
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 21 July 2021
Sec. Thoracic Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.671874
Background: The use of circulating tumor DNA (ctDNA) to reflect clinical benefits of advanced non-small cell lung cancer (NSCLC) patients during immune checkpoint inhibitor (ICI) therapy remains controversial. This study aimed to determine the association of pre-treatment and early dynamic changes of ctDNA with clinical outcomes in advanced NSCLC patients treated with ICIs.
Methods: Electronic databases (PubMed, Embase, Web of Science, and Cochrane) were systematically searched to include relevant studies published in English up to November 2020. The primary outcomes were overall survival (OS) and progression-free survival (PFS) and the secondary outcome was objective response rate (ORR) with RECIST criteria.
Results: A total of 1017 patients from 10 studies were identified. The baseline ctDNA levels (detected versus not detected) showed no significant association with clinical outcomes regarding OS (hazard ratio [HR], 1.18; 95% confidence interval [CI], 0.93-1.51), PFS (HR, 0.98; 95% CI, 0.80-1.21), and ORR (odds ratio [OR], 0.89; 95% CI, 0.54-1.46). Interestingly, when taken early longitudinal assessment of ctDNA into consideration, the early reduction of the concentration of ctDNA was associated with significant improvements of OS (HR, 0.19; 95% CI, 0.10-0.35), PFS (HR, 0.30; 95% CI, 0.22-0.41) and ORR (OR, 0.07; 95% CI, 0.03-0.18). Further subgroup analyses revealed that the reduction magnitude did not significantly impact the association between ctDNA and clinical outcomes, suggesting that both patients with decreased ctDNA or a ≥50% reduction of ctDNA was associated with improved OS, PFS and ORR.
Conclusion: Early reduction of ctDNA was associated with improved OS, PFS and ORR in advanced NSCLC patients treated with ICIs.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO, CRD42021226255.
Over the past decade, immunotherapy, targeting immune checkpoint molecules, programmed-death-1(PD-1)/PD ligand-1 (PD-L1) axis, has turned into one of the most important breakthroughs in cancer treatment, including non-small cell lung cancer (NSCLC) (1). Although treatment of NSCLC with immune checkpoint inhibitors (ICIs) can produce remarkably durable responses, a considerable proportion of patients cannot derive meaningful benefits from ICI therapy owing to drug resistance (2), disease hyper-progression (3), or immune-related adverse events (irAEs) (4).
Currently, several promising biomarkers for ICI therapy have been widely investigated, such as tumor mutation burden (TMB), PD-L1 expression, germline genotype of HLA-I, the molecular profiling of the tumor microenvironment, mutations in DNA mismatch repair and replication genes (1, 5, 6). However, most of these biomarkers are far from perfect biomarkers owing to being invasive, not always feasible, and its spatial and temporal heterogeneity (6, 7).
In response to the demand for genetic predictive and non-invasive molecular biomarkers in NSCLC, liquid biopsy, including circulating tumor DNA (ctDNA), have been developed. ctDNA, referring to a sub-set of cell-free DNA, is released by tumor cells undergoing apoptosis, necrosis, and in extracellular vesicles (exosomes) secreted from tumor cells and can be found in plasma. ctDNA is highly fragmented and ranges between 100 and 200 base pairs in size and represents genetic material from the primary tumor as well as metastases (8). ctDNA can be quantified using multiple metrics, such as mutant allele fraction or mutant allele concentration (that is, copies per milliliter) (9). The level of ctDNA in plasma has been demonstrated to correlate with tumor size (10, 11), disease stage (12), the clinical responses and prognosis of patients receiving anti-tumor treatment (13–16). The short half-life of ctDNA (17, 18), as well as the reduced and ease risk of repeating liquid biopsies relative to tissue biopsies (19) or imaging (20), enables ctDNA to be used for real-time monitoring of tumor burden in response to treatment. Although NSCLC is the cancer type for which plasma ctDNA testing has the most comprehensive and compelling evidence (21), it is still controversial regarding the role of ctDNA in predicting survival and clinical response.
ctDNA assays for the evaluation of cancers that harbor EGFR-sensitizing or EGFR-resistance mutations have already entered into clinical practice (22). A recent prospective study using ctDNA to guide matched targeted therapy in lung cancers supported the incorporation of plasma ctDNA into clinical practice (23). In addition to the direction of molecular targeted treatment, ctDNA could also potentially help monitor ICI response as the quantitative level in plasma has been demonstrated to reflect the tumor burden in patients even earlier than clinical detection (17, 24–27) and might identify response earlier than clinical detection (28, 29). However, these studies are mostly retrospective design, lacking high-level medical evidence, and some results were even inconsistent. Therefore, we conducted this meta-analysis to comprehensively investigate the predictive value of ctDNA for advanced NSCLC patients who received ICI therapy.
The authors declare that all supporting data, study materials and analytic methods within the article and the online supporting information are available to other researchers. This systematic review was performed in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (30). The PRISMA checklist is provided in Supplementary Table S1.
The systematic search of the scientific literature was performed. The search was conducted up to November 2020 in PubMed, EMBASE, Web of Science, and the Cochrane Library database. The main keywords used for the online search were “Circulating Tumor DNA,” “Carcinoma, Non-Small Cell Lung,” “Immune Checkpoint Inhibitor.” The full online search strategies were demonstrated in Supplementary Text S1. We also manually examined the references of each screened study until no additional articles could be added.
Studies were selected if they met the following inclusion criteria (1): patients with advanced NSCLC; (2) patients received ICIs alone or ICI-based therapy; (3) ctDNA was analyzed in these groups; (4) information on the clinical response or prognosis of these patients were provided; (5) if multiple studies from the same populations were available, to avoid repetition information, only the one with the largest sample size was included. Reviews, case reports, conference reports, abstracts, phase I studies and non-English publications were excluded. Endnote (Thomson Research Soft; Stanford, Connecticut, the United States) was used to select and screen the literature.
Data extraction and analyses were performed by 2 independent reviewers (HW and FZ). Any disagreement was discussed with the senior author (XC). Extracted study characteristics included: first author, publication year, country of the study, number of patients who underwent ICIs alone or combined therapy, gender, median/mean age, histological types, stage, smoking history, PD-L1 expression, ECOG PS, follow up duration, drugs, sample, extraction method, detection method, platform, detection time, most mutated genes.
The primary outcomes were overall survival (OS) and progression-free survival (PFS), then the secondary outcome included objective response rate (ORR) with RECIST criteria.
