
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 09 June 2021
Sec. Hematologic Malignancies
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.670673
This article is part of the Research TopicNovel Agents for Multiple MyelomaView all 19 articles
Human leukocyte antigen-E (HLA-E) has been putatively associated with the pathogenesis of multiple myeloma (MM). Our study first showed that HLA-E was differentially expressed on MM and normal plasma cells (39.27 ± 27.01 and 11.28 ± 0.79, respectively). Based on the median value of HLA-E expression, we further stratified MM patients into high and low-expression groups, and then found high expression of HLA-E was correlated with advanced ISS stage (p = 0.025) and high-risk cytogenetics risk stratification (p = 0.000) by the Pearson Chi-square test, suggesting that HLA-E could be considered as a biomarker for high-risk MM. Furthermore, peptide 3 (P3) from our previous study was confirmed to possess a high affinity to HLA-E positive MM cells. Taken together, HLA-E could be considered as a new marker and candidate treatment target for MM, while peptide P3 may act as a potential treatment choice for targeting MM cells.
Multiple myeloma (MM) is a common malignant hematological disease originating from plasma cells (1), and its prognosis has remarkably improved as treatment regimens have evolved into currently more popularized immunotherapies (2–6). As one type of immunotherapeutic regimens, monoclonal antibodies, such as Daratumumab (CD38 antibody), has exhibited significant treatment efficacy in both patients with MM and with relapsed/refractory MM (RRMM) (7). However, a certain percentage of MM patients have been profiled as high-risk for RRMM with much shorter progression-free survival (PFS) and overall survival (OS). Therefore, early identification of myeloma patients with a high-risk of refractory or relapse and development of targeted treatment regimen remain the priorities in the study of MM.
HLA-E is a non-classical major histocompatibility complex (MHC) class I molecule characterized by lower polymorphism, which plays a critical role in the immune response by both inhibiting and activating the function of natural killer (NK) cells (8). Studies have shown that HLA-E expression correlates with worse progression-free survival in newly diagnosed, treatment-naïve MM patients. Based on a bioinformatics analysis in our previous work, we suggest HLA-E as a potential therapeutic target for the treatment of MM (9) and designed peptides to bind HLA-E by analyzing its interaction with CD94/NKG2A. Thereafter, a peptide library was built upon the strategy of randomly replacing non-key amino acids to enhance the affinity of peptides (10), in which the top three peptides were subjected to molecular docking analysis. Subsequently, a peptide designated as P3 (NALDEYCEDKNR) was found to have the highest affinity for HLA-E, indicating that P3 could be considered as a potential inhibitor to specifically target MM cells (9). Thus, the present study aims to continue our investigation on the clinical meaning of HLA-E expression in MM patients and further explore whether peptide P3 could target HLA-E positive myeloma cells.
This study, which included 30 newly diagnosed multiple myeloma (NDMM) patients from January 1, 2018 to November 31, 2019, was approved by the ethics committee of Shengjing hospital of China Medical University (2020PS215K). Following the diagnoses of MM according to the International Myeloma Working Group (IMWG) guidelines for symptomatic MM (11) and acquiring patients’ consents, all bone marrow samples were collected. All patients were classified according to the staging criteria (12). Patients were excluded from this study if they had histories of any immune deficiency disease, transplantation or other malignant tumor, or previous immunosuppressive therapy. For the purpose of analysis, the baseline data of gender, age, clinical stage, typing, and immunoglobulin heavy chain (IgH) quantity were recorded, while bone marrow from non-malignant patients was collected for use as the control.
The expressions of HLA-E, CD138, and CD45 were determined by a flow cytometer (FACS Calibur; Becton Dickinson, San Diego, CA, USA) with mouse antihuman fluorescent monoclonal antibodies [fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin-chlorophyll-protein (Percp) and allophycocyanin (APC)]. The antibodies were purchased from BD Pharmingen (San Diego, CA, USA). After incubation with antibodies in the dark for 15 min, flow cytometry with CD138++/CD45 was performed on at least 50,000 cells for gating the viable cells. The HLA-E antigen expression was further analyzed with CellQuest software (Becton Dickinson). Target-binding peptides labeled by FITC (peptide M and peptide P3) were synthesized by Chinese Peptide (Hangzhou, China). Then, the affinity of peptides against HLA-E on the collected bone marrow from the MM patients was detected by FCM, and the binding affinity was further analyzed on the positive portion of the target-binding peptide.
