- 1Department of Clinical Trial, The Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Hangzhou, China
- 2Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou, China
- 3Department of Respiratory Disease, Taizhou Hospital, Taizhou, China
- 4The Medical Department, 3D Medicines Inc, Shanghai, China
- 5Department of Medical Oncology, Lishui Center Hospital, Lishui, China
- 6Department of Medical Oncology, Baotou Cancer Hospital, Baotou, China
- 7Department of Respiratory Disease, Ningbo Medical Center, Lihuili Eastern Hospital, Ningbo, China
- 8Department of Thoracic Disease Center, Rongjun Hospital, Jiaxing, China
- 9Department of Pulmonary Medicine, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
Background: Non-small cell lung cancer (NSCLC) patients with HER2 mutations and amplification may benefit from HER2-targeted therapy, including afatinib. However, the data regarding the clinical activity of afatinib in Chinese patients with NSCLC harboring HER2 alterations are limited.
Patients and methods: We retrospectively included metastatic NSCLC patients harboring HER2 alterations who treated with afatinib. The clinical outcomes included overall response rate (ORR), progression-free survival (PFS) and overall survival (OS). The genomic profiling data after progression on afatinib were analyzed.
Results: We included 54 patients harboring HER2 mutations and 12 patients harboring HER2 amplification. The ORR was 24% (95% CI, 16–36%), the median PFS was 3.3 months (95% CI, 2.2–4.4), and the median OS was 13.9 months (95% CI, 11.4–16.5). Patients with HER2 exon 20 mutations had numerically worse ORR (17% vs 42%), shorter PFS (2.6 vs 5.8 months, HR, 2.5; 95% CI, 1.2–5.5; P = 0.015) and OS (12.9 vs 33.3 months, HR, 4.4; 95% CI, 1.3–14.8; P = 0.009) than patients with other mutations. For HER2-amplified patients, the ORR was 33% (95% CI, 14–61%), the median PFS was 3.3 months (95% CI, 2.6–4.0), and the median OS was 13.4 months (95% CI, 0–27.6). The most frequently mutated genes in afatinib-resistant patients were TP53 (44%) and EGFR (33%). Three afatinib-resistant patients harbored secondary HER2 alterations.
Conclusions: Our results suggest that afatinib has a promising anti-tumor activity in patients with NSCLC harboring HER2 alterations. To our knowledge, this is the largest retrospective study about the clinical activity of afatinib in NSCLC patients with HER2 alterations.
Introduction
Lung cancer is one of the most common malignant tumors, causing approximate 25% of the total cancer-related deaths (1). About 85% of patients with lung cancer are histologically diagnosed as non-small cell lung cancer (NSCLC) (2). Several driver genes alterations, including EGFR (epidermal growth factor receptor) activating mutations, ALK (anaplastic lymphoma kinase) rearrangement, ROS1 (repressor of silencing 1) fusions, BRAF (B-Raf proto-oncogene, serine/threonine kinase) mutations, MET (MET proto-oncogene, receptor tyrosine kinase) alterations, and RET (ret proto-oncogene) fusions, are frequently detected in the patients with NSCLC (3). Targeted therapies based on these genes have been approved by the Food and Drug Administration (FDA), changing the treatment of NSCLC (4).
Human epidermal growth factor receptor 2 (HER2, also known as ERBB2) is a cancer driver gene, and 1.7–3% of NSCLC patients harbor HER2 mutations (5–7). Most HER2 mutations in NSCLC are present in exon 20, such as Y772_A775dup and G778_P780dup. In addition, HER2 gene amplification occurs in 3 to 14.3% of lung adenocarcinomas (7–9). HER2 activating mutations and amplification may activate tyrosine kinase and downstream signaling pathways, therefore conferring sensitivity to HER2-targeted therapy, such as trastuzumab, ado-trastuzumab (T-DM1) and tyrosine kinase inhibitors (TKIs). At present, T-DM1 is the only recommended HER2-targeted inhibitor for HER2-mutated NSCLC patients by National Comprehensive Cancer Network (NCCN) Guidelines, with an overall response rate (ORR) of 44% (10). However, no HER2-targeted therapy has been approved for patients with NSCLC harboring HER2 mutations or amplification.