To assesses the quality of non-randomized studies (31), the Newcastle-Ottawa scale was used to assess the risk of bias. The scale assesses risk of bias in the following 3 aspects: selection of the study which include adequate definition and representation of the case, comparability of groups, and ascertainment of exposure and outcome for cases and controls. Studies with score less than 4 were considered as a high risk of bias, those with scores of 4 to 6 were regarded to have an intermediate risk of bias, and scores of 7 or more represented a low risk of bias. The results displayed in Supplementary Table S2
The heterogeneity of different studies was tested by using the Q test. The HRs and ORs with 95% CIs were directly extracted from the research article or calculated using previously published methods, as proposed by Tierney et al. (32). We calculated the I2 to assess the extent of variability attributable to statistical heterogeneity across studies. I2 < 50% and P > 0.10 were interpreted as signifying low-level heterogeneity. Across the studies, if no significant heterogeneity was found, the results were combined with the fixed-effects model (Mantel–Haenszel) (33); otherwise, the random-effects model (DerSimonian-Laird) was used (34). Publication bias was assessed by visual inspection of a funnel plot, Begg’s and Egger’s tests. A sensitivity analysis was performed by serially excluding each study to determine its influence. P values were two-sided and considered significant if less than 0.05. STATA 15.1 software for Mac was used to evaluate the outcomes.
The following subgroup analyses were performed. Firstly, as already assessed, the patients were stratified with two groups depending on different cutoff value of longitudinal ctDNA dynamic. Then, we also performed subgroup analysis considering that different platforms in studies might provide heterogeneity. The details of groups and outcomes were provided in the results of subgroup analysis.
Of the 427 articles retrieved by the literature search, 97 duplicates were removed, left 330 studies available for screening. 50 studies underwent full-text review, after screening the title and abstract. Of these studies,10 pieces of literature met the inclusion criteria and were chose for the current meta-analysis (7, 35–43). The detailed flow chart is shown in Figure 1.
The basic characteristics of the studies are summarized in Table 1. A total of 1017 patients were included in the study. Data from 3 studies (7, 36, 43) analyzed the PFS, 3 studies (36, 37, 43) considered OS and 4 studies (35, 36, 39, 43) discussed the ORR with the association of baseline ctDNA. 7 studies (37–43) presented PFS, 4 studies (37–39, 43) reported OS and 6 (35, 37–39, 41, 43) studies treated ORR as one of the results in early dynamic assessment of ctDNA. There were 2 studies (36, 38), both of which had 2 independent cohorts, so we used (1) and (2) to distinguish them.
4 cohorts from 3 studies (36, 37, 43) were included for analyzing the association between baseline ctDNA and OS (Figure 2A). Considering that the results were not heterogeneous, we chose fixed effects models. There was no statistical significance between the two groups regarding OS (HR, 1.18; 95% CI, 0.93-1.51; I2<0.1%; p=0.181). Data from 3 studies (7, 36, 43) and 4 cohorts were pooled for the analysis of PFS (Figure 2B), with a total of 625 patients. Considering that the results were homogeneous, we also used fixed-effects models. There was still no evident difference between the groups in terms of PFS (HR,0.98; 95% CI, 0.80-1.21; I2 = 38.1%; p=0.865) The results of 4 studies (35, 36, 39, 43) and 5 cohorts were pooled to examine the relationship of baseline detected ctDNA and ORR (Figure 2C), with a sample size of 641 patients. The pooled OR in the detected group with ORR (OR,0.89; 95% CI,0.54-1.46; I2 = 7.6%; p=0.641) showed no significant difference between the two groups.
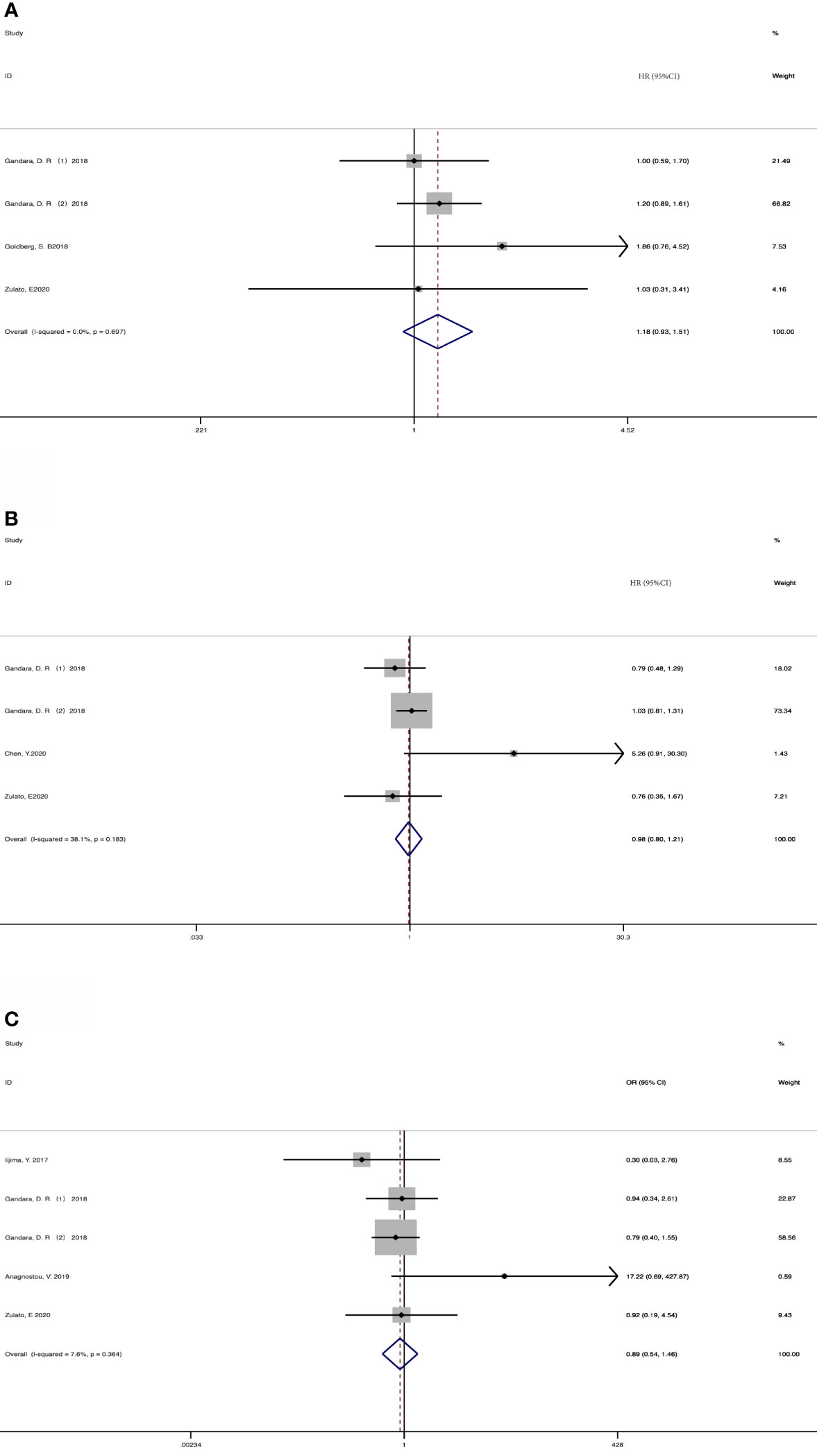
Figure 2 Meta-analysis of the associations between baseline ctDNA and (A) overall survival, (B) progression-free survival, (C) objective response rate. HR, hazard ratio; OR, odds ratio.
