
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 10 June 2021
Sec. Cancer Genetics
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.665360
This article is part of the Research Topic RNA Sequencing in Clinical Oncology for Metabolism and Immunity View all 43 articles
 Jingwen Zhang1,2
Jingwen Zhang1,2 Minjie Fu1
Minjie Fu1 Mengli Zhang1
Mengli Zhang1 Jinsen Zhang1
Jinsen Zhang1 Zunguo Du3
Zunguo Du3 Hongyi Zhang4,5*
Hongyi Zhang4,5* Wei Hua1*
Wei Hua1* Ying Mao1,6,7,8
Ying Mao1,6,7,8DDX60, an interferon (IFN)-inducible gene, plays a promotional role in many tumors. However, its function in glioma remains unknown. In this study, bioinformatic analysis (TCGA, CGGA, Rembrandt) illustrated the upregulation and prognostic value of DDX60 in gliomas. Immunohistochemical staining of clinical samples (n = 49) validated the DDX60 expression is higher in gliomas than in normal tissue (n = 20, P < 0.0001). It also could be included in nomogram as a parameter to predict the 3- and 5-year survival risk (C-index = 0.86). The biological process of DDX60 in glioma was mainly enriched in the inflammatory and immune response by GSEA and GO analysis. DDX60 expression had a positive association with most inflammatory-related functions, such as hematopoietic cell kinase (HCK) (R = 0.31), interferon (R = 0.72), STAT1 (R = 54), and a negative correlation with IgG (R = −0.24). Furthermore, DDX60 expression tends to be positively related to multiple infiltrating immune cells, while negatively related to CD56 dim nature killer cell in glioma. Some important immune checkpoints, like CTLA-4, PD-L1, EGF, CD96, and CD226, were all positively related with DDX60 (all Pearson correlation R > 0.26). The expression and correlation between DDX60, EGF, and PD-L1 were confirmed by western blot in clinical samples (n = 14, P < 0.0001) and GBM cells. These results indicated that DDX60 might have important clinical significance in glioma and could serve as a potential immune therapeutic target.
Glioma is the most common malignancy in the brain, representing more than 70% of all central nervous system (CNS) malignancies (1). Glioblastoma multiforme (GBM), the most aggressive and malignant form of glioma, has a median survival of fewer than 21 months (2) despite the progress of neurosurgical resection, chemotherapy, radiation therapy, and novel approaches such as immunotherapy. Intratumoral heterogeneity widely exists in GBM (3) and has become an obstacle for molecular targeted therapy (4). To counteract the heterogeneity, therapies targeting the cytosolic innate immune receptors retinoic-acid inducible gene I (RIG-I) have been employed to gain a good response (5).
Acting as the upstream of RIG-I in the innate immune response, DDX60 is a novel DEAD-box RNA helicase and first identified through microarray research of genes induced by measles’ virus infection in dendritic cells (DCs) (6). Through the helicase domain and ATP-binding site, DDX60 can detect abnormal intracellular nucleic acids and then induce RIG-I-dependent type I interferons (type I IFNs) and other inflammatory cytokines (6–8). Besides, DDX60 induced RIG-I-independent antiviral responses have also been demonstrated (8). Involved in RIG-I-dependent and independent innate immune responses, DDX60 has been proven to be associated with the development of tumors (9–11). It was upregulated in oral squamous cell carcinoma and correlated with poor disease-free survival (10), while downregulated in colorectal cancer and related with the initiation and progression of the disease (11). Therefore, DDX60 represents a potential target for tumor therapy. Immunotherapy and particularly immune checkpoint inhibitors, such as programmed death-ligand 1 (PD-L1) inhibitors, have revolutionized the treatment landscape of glioma (12). However, because of the heterogeneity and immunosuppression of glioma, some of the checkpoint inhibitor therapies fail to get a positive effect (1, 13), and new biomarkers for immune therapies are urgently needed. Herein, we assume that DDX60 is a novel immune therapeutic target for glioma and explore its prognostic value and biological function in glioma.
This study demonstrated that DDX60 is highly expressed in GBM and predicts poor prognosis of glioma by the Cancer Genome Atlas (TCGA), Chinese Glioma Genome Atlas (CGGA), Repository for Molecular Brain Neoplasia Data (REMBRANDT), and Gravendeel databases. Then, the correlation between DDX60 expression and inflammatory responses, immune-related molecules, infiltrating immune cells as well as checkpoint protein in glioma was also established.
Glioma patient’s clinical information and gene expression data in the TCGA, CGGA, Rembrandt, and Gravendeel databases were downloaded from GlioVis (http://gliovis.bioinfo.cnio.es/) (14). The results shown here are in whole or part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga. The expression information for DDX60 in tumor and normal tissues in multiple cancers was acquired from UALCAN (https://ualcan.path.uab.edu/).
The nomogram and calibration plots were constructed using the RMS package of R software. Pearson correlation and correlograms were generated using the circlize package and the corrgram package, respectively (15). Gene ontology (GO) analyses were employed to verify the biological processes by the R package of enrichplot and clusterProfiler (16). Gene set enrichment analysis (GSEA, http://software.broadinstitute.org/gsea/index) was performed between the DDX60 high expression group and low expression group (17). The significant difference for GSEA was verified by the normalized enrichment score (NES) and false discovery rate (FDR). The related gene pathways with P <0.05 and FDR <0.1 were visualized by Cytoscape 3.7.2 version. The R package GSVA was used to search the enrichment status of inflammatory response-associated metagenes (18). Gene set associated with the immune function was extracted from the AmiGO 2 website (http://amigo.geneontology.org/amigo) to demonstrate the role of DDX60 in the immune system in glioma. Genes with high correlation coefficients (R > 0.3 and P < 0.05) with DDX60 were selected for heatmap displays. Metagenes of immune infiltration cells were downloaded from a previous study (19) and ssGSEA analysis was conducted via GSVA R package. Venn diagrams, boxplots, and heatmaps were generated using the Venn diagrams, ggplot2, and pheatmap packages in R software.
Glioma tissues were gathered during 2019 to 2020 from patients (n = 60) who experienced craniotomy in the Department of Neurosurgery, Huashan Hospital of Fudan University. Normal brain tissues (n = 23) were obtained from traumatic brain injury patients who underwent partial resection as decompression treatment. These experimental protocols were approved by the Human Ethics Committee of Huashan Hospital and informed consent was collected from all patients.
SiRNA targeted human DDX60 (siRNA#1, CCAUCUGCCUCUUUCUCAATT; and siRNA#2, GGAUUUGAUGAGUUGGCAATT) and control siRNA were obtained from Hanbio (Shanghai, China). SiRNA knockdown of DDX60 was performed with Invitrogen Lipofectamine 2000 and standard procedures (20).
IHC staining was performed as described previously (10). The sections were incubated with rabbit anti-human DDX60 Ab (1:200, Abcam) as the primary antibody. Immune reactive score (IRS) was conducted as described (21).
The glioma and normal brain tissues were minced by scissors and homogenized in RIPA lysis buffer with proteinase inhibitors, and the homogenate was centrifuged at 13,000 g, 4°C for 10 min, and the supernatant was collected. Cell protein was extracted using RIPA lysis buffer for 20 min at 4°C. Then the 5× loading buffer was added, and the sample was boiled for 5 min. Western blot was performed as previously described (11). The primary antibodies include rabbit anti-human PD-L1 Ab (1:1,000, Abcam), anti-DDX60 Ab (1:1,000, Abcam), and anti-GAPDH (1:50,000, Proteintech). The data analysis as well as statistics was performed through ImageJ as described previously (22).
R language 3.6.2 version was employed to perform statistical analysis. A Student’s t-test was conducted to evaluate DDX60 expression differences. ‘Survival’ and ‘survminer’ packages in R were used for survival analysis. Continuous variables of the DDX60 expression were dichotomized by conducting the best cutoff values detected by the “surv_cutpoint” function of the “survminer” R package (23). the statistical significance was calculated by the log-rank test (24). Univariate and multivariate Cox proportional hazards models were performed to search hazard ratios (HRs) by R.
