- 1Department of Breast Surgery, The Sixth Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China
- 2Department of Endocrine and Breast Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Background: Breast cancer patients who achieve pathological complete response (pCR) after neoadjuvant chemotherapy (NAC) have favorable outcomes. Reliable predictors for pCR help to identify patients who will benefit most from NAC. The pretreatment serum albumin-to-alkaline phosphatase ratio (AAPR) has been shown to be a prognostic predictor in several malignancies, but its predictive value for pCR in breast cancer is still unknown. This study aims to investigate the predictive role of AAPR in breast cancer patients and develop an AAPR-based nomogram for pCR rate prediction.
Methods: A total of 780 patients who received anthracycline and taxane-based NAC from January 2012 to March 2018 were retrospectively analyzed. Univariate and multivariate analyses were performed to assess the predictive value of AAPR and other clinicopathological factors. A nomogram was developed and calibrated based on multivariate logistic regression. A validation cohort of 234 patients was utilized to further validate the predictive performance of the model. The C-index, calibration plots and decision curve analysis (DCA) were used to evaluate the discrimination, calibration and clinical value of the model.
Results: Patients with a lower AAPR (<0.583) had a significantly reduced pCR rate (OR 2.228, 95% CI 1.246-3.986, p=0.007). Tumor size, clinical nodal status, histological grade, PR, Ki67 and AAPR were identified as independent predictors and included in the final model. The nomogram was used as a graphical representation of the model. The nomogram had satisfactory calibration and discrimination in both the training cohort and validation cohort (the C-index was 0.792 in the training cohort and 0.790 in the validation cohort). Furthermore, DCA indicated a clinical net benefit from the nomogram.
Conclusions: Pretreatment serum AAPR is a potentially valuable predictor for pCR in breast cancer patients who receive NAC. The AAPR-based nomogram is a noninvasive tool with favorable predictive accuracy for pCR, which helps to make individualized treatment strategy decisions.
Introduction
Breast cancer is the most common female malignancy and is one of the leading causes of female cancer morbidity and mortality (1). Neoadjuvant chemotherapy (NAC) is a standard treatment option for most breast cancer patients, especially those with locally advanced or inoperable breast cancer. It aims to reduce disease burden and make breast cancer operable in locally advanced patients (2). For operable breast cancer, NAC makes it possible to receive breast-conserving surgery. Furthermore, NAC provides an opportunity for the assessment of chemosensitivity in vivo to guide individualized further systematic therapy based on tumor response (3). A recent meta-analysis of 52 clinical trials, including 27,895 patients, demonstrated that pathological complete response (pCR) after NAC was associated with better event-free survival and overall survival in all breast cancer molecular subtypes (4). However, the response to NAC varies among different molecular subtypes and histopathological characteristics (5). NAC might increase the risk of disease progression in chemoresistant tumors by delaying surgery. A portion of patients do not benefit from NAC and are exposed to the toxicity of the chemotherapy drugs unnecessarily. Thus, there is an urgent need to seek an effective method that can predict pCR for the identification of patients who will benefit most from NAC.
Previous studies demonstrated that various methods could be applied to predict pCR with NAC in breast cancer patients, including analysis of gene signatures, histomorphological factors and imaging features (6–8). Compared with these methods, serum samples are easy to obtain and reflect the comprehensive state of cancer patients. Various serum tumor markers have been identified as prognostic predictive factors in breast cancer patients, such as CEA, CA15-3, CA19-9 and CA125 (9, 10). It is known that systemic inflammation accelerates tumor progression (11). Inflammation-based prognostic scores, including the neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio, platelet to lymphocyte ratio and C-reactive protein/albumin ratio, have been analyzed to evaluate their prognostic predictive value in breast cancer, but the results are controversial (12, 13). Albumin (ALB) is the most abundant serum protein and is synthesized by the liver. It can be regarded as a surrogate parameter for systemic inflammation because of its important function as an immunomodulatory molecule (14). Several studies have found that ALB is a reliable factor for predicting prognosis in different types of cancer, including gynecological cancers, gastrointestinal cancers, hepatocellular carcinoma and esophageal squamous cell carcinoma (15–18). Serum alkaline phosphatase (ALP) is derived from the liver, skeletal tissue, intestine, kidney, placenta and a variety of tumors and is conventionally regarded as a serum marker of hepatobiliary pathology and fracture (19). Previous studies have suggested that ALP is associated with systemic inflammation and tumor development (20, 21). The albumin-to-alkaline phosphatase ratio (AAPR) is a combined index associated with systemic inflammation, which is calculated by dividing the ALB level by the ALP level. It was first reported as a novel prognostic indicator in hepatocellular carcinoma (22). AAPR is based on low-cost routine blood test indexes and has a better prognostic predictive effect than ALB or ALP alone (23). Patients with higher AAPR have better survival outcomes than those with lower AAPR in various cancers, including breast cancer (24). However, whether AAPR can be used as a predictor for pCR in breast cancer patients receiving NAC is still unclear.
Nomograms, graphic illustrations based on regression models, are considered comprehensive predictive tools of patient outcomes. They have been widely used in cancer prognosis prediction models. Although several AAPR-based nomograms have been developed to predict survival in various cancer patients, nomograms based on AAPR predicting the probability of pCR in breast cancer patients are still scant. In our current study, a nomogram based on AAPR and clinicopathological variables was developed and validated to predict the individual probability of pCR in breast cancer patients who received NAC.
Methods
Study Population
We retrospectively reviewed the medical records of primary breast cancer patients at the First Affiliated Hospital of Chongqing Medical University from January 2012 to March 2018. The inclusion criteria were as follows: 1) pathological diagnosis of invasive breast cancer; 2) female; 3) received NAC and surgery; 4) received at least 3 courses of treatment with TEC (docetaxel 75 mg/m2, epirubicin 75 mg/m2 and cyclophosphamide 500 mg/m2 each 21 days before surgery; and 5) serum ALB and ALP levels were measured before treatment. Patients with prior history of malignancies or without complete information were excluded. Ultimately, a total of 780 patients were included for analysis. They were randomly divided into the training cohort and the validation cohort at a ratio of 7:3 (training group: n=546, validation group: n=234). This study was reviewed and approved by the ethics committee of the First Affiliated Hospital of Chongqing Medical University.
Data Collection
Clinical characteristics collected for further analysis included age, menopausal status, courses of NAC, histological type of cancer, tumor size, clinical nodal status, histological grade, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor-2 (HER2) receptor status, Ki67 status, and serum ALB and ALP levels. The tumor size was assessed using ultrasonography by the specific ultrasound operators working in our Breast Cancer Center. ER and PR expression were defined as positive when greater than 1% of the tumor cells exhibited nuclear staining on immunohistochemistry. HER2 positivity was defined as 3+ by immunohistochemical staining or an over 2.0-fold increase by fluorescence in situ hybridization (25). The Ki67 value was defined as the percentage of Ki67-positive cells (500-1,000) among the total number of cancer cells in the invasive front of the tumor (26). ER, PR, HER2 and Ki67 were assessed by two pathologists independently. Tumors were classified into four categories based on the expression of ER, PR, and HER2: luminal subtype (ER+ and/or PR+, HER2-), luminal/HER2 subtype (ER+ and/or PR+, HER2+), HER2 enriched subtype (ER-, PR-, HER2+), and TNBC subtype (ER-, PR-, HER2-). ALB and ALP were tested along with routine plasma examinations at diagnosis. Blood samples were collected into coagulant-coated tubes after patients had fasted for at least 6 hours. ALB and ALP were analyzed by a fully automatic biochemical analyzer (Roche c701, Basel, Switzerland). Pathological complete response (pCR) was recognized as the absence of any residual tumor lesions in any excised breast tissue or lymph node according to the Miller-Payne grading system (27).
