
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 20 May 2021
Sec. Pediatric Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.668505
Purpose: To investigate the clinicopathological features and BRAF V600E mutation of melanotic neuroectodermal tumor of infancy (MNTI).
Materials and Methods: Eleven cases of MNTI diagnosed at the Department of Oral Pathology were collected. Clinicopathological characteristics were obtained from the medical records. Immunostaining was performed by immunohistochemistry (IHC). Amplification-Refractory Mutation System-qPCR (ARMS-qPCR) and Sanger Sequencing were used to detect BRAF V600E mutation.
Results: Of the 11 cases, 3 cases were female and 8 cases were male. The mean age of the first symptoms was 3.2 months (range: 1 to 6 months). Ten cases (90.9%) located in maxilla but only one (9.1%) in mandible. Most of the cases demonstrated well-defined mass with lytic bone destruction and tooth germ affecting radiologically. Histologically, MNTI was consisted of large polygonal melanin-producing epithelioid cells and small round neuroblast-like cells which arranged in irregular alveolar, tubuloglandular and fissured architecture. The epithelioid cells expressed Vim, Pan-CK, NSE and HMB45, while the smalls cells expressed Syn, NSE and scattered Vim. Most cases showed low Ki-67 index (range: <1% to 50%). None of the MNTI cases showed BRAF V600E mutation. Most cases were treated with enucleation (45.4%) or curettage (36.4%). Among the 11 cases, 6 cases had follow-up information, and 2 cases had recurrence lesions after surgery.
Conclusion: MNTI, an extremely rare tumor, mainly affects male infants with strong preference for maxilla. Distinct histopathological features and immunohistochemical profile are helpful to distinguish from other melanin-containing tumors and small round cell tumors. No BRAF V600E mutation in MNTI is detected in the present study and needs further investigations. The factors that contribute to the local recurrence of MNTI are controversial, but the close follow-up for the patients is recommended.
Melanotic neuroectodermal tumor of infancy (MNTI) is a rare neoplasm which most commonly arises in the anterior region of the maxilla and always develops during the first year of life (1). It was proposed to be of neural crest origin by Borello and Gorlin (2). Later, Hoshino et al. reported high serum levels of catecholamines in MNTI which suggested a neural crest origin of the tumor (3). The clinical manifestations of MNTI include rapid growth, light blue or pink coloration and displacement of developing teeth. MNTI predominantly affects craniofacial regions in more than 90% cases (4). The largest systematic review of 472 cases showed that 62.2% cases involved maxilla, followed by the skull (15.6%), and mandible (7.8%). MNTI shows a slight male predilection with a 1.27:1 male: female ratio (5). On a computed tomography (CT) scan, MNTI always presents as well-defined hyperdense mass. It shows radiolucent lytic lesion within bone and might have local bone destruction features (6). Although MNTI is generally regarded as a benign tumor, most of the lesions shows aggressive clinical behaviors, including rapidly expanding, destruction of surrounding bony tissues and potential of local recurrence.
Morphologically, MNTI consists of two types of cells with distinct features and different immunohistochemical profile (6). More importantly, only few studies had reported molecular alterations in MNTI, including a case with BRAF V600E mutation (7), and another case with CDKN2A mutation and RPLP1-C19MC gene fusion (8).
Because of the rarity of MNTI, most of the literature have documented single case. We present here with 11 additional cases of MNTI. The clinicopathological characteristics, immunohistochemical profile and molecular alterations, i.e. BRAF V600E mutation, of MNTI are analyzed in this study.
The study was approved by the Ethics Committee of Shanghai Ninth People’s Hospital, College of Stomatology, Shanghai Jiao Tong University School of Medicine.
Eleven cases of MNTI were retrieved from the electronic database of the Department of Oral Pathology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. All the cases were reassessed by two independent pathologists (RHX and CYZ). The clinicopathological characteristics, including sex, age, tumor location, size and follow-up information were obtained (Table 1).
Formalin-fixed paraffin-embedded (FFPE) samples were cut into 4 μm sections, and were deparaffinized in xylene and rehydrated through graded ethanol series. The immunohistochemistry procedures were performed using the DAKO AutostainerLink 48 (Agilent Technologies, California), according to the manufacturer’s recommendations. All the cases were stained with pancytokeratin (Pan-CK), HMB45, Melan-A, synaptophysin (Syn), chromogranin (CgA), Ki-67, vimentin (Vim), neuron-specific enolase (NSE), S-100 and desmin (Des) (detailed information for all the antibodies were summarized in Table 2). Signals were visualized through the AEC+ High Sensitivity Substrate Chromogen kit (K3469, Agilent Technologies, California).
Amplification-refractory mutation system-qPCR (ARMS-qPCR) was performed using BRAF V600E Mutation Detection Kit (SinoMD Gene Technology, Beijing, China) on the CFX96 machine (BioRad, Hercules, CA, USA). The sensitivity of this kit is 97.89%, and it is sensitive enough for the detection of as low as 1% BRAF V600E mutation. Briefly, the fluorescence signal of FAM using primers specific covering BRAF V600E region (determined as Ctm) and the signal of HEX using internal control primers were collected for each sample. The elevation of the HEX signal suggested that the amplification procedure was reliable. Besides, for each sample, a paired test using primers covering BRAF non-V600E region was conducted as amplification control and was determined as Ctc. The data analysis was based on the “ΔCt = (Ctm-Ctc)” method. If ΔCt ≤ 8, the sample was considered as BRAF V600E mutation positive sample. If ΔCt > 8, the sample was considered as a negative one. Except the positive control using in the kit, another two cases of Langerhans cell histiocytosis (LCH) which were previously confirmed with BRAF V600E mutation were also used as a positive control.
