
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 27 August 2021
Sec. Thoracic Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.675354
The benefits of surgical resection for patients with stage N2 limited-disease small-cell lung cancer (LD-SCLC) remain controversial. This retrospective study analyzed the survival and recurrence patterns of the patients diagnosed with pathological N2 (p-N2) LD-SCLC after radical resection. A total of 171 p-N2 LD-SCLC patients who underwent radical pulmonary resection and systematic lymphadenectomies at Shanghai Chest Hospital from July 2005 to June 2015 were enrolled. The influence of the mediastinal lymph node status (single or multiple nodes, single- or multiple-station) on the survival and recurrence patterns was retrospectively analyzed. The main recurrence sites were outside the chest cavity (54.8%) and hematogenous metastasis (67.4%). The bone and liver as initial recurrence sites had a poor prognosis, with a median overall survival (OS) of 13.100 months and 11.900 months, respectively. The median disease-free survival (DFS) of patients diagnosed with single and multiple p-N2 after surgery were 19.233 and 9.367 months (P = 0.001), and the median OS were 43.033 and 17.100 months (P < 0.001), respectively. In conclusion, recurrence occurred in the form of hematogenous metastasis mostly in the extra-thoracic part. Interestingly, patients diagnosed with single p-N2 benefited from radical resection. Surgery may be a treatment option regardless of the T stage if N2 SCLC with a single metastatic lymph node can be identified preoperatively.
Although tobacco-control measures have led to a decline in the incidence and mortality of lung cancer over the past decades, this cancer remains to be among the leading causes of cancer mortality worldwide (1, 2). Small-cell lung cancer (SCLC) accounts for 15% of all lung cancers, with a low (7%) 5-year survival (3). The disease is characterized by rapid tumor growth and early metastasis, associated with poor prognosis (4).
Two randomized phase III studies conducted in the 1960s (5) and the 1980s (6) reported negative results with surgery in limited-disease SCLC (LD-SCLC) patients and, thereafter, surgery has been discouraged. The aforementioned studies were critical in shaping treatment recommendations for LD-SCLC. Currently, the International Guidelines highlight that surgery is justified for selected stage I (T1-2,N0M0) SCLC patients, after adequate staging. Concurrent chemoradiotherapy is the standard of care in the rest of limited disease SCLC (LD-SCLC) instead of radical surgery, especially in patients diagnosed with lymph node metastasis (N1-N2) (7). However, more investigations should be done to identify the applicability of surgery to modern practice. There are few studies focused on the lymph node status of the patients diagnosed with pathological N2 (p-N2) SCLC, whether they could benefit from radical surgery or chemoradiotherapy.
Several studies (8–12) have retrospectively analyzed the prognosis and survival of patients with LD-SCLC after surgery, involving a small number of patients with p-N2. We collected a large number of cases of p-N2 LD-SCLC patients diagnosed through surgery. This study aimed to analyze the patterns of recurrence and survival of 171 Chinese p-N2 LD-SCLC patients after complete resection. It focused mainly on the relationship between lymph node status and the overall survival of the patients.
The medical history and clinical data of all patients diagnosed with SCLC through surgical pathology at Shanghai Chest Hospital, from July 2005 to June 2015, were obtained; a total of 350 cases were analyzed. There were 171 patients diagnosed with p-N2 after radical resection. EBUS-TBNA and PET-CT scan were not routinely performed as part of the preoperative staging work-up. Chest CT, abdominal B-scan ultrasonography, cranial CT or MRI, and bone scintigraphy were routinely performed, if the patients had not undergone PET-CT scan. The inclusion criteria were as follows: (i) surgically complete resection with systematic lymph node dissection adopted as a standard procedure; (ii) postoperative histopathology confirmed as p-N2 SCLC; (iii) clinical data and follow-up information were complete. The exclusion criteria were as follows: (i) patients who had incomplete resection, microscopically positive resections, and wedge resections; (ii) histological type other than SCLC. The Ethics Committee of Shanghai Chest Hospital reviewed and approved this study.
According to the result of CT, B-scan ultrasonography every 3 months in the follow-up process, the site and time of recurrence at the first progression were recorded and analyzed. The disease-free survival (DFS) was calculated as the date of surgery to the disease progression or the last follow-up visit. The overall survival (OS) was determined as the date of surgery to death or last follow-up visit. The follow-up visit ended till death or November 20, 2020.
Data was statistically analyzed using SPSS26.0 statistical software (IBM, Armonk, NY, USA). Descriptive statistics were recorded in specific numbers, percentages, medians, and ranges. The DFS and OS were obtained using the Kaplan-Meier survival methods, whose statistical results were presented in the form of median DFS, median OS, and their respective 95% confidence intervals (CIs). The log-rank test was used to compare the DFS and OS of different characteristic groups. Multivariable Cox regression model was used to identify the significant factors related to DFS and OS. P < 0.05 was considered statistically significant.
