- 1Department of Oncology, Second Affiliated Hospital of Naval Medical University, Shanghai, China
- 2Liver Cancer Institute, Zhongshan Hospital, Fudan University, Shanghai, China
- 3Department of Pathology, Second Affiliated Hospital of Naval Medical University, Shanghai, China
Background: A rare subtype of breast cancer, atypical medullary carcinoma of the breast (AMCB), shows a highly adverse prognosis compared to medullary carcinoma of the breast (MBC). The current study aimed to establish a correlated nomogram for the identification of the prognostic factors of AMCB and MBC.
Methods: Kaplan–Meier and Cox regression analyses were applied to data acquired from the Surveillance, Epidemiology and End Results (SEER) database for 2004 to 2013 to analyse tumour characteristics and overall survival. Propensity score matching (PSM) analysis was performed to determine the overall survival (OS) among those with AMCB and MBC. A predictive nomogram was created, and the concordance index (C-index) was used to predict accuracy and discriminative ability.
Results: A total of 2,001 patients from the SEER database were diagnosed with MBC between 2004 and 2013, including 147 patients diagnosed with AMCB. The number of diagnoses gradually increased in both groups. Cox analysis of multivariate and Kaplan–Meier analysis showed that older age (HR = 3.005, 95% CI 1.906–4.739) and later stage were significantly associated with poor prognosis, while cancer-directed surgery was an independent protective factor (HR = 0.252, 95% CI 0.086–0.740). In the HR-negative stratification analysis, older age (HR = 2.476, 95% CI 1.398–4.385), later stage and histological type (HR=0.381, 95% CI 0.198-0.734) were found to be independent prognostic factors for low standard survival. The log-rank analysis demonstrated significantly worse prognostic factors for patients with AMCB than for those with MBC (P = 0.004). A nomogram (C-index for survival = 0.75; 95% CI 0.69–0.81) was established from four independent prognostic factors after complete identification.
Conclusions: MBC is rare, and cancer-directed surgery, older age, and later stage are independently linked with prognosis. In the HR negative population, AMCB patients show a worse survival gain than those with MBC.
Introduction
Breast cancer is a major malignant tumour in women, ranking second in incidence among female malignant tumours. Medullary carcinoma of the breast (MBC) has been declared a special type of infiltrating breast cancer, accounting for approximately 5–7% of cases (1). The boundary of the typical medullary carcinoma of the breast (TMCB) is clearer, and a large number of lymphocytes infiltrate the interstitial substance; thus, it has a slower growth and more favourable prognosis (2). However, atypical medullary carcinoma of the breast (AMCB) is characterized by no obvious histologic boundaries and a poor prognosis.
Therefore, it is important to determine and characterize the prognostic factors for AMCB patients to facilitate diagnosis and clinical treatment. Although the cellular morphology of AMCB is essentially consistent with that of MBC, it fails to conform to the diagnostic criteria of MBC and features infiltrating tumour borders (3). Several studies have reported that the overall survival (OS) and disease-free survival (DFS) of MBC patients were closely related to age, race, local metastasis, distant metastasis, tumour size, hormone receptor status, and lymph node metastasis (4–7). In addition, HR status also affected the survival of MBC patients. Some studies found that MBC patients had a higher percentage of triple-negative status, and MBC patients with PR negativity exhibited good prognosis (8, 9). However, the prognostic factors of AMCB were not mentioned. To address this, we examined patients with AMCB and MBC of the breast cancer referring to the Surveillance, Epidemiology and End Results (SEER) database and offer a related retrospective assessment. We compared the overall survival, prognostic factors, and clinical features between MBC and AMCB patients. In addition, hormone receptor status was used as a stratification analysis to analyse the survival benefit of AMCB and MBC patients.
Materials and Methods
Patient Data
Data from patients diagnosed with breast cancer from 2004 to 2013 were downloaded using Stat version 8.2.1 of SEER. Patient data were included in this analysis if the following criteria were met: ages 18 to 79; breast cancer as the primary malignant cancer diagnosis; estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (Her2) status available; medullary carcinoma (based on ICD-O-3 8512/3) and pathological types including atypical medullary carcinoma (based on ICD-O-3 8513/3) if not specified (MBC-NOS, ICD-O-3 8510/3); and definite AJCC TNM stages; and histological grades I to IV. We excluded patients who did not have a clear tumour stage or a record of months of survival. Additionally, we also included data from patients exhibiting a history of breast cancer diagnosis prior to 2013 to ensure a sufficiently extensive follow-up period. The study was in compliance with the ethics statement of Changzheng Hospital.
Statistical Analysis
The expression status of ER and PR was combined as the hormone receptor (HR) state. The expression of HR− was further defined as ER− and PR−, while the expression of HR+ was defined as ER+ and PR+. Unclear expression of ER or PR was defined as unknown. According to the pathological type, breast cancer patients were allocated into two groups: the AMCB and MBC groups. The t-test and, where necessary, the Mann–Whitney U test were employed to compare the homogeneity of variance and continuous variables in the normal distribution. Tukey’s test and one-way ANOVA were employed to compare multiple groups. The chi-square test was used to compare the clinical and demographic characteristics of the three groups. The Kaplan–Meier method was employed to plot the survival curve, and the log-rank test was used to adjust the unadjusted overall survival rates of different histological subtypes. Overall survival was defined as the period from diagnosis to the final follow-up or the complete disappearance of the tumour. The prognostic factors were computed using the Cox proportional hazards model, where HR was the 95% confidence interval. PSM analyses were performed based on age, race, sex, grade, laterality, AJCC stage, T category, N category, local treatment of the primary tumour, ER, PR and Her2 at a 1:1 ratio to adjust for the differences among the AMCB and MBC groups. All statistical analyses were computed using SPSS version 22.0 (IBM SPSS Statistics, Chicago, IL, US), and P <0.05 was considered statistically significant.