The expression of DDX60 was upregulated in multiple cancers comparing with normal tissues, including GBM (Figure 1A). TCGA and Rembrandt dataset analysis showed that DDX60 is overexpressed in glioma (P < 0.001, Supplementary Figures 1A, B). As expected, TCGA analysis demonstrated that DDX60 in GBM is higher than in lower-grade glioma (LGG, grades II and III, P < 0.0001 and P = 0.032, respectively, Figure 1B). The CGGA and Rembrandt dataset also confirmed the lower levels of DDX60 in LGG (Supplementary Figures 1C, D). Additionally, DDX60 favored its expression in mesenchymal and classical subtype (Figure 1C and Supplementary Figure 1E), MGMT unmethylated (P < 0.001, Figure 1D), ATRX wild-type (P < 0.001, Figure 1E), TERT promoter mutated (P < 0.001, Figure 1F), and IDH wild-type gliomas (P < 0.001, Figure 1G, Supplementary Figure 1F). All the in silico analyses demonstrated the correlation between DDX60 and the malignancy of gliomas.
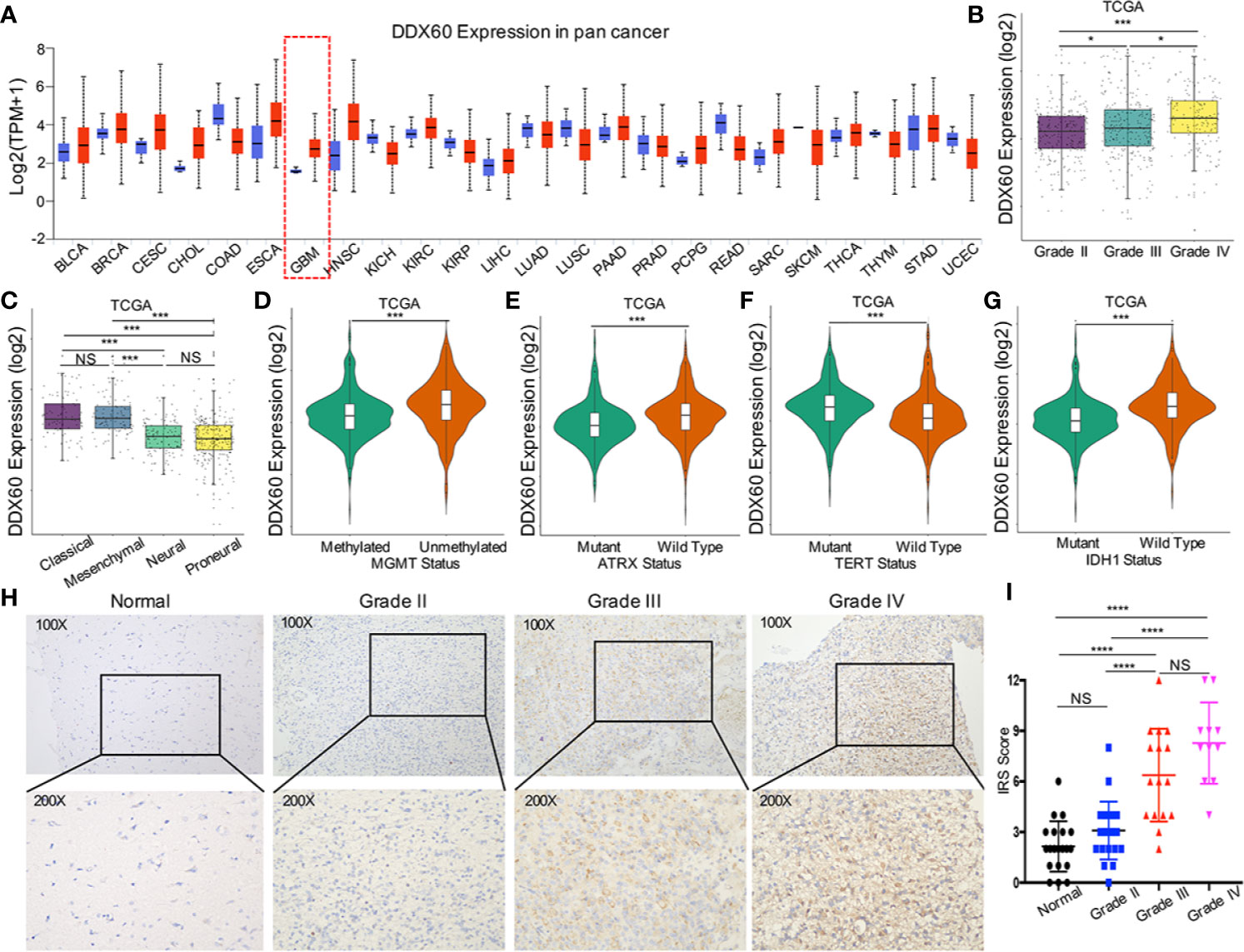
Figure 1 DDX60 expression upregulated in glioma and was correlated with glioma biomarkers. (A) The expression information for DDX60 in tumor and normal tissues in multiple cancers in UALCAN. Blue represents the normal tissue and red represents the tumor tissue. (B) DDX60 expression level increase along with WHO grade in the TCGA database. (C) Comparison between different subtypes of GBM in TCGA. (D–F) DDX60 was upregulated in ATRX wild-type group, MGMT unmethylated group and TERT expressed group based on TCGA dataset. (G) DDX60 was significantly overexpressed in IDH wild-type glioma based on TCGA dataset. (H) Representative IHC staining of DDX60 in normal brain tissue and WHO grade II–IV gliomas. (I) Immune reactive score (IRS) of DDX60 in normal brain tissue and different WHO grade gliomas, Normal (n = 20), Grade II (n = 22), Grade III (n = 16), Grade IV (n = 11). *P < 0.05, ***P < 0.001 and ****P < 0.0001. NS, not significant.
IHC analysis (Figures 1H, I) verified that DDX60 expression in normal tissue (mean IRS = 2.15, n = 20) was lower than in glioma tissue (mean IRS = 5.33, n = 49) (P < 0.0001). DDX60 was predominantly expressed in the cytoplasm of glioma cells and expressed distinctively in different WHO grades. The expression of DDX60 in grade II (mean IRS = 3.09, n = 22) was significantly lower than that in grade III (mean IRS = 6.38, n = 16, P < 0.0001) and in grade IV (mean IRS = 8.27, n = 11, P < 0.0001), while no statistical difference was found between grade III and grade IV (P = 0.077). In summary, DDX60 expression was higher in glioma than in normal tissue and increased with malignant escalation of glioma.
We further assessed the prognostic value of DDX60 in both LGG and GBM based on TCGA, CGGA, and Rembrandt datasets. Higher DDX60 expression seemed to portend a poor prognosis for GBM in TCGA (P = 0.001, Figure 2A). Likewise, a strong correlation between higher expression of DDX60 and worse OS for GBM patients was detected in CGGA and Rembrandt datasets, respectively (P = 0.0042 and P = 0.075, Figures 2B, C). Survival data in LGG were consistent with those in GBM in TCGA, CGGA, and Rembrandt, respectively (all P < 0.0001, Figures 2D–F). These outcomes demonstrated DDX60 as a negative prognostic indicator in gliomas.