This retrospective study included 171 patients with p-N2 LD-SCLC who had undergone surgery. Among these patients, 140 (81.9%) were males and 113 (66.1%) were smokers. The median age was 59 years old, with a range from 33 to 76. All patients were clinically staged based on preoperative clinical data, including 12 c-IA patients (7.0%), 2 c-IB patients (1.2%), 1 c-IIA patient (0.6%), 38 c-IIB patients (22.2%), and 118 c-IIIA patients (69.0%). According to surgical pathology, there were 50 p-T1 patients, 77 p-T2 patients, 27 p-T3 patients, and 17 p-T4 patients, accounting for 29.2%, 45.0%, 15.8%, and 9.9%, respectively. Regarding lymph node status, the number and stations of the metastatic N2 lymph nodes were reviewed. Among these 171 patients, single p-N2 (n = 69) accounted for 40.4% of all and multiple p-N2 (n = 102) accounted for 59.6%. Single-station p-N2 (n = 102) accounted for 59.6% of all and multiple-station p-N2 (n = 69) accounted for 40.4%. At the last follow-up, 36 (21.1%) patients had not relapsed.
After the operation, except for 17.5% of patients who did not receive chest radiotherapy due to personal reasons, 141 patients (82.5%) received thoracic radiation therapy (TRT). Meanwhile, 54 (31.6%) received prophylactic cranial irradiation (PCI). Also, 149 patients (87.1%) received postoperative chemotherapy on record, with platinum-etoposide as the main regimen (Table 1).
The median DFS of all the patients was 12.667 months (95%CI 8.865–16.468) (Figure 1A). The recurrence sites of all relapsed patients (78.9%) were explicitly documented, including 61 cases (45.2%) of intrathoracic recurrence and 74 cases (54.8%) of extra-thoracic recurrence (Figure 2). The median DFS in the patients with intrathoracic recurrence was 9.467 months (95%CI 6.077–12.856), slightly longer than that of patients with extra-thoracic recurrence (median DFS 9.367 months, 95%CI 7.150–11.584), but it was not statistically significant (P = 0.383) (Figure 1B).
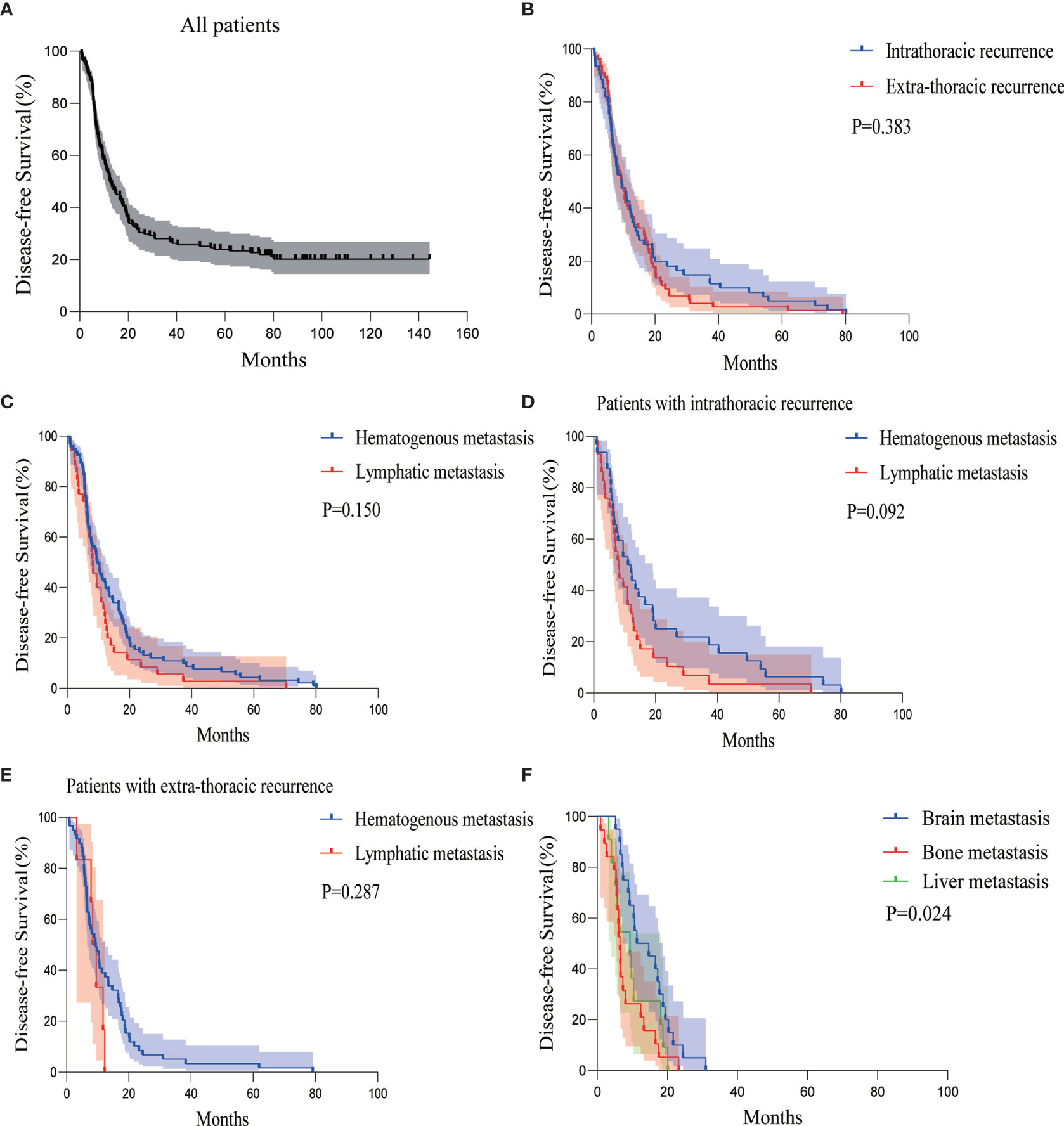
Figure 1 Kaplan-Meier curves of DFS for (A) all patients, (B) patients with intra- or extra-thoracic recurrence, (C) patients with hematogenous or lymphatic metastasis, (D) patients with intrathoracic recurrence in the form of hematogenous or lymphatic metastasis, (E) patients with extra-thoracic recurrence in the form of hematogenous or lymphatic metastasis, and (F) patients with brain, bone, or liver metastasis. DFS, disease-free survival.