Afatinib is an irreversible ERBB family inhibitor, which has been approved for EGFR-mutated lung cancer and become one of the most common therapy in NSCLC patients. In a phase II trial with 13 advanced NSCLC with HER2 exon 20 mutations, the overall response rate (ORR) of afatinib as second-line treatment was 7.7% and the median progression-free survival (PFS) was 15.9 weeks (11). Several retrospective trials revealed better activity of afatinib in patients with HER2 exon 20 mutations, with an ORR from 13 to 33% (5, 12–15). However, the interpretation of the results from all these studies were limited by the small sample sizes. In addition, the efficacy of HER2-TKI in patients with HER mutations besides HER2 exon 20 mutations and HER2 amplification has been rarely studied. Seven patients with other HER2 mutations except exon 20 mutations were enrolled into the phase II trial of T-DM1, and two of these patients had a partial response, with a S310F (exon 8) mutation and a V659E (exon 17) mutation, respectively (10). Another research showed that three of four NSCLC patients with V659E or G660R (exon 17, located in transmembrane domain) achieved responses from afatinib treatment (16).
Herein, we conducted a multicenter, retrospective study to analyze the anti-tumor activity of afatinib in patients with NSCLC harboring HER2 alterations including mutations and amplification. Furthermore, we tried to explore the potential secondary resistant mechanisms of afatinib by next generation sequencing (NGS). We present the following article/case in accordance with the STROBE reporting checklist.
Methods
Patients and Study Design
This multicenter, retrospective study included patients with non-small cell lung cancer (NSCLC) harboring HER2 alteration treated with afatinib between May 2015 and July 2019, from Zhejiang Cancer Hospital, Taizhou Hospital, Baotou Cancer Hospital, Lihuili Eastern Hospital and Rongjun Hospital. Eligible patients were 18 years or older, and had a diagnosis of stage IV NSCLC, a HER2 alteration, measurable disease as per investigator-assessed Response Evaluation Criteria in Solid Tumors (RECIST), v1.1. Patients received afatinib at a dose of 40 mg daily until disease progression or intolerable adverse events. This retrospective study was approved by the Institutional Review Board Committee of Zhejiang Cancer Hospital. Research was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments. The informed consent was waived because of the retrospective nature of this study.
Data Collection and Response Assessment
Baseline clinical information were collected from electronic medical records, including age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, tumor histology, smoking status, HER2 alteration subtype, and afatinib treatment line. These clinical data were verified independently by two oncologist physicians. Tumor size measurement according to radiologic imaging was conducted by radiologists. Best response was determined according to Response Evaluation Criteria in Solid Tumors (RECIST, v1.1). The outcomes were ORR, PFS, and overall survival (OS). ORR was defined as the proportion of patients who have a partial response (PR) or complete response (CR). PFS was defined as the time interval from initial afatinib treatment to progression or death from any cause. OS was defined as the duration from the beginning of afatinib treatment to death from any cause.
Molecular Testing
The baseline HER2 gene alterations were tested by NGS in an accredited local laboratory (for example as shown in Figure S1). Genomic profiling when progression on afatinib treatment was tested in a CLIA-accredited/CAP-certified laboratory (3D Medicines Inc., Shanghai, China). The NGS panel targeted cancer-related genes was performed on the NextSeq500 platform (Illumina, CA, USA) (17). DNA extracts (30–200 ng) were sheared to 250 bp fragments using an S220 focused-ultrasonicator (Covaris). Libraries were prepared using the KAPA Hyper Prep Kit (KAPA Biosystems) following the manufacturer’s protocol. The captured libraries were loaded onto a NextSeq500 platform for 100 bp paired-end sequencing with a mean sequencing depth of 500×.
Raw data of paired samples (an FFPE sample and its normal tissue control) were mapped to the reference human genome hg19 using the Burrows–Wheeler Aligner (v0.7.12). PCR duplicate reads were removed and sequence metrics were collected using Picard (v1.130) and SAMtools (v1.1.19), respectively. Variant calling was performed only in the targeted regions. Somatic single nucleotide variants (SNVs) were detected using an in-house developed R package to execute a variant detection model based on binomial test. Local realignment was performed to detect indels. Variants were then filtered by their unique supporting read depth, strand bias, base quality as previously described. All variants were then filtered using an automated false positive filtering pipeline to ensure sensitivity and specificity at an allele frequency (AF) of ≥1%. Single-nucleotide polymorphism (SNPs) and indels were annotated by ANNOVAR against the following databases: dbSNP (v138), 1000Genome and ESP6500 (population frequency >0.015). Only missense, stopgain, frameshift and non-frameshift indel mutations were kept. Copy number variations (CNVs) and gene rearrangements were detected. The interpretation of variants were based on American College of Medical Genetics and Genomics (ACMG) standards and guidelines.
