
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 01 July 2021
Sec. Breast Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.675955
This article is part of the Research Topic Insights in Breast Cancer: 2021 View all 13 articles
 Zhen-Yu Wu1,2
Zhen-Yu Wu1,2 Hee Jeong Kim2
Hee Jeong Kim2 Jong Won Lee2
Jong Won Lee2 Il Yong Chung2
Il Yong Chung2 Jisun Kim2
Jisun Kim2 Sae Byul Lee2
Sae Byul Lee2 Byung-Ho Son2
Byung-Ho Son2 Jin Sup Eom3
Jin Sup Eom3 Jae Ho Jeong4
Jae Ho Jeong4 Gyungyub Gong5
Gyungyub Gong5 Hak Hee Kim6
Hak Hee Kim6 Sei-Hyun Ahn2
Sei-Hyun Ahn2 BeomSeok Ko2*
BeomSeok Ko2*Background: Few data are available on the risk factors of locoregional recurrence (LRR) after neoadjuvant chemotherapy (NACT) and immediate breast reconstruction (IBR) in breast cancer. Herein, we evaluated the factors predicting LRR in a large series of patients who underwent either nipple- (NSM) or skin-sparing mastectomy (SSM) with IBR after NACT.
Methods: We retrospectively analyzed 609 breast cancer patients who underwent NACT and NSM/SSM with IBR between February 2010 and June 2017. Factors associated with an increased risk of LRR were analyzed by univariate (chi-square or Fisher’s exact test) and multivariate (Cox proportional hazard regression model) analyses.
Results: During a median follow-up of 63 months, LRR as the first event occurred in 73 patients, and the 5-year cumulative LRR rate was 10.8%. Multivariate analysis revealed post-NACT Ki67 ≥ 10% [hazard ratio (HR), 2.208; 95% confidence interval (CI), 1.295-3.765; P = 0.004], high tumor grade (HR, 1.738; 95% CI, 1.038-2.908; P = 0.035), and presence of lymphovascular invasion (LVI) (HR, 1.725; 95% CI, 1.039-2.864; P = 0.035) as independently associated with increased LRR risk. The 10-year LRR rate was 8.5% for patients with none of the three associated risk factors, 11.6% with one factor, 25.1% with two factors, and 33.7% with all three factors (P < 0.001).
Conclusions: Post-NACT Ki67 ≥ 10%, high tumor grade, and presence of LVI are independently associated with an increased risk of developing LRR after NACT and NSM/SSM with IBR. Future prospective trials are warranted to decrease the risk of LRR in patients with associated risk factors.
Neoadjuvant chemotherapy (NACT) has been established as the standard of care for locally advanced breast cancer and is now being used more often as a treatment in early-stage breast cancer (1). NACT aims to increase the rate of breast conservation; however, a large proportion of patients receiving NACT undergo mastectomy as the surgical treatment, either because breast-conserving surgery is not feasible or because of patient preference. Over the last decade, patients have begun to prefer nipple- (NSM) or skin-sparing mastectomy (SSM) combined with immediate breast reconstruction (IBR) in the treatment of breast cancer, as it provides improved aesthetic results and quality of life (2, 3). Several non-randomized studies have demonstrated that the oncologic outcomes of NSM/SSM with IBR are comparable to those of conventional mastectomy alone (4–6). Recently, NSM/SSM with IBR has also been performed in patients who receive NACT; however, data related to the long-term safety of such treatments in this patient population are still insufficient (7). In addition, locoregional recurrence (LRR) following NSM/SSM with IBR remains clinically challenging, not only because it may indicate poor prognosis (8), but also because the oncologic management of LRR may lead to loss of the initial reconstruction (9). In patients who receive NACT and breast reconstruction, the predictive value of clinicopathologic features or treatment-associated factors for LRR is unclear due to a lack of data.
In this study, we aimed to identify the factors associated with an increased risk of LRR in a large series of breast cancer patients who underwent NSM/SSM with IBR after NACT.