According to the classification of hematogenous or lymphatic metastasis, hematogenous metastasis developed in 91 patients (67.4%), whereas lymphatic metastasis developed in 35 patients (25.9%). The remaining 9 patients (6.7%) had both hematogenous and lymphatic metastasis. The median DFS of hematogenous metastasis and lymphatic metastasis group were 9.700 months (95%CI 7.151–12.249) and 8.233 months (95%CI 6.495–9.972), respectively, which was not statistically significant (P = 0.150) (Figure 1C).
There were slightly more hematogenous metastasis than lymphatic metastasis (32 versus 29 cases) among the 61 patients with intrathoracic recurrence, with a relatively similar median DFS of 11.067 months and 8.100 months, respectively (P = 0.092) (Figure 1D).
Hematogenous metastasis was more than lymphatic metastasis in the 74 patients with extra-thoracic recurrence (59 versus 6 cases, the other 9 cases had hematogenous and lymphatic metastasis synchronously). The difference in median DFS between the two groups was not statistically significant (9.367 versus 8.367 months, P = 0.287) (Figure 1E).
Among the 68 patients with extra-thoracic recurrence in the form of hematogenous metastasis with or without lymphatic tract, there were 20 patients with brain metastasis, 19 patients with bone metastasis, and 11 patients with liver metastasis. There was 1 case of simultaneous bone and liver metastasis and 1 case of systemic metastasis. The median DFS of patients with brain, bone, and liver metastasis were 11.300 months (95%CI 2.389–20.211), 6.467 months (95%CI 5.614–7.320), and 9.367 months (95%CI 4.907–13.826), respectively (Figure 1F).
There was no clear association between the recurrence patterns of intra- or extra-thoracic and hematogenous or lymphatic tracts among the patients with multiple p-N2, nor to the number of p-N2 stations. (Figures 3A, B). Patients with multiple p-N2 presented much earlier with relapse compared to those with single p-N2; the difference was not statistically significant (median DFS 9.367 versus 19.233 months, P = 0.001) (Figure 3C). The median DFS of patients with multiple-station p-N2 was significantly shorter than that of patients with single-station p-N2 (median DFS 8.100 versus 18.067 months, P < 0.001) (Figure 3D, Supplementary Figure S1).
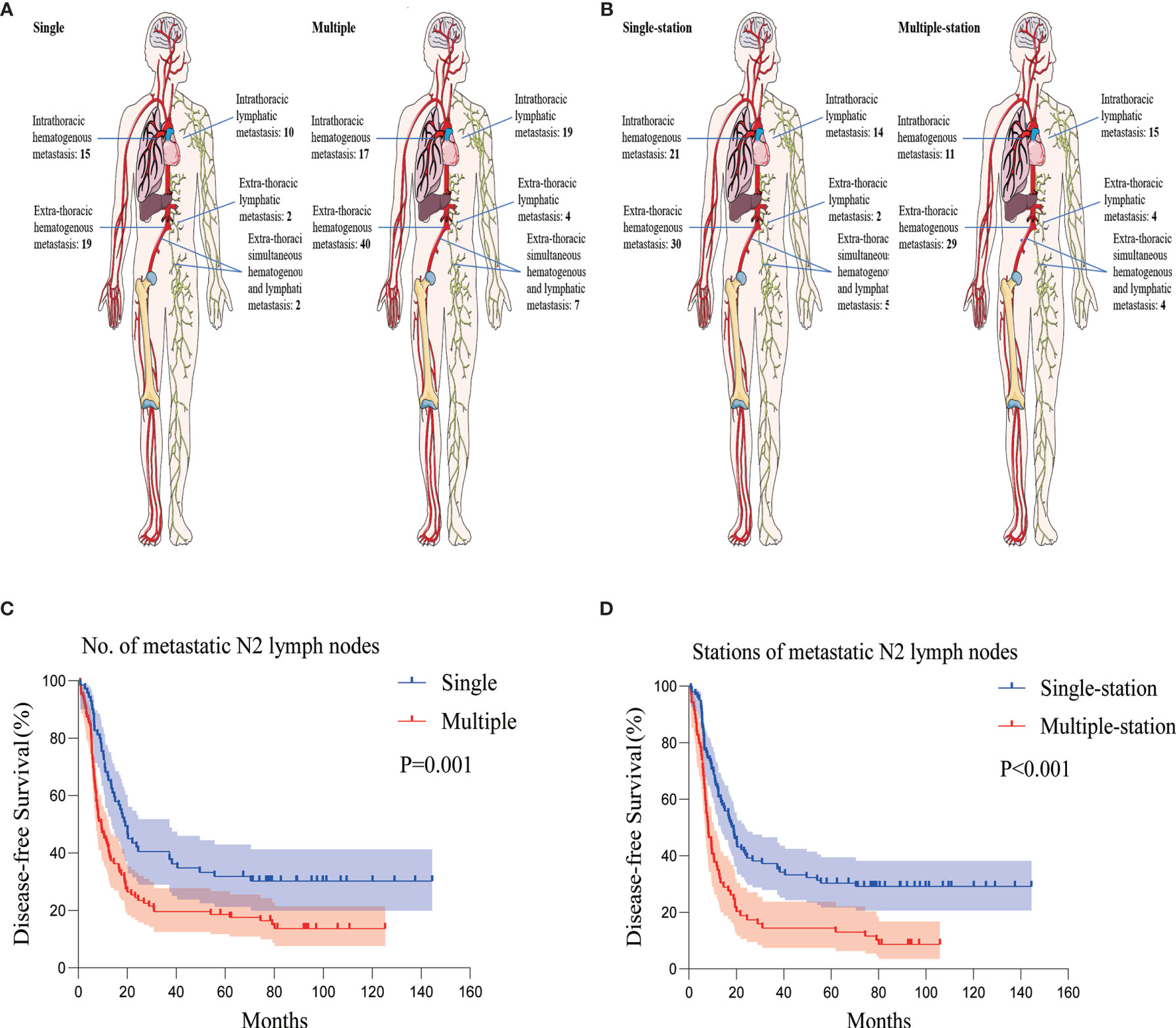
Figure 3 Recurrence patterns and Kaplan-Meier curves of DFS according to the number and stations of metastatic N2 lymph nodes. (A) Recurrence patterns of patients with single or multiple p-N2. (B) Recurrence patterns of patients with single- or multiple-station p-N2. (C) Kaplan-Meier curves of DFS for patients with single or multiple p-N2. (D) Kaplan-Meier curves of DFS for patients with single- or multiple-station p-N2. DFS, disease-free survival; p-N2, pathological N2.
