
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 01 June 2021
Sec. Cancer Imaging and Image-directed Interventions
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.665304
This article is part of the Research Topic Exploring the Potential of PSMA-PET Imaging on Personalized Prostate Cancer Treatment View all 16 articles
 Dirk Bottke1*†
Dirk Bottke1*† Jonathan Miksch2†
Jonathan Miksch2† Reinhard Thamm3
Reinhard Thamm3 Thomas Krohn4
Thomas Krohn4 Detlef Bartkowiak3
Detlef Bartkowiak3 Meinrad Beer5
Meinrad Beer5 Christian Bolenz6
Christian Bolenz6 Ambros J. Beer2
Ambros J. Beer2 Vikas Prasad2
Vikas Prasad2 Thomas Wiegel3
Thomas Wiegel3Background and Purpose: Salvage radiotherapy (SRT) is the main potentially curative treatment option for prostate cancer patients with post-prostatectomy PSA progression. Improved diagnostics by positron emission tomography/computed tomography (PET/CT) can lead to adjustments in treatment procedures (e.g. target volume of radiotherapy, androgen deprivation therapy). We analyzed the impact of 68Ga-PSMA-11-PET/CT on the target volume in early biochemical recurrence (PSA up to 0.5 ng/ml).
Patients and Methods: We retrospectively analyzed 76 patients with biochemical recurrence after radical prostatectomy in whom SRT was planned after 68Ga-PSMA-11-PET/CT. All patients had a PSA ≤0.5 ng/ml. An experienced radiation oncologist determined the radiotherapy concept, first with consideration of the PET/CT, second hypothetically based on the clinical and pathological features excluding PET/CT results.
Results: Without considering the PET/CT, all 76 patients would have been assigned to RT, 60 (79%) to the bed of the prostate and seminal vesicles alone, and 16 (21%) also to the pelvic lymph nodes because of histopathologic risk factors. Uptake indicative for tumor recurrence in 68Ga-PSMA-11-PET/CT was found in 54% of the patients. The median pre-PET/CT PSA level was 0.245 ng/ml (range 0.07–0.5 ng/ml). The results of the PET/CT led to a change in the radiotherapeutic target volume in 21 patients (28%). There were major changes in the target volume including the additional irradiation of lymph nodes or the additional or exclusive irradiation of bone metastases in 13 patients (17%). Minor changes including the additional irradiation of original seminal vesicle (base) position resulted in eight patients (11%).
Conclusion: Using 68Ga-PSMA-11-PET/CT for radiation planning, a change in the treatment concept was indicated in 28% of patients. With PET/CT, the actual extent of the tumor can be precisely determined even with PSA values of ≤0.5 ng/ml. Thus, the treatment concept can be improved and individualized. This may have a positive impact on progression free survival. Our results warrant further prospective studies.
Radical prostatectomy (RP) is considered to be a standard treatment option for patients with clinically localized prostate cancer (PCa). Nevertheless, up to 50–80% of these men develop biochemical recurrence depending on risk factors such as an advanced pathological stage, a high Gleason score or positive surgical margins (1). In case of PSA recurrence, salvage radiotherapy (SRT) is the only curative option, resulting in approximately 60% of the patients reachieving an undetectable PSA. After 5 years, 80% of these men are free from progression (2). The pre-SRT PSA level is a significant factor of progression, with more favorable results for patients with low PSA levels (0.5 ng/ml or less) (3, 4). Accordingly, European guidelines (EAU) recommend early SRT at a PSA <0.5 ng/ml (5).
At PSA levels <1 ng/ml, most imaging methods are not suitable to detect the correlate for disease progression. Therefore, up to 20% of patients with SRT to the prostate bed (with or without including original seminal vesicle) without morphological correlate will be treated locally without actual local recurrence (2).
Prostate-specific membrane antigen (PSMA) is a cell surface protein with high expression in majority of prostate cancer (6). 68Ga-PSMA has been used since 2012 as PSMA-ligand in recurrent prostate cancer (7–9). Especially at low PSA levels, the detection rate of 68Ga-PSMA-11-PET/CT is significantly higher in comparison to other imaging methods.
In a retrospective analysis of 2,533 patients with biochemical progression after RP, Afshar-Oromieh et al. found that 69% of the patients had at least one positive lesion indicating PCa recurrence. The detection rates were 43% for PSA levels ≤0.2 ng/ml, 58% for PSA >0.2 to ≤0.5 and 72% for PSA >0.5 to ≤1.0. Tumor detection was clearly associated with PSA level and higher Gleason scores (8).