This study was approved by the institutional review board (IRB) of Asan Medical Center, Seoul, Republic of Korea (No. 2017-1341). This study is a retrospective study conducted with the exemption of consent under IRB deliberation using a platform for extracting unidentified clinical information for research purposes. The medical records of all patients who underwent IBR with NSM/SSM after NACT for primary breast cancer between January 2010 and June 2017 at the Asan Medical Center, Seoul, Republic of Korea, were reviewed from a prospectively maintained database. Patients presenting with inflammatory breast cancer or synchronous distant metastasis were excluded. Patient and tumor characteristics were collected and analyzed, including age at diagnosis, tumor stage, grade, molecular subtype, histotype, lymphovascular invasion (LVI) status, presence of extensive intraductal component, post-NACT Ki67 status, and pathological multifocality/multicentricity. Tumor staging was conducted according to the 8th American Joint Committee on Cancer Staging Manual (10). Pathological complete response (pCR) was defined as no evidence of invasive cancer in the breast or axillary lymph nodes.
All patients included in this study received NACT after breast cancer diagnosis. The NACT regimens were selected at the discretion of the treating oncologist. NSM/SSM was performed by breast surgeons, and IBR was performed by plastic surgeons using autologous flaps or implants. NSM or SSM was performed according to the indications of conventional mastectomy, regardless of tumor size or tumor-to-nipple distance, as long as there was no evidence of tumor involvement in the breast skin and nipple-areola complex, clinically or on imaging. In cases of NSM, retroareolar frozen-section biopsy specimens were collected and examined intraoperatively. The nipple-areola complex was preserved if the shape, color, and palpated features of the nipple were normal, and if the nipple margin was confirmed to be tumor free on frozen-section biopsy. In cases in which the retroareolar tissue was positive for malignancy in the frozen section or permanent biopsy, the nipple with or without the areola was removed, and these cases were considered SSM. The decision to undergo adjuvant radiotherapy was made by the treating radiation oncologist after consideration of pre- and post-NACT disease stages, tumor response to NACT, and other tumor biomarkers in patients. Most patients who required adjuvant radiotherapy after evaluation underwent simultaneous irradiation of the chest wall and supraclavicular region. Adjuvant hormonal therapy was applied in patients with hormone receptor-positive disease.
Postoperatively, patients were regularly followed up every 3–6 months for the first 5 years and annually thereafter. Recurrence and metastasis were identified based on the results of the clinical examination, chest radiography, and tumor marker (CA15–3) measurements, which were taken every follow-up visit. In some cases, abnormal clinical findings were further evaluated using chest computed tomography (CT), a bone scan, ultrasonography, and/or positron emission tomography-CT. In patients suspected of LRR, fine needle aspiration, core needle, or excisional biopsy was performed for pathological confirmation. Lesions with clear evidence of distant metastasis on imaging evaluation were considered as recurrence without pathological examination.
LRRs were classified as local or regional recurrence. Local recurrence was defined as biopsy-proven recurrences in the ipsilateral skin/subcutaneous layer, chest wall, or nipple-areola complex, and regional recurrence was defined as carcinoma metastases in the ipsilateral axillary, supraclavicular, or internal mammary lymph node. Any other site of recurrence was considered distant metastasis. Patients with initial distant metastasis were excluded from the LRR group. In cases of concurrent LRR and distant metastasis, each recurrence was counted as an event. Occurrence of contralateral breast cancer was considered a new primary cancer and was not counted as a recurrence. Follow-up was calculated from the date of diagnosis.
The 5- and 10-year cumulative LRR rates were calculated using the Kaplan-Meier method and compared using the log-rank test between subgroups. The clinicopathological factors that were significant in univariate analyses (Chi-square or Fisher’s exact test) of LRR were included in the multivariate analysis using the Cox proportional hazards regression model. All statistical analyses were performed using IBM SPSS Statistics software version 24.0 for Windows (IBM Corp., Armonk, NY, USA). Two-tailed P-values < 0.05 were considered significant.
A total of 609 patients who underwent NACT and IBR with NSM/SSM for primary breast cancer were included. Patient, tumor, and treatment characteristics are shown in Table 1.