According to the univariate analysis, the median DFS of patients who had received postoperative chemotherapy was significantly longer than that of those who had not [15.033 months (95%CI 10.674–19.393) versus 6.467 months (95%CI 2.713–10.221), P < 0.001]. The median DFS of patients who had undergone TRT was significantly longer than that of those who had not [14.533 months (95%CI 10.499–18.567) versus 6.400 months (95%CI 3.179–9.621), P < 0.001].
According to the multivariate analysis, we found T stage, the number of the metastatic N2 lymph nodes, postoperative chemotherapy, TRT, and PCI were independent factors for DFS after the adjustment (Table 2).
At the last follow-up, the median OS of all the patients was 24.167 months (95%CI 19.310–29.023) (Figure 4A). There was no statistical significance in OS between patients with intrathoracic recurrence and extra-thoracic recurrence (median OS 21.500 versus 16.333 months, P = 0.057) (Figure 4B).
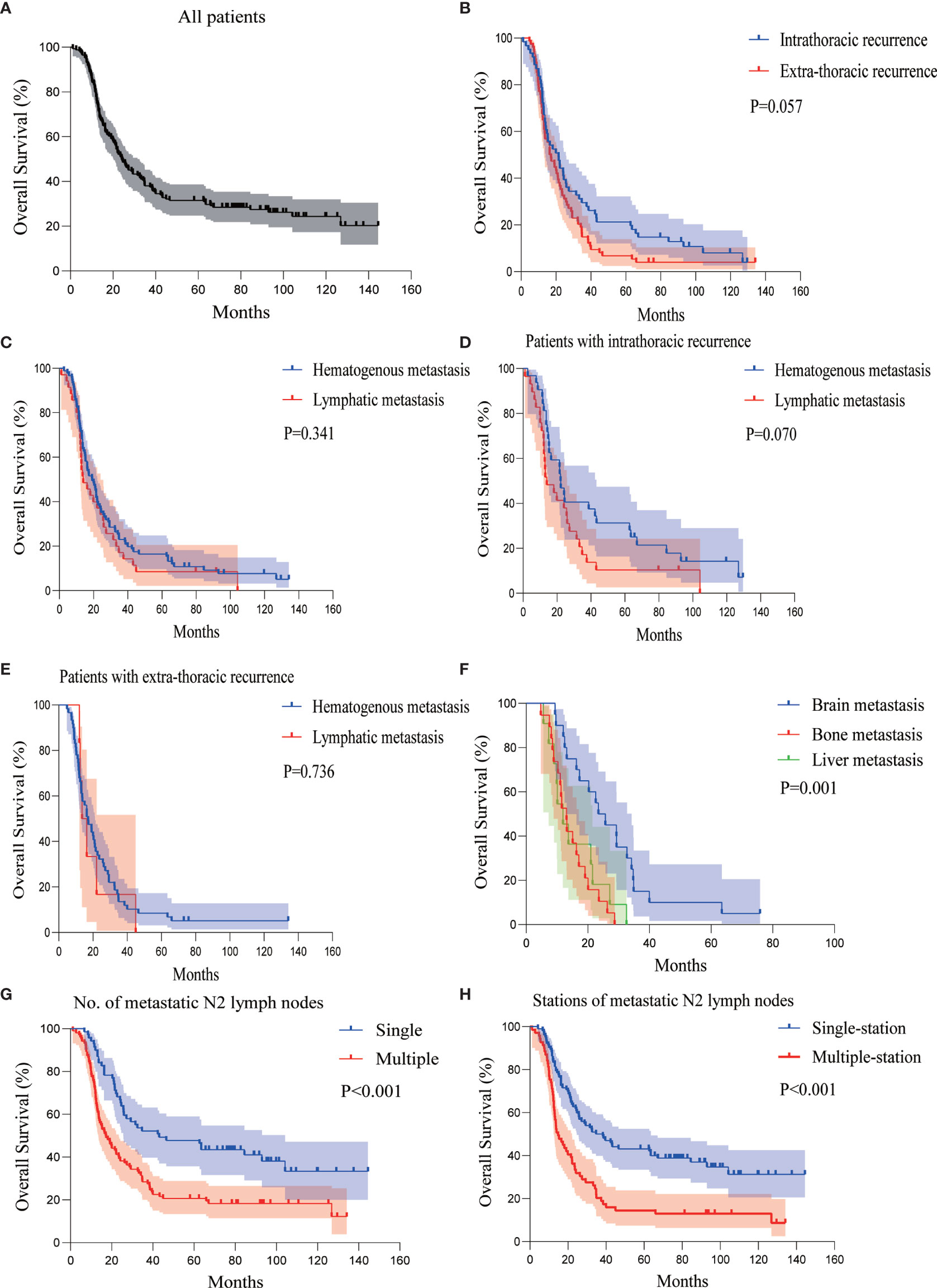
Figure 4 Kaplan-Meier curves of OS analysis for (A) all patients, (B) patients with intra- or extra-thoracic recurrence, (C) patients with hematogenous or lymphatic metastasis, (D) patients with intrathoracic recurrence in the form of hematogenous or lymphatic metastasis, (E) patients with extra-thoracic recurrence in the form of hematogenous or lymphatic metastasis, (F) patients with brain, bone, or liver metastasis, (G) patients with single or multiple metastatic N2 lymph nodes, and (H) patients with single- or multiple-station metastatic N2 lymph nodes. OS, overall survival; p-N2, pathological N2.
Similar to DFS, the OS of hematogenous and lymphatic metastasis in all patients was not statistically significant (median OS 19.133 versus 14.033 months, P = 0.341) (Figure 4C). There was no statistically significant difference in the OS of the hematogenous and lymphatic metastasis in patients with intrathoracic recurrence (median OS 22.067 versus 14.033 months, P = 0.070), similar to those with extra-thoracic recurrence (median OS 17.100 versus 13.533 months, P = 0.736) (Figures 4D, E).
The median OS of patients with brain, bone, and liver metastasis was 23.400 months (95%CI 16.607–30.193), 13.100 months (95%CI 10.446–15.754), and 11.900 months (95%CI 7.800–16.000), respectively. The presence of brain metastasis was not associated with the OS (P = 0.148), while the presence of bone metastasis was associated with a significantly worse OS (P = 0.001). However, the presence of liver metastasis