Recently, we reported a detection rate of 50% in 116 patients with PSA levels up to 0.6 ng/ml and in the PSA subgroups 0–0.2, 0.21–0.3, and 0.31–0.6 ng/ml; 24, 57, and 65%, respectively (9).
Bluemel et al. analyzed the impact of 68Ga-PSMA-11-PET/CT in patients with PSA failure and negative F-18-choline-PET/CT. Of 125 patients, 32 patients with negative F-18-choline-PET/CT received an additional 68Ga-PSMA-11-PET/CT, which detected sites of recurrence in 43.8% (10).
This new possibility of precise detection of PSMA-expressing lesions can lead to changes of tumor staging and radiation planning. Data from numerous studies are available, especially on the impact of tumor stage changes on salvage radiotherapy planning (Table 2). However, only few data are available for patients with early biochemical recurrence (PSA <0.5 ng/ml) (11).
The goal of this retrospective, single-center analysis was to assess the impact of 68Ga-PSMA-11-PET/CT on the radiotherapeutic treatment concept in biochemical recurrence up to PSA 0.5 ng/ml.
In this retrospective analysis, only patients with PSA ≤0.5 ng/ml after RP and having undergone PSMA PET/CT prior to the radiation therapy were included. Patients’ data were retrospectively analyzed from the institutional database of the Department of Nuclear Medicine and the Department of Radiation Oncology which are part of the Comprehensive Cancer Center Ulm (CCCU). Overall, 76 patients were found to fulfill the inclusion criteria. For all patients SRT was planned after 68Ga-PSMA-11-PET/CT.
An experienced radiation oncologist determined patient’s actual treatment concept with consideration of the PET/CT images. Retrospectively a hypothetical treatment concept (prescription and planning target volume [PTV] contouring) was planned based on the clinical and pathological parameters excluding PET/CT results: In pT2- and pT3a-tumors the PTV included the prostate bed and the basis of the former seminal vesicles. In pT3b-tumors, the bed of seminal vesicles was included, too. Inclusion of regional pelvic lymph nodes was considered in case of histopathologic risk factors (e.g. pN1, Gleason score ≥8).
All patients gave their written informed consent for a retrospective analysis of their data in an anonymized form. The study was approved by the local ethics committee of Ulm University (221/20-FSt/Sta).
The 68Ga-HBED-CC-PSMA complex (ABX GmbH, Radeberg, Germany) was produced as already published. For labeling the 50 mCi (1,850 MBq), iThemba LABS, South Africa 68Ge/68Ga radionuclide generator was used (12, 13).
PET/CT images were acquired by a Biograph mCT (40)S in 3D acquisition mode 63.4 ± 11.4 min after intravenous infusion of 160.3 ± 29.4 MBq 68Ga-PSMA-11. Axial, sagittal and coronal slices were reconstructed afterwards. For attenuation correction and anatomical correlation, a low-dose CT was performed. Bed positions were set weight-based taking circa 2.5 min per bed position for body scan and 2 min for scanning legs. Scans were done from the mid-thighs to the vertex in 5 to 8 bed positions resulting in 15 to 20 min for each scan (170.2 ± 39.7 mAs). Intravenous contrast (80 to 120 ml Ultravist 370, Bayer Schering Pharma, Berlin, Germany) and 15 to 20 mg of furosemide were administered in 71 (93%) and 68 patients (89%) unless contraindicated. For a diagnostic CT, scans were performed 70 s past contrast injection for the venous phase.
A tracer uptake more than the immediate surrounding tissue and not related or explained due to the physiological expression was considered as pathologic. Two experienced nuclear medicine physicians with more than 10 years of experience in PET/CT analyzed the images.
The median PSA level before PET imaging was 0.245 ng/ml (range 0.07–0.5 ng/ml). Median age was 67 years (range 47–79 years). Patient characteristics are shown in Table 1.
Pathological tracer uptake as a sign of tumor recurrence in 68Ga-PSMA-11-PET/CT was found in 54% of the patients.
Additional information from 68Ga-PSMA-11-PET/CT lead to adaptation of RT planning in 28% (n = 21) of cases. In the hypothetical scenario without considering PET/CT results all the76 patients would have received RT: 60 (79%) to the bed of the prostate and seminal vesicles alone, and 16 (21%) also to the pelvic lymph nodes because of histopathologic risk factors.