207 patients from 5 cohorts in 4 studies (37–39, 43) were included to investigate the pooled association between early reduction of ctDNA and OS. Eight cohorts from 7 studies (37–43) were included to survey the relation between early assessment of ctDNA and PFS, with a sample size of 412 patients. The pooled HR for OS with the fixed-effects model (Figure 3A) was 0.19 (95%CI, 0.10-0.35; I2<0.1%; p<0.001); the HR for PFS with random-effects model (Figure 3B) was 0.25 (95%CI, 0.16-0.40; I2 = 44.8%; p<0.001); Sensitivity analysis and Star Plot were performed because of the significant heterogeneity (Figure 4). When excluding group 1 in the study by Raja et al. (38), the heterogeneity decreased to I-squared<0.1%; with a pooled HR (Figure 3C) of 0.30 (95%CI, 0.22-0.41; p<0.001). In the early reduction ctDNA group, the pooled OR (Figure 3D) for ORR in 6 studies (35, 37–39, 41, 43) with 230 patients with fixed-effect models was 0.07 (95% CI,0.03-0.18; I2<0.1%; p<0.001).
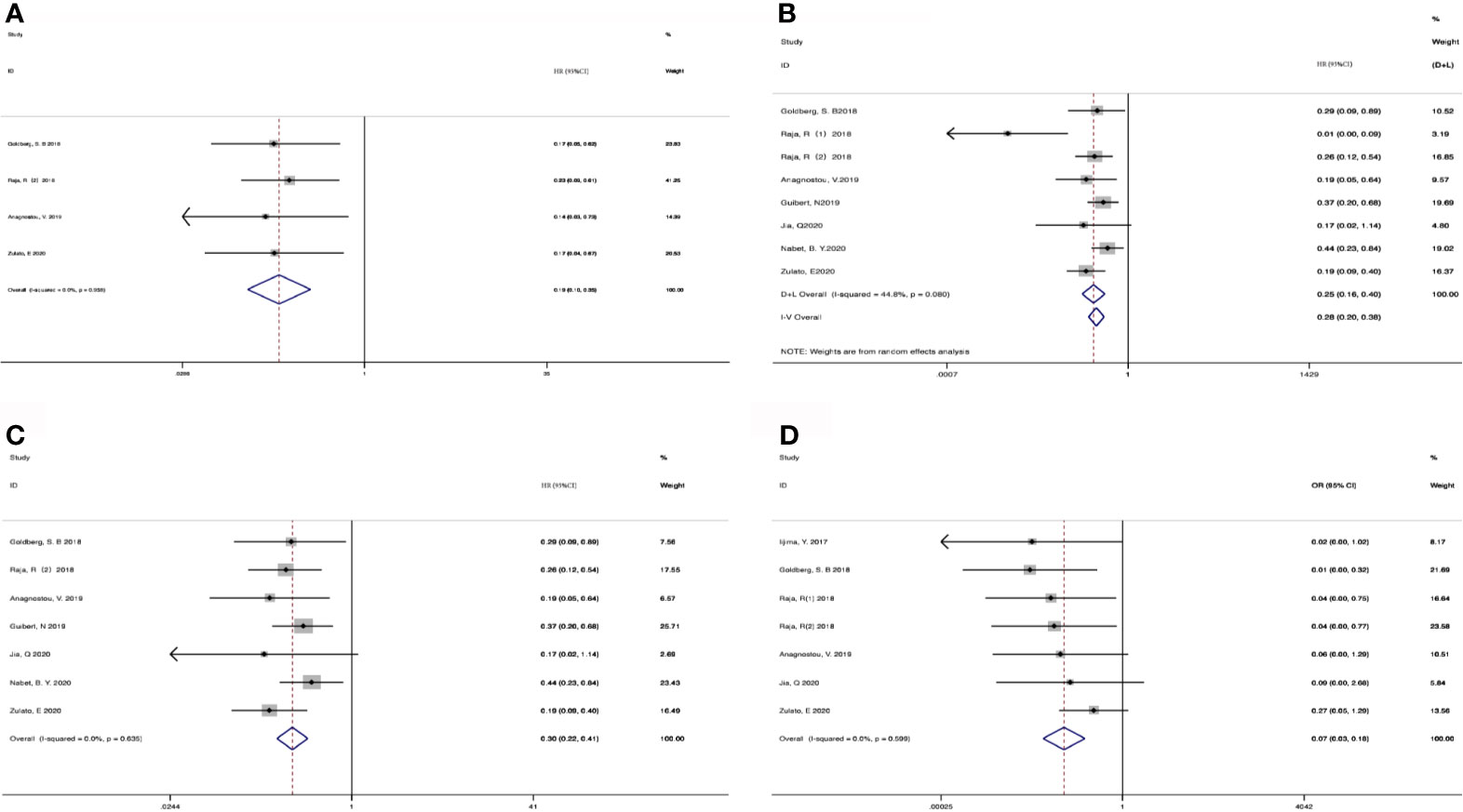
Figure 3 Meta-analysis of the associations between early reduction of ctDNA and (A) overall survival, (B, C) progression-free survival, (D) objective response rate.