Direct Sanger sequencing for BRAF V600E mutation was done by SinoMD Gene Technology Co. Ltd. (Beijing, China). Briefly, genomic DNA was extracted from FFPE samples using QIAamp DNA FFPE Tissue Kit (Cat. No. 56404, Qiagen, Hilden, Germany). The DNA was amplified by polymerase chain reaction (PCR), and then sequencing was done by capillary electrophoresis using Applied biosystem 3500 Genetic Analyzer (Waltham, MA, USA) with forward primer 5’-AGACCTCACAGTAAAAATAGGTGA-3’ and reverse primer 5’- CTGATGGGACCCACTCCATC-3’. The results were compared with the wild type BRAF sequence (NM_001354609.2). The same two cases of LCH with confirmed BRAF V600E mutation were used as a positive control.
The detailed clinical information was summarized in Table 1. Of eleven cases, three cases were female and eight cases were male. There was a 1:2.67 male predominance. The mean age of the first symptoms was 3.2 months (range: 1 to 6 months). The mean age at diagnosis was 10.6 months (range: 2 to 45 months). One patient was a recurrent case, who had surgery at the age of 45 months (Case 8 in Table 1). Ten of eleven cases (90.9%) located in maxilla but only one (9.1%) in mandible. The mean size of tumor was 3.1 cm (range: 2.0 to 6.0 cm). Interestingly, based on the Routine Blood Test, 6 patients (54.5%) showed elevated Platelet Count. Radiologically, most of the cases demonstrated well-defined mass with lytic bone destruction and tooth germ affecting in Computed Tomography (CT) imaging (Figure 1A). Based on the clinical and imaging findings, the impression of dentigerous cyst, teratoma, LCH and malignant tumor were suggested before the pathological confirmation.
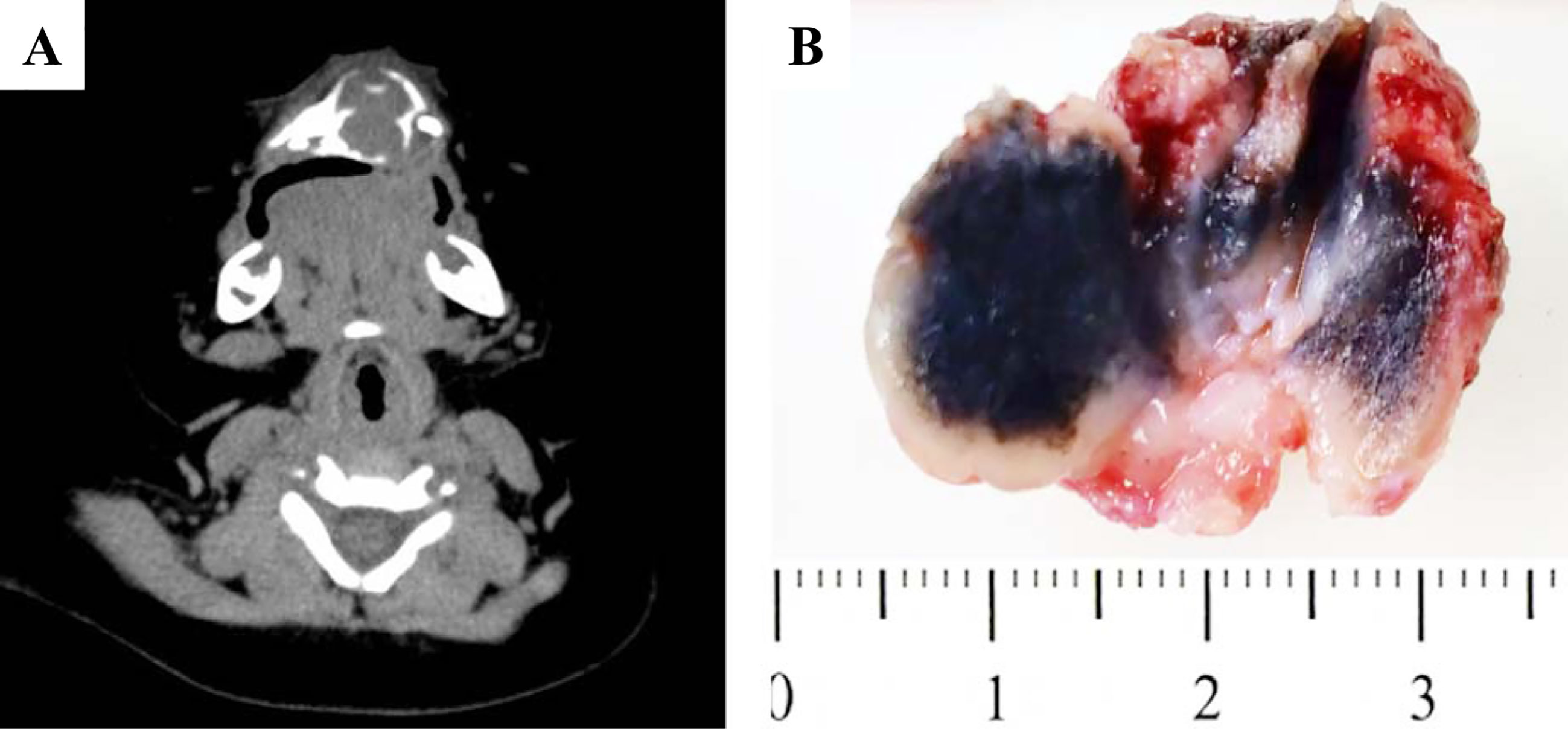
Figure 1 Gross pictures and radiological features of MNTI. (A) CT images demonstrated well-defined mass with lytic bone destruction and tooth germ affecting. (B) The tumor was firm, unencapsulated and the cut-surface was pigmented.