Statistical Analysis
The cutoff values of ALB and ALP were 40 g/L and 100 U/L, respectively, which were established based on the normal reference value. The optimal cutoff value of AAPR was determined by the maximum Youden index through receiver operating characteristic (ROC) curve analysis. The chi-square test and Fisher’s exact test were used to evaluate the differences in clinicopathological variables between the training cohort and the validation cohort. In addition, the associations between AAPR and clinicopathological characteristics in breast cancer patients were assessed by the chi-square test or Fisher’s exact test. Similarly, the relationships between pCR and clinicopathological characteristics were analyzed. The primary goal of this study was to estimate the likelihood of breast cancer patients reaching pCR after NAC. Multivariate logistic regression analysis was performed to assess the associations between clinicopathological factors and the likelihood of pCR. Odds ratios were reported with corresponding 95% confidence intervals (CIs). An individualized nomogram was constructed based on the logistic regression model with the rms package in R software. The performance of the model was evaluated by discrimination and calibration in both the training cohort and the validation cohort. By testing the concordance between the prediction probability and the actual state, the concordance index (C-index) was calculated to assess the prediction and discrimination ability of the model. Calibration of the nomogram assessed by internal validation through 1000 bootstrap resamples was shown by a calibration curve. The fitness of the model was analyzed by the Hosmer-Lemeshow test. Furthermore, decision curve analysis was applied to assess the net benefit of the nomogram.
All statistical analyses were performed by using the Statistical Package for the Social Sciences version 25.0 software (IBM Corp., Armonk, USA) and R software (version 4.0.3; https://www.R-project.org/). A two-sided p-value < 0.05 was considered statistically significant.
Results
Patient Characteristics
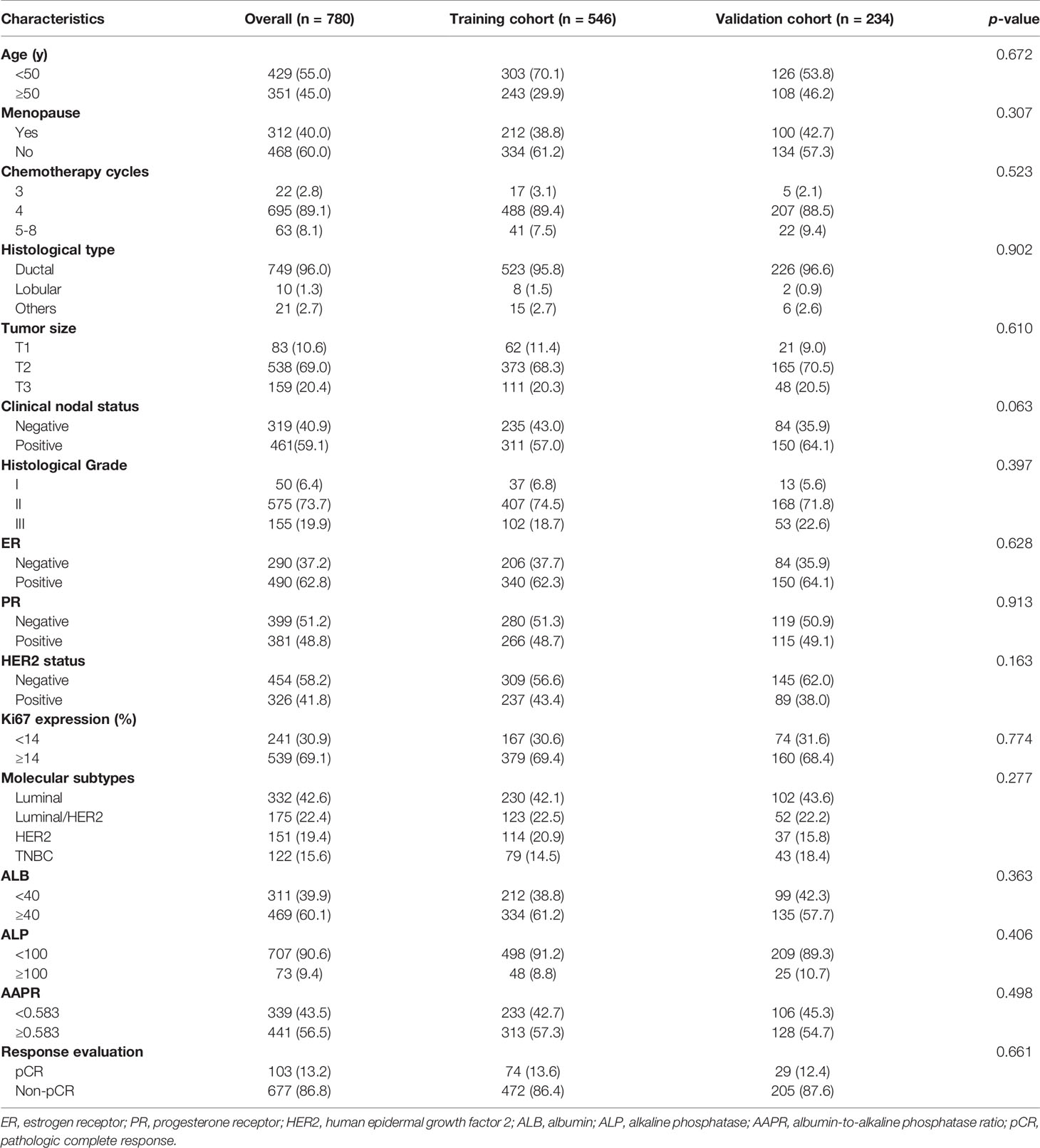