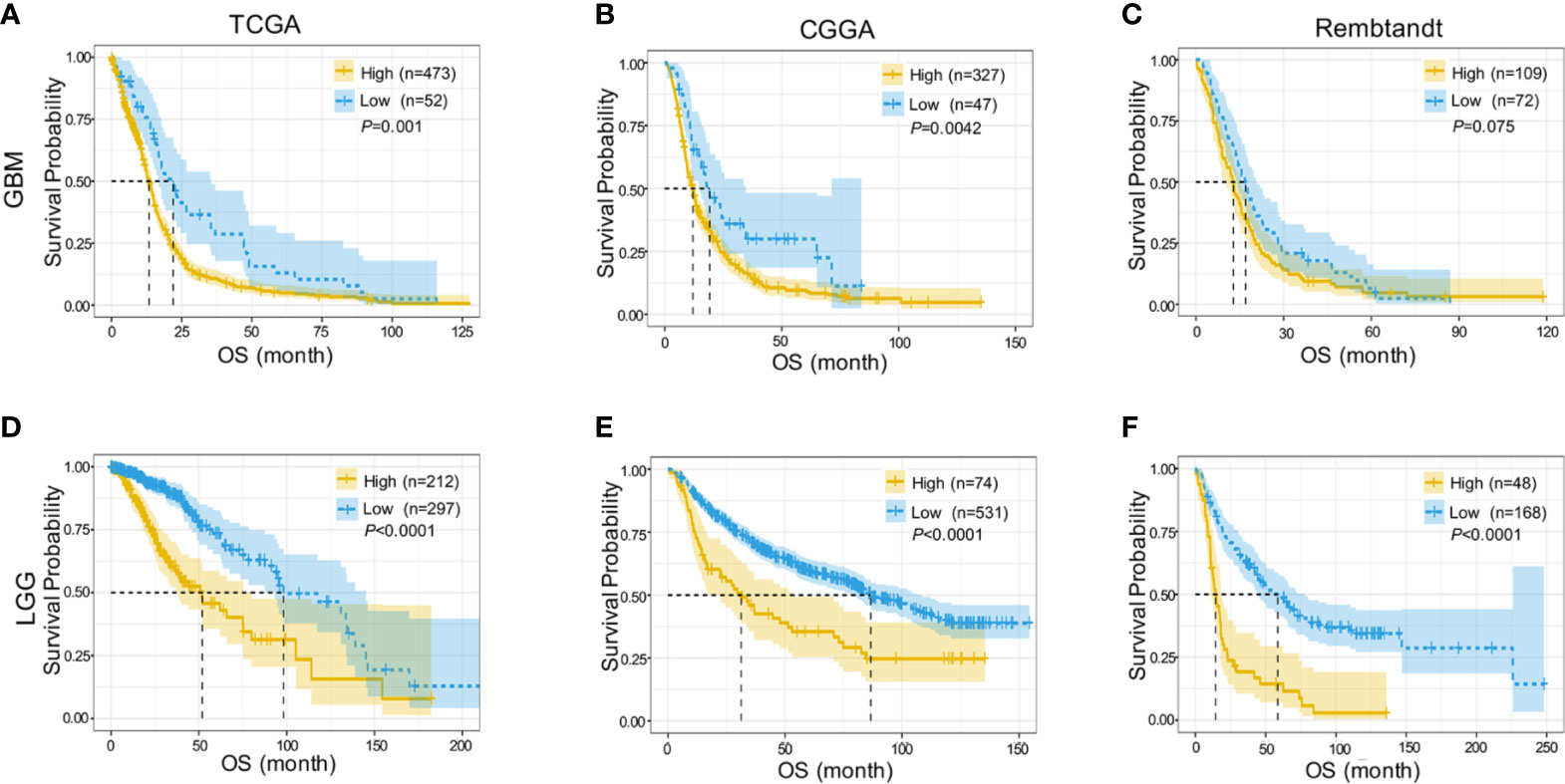
Figure 2 DDX60 predicts poor prognosis of glioma patients. (A–C) Higher DDX60 expression portended poor prognosis for GBM in TCGA database, CGGA dataset and Rembrandt dataset. (D–F) LGG patients with increased expression of DDX60 also get poor prognosis in TCGA database, CGGA dataset and Rembrandt dataset.
Univariate (HR = 1.623, P < 0.0001) and multivariate (HR = 1.1937, P = 0.024) Cox regression analyses were then performed, and factors related to the prognosis of gliomas were selected (Table 1). The prognostic nomogram with a risk classification system for 3- and 5-year survival rates of glioma based on TCGA was established (n = 596, Figure 3A). This nomogram integrated all significant independent variables including DDX60, and the C-index for OS prediction was 0.86. The calibration plot for the probability of survival at 3 or 5 years based on the two independent cohorts of CGGA (n = 960) and Gravendeel (n = 216) showed optimal conformity between the prediction by nomogram and actual observation (Figures 3B–E). The demographics and clinical characteristics of patients with glioma in primary and validation cohort were in Supplementary Table 1.
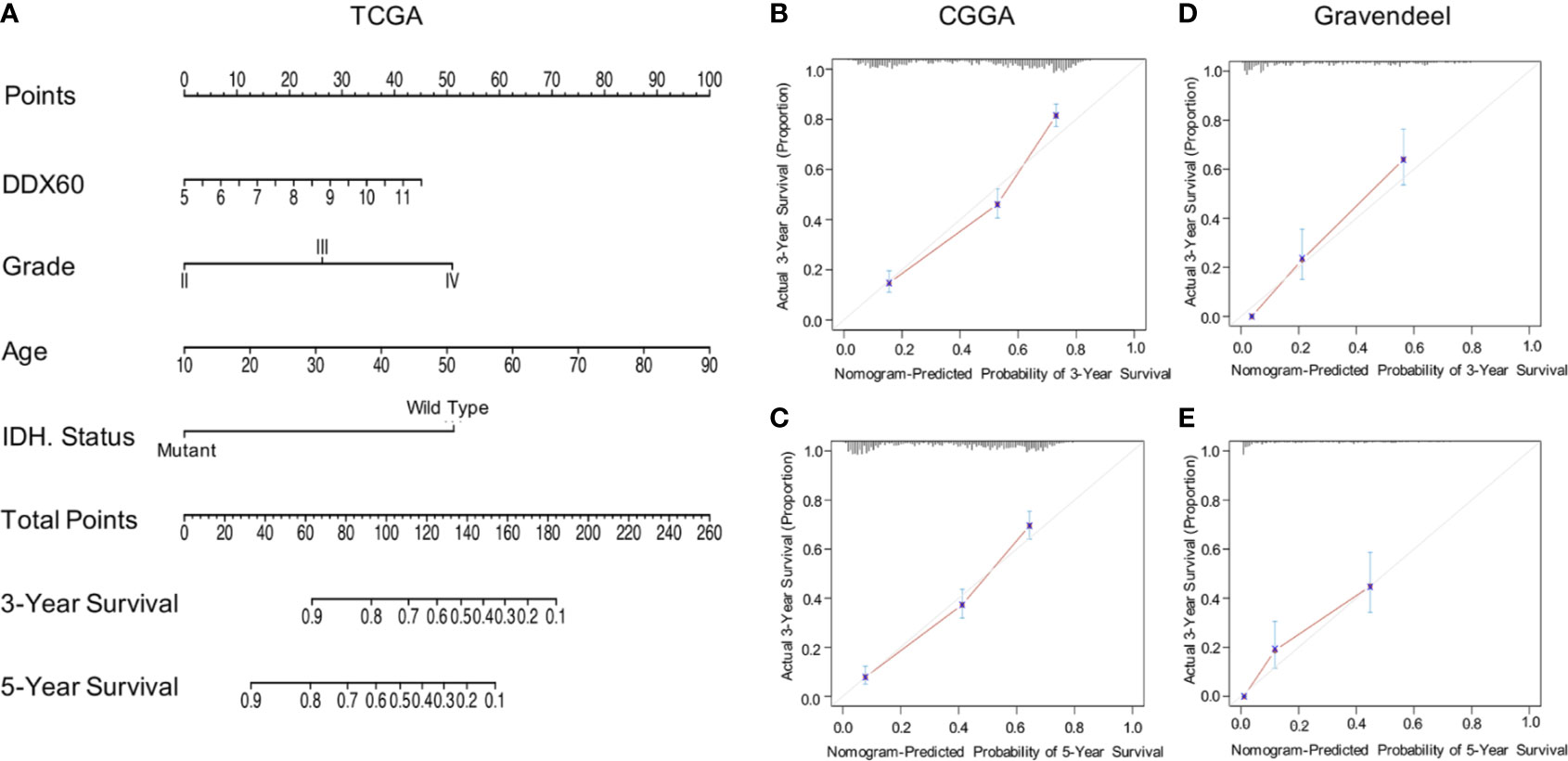
Figure 3 DDX60-related prediction nomogram. (A) Nomogram for predicting 3- or 5-year survival in glioma patients. The top row represents the point value for each variable. Rows 2–5 display the variables included in the nomogram. Each variable fits to a point value based on glioma characteristics. The Total Points axis equals to the sum of the point values, and the lines downward to the total points is used to establish the liability of 3- or 5-year survival. (B, C) Calibration curves for predicting patient survival in CGGA dataset at 3 and 5 years. (D, E) The Gravendeel Dataset was also used as the validation cohort to show calibration curves for predicting patient survival at 3 and 5 years.
DDX60 is known as an IFN-inducible gene (6). To verify the function of DDX60 in gliomas, 775 genes were identified in the intersection of the three datasets through Pearson’s correlation (|R| > 0.3, Figure 4A, Supplementary Table 2). Gene ontology (GO) analysis illustrated that DDX60 was involved in multiple biological processes, including immune response, defense response to other organisms, cytokine-mediated signaling pathway (Figure 4B). Meanwhile, GSEA verified the gene signatures were mainly enriched in the inflammatory response and immune response (Figures 4C–E). The Cytoscape of enrichment map displayed that enriched terms are centrally attached to the immune response as well as inflammatory response (Figure 4F).