was associated with worse OS (P = 0.039). The brain metastasis group versus the bone metastasis group and brain metastasis group versus liver metastasis group had P < 0.001 and P = 0.006, respectively. The difference in the bone metastasis group versus the liver metastasis group was not significant (P = 0.554) (Figure 4F). This indicates that bone and liver metastasis was associated with a relatively poor prognosis compared with brain metastasis.
Analysis of the lymph node status revealed that the number and sites of metastatic p-N2 were strongly associated with OS. Similar to DFS, the OS was significantly shorter in patients with two or more p-N2 (median OS 17.100 months) compared to those with a single metastatic p-N2 (median OS 43.033 months), P < 0.001 (Figure 4G). The median OS of patients with single- and multiple-station p-N2 were 34.667 and 14.767 months, P < 0.001 (Figure 4H, Supplementary Figure S2).
According to the univariate analysis, the median OS of patients who had received postoperative chemotherapy and those who had not were 26.300 months (95%CI 19.043–33.557) and 14.033 months (95%CI 10.356–17.711), respectively (P = 0.004). The median OS of patients who had undergone TRT and those who had not were 25.933 months (95%CI 18.227–33.639) and 10.000 months (95%CI 3.648–16.352), respectively (P = 0.004).
According to the multivariate analysis, T stage, the number of the metastatic N2 lymph nodes, postoperative chemotherapy, TRT, and PCI were independent factors for OS after the adjustment (Table 3).
The objective of the retrospective study was to observe the survival and recurrent features of p-N2 LD-SCLC patients after surgical resection. As far as we know, there are few studies in this area.
Concurrent chemoradiotherapy has been served as the cornerstone of treatment for LD-SCLC instead of surgery for decades except the selected stage I (T1-2,N0M0) patients, which is mainly based on the findings of two randomized controlled trials conducted more than 20 years ago (5, 6). As shown in CONVERT trial (13), for LD-SCLC patients who accepted concurrent chemoradiotherapy, median OS ranged from 25 to 30 months, which is not satisfying. Is there still a role for surgery in LD-SCLC patients with lymph node metastasis?
There have been few randomized clinical trials on LD-SCLC surgery in the last decade. Meanwhile, some evidence comes from prospective non-randomized studies and retrospective analysis (14–17), but they were generally conducted on few patients. Advancements in the stratification of mediastinal lymph nodes and surgical techniques call into question the applicability of surgery to modern practice.