Results
Demographic Analysis of Patients With AMCB and MBC
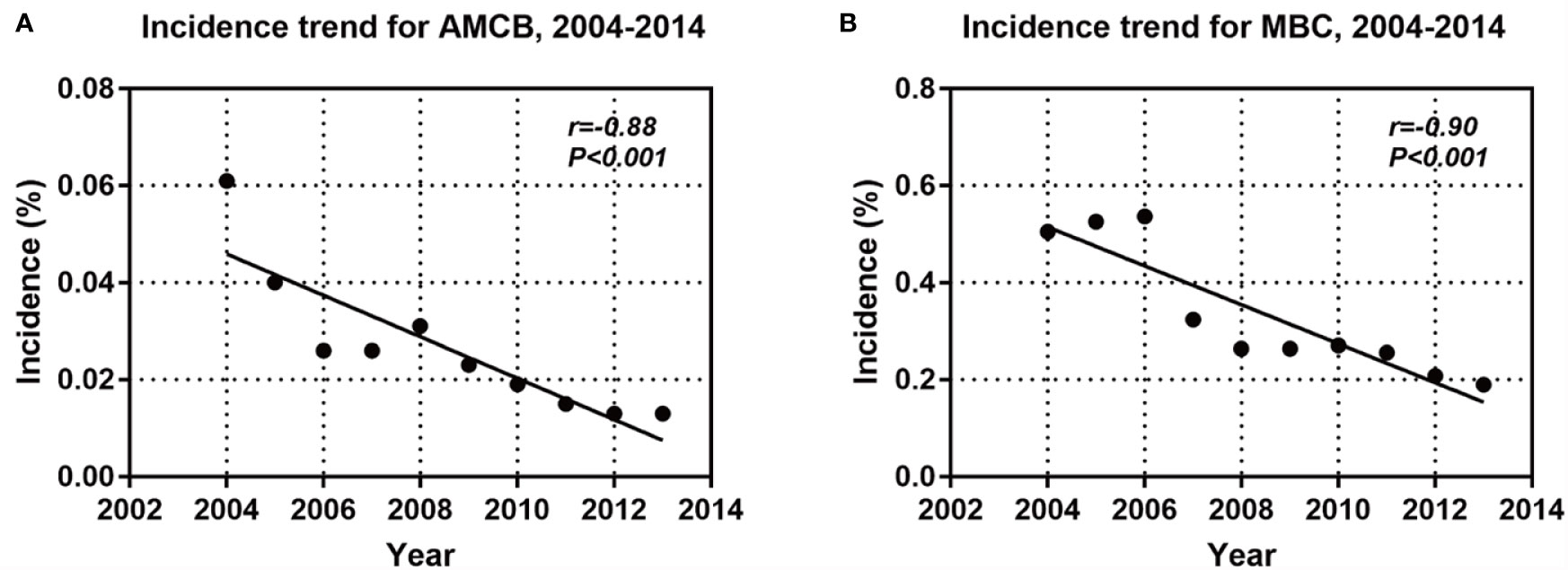
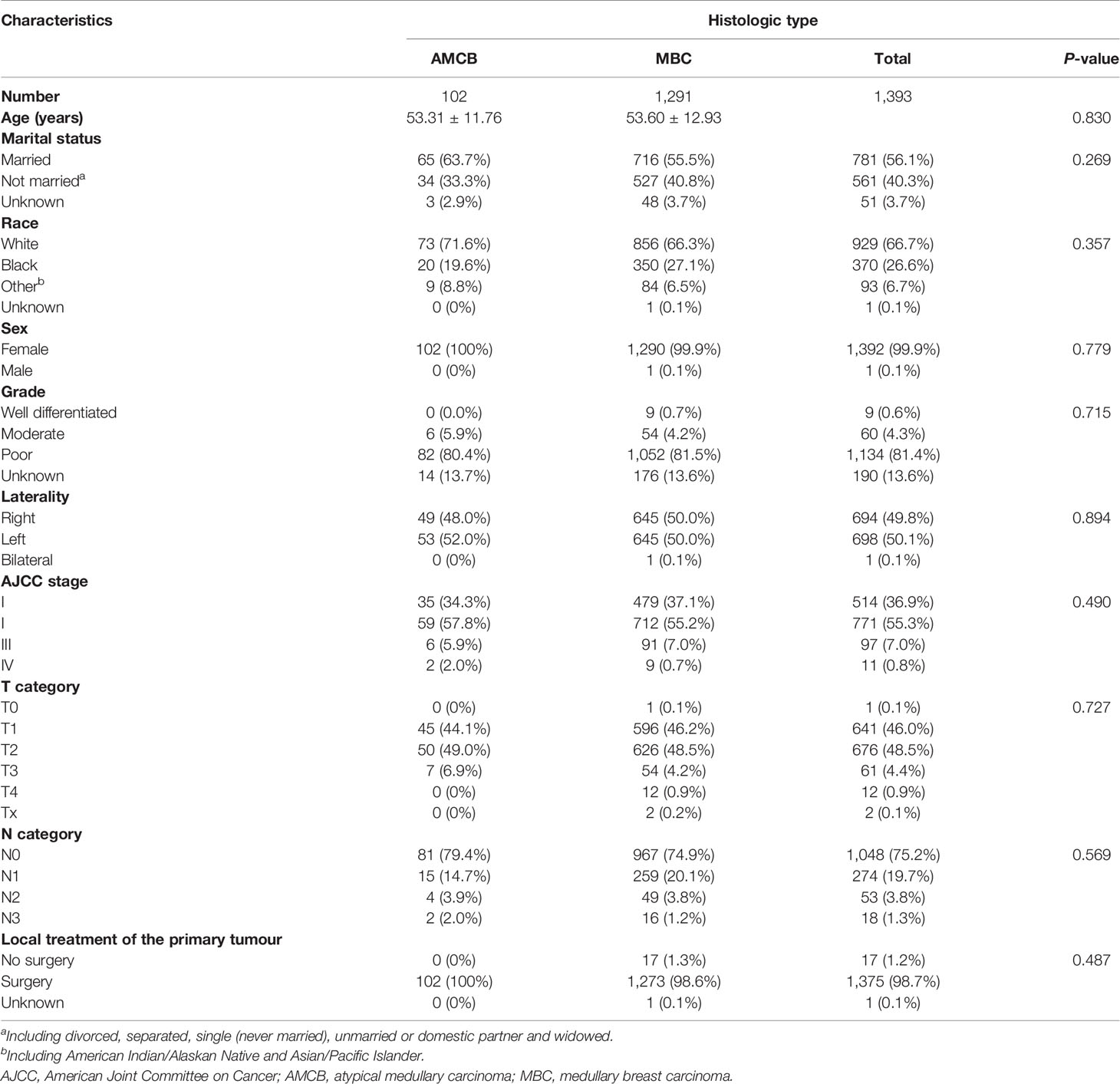
A total of 2,001 eligible patients, including 1,863 MBC patients and 147 AMCB patients, were included in our study based on the inclusion criteria. The clinical and demographic characteristics of all patients in the SEER database with Histologic Type of Breast Cancer from 2004–2013 are summarized in Table 1. The X-tile analysis revealed that the best possible cut-off value to assess prognostic factors for age was 71 years. The comparison of MBC and AMCB showed no substantial differences in race, sex, age, degree of differentiation, tumour size, AJCC stage, lymph node metastasis, Her2 status, HR status, or surgical method. Similar to MBC, the rate of incidence of AMCB was high in triple-negative breast cancer. In molecular subtypes, three categories were defined (Table 1). Regarding Her2 status, the frequency of Her2-negative cases was higher in the MBC group than in the AMCB group (24.6% vs 17.7%, P = 0.108). Overall, the number of Her2-negative patients was higher than that of Her2-positive patients (24.1% vs 3.1%). However, 72.8% of cases were characterized as unknown, with neither Her2 negativity nor Her2 positivity. Due to limited data, the comparative analysis of the treatment method did not generate statistically significant results. Between 2004 and 2013, the overall incidence rate trends of AMCB and MBC decreased (AMBC: r = −0.88 and MBC: r = −0.90, P <0.001) (Figure 1).
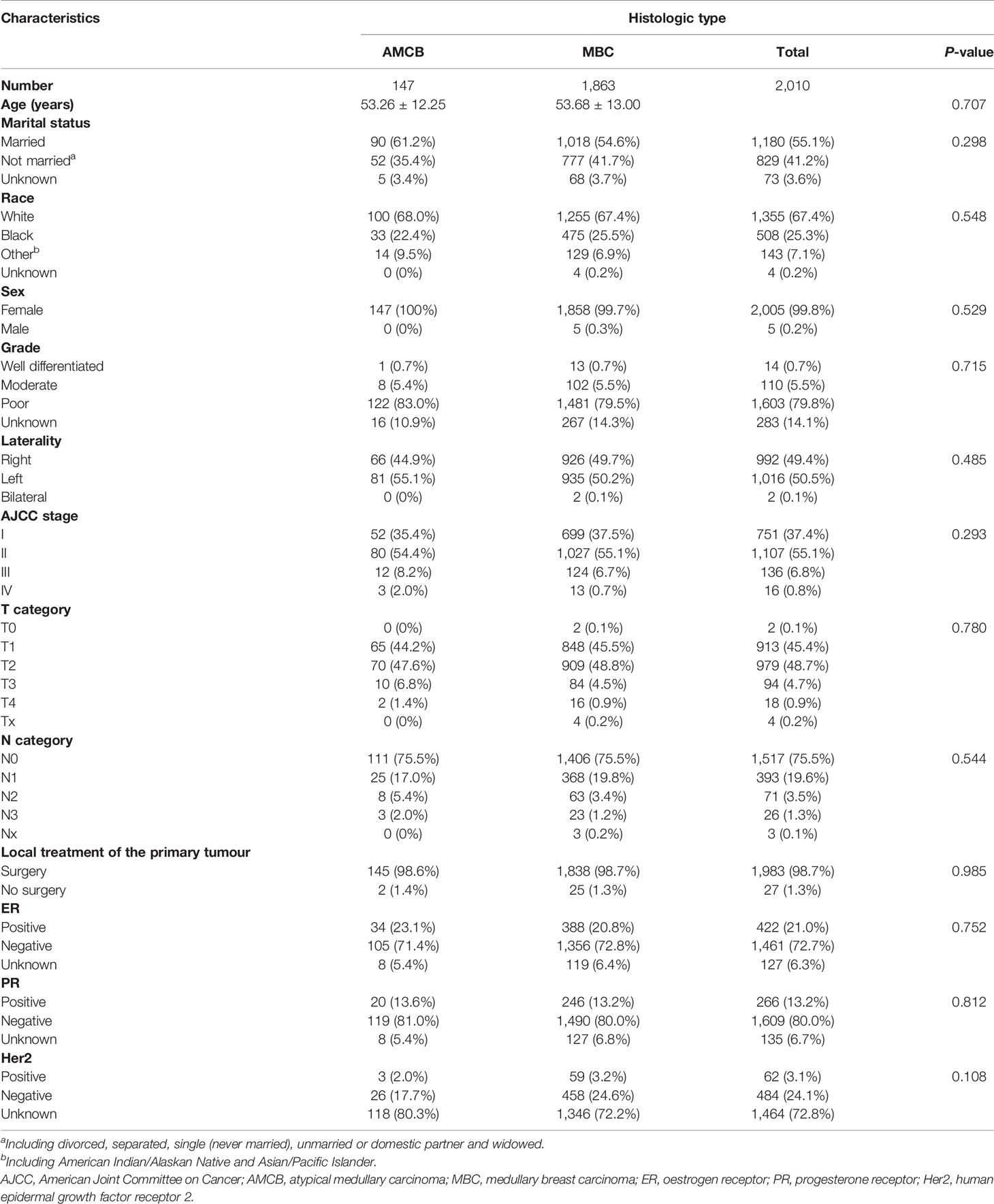
Table 1 Patient characteristics with histologic type of breast cancer in the SEER database, 2004–2013.
