrer J, et al. COX-2 Is Expressed in Human Pulmonary, Colonic, and Mammary Tumors. Cancer (2000) 89:2637–45. doi: 10.1002/1097-0142(20001215)89:12<2637::AID-CNCR17>3.0.CO;2-B
17. Tiwari N, Wang CC, Brochetta C, Ke G, Vita F, Qi Z, et al. VAMP-8 Segregates Mast Cell-Preformed Mediator Exocytosis From Cytokine Trafficking Pathways. Blood (2008) 111:3665–74. doi: 10.1182/blood-2007-07-103309
18. Xu Z, Liu J, Gu L, Ma X, Huang B, Pan X. Research Progress on the Reproductive and Non-Reproductive Endocrine Tumors by Estrogen-Related Receptors. J Steroid Biochem Mol Biol (2016) 158:22–30. doi: 10.1016/j.jsbmb.2016.01.008
19. Bhat-Nakshatri P, Wang G, Appaiah H, Luktuke N, Carroll JS, Geistlinger TR, et al. AKT Alters Genome-Wide Estrogen Receptor α Binding and Impacts Estrogen Signaling in Breast Cancer. Mol Cell Biol (2008) 28:7487–503. doi: 10.1128/mcb.00799-08
20. Park S, Song J, Joe CO, Shin I. Akt Stabilizes Estrogen Receptor α With the Concomitant Reduction in its Transcriptional Activity. Cell Signal (2008) 20:1368–74. doi: 10.1016/j.cellsig.2008.03.004
21. Sanchez M, Sauvé K, Picard N, Tremblay A. The Hormonal Response of Estrogen Receptor β is Decreased by the Phosphatidylinositol 3-Kinase/Akt Pathway via a Phosphorylation-Dependent Release of CREB-Binding Protein. J Biol Chem (2007) 282:4830–40. doi: 10.1074/jbc.M607908200
22. Mafuvadze B, Liang Y, Besch-Williford C, Zhang X, Hyder SM. Apigenin Induces Apoptosis and Blocks Growth of Medroxyprogesterone Acetate-Dependent BT-474 Xenograft Tumors. Horm Cancer (2012) 3:160–71. doi: 10.1007/s12672-012-0114-x
23. Seo HS, Choi HS, Kim SR, Choi YK, Woo SM, Shin I, et al. Apigenin Induces Apoptosis via Extrinsic Pathway, Inducing P53 and Inhibiting STAT3 and Nfκb Signaling in HER2-Overexpressing Breast Cancer Cells. Mol Cell Biochem (2012) 366:319–34. doi: 10.1007/s11010-012-1310-2
24. Ellis MJ, Perou CM. The Genomic Landscape of Breast Cancer as a Therapeutic Roadmap. Cancer Discov (2013) 3:27–34. doi: 10.1158/2159-8290.CD-12-0462
25. Komi DEA, Mortaz E, Amani S, Tiotiu A, Folkerts G, Adcock IM. The Role of Mast Cells in IgE-Independent Lung Diseases. Clin Rev Allergy Immunol (2020) 58:377–87. doi: 10.1007/s12016-020-08779-5
26. Marshall JS, Jawdat DM. Mast Cells in Innate Immunity. J Allergy Clin Immunol (2004) 114:21–7. doi: 10.1016/j.jaci.2004.04.045
27. Carroll SY, O’Mahony SM, Grenham S, Cryan JF, Hyland NP. Disodium Cromoglycate Reverses Colonic Visceral Hypersensitivity and Influences Colonic Ion Transport in a Stress-Sensitive Rat Strain. PloS One (2013) 8:e84718. doi: 10.1371/journal.pone.0084718
28. Theoharides TC, Conti P. Mast Cells: The JEKYLL and HYDE of Tumor Growth. Trends Immunol (2004) 25:235–41. doi: 10.1016/j.it.2004.02.013
29. Kuil J, Van Wandelen LTM, De Mol NJ, Liskamp RMJ. Switching Between Low and High Affinity for the Syk Tandem SH2 Domain by Irradiation of Azobenzene Containing ITAM Peptidomimetics. J Pept Sci (2009) 15:685–91. doi: 10.1002/psc.1173
30. Beavitt S-JE, Harder KW, Kemp JM, Jones J, Quilici C, Casagranda F, et al. Lyn-Deficient Mice Develop Severe, Persistent Asthma: Lyn Is a Critical Negative Regulator of Th2 Immunity. J Immunol (2005) 175:1867–75. doi: 10.4049/jimmunol.175.3.1867
31. Kurashima Y, Amiya T, Fujisawa K, Shibata N, Suzuki Y, Kogure Y, et al. The Enzyme Cyp26b1 Mediates Inhibition of Mast Cell Activation by Fibroblasts to Maintain Skin-Barrier Homeostasis. Immunity (2014) 40:530–41. doi: 10.1016/j.immuni.2014.01.014