Statistical Analyses
All statistical analyses were conducted using the SPSS statistical package, version 20.0 (SPSS Inc®, Chicago, Illinois, USA) and GraphPad prism v6 (GraphPad, La Jolla, CA, USA). The PFS and OS were estimated by Kaplan–Meier curves, with P value determined by a log-rank test. And we calculated hazard ratio (HR) and its 95% confidence intervals (CIs) by Cox regression. Univariate and multivariate analyses were performed by Cox proportional hazard model. A two-sided P <.05 was considered statistically significant.
Results
Baseline Characteristics
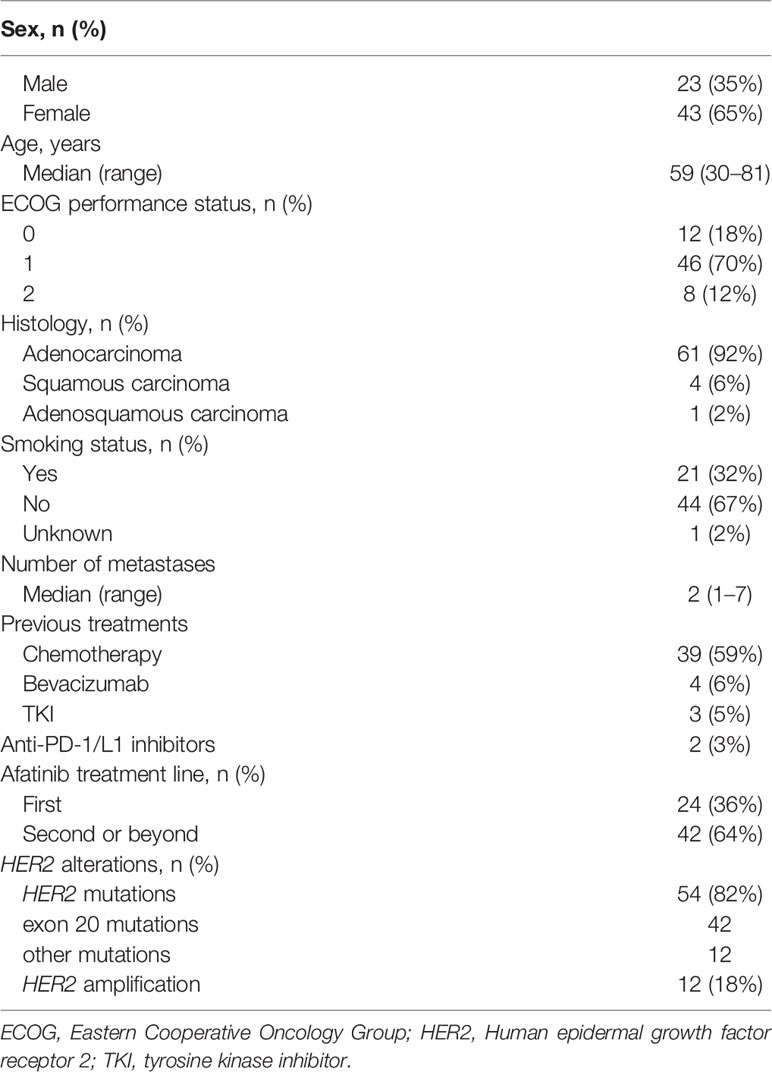
A total of 66 patients with lung cancer were included in this retrospective study. The baseline characteristics are shown in Table 1. The median age was 59 years (range, 30–81), and 65% (43/66) were female. Eight patients (12%) had an Eastern Cooperative Oncology Group (ECOG) performance status of 2 and the rest were ECOG 0–1. Most patients were adenocarcinoma (92%, 61/66) and non-smokers (67%, 44/66). Ten (15%) patients had brain metastases. All the patients received afatinib as a single agent. The median line of afatinib treatment was 2 (range, 1–7). Twenty-four patients (36%, 24/66) received afatinib as first-line therapy, and 42 patients (64%, 42/66) as second-line or beyond therapy (Table 1). The median follow-up period was 13.9 months (range: 2.1–39.5).