Survival and Prognostic Factors of AMCB and MBC Patients
The overall survival of MBC and AMCB patients was estimated using the Kaplan–Meier estimator. The survival curve was stratified by race, sex, age at diagnosis, laterality, AJCC stage, primary tumour size, primary tumour differential grade, surgery for the primary tumour, lymph node status, HR status, and histological type (Figure 2). As illustrated, older-aged patients had poorer survival (5-year overall survival rate: 83.3% vs 94.9%). The outcomes were extremely poor for patients with advanced cancer stages; the prognostic factors were the worst (27.5% 5-year overall survival rate) for MBC and AMCB patients with stage IV cancer, while the 5-year overall survival rates for stage I, II, and III cancers were 75.3, 94.5, and 97.3%, respectively (P <0.01, Figures 2A, E). Similarly, the overall survival rate of MBC and AMCB patients with smaller tumour sizes was significantly higher than that of patients with larger tumour sizes (P <0.01). The prognostic factors for MBC and AMCB patients were much worse when breast cancer cells were detected in lymph nodes (P <0.01) (Figures 2F, G). Figure 2H shows that the median overall survival for the MBC and AMCB patients significantly improved after cancer-directed surgery (P <0.01, mOS: 16.5 m vs 28.0 m). In the histological type analysis for OS, patients with MBC had better survival than those with AMCB (P = 0.013). The 5-year overall survival rates of MBC and AMCB patients were 94.3 and 87.8%, respectively.
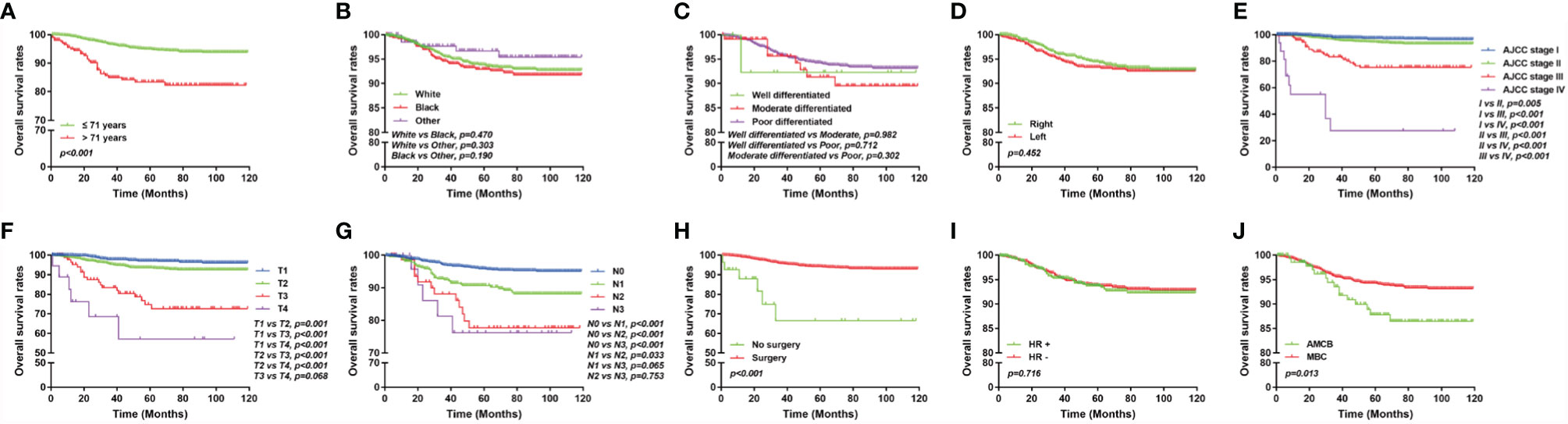
Figure 2 Overall survival of MBC and AMCB patients using the Kaplan–Meier estimator stratified by (A) age at diagnosis; (B) race; (C) grade; (D) laterality; (E) AJCC stage; (F) primary tumour size; (G) lymph node status; (H) surgery for primary tumour; (I) HR status; and (J) histological type.
The Cox regression models in both forms, i.e., univariate and multivariate analyses, were applied to the overall survival results to further analyse the prognostic factors. According to univariate factor analysis, as shown in Table 2, older age, larger tumour size, later stage, lymph node metastasis, and histological type of AMCB were significantly related to worse prognosis (P <0.01). Cancer-directed surgery was found to be significantly related to extended overall survival (P <0.01). After adjusting the multivariate analysis, as shown in Table 2, the independent prognostic factors of poorer survival in MBC and AMCB patients were older age and later stage only. However, cancer-directed surgery was found to be an independent protective factor that reduced the probability of death by 74.8% (HR = 0.252, 95% CI 0.086–0.740) in MBC and AMCB patients.