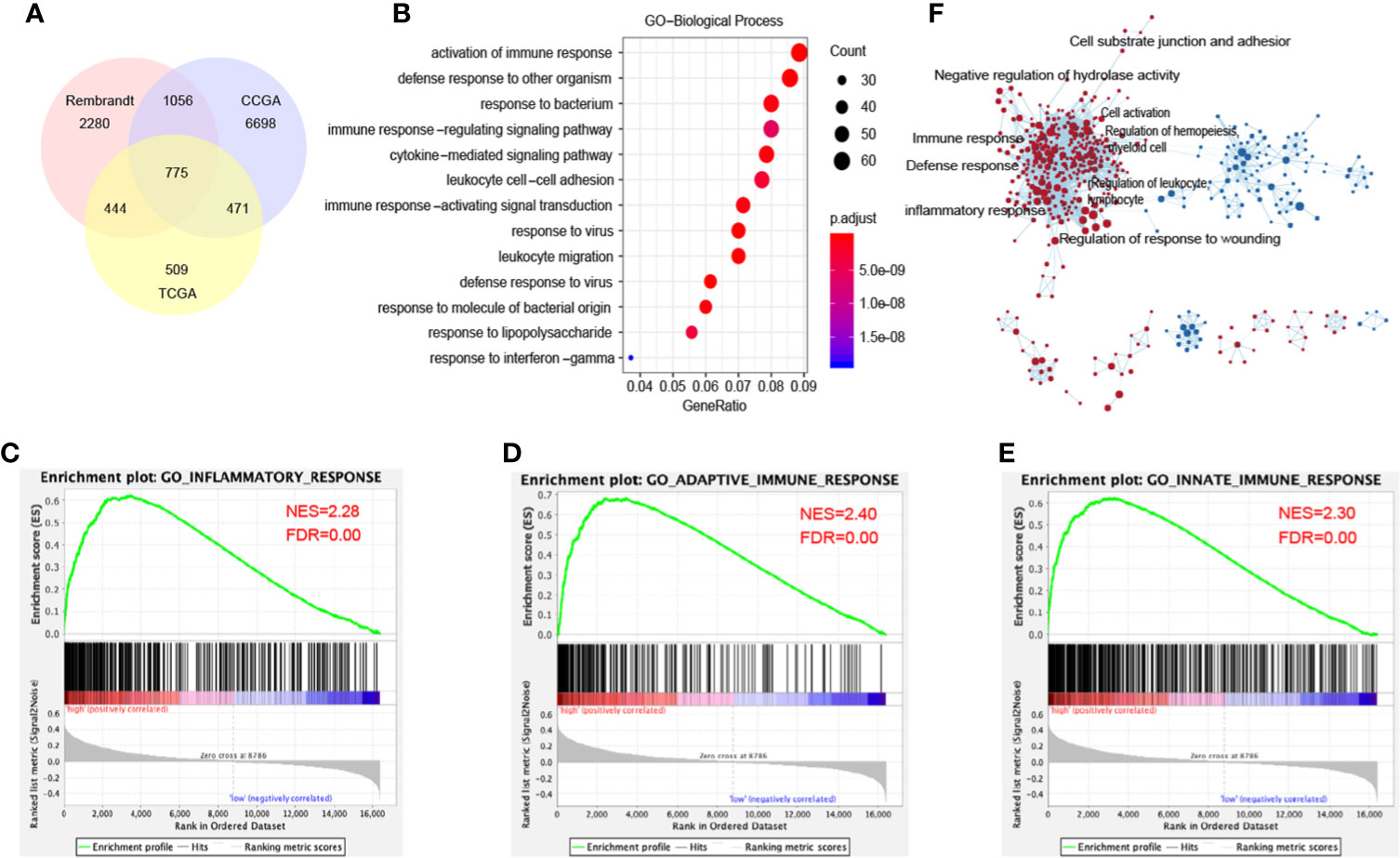
Figure 4 DDX60-related biological processes in glioma. (A) 775 related genes of DDX60 were chosen in glioma from the TCGA, CGGA, and Rembrandt databases based on Pearson’s correlation analysis (|R| > 0.3). (B) The term of immune response enriched most through gene ontology (GO) analysis on biological processes (BP). (C–E) GSEA employed to verify the gene signatures, mainly included inflammatory response, adoptive immune response, and innate immune response. (F) The cytoscape of enrichment map results. Nodes represent gene-sets, which were automatically arranged so that highly similar gene-sets are placed close together, and node size represents the number of genes in the gene-set.
To better understand DDX60-related inflammatory responses in glioma, seven metagenes including 105 genes (Supplementary Table 3) associated with multiple types of inflammation and immune functions were chosen (25). Clustering based on TCGA and CGGA showed that all clusters have a positive correlation with DDX60 expression level apart from IgG (Figures 5A, B). Correlograms show that DDX60 expression level had a positive association with hematopoietic cell kinase (HCK), interferon, lymphocyte-specific protein tyrosine kinase (LCK), major histocompatibility complex class-I (MHC-I), major histocompatibility complex class-II (MHC-II), and STAT1, while it had a negative correlation with IgG (Figures 5C, D). Among the gene set associated with the immune function, 103 out of 105 genes in TCGA and 145 out of 149 genes in CGGA were significantly positively associated with DDX60 (Supplementary Figure 2, Supplementary Tables 4 and 5). Collectively, a strong association between DDX60 expression patterns and immune functions has been found in glioma.
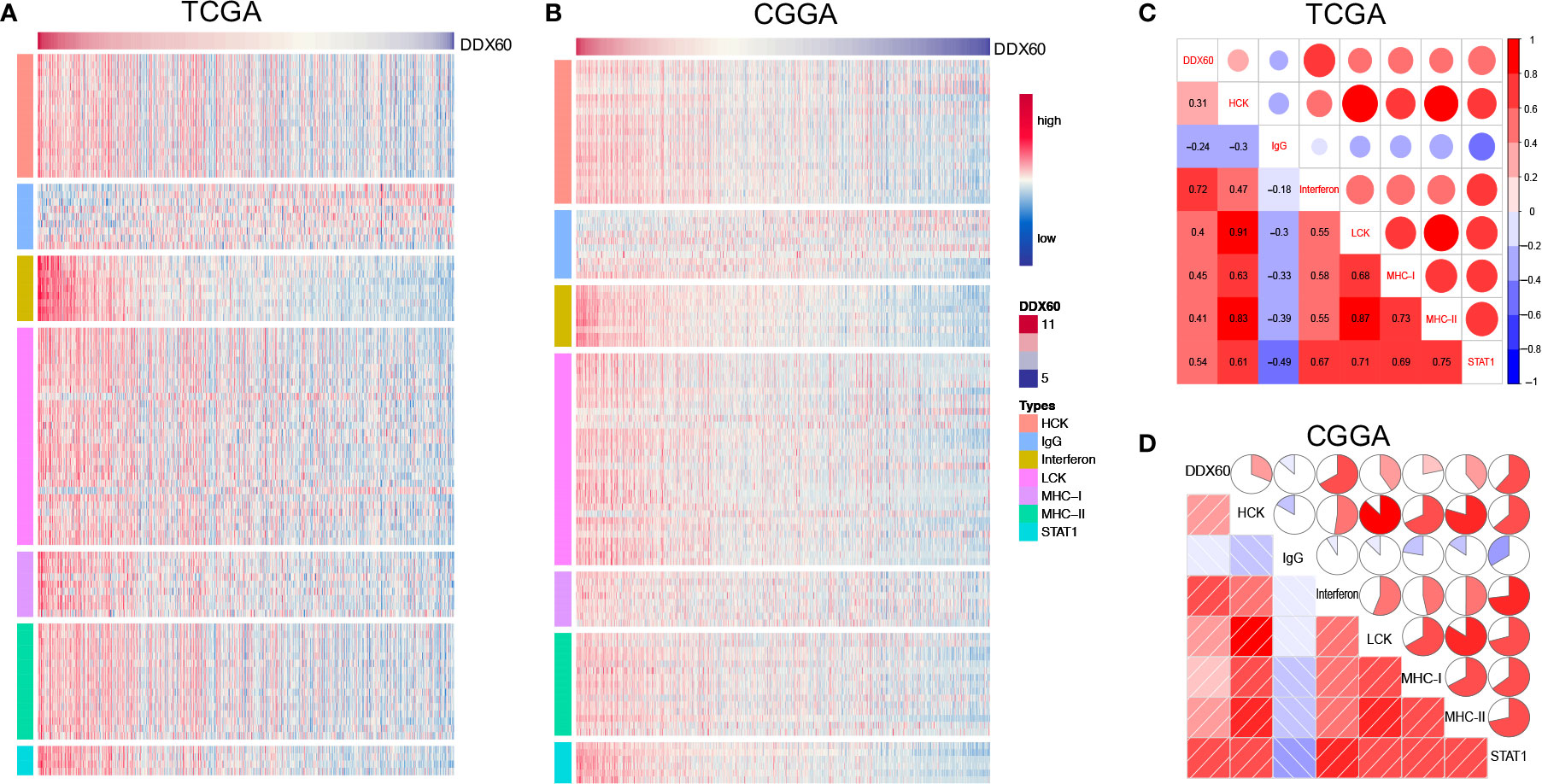
Figure 5 DDX60-related inflammatory responses in glioma. (A, B) Heatmap of the correlation between DDX60 and metagenes based on TCGA and CGGA. (C, D) Correlogram showed the association between DDX60 and seven inflammatory-related metagenes in TCGA and CGGA datasets.