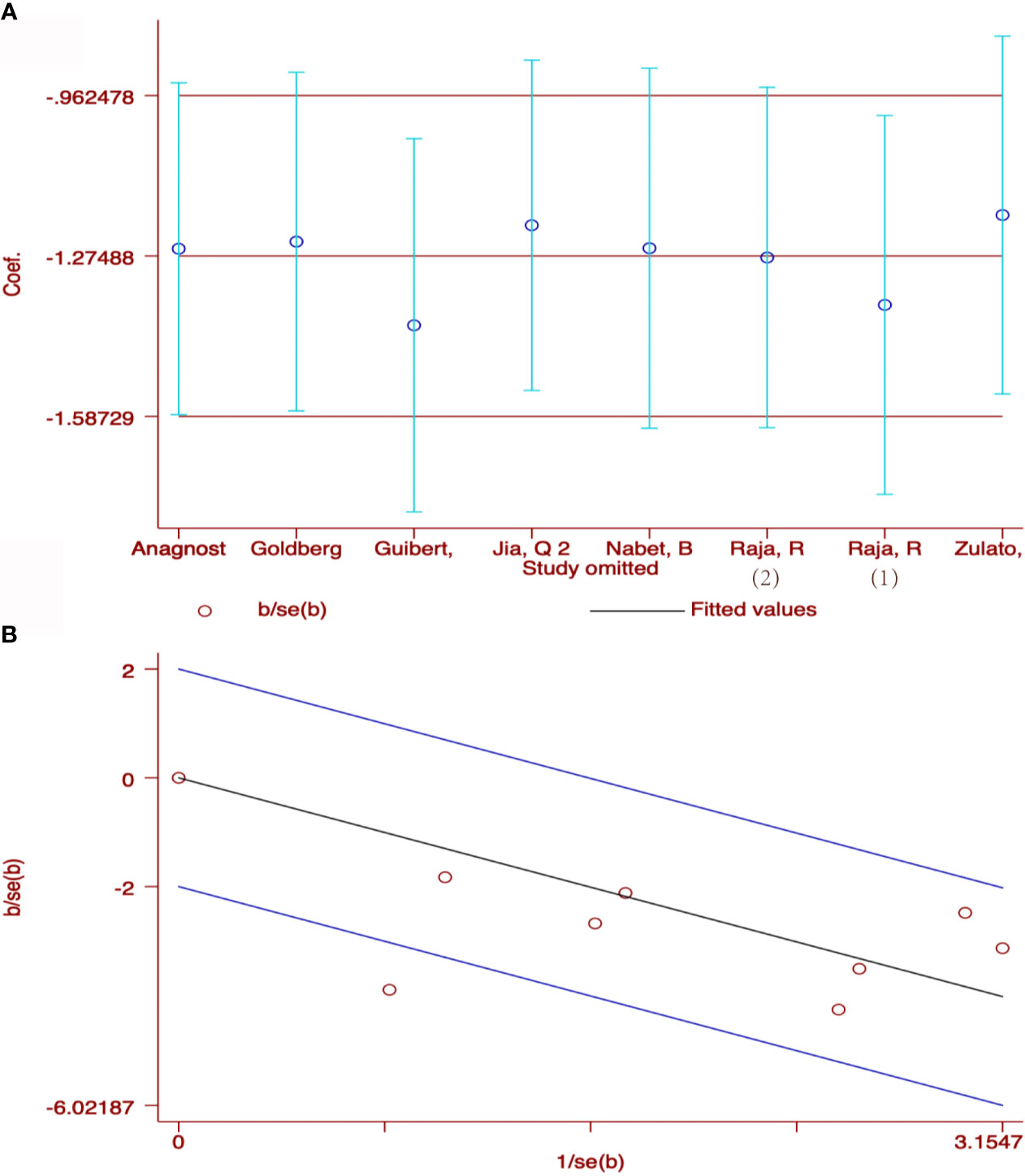
Figure 4 Sensitivity analysis (A) and Star Plot (B) of the included literatures on the reduction of ctDNA with PFS as the result.
Taking into account these studies, the authors selected different degrees of the reduction of ctDNA during treatment as the cutoff to define the positive group. Therefore, we further adopted subgroup analysis to divide the studies that dropped into the positive group and those with different degrees of decline into two subgroups: a decline of >50% as the threshold to define ctDNA response (subgroup 1) vs others; decreased vs others (subgroup 2). 73 patients from two studies (37, 39) were included in subgroup 1 with a HR (Figure 5A) of 0.16 for OS (95%CI, 0.06-0.43; I2 <0.1%, p<0.001), 106 patients from two studies were included in subgroup 2 with a HR (Figure 5A) of 0.21 for OS (95%CI, 0.10-0.46; I2 <0.1%, p<0.001). 181 patients from 4 studies (37, 39, 41, 42) were included in subgroup 1 with a HR (Figure 5B) of 0.33 for PFS (95%CI, 0.20-0.54; I2 <0.1%, p<0.001). 203 patients from 3 studies (38, 40, 43) were included in subgroup 2 with a HR (Figure 5B) of 0.28 for PFS (95% CI, 0.18-0.41; I2 <0.1%, p<0.001). When regarding ORR, 73 patients from 2 studies (37, 39) were included in subgroup 1 with an OR (Figure 5C) of 0.03 (95%CI,0.01-0.25; I2<0.1%, p=0.001), and 157 patients from 4 studies (35, 38, 41, 43) in subgroup 2 with an OR (Figure 5C) of 0.09 (95%CI,0.03-0.26 I2 <0.1%, p<0.001). Considering that most studies included used illumina platform, the subgroup was utilized to analyze the difference of illumina platform vs other platforms (Supplement Figure S1). The results still maintained coherence in different platforms both in baseline detection and dynamic observation, showing consistent with previous research (44).
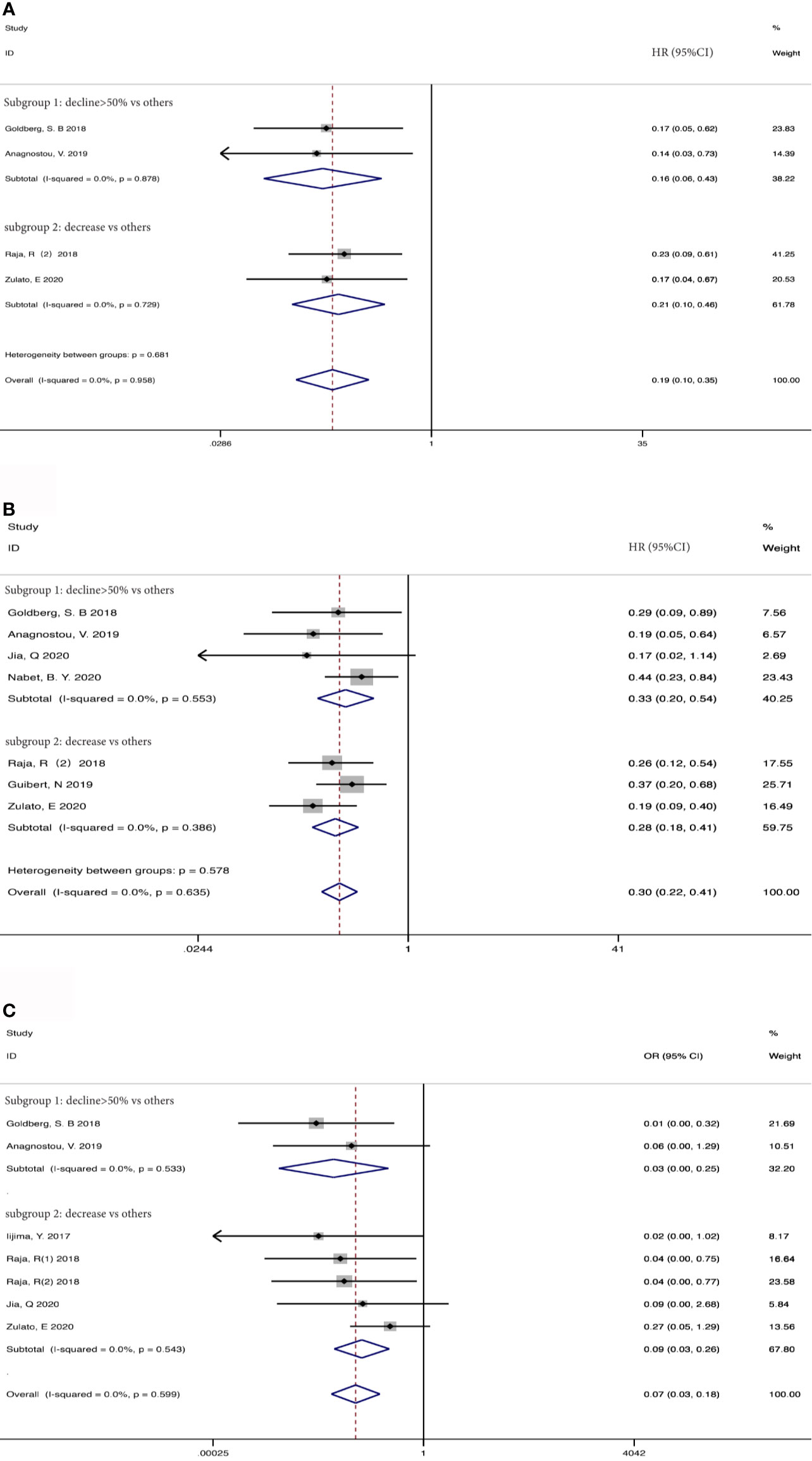
Figure 5 Subgroup analysis the associations between early decrease of ctDNA and (A) overall survival, (B) progression-free survival, (C) objective response rate.
