- 1Department of Radiology, University Hospital, LMU Munich, Munich, Germany
- 2Department of Radiation Oncology, University Hospital, LMU Munich, Munich, Germany
- 3Department of Medicine III, University Hospital, LMU Munich, Munich, Germany
- 4Department of Radiology and Nuclear Medicine, University Medical Centre Mannheim, Medical Faculty Mannheim-University of Heidelberg, Mannheim, Germany
Background: In certain malignancies, patients with oligometastatic disease benefit from radical ablative or surgical treatment. The SABR-COMET trial demonstrated a survival benefit for oligometastatic patients randomized to local stereotactic ablative radiation (SABR) compared to patients receiving standard care (SC) alone. Our aim was to determine the cost-effectiveness of SABR.
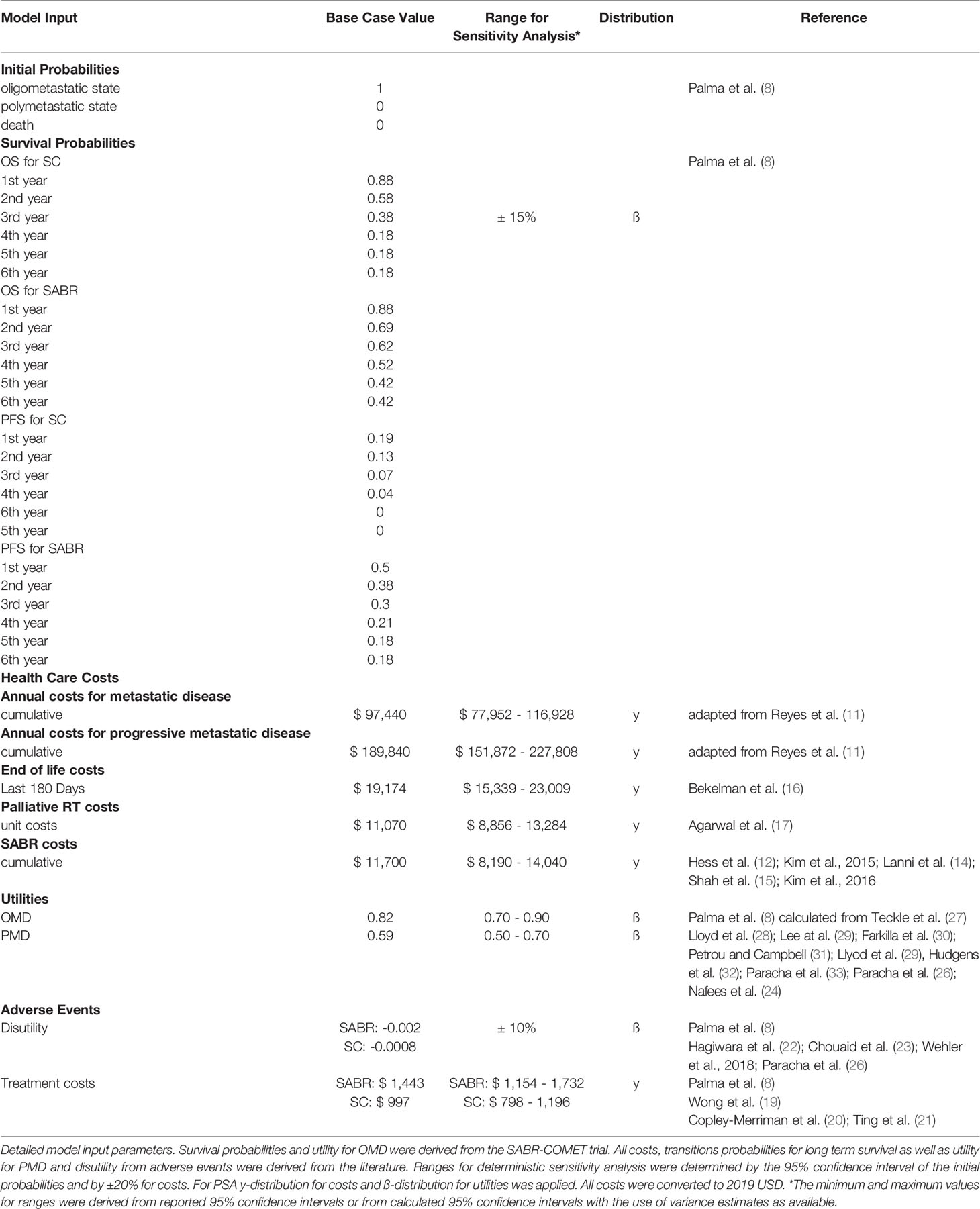
Materials and Methods: A decision model based on partitioned survival simulations estimated costs and quality-adjusted life years (QALY) associated with both strategies in a United States setting from a health care perspective. Analyses were performed over the trial duration of six years as well as a long-term horizon of 16 years. Model input parameters were based on the SABR-COMET trial data as well as best available and most recent data provided in the published literature. An annual discount of 3% for costs was implemented in the analysis. All costs were adjusted to 2019 US Dollars according to the United States Consumer Price Index. SABR costs were reported with an average of $11,700 per treatment. Deterministic and probabilistic sensitivity analyses were performed. Incremental costs, effectiveness, and cost-effectiveness ratios (ICER) were calculated. The willingness-to-pay (WTP) threshold was set to $100,000/QALY.