Metagenes (Supplementary Table 6) (19) were delineated in heatmaps to show the correlation between DDX60 expression and 28 infiltrating immune cell populations (Figures 6A, B and Supplementary Figure 3A). The top five DDX60-related immune cells in TCGA were effector memory CD8 T cell (CD8+ TEM), natural killer cell (NK), natural killer T cell (NKT), plasmacytoid dendritic cell (pDC), and activated dendritic cell (aDC) (Figure 6C). Correlation matrixes of the top five most related immune cells in the CGGA and Rembrandt dataset were also displayed (Figure 6D and Supplementary Figure 3B), and all the Pearson’s correlation coefficient (R) and P-values were listed (Supplementary Table 7). In summary, DDX60 expression tends to be positively related to most infiltrating immune cells, while negatively related with CD56 dim nature killer cell in glioma.
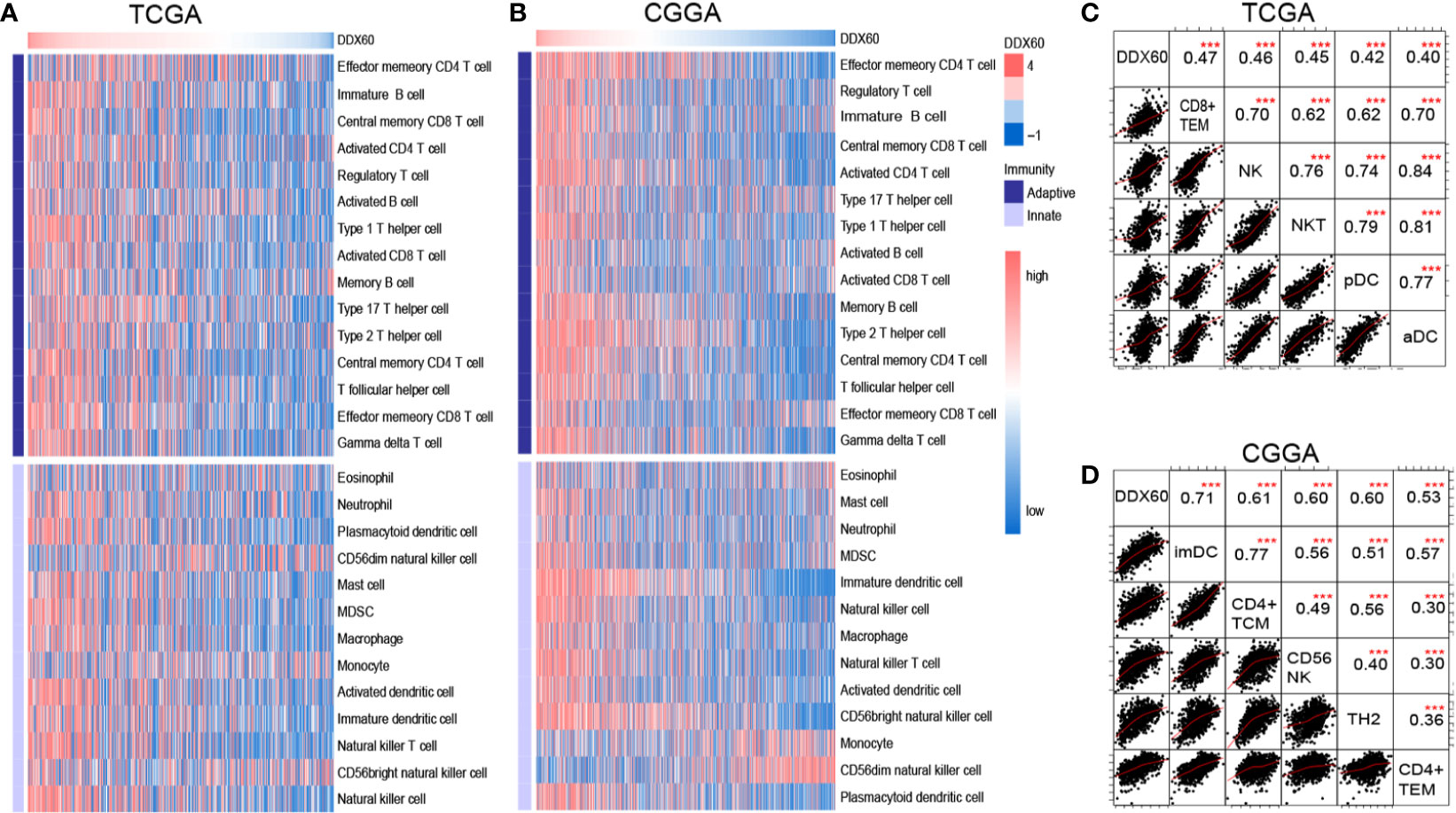
Figure 6 DDX60 is closely correlated with immune cells in the glioma microenvironment. (A, B) Heatmap based on TCGA and CGGA dataset visualizing the relationship between DDX60 and 28 infiltrating immune cell populations. (C, D) Correlation matrixes of the top five most related immune cells with DDX60 in TCGA and CGGA datasets. ***P < 0.001.
The correlation between DDX60 and some important checkpoint proteins like CTLA-4, PD-L1, EGF, CD226, and CD96 was assessed in the TCGA and CGGA datasets. Circos plots showed the strong positive association between DDX60 and these five immune check point-related genes in all grade gliomas (Figures 7A–D). The correlation coefficients (R) between DDX60 and immune checkpoint genes were shown in Table 2. Among these genes, PD-L1 showed the strongest positive correlation with DDX60 in the TCGA dataset (glioma R = 0.54, GBM R = 0.45). Western blot analysis with clinical samples and DDX60 knockdown glioma cells demonstrated that DDX60 protein expression was correlated with PD-L1 (P < 0.0001, R = 0.86) and EGF (P = 0.002, R = 0.56) (Figures 7E–H). The relatively density of PD-L1 and EGF of western blot for glioma tissue were shown in supplementary Figure 4. Results predicted the possible synergistic effects of DDX60 with these checkpoint genes.