As shown in Figure 6, the funnel plots were almost symmetrical and the test results indicated that no publication bias existed regarding the HRs for OS (Begg’s test, p=1.000; Egger’s test, p=0.802), PFS (Begg’s test, p=0.734; Egger’s test, p=0.699) and OR for ORR (Begg’s test, p= 0.806; Egger’s test, p=0.493) in baseline detected ctDNA. However, there are inconsistent results when taking early ctDNA dynamics into consideration, with HRs for OS (Begg’s test, p=0.308; Egger’s test, p=0.023) or PFS (Begg’s test, p=0.230; Egger’s test, p=0.160) and OR for ORR (Begg’s test, p= 0.548; Egger’s test, p=0.009), suggesting that publication bias might exist among these studies.
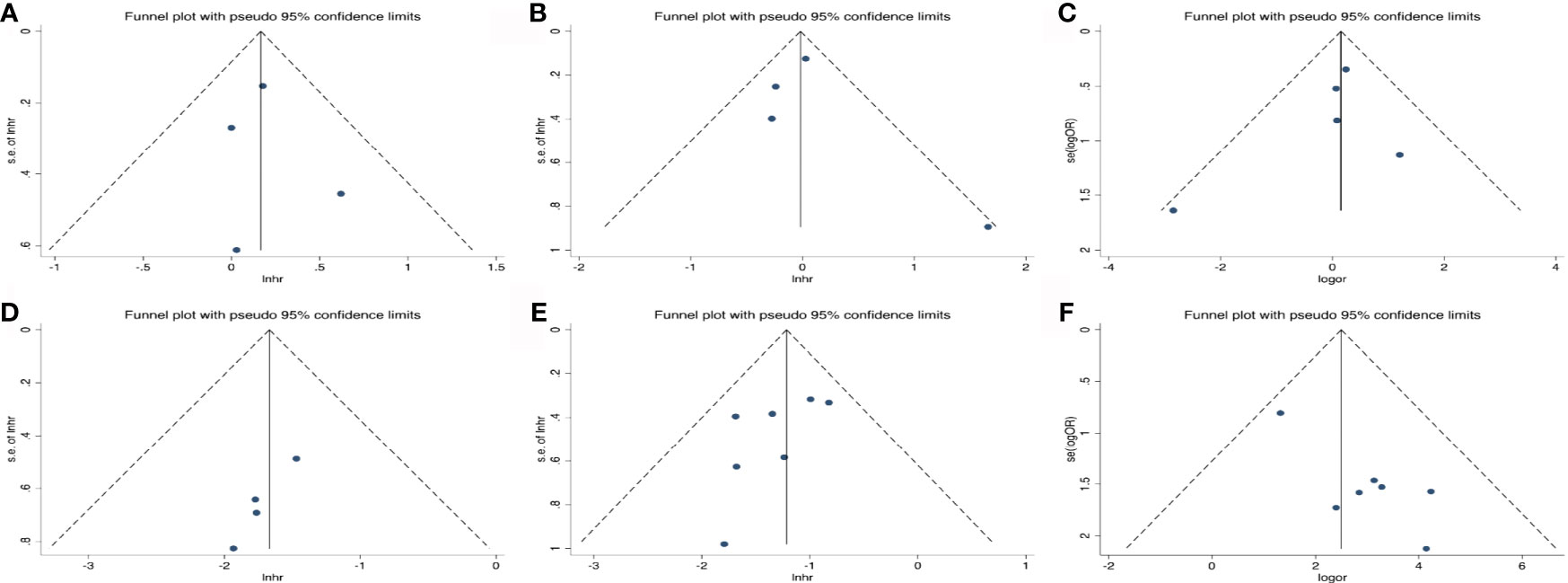
Figure 6 Publication bias for the association of detected baseline ctDNA with (A) overall survival, (B) progression-free survival, (C) objective response rate and (D) the relationship of early decreased ctDNA with OS; (E) PFS; (F) ORR.
To our knowledge, this was the first meta-analysis to comprehensively investigate predictive significance of ctDNA in advanced NSCLC patients treated with ICI therapy. The current study found that early reduction of ctDNA was associated with improved PFS, OS and ORR while there was no significant association between baseline ctDNA and clinical outcomes.
The concept of monitoring tumor burden and therefore biological effects of treatment by analyzing circulating biomarkers has been known for long, but only recently the availability of techniques able to detect ctDNA has opened new perspectives (17, 45). In lung cancer, the first experience concern EGFR-mutated disease (46). The use of ctDNA as a quantitative biomarker for assessment of ICI response has been investigated in prior studies (47–50), most of which have focused on tracking driver mutations in patients using digital PCR or allele-specific PCR. Even previous studies concluded that pretreatment ctDNA level appears to be an independent, inversely prognostic variable across tumor types, characterized by an association with OS and other known prognostic variables, but not with ORR (51). In this meta-analysis, we did not find a significant association between baseline ctDNA and clinical outcomes. Compared with tumor tissue, the abundance of tumor DNA in plasma samples is relatively low, which poses a great challenge to the sensitivity of plasma detection. False-negative results may occur due to insufficient plasma ctDNA content or lacking of inclusion of altered genes in the targeted NGS panel (21). On the other hand, false-positive results may also occur because of germline variants or the presence of somatic mutations in hematopoietic stem cells owing to clonal hematopoiesis, although most plasma ctDNA assays use matched sequencing of white blood cells (52–54), there still be sequencing errors and artifacts (55). A recent study found that high allele frequency blood TMB was strongly correlated with the ctDNA amount (56). However, high allele frequency blood TMB was a negative prognostic factor rather than a predictive factor (56), this may partially explain that there was no significant association between baseline ctDNA and clinical outcomes.