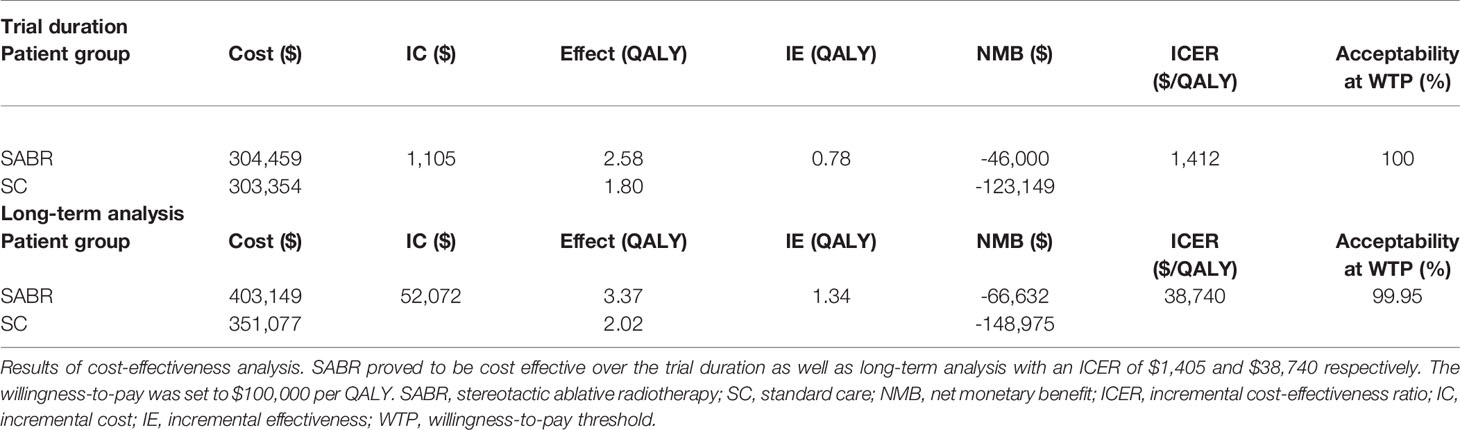
Results: Based on increased overall and progression-free survival, the SABR group showed 0.78 incremental QALYs over the trial duration and 1.34 incremental QALYs over the long-term analysis. Treatment with SABR led to a marginal increase in costs compared to SC alone (SABR: $304,656; SC: $303,523 for 6 years; ICER $1,446/QALY and SABR: $402,888; SC: $350,708 for long-term analysis; ICER $38,874/QALY). Therapy with SABR remained cost-effective until treatment costs of $88,969 over the trial duration (i.e. 7.6 times the average cost). Sensitivity analysis identified a strong model impact for ongoing annual costs of oligo- and polymetastatic disease states.
Conclusion: Our analysis suggests that local treatment with SABR adds QALYs for patients with certain oligometastatic cancers and represents an intermediate- and long-term cost-effective treatment strategy.
Introduction
Metastatic cancers are considered incurable in a variety of tumor entities. The treatment of choice is systemic therapy. The state of oligometastatic disease (OMD) was introduced in the mid 90s as a subcategory of metastatic cancer. With only a limited number of metastases confined to a few organs, this state may represent a less aggressive tumor biology and open the possibility of treatment in a curative intent (1). However, the oligometastatic state is not fully defined and established (2), and studies regarding treatment are still unfolding (3).
Treatment options include ablative surgery, stereotactic ablative radiotherapy (SABR) and other local ablative procedures like thermal ablation and radioablation, which show different efficacy depending on anatomic location (4). Considering treatment of several metastases in different locations with particularities of their anatomy and composition, SABR has proven to be a targeted treatment option with only few side effects (5, 6) and sufficient local tumor control (7).
The SABR-COMET trial is one of the first phase II trials to compare treatment of patients with one to five metastases of varying tumor entities with standard care (SC) to additional SABR (SABR) (8). The trial demonstrated that combined treatment extended progression-free survival (PFS) and overall survival (OS), all while maintaining quality of life (QoL).
Given this new local treatment option, our aim was to determine the cost-effectiveness of SABR compared to SC, taking into account PFS, OS and QoL.
Methods
Model Structure
Our analysis followed recommendations of the Second Panel on Cost-Effectiveness in Health and Medicine (9). We developed a partitioned survival model using decision-analytic software (Treeage Healthcare Pro 2020, Version 20.1.2-v20200326; Treeage, Williamstown, MA) to assess the cost-effectiveness of SABR versus SC over the trial duration of 6 years, using a cycle length of 1 month. Furthermore, long-term survival data was obtained from the Surveillance, Epidemiology, and End Results (SEER) Program (10). The partitioned survival analysis model allows to simulate a patient cohort over time as patients advance along mutually exclusive health states. During each cycle, patients could therefore remain in the oligometastatic state, progress to the polymetastatic disease (PMD) state or die. The only absorbing state was death.
Model Input Parameters
Progression and Survival Probabilities
All individuals started in the oligometastatic state. Monthly overall and progression-free survival rates were derived from the Kaplan-Meier analysis of the SABR-COMET trial (Supplementary Figure 1). Therefore, no adjustment for the age-related death rate was necessary. For modeling long-term survival, we referred to the Surveillance, Epidemiology, and End Results Program (SEER) using the SEER*Explorer. OS data were pooled from the database for the metastatic stage of the most frequent cancer entities in the SABR-COMET trial (breast, colorectal, lung, prostate) and fitted with respect to the proportion in the study population. The OS course in the SEER data was used to extrapolate the trial OS curve beyond the trial period. In detail, the curve was expanded beginning from the latest reported OS percentage from the trial and continued with the SEER survival curve at that same percentage. Because of missing data in terms of PFS, we also applied this data to extrapolate the long-term course of PFS; for this we additionally assumed the same proportionality of OS to PFS as in the SABR COMET trial (Supplementary Table 1). An overview of the model structure is shown in Figure 1.