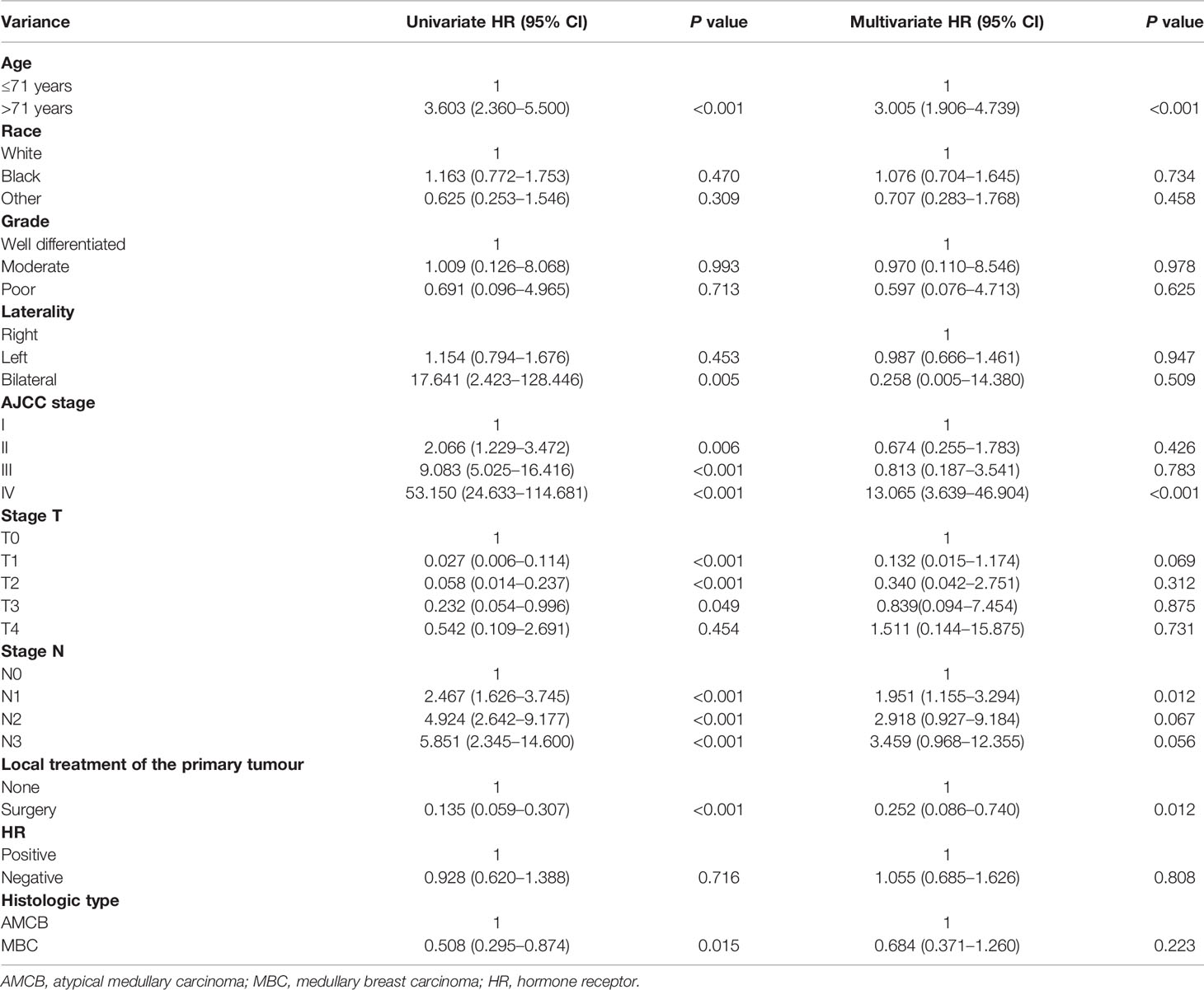
Table 2 Univariate and multivariate Cox proportional hazard analyses of the association of clinical characteristics with overall survival rates in patients with AMCB and MBC.
PSM analysis was performed to adjust for the unmatching cohort, and a total of 147 AMCB patients were matched with 147 MBC patients (1:1) (Table 3). According to univariate and multivariate analyses, as shown in Table 4, compared with MBC, histological type of AMCB was identified as an independent risk factor (HR = 4.767, 95% CI 3.408–6.669).
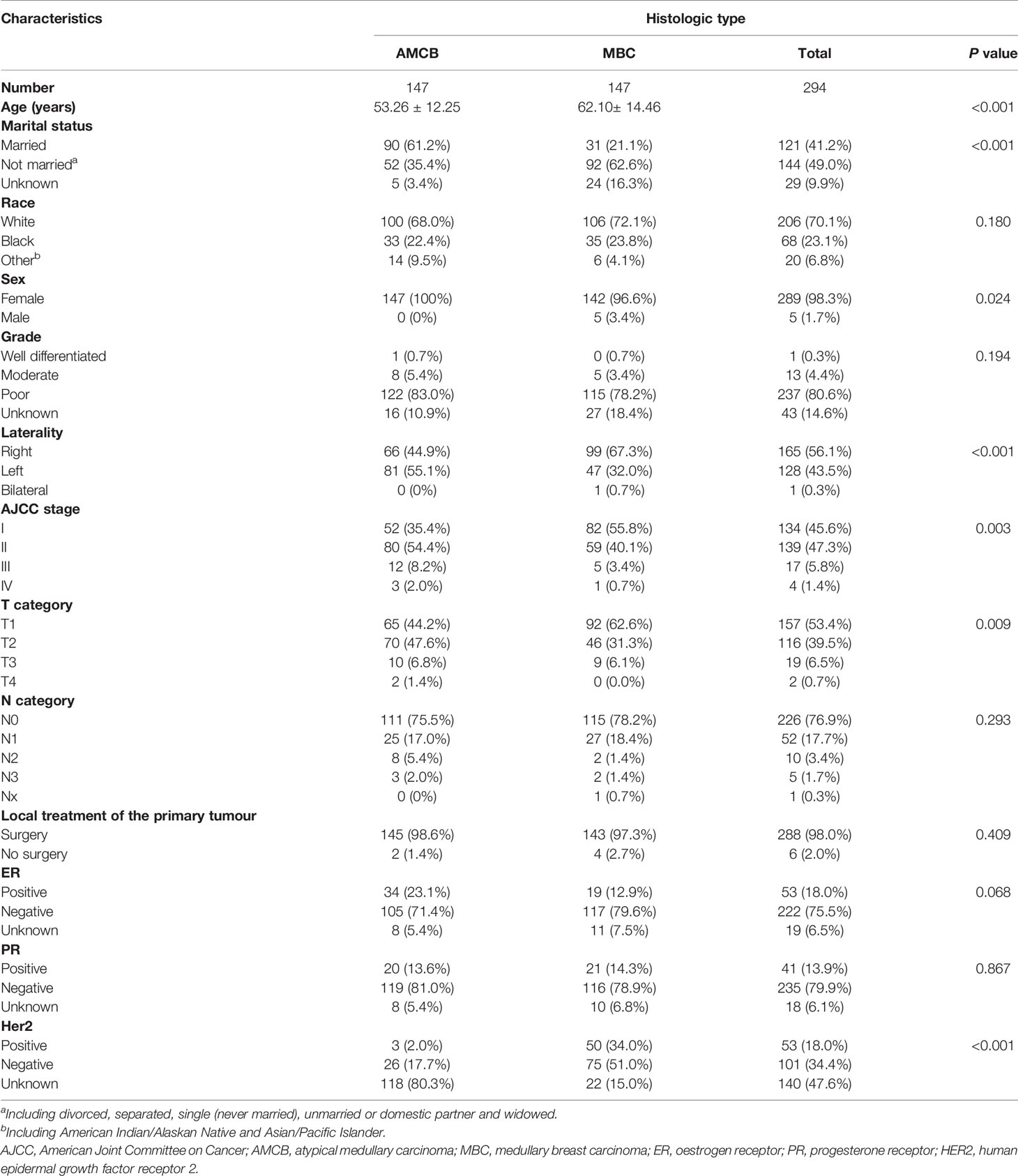
Table 3 Patient characteristics with histologic type of breast cancer after propensity score matching in the SEER database, 2004–2013.