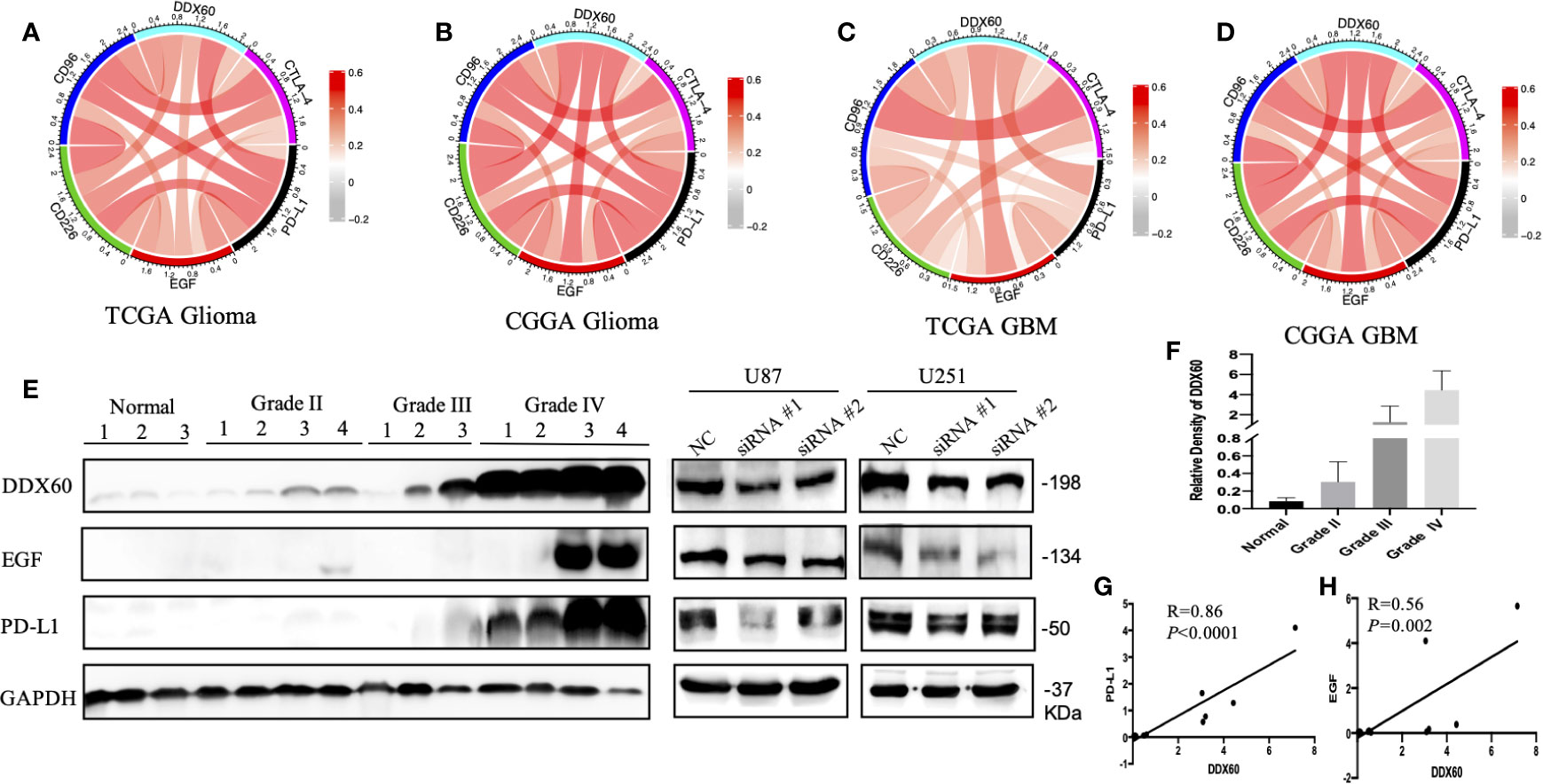
Figure 7 Relationship between DDX60 and checkpoint markers in glioma. (A–D) The associations between DDX60 and immune check point-related genes including CTLA-4, PD-L1, EGF, CD96, and CD226 based on TCGA and CGGA datasets were presented. (E) The level of DDX60, PD-L1 and EGF protein expression in normal brain tissue, WHO grade II–IV glioma tissue and DDX60 knockdown glioma cells were shown by western blot. (F) The relative density of DDX60 of western blot. (G) A strong association between DDX60 and PD-L1 (R = 0.86, P < 0.0001) according to the gray-scale analysis of the western blot. (H) The expression correlation between EGF and DDX60 (R = 0.56, P = 0.002).
Although novel therapies such as immunotherapy have been used, less progress has been made in overall survival (OS) in GBM patients (26). Within-tumor heterogeneity is a major driver of progression, recurrence, and therapeutic resistance of GBM (27). Therefore, more biomarkers are needed to be identified to accurately access the prognosis of GBM patients and individualize treatment strategies.
DDX60 is a DEAD-box RNA helicase and has been proved upregulated in melanoma (28) and oral squamous cell carcinoma (OSCC) (10) while deregulating in colorectal cancer (11). Herein, we demonstrated that higher expression of DDX60 was correlated with high-grade glioma. Moreover, we observed that DDX60 was significantly overexpressed in glioma with IDH wild-type, ATRX wild-type, MGMT unmethylated as well as TERT promoter mutated. These findings suggest that gliomas with high DDX60 expression have increased malignancy and insensitive to chemotherapy (29).
A previous study has shown that high expression of DDX60 was significantly associated with poor survival in lip squamous cell carcinoma (10). As expected, this study demonstrated that high DDX60 expression was associated with poor prognosis both in LGG and GBM. As the nomogram could show better performance than conventional staging systems and more precise prognostic forecast in some tumors (30, 31), we identified DDX60 as a prognostic marker of glioma and built a nomogram with a risk classification system. The four parameters included in the nomogram complied with clinical relevance and Cox analysis (32). Studies have shown that gender, age, WHO grade, and IDH status are related to the prognosis of glioma (33–35). However, our univariate and multivariate Cox analyses did not find a correlation between sex and prognosis of glioma. Thus, gender was excluded from the visualization of the nomogram. The calibration plots of the two external validation cohorts were highly fitted, illustrating that the nomogram performed well in predicting 3- or 5-year survival for glioma patients.
GO and GSEA of DDX60 in this research showed that immune and inflammatory responses were the most enriched terms. It has been indicated that inflammation regulates various stages of the tumor process, such as promotion and invasion (36). Different proinflammatory mediators induced by inflammation promote tumor progression by regulating chemokines, cascades of cytokines, adhesion, and pro-angiogenic activities (37). DDX60 is an IFN-inducible gene, and its ectopic expression can promote RIG-I RNA-binding activity, causing RIG-I-mediated type I IFN expression (6). Type I IFNs (IFN-alpha and IFN-beta) are a family of cytokines with a diverse cellular processes such as regulation of inflammatory and immune responses (38). Through mediating type I IFNs, DDX60 can also activate STAT1and upregulate MHC-I (39); these results are compatible with our findings. Furthermore, we also found that DDX60 was positively associated with LCK and HCK while negatively associated with IgG response; these results refined the mechanism of DDX60 in the inflammatory response of glioma.
Multiple non-neoplastic cells exist in the GBM microenvironment, such as infiltrating immune cells (40). The immune surveillance of these immune cells would be converted to detrimental function when the immune system is overwhelmed by cancer burden during tumor development (40). The high correlation between infiltrating immune cells and DDX60 expression has been demonstrated in this study, such as type 17 T helper cell (Th17) and macrophages. Previous research had verified that Th17 cells in the GBM microenvironment may participate in immune suppression via TGF-β1-induced IL-10 secretion (41). Tumor-associated macrophages (TAMs) in GBM have been proven to be the dominant infiltrating immune cell population and engaged in interactions with tumor cells to aid tumor infiltration and proliferation (42). These results suggest that DDX60 might involve in immunosuppression by mediating immune cells in glioma.
CNS used to be considered as “immunologically privileged” in the past decades. However, as more and more researchers have verified that the leukocyte lymphatics not only be present in CNS but also have the ability to transport antigens to cervical lymph nodes (43–46), researchers agree that CNS is more likely “immunologically unique” rather than “immunologically privileged”. These provide a basis for glioma immunotherapy. Undoubtedly, immunotherapy holds a bright future for the treatment of glioma. However, it seems difficult to achieve stable and better outcomes for immunotherapy in clinical. This can be mostly attributed to the tumor heterogeneity of glioma (47, 48). New biomarkers which can predict and monitor immunotherapy response have become urgently needed. In this study, DDX60 was not only a prognosis prediction for glioma patients but also an indicator of the immune microenvironment of glioma and might become a novel biomarker and potential therapeutic target.
Checkpoint inhibitors, advancing rapidly in recent years, have been the immunotherapy most advanced in clinical use. Among them, death protein 1 (PD-1) and PD-L1 are the most broadly studied (49). PD-L1 is widely expressed on the GBM infiltrating T cells and is a negative prognosticator for GBM outcome (50). PD-1 combining with PD-L1, negatively modulates T cell receptor-induced signaling transduction, blocks the activation of cytotoxic T cell, and inhibits the producing of inflammatory factors, causing T cell inability (49). Many clinical trials in GBM are evaluating anti-PD-L1 agents such as Durvalumab, Atezolizumab, Avelumab, alone or combined with other therapies (51). However, not all the research studies on anti-PD-L1 agents come out with a meaningful benefit (51). Thus, new biomarkers that can accurately predict the efficacy of PD-L1 inhibitor therapy are urgently needed. In this study, we demonstrated that the correlation coefficients (R) between PD-L1 and DDX60 were 0.54 in the TCGA glioma dataset and 0.61 in the CGGA glioma dataset. We further performed western blot both in patient tissues and glioma cell lines to verify the strong correlation between PD-L1 and DDX60 (P < 0.0001, R = 0.86). The mechanism of the positive correlation between DDX60 and PD-L1 might lie in the IFN/PD-L1 axis. As an IFN-inducible gene, the ectopic expression of DDX60 can improve RIG-I RNA-binding activity, causing RIG-I-mediated IFN expression (6). A previous study has shown that IFN was a crucial factor of PD-L1 expression in the glioma model (52). Thus, the upregulation of DDX60 might lead to a higher expression level of PD-L1. These results implied that glioma patients with higher DDX60 expression might benefit more from PD-L1 blocker therapy.