The Mann–Whitney U test was used to compare the difference between non-normal distribution data, while the Pearson chi-square test was employed to compare the correlation between HLA-E expression and clinic-pathologic parameters. MM patients with HLA-E expression were divided into high-expression and low-expression groups based on the mean value. SPSS24.0 (Chicago, IL, USA) and GraphPad PRISM 6.0 (La Jolla, CA, USA) were used for statistical analysis, and p < 0.05 was considered statistically significant.
A total of 30 patients diagnosed with MM, according to IMWG guidelines, were evaluated, including 18 males and 12 females with a median age of 65 years (47-83 years). All patients received Bortezomib-based regime as the standard chemotherapy. The general characteristics of these MM patients are summarized in Table 1. Additionally, bone marrow from seven patients with a non-malignant hematological disease were selected as a control for the present study. High-risk cytogenetic features of MM patients were detected by fluorescence in situ hybridization (FISH).
HLA-E was detected in 30 MM patients and in 7 non-malignancy control patients by FCM. In MM patients, CD138 and CD38 were strongly positive in abnormal plasma cells, thus indicating that the CD138 antigen can be used to identify MM cells. According to the quantitative analysis of FCM results, the mean fluorescence intensity of HLA-E was 39.27 ± 27.01 (15.4-152.61) in MM cells and 11.28 ± 0.79 (8.82-14.33) in control cells with positive CD138. These results show that HLA-E was highly expressed in MM patients (p < 0.05) (Figure 1).
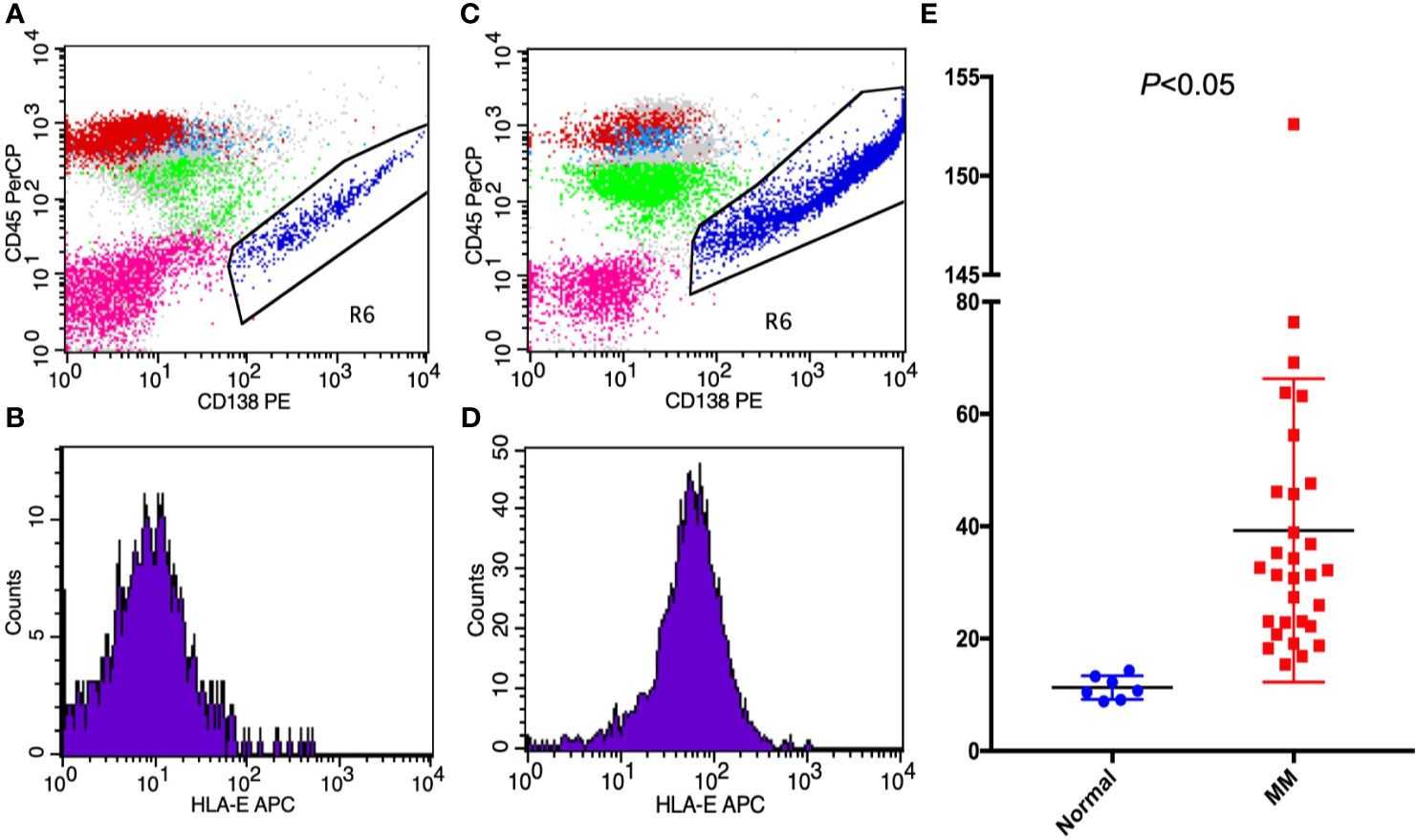
Figure 1 The expressions of (A, B) HLA-E on non-malignant hematological patients; (C, D) HLA-E in MM patients; and (E) HLA-E between MM patients and non-malignant patients presented by mean fluorescence intensity.