We have defined major and minor changes. Major changes included the additional or exclusive irradiation of lymph nodes or the additional or exclusive irradiation of bone metastases based on the PET/CT. Minor changes included the additional irradiation of original seminal vesicle (base) position (Figure 1). Due to PET/CT, major changes were necessary in 13 patients (17%) and minor changes in eight patients (11%) (Figures 2 and 3). Postoperative prostate bed ± vesicle base position was irradiated with 72–74 Gy (median 72 Gy) and-positive lymph nodes with 60 Gy (range 50.4–66.6 Gy).
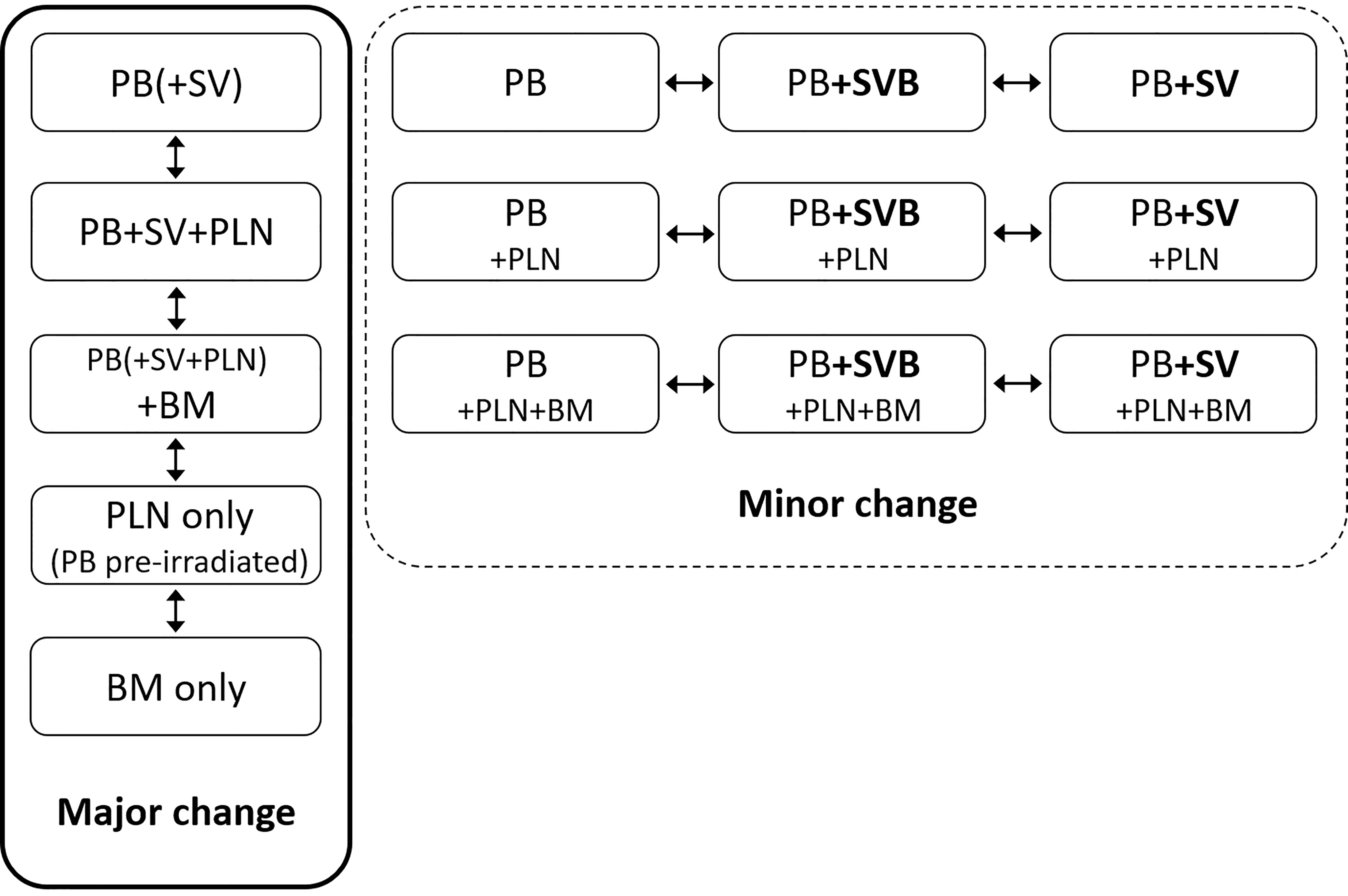
Figure 1 Definition of major and minor changes of radiotherapeutic treatment concept. PB, postoperative prostate bed; SV, original seminal vesicle position; SVB, original seminal vesicle base position; PLN, pelvic lymph nodes; BM, bone metastasis; NC, no change.
