- 1Department of Hematology/Oncology, Fox Chase Cancer Center, Philadelphia, PA, United States
- 2Department of Biostatistics and Bioinformatics, Fox Chase Cancer Center, Philadelphia, PA, United States
- 3Guardant Health, Redwood City, CA, United States
By Ghatalia P, Smith CH, Winer A, Gou J, Kiedrowski LA, Slifker M, Saltzberg PD, Bubes N, Anari FM, Kasireddy V, Varshavsky A, Liu Y, Ross EA and El-Deiry WS (2019). Front. Oncol. 8:652. doi: 10.3389/fonc.2018.00652
In the original article, there was a mistake in Figure 3 as published. Incorrect CT scans were provided for two patients, while another patient’s CT scan was erroneously duplicated. The error was previously missed by the authors and the reviewers.
The corrected Figure 3 appears below.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
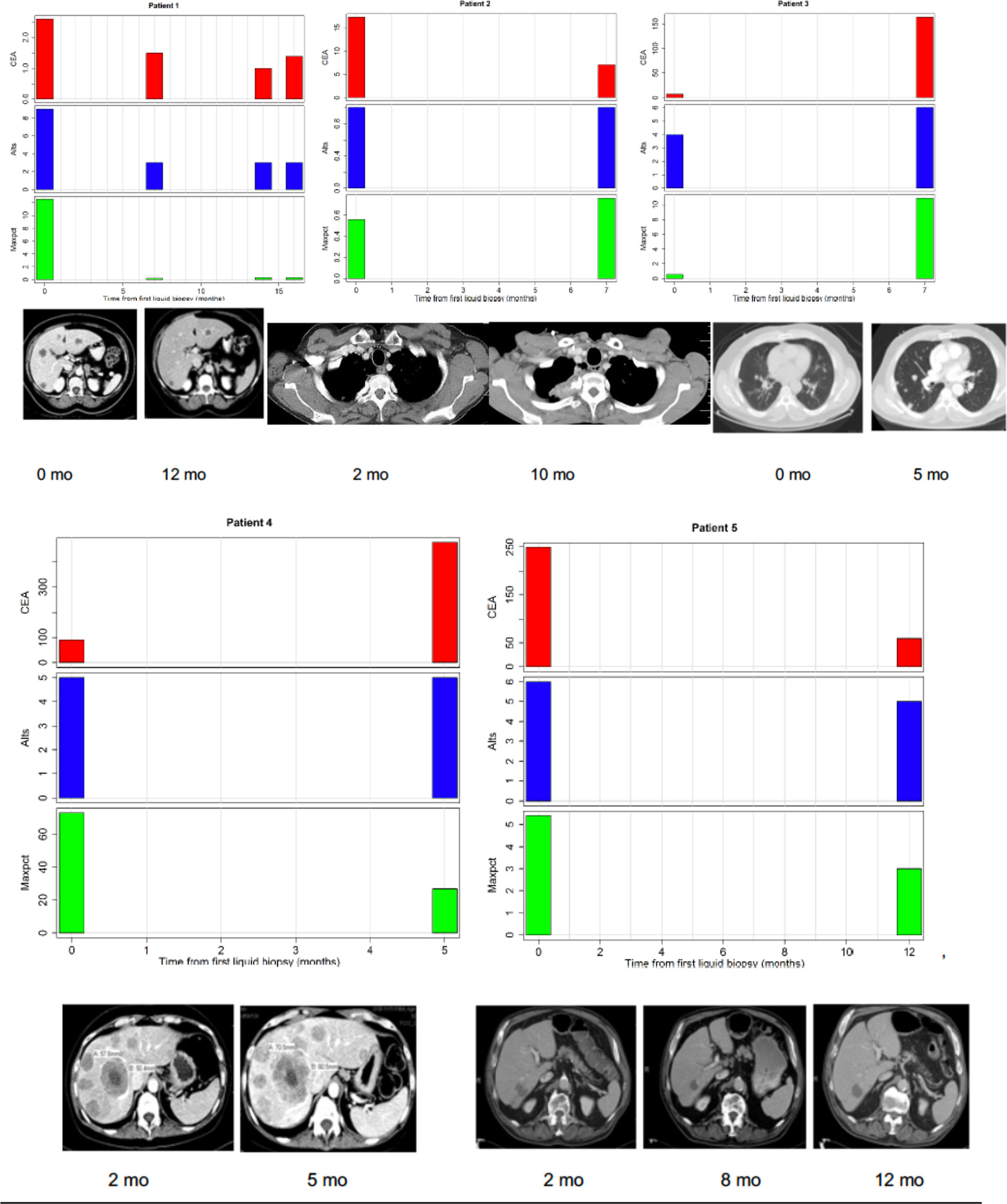
Figure 3 Correlations between tumor burden as assessed by radiographic imaging (CT scans) or CEA (tumor marker) and liquid biopsy mutation parameters (alts or number of alterations/number of mutated genes and maxpct or maximal allele frequency of mutated allele). (Top Left) Maxpct, CEA, and alts follow a downward trend as disease on CT scan improves. Center: With growth of mediastinal mass on CT, note rise in maxpct, CEA, and alts. (Top Right) As lung disease worsens on CT, maxpct, CEA, and alts increase. (Bottom Left) Despite increasing tumor on CT scan and rising CEA, maxpct did not rise. Liquid biopsy did contain APC and TP53 mutations, indicating presence of ctDNA. (Bottom Right) Liver metastases decreased between 1/2017 and 7/2017 and then increased in 11/2017. Allele freq. low (2–4%) probably due to low disease burden on CT.
Keywords: liquid biopsy, precision oncology, molecular target, tumor heterogeneity, drug resistance, tumor burden, cfDNA
Citation: Ghatalia P, Smith CH, Winer A, Gou J, Kiedrowski LA, Slifker M, Saltzberg PD, Bubes N, Anari FM, Kasireddy V, Varshavsky A, Liu Y, Ross EA and El-Deiry WS (2021) Corrigendum: Clinical Utilization Pattern of Liquid Biopsies (LB) to Detect Actionable Driver Mutations, Guide Treatment Decisions and Monitor Disease Burden During Treatment of 33 Metastatic Colorectal Cancer (mCRC) Patients (pts) at a Fox Chase Cancer Center GI Oncology Subspecialty Clinic. Front. Oncol. 11:674782. doi: 10.3389/fonc.2021.674782
Received: 18 March 2021; Accepted: 18 March 2021;
Published: 07 May 2021.
Approved by: Frontiers Editorial Office, Frontiers Media SA, Switzerland
Copyright © 2021 Ghatalia, Smith, Winer, Gou, Kiedrowski, Slifker, Saltzberg, Bubes, Anari, Kasireddy, Varshavsky, Liu, Ross and El-Deiry. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wafik S. El-Deiry, d2FmaWsuZWxkZWlyeUBnbWFpbC5jb20=
 Pooja Ghatalia1
Pooja Ghatalia1 Arthur Winer
Arthur Winer Lesli A. Kiedrowski
Lesli A. Kiedrowski Michael Slifker
Michael Slifker Fern M. Anari
Fern M. Anari Wafik S. El-Deiry
Wafik S. El-Deiry