Interestingly, in this meta-analysis, patients with the reduced dynamic ctDNA obviously obtained more clinical benefits from ICI therapy. The early reduction of ctDNA may reflect an early response of tumors to effective treatment. This was consistent with previous studies, the amount of ctDNA may be an independent factor to predict the efficacy of patients receiving ICIs, and combined with other predictive indicators can better distinguish potential benefit populations (42). Subgroup analysis also indicated that a decline of >50% as a threshold to define ctDNA response and even achievement of undetectable ctDNA may prove to be a stronger predictor of long-term response, compared with just defining ctDNA response as decrease during ICIs therapy, and may identify patients who comprise the “tail” of the survival curve (57). Nevertheless, it still needs prospective studies to confirm these findings. Interestingly, some studies report a transient spike preceding a decline in ctDNA levels in a subset of patients, likely reflecting DNA release as tumor cells are killed. It would be important to avoid misinterpreting such a spike as disease progression (37, 58, 59).
There still were some limitations in our analysis. Firstly, all data were extracted from retrospective or post-hoc analysis studies. Secondly, the quality of data was heterogeneous as several pieces of important information such as prior therapy were not consistently reported. Finally, a high proportion of patients in these studies had KRAS and TP53 mutations. As TP53 and KRAS mutations showed remarkable clinical benefits from ICIs (60), it remains undermined that whether detecting TP53 and KRAS in ctDNA for predicting the response to ICIs was as effective as total ctDNA.
In summary, this current study suggested that early reduction of ctDNA was associated with improved PFS, OS and ORR in advanced NSCLC patients treated with ICIs. Further large-scale and rigorously designed prospective studies are still warranted to verify its clinical value. In the future, clinical trials will be conducted in NSCLC patients to determine whether ctDNA could be selected as a significant factor to monitor clinical response to ICI therapy.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
HW and FZ collected the relevant papers and data and drafted the manuscript text. HW, MQ and XL performed statistical analyses. HW, CZ, and LC performed the quality assessment. XC and CCZ gave critical comments and revised the paper. All authors contributed to the article and approved the submitted version.
This study was supported in part by grants from the Shanghai “Rising Stars of Medical Talent” Youth Development Program (to FZ) National Natural Science Foundation of China (No. 81703020), Medical Guidance Project of Shanghai Science and Technology Commission (No. 17411969200), and Shanghai Innovative Collaboration Project (No. 2020CXJQ02).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.671874/full#supplementary-material
Supplementary Figure 1 | Subgroup analysis of the different platforms in baseline detection with (A) overall survival, (B) progression-free survival, (C) objective response rate and in dynamic ctDNA with (D) overall survival, (E) progression-free survival, (F) objective response rate. HR, hazard ratio; OR, odds ratio.
1. Zhou F, Qiao M, Zhou CC. The Cutting-Edge Progress of Immune-Checkpoint Blockade in Lung Cancer. Cell Mol Immunol (2020) 18(2):279–93. doi: 10.1038/s41423-020-00577-5
2. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell (2017) 168(4):707–23. doi: 10.1016/j.cell.2017.01.017
3. Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors After Immunotherapy: Analysis of Genomic Alterations Associated With Accelerated Growth Rate. Clin Cancer Res Off J Am Assoc Cancer Res (2017) 23(15):4242–50. doi: 10.1158/1078-0432.CCR-16-3133
4. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol (2016) 2(10):1346–53. doi: 10.1001/jamaoncol.2016.1051
5. Hallqvist A, Rohlin A, Raghavan S. Immune Checkpoint Blockade and Biomarkers of Clinical Response in non-Small Cell Lung Cancer. Scand J Immunol (2020) 92(6):e12980. doi: 10.1111/sji.12980
6. Galon J, Bruni D. Approaches to Treat Immune Hot, Altered and Cold Tumours With Combination Immunotherapies. Nat Rev Drug Discov (2019) 18(3):197–218. doi: 10.1038/s41573-018-0007-y
7. Chen Y, Li XB, Liu GF, Chen SF, Xu MY, Song LL, et al. ctDNA Concentration, MIKI67 Mutations and Hyper-Progressive Disease Related Gene Mutations Are Prognostic Markers for Camrelizumab and Apatinib Combined Multiline Treatment in Advanced NSCLC. Front Oncol (2020) 10:1706–17. doi: 10.3389/fonc.2020.01706
8. Pessoa LS, Heringer M, Ferrer VP. ctDNA as a Cancer Biomarker: A Broad Overview. Crit Rev Oncol Hematol (2020) 155:103109. doi: 10.1016/j.critrevonc.2020.103109
9. Parkinson CA, Gale D, Piskorz AM, Biggs H, Hodgkin C, Addley H, et al. Exploratory Analysis of TP53 Mutations in Circulating Tumour DNA as Biomarkers of Treatment Response for Patients With Relapsed High-Grade Serous Ovarian Carcinoma: A Retrospective Study. PloS Med (2016) 13(12):e1002198. doi: 10.1371/journal.pmed.1002198
10. Thierry AR, Mouliere F, Gongora C, Ollier J, Robert B, Ychou M, et al. Origin and Quantification of Circulating DNA in Mice With Human Colorectal Cancer Xenografts. Nucleic Acids Res (2010) 38(18):6159–75. doi: 10.1093/nar/gkq421
11. Kamat AA, Bischoff FZ, Dang D, Baldwin MF, Han LY, Lin YG, et al. Circulating Cell-Free DNA: A Novel Biomarker for Response to Therapy in Ovarian Carcinoma. Cancer Biol Ther (2006) 5(10):1369–74. doi: 10.4161/cbt.5.10.3240
12. Bettegowda C, Sausen M, Leary R, Kinde I, Wang Y, Agrawal N, et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci Trans Med (2014) 6:224ra24. doi: 10.1126/scitranslmed.3007094