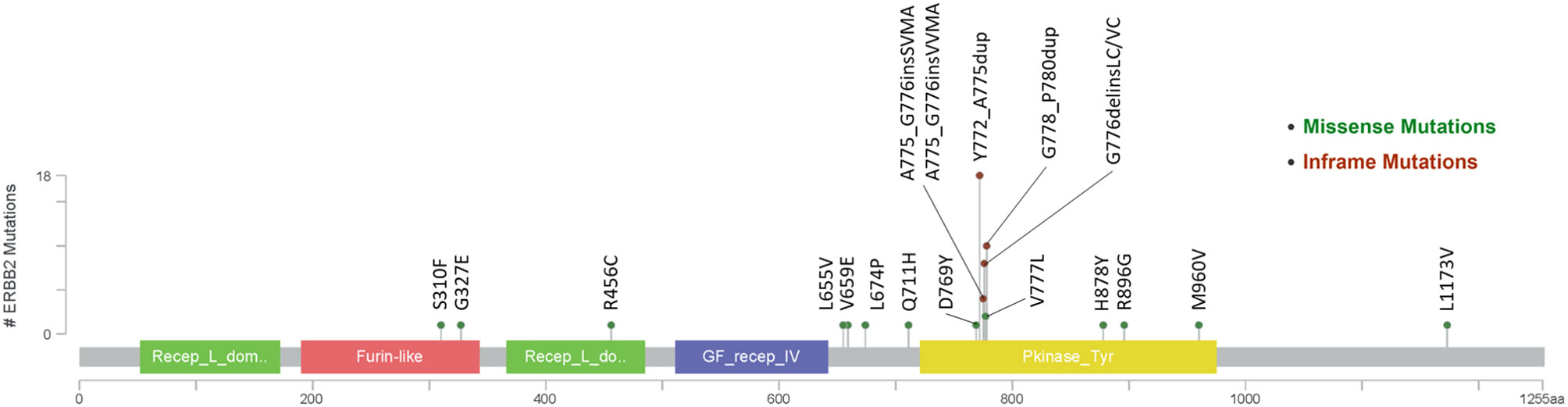
Fifty-four patients (82%) harbored mutations in HER2 gene (Figure 1), most of which were identified in exon 20 (78%, 42/54). In addition, twelve patients carried HER2 amplification. Among the patients with HER2-mutated lung cancer, the most common mutation was Y772_A775dup (33%, 18/54), followed by G778_P780dup (19%, 10/54) and G776delinsVC/LC (15%, 8/54).
Clinical Activity of Afatinib in NSCLC Patients With HER2 Alterations
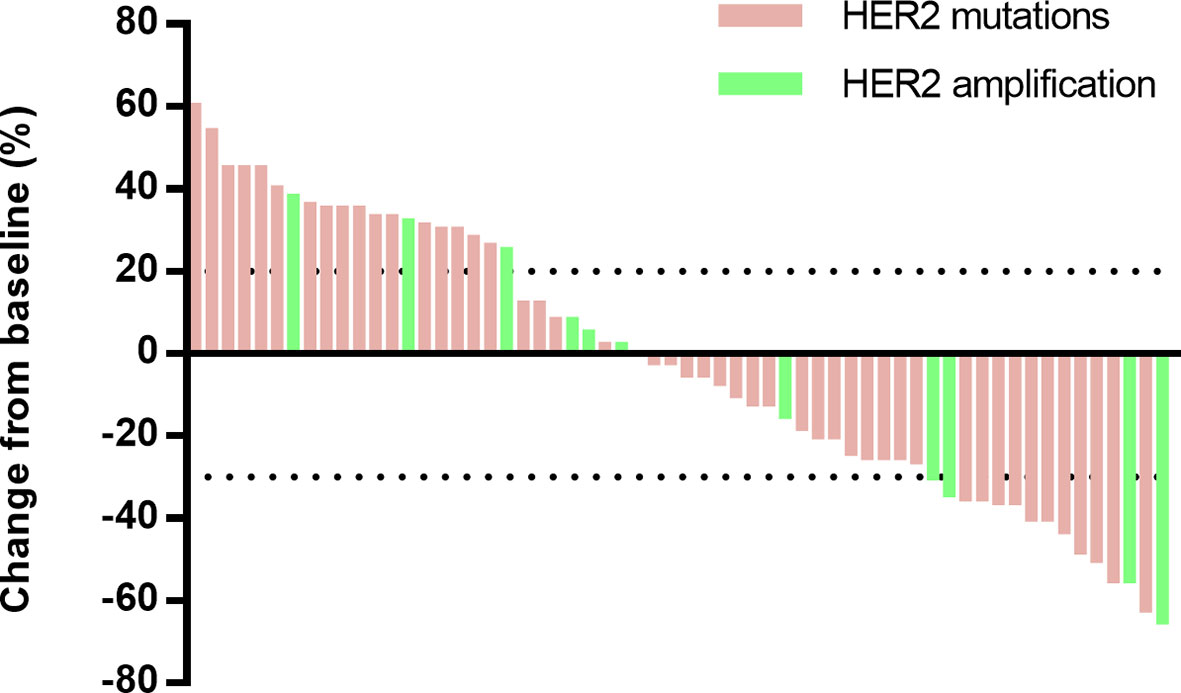
The responses to afatinib were evaluated according to RECIST 1.1 (Figure 2), and the best response to afatinib was partial response (PR) in 16 patients (24%), stable disease (SD) in 24 patients (36%), and progressive disease (PD) in 26 patients (39%, Table 2). The ORR was 24% (95% CI, 16–36%), and disease control rate (DCR) was 61% (95% CI, 49–72%).