Grossly, the tumor was firm, unencapsulated and the cut-surface was pigmented (Figure 1B). Morphologically, the tumor was consisted of two types of cells which arranged in irregular alveolar, tubuloglandular and fissured architecture, with cords and strands intersected by dense fibroblastic stroma (Figure 2). The large polygonal epithelioid cells were melanin-producing and showed vesicular nuclei, prominent nucleoli and eosinophilic cytoplasm. The small round neuroblast-like cells were surrounded by the epithelioid cells and showed hyperchromatic nuclei and scanty cytoplasm (Figures 2A–C). The proportion of the epithelioid cells varied from patient to patient, with a mean of 51% (range: 20% to 95%) (Table 3). In some cases, most of the tumor cells were large melanin-producing epithelioid cells (Figures 2D–F), while other cases were predominant with small neuroblast-like cells (Figures 2G–I).
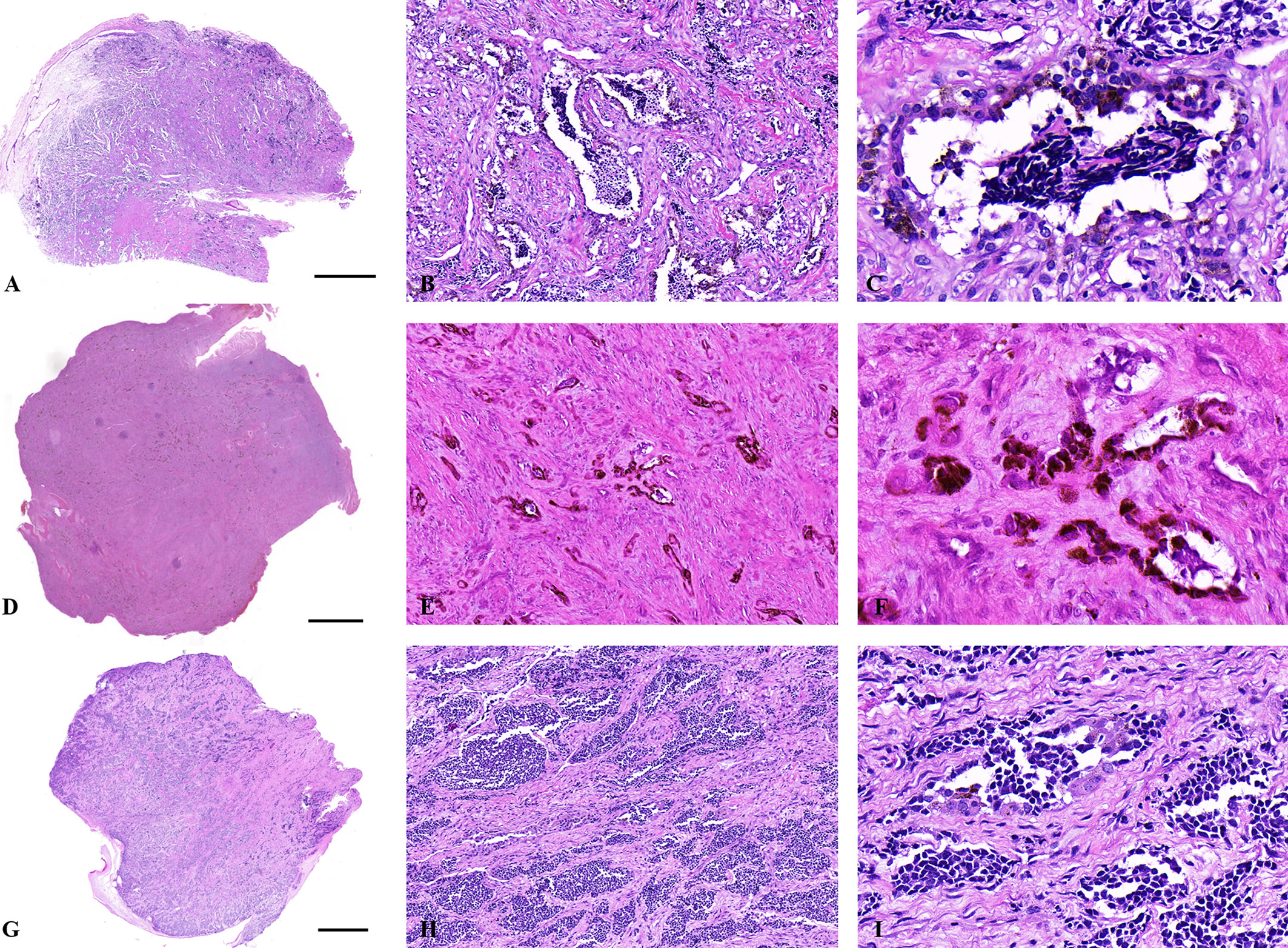
Figure 2 Histological features of MNTIs. (A–C) The tumor was consisted of two types of cells which arranged in irregular alveolar, tubuloglandular and fissured architecture, with cords and strands intersected by dense fibroblastic stroma. (D–F) this case was predominant with large melanin-producing epithelioid cells. (G–I) this case was predominant with small neuroblast-like cells. Hematoxylin-Eosin staining. Scale bar: 2000μm. (B, E, H) x100. (C, F, I) x400.