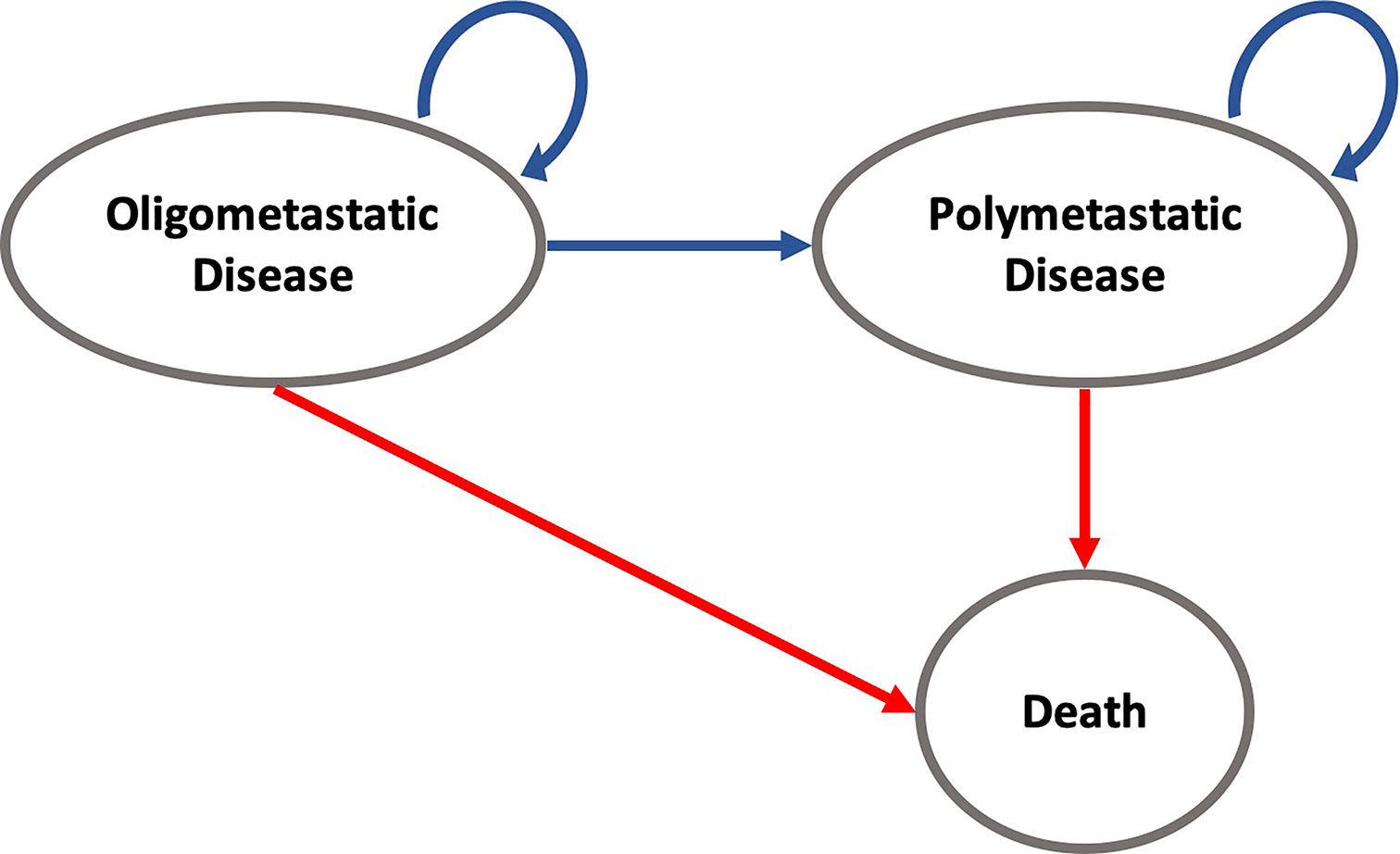
Figure 1 State-transition diagram for modeling cost and effectiveness for the SABR and SC strategies over time intervals. For example, patients in the oligometastatic disease state can either remain in the oligometastatic state, transition to the polymetastatic disease state, or die. Death is an absorbing state and will discontinue the individual simulation.
Costs
The analysis was performed in a United States setting from a health care perspective. The ongoing treatment costs for standard care of OMD and PMD states were derived from Reyes et al. (11) and accumulated. These accumulated cost data were used to reflect average annual health expenditures for the patient population that was investigated in the SABR-COMET trial. It further allowed to model the differences in therapy costs during the time intervals spent in the OMD or PMD state. 55% of patients in the SABR group as well as 63.6% of patients in the SC group received systemic therapy. Because of missing information in terms of drug administration, costs were distributed proportionally in the OMD and PMD group. Costs for single treatment of SABR were pooled from assorted papers comprising different fraction numbers and localization of treatment (12–16). Costs for palliative radiotherapy were derived from Medicare coverage data (17).
23 patients in the SC group and 16 patients in the SABR group obtained salvage radiotherapy. 9 patients of the SABR group received additional salvage SABR. Total costs for additional radiotherapy were accumulated per group and factored in as cost items at the beginning of the simulation as data concerning the time of administration was not available; this approximation will slightly alter the costs as these would not be discounted before the actual time of administration. An additional cumulative single time cost was added for the last 180 days of treatment before death (18).
Therapy-related adverse events higher than or equal to grade 2 occurred in 19 patients in the SABR group and 3 patients in the SC group. Costs for treatment (19–21) and disutility (22–26) were pooled from the literature and added as one-time cost and disutility at the beginning of the analysis. An overview of the input parameters is given in Table 1. An annual discount of 3% for costs was implemented in the analysis according to current recommendations (9). All costs were adjusted to 2019 US Dollars according to the United States Consumer Price Index.
Utilities
Therapy effectiveness was measured in quality-adjusted life years (QALYs), calculated by multiplying years spent in OMD and PMD states by assigned utility weights. Utility weights for OMD were obtained from the FACT-G-Score used in the SABR-COMET trial and converted to EQ-5D according to Teckle et al. (27). Utility weights for PMD were derived from the literature (24, 26, 28–34). A discount of 3% for utilities was implemented in the analysis (9).
Cost-Effectiveness Analysis
Treatment strategies were compared in terms of net monetary benefits, incremental costs, incremental effectiveness, and incremental cost-effectiveness ratios (ICERs). The willingness-to-pay was set to $100,000 per QALY as in recent studies (35). Net monetary benefits combine costs and effectiveness in one measure: net monetary benefit = (effectiveness × willingness-to-pay) minus costs.
Sensitivity Analysis
We used comprehensive deterministic and probabilistic sensitivity analysis (PSA) to test the robustness of the model. Deterministic one-way sensitivity analysis was performed to identify variables that significantly influence the model outcomes. The ranges for deterministic sensitivity analysis were determined by the 95% confidence interval of the initial probabilities and by ±20% for costs. Moreover, PSA allows simultaneous alteration of multiple model input parameters using distributions according to probability density functions for second order Monte Carlo simulation runs (n=10,000) (36). The model input parameters were assigned appropriate distributions as indicated in Table 1. Utilities were varied with a beta distribution. Treatment costs were modeled by gamma distribution. Beta distributions were used for disutilities as well as PFS and OS data.
Results
Base Case Analysis
In the base case analysis of the total study population over the trial duration of 6 years, SABR led to an increased effectiveness of 0.78 QALY at increased costs of $1,133. The ICER was $1,446 per QALY. When additional long-term SEER data were applied, SABR led to an increased effectiveness of 1.34 QALY at additional costs of $52,180. The corresponding ICER was $38,874 per QALY. Adverse events only had a minor effect on our results with a loss of 0.002 QALYs for SABR and 0.0008 QALYs for SC. Incremental costs for treatment of adverse events amounted to $1,443 for the SABR group and $997 for the SC arm.
Deterministic Sensitivity Analysis
The results of the deterministic one-way sensitivity analysis are presented in Figure 2. Costs of systemic therapy of PMD and OMD possessed the strongest impact on ICER regarding the trial duration as well as costs of OMD state on long-term survival. Higher costs of OMD state and lower costs of PMD led to unfavorable ICER values whereas lower costs for therapy of OMD state and higher costs of PMD state led to favorable ICER values. These effects were reversed for the SC strategy. SABR remained cost-effective even when the costs for SABR and salvage SABR were increased 7.6 times during the trial duration and stayed cost-effective when raised up to 8 times for long-term survival (see Figure 3).