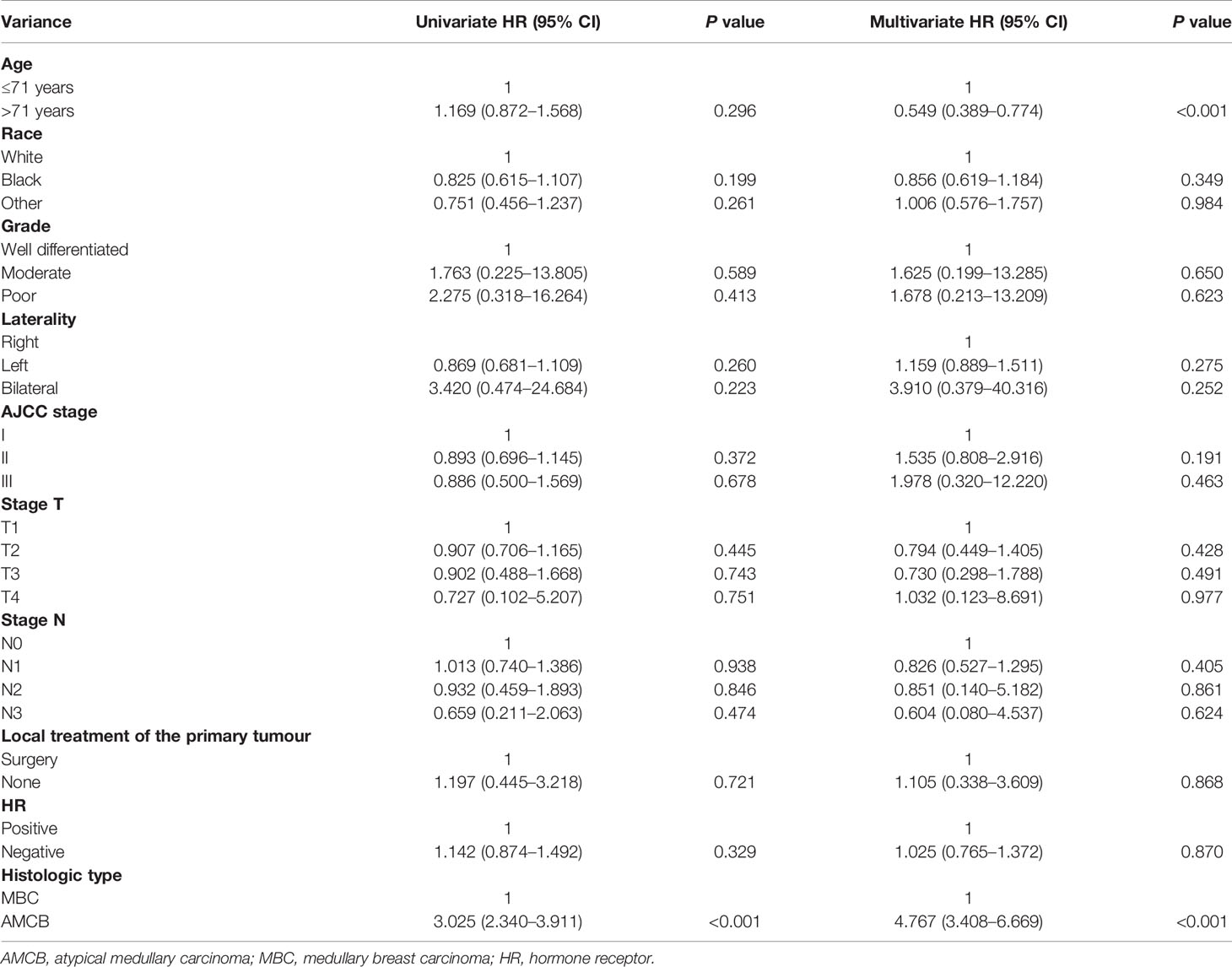
Table 4 Univariate and multivariate Cox proportional hazard analyses of the association of clinical characteristics with overall survival rates in patients with AMCB and MBC after propensity score matching.
Baseline Characteristics and Survival Benefits in the Hormone Receptor Subgroups
We analyzed the characteristics of the patients belonging to the HR-negative subgroup, which included 1,291 MBC and 102 AMCB patients (Table 5). The X-tile analysis revealed that the best possible cut-off value to assess prognostic factors for age was 71 years. The results were consistent with the entire population, and the comparison of MBC and AMCB showed no significant differences in sex, race, age, AJCC stage, degree of differentiation, tumour size, lymph node metastasis, or surgical method. The results of the Kaplan–Meier estimator and the Cox regression including univariate and multivariate analyses showed that later stage, older age, and histological type of AMCB were independently related to shortened OS, while surgery was independently related to prolonged overall survival (P <0.01, Figure 3 and Table 6). The MBC group showed a better survival benefit than the AMCB group, with a lower hazard ratio of 0.38 (95% CI, 0.198–0.734, P = 0.004).
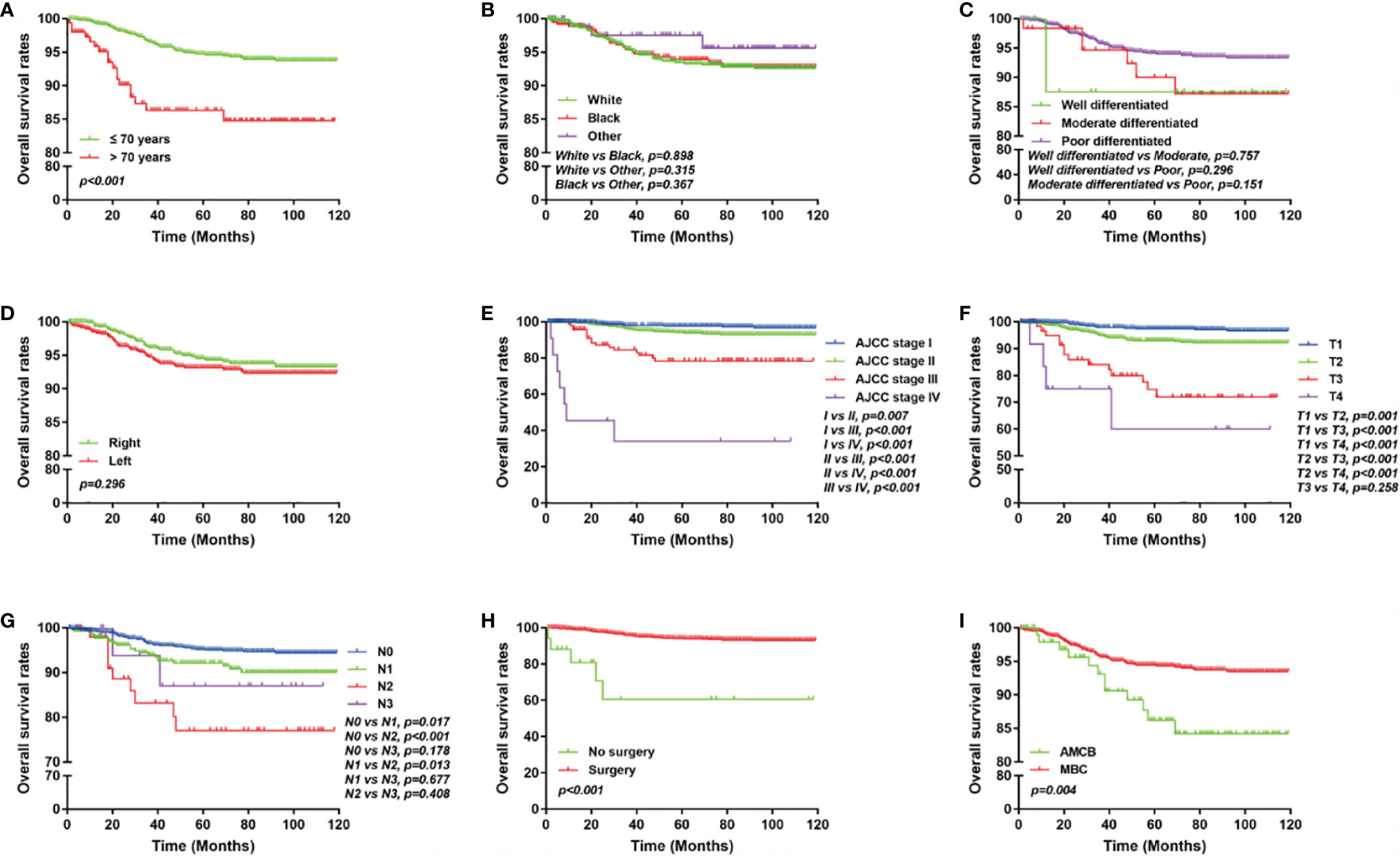
Figure 3 Overall survival of MCB and AMCB patients using the Kaplan–Meier estimator with HR negativity stratified by (A) age at diagnosis; (B) race; (C) grade; (D) laterality; (E) AJCC stage; (F) primary tumour size; (G) lymph node status; (H) surgery for primary tumour; (I) and histological type.