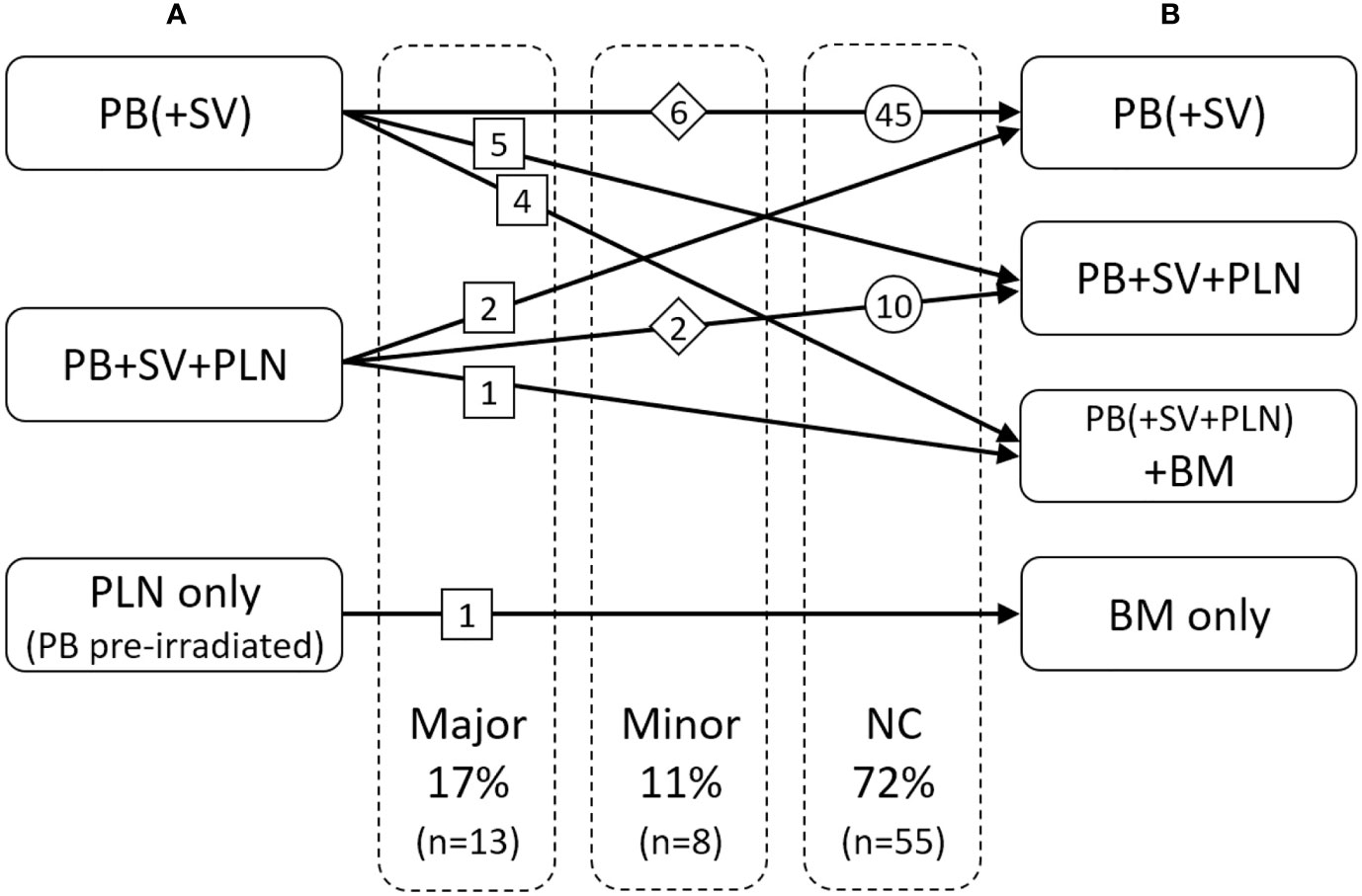
Figure 2 Changes of radiotherapeutic treatment concept: (A) based on the clinical and pathological situation without PET/CT, (B) with consideration of the PET/CT. PB, postoperative prostate bed; SV, original seminal vesicle position; PLN, pelvic lymph nodes; BM, bone metastasis.