The relationship between HLA-E expression and age, gender, stage, and cytogenetic risk stratification in the 30 patients with MM was further analyzed. Considering the median expression value of HLA-E protein of 31.77, median age of 65 years old, and R-ISS staging system, the MM patients were stratified into high-expression and low-expression groups, older and younger age groups, and early stage and advanced stage groups, respectively. Furthermore, the early stage was divided into stages I and II, which included 12 patients, and the advanced stage was categorized as stage III with 18 patients. Patients were also divided into two groups based on cytogenetic risk. The Pearson Chi-square test showed that high HLA-E expression was correlated with advanced ISS stage (p = 0.025) and high cytogenetic risk (p = 0.000) (Table 2). Therefore, the high expression of HLA-E in advanced stage, high-risk MM patients may predict poor prognosis, indicating that HLA-E could be considered as a treatment target, especially for high-risk MM patients.
As mentioned, the results show that HLA-E was highly expressed on myeloma cells. In our previous work, the Molecular Operating Environment (MOE) software was employed to screen four target-binding peptides, namely M and P1-P3, for their affinity to HLA-E. Furthermore, these four peptides were synthesized and labeled with FITC fluorescein, for which the amino acid sequences are listed in Figure 2. The results from our previous work also indicate that P3 specifically binds to HLA-E highly expressed in cell lines with the highest affinity compared to the three other peptides. The purity and molecular weights of these peptides are provided in Supplementary Material. As shown in Figure 3A, CD138+HLA-E+ and CD138+HLA-E- myeloma cells were cultured with peptide M and P3. It was found that both peptide M and P3 could interact with CD138+HLA-E+ cells but not with CD138+HLA-E- myeloma cells. Specifically, the proportions of FITC-labeled peptide M and P3 on CD138+HLA-E+ cells were 21.97% and 53.1%, respectively (Figures 3B, D), but were only 3.17% and 1.65% on CD138+HLA-E- cells (Figures 3C, E).