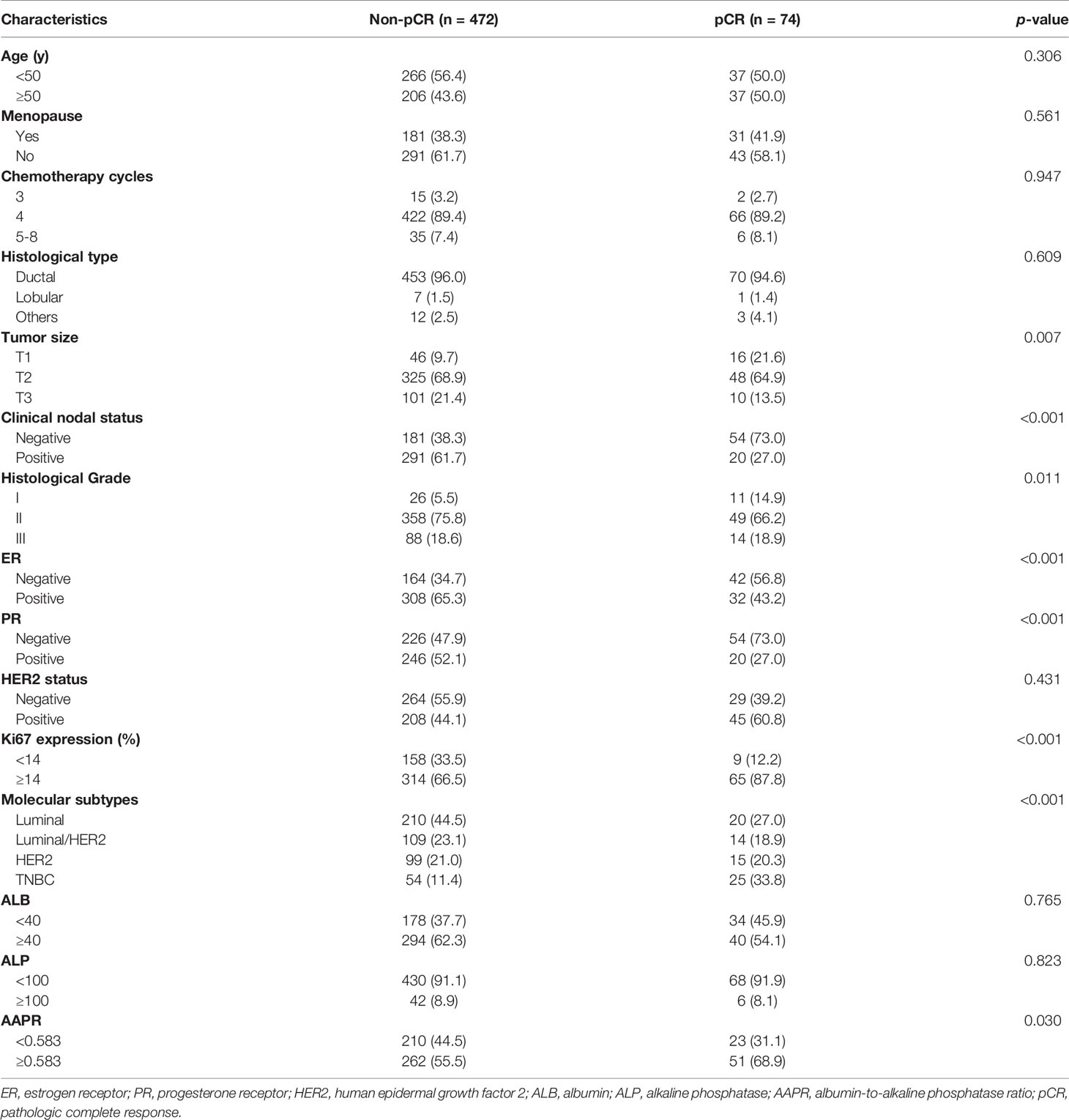
A total of 780 eligible patients were enrolled in this study according to the inclusion criteria. They were randomly divided into the training cohort and the validation cohort at a ratio of 7:3 to develop and validate the predictive model. The mean age of all patients at baseline was 49.0 ± 9.1 years (range 20.0-72.0 years), and 60.0% (n=468) of them were premenopausal. Most of the patients (n=749, 96.0%) were diagnosed with invasive lobular carcinoma. The mean tumor size was 4.0 ± 2.1 cm at baseline, while it was reduced to 2.1 ± 1.7 cm after NAC. Moreover, 461 (59.1%) patients had node-positive disease at baseline. The proportions of ER-positive, PR-positive, HER2-positive patients were 62.8% (n=490), 48.8% (n=381) and 41.8% (n=326), respectively, among 780 patients. More than half of the patients (n=539, 69.1%) had Ki67 expression ≥ 14%. In addition, 90.6% (n=707) of patients had normal serum ALP levels, while 60.1% (n=469) of patients had normal serum ALB levels. According to the Miller-Payne grading system, 103 (13.2%) patients were evaluated as having pCR after NAC. As shown in Table 1, no significant difference was observed in clinicopathological factors between the training cohort and the validation cohort.
Associations Between AAPR and Clinicopathological Characteristics
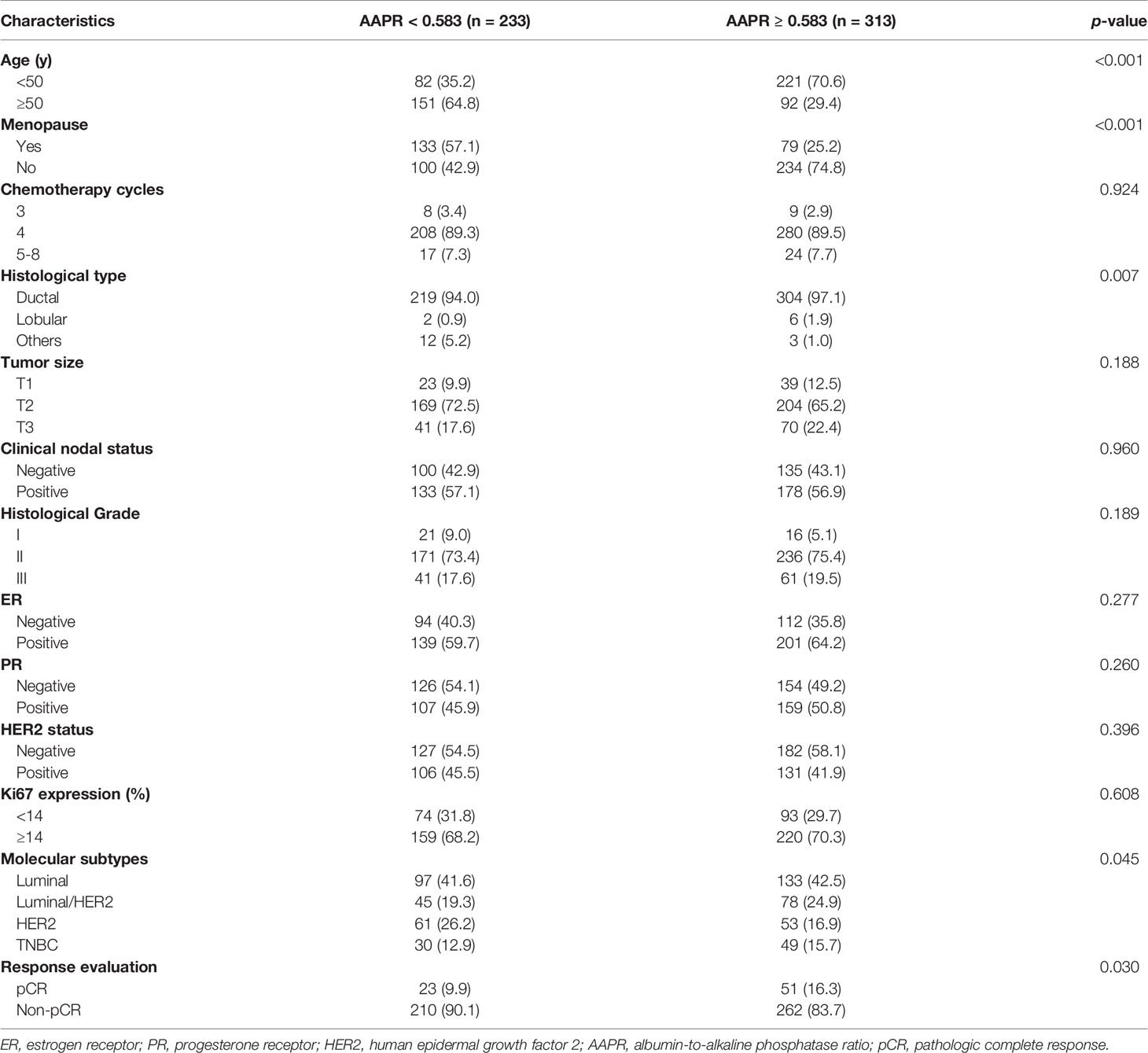
The relationships between AAPR and clinicopathological characteristics in the training cohort were assessed (Table 2). The optimal cutoff value of AAPR was 0.583, according to the ROC curve. Following the cutoff value, 233 (42.7%) patients were included in the high-AAPR group (AAPR<0.583), while the other 313 (57.3%) patients were included in the low-AAPR group (AAPR≥0.583). The results revealed that AAPR level was significantly associated with age (p<0.001), menopausal status (p<0.001), histological type (p=0.007), molecular subtypes (p=0.045) and pCR (p=0.030). No differences were observed in chemotherapy cycles, tumor size, clinical nodal status, histological grade, ER, PR, HER2 or Ki67 between the two groups.