The main recurrence site of p-N2 SCLC after surgical resection was the extra-thoracic cavity. Stish et al. (8) in 2015 investigated the recurrence patterns and long-term outcomes of LD-SCLC after surgery in 54 patients (including only 8 cases with N2); the main recurrence site was in the thoracic cavity. On the contrary, we analyzed data from 171 p-N2 LD-SCLC patients and concluded that more p-N2 patients had recurrence outside than inside the chest. One possible reason is that the sample sizes of the two studies were different. Also, we mainly observed the prognosis of p-N2 patients, while they focused on p-N0 patients (59%) and only 8 cases were p-N2. Additionally, 82.5% of patients in our study received adjuvant TRT compared to 7% of in their study, which may reduce the recurrence rate in the thoracic cavity.
Adjuvant radiotherapy is the primary locoregional treatment modality and is often followed by chemotherapy. According to a meta-analysis, adjuvant TRT could increase the local control rate of unresected LD-SCLC by 25.3% and increase the 2-year survival rate to 20% (18). There are clear benefits of radiation therapy for LD-SCLC patients with p-N2 who had undergone radical surgery (19). In our study, the 2-year survival rate and 5-year survival rate of p-N2 patients who received TRT after complete resection were approximately 54% and 34%, respectively, which were better than those who did not receive TRT (P = 0.004) with or without chemotherapy. In recent years, “the abscopal effect” in non-small cell lung cancer caused concern, referring to an underlying immune response dependent on the microenvironment playing a significant role in trigging systemic antitumor effects after receiving radiotherapy combined with immune checkpoint inhibitors (20–22). However, the abscopal effect of radiotherapy on SCLC is still in research. Whether the resected LD-SCLC patients could benefit from immune checkpoint inhibitors combined with radiotherapy or not remains unknown.
Compared with lymphatic metastasis, patients who suffered from hematogenous metastasis accounted for the majority (67.4%). Both intrathoracic and extra-thoracic recurrences were mainly hematogenous metastasis (32 out of 61 cases and 59 out of 74 cases). Local radiotherapy may reduce the chance of local lymphatic recurrence, but the results showed that hematogenous metastasis was more prone to occur, especially the distant hematogenous metastasis. Furthermore, there was no statistical difference in the OS of patients who developed hematogenous or lymphatic metastasis regardless of TRT or not (P = 0.341). The recurrence patterns do not seem to be related to the survival benefits of local radiotherapy given that p-N2 SCLC patients can benefit from postoperative local radiotherapy.
Patients suffering from bone and liver metastasis had poor prognosis and survival compared with patients suffering from brain metastasis (P = 0.001). Patients with bone metastasis had the poorest DFS (median DFS 6.467 months) with a median OS of 13.100 months. However, it was better than those suffering from liver metastasis. One reason may be that patients with bone metastasis can be treated with phosphate-containing drugs or even with local radiotherapy, which may help to achieve a relatively longer OS regardless of early recurrence. Patients with liver metastasis were associated with the poorest survival (median OS 11.900 months). There are generally few local treatments for liver metastasis, and the liver has a dual blood supply, which makes the OS worsen. The poor prognosis and survival presented by bone and liver metastasis of N2 LD-SCLC after surgical resection raise attention on monitoring and intervening in the metastasis in time.
Zhao et al. (9) analyzed the prognostic factors of LD-SCLC patients after surgical resection using multivariate analysis and found that complete resection, cigarette index, lymph node metastatic rate (the number of lymph nodes involved divided by the total number of resected lymph nodes) etc. were independent prognostic factors. However, their study regarded all postoperative patients (N0-N2). Subgroup analyses in several studies (11, 12, 23, 24) suggested that chemoradiotherapy after surgery may confer a survival advantage for patients with N2 in LD-SCLC. However, the studies did not analyze single node or single-station p-N2 and were involved small sample sizes.
Given that none of the above studies analyzed the specific p-N2 status in terms of postoperative pathology, we examined the relationship between the number, stations of p-N2, and the prognosis of the LD-SCLC patients who underwent radical resections. The results showed that the number of stations of metastatic p-N2 neither affected the first recurrence site nor did it affect hematogenous or lymphatic metastasis. However, the number of metastatic p-N2 was strongly associated with the time to recurrence (P = 0.001) and overall survival (P < 0.001). The prognosis of multiple p-N2 was poor (median DFS 9.367 months; median OS 17.100 months). Notably, the median OS of patients with single p-N2 was 43.033 months, which was significantly higher compared with concurrent chemoradiotherapy as shown in clinical trials conducted by Sundstrom et al. and Takada et al. (25, 26); however, the above studies regarded patients with LD-SCLC as a whole without specifically mentioning p-N2. This suggests a need to consider different treatment options for single and multiple p-N2 patients and shows that chemoradiotherapy as standard treatment for LD-SCLC patients with single p-N2 is debatable.
Similarly, the number of metastatic stations of p-N2 did not correlate to the recurrence patterns but was related to the time to relapse and the overall survival. Patients with multiple-station p-N2 had a worse prognosis compared with patients with single-station p-N2 (median DFS 18.067 versus 8.100 months, P < 0.001; median OS 34.667 versus 14.767 months, P < 0.001). This indicates that a favorable prognosis may occur when mediastinal lymph node metastasis is limited to a single station p-N2.
This study intended to evaluate which characteristics of patients would be crucial to the recurrence patterns of these p-N2 SCLC patients after surgery, but unfortunately, no results were obtained.