Besides, the correlation between DDX60 and some other immune checkpoint genes such as cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), epidermal growth factor (EGF), CD226, and CD96 was shown (Figure 7, Table 2). As the correlation coefficients (R) between EGF and DDX60 were >0.5 in both CGGA glioma and CGGA GBM database, western blot was then employed to demonstrate the strong association between EGF and DDX60 (P = 0.002, R = 0.56). Thus, these results illustrate the predictive significance and potential synergistic responses of DDX60 to immune checkpoint treatments.
There have been reports that DDX60 was overexpressed in other types of cancers (10, 11), but most of them did not further explore the intrinsic mechanisms. Thus, the novelties of this paper lie not merely in the findings of prognostically significant of DDX60 in glioma, but also in the mechanism of DDX60 on glioma. Our research verified the strong association between DDX60 and glioma immune microenvironment, clarified the mechanism of DDX60, and proposed that DDX60 might become a novel biomarker for immunotherapy.
In conclusion, these results would widen our knowledge of the expression and prognostic value of DDX60 in gliomas. Furthermore, as a potential therapeutic target, DDX60 is positively correlated with PD-L1 and other checkpoints. Thus, these findings will help to optimize the immunotherapy in glioma.
Publicly available datasets were analyzed in this study. This data can be found here: http://gliovis.bioinfo.cnio.es/.
The studies involving human participants were reviewed and approved by the Human Ethics Committee of Huashan Hospital. The patients/participants provided their written informed consent to participate in this study.
JingZ designed and conducted the study and drafted the original manuscript. MF, JinZ, and MZ helped collect the databases. ZD helped conduct the IHC and IRS. WH, HZ, and YM supervised the study. WH revised the manuscript. All authors contributed to the article and approved the submitted version.
The study was funded by Shanghai Science and Technology Commission (17430750200), the National Natural Science Foundation of China (82072785, 82072784), Join Breakthrough Project for New Frontier Technologies of Shanghai Hospital Development Center (SHDC12016120).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank the reviewers and editor for their valuable comments.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.665360/full#supplementary-material
GBM, glioblastoma multiforme; OS, overall survival; MHC I, major histocompatibility complex class I; GO, Gene ontology; BP, biological processes; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; CTLA-4, cytotoxic T-lymphocyte-associated antigen-4; Rembrandt, Repository for Molecular Brain Neoplasia Data; TCGA, The Cancer Genome Atlas; HRs, hazard ratios.
1. Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, et al. Cbtrus Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009-2013. Neuro Oncol (2016) 18(suppl_5):v1–75. doi: 10.1093/neuonc/now207
2. Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain Immunology and Immunotherapy in Brain Tumours. Nat Rev Cancer (2020) 20(1):12–25. doi: 10.1038/s41568-019-0224-7
3. Friese MA, Steinle A, Weller M. The Innate Immune Response in the Central Nervous System and its Role in Glioma Immune Surveillance. Onkologie (2004) 27(5):487–91. doi: 10.1159/000080371
4. Binder ZA, Thorne AH, Bakas S, Wileyto EP, Bilello M, Akbari H, et al. Epidermal Growth Factor Receptor Extracellular Domain Mutations in Glioblastoma Present Opportunities for Clinical Imaging and Therapeutic Development. Cancer Cell (2018) 34(1):163–77.e7. doi: 10.1016/j.ccell.2018.06.006
5. Glas M, Coch C, Trageser D, Dassler J, Simon M, Koch P, et al. Targeting the Cytosolic Innate Immune Receptors RIG-I and MDA5 Effectively Counteracts Cancer Cell Heterogeneity in Glioblastoma. Stem Cells (2013) 31(6):1064–74. doi: 10.1002/stem.1350
6. Miyashita M, Oshiumi H, Matsumoto M, Seya T. DDX60, a DEXD/H Box Helicase, is a Novel Antiviral Factor Promoting RIG-I-like Receptor-Mediated Signaling. Mol Cell Biol (2011) 31(18):3802–19. doi: 10.1128/MCB.01368-10
7. Gustafson EA, Wessel GM. DEAD-Box Helicases: Posttranslational Regulation and Function. Biochem Biophys Res Commun (2010) 395(1):1–6. doi: 10.1016/j.bbrc.2010.02.172
8. Oshiumi H, Miyashita M, Okamoto M, Morioka Y, Okabe M, Matsumoto M, et al. Ddx60 Is Involved in RIG-I-Dependent and Independent Antiviral Responses, and Its Function Is Attenuated by Virus-Induced Egfr Activation. Cell Rep (2015) 11(8):1193–207. doi: 10.1016/j.celrep.2015.04.047
9. Grunvogel O, Esser-Nobis K, Reustle A, Schult P, Muller B, Metz P, et al. Ddx60l Is an Interferon-Stimulated Gene Product Restricting Hepatitis C Virus Replication in Cell Culture. J Virol (2015) 89(20):10548–68. doi: 10.1128/JVI.01297-15
10. Fu TY, Wu CN, Sie HC, Cheng JT, Lin YS, Liou HH, et al. Subsite-Specific Association of DEAD Box RNA Helicase DDX60 With the Development and Prognosis of Oral Squamous Cell Carcinoma. Oncotarget (2016) 7(51):85097–108. doi: 10.18632/oncotarget.13197
11. He L, Liu Y, Lai W, Tian H, Chen L, Xie L, et al. DNA Sensors, Crucial Receptors to Resist Pathogens, are Deregulated in Colorectal Cancer and Associated With Initiation and Progression of the Disease. J Cancer (2020) 11(4):893–905. doi: 10.7150/jca.34188
12. Xue S, Hu M, Iyer V, Yu J. Blocking the PD-1/PD-L1 Pathway in Glioma: A Potential New Treatment Strategy. J Hematol Oncol (2017) 10(1):81. doi: 10.1186/s13045-017-0455-6
13. Woehrer A, Bauchet L, Barnholtz-Sloan JS. Glioblastoma Survival: has it Improved? Evidence From Population-Based Studies. Curr Opin Neurol (2014) 27(6):666–74. doi: 10.1097/WCO.0000000000000144
14. Bowman RL, Wang Q, Carro A, Verhaak RG, Squatrito M. GlioVis Data Portal for Visualization and Analysis of Brain Tumor Expression Datasets. Neuro Oncol (2017) 19(1):139–41. doi: 10.1093/neuonc/now247
15. Gu Z, Gu L, Eils R, Schlesner M, Brors B. Circlize Implements and Enhances Circular Visualization in R. Bioinformatics (2014) 30(19):2811–2. doi: 10.1093/bioinformatics/btu393
16. Yu G, Wang LG, Han Y, He QY. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS (2012) 16(5):284–7. doi: 10.1089/omi.2011.0118
17. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc Natl Acad Sci U S A (2005) 102(43):15545–50. doi: 10.1073/pnas.0506580102
18. Hanzelmann S, Castelo R, Guinney J. GSVA: Gene Set Variation Analysis for Microarray and RNA-seq Data. BMC Bioinf (2013) 14:7. doi: 10.1186/1471-2105-14-7
19. Charoentong P, Finotello F, Angelova M, Mayer C, Efremova M, Rieder D, et al. Pan-Cancer Immunogenomic Analyses Reveal Genotype-Immunophenotype Relationships and Predictors of Response to Checkpoint Blockade. Cell Rep (2017) 18(1):248–62. doi: 10.1016/j.celrep.2016.12.019