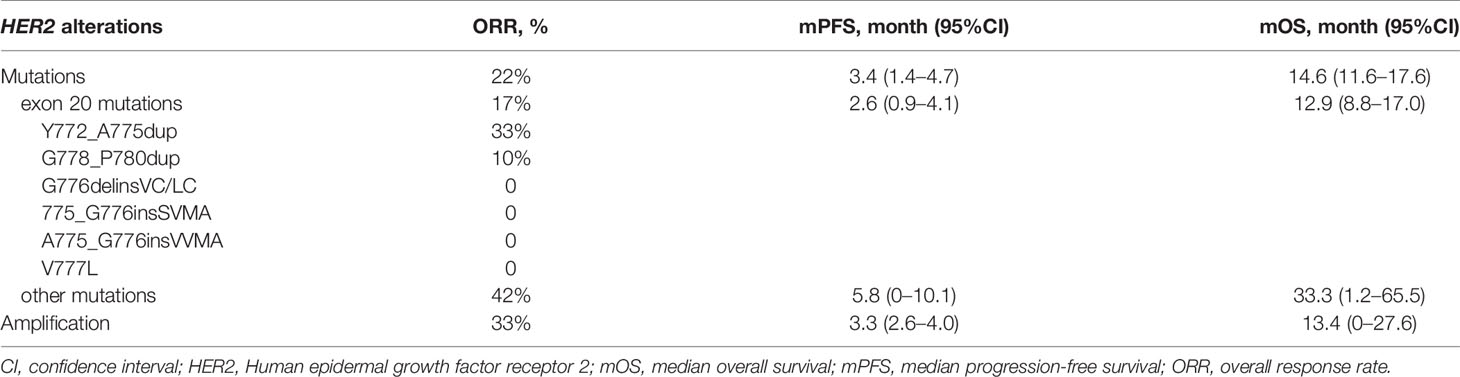
As data cutoff, 62 (94%) out of 66 patients had died or had disease progression and 40 (61%) patients had died. The median PFS was 3.3 months (95% CI, 2.2–4.4), and the median OS was 13.9 months (95% CI, 11.4–16.5) (Table 2 and Figure 3). The median duration of response was 7.1 months (95%CI, 6.4–7.7 months). Furthermore, we deeply analyzed the efficacy of afatinib in patients with different HER2 alterations. Among the patients with a HER2 mutation, the ORR, mPFS and mOS were 22%, 3.4 months (95% CI, 1.4–4.7) and 14.6 months (95% CI, 11.6–17.6), similar with the whole cohort (Table 3). In addition, four of the patients with HER2 amplification (33%, 4/12) achieved a PR (Figure 2). The mPFS and mOS of these patients were respectively 3.3 months (95% CI, 2.6–4.0) and 13.4 months (95% CI, 0–27.6), comparable to those of the patients with a HER2 mutation (Figures 3C, D).