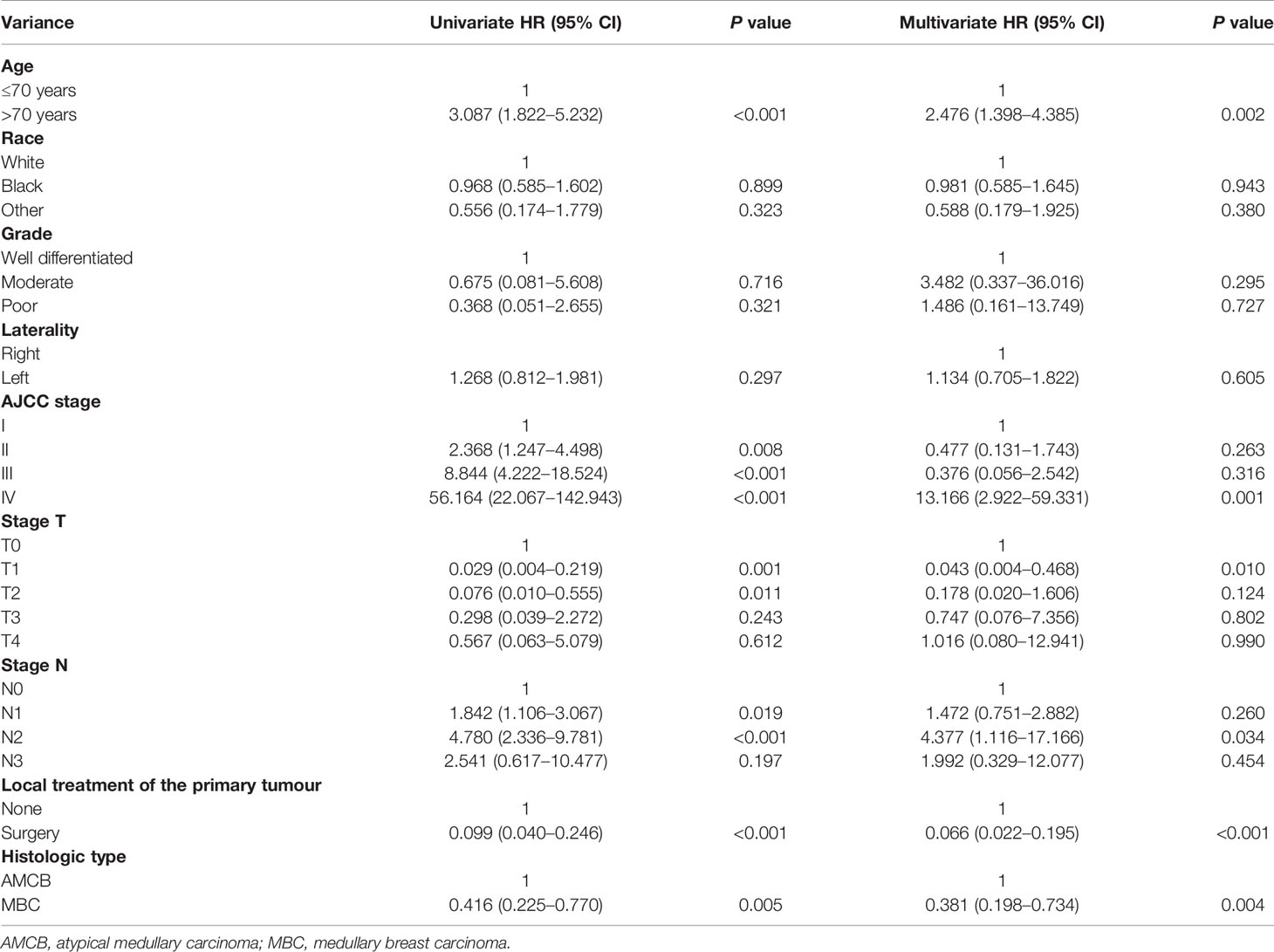
Table 6 Univariate and multivariate Cox proportional hazard analyses of the association of clinical characteristics with overall survival rates in HR (−) patients with AMCB and MBC.
Prognostic Nomogram for AMCB and MBC Patients With Hormone Receptor Negativity
A nomogram based on the prognostic factors that combined all significant independent variables for overall survival in the MBC and AMCB groups with HR negativity is shown in Figure 4. All patients were divided into three groups based on their age using the X-tile program, and the probabilities of 1-, 3-, or 5-year overall survival were determined. The optimal cut-points were 54 and 70 years. To determine the survival of MBC and AMCB patients more accurately with HR negativity, a nomogram based on the prognostic factors that included all significant independent variables in a multivariate Cox analysis was created (Table 4). Univariate and multivariate Cox analyses were used to calculate the association of the overall survival rate with the clinical characteristics of HR− patients with MBC and AMCB. The C-index for overall survival was found to be 0.75 (95% CI 0.69–0.81). As shown in Figures 4B–D, the actual observation and the prediction by the nomogram displayed optimal agreement based on the calibration plot for the probability of overall survival at 1, 3, or 5 years.
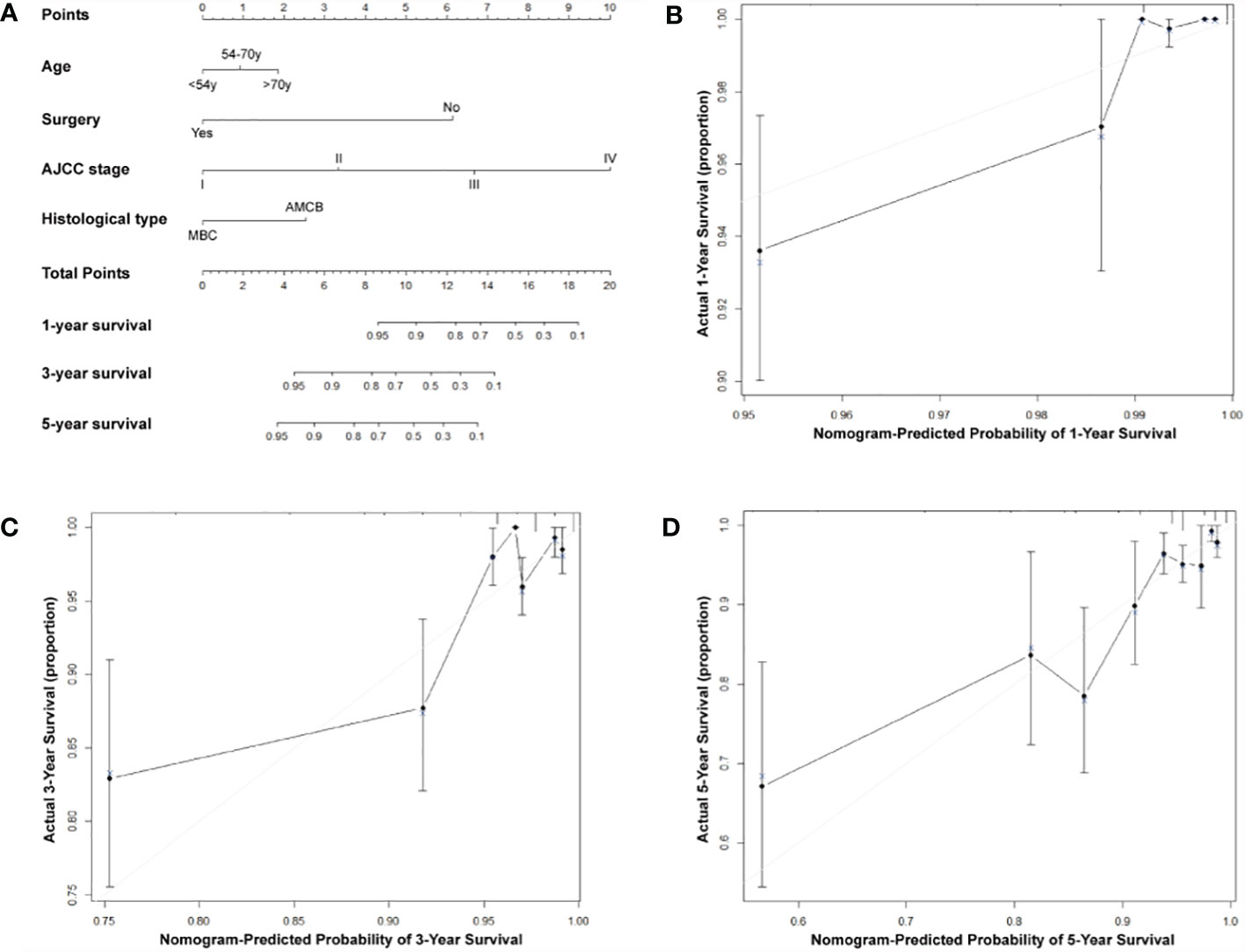
Figure 4 Predictive nomogram assessed by clinical characteristics for 1-, 3-, and 5-year overall survival in MBC and AMCB patients who were HR-negative. (A) The probability of 1-, 3-, or 5-year overall survival was estimated by connecting the probability scale to the total point scale through a vertical line. The calibration curves for overall survival at 1 year (B), 3 years (C), and 5 years (D) are shown.