13. Yi X, Ma J, Guan Y, Chen R, Yang L, Xia X. The Feasibility of Using Mutation Detection in ctDNA to Assess Tumor Dynamics. Int J Cancer (2017) 140(12):2642–7. doi: 10.1002/ijc.30620
14. Ossandon MR, Agrawal L, Bernhard EJ, Conley BA, Dey SM, Divi RL, et al. Circulating Tumor DNA Assays in Clinical Cancer Research. J Natl Cancer Inst (2018) 110(9):929–34. doi: 10.1093/jnci/djy105
15. Pantel K, Alix-Panabières C. Liquid Biopsy and Minimal Residual Disease - Latest Advances and Implications for Cure. Nat Rev Clin Oncol (2019) 16(7):409–24. doi: 10.1038/s41571-019-0187-3
16. Cabel L, Proudhon C, Romano E, Girard N, Lantz O, Stern MH, et al. Clinical Potential of Circulating Tumour DNA in Patients Receiving Anticancer Immunotherapy. Nat Rev Clin Oncol (2018) 15(10):639–50. doi: 10.1038/s41571-018-0074-3
17. Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating Mutant DNA to Assess Tumor Dynamics. Nat Med (2008) 14(9):985–90. doi: 10.1038/nm.1789
18. Yao W, Mei C, Nan X, Hui L. Evaluation and Comparison of In Vitro Degradation Kinetics of DNA in Serum, Urine and Saliva: A Qualitative Study. Gene (2016) 590(1):142–8. doi: 10.1016/j.gene.2016.06.033
19. Overman MJ, Modak J, Kopetz S, Murthy R, Yao JC, Hicks ME, et al. Use of Research Biopsies in Clinical Trials: Are Risks and Benefits Adequately Discussed? J Clin Oncol (2013) 31(1):17–22. doi: 10.1200/JCO.2012.43.1718
20. Fazel R, Krumholz HM, Wang Y, Ross JS, Chen J, Ting HH, et al. Exposure to Low-Dose Ionizing Radiation From Medical Imaging Procedures. N Engl J Med (2009) 361(9):849–57. doi: 10.1056/NEJMoa0901249
21. Cheng ML, Pectasides E, Hanna GJ, Parsons HA, Choudhury AD, Oxnard GR. Circulating Tumor DNA in Advanced Solid Tumors: Clinical Relevance and Future Directions. CA Cancer J Clin (2020) 71(2):176–90. doi: 10.3322/caac.21650
22. Rolfo C, Mack PC, Scagliotti GV, Baas P, Barlesi F, Bivona TG, et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper From The IASLC. J Thorac Oncol (2018) 13(9):1248–68. doi: 10.1016/j.jtho.2018.05.030
23. Sabari JK, Offin M, Stephens D, Ni A, Lee A, Pavlakis N, et al. A Prospective Study of Circulating Tumor DNA to Guide Matched Targeted Therapy in Lung Cancers. J Natl Cancer Inst (2019) 111(6):575–83. doi: 10.1093/jnci/djy156
24. Forshew T, Murtaza M, Parkinson C, Gale D, Tsui DW, Kaper F, et al. Noninvasive Identification and Monitoring of Cancer Mutations by Targeted Deep Sequencing of Plasma DNA. Sci Transl Med (2012) 4(136):136ra68. doi: 10.1126/scitranslmed.3003726
25. Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, et al. Analysis of Circulating Tumor DNA to Monitor Metastatic Breast Cancer. N Engl J Med (2013) 368(13):1199–209. doi: 10.1056/NEJMoa1213261
26. Newman AM, Bratman SV, To J, Wynne JF, Eclov NC, Modlin LA, et al. An Ultrasensitive Method for Quantitating Circulating Tumor DNA With Broad Patient Coverage. Nat Med (2014) 20(5):548–54. doi: 10.1038/nm.3519
27. Gray ES, Rizos H, Reid AL, Boyd SC, Pereira MR, Lo J, et al. Circulating Tumor DNA to Monitor Treatment Response and Detect Acquired Resistance in Patients With Metastatic Melanoma. Oncotarget (2015) 6(39):42008–18. doi: 10.18632/oncotarget.5788
28. Schreuer M, Meersseman G, Van Den Herrewegen S, Jansen Y, Chevolet I, Bott A, et al. Quantitative Assessment of BRAF V600 Mutant Circulating Cell-Free Tumor DNA as a Tool for Therapeutic Monitoring in Metastatic Melanoma Patients Treated With BRAF/MEK Inhibitors. J Transl Med (2016) 14:95. doi: 10.1186/s12967-016-0852-6
29. Marchetti A, Palma JF, Felicioni L, De Pas TM, Chiari R, Del Grammastro M, et al. Early Prediction of Response to Tyrosine Kinase Inhibitors by Quantification of EGFR Mutations in Plasma of NSCLC Patients. J Thorac Oncol (2015) 10(10):1437–43. doi: 10.1097/JTO.0000000000000643
30. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Int J Surg (2010) 8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007
31. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. Ottawa: Ottawa Hospital Research Institute (2011). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
32. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical Methods for Incorporating Summary Time-to-Event Data Into Meta-Analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
33. Overton R. A Comparison of Fixed-Effects and Mixed (Random-Effects) Models for Meta-Analysis Tests of Moderator Variable Effects. Psychol Methods (1998) 3:354–79. doi: 10.1037/1082-989X.3.3.354
34. DerSimonian R, Kacker R. Random-Effects Model for Meta-Analysis of Clinical Trials: An Update. Contemp Clin Trials (2007) 28(2):105–14. doi: 10.1016/j.cct.2006.04.004
35. Iijima Y, Hirotsu Y, Amemiya K, Ooka Y, Mochizuki H, Oyama T, et al. Very Early Response of Circulating Tumour–Derived DNA in Plasma Predicts Efficacy of Nivolumab Treatment in Patients With Non–Small Cell Lung Cancer. Eur J Cancer (2017) 86:349–57. doi: 10.1016/j.ejca.2017.09.004
36. Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, et al. Blood-Based Tumor Mutational Burden as a Predictor of Clinical Benefit in non-Small-Cell Lung Cancer Patients Treated With Atezolizumab. Nat Med (2018) 24(9):1441–8. doi: 10.1038/s41591-018-0134-3
37. Goldberg SB, Narayan A, Kole AJ, Decker RH, Teysir J, Carriero NJ, et al. Early Assessment of Lung Cancer Immunotherapy Response via Circulating Tumor DNA. Clin Cancer Res (2018) 24(8):1872–80. doi: 10.1158/1078-0432.Ccr-17-1341
38. Raja R, Kuziora M, Brohawn PZ, Higgs BW, Gupta A, Dennis PA, et al. Early Reduction in ctDNA Predicts Survival in Patients With Lung and Bladder Cancer Treated With Durvalumab. Clin Cancer Res (2018) 24(24):6212–22. doi: 10.1158/1078-0432.Ccr-18-0386
39. Anagnostou V, Forde PM, White JR, Niknafs N, Hruban C, Naidoo J, et al. Dynamics of Tumor and Immune Responses During Immune Checkpoint Blockade in Non–Small Cell Lung Cancer. Cancer Res (2019) 79(6):1214–25. doi: 10.1158/0008-5472.CAN-18-1127