The results suggest a benefit of surgical resection for selected p-N2 LD-SCLC, especially that a favorable prognosis may occur when mediastinal lymph node metastasis is limited regardless of T stage. However, the main limitation of this study is the single-center retrospective nature, which leads to inevitable bias in data collection and patient selection. Healthier patients with fewer comorbid conditions might be disproportionally selected over sicker patients to undergo surgical resection. The potential bias and the lack of prospective validation must be considered when interpreting the results of the study.
In conclusion, in patients with p-N2 LD-SCLC who had undergone radical surgery and adjuvant therapy, the first recurrence site is extra-thoracic and hematogenous metastasis is the main form. The prognosis of bone and liver metastasis is poor. Moreover, although lymph node status seems not related to the recurrence patterns, patients with multiple p-N2 and multiple-station p-N2 may have suboptimal DFS and OS. Interestingly, LD-SCLC patients with single p-N2 exhibit good prognosis after surgical resection. Surgery may be a treatment option if LD-SCLC with a single metastatic N2 lymph node can be identified preoperatively.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Shanghai Chest Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
LY: data curation, formal analysis, investigation, methodology, software, roles/writing—original draft, and writing—review and editing. JX: data curation, formal analysis, investigation, methodology, and software. RQ: data curation, investigation, and methodology. HZ: project administration, supervision, validation, and visualization. BH: project administration, resources, supervision, validation, and visualization. RZ: conceptualization, data curation, project administration, resources, supervision, validation, visualization, and writing—review and editing. All authors contributed to the article and approved the submitted version.
This work was supported by the Science and Technology Innovation Program of Shanghai [20Y11913800] and Nurture projects for basic research of Shanghai Chest Hospital [2020YNJCM04]. The funder did not participate in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.675354/full#supplementary-material
Supplementary Figure S1 | Scatter plot of DFS for relapsed patients. DFS, disease-free survival. p-N2, pathological N2.
Supplementary Figure S2 | Scatter plot of OS for dead patients. OS, overall survival. p-N2, pathological N2.
CI, confidence interval; DFS, disease-free survival; LD-SCLC, limited-disease small-cell lung cancer; OS, overall survival; PCI, prophylactic cranial irradiation; p-N2, pathological N2; SCLC, small-cell lung cancer; TRT, thoracic radiation therapy.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin (2015) 65(2):87–108. doi: 10.3322/caac.21262
2. van Meerbeeck JP, Fennell DA, De Ruysscher DKM. Small-Cell Lung Cancer. Lancet (2011) 378(9804):1741–55. doi: 10.1016/s0140-6736(11)60165-7
3. Gazdar AF, Bunn PA, Minna JD. Small-Cell Lung Cancer: What We Know, What We Need to Know and the Path Forward. Nat Rev Cancer (2017) 17(12):725–37. doi: 10.1038/nrc.2017.87
4. Rudin CM, Poirier JT. Small-Cell Lung Cancer in 2016: Shining Light on Novel Targets and Therapies. Nat Rev Clin Oncol (2017) 14(2):75–6. doi: 10.1038/nrclinonc.2016.203
5. Fox W, Scadding JG. Medical Research Council Comparative Trial of Surgery and Radiotherapy for Primary Treatment of Small-Celled or Oat-Celled Carcinoma of Bronchus. Lancet (1973) 302(7820):63–5. doi: 10.1016/s0140-6736(73)93260-1
6. Lad T, Piantadosi S, Thomas P, Payne D, Ruckdeschel J, Giaccone G. A Prospective Randomized Trial to Determine the Benefit of Surgical Resection of Residual Disease Following Response of Small Cell Lung Cancer to Combination Chemotherapy. Chest (1994) 106(6 Suppl):320S–3S. doi: 10.1378/chest.106.6_supplement.320s
7. NCCN Clinical Practice Guidelines in Oncolog: Small Cell Lung Cancer Version 1.2021 (Accessed August 19, 2020).
8. Stish BJ, Hallemeier CL, Olivier KR, Harmsen WS, Allen MS, Garces YI. Long-Term Outcomes and Patterns of Failure After Surgical Resection of Small-Cell Lung Cancer. Clin Lung Cancer (2015) 16(5):e67–73. doi: 10.1016/j.cllc.2015.02.004
9. Zhao X, Kallakury B, Chahine JJ, Hartmann D, Zhang Y, Chen Y, et al. Surgical Resection of SCLC: Prognostic Factors and the Tumor Microenvironment. J Thorac Oncol (2019) 14(5):914–23. doi: 10.1016/j.jtho.2019.01.019
10. Granetzny A, Boseila A, Wagner W, Krukemeyer G, Vogt U, Hecker E, et al. Surgery in the Tri-Modality Treatment of Small Cell Lung Cancer. Stage-Dependent Survival. Eur J Cardiothorac Surg (2006) 30(2):212–6. doi: 10.1016/j.ejcts.2006.05.002