The median age at diagnosis was 42 years (range, 23-72 years). The majority (89.7%) of patients received anthracycline-based (with or without taxane) NACT. NSM was performed in 370 (60.8%) patients and SSM in 239 (39.2%). Four hundred and twenty (69%) patients underwent autologous flap reconstruction, and 189 (31%) patients underwent implant-based reconstruction. Adjuvant radiotherapy was administrated in 316 (51.9%) patients. Among the 223 patients with human epidermal growth factor receptor 2 (HER2)-positive disease, 219 (98.2%) received adjuvant trastuzumab. On follow-up, pCR was observed in 79 (13%) patients.
The median follow-up period was 63 months (range, 11-135 months). LRR as the first event occurred in 73 patients, and the 5-year cumulative LRR rate was 10.8%. Among these, isolated LRR occurred in 55 patients (75.3%) and concurrent LRR with distant metastasis occurred in 18 (24.7%). Table 2 summarizes the oncologic outcomes of the entire cohort. The median time to LRR was 35 months (range, 7-76 months). Patients with isolated LRR as the first event showed a significantly lower 10-year overall survival rate than those without LRR (64.7% vs. 90.2%; log-rank P = 0.035). Table 3 shows the incidence rates of LRR according to various clinicopathological and treatment factors. The following factors were significantly associated with increased rates of LRR in the univariate analysis: age at diagnosis ≤ 40 years, pathological T stage, pathological nodal status, pCR status, tumor grade, LVI, and post-NACT Ki67 status. Of these, post-NACT Ki67 ≥ 10% [hazard ratio (HR), 2.208; 95% confidence interval (CI), 1.295-3.765; P = 0.004], high tumor grade (HR, 1.738; 95% CI, 1.038-2.908; P = 0.035), and presence of LVI (HR, 1.725; 95% CI, 1.039-2.864; P = 0.035) were independently associated with reduced LRR-free survival in the multivariate analysis (Table 4).
Figure 1 shows the Kaplan-Meier curves for LRR risk, according to the number of independent risk factors. The 10-year rate of LRR was 8.5% for patients with none of the three independent risk factors (n = 197, 32.3%), 11.6% for those with one risk factor (n = 226, 37.1%), 25.1% for those with two risk factors (n = 144, 23.6%), and 33.7% for those with all three risk factors (n = 42, 6.9%; log-rank P < 0.001).
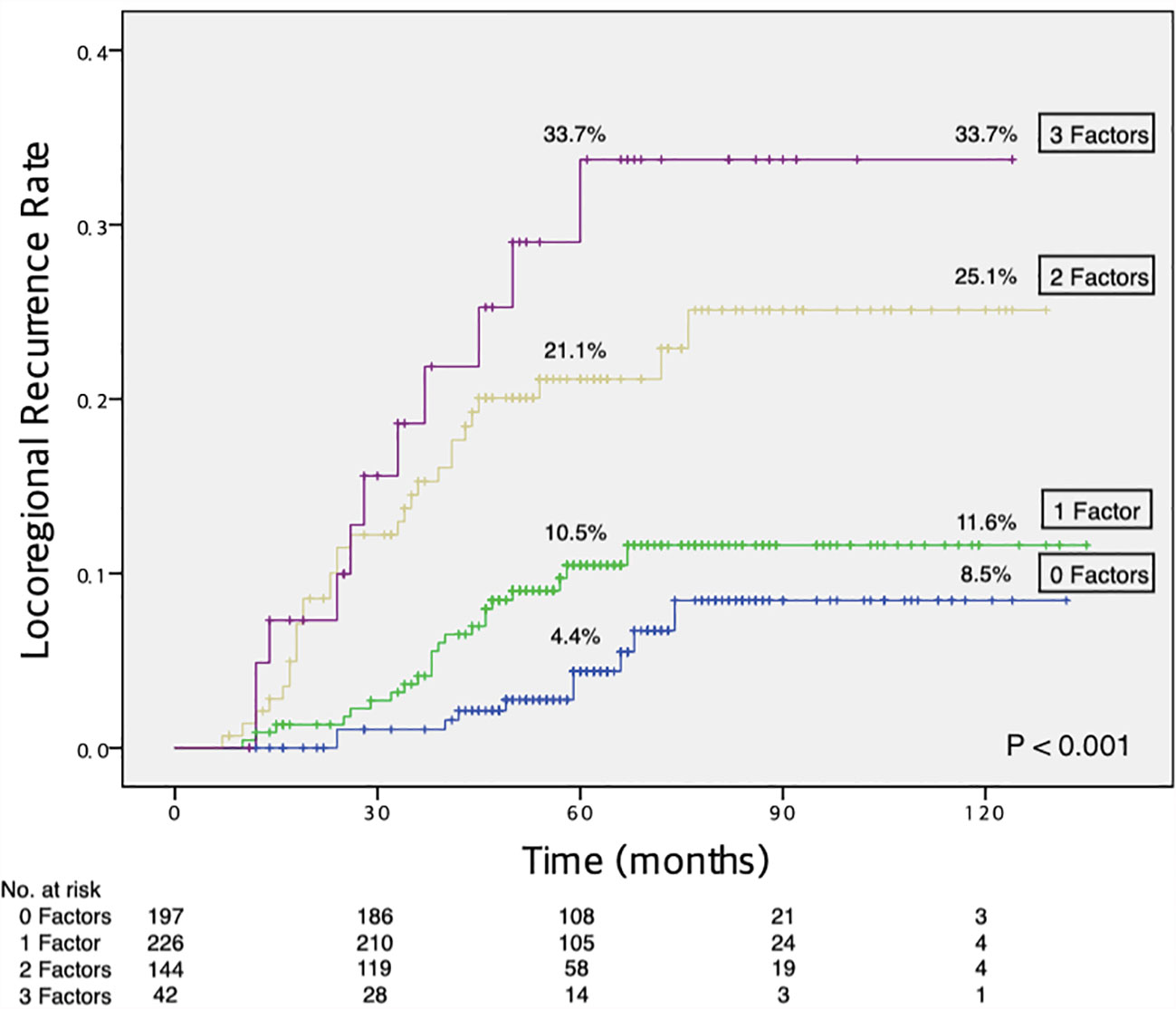
Figure 1 Increased risk of LRR with an increasing number of independent risk factors. LRR, locoregional recurrence.