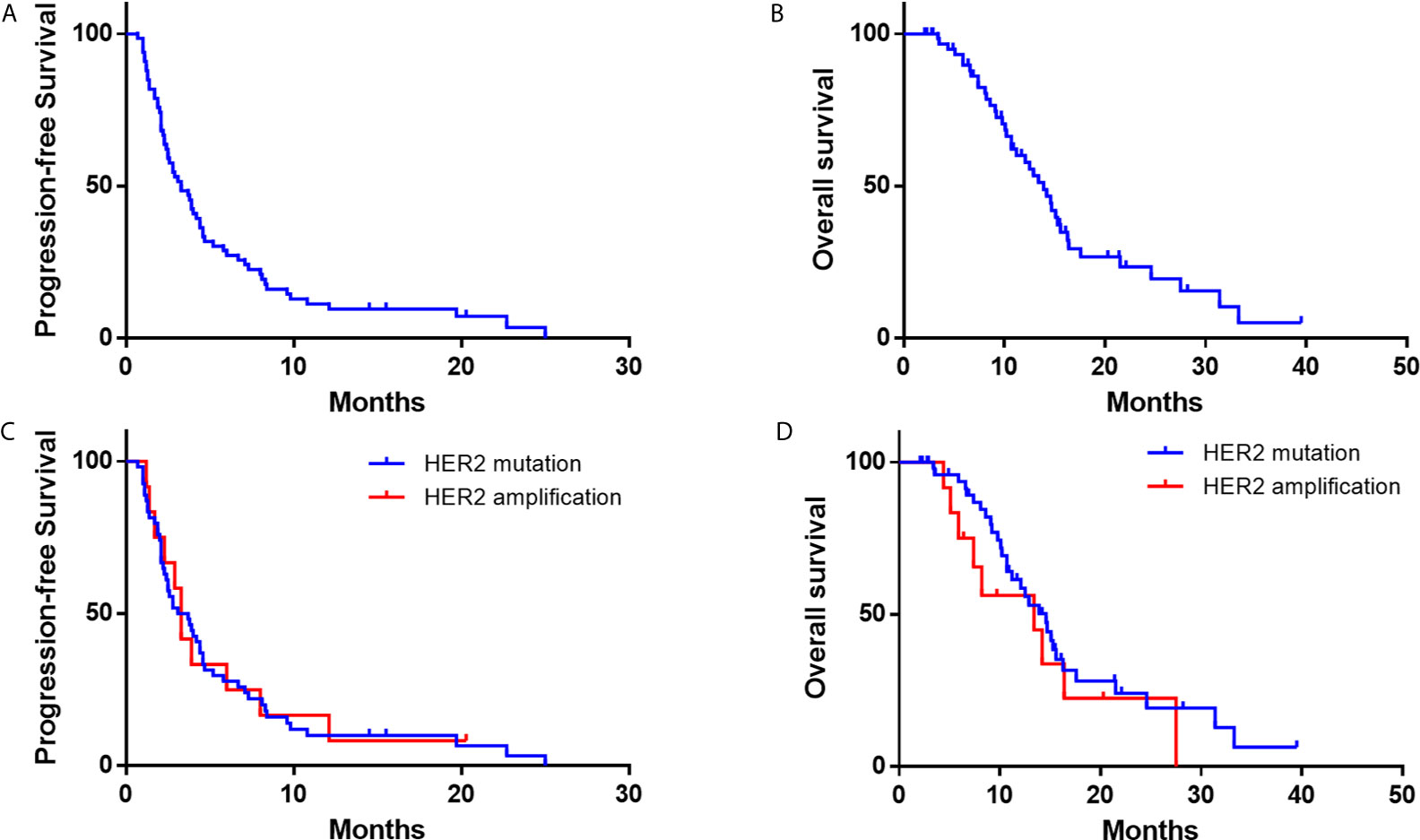
Figure 3 Kaplan–Meier estimates of progression-free survival and overall survival of NSCLC Patients with HER2 alterations. (A, B) progression-free survival and overall survival of the whole cohort; (C, D) progression-free survival and overall survival according to HER2 mutations or amplification.
Since HER2 exon 20 mutation is the most common mutation for HER2 in patients with NSCLC, we further compared the outcomes of patients with exon 20 mutation and other mutations. As for HER2 exon 20 mutations, the total ORR was 17%, and the ORRs of the patients with Y772_A775dup mutation and G778_P780dup were 33 and 10%, respectively, while the ORR was 0% in patients with other exon 20 mutations including G776delinsVC/LC, A775_G776insSVMA, A775_G776insVVMA, and V777L (Table 3). In patients with other HER2 mutations, five (42%) out of 12 patients achieved a PR (L655V [exon 17], H878Y [exon 21], R896G [exon 22], M960V [exon 24] and L1173V [exon 27]). Patients with HER2 exon 20 mutations had worse PFS (2.6 vs 5.8 months, HR, 2.5; 95% CI, 1.2–5.5; P = 0.015) and OS (12.9 vs 33.3 months, HR, 4.4; 95% CI, 1.3–14.8; P = 0.009) than patients with other HER2 mutations (Figure S2).