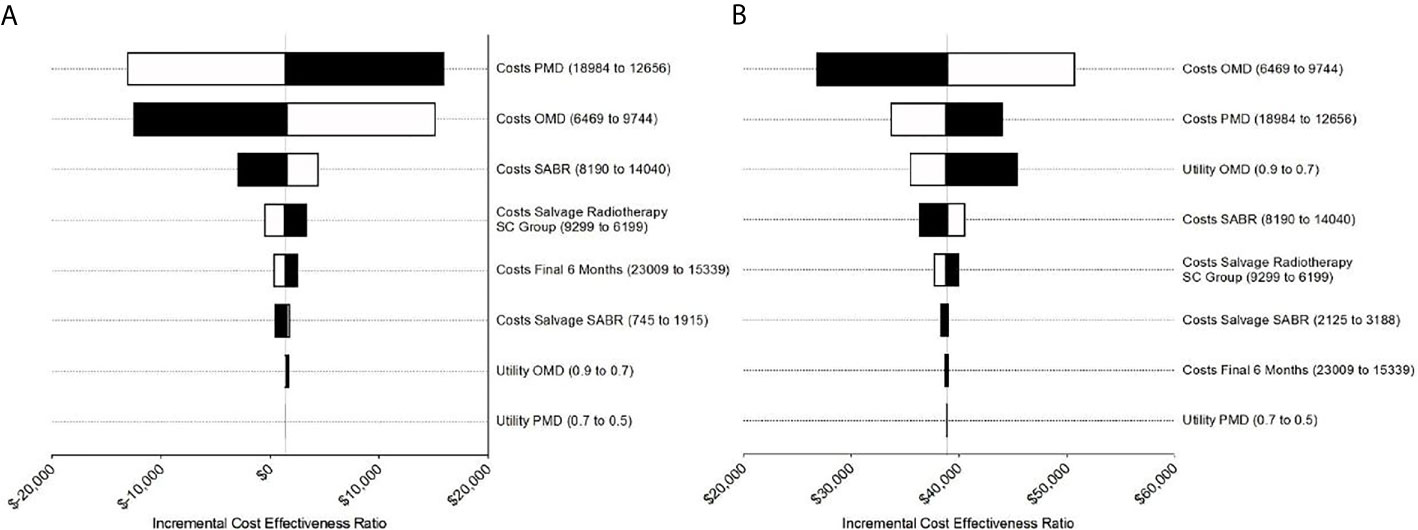
Figure 2 Tornado diagrams for the sensitivity analysis during (A) the trial duration and (B) long-term simulation extrapolated based on SEER survival data. (A) Costs for PMD and OMD demonstrated the strongest impact on ICER during trial duration. (B) For long-term analysis costs for PMD influenced ICER the most followed by costs for PMD and utility for OMD.
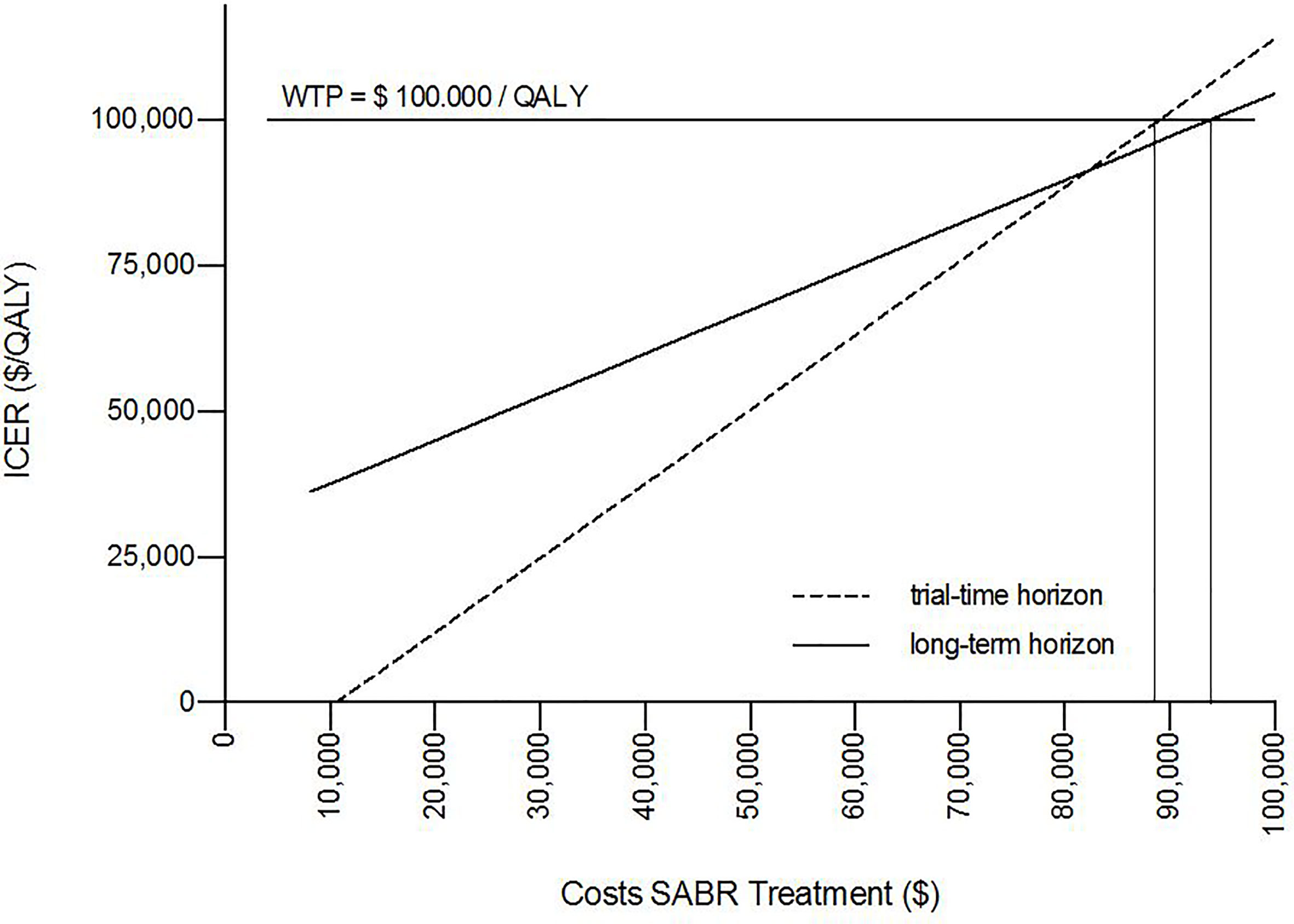
Figure 3 One-way sensitivity analysis proved cost-effectiveness for SABR up to unit costs of $88,696 over the trial duration and $93,750 for long-term survival for a willingness-to-pay (WTP) threshold of $100,000/QALY.