Predictors of pCR
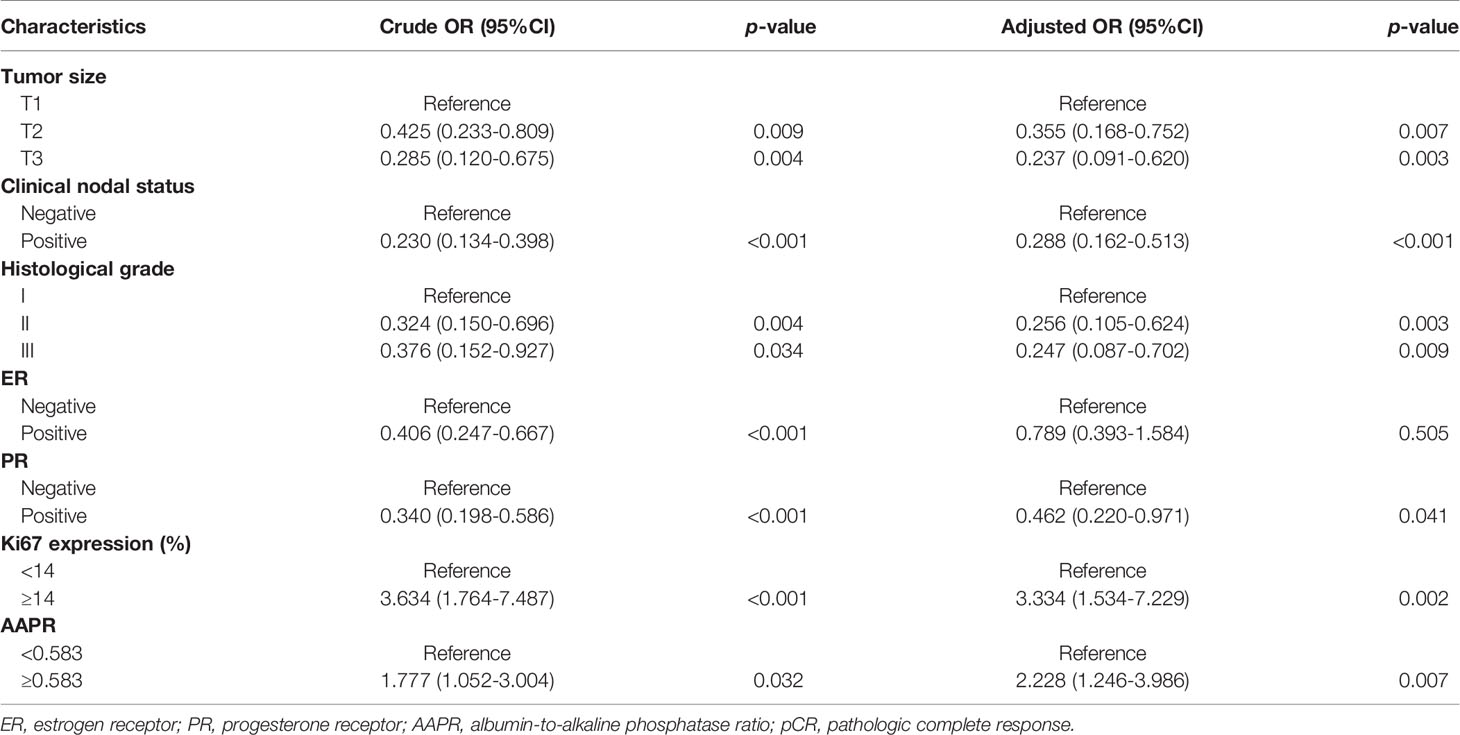
In univariate analysis of the training cohort (Table 3), pCR was significantly correlated with tumor size, clinical nodal status, histological grade, ER, PR, Ki67, molecular subtypes and AAPR. Multivariate logistic regression models were applied to adjust for potential confounders. Variables with p < 0.05 in univariate analysis were included in multivariable models. To avoid the influence of multicollinearity between ER and molecular subtypes, only one of them was applied to the final model. Tumor size, clinical nodal status, histological grade, PR, Ki67 and AAPR were indicated as independent predictors for pCR in breast cancer patients who received NAC. Compared with patients with lower AAPR (AAPR<0.583), the probability of pCR in those with higher AAPR was 2.228-fold higher (95% CI 1.246-3.986, p=0.007). Patients with larger and higher historical grade tumors were less likely to achieve pCR (adjusted OR 0.355, 95% CI 0.168-0.752, p=0.007 for T2; adjusted OR 0.237, 95% CI 0.091-0.620, p=0.003 for T3; adjusted OR 0.256, 95% CI 0.105-0.624, p=0.003 for Grade II; adjusted OR 0.247, 95% CI 0.087-0.702, p=0.009 for Grade III) (Table 4). Patients with node-positive and PR-positive diseases had more difficulty achieving pCR than those with node-negative and PR-negative diseases (adjusted OR 0.288, 95% CI 0.162-0.513, p<0.001 for node-negative status; adjusted OR 0.462, 95% CI 0.220-0.971, p=0.041 for PR-negative status). Moreover, the probability of pCR in patients with higher Ki67 levels was 3.334-fold (95% CI 1.534-7.229, p=0.002) higher than that in patients with lower Ki67 levels.
Considering the high heterogeneity of breast cancer, univariate and multivariate analyses were performed in different subtypes. As shown in Supplementary Tables 1, 2, tumor size, histological grade, Ki67 and AAPR were indicated as independent predictors for pCR in the luminal subtype. In the luminal/HER2 subtype, the independent predictors were clinical nodal status, histological grade and Ki67 (Supplementary Tables 3, 4). In the HER2 enriched subtype, only clinical nodal status and histological grade were statistically significant in multivariate analysis (Supplementary Tables 4, 5). In the TNBC subtype, tumor size, clinical nodal status and AAPR were identified as independent predictors for pCR (Supplementary Tables 7, 8). The results demonstrated that the probability of pCR in patients with higher AAPR was 3.245-fold higher (95% CI 1.055-9.980, p=0.040) in the luminal subtype and 2.868-fold higher (95% CI 1.048-7.849, p=0.040) in the TNBC subtype when compared with those with lower AAPR (AAPR<0.583). It is consistent with the results obtained in the overall analysis. However, AAPR was not correlated with the pCR rate in the luminal/HER2 (p=0.215) and the HER2 enriched (p=0.853) subtype.
Development and Validation of the Nomogram
A nomogram was constructed based on the multivariate regression analysis of the training cohort. In Figure 1, tumor size, clinical nodal status, histological grade, PR, Ki67 and AAPR were used to calculate points based on the points scale axis. The sum of all these points provides a total point to estimate the probability of pCR.

Figure 1 The AAPR-based nomogram for predicting the probability of pCR after NAC in breast cancer patients. PR, progesterone receptor; AAPR, albumin-to-alkaline phosphatase ratio; pCR, pathologic complete response.