Immunohistochemically, the large polygonal epithelioid cell and the small neuroblast-like cells had distinct phenotype. The epithelioid cells of all the tumors expressed Vim, Pan-CK, NSE and HMB45, while the smalls cells expressed Syn (11/11, 100.0%), NSE (11/11, 100.0%) and CgA (4/11, 36.4%). We found that the small neuroblast-like cells showed scattered staining for Vim and all the cases were negative for S-100, Melan-A and Des (Figure 3). The Ki-67 index ranged from <1% to 50%. Most of the cases showed low Ki-67 index, i.e. 6 cases (54.5%) with <1%, 2 cases (18.2%) with 2%, 1 case (9.1%) with 5%, 1 case (9.1%) with 40% and 1 case (9.1%) with 50%. The detailed immunohistochemical profile were summarized in Table 3.
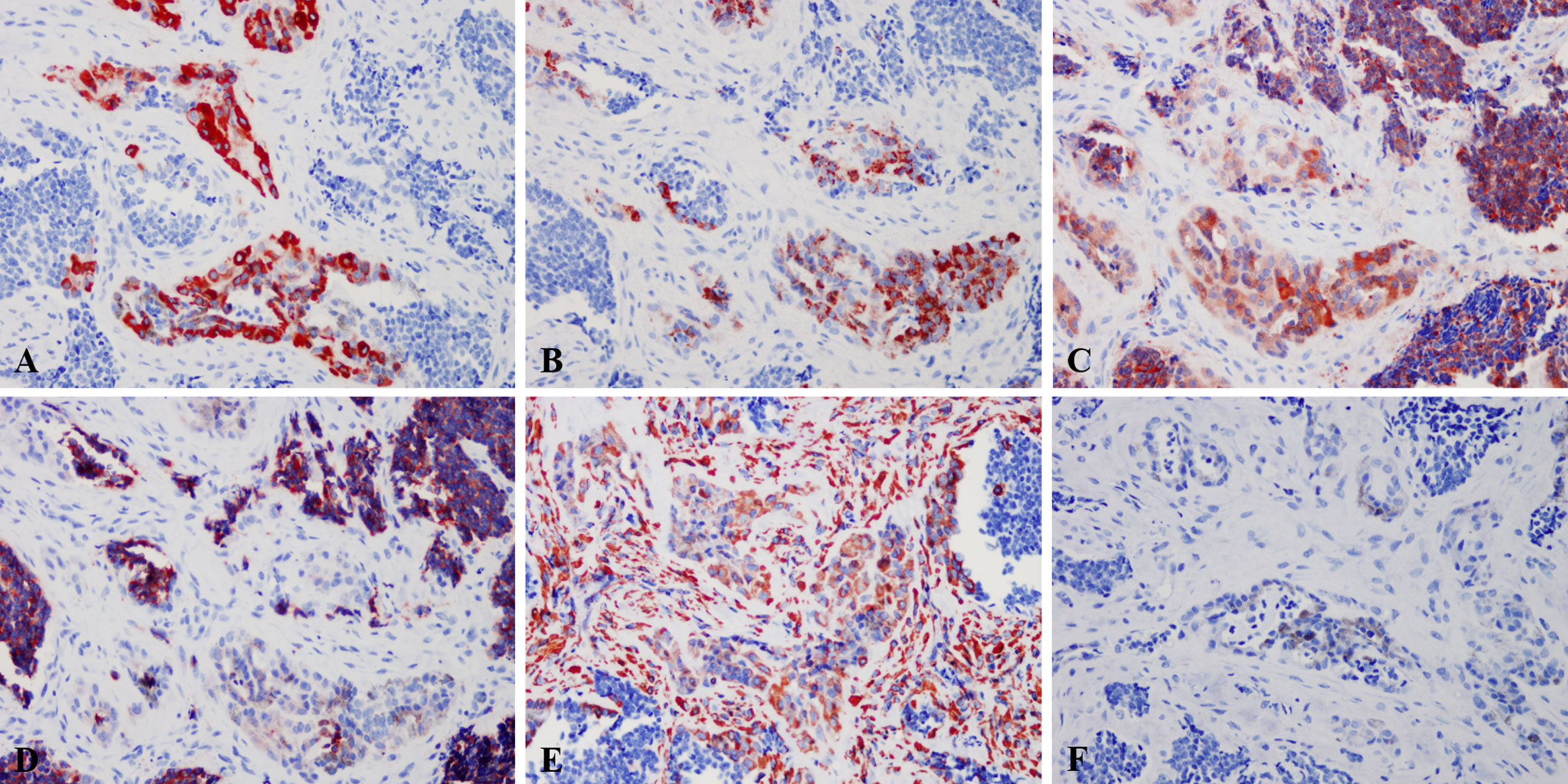
Figure 3 Immunohistochemical profile of MNTI. The epithelioid cells expressed Pan-CK (A), HMB45 (B), NSE (C), while the smalls cells strongly expressed NSE (C), Syn (D). The large cells were also positive for Vim, while the small cells only showed scattered positivity (E). Both large and small cells were negative for S-100 (F). Immunohistochemistry, x400.