Probabilistic Sensitivity Analysis
Overall, SABR was cost-effective in 100% of Monte Carlo simulation runs with an ICER of $1,105 per QALY during the trial duration and $38,740 per QALY for long-term survival in 99.95% of Monte Carlo simulation runs, indicating robustness of the model. In 47% of simulations, SABR was the dominant strategy when analyzed with SABR-COMET data, meaning that it provided better outcomes at lower costs.
The mean incremental effectiveness was positive, meaning that SABR on average led to increased QALYs. Moreover, the mean values for the ICERs were below the willingness-to-pay threshold. The detailed results of the PSA are shown in Table 2.
Discussion
This study evaluated the economic impact of SABR in the treatment of oligometastatic cancer patients. The analysis indicates that SABR is a cost-effective treatment option compared to SC alone. Additional costs of SABR were partly amortized due to longer progression-free survival in the OMD state, which was accompanied with lower treatment costs of systemic treatment. As expected, DSA demonstrated a relevant impact of treatment costs on the ICER. Yet even with an increase in SABR treatment costs up to about sevenfold, the SABR treatment strategy remained cost-effective.
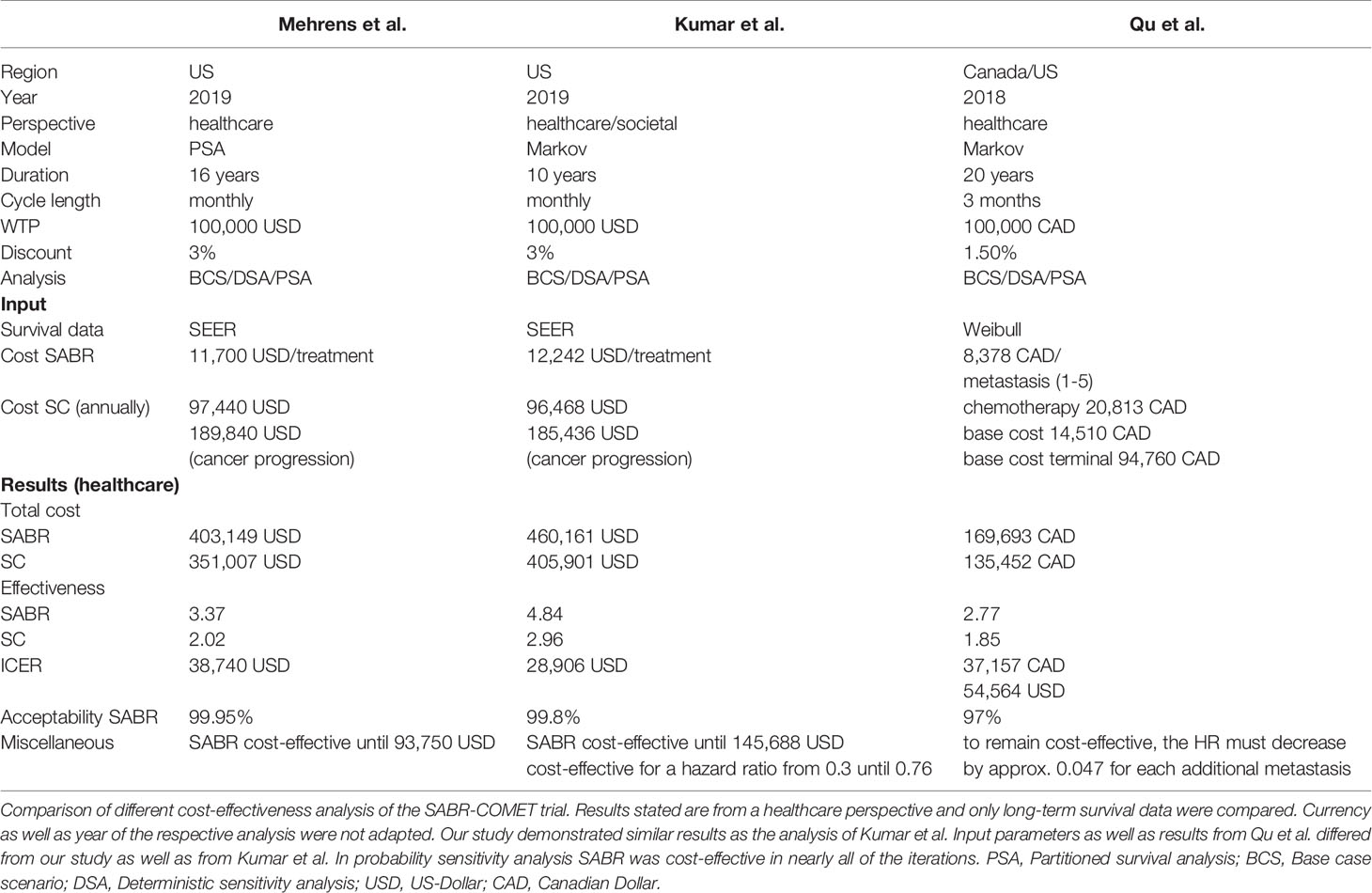
The SABR-COMET trial is the first basket study to prove survival benefits of SABR treatment in patients with OMD across different cancer entities. Previous cost-effectiveness analysis indicated cost-effectiveness for SABR in oligometastatic prostate cancer and NSCLC (37, 38). Recently, two economic analyse have also analyzed the cost-effectiveness of the SABR-COMET trial (39, 40). Kumar et al. (39) assessed that treatment with SABR is cost-effective in 99.8% of cases at a WTP threshold of $100,000 per QALY, with an ICER of $28,906 per QALY in a US health care setting after a 10-year horizon. Qu et al. (40) showed that SABR is cost-effective over a lifetime horizon in 97% of cases at a WTP threshold of $100,000 per QALY with an ICER of $54,564 per QALY. Kumar et al. used SEER data for long-term analysis over 10 years in total with an increased ICER of $79,406 per QALY if costs for treatment were continued. A detailed comparison of methods and results of these studies is provided in Table 3. These data provide external validation and demonstrate robustness of the cost-effectiveness of SABR. Similar to our analysis, Kumar et al. showed cost-effectiveness for SABR up to a 10-fold increase in treatment costs.