32. Lahortiga I, Akin C, Cools J, Wilson TM, Mentens N, Arthur DC, et al. Activity of Imatinib in Systemic Mastocytosis With Chronic Basophilic Leukemia and a PRKG2-PDGFRB Fusion. Haematologica (2008) 93:49–56. doi: 10.3324/haematol.11836
33. Shmukler BE, Rivera A, Bhargava P, Nishimura K, Hsu A, Kim EH, et al. Combined Genetic Disruption of K-Cl Cotransporters and Gardos Channel KCNN4 Rescues Erythrocyte Dehydration in the SAD Mouse Model of Sickle Cell Disease. Blood Cells Mol Dis (2019) 79:102346. doi: 10.1016/j.bcmd.2019.102346
34. Hiragun T, Morita E, Mihara S, Tanaka T, Gyotoku E, Kameyoshi Y, et al. Leukemia Inhibitory Factor Enhances Mast Cell Growth in a Mast Cell/Fibroblast Co-Culture System Through Stat3 Signaling Pathway of Fibroblasts. FEBS Lett (2000) 487:219–23. doi: 10.1016/S0014-5793(00)02355-3
35. Lawson JA, Adams WJ, Morris DL. Ranitidine and Cimetidine Differ in Their In Vitro and In Vivo Effects on Human Colonic Cancer Growth. Br J Cancer (1996) 73:872–6. doi: 10.1038/bjc.1996.155
36. Falus A, Pós Z, Darvas Z. Histamine in Normal and Malignant Cell Proliferation. Adv Exp Med Biol (2010) 709:109–23. doi: 10.1007/978-1-4419-8056-4_11
37. Tomita K, Okabe S. Exogenous Histamine Stimulates Colorectal Cancer Implant Growth via Immunosuppression in Mice. J Pharmacol Sci (2005) 97:116–23. doi: 10.1254/jphs.FP0040691
38. Zhang E, Zhang Y, Fan Z, Cheng L, Han S, Che H. Apigenin Inhibits Histamine-Induced Cervical Cancer Tumor Growth by Regulating Estrogen Receptor Expression. Molecules (2020) 25:1960. doi: 10.3390/molecules25081960
39. Chan KKL, Wei N, Liu SS, Xiao-Yun L, Cheung AN, Ngan HYS. Estrogen Receptor Subtypes in Ovarian Cancer: A Clinical Correlation. Obstet Gynecol (2008) 111:144–51. doi: 10.1097/01.AOG.0000296715.07705.e9
40. Shupnik MA. Crosstalk Between Steroid Receptors and the c-Src-Receptor Tyrosine Kinase Pathways: Implications for Cell Proliferation. Oncogene (2004) 23:7979–89. doi: 10.1038/sj.onc.1208076
41. Thomas C, Gustafsson JA. The Different Roles of ER Subtypes in Cancer Biology and Therapy. Nat Rev Cancer (2011) 11:597–08. doi: 10.1038/nrc3093
42. Jefferson WN, Doerge D, Padilla-Banks E, Woodling KA, Kissling GE, Newbold R. Oral Exposure to Genistin, the Glycosylated Form of Genistein, During Neonatal Life Adversely Affects the Female Reproductive System. Environ Health Perspect (2009) 117:1883–9. doi: 10.1289/ehp.0900923
43. Gong P, Madak-Erdogan Z, Li J, Cheng J, Greenlief CM, Helferich W, et al. Transcriptomic Analysis Identifies Gene Networks Regulated by Estrogen Receptor α (Erα) and Erβ That Control Distinct Effects of Different Botanical Estrogens. Nucl Recept Signal (2014) 12:e001. doi: 10.1621/nrs.12001
Keywords: ovarian cancer, mast cells, histamine, estrogen receptor, apigenin
Citation: Liu M, Zhang Y, Xu Q, Liu G, Sun N, Che H and He T (2021) Apigenin Inhibits the Histamine-Induced Proliferation of Ovarian Cancer Cells by Downregulating ERα/ERβ Expression. Front. Oncol. 11:682917. doi: 10.3389/fonc.2021.682917
Received: 26 April 2021; Accepted: 28 July 2021;
Published: 08 September 2021.
Edited by:
Fuhai Li, Washington University in St. Louis, United StatesReviewed by:
Rajeev K. Singla, Sichuan University, ChinaCopyright © 2021 Liu, Zhang, Xu, Liu, Sun, Che and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huilian Che, Y2hlaHVpbGlhbkBjYXUuZWR1LmNu; Tao He, c2NoZTEyM0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.