The frequency of BRAF V600E mutation was reported as high as 50%-80% in melanoma (9). Based on the similarities between MNTI and melanoma, such as derivation from the neural crest cells and the presence of melanin-producing cells, Gomes et al. (7) suggested that MNTI might also harbor BRAF V600E mutation and they found 1 case with BRAF V600E mutation. Here we performed ARMS-qPCR and direct Sanger Sequencing in all the 11 cases of MNTI and 2 case of LCH. The latter was used as positive control. All the 11 cases of MNTI did not harbor BRAF V600E mutation while 2 cases of LCH were positive for BRAF V600E mutation (Figure 4).
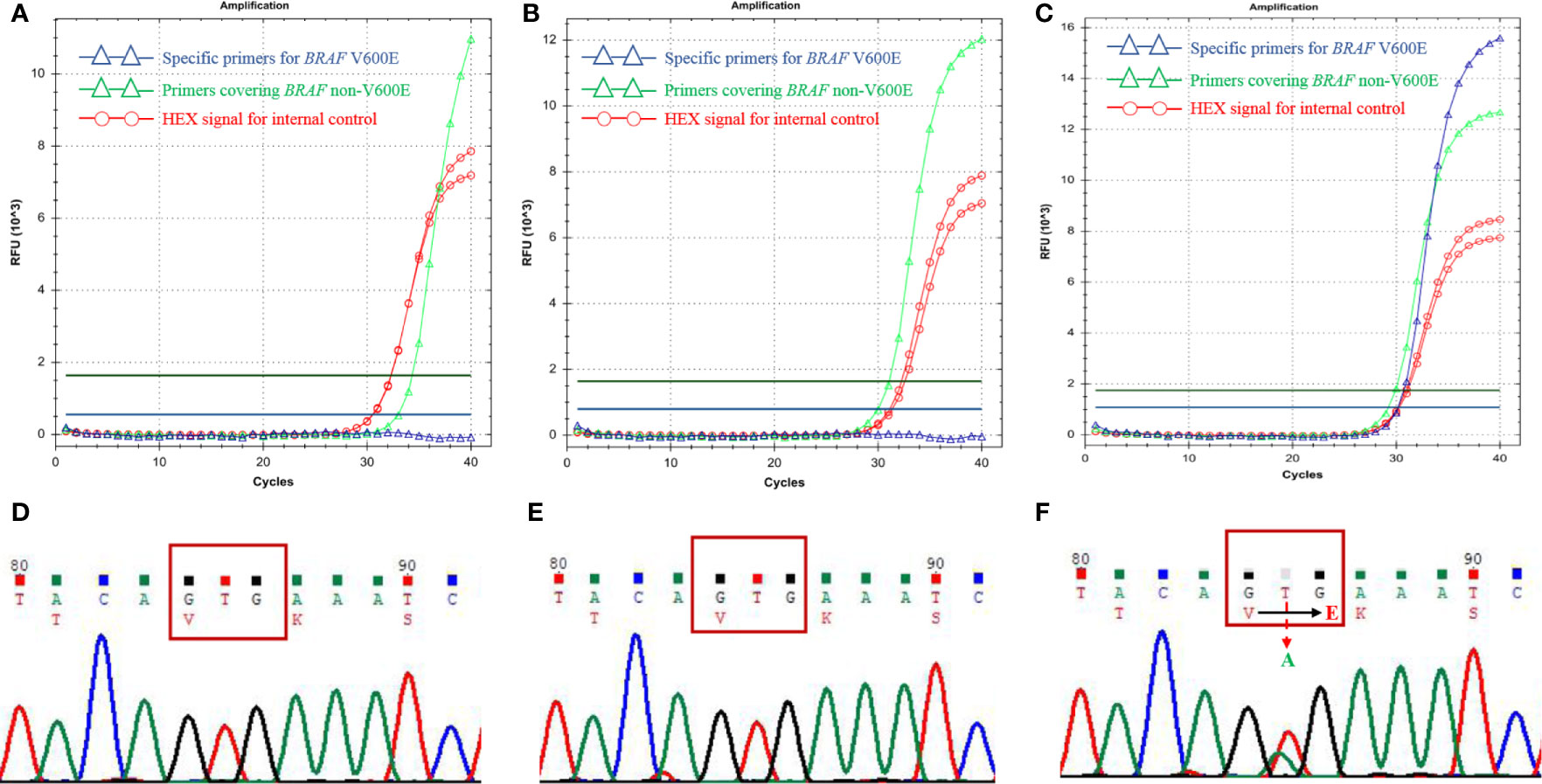
Figure 4 BRAF V600E mutation detections in MNTIs and LCH. Detections performed by ARMS-qPCR and Sanger Sequencing showed Case 1 (A, D) and Case 11 (B, E) were negative for BRAF V600E mutation, while the positive control, LCH case was positive for BRAF V600E mutation (C, F).
Of eleven cases, 5 cases were treated with enucleation, 4 cases were treated curettage, the recurrent case were treated with partial resection of maxilla, and one case were performed with biopsy. 6 cases had follow-up information. 2 cases had recurrence lesions one month and 77 months after surgery, respectively. The proportion of the epithelioid cells of those two recurrent cases was 20% and 50% respectively. The Ki-67 index of those two recurrent cases was <1% and 50% respectively. The surgical mode for these two cases which developed recurrence was curettage. The detailed treatment and follow-up information were summarized in Table 1.