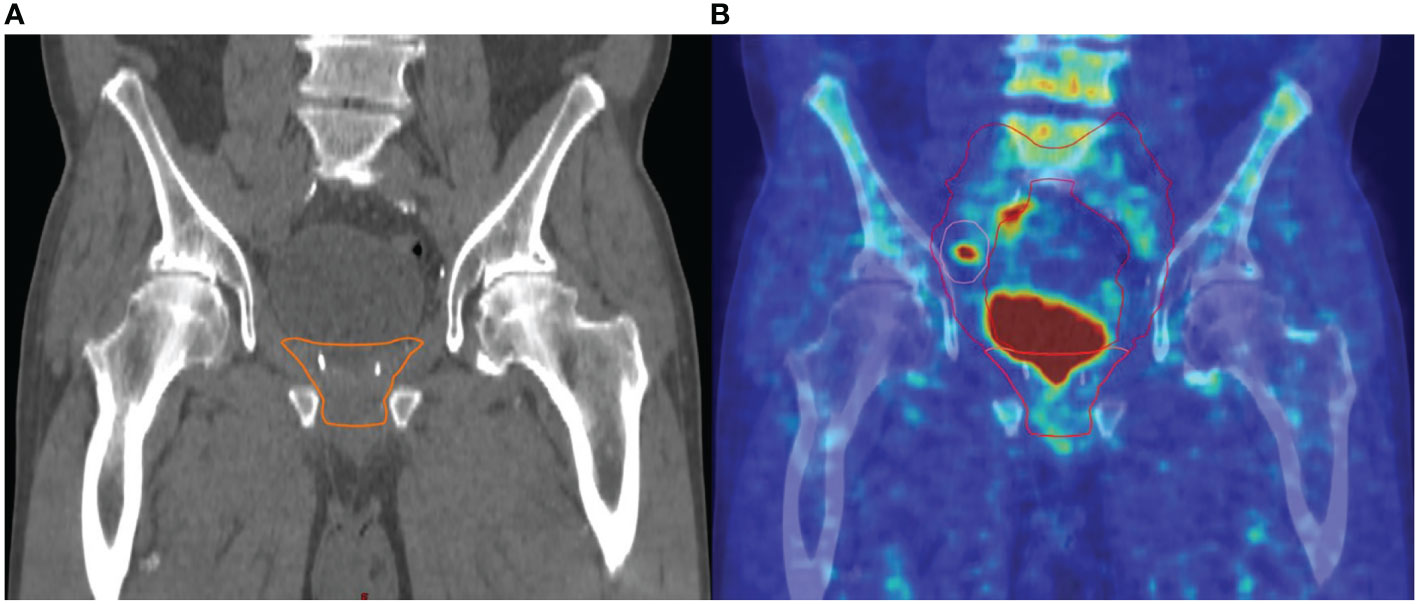
Figure 3 Example of major change of the radiotherapeutic treatment concept: (A) target volume without pelvic lymph nodes based on the clinical and pathological situation without PET/CT, (B) target volume with consideration of the PET/CT: additional irradiation of pelvic lymph nodes based on a PET/CT-positive right iliac lymph node metastasis.
Based on the PET/CT, in six patients (8%), bone metastases were detected and were additionally irradiated with median 45 Gy (range 39–54 Gy).
PSA values 6 months after completion of SRT were available in 54 patients, out of which 47 (87%) patients achieved a PSA response (Figure 4).
In our retrospective study 68Ga-PSMA-11-PET/CT led to changes of the RT target volume in 28% of patients with PSA ≤0.5 ng/ml after RP.
Detecting the site of prostate cancer recurrence is crucial for a successful treatment planning. In the presence of distant metastases, a prostate bed RT is not indicated. On the other hand, in the event of an exclusive loco-regional recurrence, a long-term ADT could be avoided or at least delayed by SRT. In the pre-PSMA era, most patients with PSA-failure had to undergo “blind” SRT under the assumption of local recurrence limited to the prostatic bed only.
It is equally imperative to stress that a negative PSMA-PET should not delay early SRT as the sensitivity of PSMA PET/CT for detection of micrometastases is questionable. Therefore, in the absence of any suspicious findings on PSMA-PET/CT, early SRT of the prostate bed should be offered without time delay (14).
On the other side of the spectrum, the possibility to visualize PSMA avid lesions at an early stage prior to the SRT offers the unique possibility of individualization of treatment planning as is shown in our study. 68Ga-PSMA-11-PET/CT led to changes of the radiation concept in 28% of all cases. All patients had a PSA <0.5ng/ml, impressively demonstrating the great potential of this imaging approach.
The impact of PSMA-PET/CT on SRT planning is extensively evaluated in several studies, albeit in heterogeneous patient population as is evident from Table 2. In most studies the median PSA value was significantly higher than in our study (median PSA 0.245 ng/ml).