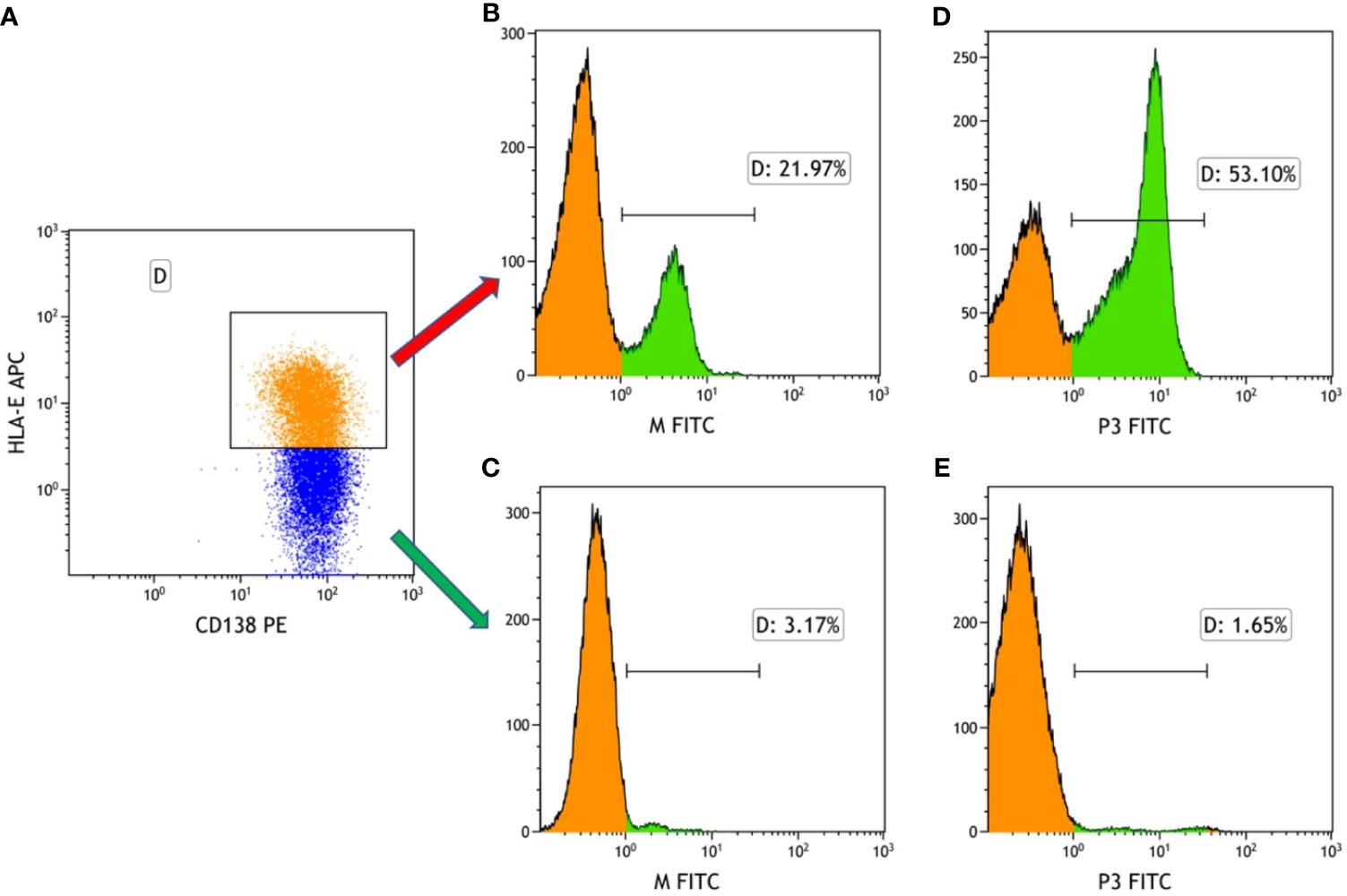
Figure 3 The binding frequency of HLA-E-targeted binding peptides to the bone marrow cells from the patients with NDMM. (A) Myeloma cells were divided into HLA-E+ cells and HLA-E- cells. (B) The binding affinity of peptide M to HLA-E positive myeloma cells was 21.97%. (C) The affinity of peptide M to HLA-E negative myeloma cells was 3.17%. (D) The binding affinity of peptide P3 to HLA-E positive myeloma cells was 53.10%. (E) The binding affinity of peptide M to HLA-E negative myeloma cells was 1.65%.