MNTI is an extremely rare neoplasm occurring frequently in male infant younger than 1-year old. Variable names, including melanotic progonoma (10), pigmented congenital epulis (11), retinal anlage tumor (12), etc. have been used for this tumor since 1918. However, all those synonyms are obsolete and not recommended. The name of MNTI is widely accepted now and the tumor is proposed to be of neural crest origin because the tumor produces VMA (2) and has heterogeneous cellular phenotype microscopically which explains by the mesodermal and ectodermal morphological features displayed by neural crest cells at different stages of their ontogeny (13).
Clinically, MNTI may appear as a rapid expansive, lobulated, but non-ulcerated and painless mass. It always presents with light blue or pink appearance (14). In the current study, MNTI mainly affects the maxilla of male infants, which is consistent with the literature (5, 15). Besides the maxilla, other sites, including skull, mandible (16), and noncranial regions, such as brain, epididymis (2), mediastinum, femur and ovary (4, 17) may be affected. Moreau et al. reported 11 cases of MNTI and found that the mean age of the first symptoms and at diagnosis was 2.8 months and 3.2 months, respectively (15). The current study showed similar results, the mean age of the first symptoms and at diagnosis was 3.2 months and 10.6 months, respectively. It should be noticed that there was a time lag between the onset of symptoms and the diagnosis. The reason for this might be that some patients received surgeries at older age because it was not tolerated at a younger age. One limitation for the current study was that none of the cases were subjected to urinary VMA level detection. We could not acquire this information from this cohort. Interestingly, we identified a proportion of cases in this cohort which showed elevated Platelet Count based on the Routine Blood Test. However, it needed more studies and detections before drawing a reliable conclusion.
MNTI is categorized as one type of benign maxillofacial bone and cartilage tumors in 4th edition of WHO Classification of Head and Neck Tumors. However, radiologically, MNTI always presented with well circumscribed hypodense mass, but may show excessive bone destruction and displacement of dental follicles under CT scan (13). The present case showed a well-defined, unilocular, osteolytic lesion causing expansion and destruction of the maxillary cortical bone, which was in line with the previous publications. The age, tumor location and radiological findings may be suggestive of this tumor. However, we should notice that in the current study, most case were clinically diagnosed with teratoma, cyst or LCH preliminary, only one case were diagnosed as MNTI because this was a recurrent case. Thus, pathological examination is required for a definitive diagnosis.
Late diagnosis might lead to the difficulty in radical resection, because the tumor involves the adjacent structures with rapid invasive growth (4). Once suspected to be MNTI, pathological confirmation might be performed as soon as possible. Histologically, two types of cells with distinct morphological and immunohistochemical phenotype could be seen within this tumor. The tumor cells are arranged in irregular alveolar, tubuloglandular and fissured architecture, with cords and strands intersected by dense fibroblastic stroma. The large melanin-producing polygonal epithelioid cells had vesicular nuclei, prominent nucleoli and eosinophilic cytoplasm, while the small round neuroblast-like cells were surrounded by the epithelioid cells and showed hyperchromatic nuclei and scanty cytoplasm (6). In the present study, the proportion of these two types of cells varied case by case. When a case was predominant with large epithelioid cells (18) or small neuroblast-like cells (19), immunohistochemistry should be performed to confirm the diagnosis because the two type of cells had distinct immunohistochemical phenotype and some of the markers could indicated the presence of the specific type of the cells. In the current study, both large and small cells expressed NSE and Vim, even though that only few small cells expressed Vim. The epithelioid cells were also positive for Pan-CK, HMB45 and the small neuroblast-like cells were positive for Syn. All the tumors were negative for S-100, Melan-A and Des. The immunohistochemical results were in line with the previous publications (6). Those distinct immunohistochemical profile could help distinguishing with other melanin-containing tumors or small round cell tumors, such as melanoma, neuroblastoma, Ewing sarcoma and rhabdomyosarcoma.
The investigations of molecular alterations of MNTI were limited. In 1998, Khoddami et al. (20) studied three typical cases of MNTI and tried to find out the genetic link between MNTI and other small round cell tumors with well-characterized molecular alterations, including MYCN gene amplification, deletion of 1p, the presence of the t(11;22)(q24;q12) and the t(11;22)(p13;q12) translocations. Unfortunately, none of the cases yielded positive results. Melanoma showed a high frequency of BRAF V600E mutation. Gomes et al. (7) performed BRAF V600E mutation detection in three cases of MNTI based on the hypothesis of the similarities with melanoma. They confirmed 1 case with BRAF V600E mutation. Based on those findings, we tried to elucidate the BRAF V600E mutation status in the present cohort. As the pigment can interfere with the fluorescence in the qPCR assay, to ensure the accuracy of the detection, internal and amplification control were included in the ARMS-qPCR experiments. Sanger Sequencing method was used to confirm the results, we also included 2 cases of LCH with confirmed BRAF V600E mutation as positive control. As the results showed, none of the MNTI cases harbored BRAF V600E mutation. The result was inconsistent with Gomes et al.’s study, the tumor enrichment prior to DNA extraction in their investigation might partly explain the mutation detection in one case. However, it had been noticed that another two cases in recent publications were also negative for BRAF V600E mutation (18, 21). Paraffin-embedded samples could influence the molecular analysis, further studies on molecular alterations should be conducted using fresh or frozen samples, if possible. Though all those results were based on limited cases, the frequency of BRAF V600E mutation in MNTI and the individualized treatment plan that might be developed due to this mutation needed more explorations. It had been reported that besides BRAF, other mutated genes, including CDKN2A, NRAS, TP53, NF1 and SF3B1 played significant role in different types of melanoma. The results suggested that those genetic alterations might be also candidates for MNTI, and needed further investigations (22). A better understanding of the molecular biology of MNTI is warranted to improve the efficiency of current treatment strategies, especially for those patients with recurrent or metastatic diseases.