The predictive accuracy of the nomogram for the pCR rate of breast cancer patients who received NAC measured by the C-index was 0.792 (95% CI 0.737-0.848) in the training cohort and 0.790 (95% CI 0.701-0.880) in the validation cohort (Figures 2A, B). In addition, the calibration curves for pCR demonstrated a satisfactory fit between the prediction and the actual values (Figures 2C, D). As shown in Figures 2E, F, decision curves were illustrated for the constructed nomogram. It suggested that, for predicted probability thresholds between 0 and 60% the model-based decision was superior to either the treat-none or the treat-all-patients scheme.
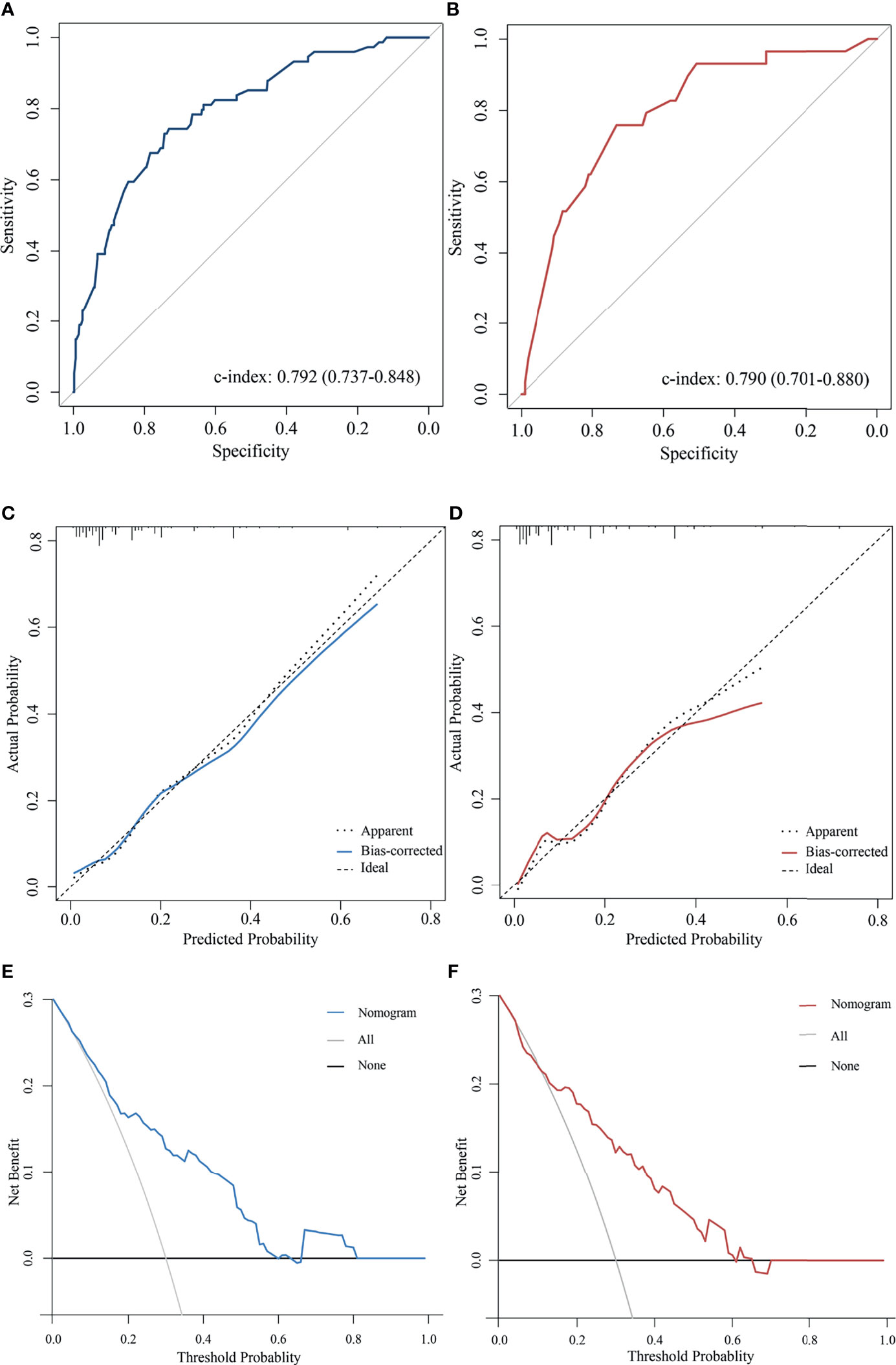
Figure 2 Validation the predictive value of the AAPR-based nomogram. The ROC curves for the nomogram model in (A) the training cohort and (B) validation cohort. The calibration plots for the nomogram model in (C) the training cohort and (D) validation cohort. The decision curves show the net-benefit of using the nomogram in (E) the training cohort and (F) validation cohort.
Discussion
NAC was first introduced in the 1970s (28). It is now widely used among breast cancer patients. Patients attaining pCR after NAC have better survival outcomes regardless of molecular subtype (4); however, the tumor response to NAC varies. Therefore, an accurate prediction assessment for pCR after NAC in breast cancer patients has great clinical significance. In the present study, the predictive value of AAPR for the probability of pCR was analyzed in breast cancer patients who received NAC. The results demonstrate that AAPR is an independent predictive factor. Pretreatment AAPR under 0.583 is associated with a lower pCR rate. More importantly, a novel AAPR-based nomogram was constructed to quantify the probability of pCR, which has promising prospects for clinical use.
In recent years, an accumulating body of research has found that serum parameters can be utilized as predictive factors in breast cancer, such as serum fibrinogen, D-dimer, lipid profiles, lymphocyte to monocyte ratio and platelet to lymphocyte ratio (12, 29). ALB and ALP are two accessible routine laboratory indexes. ALB is a globular, water-soluble protein that is exclusively produced and secreted by hepatocytes. Since ALB accounts for approximately half of the total serum protein, it is the most abundant protein in serum (14). Previous studies have confirmed that hypoalbuminemia is associated with inflammation and malnutrition during cancer development and progression (30). This may be explained by reduced synthesis, increased consumption and loss of serum ALB (31). ALB also contributes to balancing cell proliferation and metabolism (32). Hypoalbuminemia may reflect impairment of immunity and affect the response to anticancer treatment (33). Several studies have demonstrated that pretreatment ALB is a prognostic factor in various cancers, including lung, pancreatic, gastric, and colorectal cancers (34). ALP is a hydrolytic enzyme that dephosphorylates different types of molecules, including nucleotides, proteins, and alkaloids (35). It plays an anti-inflammatory and tissue-protective role by enhancing the conversion of ATP into adenosine and increasing the level of adenosine (36). A previous study demonstrated that the activity of ALP is associated with cancer cell death, migration and mesenchymal-to-epithelial transition (21). It was reported that a heavy tumor burden and tumor metastasis might result in the elevation of ALP. Higher serum ALP levels were demonstrated to be related to a worse prognosis in nasopharyngeal carcinoma, prostate cancer and colorectal cancer (37–39). Chen et al. (40) reported that the pretreatment serum ALP level was an independent unfavorable prognostic factor for disease-free survival and overall survival in TNBC patients. AAPR is a novel, accessible, low-cost and noninvasive index that is calculated based on ALB and ALP and potentially reflects systematic inflammation and nutrition status. It was first reported as a prognostic factor in hepatocellular carcinoma and has already been identified as a prognostic predictor in various cancers (22). Kim et al. (33) reported that a high AAPR was related to better overall survival, progression-free survival, and locoregional relapse-free survival in nasopharyngeal carcinoma. Li et al. (23) also reported that AAPR was an independent predictive factor for PFS in small-cell lung cancer. A retrospective study enrolled 746 nonmetastatic breast cancer patients and suggested that a lower AAPR was related to shorter OS (41).