In contrast to our study, Kumar et al. assumed treatment with SC for all patients and did not include costs for salvage or palliative radiotherapy. Qu et al. used data directly from the SABR-COMET trial, which is not publicly available in its entirety. Moreover, the discount rate was adapted according to Canadian guidelines for the Economic Evaluation of Health Technologies with 1.5% and not 3% as in Kumar et al. and our study. Qu et al. report a non-linear relationship between the number of lesions and the PFS hazard ratio (HR) with the need of decreasing the HR by 0.047 for each additional metastasis to maintain cost-effectiveness for SABR.
Further studies including phase III trials are required to validate the results. Several studies are ongoing at the moment. These include the phase III of the SABR-COMET trial, namely SABR-COMET-3 (41) and SABR-COMET-10 (42), investigating the impact of SABR on patients with 1-3 metastases or 4-10 metastases respectively. By analyzing these two subpopulations, Palma et al. will help to clarify the uncertainty up to which number of metastases patients benefit from SABR. Further phase III trials include the SARON study comparing SC versus SABR and SC for oligometastatic NSCLC with 1-5 metastases in up to a maximum of 3 organs (43), NRG-BR002 investigating systemic therapy versus SABR or surgery combined with systemic therapy in breast cancer with less than 4 metastases (44), and the HALT trial examining the effect of SABR under tyrosine kinase inhibitor (TKI) therapy versus TKI treatment alone in metastatic disease with equal to or less than 3 sites of metastases (45).
The study results should be interpreted with knowledge of the following limitations. First, the current state of evidence on SABR in OMD is still limited by the sample size of the underlying trial; current phase III trials are ongoing. Second, the FACT-G score was stated only for whole populations of study groups. No distinction was made between progression-free and progressive patients. Data on progression-related decrease in QoL were not publicly available from the SABR-COMET study. Third, no information was provided concerning which patients received systemic therapy. Therefore, in our analysis we used the same percentage for treatment with systemic therapy in the progression-free as well as the progressive patient group to avoid introducing any bias. Fourth, because of rapidly changing treatment regimens, specifying a cost for systemic treatment may remain a source of inaccuracy.
Fifth, missing information on which treatment was administered and the inclusion of diverse tumor entities represents a challenge for precise estimation of costs for systemic cancer treatment. This may influence cost-effectiveness as one-dimensional sensitivity analysis demonstrated a great impact of costs for systemic treatment on the ICER. We therefore chose a restrictive approach for our cost-effectiveness analysis, which still indicated cost-effectiveness for the SABR group. Sixth, long-term survival data was obtained from SEER-Program with only OS being available. We deployed these data to also model PFS. Moreover, changes in systemic therapy with more efficient treatments (46, 47) as well as technical advances in planning and performing SABR with accompanying reduction of costs (7) have to be taken into account to obtain an authentic cost estimate in the future.
In conclusion, local treatment with SABR adds QALYs for patients with oligometastatic disease across selected cancer entities in SABR-COMET and represents an intermediate- and long-term cost-effective treatment strategy.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://pubmed.ncbi.nlm.nih.gov/32484754/ DOI: 10.1200/JCO.20.00818.
Author Contributions
DM: Investigation, Data Curation, Formal analysis, Writing - Original Draft, Software. MU: Validation, Writing - Review and Editing. SC: Validation, Writing - Review and Editing. K-MN Validation, Writing - Review and Editing. FM: Validation, Writing - Review and Editing. CW: Validation, Writing - Review and Editing. MF: Validation, Writing - Review and Editing. MW: Validation, Writing - Review and Editing. MS: Validation, Writing - Review and Editing. JeR: Validation, Writing - Review and Editing. JoR: Validation, Writing - Review and Editing. WK: Conceptualization, Methodology, Validation, Supervision, Project administration. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.667993/full#supplementary-material
References
1. Pitroda SP, Khodarev NN, Huang L, Uppal A, Wightman SC, Ganai S, et al. Integrated Molecular Subtyping Defines a Curable Oligometastatic State in Colorectal Liver Metastasis. Nat Commun (2018) 9(1):1793. doi: 10.1038/s41467-018-04278-6
2. Lievens Y, Guckenberger M, Gomez D, Hoyer M, Iyengar P, Kindts I, et al. Defining Oligometastatic Disease From a Radiation Oncology Perspective: An ESTRO-ASTRO Consensus Document. Radiother Oncol (2020) 148:157–66. doi: 10.1016/j.radonc.2020.04.003
3. Palma DA, Salama JK, Lo SS, Senan S, Treasure T, Govindan R, et al. The Oligometastatic State—Separating Truth From Wishful Thinking. Nat Rev Clin Oncol (2014) 11(9):549–57. doi: 10.1038/nrclinonc.2014.96
4. Palma DA, Bauman GS, Rodrigues GB. Beyond Oligometastases. Int J Radiat Oncol Biol Phys (2020) 107(2):253–6. doi: 10.1016/j.ijrobp.2019.12.023
5. Arnett ALH, Mou B, Owen D, Park SS, Nelson K, Hallemeier CL, et al. Long-Term Clinical Outcomes and Safety Profile of SBRT for Centrally Located Nsclc. Adv Radiat Oncol (2019) 4(2):422–8. doi: 10.1016/j.adro.2019.01.002
6. Baumann BC, Wei J, Plastaras JP, Lukens JN, Damjanov N, Hoteit M, et al. Stereotactic Body Radiation Therapy (SBRT) for Hepatocellular Carcinoma: High Rates of Local Control With Low Toxicity. Am J Clin Oncol (2018) 41(11):1118–24. doi: 10.1097/COC.0000000000000435
7. Tsang MWK. Stereotactic Body Radiotherapy: Current Strategies and Future Development. J Thoracic Dis (2016) 8(Suppl 6):S517–27. doi: 10.21037/jtd.2016.03.14
8. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol (2020) JCO2000818. doi: 10.1101/2020.03.26.20044305
9. Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA (2016) 316(10):1093–103. doi: 10.1001/jama.2016.12195
10. Seer*Explorer: An Interactive Website for SEER Cancer Statistics. Surveillance Research Program, National Cancer Institute. Available at: https://seer.cancer.gov/explorer/.