20. Zietzer A, Hosen MR, Wang H, Goody PR, Sylvester M, Latz E, et al. The RNA-binding Protein hnRNPU Regulates the Sorting of microRNA-30c-5p Into Large Extracellular Vesicles. J Extracell Vesicles (2020) 9(1):1786967. doi: 10.1080/20013078.2020.1786967
21. Lemke D, Pfenning PN, Sahm F, Klein AC, Kempf T, Warnken U, et al. Costimulatory Protein 4igB7H3 Drives the Malignant Phenotype of Glioblastoma by Mediating Immune Escape and Invasiveness. Clin Cancer Res (2012) 18(1):105–17. doi: 10.1158/1078-0432.CCR-11-0880
22. Stanic J, Carta M, Eberini I, Pelucchi S, Marcello E, Genazzani AA, et al. Rabphilin 3A Retains NMDA Receptors At Synaptic Sites Through Interaction With GluN2A/PSD-95 Complex. Nat Commun (2015) 6:10181. doi: 10.1038/ncomms10181
23. Li X, Yuan Y, Ren J, Shi Y, Tao X. Incremental Prognostic Value of Apparent Diffusion Coefficient Histogram Analysis in Head and Neck Squamous Cell Carcinoma. Acad Radiol (2018) 25(11):1433–8. doi: 10.1016/j.acra.2018.02.017
24. Kassambara A, Moreaux J. Analysis of Global Gene Expression Profiles. Methods Mol Biol (2018) 1792:157–66. doi: 10.1007/978-1-4939-7865-6_11
25. Rody A, Holtrich U, Pusztai L, Liedtke C, Gaetje R, Ruckhaeberle E, et al. T-Cell Metagene Predicts a Favorable Prognosis in Estrogen Receptor-Negative and HER2-positive Breast Cancers. Breast Cancer Res (2009) 11(2):R15. doi: 10.1186/bcr2234
26. Jain KK. A Critical Overview of Targeted Therapies for Glioblastoma. Front Oncol (2018) 8:419. doi: 10.3389/fonc.2018.00419
27. Friedmann-Morvinski D. Glioblastoma Heterogeneity and Cancer Cell Plasticity. Crit Rev Oncog (2014) 19(5):327–36. doi: 10.1615/CritRevOncog.2014011777
28. Znidar K, Bosnjak M, Cemazar M, Heller LC. Cytosolic DNA Sensor Upregulation Accompanies DNA Electrotransfer in B16.F10 Melanoma Cells. Mol Ther Nucleic Acids (2016) 5(6):e322. doi: 10.1038/mtna.2016.34
29. Bell EH, Zhang P, Fisher BJ, Macdonald DR, McElroy JP, Lesser GJ, et al. Association of MGMT Promoter Methylation Status With Survival Outcomes in Patients With High-Risk Glioma Treated With Radiotherapy and Temozolomide: An Analysis From the NRG Oncology/Rtog 0424 Trial. JAMA Oncol (2018) 4(10):1405–9. doi: 10.1001/jamaoncol.2018.1977
30. Wang C, Yang C, Wang W, Xia B, Li K, Sun F, et al. A Prognostic Nomogram for Cervical Cancer After Surgery From SEER Database. J Cancer (2018) 9(21):3923–8. doi: 10.7150/jca.26220
31. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic Nomogram for Intrahepatic Cholangiocarcinoma After Partial Hepatectomy. J Clin Oncol (2013) 31(9):1188–95. doi: 10.1200/JCO.2012.41.5984
32. Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A Prospective Natural-History Study of Coronary Atherosclerosis. N Engl J Med (2011) 364(3):226–35. doi: 10.1056/NEJMoa1002358
33. Rasmussen BK, Hansen S, Laursen RJ, Kosteljanetz M, Schultz H, Norgard BM, et al. Epidemiology of Glioma: Clinical Characteristics, Symptoms, and Predictors of Glioma Patients Grade I-IV in the the Danish Neuro-Oncology Registry. J Neurooncol (2017) 135(3):571–9. doi: 10.1007/s11060-017-2607-5
34. Lapointe S, Perry A, Butowski NA. Primary Brain Tumours in Adults. Lancet (2018) 392(10145):432–46. doi: 10.1016/S0140-6736(18)30990-5
35. Ostrom QT, Coleman W, Huang W, Rubin JB, Lathia JD, Berens ME, et al. Sex-Specific Gene and Pathway Modeling of Inherited Glioma Risk. Neuro Oncol (2019) 21(1):71–82. doi: 10.1093/neuonc/noy135
36. Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell (2010) 140(6):883–99. doi: 10.1016/j.cell.2010.01.025
37. Coussens LM, Werb Z. Inflammation and Cancer. Nature (2002) 420(6917):860–7. doi: 10.1038/nature01322
38. Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, et al. Apoptosis and Interferons: Role of Interferon-Stimulated Genes as Mediators of Apoptosis. Apoptosis (2003) 8(3):237–49. doi: 10.1023/A:1023668705040
39. Galani V, Papadatos SS, Alexiou G, Galani A, Kyritsis AP. In Vitro and In Vivo Preclinical Effects of Type I Ifns on Gliomas. J Interferon Cytokine Res (2017) 37(4):139–46. doi: 10.1089/jir.2016.0094
40. Chen Z, Hambardzumyan D. Immune Microenvironment in Glioblastoma Subtypes. Front Immunol (2018) 9:1004. doi: 10.3389/fimmu.2018.01004
41. Paladugu M, Thakur A, Lum LG, Mittal S, Parajuli P. Generation and Immunologic Functions of Th17 Cells in Malignant Gliomas. Cancer Immunol Immunother (2013) 62(1):75–86. doi: 10.1007/s00262-012-1312-7
42. Hu F, Dzaye O, Hahn A, Yu Y, Scavetta RJ, Dittmar G, et al. Glioma-Derived Versican Promotes Tumor Expansion Via Glioma-Associated Microglial/Macrophages Toll-like Receptor 2 Signaling. Neuro Oncol (2015) 17(2):200–10. doi: 10.1093/neuonc/nou324
43. Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A Dural Lymphatic Vascular System That Drains Brain Interstitial Fluid and Macromolecules. J Exp Med (2015) 212(7):991–9. doi: 10.1084/jem.20142290
44. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and Functional Features of Central Nervous System Lymphatic Vessels. Nature (2015) 523(7560):337–41. doi: 10.1038/nature14432
45. Eide PK, Vatnehol SAS, Emblem KE, Ringstad G. Magnetic Resonance Imaging Provides Evidence of Glymphatic Drainage From Human Brain to Cervical Lymph Nodes. Sci Rep (2018) 8(1):7194. doi: 10.1038/s41598-018-25666-4
46. Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, et al. CNS Lymphatic Drainage and Neuroinflammation are Regulated by Meningeal Lymphatic Vasculature. Nat Neurosci (2018) 21(10):1380–91. doi: 10.1038/s41593-018-0227-9
47. Cancer Genome Atlas Research N. Comprehensive Genomic Characterization Defines Human Glioblastoma Genes and Core Pathways. Nature (2008) 455(7216):1061–8. doi: 10.1038/nature07385
48. Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, Idh1, EGFR, and NF1. Cancer Cell (2010) 17(1):98–110. doi: 10.1016/j.ccr.2009.12.020
49. Qi Y, Liu B, Sun Q, Xiong X, Chen Q. Immune Checkpoint Targeted Therapy in Glioma: Status and Hopes. Front Immunol (2020) 11:578877. doi: 10.3389/fimmu.2020.578877
50. Nduom EK, Wei J, Yaghi NK, Huang N, Kong LY, Gabrusiewicz K, et al. Pd-L1 Expression and Prognostic Impact in Glioblastoma. Neuro Oncol (2016) 18(2):195–205. doi: 10.1093/neuonc/nov172
51. Khasraw M, Reardon DA, Weller M, Sampson JH. Pd-1 Inhibitors: do They Have a Future in the Treatment of Glioblastoma? Clin Cancer Res (2020) 26(20):5287–96. doi: 10.1158/1078-0432.CCR-20-1135
Keywords: DDX60, glioma, biomarkers, immune checkpoints, PD-L1
Citation: Zhang J, Fu M, Zhang M, Zhang J, Du Z, Zhang H, Hua W and Mao Y (2021) DDX60 Is Associated With Glioma Malignancy and Serves as a Potential Immunotherapy Biomarker. Front. Oncol. 11:665360. doi: 10.3389/fonc.2021.665360
Received: 08 February 2021; Accepted: 24 May 2021;
Published: 10 June 2021.
Edited by:
Xiaoming Xing, The Affiliated Hospital of Qingdao University, ChinaCopyright © 2021 Zhang, Fu, Zhang, Zhang, Du, Zhang, Hua and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Hua, aHNfaHVhd2VpQDEyNi5jb20=; Hongyi Zhang, emhhbmdoeXRzQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.