The results of these previous studies suggested that a high AAPR was associated with better survival outcomes. However, the predictive value of AAPR for pCR possibility in breast cancer patients who received NAC remains unknown. In the present study, the optimal cutoff value of AAPR was 0.583 with the maximum Youden index. Initially, we evaluated the relationship between AAPR and breast cancer characteristics, and our results suggested that AAPR was significantly associated with age (p<0.001), menopausal status (p<0.001), histological type (p=0.007), molecular subtypes (p=0.045) and pCR (p=0.030). Our further analysis focused on assessing the predictive value of clinicopathological factors. We found that the pretreatment ALB and ALP levels of most patients were within the normal range. Moreover, the univariate analysis indicated that neither pretreatment ALB nor ALP could be used as a predictor for pCR in breast cancer patients. Nevertheless, AAPR is an independent predictive factor, and patients with low AAPR had a significantly lower probability of achieving pCR.
Multivariate analysis also suggested that tumor size, clinical nodal status, histological grade, PR and Ki67 expression were independent predictive factors. A retrospective study by Briete et al. (n= 2366) demonstrated that lower T-stages had significantly higher pCR rates than higher T-stages (42), and a study by Bonadonna et al. (n = 165) showed that the tumor response was inversely proportional to initial tumor size for tumors larger than 3 cm (43). These results emphasized the importance to consider tumor size when estimating the chance of pCR in breast cancer patients. The CTNeoBC pooled analysis included 11955 patients suggested that the frequency of pCR in patients with clinical-nodal-positive and hormone-receptor-positive tumors was low (44). Ki67 expression was related to tumor cell proliferation, several studies revealed that patients with higher Ki67 expression were more likely to achieve pCR (45–47).Most of the above indicators are consistent with the existing literature. However, there was no significant correlation between HER2 status and pCR, which is inconsistent with previous studies (4). Moreover, the overall pCR rate in the current study was 13.2%, which is lower than that in some previous large-scale studies (20.4-21.1%) (4, 48). The NOAH trial and the NeoSphere trial demonstrated the addition of neoadjuvant trastuzumab and pertuzumab to neoadjuvant chemotherapy significantly improved the pCR rate in the HER2-positive disease (49, 50). However, 97% of HER2-positive patients refused anti-HER2 therapy in our study owing to the high costs. The absence of neoadjuvant anti-HER2 therapy has a great impact on the pCR rate of HER2-positive patients, which may result in the relatively lower pCR rate and the insignificant predictive value of AAPR in the luminal/HER2 and the HER2 enriched subtypes. This phenomenon also confirmed the importance of HER2-targeted therapy during NAC in HER2-positive patients. To the best of our knowledge, this is the first retrospective study conducted to analyze the predictive value of AAPR for pCR in breast cancer patients who received NAC. Based on the above results, a serum AAPR-based nomogram was developed and validated to quantitatively estimate the pCR probability in patients who received NAC.
However, there are several limitations. First, this study was a retrospective study conducted at a single center. The training cohort and validation cohort minimize the possible selection bias, but the optimal cutoff value of AAPR and nomogram require further external validation. In addition, only 3% of HER2-positive patients received trastuzumab therapy during NAC due to financial issues, which may have affected the pCR rate and the predictive role of AAPR in the HER2-positive patients. Third, although this study included clinicopathological information as comprehensively as possible from medical records, some valuable factors may still exist that were not available in our analysis. It is necessary to further analyze the predictive role of AAPR in the HER2-positive subgroup with adequately treated patients in the future. Larger multicenter prospective clinical studies are needed to improve and validate the AAPR-based nomogram in breast cancer patients with different molecular subtypes and treated with more innovative therapeutic modalities. Laboratory experiments are required to explore the mechanism of the predictive capability of AAPR for pCR in breast cancer patients.
Conclusion
In conclusion, the present study demonstrated that pretreatment serum AAPR, tumor size, clinical nodal status, histological grade, PR and Ki67 expression were independent predictive factors for pCR in breast cancer patients treated with NAC. The AAPR-based nomogram can accurately estimate pCR probability and helps to determine individual treatment strategies.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
This study was reviewed and approved by the ethics committee of the First Affiliated Hospital of Chongqing Medical University. Written informed consent was obtained from all patients included in the study.
Author Contributions
FQ conceived the study, conducted most of the data analysis and drafted the manuscript. ZL, SQL, and XZ participated in the data analysis. XF, XH, and QL participated in the figure production. SCL provided the original data and made detailed revisions to the manuscript. HL guided the entire analysis process and determined the direction of the research for each section. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Science and Technology Planning Project of Guangzhou (No. 805275295029), the Natural Science Foundation of Guangdong Province (No.2020A1515010425), and the China Postdoctoral Science Foundation (No. 2020M672986, 2020M670108ZX).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.681905/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Gralow J, Burstein H, Wood W, Hortobagyi G, Gianni L, von Minckwitz G, et al. Preoperative Therapy in Invasive Breast Cancer: Pathologic Assessment and Systemic Therapy Issues in Operable Disease. J Clin Oncol: Off J Am Soc Clin Oncol (2008) 26(5):814–9. doi: 10.1200/jco.2007.15.3510
3. Bardia A, Baselga J. Neoadjuvant Therapy as a Platform for Drug Development and Approval in Breast Cancer. Clin Cancer Res: An Off J Am Assoc Cancer Res (2013) 19(23):6360–70. doi: 10.1158/1078-0432.Ccr-13-0916