11. Reyes C, Engel-Nitz NM, DaCosta Byfield S, Ravelo A, Ogale S, Bancroft T, et al. Cost of Disease Progression in Patients With Metastatic Breast, Lung, and Colorectal Cancer. (1549-490X (Electronic)). doi: 10.1634/theoncologist.2018-0018
12. Hess G, Barlev A, Chung K, Hill JW, Fonseca E. Cost of Palliative Radiation to the Bone for Patients With Bone Metastases Secondary to Breast or Prostate Cancer. Radiat Oncol (2012) 7(1):168. doi: 10.1186/1748-717X-7-168
13. Kim H, Rajagopalan MS, Beriwal S, Huq MS, Smith KJ. Cost-Effectiveness Analysis of Single Fraction of Stereotactic Body Radiation Therapy Compared With Single Fraction of External Beam Radiation Therapy for Palliation of Vertebral Bone Metastases. Int J Radiat Oncol Biol Phys (2015) 91(3):556–63. doi: 10.1016/j.ijrobp.2014.10.055
14. Lanni TB Jr., Grills IS, Kestin LL, Robertson JM. Stereotactic Radiotherapy Reduces Treatment Cost While Improving Overall Survival and Local Control Over Standard Fractionated Radiation Therapy for Medically Inoperable Non-Small-Cell Lung Cancer. Am J Clin Oncol (2011) 34(5):494–8. doi: 10.1097/COC.0b013e3181ec63ae
15. Shah A, Hahn SM, Stetson RL, Friedberg JS, Pechet TTV, Sher DJ. Cost-Effectiveness of Stereotactic Body Radiation Therapy Versus Surgical Resection for Stage I Non–Small Cell Lung Cancer. Cancer (2013) 119(17):3123–32. doi: 10.1002/cncr.28131
16. Kim H, Gill B, Beriwal S, Huq MS, Roberts MS, Smith KJ. Cost-Effectiveness Analysis of Stereotactic Body Radiation Therapy Compared With Radiofrequency Ablation for Inoperable Colorectal Liver Metastases. Int J Radiat Oncol Biol Phys (2016) 95(4):1175–83. doi: 10.1016/j.ijrobp.2016.02.045
17. Agarwal A, Dayal A, Kircher SM, Chen RC, Royce TJ. Analysis of Price Transparency Via National Cancer Institute–Designated Cancer Centers’ Chargemasters for Prostate Cancer Radiation Therapy. JAMA Oncol (2020) 6(3):409–12. doi: 10.1001/jamaoncol.2019.5690
18. Bekelman JE, Halpern SD, Blankart CR, Bynum JP, Cohen J, Fowler R, et al. Comparison of Site of Death, Health Care Utilization, and Hospital Expenditures for Patients Dying With Cancer in 7 Developed Countries. Jama (2016) 315(3):272–83. doi: 10.1001/jama.2015.18603
19. Wong W, Yim YM, Kim A, Cloutier M, Gauthier-Loiselle M, Gagnon-Sanschagrin P, et al. Assessment of Costs Associated With Adverse Events in Patients With Cancer. PloS One (2018) 13(4):e0196007. doi: 10.1371/journal.pone.0196007
20. Copley-Merriman C, Stevinson K, Liu FX, Wang J, Mauskopf J, Zimovetz EA, et al. Direct Costs Associated With Adverse Events of Systemic Therapies for Advanced Melanoma: Systematic Literature Review. Med (Baltimore) (2018) 97(31):e11736. doi: 10.1097/MD.0000000000011736
21. Ting J, Tien Ho P, Xiang P, Sugay A, Abdel-Sattar M, Wilson L. Cost-Effectiveness and Value of Information of Erlotinib, Afatinib, and Cisplatin-Pemetrexed for First-Line Treatment of Advanced Egfr Mutation-Positive non-Small-Cell Lung Cancer in the United States. Value Health (2015) 18(6):774–82. doi: 10.1016/j.jval.2015.04.008
22. Hagiwara Y, Shiroiwa T, Shimozuma K, Kawahara T, Uemura Y, Watanabe T, et al. Impact of Adverse Events on Health Utility and Health-Related Quality of Life in Patients Receiving First-Line Chemotherapy for Metastatic Breast Cancer: Results From the SELECT BC Study. Pharmacoeconomics (2018) 36(2):215–23. doi: 10.1007/s40273-017-0580-7
23. Chouaid C, Agulnik J, Goker E, Herder GJ, Lester JF, Vansteenkiste J, et al. Health-Related Quality of Life and Utility in Patients With Advanced non-Small-Cell Lung Cancer: A Prospective Cross-Sectional Patient Survey in a Real-World Setting. J Thorac Oncol (2013) 8(8):997–1003. doi: 10.1097/JTO.0b013e318299243b
24. Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health State Utilities for non Small Cell Lung Cancer. Health Qual Life Outcomes (2008) 6:84. doi: 10.1186/1477-7525-6-84
25. Lachaine J, Mathurin K, Barakat S, Couban S. Economic Evaluation of Arsenic Trioxide Compared to All-Trans Retinoic Acid + Conventional Chemotherapy for Treatment of Relapsed Acute Promyelocytic Leukemia in Canada. Eur J Haematol (2015) 95(3):218–29. doi: 10.1111/ejh.12475
26. Paracha N, Abdulla A, MacGilchrist KS. Systematic Review of Health State Utility Values in Metastatic Non-Small Cell Lung Cancer With a Focus on Previously Treated Patients. Health Qual Life Outcomes (2018) 16(1):179. doi: 10.1186/s12955-018-0994-8
27. Teckle P, McTaggart-Cowan H, Van der Hoek K, Chia S, Melosky B, Gelmon K, et al. Mapping the FACT-G Cancer-Specific Quality of Life Instrument to the EQ-5D and SF-6D. (1477-7525 (Electronic)). doi: 10.12968/ijpn.1997.3.5.275
28. Lloyd AJ, Kerr C, Penton J, Knerer G. Health-Related Quality of Life and Health Utilities in Metastatic Castrate-Resistant Prostate Cancer: A Survey Capturing Experiences From a Diverse Sample of UK Patients. Value Health (2015) 18(8):1152–7. doi: 10.1016/j.jval.2015.08.012
29. Lee JY, Ock M, Jo MW, Son WS, Lee HJ, Kim SH, et al. Estimating Utility Weights and Quality-Adjusted Life Year Loss for Colorectal Cancer-Related Health States in Korea. Sci Rep (2017) 7(1):5571. doi: 10.1038/s41598-017-06004-6