Although previous studies have investigated predictive factors of LRR after NACT in conventional mastectomy or breast-conserving surgery (11–15), little data regarding the risk factors of LRR after NACT for NSM/SSM with IBR exists. In this study, we identified the 5-year LRR rate (10.8%) and factors predicting LRR in breast cancer patients who underwent NSM/SSM with IBR after receiving NACT. Post-NACT Ki67 ≥ 10%, high tumor grade, and presence of LVI were independent risk factors for LRR in the current setting. Notably, the 10-year LRR rate reached 33.7% in patients with all three risk factors and was 8.5% in patients with none of these factors.
NSM/SSM with IBR has become an important surgical strategy in modern breast cancer care. This surgical procedure, particularly NSM with IBR, can provide significantly improved aesthetic results, patient satisfaction, and/or psychosocial/sexual well-being (2, 3, 16). A recent analysis from the National Cancer Database of the American College of Surgeons and the American Cancer Society showed an increasing trend toward the application of NSM in patients with advanced disease, particularly in those who received NACT, and highlighted the importance of further prospective trials to validate the evidence of oncologic safety of this procedure (7). The current National Comprehensive Cancer Network (NCCN) guidelines recommend that NSM/SSM should be performed by an experienced breast surgery team working in a multidisciplinary fashion, according to specific clinical features and selected criteria (17). In case of NSM, NCCN guidelines include some cases of locally advanced invasive breast cancers, provided there is complete clinical response after NACT and no nipple involvement. Furthermore, assessment of nipple margin during surgery is mandatory (17). Several studies have reported on the feasibility of this approach in patients who receive NACT, and the LRR rates ranged between 3.2% and 10.3% (18–22). However, the majority of the studies involved a relatively small sample size and short follow-up durations. In the current study, with a median follow-up of 63 months, we found a 5-year cumulative LRR rate of 10.8% for the entire cohort. The LRR rate of our cohort appears acceptable in consideration of the previously reported LRR rates, which ranged from 6.0% to 21.0% after NACT and mastectomy with or without reconstruction (14, 23–26).