At present, the most common treatment protocol is surgery (16, 23). In the present study, enucleation was the predominant treatment (45.4%), followed by curettage (36.4%), partial resection of maxilla (9.1%) and biopsy (9.1%). Chemotherapy and radiation therapy, either alone or in combination with surgery had also been proposed (5, 24). Because MNTI was consisted of large epithelioid cells and neuroblast-like cells, when it was predominant with neuroblast-like cells, chemotherapy has been suggested as the primary treatment mode (25). Adjuvant chemotherapy was also conducted to avoid recurrence after incomplete surgery (19). Targeted therapy should base on the definitive genetic alterations, which had been inconclusive for MNTI until now.
Unfortunately, the biological behavior of MNTI is hard to be predicted by histopathological features, or immunohistochemical markers. Age of diagnosis (5), gender (4, 5), localization in the maxilla, and R1/R2 resection (4), curettage (16), predominance of a neuroblast-like cell component (26) and Ki-67 index (14) were considered as indicators for high risk of local recurrence. However, those results were controversial because a recent publication reported that none of these factors seemed to be associated with a higher risk of recurrence (15). In the present study, it seemed that only the surgical mode, i.e. curettage showed a correlation with the local recurrence. However, the results in the present study should be considered with caution and a lager sample size of patients were needed to confirm our results and allow statistically relevant conclusions to be drawn.
MNTI, although an extremely rare tumor, it should be added to the differential diagnosis of pediatric population with rapidly growing head and neck lesions. It mainly affects male infants younger than 1-year old with strong preference for maxilla. Distinct histopathological features and immunohistochemical profile are helpful to distinguish from other melanin-containing tumor and small round cell tumors. BRAF V600E mutation in MNTI is very rare and needs further investigations. The factors that contribute to the local recurrence of MNTI are controversial, but the close follow-up for the patients are recommended.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee of Shanghai Ninth People’s Hospital, College of Stomatology, Shanghai Jiao Tong University School of Medicine. Written informed consent for participation was not provided by the participants’ legal guardians/next of kin because this is a retrospective study and all the data are anonymous, and the requirement for written consent was therefore waived.
Study conception and design: JL and ZT. Acquisition of data: RHX and CYZ. Analysis and interpretation of data: ZT and JJS. Drafting and revising the article: RHX, LZW, and YHH. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (81872187 and 81702694), Postdoctoral Program of Shanghai Ninth People’s Hospital and the SHIPM-mu fund No. JC201901 from Shanghai Institute of Precision Medicine, Ninth People’s Hospital Shanghai Jiao Tong University School of Medicine and CAMS Innovation Fund for Medical Sciences (CIFMS) (Project No. 2019-I2M-5-037). The funders had no role in study design, data collection, analysis and interpretation, decision to submit the article for publication, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Sharma P, Yadav AK, Goyal S, Mandal AK. Melanotic neuroectodermal tumor of infancy: A rare entity. J Oral Maxillofac Pathol (2019) 23:134–7. doi: 10.4103/jomfp.JOMFP_237_16
2. Borello ED, Gorlin RJ. Melanotic neuroectodermal tumor of infancy–a neoplasm of neural crese origin. Report of a case associated with high urinary excretion of vanilmandelic acid. Cancer (1966) 19:196–206. doi: 10.1002/1097-0142(196602)19:2<196::AID-CNCR2820190210>3.0.CO;2-6
3. Hoshino S, Takahashi H, Shimura T, Nakazawa S, Naito Z, Asano G. Melanotic neuroectodermal tumor of infancy in the skull associated with high serum levels of catecholamine. Case report. J Neurosurg (1994) 80:919–24. doi: 10.3171/jns.1994.80.5.0919
4. Kruse-Losler B, Gaertner C, Burger H, Seper L, Joos U, Kleinheinz J. Melanotic neuroectodermal tumor of infancy: systematic review of the literature and presentation of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod (2006) 102:204–16. doi: 10.1016/j.tripleo.2005.08.010
5. Rachidi S, Sood AJ, Patel KG, Nguyen SA, Hamilton H, Neville BW, et al. Melanotic Neuroectodermal Tumor of Infancy: A Systematic Review. J Oral Maxillofac Surg (2015) 73:1946–56. doi: 10.1016/j.joms.2015.03.061
6. Soles BS, Wilson A, Lucas DR, Heider A. Melanotic Neuroectodermal Tumor of Infancy. Arch Pathol Lab Med (2018) 142:1358–63. doi: 10.5858/arpa.2018-0241-RA
7. Gomes CC, Diniz MG, De Menezes GH, Castro WH, Gomez RS. BRAFV600E Mutation in Melanotic Neuroectodermal Tumor of Infancy: Toward Personalized Medicine? Pediatrics (2015) 136:e267–9. doi: 10.1542/peds.2014-3331