Our results are in line with the retrospective study published by Farolfi et al. (11), to our knowledge the only work that has also studied patients with low PSA limit of 0.5 ng/ml. 119 patients with a median PSA of 0.32 ng/ml (range 0.2–0.5 ng/ml) were evaluated. 68Ga-PSMA-11-PET/CT was positive in 41 patients (34.4%). Pathological PSMA uptake was detected in the prostate bed (three patients), in the pelvic lymph nodes (21 patients), in the retroperitoneal lymph nodes (four patients) and in bone (21 patients). The initial planned radiation concept was changed in 36 patients (30.2%) due to the PET/CT results.
In contrast, a multicenter post-hoc analysis of 270 patients with biochemical recurrence after RP with a PSA ≤1 ng/ml (median 0.48 ng/ml) showed that PSMA-PET/CT had a major impact in 19% of patients (22). A major impact was defined as PSMA-PET/CT-positive disease outside planning target volumes expanded from clinical target volumes (CTV) covering both the prostate bed and pelvic lymph nodes. The two most common PET-positive locations outside the CTV were bone (44%) and lymph nodes (31%). These results show the need for an earlier PSMA-PET/CT (22).
Habl et al. analyzed staging changes due to 68Ga-PSMA-11-PET and its impact on RT procedure in 100 patients after radical prostatectomy. Median PSA level was 1.0 ng/ml (range 0.12–14.7 ng/ml). 29 patients had initial pN1 disease. In 76 patients, at least one pathological PSMA uptake was found. Of these, 80% showed no morphological correlate in the corresponding CT or MRI. The tumor stage was changed in 43% of the patients. Due to the PSMA-PET/CT imaging, initial RT planning was modified in 59% of all cases. An additional simultaneous integrated boost to the prostate bed or lymph nodes was given to 32 and 63%, respectively. Ten patients received stereotactic body radiation therapy to single bone metastases (19).
Our study has some limitations due to the retrospective and monocentric approach. In addition, a biopsy of PET-positive lesions is often not feasible, particularly in patients with recurrent prostate cancer. Therefore, the lack of histological validation is a common limitation in many imaging studies.
Our results showed that 68Ga-PSMA-11-PET/CT is a valuable tool in the RT planning procedure of patients with recurrent PCa after radical prostatectomy even at PSA levels ≤0.5 ng/ml. They support the implementation of this imaging procedure in routine practice for biochemical progression after RP.
However, it remains unclear whether the use of PSMA-PET/CT in planning SRT could improve outcomes.
In September 2018, Calais et al. initiated a randomized phase III trial to determine whether oncological outcomes can be improved by PSMA-PET/CT in patients with early biochemical recurrence following RP. A total of 193 patients will be randomized to standard SRT (without PSMA-PET/CT) or PET scan prior to SRT planning. The primary endpoint is the biochemical progression-free survival after SRT (25).
In the future, the superior soft-tissue contrast of PET/MRI may be able to further improve the detection of pelvic tumor lesions. First results showed that the detection rate was higher than the published results for PET/CT (26). Up to now, however, the availability of PET/MRI is very low.
68Ga-PSMA-11-PET/CT showed a high impact on radiation therapy procedure in patients with biochemically recurrent prostate cancer at PSA levels <0.5 ng/ml. With 28% changes in radiotherapy planning, 68Ga-PSMA-11-PET/CT is an important tool in guiding radiation treatment in this patient group. However, clinical data about the outcome of those treated patients have to be awaited.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
All patients gave their written informed consent for a retrospective analysis of their data in an anonymized form. The study was approved by the local ethics committee of Ulm University (221/20- FSt/Sta).
RT, TW, and AB contributed to conception and design of the study. JM, RT, and DBo organized the database. DBo performed the statistical analysis and wrote the first draft of the manuscript. JM, TK, DBa, MB, CB, AB, VP, and TW wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We want to thank the PET-team and Radiopharmacy unit for their excellent work. Ambros Beer, Meinrad Beer and Christian Bolenz are members of the i2SOUL (innovative imaging in surgical oncology Ulm) consortium, which we thank for support.