The occurrence of breast cancer LRR is an important determinant of adverse survival outcomes (8, 27–29). In our study, isolated LRR as the first event in patients who underwent NSM/SSM with IBR after NACT was associated with a poor 10-year overall survival rate. In addition, patients with isolated LRR often required oncologic management, including surgical excision of the recurrent tumor, which could result in loss of the initial reconstruction (9). Therefore, identifying risk factors for LRR in the current setting is imperative for optimal locoregional management and patient surveillance strategies. However, investigating risk factors for recurrence after NACT remains a challenge because of the high frequency of inconsistent disease status in patients between before and after neoadjuvant treatment. Previous studies have described several clinical and pathological factors of LRR after NACT. The National Surgical Adjuvant Breast and Bowel Project (NSABP) study, including NSABP B-18 and NSABP B-27 data, identified that young age (< 50 years), clinical tumor size (> 5 cm), clinical node status (cN+), and pCR status (ypT+ or ypN+) were predictive of an increased risk of LRR after NACT in patients who underwent mastectomy and breast conservation therapy (11). The authors developed a nomogram using these factors to predict the risk of LRR and guide optimal administration of adjuvant radiotherapy (11); however, histopathological characteristics such as molecular subtype, tumor grade, LVI, and Ki67 index were not analyzed in that study (11). One study by the European Organization for Research and Treatment of Cancer 10994/BIG 1-00 revealed that triple-negative or HER2-positive subtype and lack of pathologic response were associated with increased LRR after NACT (12). However, Ki67 index, tumor grade, and LVI were not analyzed in that study (12). Our current study investigated the risk factors of LRR exclusively in patients who underwent NSM/SSM with IBR after NACT and involved several prognostic factors not included in the aforementioned studies that used prospective data. Moreover, in our multivariate analysis, post-NACT Ki67 index, tumor grade, and LVI independently influenced LRR. In our univariate analysis, factors including age at diagnosis, pathological T stage, pathological node stage, and pCR status were associated with LRR rates; however, after multivariate analysis these factors were no longer significant. Notably, the role of post-NACT Ki67, tumor grade, and LVI in LRR risk has previously been suggested in smaller retrospective studies (13–15). In a study by Yamazaki et al., 217 patients who underwent NACT and breast-conserving surgery were analyzed, and post-NACT Ki67 > 20%, triple-negative subtype, the presence of LVI, and high tumor grade were found to be significant prognostic factors of LRR (13). However, these factors were identified in a univariate analysis, and no multivariate analysis was conducted (13). In another retrospective study by Wang et al. that included 217 patients with cT1-2N0-1 who underwent NACT and mastectomy, the 5-year LRR rate was 12%, and LVI, tumor grade, and ypN stage were independent prognostic factors of LRR in multivariate analysis (14). However, no data on the Ki67 index were presented in that study (14). In a previous retrospective study including 319 NSM cases after NACT conducted at our center demonstrated that post-NACT Ki67 index was the only independent risk factor for LRR in multivariate analysis (30). Our results on factors correlated with higher LRR risk after NACT are in line with those of previous reports (13–15, 30). In addition, we quantified LRR risk according to the number of independent risk factors and found that the 10-year LRR rate was 8.5% in patients with none of the three independent risk factors, while patients with one, two, or, three of these factors had 10-year LRR rates of 11.6%, 25.1%, and 33.7%, respectively. This risk stratification of LRR may aid in selecting patients who can benefit from further investigation of locoregional management (i.e., adjuvant radiotherapy) strategies in the current setting.