Immune function plays an important role in MM (13), whereby the absolute lymphocyte count (ALC) is related to the prognosis of MM patients (14). Therefore, a high level of ALC in NDMM patients leads to a better prognosis even in the new immunotherapy era. Although HLA-E has been screened as the key membrane antigen in the development of MM by the bioinformatics method (9), the clinical meaning of the different expressions of HLA-E in MM patients remains unknown. In the present study, we found that the HLA-E protein is highly expressed on MM cells and is linked to high-risk MM. Thus, HLA-E could be considered as both a marker of high-risk MM and a targeted candidate in a new treatment regimen for MM patients.
As a non-classical major histocompatibility complex (MHC) molecule, HLA-E can interact with NK cells and T cells (15, 16). It has been suggested that the overexpression of HLA-E on cells could inhibit the immune clearance function (17). On some tumors, including MM, inflammatory cells and senescent cells with highly expressed HLA-E could escape the NK and T-cell immune surveillance and stay alive in the host body (18–21). Herein, we found that HLA-E was expressed much higher on MM cells than normal plasma cells, especially in NDMM patients. While cytogenetic risk factors are typically used to predict the prognosis of MM patients (22), introducing new medication, such as protease inhibitor, immunomodulatory agents, and monoclonal antibodies, could significantly improve prognoses (7, 23). However, some patients still suffer from disease progression despite these new therapies. Therefore, new prognostic markers and novel therapeutic targets should be investigated for identifying the patients with high-risk MM and improving treatment efficacy.
Furthermore, we divided MM patients into two groups based on the median HLE-A expression value of 31.77. On one hand, no statistical difference existed between the different age and sex groups. On the other hand, the group with a high risk of MM and the standard-risk group showed a significant difference in the expression of HLA-E. Comparatively, HLA-E was expressed much higher in the high-risk group than the low-risk one. This indicates that HLA-E could be considered as a marker for predicting whether the patients have high-risk myeloma or not. Besides, HLA-E might be taken as a potential treatment target for MM, especially for high-risk patients. Since HLA-E overexpression could inhibit the immune clearance function of NK cells, we propose that MM cells could be eliminated by either targeting HLA-E or inhibiting the interaction between HLA-E and NKG2A using peptide P3 (15, 24, 25). In our future work, we aim to examine the effect of peptide P3 on recovering the killing function of NK cells by inhibiting the interaction between HLA-E and NKG2A. Yet, if this recovery cannot be done by peptide P3, HLA-E could be considered as a target to find MM cells, and then a peptide drug conjugate could be produced to target MM cells.
Target-binding peptides M and P1-3, which were designed and synthesized in our previous work, could interact with the HLA-E protein in MM cell lines (9). Comparatively, peptide M exhibited the lowest binding affinity with HLA-E, while P3 showed the highest affinity. The present study further verified this finding in the bone marrow of MM patients. The results confirm that peptide P3 could bind to HLA-E positive cells but cannot interact with HLA-E negative cells in bone marrow.