Discussion
Medullary carcinoma is a distinct subgroup of breast cancers that makes up less than 5% of all advanced breast cancers. It has been considered that medullary breast carcinoma has a better prognosis than other common subtypes of histological breast cancer (10). In 1977, Ridolfi clarified the definition of MBC and proposed clear diagnostic criteria (six basic criteria) for the identification and diagnosis of medullary carcinoma (11). AMCB was defined to satisfy the first criterion of Ridolfi but not the remaining criteria. Through these precise diagnostic criteria, some institutions have reclassified breast medullary carcinoma (12) according to histopathological measures. Hormone receptors such as ER and PR are predictive prognostic factors and can serve as the foundations of a patient’s treatment for breast cancer. In previous studies, MBC was observed to have the lowest frequencies of ER, PR, and Her/neu-2 expression (13). ER positivity is infrequent in MBC, which is why previous studies reported only 547 patients (16.3%) with ER+ tumours. A positive ER in MBC patients is accompanied by the worst overall survival (5). Pinto et al. found no survival difference in patients with ER+/Her2/neu+ carcinomas and ER−/Her2/neu− carcinomas, indicating resistance to hormone therapy (14). Identification and characterization of prognostic factors for MBC patients will help in dictating diagnosis and suggesting more applicable treatments. In the current study, we tried to assess the clinicopathological features and prognostic factors of MBC and AMCB using data from patients in the SEER database from 2004 to 2013. MBC has many prognostic variables in common with ductal carcinoma infiltration. They both involve increasing age, the growing size of the tumour, metastasis at the lymph node, and the existence of cancer cells in distant lymph nodes, all of which shorten overall survival. Previous studies have identified numerous distinctive factors that are exclusively prognostic of survival in MBC (7, 15). Several factors, such as age, marital status, tumour size, stage, lymph node status, subtype of breast cancer, and radiation therapy, were substantially linked to overall survival in MBC (5). Our findings suggest that AMCB has a worse prognosis than MBC. Importantly, according to multivariate regression analysis, the prognosis of AMCB has close associations with age, stage, tumour size, surgical type, and hormone receptor positivity. In our research, the ratio of high-grade (grade III/IV) AMCB was 83.0%. Therefore, AMCB was considered a more aggressive tumour and was predominantly determined to be a high-grade tumour, similar to other studies (16). Moreover, the prevalence rate of HR-negative AMCB was 81.0%, which was more prevalent than HR-positive AMCB (13.6%), similar to other studies (17). According to further stratification studies, patients with hormone receptor-positive AMCB and MBC showed similar OS. In hormone receptor-negative patients, the prognosis of AMCB was significantly worse than that of MBC (data not shown). It was shown that the hormone receptor can be a clear independent prognostic factor for AMCB. It is important to realize that the clinicopathological features and prognostic factors of AMCB can serve as a reference for patients to provide more accurate clinical treatment.
Reported studies have indicated that the prognosis of MBC is better than that of IDC. Huober et al. found that the overall survival rates and 14-year distant recurrence-free intervals for invasive ductal tumours and medullary tumours of the full cohort were 57, 66, 64 and 76%, respectively (16). Similar findings were also found in the report of Dongjun Dai et al. (18). However, the results of the AMCB comparison between MBC and IDC were different. MBC occurs in patients of younger age; however, there was variation in the results of previous studies (5, 6, 19). Ethnic variations may play a role in such differences. Compared with patients who had an atypical characteristic, Rakha et al. (20) achieved better survival rates in patients with the typical characteristics of MBC. However, they found that the difference was not statistically significant. Conversely, Aksoy et al. (8) found that patients with atypical MBC presented significantly superior rates of recurrence and RFS. This may be associated with a smaller number of selected cases, and these patients have less tumour growth and angiogenesis with fewer lymphocytes and mononuclear infiltration. Some studies of immunohistochemical staining and gene expression analysis revealed that MBCs expressed a substantially higher fraction of triple-negative subtypes (ER, PR, and Her2) (21, 22). Considering local invasion, IDC has a more aggressive manner than MBC, and a previous study showed that MBC expressed a higher negative rate of lymph nodes than IDC (75.0% vs 47.9%, P = 0.0014) (23). In our study, the clinical features of 2,001 patients with MBC were analysed, and hormone receptor-negative cases comprised the majority. Her2 expression status was not included in this study as a prognostic factor. This was related to the number of cases that did not report Her2 expression before the 2010 SEER database. In our research, the prognosis of hormone receptor-negative AMCB patients was significantly worse than that of MBC patients. This was similar to the study of Shokouh et al. (24) and referred to the relationship of Ki67, Her2, p53, ER, and PR status and breast carcinoma subtypes. These previous studies have confirmed the possibility that hormone receptors will be used as prognostic indicators for AMCB.
The SEER database provides extensive data on breast cancer patients, which makes our study more convenient. However, there are limits to what we are able to do using this information. It is well known that there are no Her2 expression data in the SEER database prior to 2010. Thus, we are not able to group patients according to the 2015 St. Gallen consensus breast cancer classification; we can only separate the hormone receptor status into a single stratified study of the prognosis. In addition, different treatments for breast cancer have different effects on prognosis. The SEER database lacks data about patients who have received targeted therapy, endocrine therapy, and chemotherapy. Previous studies also used the SEER database and demonstrated that MBCs had distinctive clinicopathological characteristics, such as higher grade, larger tumour size, advanced stage, younger age at diagnosis, and a higher proportion of triple-negative breast cancer (7). The SEER database is more convenient for a specific pathologic review of specimens than for histological diagnosis.
In conclusion, the Cox analysis of multivariate and Kaplan–Meier analyses revealed that the prognostic factors of AMCB were worse than those of MBC in the hormone receptor-negative cohort. The prognostic factors were related to age, stage, and cancer-directed surgery. Finally, a novel nomogram based on the prognostic factors that combined all significant independent variables was established for the overall survival of AMCB patients and could be used to suggest a more applicable treatment.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/data/.
Ethics Statement
Our study was approved by the Ethics Committee of Changzheng Hospital. All patients provided written informed consent prior to enrolment in the study.
Author Contributions
WQ, FQ, and MG participated in the conception and design of the study. FQ, LZW, and Y-SZ performed the statistical and bioinformatics analyses. WQ, FQ, MG, and Y-SZ coordinated, drafted, revised and finalized the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
SEER database, Surveillance, Epidemiology and End Results database; AJCC, American Joint Committee on Cancer; AMCB, atypical medullary carcinoma of the breast (MBC); MBC, medullary carcinoma of the breast; ER, estrogen receptor; PR, progesterone receptor; Her2, human epidermal growth factor receptor 2.