11. Salzer GM, Müller LC, Huber H, Denz H, Gasser R, Frommhold H, et al. Operation for N2 Small Cell Lung Carcinoma. Ann Thorac Surg (1990) 49(5):759–62. doi: 10.1016/0003-4975(90)90016-Y
12. Che K, Shen H, Qu X, Pang Z, Jiang Y, Liu S, et al. Survival Outcomes for Patients With Surgical and Non-Surgical Treatments in Stages I-III Small-Cell Lung Cancer. J Cancer (2018) 9(8):1421–9. doi: 10.7150/jca.23583
13. Faivre-Finn C, Snee M, Ashcroft L, Appel W, Barlesi F, Bhatnagar A, et al. Concurrent Once-Daily Versus Twice-Daily Chemoradiotherapy in Patients With Limited-Stage Small-Cell Lung Cancer (CONVERT): An Open-Label, Phase 3, Randomised, Superiority Trial. Lancet Oncol (2017) 18(8):1116–25. doi: 10.1016/s1470-2045(17)30318-2
14. Tsuchiya R, Suzuki K, Ichinose Y, Watanabe Y, Yasumitsu T, Ishizuka N, et al. Phase II Trial of Postoperative Adjuvant Cisplatin and Etoposide in Patients With Completely Resected Stage I-IIIa Small Cell Lung Cancer: The Japan Clinical Oncology Lung Cancer Study Group Trial (JCOG9101). J Thorac Cardiovasc Surg (2005) 129(5):977–83. doi: 10.1016/j.jtcvs.2004.05.030
15. Casiraghi M, Sedda G, Del Signore E, Piperno G, Maisonneuve P, Petrella F, et al. Surgery for Small Cell Lung Cancer: When and How. Lung Cancer (2021) 152:71–7. doi: 10.1016/j.lungcan.2020.12.006
16. Takenaka T, Takenoyama M, Inamasu E, Yoshida T, Toyokawa G, Nosaki K, et al. Role of Surgical Resection for Patients With Limited Disease-Small Cell Lung Cancer. Lung Cancer (2015) 88(1):52–6. doi: 10.1016/j.lungcan.2015.01.010
17. Brock MV, Hooker CM, Syphard JE, Westra W, Xu L, Alberg AJ, et al. Surgical Resection of Limited Disease Small Cell Lung Cancer in the New Era of Platinum Chemotherapy: Its Time has Come. J Thorac Cardiovasc Surg (2005) 129(1):64–72. doi: 10.1016/j.jtcvs.2004.08.022
18. Warde P, Payne D. Does Thoracic Irradiation Improve Survival and Local Control in Limited-Stage Small-Cell Carcinoma of the Lung? A Meta-Analysis. J Clin Oncol (1992) 10(6):890–5. doi: 10.1200/JCO.1992.10.6.890
19. Wong AT, Rineer J, Schwartz D, Schreiber D. Assessing the Impact of Postoperative Radiation Therapy for Completely Resected Limited-Stage Small Cell Lung Cancer Using the National Cancer Database. J Thorac Oncol (2016) 11(2):242–8. doi: 10.1016/j.jtho.2015.10.011
20. Takahashi J, Nagasawa S. Immunostimulatory Effects of Radiotherapy for Local and Systemic Control of Melanoma: A Review. Int J Mol Sci (2020) 21(23):9324. doi: 10.3390/ijms21239324
21. Walle T, Martinez Monge R, Cerwenka A, Ajona D, Melero I, Lecanda F. Radiation Effects on Antitumor Immune Responses: Current Perspectives and Challenges. Ther Adv Med Oncol (2018) 10:1758834017742575. doi: 10.1177/1758834017742575
22. Rodriguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol (2018) 39(8):644–55. doi: 10.1016/j.it.2018.06.001
23. Combs SE, Hancock JG, Boffa DJ, Decker RH, Detterbeck FC, Kim AW. Bolstering the Case for Lobectomy in Stages I, II, and IIIA Small-Cell Lung Cancer Using the National Cancer Data Base. J Thorac Oncol (2015) 10(2):316–23. doi: 10.1097/JTO.0000000000000402
24. Schreiber D, Rineer J, Weedon J, Vongtama D, Wortham A, Kim A, et al. Survival Outcomes With the Use of Surgery in Limited-Stage Small Cell Lung Cancer: Should Its Role be Re-Evaluated? Cancer (2010) 116(5):1350–7. doi: 10.1002/cncr.24853
25. Sundstrom S, Bremnes RM, Kaasa S, Aasebo U, Hatlevoll R, Dahle R, et al. Cisplatin and Etoposide Regimen Is Superior to Cyclophosphamide, Epirubicin, and Vincristine Regimen in Small-Cell Lung Cancer: Results From a Randomized Phase III Trial With 5 Years' Follow-Up. J Clin Oncol (2002) 20(24):4665–72. doi: 10.1200/JCO.2002.12.111
26. Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, et al. Phase III Study of Concurrent Versus Sequential Thoracic Radiotherapy in Combination With Cisplatin and Etoposide for Limited-Stage Small-Cell Lung Cancer: Results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol (2002) 20(14):3054–60. doi: 10.1200/JCO.2002.12.071
Keywords: small cell lung cancer, limited disease, surgery, mediastinal lymph nodes, prognosis
Citation: Yu L, Xu J, Qiao R, Zhong H, Han B and Zhong R (2021) Patterns of Recurrence and Survival Rate After Complete Resection of Pathological Stage N2 Small-Cell Lung Cancer. Front. Oncol. 11:675354. doi: 10.3389/fonc.2021.675354
Received: 03 March 2021; Accepted: 06 August 2021;
Published: 27 August 2021.
Edited by:
Antonio Calles, Gregorio Marañón Hospital, SpainReviewed by:
Masafumi Yamaguchi, Kitakyushu Municipal Medical Center, JapanCopyright © 2021 Yu, Xu, Qiao, Zhong, Han and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baohui Han, MTg5MzA4NTgyMTZAMTYzLmNvbQ==; Runbo Zhong, dG9uaWNfY2h1bmdAMTM5LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.