4. Spring L, Fell G, Arfe A, Sharma C, Greenup R, Reynolds K, et al. Pathologic Complete Response After Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-Analysis. Clin Cancer Res: An Off J Am Assoc Cancer Res (2020) 26(12):2838–48. doi: 10.1158/1078-0432.Ccr-19-3492
5. von Minckwitz G, Untch M, Blohmer J, Costa S, Eidtmann H, Fasching P, et al. Definition and Impact of Pathologic Complete Response on Prognosis After Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. J Clin Oncol: Off J Am Soc Clin Oncol (2012) 30(15):1796–804. doi: 10.1200/jco.2011.38.8595
6. Li Z, Zhang Y, Zhang Z, Zhao Z. Lv Q. A Four-Gene Signature Predicts the Efficacy of Paclitaxel-Based Neoadjuvant Therapy in Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer. J Cell Biochem (2019) 120(4):6046–56. doi: 10.1002/jcb.27891
7. Jung Y, Hyun C, Jin M, Park I, Chung Y, Shim B, et al. Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. J Breast Cancer (2016) 19(3):261–7. doi: 10.4048/jbc.2016.19.3.261
8. Chen S, Shu Z, Li Y, Chen B, Tang L, Mo W, et al. Machine Learning-Based Radiomics Nomogram Using Magnetic Resonance Images for Prediction of Neoadjuvant Chemotherapy Efficacy in Breast Cancer Patients. Front Oncol (2020) 10:1410. doi: 10.3389/fonc.2020.01410
9. Wu S, He Z, Zhou J, Sun J, Li F, Lin Q, et al. Serum Levels of CEA and CA15-3 in Different Molecular Subtypes and Prognostic Value in Chinese Breast Cancer. Breast (Edinburgh Scotland) (2014) 23(1):88–93. doi: 10.1016/j.breast.2013.11.003
10. Wang W, Xu X, Tian B, Wang Y, Du L, Sun T, et al. The Diagnostic Value of Serum Tumor Markers CEA, CA19-9, CA125, CA15-3, and TPS in Metastatic Breast Cancer. Clinica Chimica Acta; Int J Clin Chem (2017) 470:51–5. doi: 10.1016/j.cca.2017.04.023
11. Greten F, Grivennikov S. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity (2019) 51(1):27–41. doi: 10.1016/j.immuni.2019.06.025
12. Zhang F, Huang M, Zhou H, Chen K, Jin J, Wu Y, et al. A Nomogram to Predict the Pathologic Complete Response of Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer Based on Simple Laboratory Indicators. Ann Surg Oncol (2019) 26(12):3912–9. doi: 10.1245/s10434-019-07655-7
13. Marín Hernández C, Piñero Madrona A, Gil Vázquez P, Galindo Fernández P, Ruiz Merino G, Alonso Romero J, et al. Usefulness of Lymphocyte-to-Monocyte, Neutrophil-to-Monocyte and Neutrophil-to-Lymphocyte Ratios as Prognostic Markers in Breast Cancer Patients Treated With Neoadjuvant Chemotherapy. Clin Trans Oncol: Off Publ Fed Spanish Oncol Societies Natl Cancer Institute Mexico (2018) 20(4):476–83. doi: 10.1007/s12094-017-1732-0
14. Bernardi M, Angeli P, Claria J, Moreau R, Gines P, Jalan R, et al. Albumin in Decompensated Cirrhosis: New Concepts and Perspectives. Gut (2020) 69(6):1127–38. doi: 10.1136/gutjnl-2019-318843
15. Lin J, Lin J, Xie J, Wang J, Lu J, Chen Q, et al. Prognostic Importance of the Preoperative Modified Systemic Inflammation Score for Patients With Gastric Cancer. Gastric Cancer: Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2019) 22(2):403–12. doi: 10.1007/s10120-018-0854-6
16. Fuke H, Sugimoto K, Shiraki K, Tanaka J, Beppu T, Yoneda K, et al. Predictive Factors for Distant Recurrence of HCV-Related Hepatocellular Carcinoma After Radiofrequency Ablation Combined With Chemoembolization. Alimentary Pharmacol Ther (2008) 27(12):1253–60. doi: 10.1111/j.1365-2036.2008.03627.x
17. Liu D, Li F, Jia W. Cumulative Scores Based on Plasma D-Dimer and Serum Albumin Levels Predict Survival in Esophageal Squamous Cell Carcinoma Patients Treated With Transthoracic Esophagectomy. Chin J Cancer (2016) 35:11. doi: 10.1186/s40880-015-0062-2
18. Stavraka C, Pinato D, Turnbull S, Flynn M, Forster M, O’Cathail S, et al. Developing an Objective Marker to Optimize Patient Selection and Predict Survival Benefit in Early-Phase Cancer Trials. Cancer (2014) 120(2):262–70. doi: 10.1002/cncr.28381
19. Haarhaus M, Brandenburg V, Kalantar-Zadeh K, Stenvinkel P, Magnusson P. Alkaline Phosphatase: A Novel Treatment Target for Cardiovascular Disease in CKD. Nat Rev Nephrol (2017) 13(7):429–42. doi: 10.1038/nrneph.2017.60
20. Damera S, Raphael K, Baird B, Cheung A, Greene T, Beddhu S. Serum Alkaline Phosphatase Levels Associate With Elevated Serum C-Reactive Protein in Chronic Kidney Disease. Kidney Int (2011) 79(2):228–33. doi: 10.1038/ki.2010.356
21. Rao S, Snaith A, Marino D, Cheng X, Lwin S, Orriss I, et al. Tumour-Derived Alkaline Phosphatase Regulates Tumour Growth, Epithelial Plasticity and Disease-Free Survival in Metastatic Prostate Cancer. Br J Cancer (2017) 116(2):227–36. doi: 10.1038/bjc.2016.402
22. Chan A, Chan S, Mo F, Wong G, Wong V, Cheung Y, et al. Albumin-to-Alkaline Phosphatase Ratio: A Novel Prognostic Index for Hepatocellular Carcinoma. Dis Markers (2015) 2015:564057. doi: 10.1155/2015/564057
23. Li B, Jiang C, Wang R, Zou B, Xie P, Li W, et al. Prognostic Value of a Nomogram Based on the Dynamic Albumin-to-Alkaline Phosphatase Ratio for Patients With Extensive-Stage Small-Cell Lung Cancer. OncoTargets Ther (2020) 13:9043–57. doi: 10.2147/ott.S262084
24. Tian G, Li G, Guan L, Yang Y, Li N. Pretreatment Albumin-to-Alkaline Phosphatase Ratio as a Prognostic Indicator in Solid Cancers: A Meta-Analysis With Trial Sequential Analysis. Int J Surg (London England) (2020) 81:66–73. doi: 10.1016/j.ijsu.2020.07.024
25. Wolff A, Hammond M, Allison K, Harvey B, Mangu P, Bartlett J, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol: Off J Am Soc Clin Oncol (2018) 36(20):2105–22. doi: 10.1200/jco.2018.77.8738
26. Dowsett M, Nielsen T, A’Hern R, Bartlett J, Coombes R, Cuzick J, et al. Assessment of Ki67 in Breast Cancer: Recommendations From the International Ki67 in Breast Cancer Working Group. J Natl Cancer Institute (2011) 103(22):1656–64. doi: 10.1093/jnci/djr393
27. Ogston K, Miller I, Payne S, Hutcheon A, Sarkar T, Smith I, et al. A New Histological Grading System to Assess Response of Breast Cancers to Primary Chemotherapy: Prognostic Significance and Survival. Breast (Edinburgh Scotland) (2003) 12(5):320–7. doi: 10.1016/s0960-9776(03)00106-1
28. Rubens R, Sexton S, Tong D, Winter P, Knight R, Hayward J. Combined Chemotherapy and Radiotherapy for Locally Advanced Breast Cancer. Eur J Cancer (1980) 16(3):351–6. doi: 10.1016/0014-2964(80)90352-7
29. Qu F, Chen R, Peng Y, Ye Y, Tang Z, Wang Y, et al. Assessment of the Predictive Role of Serum Lipid Profiles in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. J Breast Cancer (2020) 23(3):246–58. doi: 10.4048/jbc.2020.23.e32