References
1. Perkins GH, Green MC, Middleton LP, Gior da no SH, Garcia SM, Strom EA, et al. Hortobagyi, Medullary Breast Carcinoma: Outcomes and Prognosis With the Utilization of Chemotherapy. J Clin Oncol (2004) 22:671–1. doi: 10.3816/CLM.2004.n.025
2. Pavoni E, Vaccaro P, Pucci A, Monteriù G, Beghetto E, Barca S, et al. Minenkova, Identification of a Panel of Tumor-Associated Antigens From Breast Carcinoma Cell Lines, Solid Tumors and Testis cDNA Libraries Displayed on Lambda Phage. BMC Cancer (2004) 4:78. doi: 10.1186/1471-2407-4-78
3. Mateo AM, Pezzi TA, Sundermeyer M, Kelley CA, Klimberg VS, Pezzi CM. Atypical Medullary Carcinoma of the Breast Has Similar Prognostic Factors and Survival to Typical Medullary Breast Carcinoma: 3,976 Cases From the National Cancer Data Base. J Surg Oncol (2006) 114:533–6. doi: 10.1002/jso.24367
4. Malyuchik SS, Kiyamova RG. Medullary Breast Carcinoma. Exp Oncol (2008) 30:96–101. doi: 10.1089/153685903321948067
5. Wang XX, Jiang YZ, Liu XY, Li JJ, Song CG, Shao ZM. Difference in Characteristics and Outcomes Between Medullary Breast Carcinoma and Invasive Ductal Carcinoma: A Population Based Study From SEER 18 Database. Oncotarget (2016) 7:22665–73. doi: 10.18632/oncotarget.8142
6. Park I, Kim J, Kim M, Bae SY, Lee SK, Kil WH, et al. Comparison of the Characteristics of Medullary Breast Carcinoma and Invasive Ductal Carcinoma. J Breast Cancer (2013) 16:417–25. doi: 10.4048/jbc.2013.16.4.417
7. Martinez SR, Beal SH, Canter RJ, Chen SL, Khatri VP, Bold RJ. Medullary Carcinoma of the Breast: A Population-Based Perspective. Med Oncol (2011) 28:738–44. doi: 10.1007/s12032-010-9526-z
8. Aksoy A, Odabas H, Kaya S, Bozkurt O, Degirmenci M, Topcu TO, et al. Hormone Receptor Status and Survival of Medullary Breast Cancer Patients. A Turkish Cohort. Saudi Med J (2017) 38:156–62. doi: 10.15537/smj.2017.2.18055
9. Mitchell AL, Gandhi A, Scott-Coombes D, Perros P. Management of Thyroid Cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol (2016) 130(S2):S150–60. doi: 10.1017/S0022215116000578
10. Moore OS Jr., Foote FW Jr. The Relatively Favorable Prognosis of Medullary Carcinoma of the Breast. Cancer (1949) 2:635–42. doi: 10.1002/1097-0142(194907)2:43.0.CO;2-Q
11. Ridolfi RL, Rosen PP, Port A, Kinne D, Miké V. Medullary Carcinoma of the Breast: A Clinicopathologic Study With 10 Year Follow-Up. Cancer (1977) 40:1365–85. doi: 10.1002/1097-0142(197710)40:4<1365::aid-cncr2820400402>3.0.co;2-n
12. Bae SY, Lee SK, Koo MY, Hur SM, Choi MY, Cho DH, et al. The Prognoses of Metaplastic Breast Cancer Patients Compared to Those of Triple-Negative Breast Cancer Patients. Breast Cancer Res Treat (2011) 126:471–8. doi: 10.1007/s10549-011-1359-8
13. Romaniuk A, Lyndin M, Sikora V, Lyndina Y, Panasovska K. Histological and Immunohistochemical Features of Medullary Breast Cancer. Folia Med Cracov (2015) 55:41–8. doi: 10.1007/s10549-011-1359-8
14. Pinto AE, André S, Pereira T, Nóbrega S, Soares J. C-erbB-2 Oncoprotein Overexpression Identifies a Subgroup of Estrogen Receptor Positive (ER+) Breast Cancer Patients With Poor Prognosis. Ann Oncol (2001) 12:525–33. doi: 10.1007/BF00617471
15. Kleer CG. Carcinoma of the Breast With Medullary-Like Features: Diagnostic Challenges and Relationship With BRCA1 and EZH2 Functions. Arch Pathol Lab Med (2009) 133:1822–5. doi: 10.1074/jbc.M511097200
16. Huober J, Gelber S, Goldhirsch A, Coates AS, Viale G, Öhlschlegel C, et al. Prognosis of Medullary Breast Cancer: Analysis of 13 International Breast Cancer Study Group (IBCSG) Trials. Ann Oncol (2012) 23:2843–51. doi: 10.1093/annonc/mds105
17. Hashmi AA, Edhi MM, Naqvi H, Faridi N, Khurshid A, Khan M. Clinicopathologic Features of Triple Negative Breast Cancers: An Experience From Pakistan. Diagn Pathol (2014) 9:43. doi: 10.1186/1746-1596-9-43
18. Dai D, Shi R, Wang Z, Zhong Y, Shin VY, Jin H, et al. Competing Risk Analyses of Medullary Carcinoma of Breast in Comparison to Infiltrating Ductal Carcinoma. Sci Rep (2020) 10(1):560. doi: 10.1038/s41598-019-57168-2
19. Zangouri VMD, Akrami MMD, Tahmasebi SMD, Talei AMD, Ghaeini AMD. Hesarooeih, Medullary Breast Carcinoma and Invasive Ductal Carcinoma: A Review Study. Iran J Med Sci (2018) 43:365–71. doi: 10.21859/mci-supp-100
20. Rakha EA, Aleskandarany M, El-Sayed ME, Blamey RW, Elston CW, Ellis IO, et al. The Prognostic Significance of Inflammation and Medullary Histological Type in Invasive Carcinoma of the Breast. Eur J Cancer (Oxford Engl 1990) (2009) 45:1780–7. doi: 10.1016/j.ejca.2009.02.014
21. Cao AY, He M, Huang L, Shao ZM, Di GH. Clinicopathologic Characteristics at Diagnosis and the Survival of Patients With Medullary Breast Carcinoma in China: A Comparison With Infiltrating Ductal Carcinoma-Not Otherwise Specified. World J Surg Oncol (2013) 11:91–1. doi: 10.1186/1477-7819-11-91
22. Bertucci F, Finetti P, Cervera N, Charafe-Jauffret E, Mamessier E, Adélaïde J, et al. Gene Expression Profiling Shows Medullary Breast Cancer Is a Subgroup of Basal Breast Cancers. Cancer Res (2006) 66:4636–44. doi: 10.1158/0008-5472.CAN-06-0031
23. Flucke U, Flucke MT, Hoy L, Breuer E, Goebbels R, Rhiem K, et al. Distinguishing Medullary Carcinoma of the Breast From High-Grade Hormone Receptor-Negative Invasive Ductal Carcinoma: An Immunohistochemical Approach. Histopathology (2010) 56:852–9. doi: 10.1111/j.1365-2559.2010.03555.x
24. Shokouh TZ, Ezatollah A, Barand P. Interrelationships Between Ki67, HER2/neu, P53, ER, and PR Status and Their Associations With Tumor Grade and Lymph Node Involvement in Breast Carcinoma Subtypes: Retrospective-Observational Analytical Study. Medicine (Baltimore) (2015) 94:e1359–9. doi: 10.1097/MD.0000000000001359
Keywords: hormone receptor status, breast cancer, medullary carcinoma, nomogram, atypical medullary carcinoma of the breast
Citation: Qin WX, Qi F, Guo M, Wang L and Zang Y-S (2021) Hormone Receptor Status May Impact the Survival Benefit Between Medullary Breast Carcinoma and Atypical Medullary Carcinoma of the Breast: A Population-Based Study. Front. Oncol. 11:677207. doi: 10.3389/fonc.2021.677207
Received: 07 March 2021; Accepted: 17 June 2021;
Published: 06 July 2021.
Edited by:
Pu Li, Shanghai Jiao Tong University, ChinaReviewed by:
Yeon Hee Park, Sungkyunkwan University, South KoreaMasoud Sadeghi, Kermanshah University of Medical Sciences, Iran
Jia-Yi Chen, Shanghai Jiao Tong University, China
Copyright © 2021 Qin, Qi, Guo, Wang and Zang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan-Sheng Zang, ZG9jdG9yemFuZ3lzQDE2My5jb20=
†These authors have contributed equally to this work
 Wenxing Qin1†
Wenxing Qin1† Yuan-Sheng Zang
Yuan-Sheng Zang