40. Guibert N, Jones G, Beeler JF, Plagnol V, Morris C, Mourlanette J, et al. Targeted Sequencing of Plasma Cell-Free DNA to Predict Response to PD1 Inhibitors in Advanced Non-Small Cell Lung Cancer. Lung Cancer (2019) 137:1–6. doi: 10.1016/j.lungcan.2019.09.005
41. Jia Q, Chiu L, Wu S, Bai J, Peng L, Zheng L, et al. Tracking Neoantigens by Personalized Circulating Tumor DNA Sequencing During Checkpoint Blockade Immunotherapy in Non-Small Cell Lung Cancer. Adv Sci (Weinh) (2020) 7(9):1903410. doi: 10.1002/advs.201903410
42. Nabet BY, Esfahani MS, Moding EJ, Hamilton EG, Chabon JJ, Rizvi H, et al. Noninvasive Early Identification of Therapeutic Benefit From Immune Checkpoint Inhibition. Cell (2020) 183(2):363–76.e13. doi: 10.1016/j.cell.2020.09.001
43. Zulato E, Attili I, Pavan A, Nardo G, Del Bianco P, Bragadin AB, et al. Early Assessment of KRAS Mutation in cfDNA Correlates With Risk of Progression and Death in Advanced Non-Small-Cell Lung Cancer. Br J Cancer (2020) 123(1):81–91. doi: 10.1038/s41416-020-0833-7
44. Boland JF, Chung CC, Roberson D, Mitchell J, Zhang X, Im KM, et al. The New Sequencer on the Block: Comparison of Life Technology’s Proton Sequencer to an Illumina HiSeq for Whole-Exome Sequencing. Hum Genet (2013) 132(10):1153–63. doi: 10.1007/s00439-013-1321-4
45. Alix-Panabieres C, Pantel K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov (2016) 6(5):479–91. doi: 10.1158/2159-8290.CD-15-1483
46. Sacher AG, Paweletz C, Dahlberg SE, Alden RS, O’Connell A, Feeney N, et al. Prospective Validation of Rapid Plasma Genotyping for the Detection of EGFR and KRAS Mutations in Advanced Lung Cancer. JAMA Oncol (2016) 2(8):1014–22. doi: 10.1001/jamaoncol.2016.0173
47. Lipson EJ, Velculescu VE, Pritchard TS, Sausen M, Pardoll DM, Topalian SL, et al. Circulating Tumor DNA Analysis as a Real-Time Method for Monitoring Tumor Burden in Melanoma Patients Undergoing Treatment With Immune Checkpoint Blockade. J Immunother Cancer (2014) 2(1):42. doi: 10.1186/s40425-014-0042-0
48. Xi L, Pham TH, Payabyab EC, Sherry RM, Rosenberg SA, Raffeld M. Circulating Tumor DNA as an Early Indicator of Response to T-Cell Transfer Immunotherapy in Metastatic Melanoma. Clin Cancer Res (2016) 22(22):5480–6. doi: 10.1158/1078-0432.Ccr-16-0613
49. Cabel L, Riva F, Servois V, Livartowski A, Daniel C, Rampanou A, et al. Circulating Tumor DNA Changes for Early Monitoring of Anti-PD1 Immunotherapy: A Proof-of-Concept Study. Ann Oncol (2017) 28(8):1996–2001. doi: 10.1093/annonc/mdx212
50. Lee JH, Long GV, Boyd S, Lo S, Menzies AM, Tembe V, et al. Circulating Tumour DNA Predicts Response to Anti-PD1 Antibodies in Metastatic Melanoma. Ann Oncol (2017) 28(5):1130–6. doi: 10.1093/annonc/mdx026
51. Zhang Q, Luo J, Wu S, Si H, Gao C, Xu W, et al. Prognostic and Predictive Impact of Circulating Tumor DNA in Patients With Advanced Cancers Treated With Immune Checkpoint Blockade. Cancer Discov (2020) 10(12):1842–53. doi: 10.1158/2159-8290.CD-20-0047
52. Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, et al. Clonal Hematopoiesis of Indeterminate Potential and its Distinction From Myelodysplastic Syndromes. Blood (2015) 126(1):9–16. doi: 10.1182/blood-2015-03-631747
53. Razavi P, Li BT, Brown DN, Jung B, Hubbell E, Shen R, et al. High-Intensity Sequencing Reveals the Sources of Plasma Circulating Cell-Free DNA Variants. Nat Med (2019) 25(12):1928–37. doi: 10.1038/s41591-019-0652-7
54. Coombs CC, Zehir A, Devlin SM, Kishtagari A, Syed A, Jonsson P, et al. Therapy-Related Clonal Hematopoiesis in Patients With Non-Hematologic Cancers Is Common and Associated With Adverse Clinical Outcomes. Cell Stem Cell (2017) 21(3):374–82.e4. doi: 10.1016/j.stem.2017.07.010
55. El-Deiry WS, Goldberg RM, Lenz HJ, Shields AF, Gibney GT, Tan AR, et al. The Current State of Molecular Testing in the Treatment of Patients With Solid Tumors, 2019. CA Cancer J Clin (2019) 69(4):305–43. doi: 10.3322/caac.21560
56. Wang ZJ, Duan JC, Wang GQ, Zhao J, Xu JC, Han JF, et al. Allele Frequency-Adjusted Blood-Based Tumor Mutational Burden as a Predictor of Overall Survival for Patients With NSCLC Treated With PD-(L)1 Inhibitors. J Thoracic Oncol (2020) 15(4):556–67. doi: 10.1016/j.jtho.2019.12.001
57. Hellmann MD, Kris MG, Rudin CM. Medians and Milestones in Describing the Path to Cancer Cures: Telling "Tails". JAMA Oncol (2016) 2(2):167–8. doi: 10.1001/jamaoncol.2015.4345
58. Deshpande S, Tytarenko RG, Wang Y, Boyle EM, Ashby C, Schinke CD, et al. Monitoring Treatment Response and Disease Progression in Myeloma With Circulating Cell-Free DNA. Eur J Haematol (2020) 106(2):230–40. doi: 10.1111/ejh.13541
59. Husain H, Melnikova VO, Kosco K, Woodward B, More S, Pingle SC, et al. Monitoring Daily Dynamics of Early Tumor Response to Targeted Therapy by Detecting Circulating Tumor DNA in Urine. Clin Cancer Res (2017) 23(16):4716–23. doi: 10.1158/1078-0432.Ccr-17-0454
Keywords: non-small-cell lung cancer, immune checkpoint inhibitor, circulating tumor DNA, biomarker, survival
Citation: Wang H, Zhou F, Qiao M, Li X, Zhao C, Cheng L, Chen X and Zhou C (2021) The Role of Circulating Tumor DNA in Advanced Non-Small Cell Lung Cancer Patients Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Oncol. 11:671874. doi: 10.3389/fonc.2021.671874
Received: 26 February 2021; Accepted: 02 July 2021;
Published: 21 July 2021.
Edited by:
Ashish Saxena, Weill Cornell Medical Center, United StatesReviewed by:
Xabier Mielgo Rubio, Hospital Universitario Fundación Alcorcón, SpainCopyright © 2021 Wang, Zhou, Qiao, Li, Zhao, Cheng, Chen and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caicun Zhou, Y2FpY3VuemhvdV9kckAxNjMuY29t; Xiaoxia Chen, Y2hlZXRvc194eEAxMjYuY29t; Fei Zhou, ZmVpLnpob3VAdG9uZ2ppLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.