1. Wiegel T, Bartkowiak D, Bottke D, Bronner C, Steiner U, Siegmann A, et al. Adjuvant Radiotherapy Versus Wait-and-See After Radical Prostatectomy: 10-Year Follow-Up of the ARO 96-02/AUO AP 09/95 Trial. Eur Urol (2014) 66:243–50. doi: 10.1016/j.eururo.2014.03.011
2. Wiegel T, Lohm G, Bottke D, Höcht S, Miller K, Siegmann A, et al. Achieving an Undetectable PSA After Radiotherapy for Biochemical Progression After Radical Prostatectomy Is an Independent Predictor of Biochemical Outcome – Results of a Retrospective Study. Int J Radiat Oncol Biol Phys (2009) 73:1009–16. doi: 10.1016/j.ijrobp.2008.06.1922
3. Tendulkar RD, Agrawal S, Gao T, Efstathiou JA, Pisansky TM, Michalski JM, et al. Contemporary Update of a Multi-Institutional Predictive Nomogram for Salvage Radiotherapy After Radical Prostatectomy. J Clin Oncol (2016) 34:3648–54. doi: 10.1200/JCO.2016.67.9647
4. Bottke D, Bartkowiak D, Siegmann A, Thamm R, Böhmer D, Budach V, et al. Effect of Early Salvage Radiotherapy At PSA < 0.5 ng/ml and Impact of Post-SRT PSA Nadir in Post-Prostatectomy Recurrent Prostate Cancer. Prostate Cancer Prostatic Dis (2019) 22:344–9. doi: 10.1038/s41391-018-0112-3
5. Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol (2017) 71:630–42. doi: 10.1016/j.eururo.2016.08.002
6. Ghosh A, Heston WD. Tumor Target Prostate Specific Membrane Antigen (PSMA) and Its Regulation in Prostate Cancer. J Cell Biochem (2004) 91:528–39. doi: 10.1002/jcb.10661
7. Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of Hybrid 68Ga-PSMA Ligand PET/CT in 248 Patients With Biochemical Recurrence After Radical Prostatectomy. J Nucl Med (2015) 56:668–74. doi: 10.2967/jnumed.115.154153
8. Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic Performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in Patients With Recurrent Prostate Cancer: Evaluation in 1007 Patients. Eur J Nucl Med Mol Imaging (2017) 44:1258–68. doi: 10.1007/s00259-017-3711-7
9. Miksch J, Bottke D, Krohn T, Thamm R, Bartkowiak D, Solbach C, et al. Interobserver Variability, Detection Rate, and Lesion Patterns of (68)Ga-PSMA-11-PET/CT in Early-Stage Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy. Eur J Nucl Med Mol Imaging (2020) 47:2339–47. doi: 10.1007/s00259-020-04718-w
10. Bluemel C, Krebs M, Polat B, Linke F, Eiber M, Samnick S, et al. 68Ga-PSMA-PET/CT in Patients With Biochemical Prostate Cancer Recurrence and Negative 18F-Choline-PET/CT. Clin Nucl Med (2016) 41:515–21. doi: 10.1097/RLU.0000000000001197
11. Farolfi A, Ceci F, Castellucci P, Graziani T, Siepe G, Lambertini A, et al. (68)Ga-Psma-11 PET/CT in Prostate Cancer Patients With Biochemical Recurrence After Radical Prostatectomy and PSA <0.5 Ng/Ml. Efficacy and Impact on Treatment Strategy. Eur J Nucl Med Mol Imaging (2019) 46:11–9. doi: 10.1007/s00259-018-4066-4
12. Eder M, Schäfer M, Bauder-Wüst U, Hull WE, Wängler C, Mier W, et al. 68Ga-Complex Lipophilicity and the Targeting Property of a Urea-Based PSMA Inhibitor for PET Imaging. Bioconjug Chem (2012) 23:688–97. doi: 10.1021/bc200279b
13. Schäfer M, Bauder-Wüst U, Leotta K, Zoller F, Mier W, Haberkorn U, et al. A Dimerized Urea-Based Inhibitor of the Prostate-Specific Membrane Antigen for 68Ga-PET Imaging of Prostate Cancer. EJNMMI Res (2012) 2:23. doi: 10.1186/2191-219X-2-23
14. Mottet N, Cornford P, van den Bergh RCN, Briers E, De Santis M, Fanti S, et al. Eau Guidelines on Prostate Cancer (2020). Available at: https://uroweb.org/guideline/prostate-cancer/.