The current study was limited by its retrospective, single-center design, and the study population was heterogeneous for clinicopathological and treatment characteristics. Detailed analysis of the relationship between different adjuvant radiotherapy regimens and LRR, as well as the rate of reconstruction failure, could not be conducted in this study because relevant data were not available. In addition, a relatively small number of patients and LRR events were included in certain subgroups of interest, which might have affected the statistical power of the results.
In conclusion, post-NACT Ki67 ≥ 10%, high tumor grade, and presence of LVI are independently associated with a high risk of developing LRR after NACT and NSM/SSM with IBR. Future prospective trials are warranted to decrease the risk of LRR in patients with associated risk factors.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
This study was approved by the institutional review board of Asan Medical Center, Seoul, Korea (No. 2017-1341). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Z-YW and BK: conception and design. S-HA and BK: administrative support. Z-YW, HK, JL, IC, JK, SL, B-HS, JE, JJ, GG, HK, and BK: data collection. Z-YW: data processing, analysis and manuscript writing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Early Breast Cancer Trialists’ Collaborative Group. Long-Term Outcomes for Neoadjuvant Versus Adjuvant Chemotherapy in Early Breast Cancer: Meta-Analysis of Individual Patient Data From Ten Randomised Trials. Lancet Oncol (2018) 19:27–39. doi: 10.1016/S1470-2045(17)30777-5
2. Bailey CR, Ogbuagu O, Baltodano PA, Simjee UF, Manahan MA, Cooney DS, et al. Quality-Of-Life Outcomes Improve With Nipple-Sparing Mastectomy and Breast Reconstruction. Plast Reconstr Surg (2017) 140:219–26. doi: 10.1097/PRS.0000000000003505
3. Galimberti V, Vicini E, Corso G, Morigi C, Fontana S, Sacchini V, et al. Nipple-Sparing and Skin-Sparing Mastectomy: Review of Aims, Oncological Safety and Contraindications. Breast (2017) 34 Suppl 1:S82–4. doi: 10.1016/j.breast.2017.06.034
4. Lee SB, Lee JW, Kim HJ, Ko BS, Son BH, Eom JS, et al. Long-Term Outcomes of Patients With Breast Cancer After Nipple-Sparing Mastectomy/Skin-Sparing Mastectomy Followed by Immediate Transverse Rectus Abdominis Musculocutaneous Flap Reconstruction: Comparison With Conventional Mastectomy in a Single Center Study. Med (Baltimore) (2018) 97:e0680. doi: 10.1097/MD.0000000000010680
5. Shimo A, Tsugawa K, Tsuchiya S, Yoshie R, Tsuchiya K, Uejima T, et al. Oncologic Outcomes and Technical Considerations of Nipple-Sparing Mastectomies in Breast Cancer: Experience of 425 Cases From a Single Institution. Breast Cancer (Auckl) (2016) 23:851–60. doi: 10.1007/s12282-015-0651-6
6. Adam H, Bygdeson M, de Boniface J. The Oncological Safety of Nipple-Sparing Mastectomy - a Swedish Matched Cohort Study. Eur J Surg Oncol (2014) 40:1209–15. doi: 10.1016/j.ejso.2014.07.037
7. Wong SM, Chun YS, Sagara Y, Golshan M, Erdmann-Sager J. National Patterns of Breast Reconstruction and Nipple-Sparing Mastectomy for Breast Cancer, 2005-2015. Ann Surg Oncol (2019) 26:3194–203. doi: 10.1245/s10434-019-07554-x