30. Diakos C, Charles K, McMillan D, Clarke S. Cancer-Related Inflammation and Treatment Effectiveness. Lancet Oncol (2014) 15(11):e493–503. doi: 10.1016/s1470-2045(14)70263-3
31. Eckart A, Struja T, Kutz A, Baumgartner A, Baumgartner T, Zurfluh S, et al. Relationship of Nutritional Status, Inflammation, and Serum Albumin Levels During Acute Illness: A Prospective Study. Am J Med (2020) 133(6):713–22.e7. doi: 10.1016/j.amjmed.2019.10.031
32. Nojiri S, Joh T. Albumin Suppresses Human Hepatocellular Carcinoma Proliferation and the Cell Cycle. Int J Mol Sci (2014) 15(3):5163–74. doi: 10.3390/ijms15035163
33. Kim J, Keam B, Heo D, Han D, Rhee C, Kim J, et al. The Prognostic Value of Albumin-to-Alkaline Phosphatase Ratio Before Radical Radiotherapy in Patients With Non-Metastatic Nasopharyngeal Carcinoma: A Propensity Score Matching Analysis. Cancer Res treatment: Off J Korean Cancer Assoc (2019) 51(4):1313–23. doi: 10.4143/crt.2018.503
34. Gupta D, Lis C. Pretreatment Serum Albumin as a Predictor of Cancer Survival: A Systematic Review of the Epidemiological Literature. Nutr J (2010) 9:69. doi: 10.1186/1475-2891-9-69
35. Cauwels A, Rogge E, Vandendriessche B, Shiva S, Brouckaert P. Extracellular ATP Drives Systemic Inflammation, Tissue Damage and Mortality. Cell Death Dis (2014) 5:e1102. doi: 10.1038/cddis.2014.70
36. Peters E, Geraci S, Heemskerk S, Wilmer M, Bilos A, Kraenzlin B, et al. Alkaline Phosphatase Protects Against Renal Inflammation Through Dephosphorylation of Lipopolysaccharide and Adenosine Triphosphate. Br J Pharmacol (2015) 172(20):4932–45. doi: 10.1111/bph.13261
37. Li G, Gao J, Tao Y, Xu B, Tu Z, Liu Z, et al. Increased Pretreatment Levels of Serum LDH and ALP as Poor Prognostic Factors for Nasopharyngeal Carcinoma. Chin J Cancer (2012) 31(4):197–206. doi: 10.5732/cjc.011.10283
38. Sonpavde G, Pond G, Berry W, de Wit R, Armstrong A, Eisenberger M, et al. Serum Alkaline Phosphatase Changes Predict Survival Independent of PSA Changes in Men With Castration-Resistant Prostate Cancer and Bone Metastasis Receiving Chemotherapy. Urologic Oncol (2012) 30(5):607–13. doi: 10.1016/j.urolonc.2010.07.002
39. Maisano R, Azzarello D, Del Medico P, Maisano M, Bottari M, Egitto G, et al. Alkaline Phosphatase Levels as a Prognostic Factor in Metastatic Colorectal Cancer Treated With the FOLFOX 4 Regimen: A Monoinstitutional Retrospective Study. Tumori (2011) 97(1):39–42. doi: 10.1177/030089161109700108
40. Chen B, Dai D, Tang H, Chen X, Ai X, Huang X, et al. Pre-Treatment Serum Alkaline Phosphatase and Lactate Dehydrogenase as Prognostic Factors in Triple Negative Breast Cancer. J Cancer (2016) 7(15):2309–16. doi: 10.7150/jca.16622
41. Long Z, Hua X, Zhang W, Lv S, Deng J, Guo L, et al. Prognostic Impact of the Pretreatment Albumin to Alkaline Phosphatase Ratio for Nonmetastatic Breast Cancer Patients. Cancer Manage Res (2019) 11:4809–14. doi: 10.2147/cmar.S200759
42. Goorts B, van Nijnatten T, de Munck L, Moossdorff M, Heuts E, de Boer M, et al. Clinical Tumor Stage Is the Most Important Predictor of Pathological Complete Response Rate After Neoadjuvant Chemotherapy in Breast Cancer Patients. Breast Cancer Res Treat (2017) 163(1):83–91. doi: 10.1007/s10549-017-4155-2
43. Bonadonna G, Veronesi U, Brambilla C, Ferrari L, Luini A, Greco M, et al. Primary Chemotherapy to Avoid Mastectomy in Tumors With Diameters of Three Centimeters or More. J Natl Cancer Institute (1990) 82(19):1539–45. doi: 10.1093/jnci/82.19.1539
44. Cortazar P, Zhang L, Untch M, Mehta K, Costantino J, Wolmark N, et al. Pathological Complete Response and Long-Term Clinical Benefit in Breast Cancer: The Ctneobc Pooled Analysis. Lancet (London England) (2014) 384(9938):164–72. doi: 10.1016/s0140-6736(13)62422-8
45. Chen R, Ye Y, Yang C, Peng Y, Zong B, Qu F, et al. Assessment of the Predictive Role of Pretreatment Ki-67 and Ki-67 Changes in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy According to the Molecular Classification: A Retrospective Study of 1010 Patients. Breast Cancer Res Treat (2018) 170(1):35–43. doi: 10.1007/s10549-018-4730-1
46. Yan S, Wang W, Zhu B, Pan X, Wu X, Tao W. Construction of Nomograms for Predicting Pathological Complete Response and Tumor Shrinkage Size in Breast Cancer. Cancer Manage Res (2020) 12:8313–23. doi: 10.2147/cmar.S270687
47. Pu S, Wang K, Liu Y, Liao X, Chen H, He J, et al. Nomogram-Derived Prediction of Pathologic Complete Response (Pcr) in Breast Cancer Patients Treated With Neoadjuvant Chemotherapy (NCT). BMC Cancer (2020) 20(1):1120. doi: 10.1186/s12885-020-07621-7
48. Werutsky G, Untch M, Hanusch C, Fasching P, Blohmer J, Seiler S, et al. Locoregional Recurrence Risk After Neoadjuvant Chemotherapy: A Pooled Analysis of Nine Prospective Neoadjuvant Breast Cancer Trials. Eur J Cancer (Oxford England: 1990) (2020) 130:92–101. doi: 10.1016/j.ejca.2020.02.015
49. Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant Chemotherapy With Trastuzumab Followed by Adjuvant Trastuzumab Versus Neoadjuvant Chemotherapy Alone, in Patients With HER2-Positive Locally Advanced Breast Cancer (the NOAH Trial): A Randomised Controlled Superiority Trial With a Parallel HER2-Negative Cohort. Lancet (London England) (2010) 375(9712):377–84. doi: 10.1016/s0140-6736(09)61964-4
50. Gianni L, Pienkowski T, Im Y, Roman L, Tseng L, Liu M, et al. Efficacy and Safety of Neoadjuvant Pertuzumab and Trastuzumab in Women With Locally Advanced, Inflammatory, or Early HER2-Positive Breast Cancer (Neosphere): A Randomised Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol (2012) 13(1):25–32. doi: 10.1016/s1470-2045(11)70336-9
Keywords: breast cancer, albumin-to-alkaline phosphatase ratio, neoadjuvant chemotherapy, nomogram, pathological complete response
Citation: Qu F, Li Z, Lai S, Zhong X, Fu X, Huang X, Li Q, Liu S and Li H (2021) Construction and Validation of a Serum Albumin-to-Alkaline Phosphatase Ratio-Based Nomogram for Predicting Pathological Complete Response in Breast Cancer. Front. Oncol. 11:681905. doi: 10.3389/fonc.2021.681905
Received: 17 March 2021; Accepted: 21 September 2021;
Published: 08 October 2021.
Edited by:
Shengtao Zhou, Sichuan University, ChinaReviewed by:
Shifang Ren, Fudan University, ChinaDonatella Santini, Sant’Orsola-Malpighi Polyclinic, Italy
Copyright © 2021 Qu, Li, Lai, Zhong, Fu, Huang, Li, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengchun Liu, bGl1c2hlbmdjaHVuMTk2OEAxNjMuY29t; Haiyan Li, bGloeTI3QG1haWwuc3lzdS5lZHUuY24=
 Fanli Qu
Fanli Qu Zongyan Li
Zongyan Li Shengqing Lai1
Shengqing Lai1 Shengchun Liu
Shengchun Liu Haiyan Li
Haiyan Li