30. Farkkila N, Sintonen H, Saarto T, Jarvinen H, Hanninen J, Taari K, et al. Health-Related Quality of Life in Colorectal Cancer. Colorectal Dis (2013) 15(5):e215–22. doi: 10.1111/codi.12143
31. Petrou S, Campbell N. Stabilisation in Colorectal Cancer. Int J Palliat Nurs (1997) 3(5):275–80. doi: 10.12968/ijpn.1997.3.5.275
32. Hudgens S T-SG, De Courcy J, Kontoudis I, Tremblay G, Forsythe A, Lloyd A. Real-World Evidence on Health States Utilities in in Metastatic Breast Cancer Patients: Data From a Retrospective Patient Record Form Study and A Cross-Sectional Patient Survey. Value Health (2016) 19(3):A157. doi: 10.1016/j.jval.2016.03.1466
33. Paracha N, Thuresson PO, Moreno SG, MacGilchrist KS. Health State Utility Values in Locally Advanced and Metastatic Breast Cancer by Treatment Line: A Systematic Review. Expert Rev Pharmacoecon Outcomes Res (2016) 16(5):549–59. doi: 10.1080/14737167.2016.1222907
34. Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health State Utilities for Metastatic Breast Cancer. Br J Cancer (2006) 95(6):683–90. doi: 10.1038/sj.bjc.6603326
35. Cameron D, Ubels J, Norström F. On What Basis are Medical Cost-Effectiveness Thresholds Set? Clashing Opinions and an Absence of Data: A Systematic Review. Global Health Action (2018) 11(1):1447828–1447828. doi: 10.1080/16549716.2018.1447828
36. Sheppard CW. Computer Simulation of Stochastic Processes Through Model-Sampling (Monte Carlo) Techniques. FEBS Lett (1969) 2 Suppl 1:S14–s21. doi: 10.1016/0014-5793(69)80071-2
37. Lester-Coll NH, Decker RH, Yu JB. Cost-Effectiveness Analysis of Stereotactic Body Radiation Therapy for Pulmonary Oligometastases. Int J Radiat Oncol Biology Phys (2014) 90(1):S585–6. doi: 10.1016/j.ijrobp.2014.05.1761
38. Parikh NR, Nickols NG, Rettig M, King CR, Raldow AC, Steinberg ML, et al. Cost-Effectiveness of Metastasis-Directed Therapy in the Setting of Oligometastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol (2019) 37(7_suppl):147–7. doi: 10.1200/JCO.2019.37.7_suppl.147
39. Kumar A, Straka C, Courtney PT, Vitzthum L, Riviere P, Murphy JD. Cost-Effectiveness Analysis of Stereotactic Ablative Radiation Therapy in Patients With Oligometastatic Cancer. Int J Radiat Oncol Biol Phys (2020). doi: 10.1016/j.ijrobp.2020.09.045
40. Qu XM, Chen Y, Zaric GS, Senan S, Olson RA, Harrow S, et al. Is SABR Cost-Effective in Oligometastatic Cancer? An Economic Analysis of the SABR-COMET Randomized Trial. Int J Radiat Oncol Biol Phys (2020). doi: 10.1016/j.ijrobp.2020.12.001
41. Olson R, Mathews L, Liu M, Schellenberg D, Mou B, Berrang T, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of 1-3 Oligometastatic Tumors (SABR-COMET-3): Study Protocol for a Randomized Phase III Trial. BMC Cancer (2020) 20(1):380. doi: 10.1186/s12885-020-06876-4
42. Palma DA, Olson R, Harrow S, Correa RJM, Schneiders F, Haasbeek CJA, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of 4-10 Oligometastatic Tumors (SABR-COMET-10): Study Protocol for a Randomized Phase III Trial. BMC Cancer (2019) 19(1):816. doi: 10.1186/s12885-019-5977-6
43. Conibear J, Chia B, Ngai Y, Bates AT, Counsell N, Patel R, et al. Study Protocol for the SARON Trial: A Multicentre, Randomised Controlled Phase III Trial Comparing the Addition of Stereotactic Ablative Radiotherapy and Radical Radiotherapy With Standard Chemotherapy Alone for Oligometastatic Non-Small Cell Lung Cancer. BMJ Open (2018) 8(4):e020690. doi: 10.1136/bmjopen-2017-020690
44. Chmura SJ WK, Al-Hallaq HA, Borges VF, Jaskowiak NT, Matuszak M, Milano MT, et al. NRG-BR002: A Phase IIR/III Trial of Standard of Care Therapy With or Without Stereotactic Body Radiotherapy (SBRT) and/or Surgical Ablation for Newly Oligometastatic Breast Cancer (NCT02364557). J Clin Oncol (2019) 35(TPS1117-TPS1117 ). doi: 10.1200/JCO.2019.37.15_suppl.TPS1117
45. McDonald F GM, Popat S, Andratschke N, Kilburn L, Toms C, Bliss J. HALT: Targeted Therapy Beyond Progression With or Without Dose-Intensified Radiotherapy in Oligoprogressive Disease in Oncogene Addicted Lung Tumours. . Lung Cancer (2017) 103:57. doi: 10.1016/S0169-5002(17)30175-7
46. Wartman LD. The Future of Cancer Treatment Using Precision Oncogenomics. Cold Spring Harbor Mol Case Stud (2018) 4(2):a002824. doi: 10.1101/mcs.a002824
Keywords: OMD, cost-effectiveness (economics), radiation therapy (radiotherapy), cancer, SABR
Citation: Mehrens D, Unterrainer M, Corradini S, Niyazi M, Manapov F, Westphalen CB, Froelich MF, Wildgruber M, Seidensticker M, Ricke J, Rübenthaler J and Kunz WG (2021) Cost-Effectiveness Analysis of Local Treatment in Oligometastatic Disease. Front. Oncol. 11:667993. doi: 10.3389/fonc.2021.667993
Received: 15 February 2021; Accepted: 31 May 2021;
Published: 15 June 2021.
Edited by:
Rupesh Kotecha, Baptist Hospital of Miami, United StatesReviewed by:
Vivek Verma, Allegheny General Hospital, United StatesMoshe Schaffer, Ben-Gurion University of the Negev, Israel
Erqi Pollom, Stanford University, United States
Copyright © 2021 Mehrens, Unterrainer, Corradini, Niyazi, Manapov, Westphalen, Froelich, Wildgruber, Seidensticker, Ricke, Rübenthaler and Kunz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wolfgang G. Kunz, d29sZmdhbmcua3VuekBtZWQubG11LmRl
 Dirk Mehrens
Dirk Mehrens Marcus Unterrainer1
Marcus Unterrainer1 Wolfgang G. Kunz
Wolfgang G. Kunz