The results of this study reveal the overexpression of HLA-E on MM cells, especially of high-risk patients, and the high binding frequency of peptide P3 to HLA-E on MM patients in vitro. From a therapeutic perspective, HLA-E can be considered as an effective targeting therapy against MM cells, while P3 specifically binds with HLA-E. Consequently, this interrupts the interaction between HLA-E and the inhibitory receptor NKG2A, providing a promising strategy to improve the immune clearance of MM cells.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by ethics committee in Shengjing hospital, 2020PS215K. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HW and YY conceived and designed the study. YY and GZ performed the experiment and collected the data. GZ and ZL performed the data analysis and statistical analysis. YY, HW, and GZ revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the 345 Talent Project in Shengjing Hospital of China Medical University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank all the patients recruited from Shengjing Hospital of China Medical University for participating in the research that made this study possible. Also, we thank the colleagues, especially Fang Chen, who participated in this research.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.670673/full#supplementary-material
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin (2017) 67:7–30. doi: 10.3322/caac.21387
2. Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued Improvement in Survival in Multiple Myeloma: Changes in Early Mortality and Outcomes in Older Patients. Leukemia (2014) 28:1122–8. doi: 10.1038/leu.2013.313
3. Yanamandra U, Kumar SK. Minimal Residual Disease Analysis in Myeloma - When, Why and Where. Leuk Lymphoma (2018) 59:1772–84. doi: 10.1080/10428194.2017.1386304
4. Mateos M-V, Cavo M, Blade J, Dimopoulos MA, Suzuki K, Jakubowiak A, et al. Overall Survival With Daratumumab, Bortezomib, Melphalan, and Prednisone in Newly Diagnosed Multiple Myeloma (ALCYONE): A Randomised, Open-Label, Phase 3 Trial. Lancet (2020) 395:132–41. doi: 10.1016/S0140-6736(19)32956-3
5. Rasche L, Weinhold N, Morgan GJ, van Rhee F, Davies FE. Immunologic Approaches for the Treatment of Multiple Myeloma. Cancer Treat Rev (2017) 55:190–9. doi: 10.1016/j.ctrv.2017.03.010
6. Cho S-F, Lin L, Xing L, Yu T, Wen K, Anderson KC, et al. Monoclonal Antibody: A New Treatment Strategy Against Multiple Myeloma. Antibodies (Basel) (2017) 6:18. doi: 10.3390/antib6040018
7. Spencer A, Lentzsch S, Weisel K, Avet-Loiseau H, Mark TM, Spicka I, et al. Daratumumab Plus Bortezomib and Dexamethasone Versus Bortezomib and Dexamethasone in Relapsed or Refractory Multiple Myeloma: Updated Analysis of CASTOR. Haematologica (2018) 103:2079–87. doi: 10.3324/haematol.2018.194118
8. Strong RK, Holmes MA, Li P, Braun L, Lee N, Geraghty DE. HLA-E Allelic Variants. Correlating Differential Expression, Peptide Affinities, Crystal Structures, and Thermal Stabilities. J Biol Chem (2003) 278:5082–90. doi: 10.1074/jbc.M208268200
9. Yang Y, Sun M, Yu Z, Liu J, Yan W, Liu Z, et al. Designing High Affinity Target-Binding Peptides to HLA-E: A Key Membrane Antigen of Multiple Myeloma. Aging (Albany NY) (2020) 12:20457–70. doi: 10.18632/aging.103858
10. Kobayashi K, Mizuguchi T, Hattori Y, Ohara N, Ninomiya R, Iida M, et al. Effects of Replacement and Addition of an Amino Acid Contained in a Cyclic Peptide Corresponding to a β-Hairpin Loop Sequence of Human EGF Receptor. J Pept Sci (2017) 23:581–6. doi: 10.1002/psc.3004
11. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M-V, et al. International Myeloma Working Group Updated Criteria for the Diagnosis of Multiple Myeloma. Lancet Oncol (2014) 15:e538–48. doi: 10.1016/S1470-2045(14)70442-5