8. Barnes DJ, Hookway E, Athanasou N, Kashima T, Oppermann U, Hughes S, et al. A germline mutation of CDKN2A and a novel RPLP1-C19MC fusion detected in a rare melanotic neuroectodermal tumor of infancy: a case report. BMC Cancer (2016) 16:629. doi: 10.1186/s12885-016-2669-3
9. Chavda J, Bhatt H. Systemic review on B-Raf(V600E) mutation as potential therapeutic target for the treatment of cancer. Eur J Med Chem (2020) 206:112675. doi: 10.1016/j.ejmech.2020.112675
10. Blanc WA, Rosenblatt P, Wolff JA. Melanotic progonoma (retinal anlage tumor) of the shoulder in an infant; a case report. Cancer (1958) 11:959–63. doi: 10.1002/1097-0142(195809/10)11:5<959::AID-CNCR2820110513>3.0.CO;2-S
11. Henry TC, Bodian M. A case of pigmented congenital epulis. Br J Surg (1960) 47:574–6. doi: 10.1002/bjs.18004720524
12. Martin H, Foote FW Jr. A third case of so-called retinal anlage tumor. Cancer (1951) 4:86–91. doi: 10.1002/1097-0142(195101)4:1<86::AID-CNCR2820040108>3.0.CO;2-B
13. Santos HBP, Luna AHB, Goes PEM, Sverzut AT, Nonaka CFW, Alves PM. Clinical and immunohistochemical study of melanotic neuroectodermal tumor of infancy in the maxilla. Einstein (Sao Paulo) (2018) 16:eRC4025. doi: 10.1590/s1679-45082018rc4025
14. Barrett AW, Morgan M, Ramsay AD, Farthing PM, Newman L, Speight PM. A clinicopathologic and immunohistochemical analysis of melanotic neuroectodermal tumor of infancy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod (2002) 93:688–98. doi: 10.1067/moe.2002.124000
15. Moreau A, Galmiche L, Minard-Colin V, Rachwalski M, Belhous K, Orbach D, et al. Melanotic neuroectodermal tumor of infancy (MNTI) of the head and neck: A French multicenter study. J Craniomaxillofac Surg (2018) 46:201–6. doi: 10.1016/j.jcms.2017.12.001
16. Chrcanovic BR, Gomez RS. Melanotic neuroectodermal tumour of infancy of the jaws: an analysis of diagnostic features and treatment. Int J Oral Maxillofac Surg (2019) 48:1–8. doi: 10.1016/j.ijom.2018.08.006
17. Gaiger de Oliveira M, Thompson LD, Chaves AC, Rados PV, da Silva Lauxen I, Filho MS. Management of melanotic neuroectodermal tumor of infancy. Ann Diagn Pathol (2004) 8:207–12. doi: 10.1053/j.anndiagpath.2004.04.003
18. Moreau A, Galmiche L, Belhous K, Franchi G, Couloigner V, Nevoux J, et al. Prenatal Diagnosis of a Melanotic Neuroectodermal Tumor of Infancy (MNTI): A Case Report With a Favorable Outcome After Chemotherapy Failure and Incomplete Resection. J Pediatr Hematol Oncol (2018) 40:320–4. doi: 10.1097/MPH.0000000000000982
19. Neven J, Hulsbergen-van der Kaa C, Groot-Loonen J, de Wilde PC, Merkx MA. Recurrent melanotic neuroectodermal tumor of infancy: a proposal for treatment protocol with surgery and adjuvant chemotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod (2008) 106:493–6. doi: 10.1016/j.tripleo.2008.02.001
20. Khoddami M, Squire J, Zielenska M, Thorner P. Melanotic neuroectodermal tumor of infancy: a molecular genetic study. Pediatr Dev Pathol (1998) 1:295–9. doi: 10.1007/s100249900042
21. Creytens D, Ferdinande L, Lecoutere E, van Dorpe J. Melanotic neuroectodermal tumour of infancy presenting as an undifferentiated round cell tumour in the soft tissue of the forearm. Pathology (2017) 49:87–90. doi: 10.1016/j.pathol.2016.10.006
22. Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. Whole-genome landscapes of major melanoma subtypes. Nature (2017) 545:175–80. doi: 10.1038/nature22071
23. Rickart AJ, Drummond-Hay V, Suchak A, Sadiq Z, Sebire NJ, Slater O, et al. Melanotic neuroectodermal tumour of infancy: Refining the surgical approach. Int J Oral Maxillofac Surg (2019) 48:1307–12. doi: 10.1016/j.ijom.2019.02.011
24. Maroun C, Khalifeh I, Alam E, Akl PA, Saab R, Moukarbel RV. Mandibular melanotic neuroectodermal tumor of infancy: a role for neoadjuvant chemotherapy. Eur Arch Otorhinolaryngol (2016) 273:4629–35. doi: 10.1007/s00405-016-4066-6
25. Woessmann W, Neugebauer M, Gossen R, Blutters-Sawatzki R, Reiter A. Successful chemotherapy for melanotic neuroectodermal tumor of infancy in a baby. Med Pediatr Oncol (2003) 40:198–9. doi: 10.1002/mpo.10135
Keywords: MNTI, clinicopathological features, BRAF, mutation, recurrence
Citation: Xia R-H, Zhang C-Y, Wang L-Z, Hu Y-H, Sun J-J, Tian Z and Li J (2021) Melanotic Neuroectodermal Tumor of Infancy: A Clinicopathological and BRAF V600E Mutation Study of 11 Cases. Front. Oncol. 11:668505. doi: 10.3389/fonc.2021.668505
Received: 16 February 2021; Accepted: 29 March 2021;
Published: 20 May 2021.
Edited by:
Carolina Cavalieri Gomes, Federal University of Minas Gerais, BrazilReviewed by:
Marina G. Diniz, Federal University of Minas Gerais, BrazilCopyright © 2021 Xia, Zhang, Wang, Hu, Sun, Tian and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiang Li, bGlqaWFuZzE4MjAwMEAxMjYuY29t
Zhen Tian, dGlhbjAzMDRfY25AMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.