8. van Tienhoven G, Voogd AC, Peterse JL, Nielsen M, Andersen KW, Mignolet F, et al. Prognosis After Treatment for Loco-Regional Recurrence After Mastectomy or Breast Conserving Therapy in Two Randomised Trials (EORTC 10801 and DBCG-82tm). EORTC Breast Cancer Cooperative Group and the Danish Breast Cancer Cooperative Group. Eur J Cancer (1999) 35:32–8. doi: 10.1016/s0959-8049(98)00301-3
9. Mirzabeigi MN, Rhemtulla IA, McDonald ES, Sataloff DM, Kovach SJ, Wu LC, et al. Locoregional Cancer Recurrence After Breast Reconstruction: Detection, Management, and Secondary Reconstructive Strategies. Plast Reconstr Surg (2019) 143:1322–30. doi: 10.1097/PRS.0000000000005522
11. Mamounas EP, Anderson SJ, Dignam JJ, Bear HD, Julian TB, Geyer CE Jr., et al. Predictors of Locoregional Recurrence After Neoadjuvant Chemotherapy: Results From Combined Analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol (2012) 30:3960–6. doi: 10.1200/JCO.2011.40.8369
12. Gillon P, Touati N, Breton-Callu C, Slaets L, Cameron D, Bonnefoi H. Factors Predictive of Locoregional Recurrence Following Neoadjuvant Chemotherapy in Patients With Large Operable or Locally Advanced Breast Cancer: An Analysis of the EORTC 10994/BIG 1-00 Study. Eur J Cancer (2017) 79:226–34. doi: 10.1016/j.ejca.2017.04.012
13. Yamazaki N, Wada N, Yamauchi C, Yoneyama K. High Expression of Post-Treatment Ki-67 Status is a Risk Factor for Locoregional Recurrence Following Breast-Conserving Surgery After Neoadjuvant Chemotherapy. Eur J Surg Oncol (2015) 41:617–24. doi: 10.1016/j.ejso.2015.01.036
14. Wang X, Xu L, Yin Z, Wang D, Wang Q, Xu K, et al. Locoregional Recurrence-Associated Factors and Risk-Adapted Postmastectomy Radiotherapy for Breast Cancer Staged in Ct1-2N0-1 After Neoadjuvant Chemotherapy. Cancer Manag Res (2018) 10:4105–12. doi: 10.2147/CMAR.S173628
15. Werutsky G, Untch M, Hanusch C, Fasching PA, Blohmer JU, Seiler S, et al. Locoregional Recurrence Risk After Neoadjuvant Chemotherapy: A Pooled Analysis of Nine Prospective Neoadjuvant Breast Cancer Trials. Eur J Cancer (2020) 130:92–101. doi: 10.1016/j.ejca.2020.02.015
16. Romanoff A, Zabor EC, Stempel M, Sacchini V, Pusic A, Morrow M. A Comparison of Patient-Reported Outcomes After Nipple-Sparing Mastectomy and Conventional Mastectomy With Reconstruction. Ann Surg Oncol (2018) 25:2909–16. doi: 10.1245/s10434-018-6585-4
17. National Comprehensive Cancer Network. Breast Cancer (Version 3.2020). Available at: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (Accessed May 7, 2020).
18. Wengler CA, Valente SA, Al-Hilli Z, Woody NM, Muntean JH, Abraham J, et al. Determinants of Short and Long Term Outcomes in Patients Undergoing Immediate Breast Reconstruction Following Neoadjuvant Chemotherapy. J Surg Oncol (2017) 116:797–802. doi: 10.1002/jso.24741
19. Santoro S, Loreti A, Cavaliere F, Costarelli L, La Pinta M, Manna E, et al. Neoadjuvant Chemotherapy is Not a Contraindication for Nipple Sparing Mastectomy. Breast (2015) 24:661–6. doi: 10.1016/j.breast.2015.08.001
20. Agresti R, Sandri M, Gennaro M, Bianchi G, Maugeri I, Rampa M, et al. Evaluation of Local Oncologic Safety in Nipple-Areola Complex-Sparing Mastectomy After Primary Chemotherapy: A Propensity Score-Matched Study. Clin Breast Cancer (2017) 17:219–31. doi: 10.1016/j.clbc.2016.12.003
21. Burdge EC, Yuen J, Hardee M, Gadgil PV, Das C, Henry-Tillman R, et al. Nipple Skin-Sparing Mastectomy is Feasible for Advanced Disease. Ann Surg Oncol (2013) 20:3294–302. doi: 10.1245/s10434-013-3174-4