15. van Leeuwen PJ, Stricker P, Hruby G, Kneebone A, Ting F, Thompson B, et al. (68) Ga-PSMA has a High Detection Rate of Prostate Cancer Recurrence Outside the Prostatic Fossa in Patients Being Considered for Salvage Radiation Treatment. BJU Int (2016) 117:732–9. doi: 10.1111/bju.13397
16. Sterzing F, Kratochwil C, Fiedler H, Katayama S, Habl G, Kopka K, et al. (68)Ga-PSMA-11 PET/CT: A New Technique With High Potential for the Radiotherapeutic Management of Prostate Cancer Patients. Eur J Nucl Med Mol Imaging (2016) 43:34–41. doi: 10.1007/s00259-015-3188-1
17. Bluemel C, Linke F, Herrmann K, Simunovic I, Eiber M, Kestler C, et al. Impact of (68)Ga-PSMA PET/CT on Salvage Radiotherapy Planning in Patients With Prostate Cancer and Persisting PSA Values or Biochemical Relapse After Prostatectomy. EJNMMI Res (2016) 6:78. doi: 10.1186/s13550-016-0233-4
18. Albisinni S, Artigas C, Aoun F, Biaou I, Grosman J, Gil T, et al. Clinical Impact of (68) Ga-Prostate-Specific Membrane Antigen (PSMA) Positron Emission Tomography/Computed Tomography (PET/CT) in Patients With Prostate Cancer With Rising Prostate-Specific Antigen After Treatment With Curative Intent: Preliminary Analysis of a Multidisciplinary Approach. BJU Int (2017) 120:197–203. doi: 10.1111/bju.13739
19. Habl G, Sauter K, Schiller K, Dewes S, Maurer T, Eiber M, et al. (68)Ga-PSMA-PET for Radiation Treatment Planning in Prostate Cancer Recurrences After Surgery: Individualized Medicine or New Standard in Salvage Treatment. Prostate (2017) 77:920–7. doi: 10.1002/pros.23347
20. Koerber SA, Will L, Kratochwil C, Haefner MF, Rathke H, Kremer C, et al. (68)Ga-PSMA-11 PET/CT in Primary and Recurrent Prostate Carcinoma: Implications for Radiotherapeutic Management in 121 Patients. J Nucl Med (2018) 60:234–40. doi: 10.2967/jnumed.118.211086
21. Frenzel T, Tienken M, Abel M, Berliner C, Klutmann S, Beyersdorff D, et al. The Impact of [(68)Ga]PSMA I&T PET/CT on Radiotherapy Planning in Patients With Prostate Cancer. Strahlenther Onkol (2018) 194:646–54. doi: 10.1007/s00066-018-1291-5
22. Calais J, Czernin J, Cao M, Kishan AU, Hegde JV, Shaverdian N, et al. (68)Ga-PSMA-11 PET/CT Mapping of Prostate Cancer Biochemical Recurrence After Radical Prostatectomy in 270 Patients With a PSA Level of Less Than 1.0 ng/mL: Impact on Salvage Radiotherapy Planning. J Nucl Med (2018) 59:230–7. doi: 10.2967/jnumed.117.201749
23. Schmidt-Hegemann NS, Eze C, Li M, Rogowski P, Schaefer C, Stief C, et al. Impact of (68)Ga-PSMA PET/CT on the Radiotherapeutic Approach to Prostate Cancer in Comparison to CT: A Retrospective Analysis. J Nucl Med (2019) 60:963–70. doi: 10.2967/jnumed.118.220855
24. Boreta L, Gadzinski AJ, Wu SY, Xu M, Greene K, Quanstrom K, et al. Location of Recurrence by Gallium-68 PSMA-11 PET Scan in Prostate Cancer Patients Eligible for Salvage Radiotherapy. Urology (2019) 129:163–4. doi: 10.1016/j.urology.2018.12.055
25. Calais J, Czernin J, Fendler WP, Elashoff D, Nickols NG. Randomized Prospective Phase III Trial of (68)Ga-PSMA-11 PET/CT Molecular Imaging for Prostate Cancer Salvage Radiotherapy Planning [PSMA-SRT]. BMC Cancer (2019) 19:18. doi: 10.1186/s12885-019-5297-x
Keywords: prostate cancer, biochemical recurrence, early salvage radiotherapy, positron emission tomography (PET), PSMA
Citation: Bottke D, Miksch J, Thamm R, Krohn T, Bartkowiak D, Beer M, Bolenz C, Beer AJ, Prasad V and Wiegel T (2021) Changes of Radiation Treatment Concept Based on 68Ga-PSMA-11-PET/CT in Early PSA-Recurrences After Radical Prostatectomy. Front. Oncol. 11:665304. doi: 10.3389/fonc.2021.665304
Received: 07 February 2021; Accepted: 03 May 2021;
Published: 01 June 2021.
Edited by:
Trevor Royce, University of North Carolina at Chapel Hill, United StatesReviewed by:
Panayiotis Mavroidis, University of North Carolina at Chapel Hill, United StatesCopyright © 2021 Bottke, Miksch, Thamm, Krohn, Bartkowiak, Beer, Bolenz, Beer, Prasad and Wiegel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dirk Bottke, ZGlyay5ib3R0a2VAeC1jYXJlLmRl
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.