12. Greipp PR, San Miguel J, Durie BGM, Crowley JJ, Barlogie B, Bladé J, et al. International Staging System for Multiple Myeloma. J Clin Oncol (2005) 23:3412–20. doi: 10.1200/JCO.2005.04.242
13. Shah N, Aiello J, Avigan DE, Berdeja JG, Borrello IM, Chari A, et al. The Society for Immunotherapy of Cancer Consensus Statement on Immunotherapy for the Treatment of Multiple Myeloma. J Immunother Cancer (2020) 8:e000734. doi: 10.1136/jitc-2020-000734
14. Yang Y, Liu Z, Wang H. Peripheral Absolute Lymphocyte Count: An Economical and Clinical Available Immune-Related Prognostic Marker for Newly Diagnosed Multiple Myeloma. Med Sci Monit (2020) 26:e923716. doi: 10.12659/MSM.923716
15. Abd Hamid M, Wang R-Z, Yao X, Fan P, Li X, Chang X-M, et al. Enriched HLA-E and CD94/NKG2A Interaction Limits Antitumor CD8+ Tumor-Infiltrating T Lymphocyte Responses. Cancer Immunol Res (2019) 7:1293–306. doi: 10.1158/2326-6066.CIR-18-0885
16. Braud VM, Allan DS, O’Callaghan CA, Söderström K, D’Andrea A, Ogg GS, et al. HLA-E Binds to Natural Killer Cell Receptors CD94/NKG2A, B, and C. Nature (1998) 391:795–9. doi: 10.1038/35869
17. Ravindranath MH, Filippone EJ, Devarajan A, Asgharzadeh S. Enhancing Natural Killer and CD8+ T Cell-Mediated Anticancer Cytotoxicity and Proliferation of CD8+ T Cells With HLA-E Monospecific Monoclonal Antibodies. Monoclon Antib Immunodiagn Immunother (2019) 38:38–59. doi: 10.1089/mab.2018.0043
18. Carlsten M, Namazi A, Reger R, Levy E, Berg M, St. Hilaire C, et al. Bortezomib Sensitizes Multiple Myeloma to NK Cells Via ER-Stress-Induced Suppression of HLA-E and Upregulation of DR5. Oncoimmunology (2018) 8:e1534664. doi: 10.1080/2162402X.2018.1534664
19. Pereira BI, Devine OP, Vukmanovic-Stejic M, Chambers ES, Subramanian P, Patel N, et al. Senescent Cells Evade Immune Clearance Via HLA-E-Mediated NK and CD8+ T Cell Inhibition. Nat Commun (2019) 10:2387. doi: 10.1038/s41467-019-10335-5
20. Goel R, Kabeerdoss J, Mohan H, Danda S, Jayaseelan V, Kumar TS, et al. Soluble-HLA-E: A Follow Up Biomarker in Takayasu Arteritis, Independent of HLA-E Genotype. Int J Rheum Dis (2018) 21:532–40. doi: 10.1111/1756-185X.13027
21. Costa Arantes DA, Gonçalves AS, Jham BC, Duarte ECB, de Paula ÉC, de Paula HM, et al. Evaluation of HLA-G, Hla-E, and PD-L1 Proteins in Oral Osteosarcomas. Oral Surg Oral Med Oral Pathol Oral Radiol (2017) 123:e188–96. doi: 10.1016/j.oooo.2016.12.002
22. Rajkumar SV. Multiple Myeloma: 2018 Update on Diagnosis, Risk-Stratification and Management. Am J Hematol (2018) 93:981–1114. doi: 10.1002/ajh.25117
23. Rosiñol L, Oriol A, Rios R, Sureda A, Blanchard MJ, Hernández MT, et al. Bortezomib, Lenalidomide, and Dexamethasone as Induction Therapy Prior to Autologous Transplant in Multiple Myeloma. Blood (2019) 134:1337–45. doi: 10.1182/blood.2019000241
24. Mahaweni NM, Ehlers FAI, Bos GMJ, Wieten L. Tuning Natural Killer Cell Anti-Multiple Myeloma Reactivity by Targeting Inhibitory Signaling Via KIR and NKG2A. Front Immunol (2018) 9:2848. doi: 10.3389/fimmu.2018.02848
Keywords: HLA-E, high risk, multiple myeloma, clinical outcomes, target-binding peptide
Citation: Yang Y, Liu Z, Wang H and Zhang G (2021) HLA-E Binding Peptide as a Potential Therapeutic Candidate for High-Risk Multiple Myeloma. Front. Oncol. 11:670673. doi: 10.3389/fonc.2021.670673
Received: 22 February 2021; Accepted: 17 May 2021;
Published: 09 June 2021.
Edited by:
Claudio Cerchione, Istituto Scientifico Romagnolo per lo Studio e il Trattamento dei Tumori (IRCCS), ItalyReviewed by:
Guru Prasad Maiti, Oklahoma Medical Research Foundation, United StatesCopyright © 2021 Yang, Liu, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guojun Zhang, emhhbmdnakBzai1ob3NwaXRhbC5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.