22. Peled AW, Wang F, Foster RD, Alvarado M, Ewing CA, Sbitany H, et al. Expanding the Indications for Total Skin-Sparing Mastectomy: Is it Safe for Patients With Locally Advanced Disease? Ann Surg Oncol (2016) 23:87–91. doi: 10.1245/s10434-015-4734-6
23. Krug D, Lederer B, Seither F, Nekljudova V, Ataseven B, Blohmer JU, et al. Post-Mastectomy Radiotherapy After Neoadjuvant Chemotherapy in Breast Cancer: A Pooled Retrospective Analysis of Three Prospective Randomized Trials. Ann Surg Oncol (2019) 26:3892–901. doi: 10.1245/s10434-019-07635-x
24. Sun Y, Liao M, He L, Zhu C. Comparison of Breast-Conserving Surgery With Mastectomy in Locally Advanced Breast Cancer After Good Response to Neoadjuvant Chemotherapy: A PRISMA-Compliant Systematic Review and Meta-Analysis. Med (Baltimore) (2017) 96:e8367. doi: 10.1097/MD.0000000000008367
25. Wright JL, Takita C, Reis IM, Zhao W, Saigal K, Wolfson A, et al. Predictors of Locoregional Outcome in Patients Receiving Neoadjuvant Therapy and Postmastectomy Radiation. Cancer (2013) 119:16–25. doi: 10.1002/cncr.27717
26. Aurilio G, Bagnardi V, Graffeo R, Nolè F, Petit JY, Locatelli M, et al. Does Immediate Breast Reconstruction After Mastectomy and Neoadjuvant Chemotherapy Influence the Outcome of Patients With non-Endocrine Responsive Breast Cancer? Anticancer Res (2014) 34:6677–83.
27. Rouzier R, Extra JM, Carton M, Falcou MC, Vincent-Salomon A, Fourquet A, et al. Primary Chemotherapy for Operable Breast Cancer: Incidence and Prognostic Significance of Ipsilateral Breast Tumor Recurrence After Breast-Conserving Surgery. J Clin Oncol (2001) 19:3828–35. doi: 10.1200/JCO.2001.19.18.3828
28. Wapnir IL, Anderson SJ, Mamounas EP, Geyer CE Jr., Jeong JH, Tan-Chiu E, et al. Prognosis After Ipsilateral Breast Tumor Recurrence and Locoregional Recurrences in Five National Surgical Adjuvant Breast and Bowel Project Node-Positive Adjuvant Breast Cancer Trials. J Clin Oncol (2006) 24:2028–37. doi: 10.1200/JCO.2005.04.3273
29. Anderson SJ, Wapnir I, Dignam JJ, Fisher B, Mamounas EP, Jeong JH, et al. Prognosis After Ipsilateral Breast Tumor Recurrence and Locoregional Recurrences in Patients Treated by Breast-Conserving Therapy in Five National Surgical Adjuvant Breast and Bowel Project Protocols of Node-Negative Breast Cancer. J Clin Oncol (2009) 27:2466–73. doi: 10.1200/JCO.2008.19.8424
Keywords: breast cancer, immediate breast reconstruction, skin-sparing mastectomy, nipple-sparing mastectomy, neoadjuvant chemotherapy, locoregional recurrence, risk factor
Citation: Wu Z-Y, Kim HJ, Lee JW, Chung IY, Kim J, Lee SB, Son B-H, Eom JS, Jeong JH, Gong G, Kim HH, Ahn S-H and Ko B (2021) Factors Predicting Locoregional Recurrence After Neoadjuvant Chemotherapy and Nipple-Sparing/Skin-Sparing Mastectomy With Immediate Breast Reconstruction. Front. Oncol. 11:675955. doi: 10.3389/fonc.2021.675955
Received: 04 March 2021; Accepted: 18 June 2021;
Published: 01 July 2021.
Edited by:
San-Gang Wu, First Affiliated Hospital of Xiamen University, ChinaReviewed by:
Gilles Houvenaeghel, Institut Paoli-Calmettes (IPC), FranceCopyright © 2021 Wu, Kim, Lee, Chung, Kim, Lee, Son, Eom, Jeong, Gong, Kim, Ahn and Ko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: BeomSeok Ko, c3Bkb2N0b3Jrb0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.