We also performed subgroup analysis according afatinib treatment lines. The ORR was 42% in patients who received afatinib as first-line treatment compared with 14% in those who received afatinib as secondary-line or beyond treatment. Patients who received afatinib as secondary-line or beyond treatment had shorter PFS and OS compared with patients who received afatinib as first-line treatment (mPFS = 2.7 vs 4.7 months; OS = 11.2 vs 15.6 months; Figure S3). Multivariate analysis showed that afatinib treatment line and brain metastasis were associated with PFS (P = 0.026 and 0.017, respectively), and ECOG performance status was associated with OS (P = 0.046) (Tables S1 and S2).
Potential Biomarkers for Resistance to Afatinib
To reveal potential biomarkers of resistance to afatinib, NGS was performed from blood or tissue samples of nine patients after progression on afatinib treatment. Pathogenic and likely pathogenic mutations were analyzed. We observed most patients (78%, 7/9) still harbored HER2 alterations after afatinib treatment (Table S3). Among these patients, three patients harbored secondary HER2 alterations (p.G776delinsLC [patient 3], Y772_A775dup [patient 4], and amplification [patient 6], Table S3). The most frequently mutated genes in afatinib-resistant patients were TP53 (44%) and EGFR (33%). Besides, one patient carried a NRAS mutation and another patient had no HER2 alteration nor other pathogenic mutation when progression on afatinib (Table S3).
Discussion
In the present study, afatinib showed promising anti-tumor activity in patients with NSCLC harboring HER2 alterations including HER2 exon 20 mutations, other mutations and HER2 amplification. To our knowledge, this is the largest retrospective study on clinical activity of afatinib in NSCLC patients with HER2 alterations.
Most previous studies focused on HER2 exon 20 insertions. Recent studies reported that NSCLC patients with HER2 exon 20 insertions had an ORR of 13–19% from afatinib treatment (14, 15, 18). The sole to date prospective study (11) on afatinib in NSCLC patients with HER2 exon 20 insertions only enrolled 13 patients, with a modest clinical outcomes (ORR = 7.7%). In the largest cohort of NSCLC patients with HER2 exon 20 insertions, Mazières et al. (12) reported clinical activity of chemotherapy and HER2-targeted drugs. The ORR of the patients (N = 29) treated with TKIs (neratinib, afatinib, and lapatinib) was 7.4%. Among the patients (N = 11) who were treated with afatinib, the ORR was 18.2%. In the present study, most HER2 mutations were exon 20 insertions (61%), which was similar with previous studies. We observed an ORR of 17% in these patients, which was comparable with previous retrospective studies. Of the patents with most common Y772_A775dup mutation, ORR was 33%, which suggests that these patients might have better clinical outcome from afatinib.
Moreover, we found HER2 other mutations except exon 20 mutations were also sensitive to afatinib. Five (42%) of these patients achieved response from afatinib treatment. Among these patients who were response to afatinib, one patient with a L655V (exon 17) mutation had a PFS of 8.1 months. L655V (exon 17) is located in transmembrane domain (TMD) that is important to stabilize the active HER2 homodimer (19). And L655V is close to V659/G660, which were demonstrated to be sensitive to afatinib (16). One lung squamous cell carcinoma patient with a HER2 R896G (exon 22) mutation had a long PFS of 14.5 months, which was recently reported as a case report (20). Another patient with a M960V (exon 24) mutation received afatinib as third-line therapy, and achieved a PR and a PFS of 7.1 months. The other two patients respectively harbored H878Y (exon 21) and L1173V (exon 27), and the PFS were 22.7 and 25.0 months, respectively. These results suggest that the patients with HER2 other mutations except exon 20 mutations could also benefit from HER2-targeted inhibitors.
So far, the standard care for NSCLC patients with HER2 amplification is chemotherapy. Although T-DM1 is recommended by NCCN Guidelines for HER2-mutated NSCLC patients, no HER2-targerd inhibitors are approved for NSCLC patients with HER2 mutations or amplification. In a phase II trial of dacomitinib in lung cancer patients with HER2 alterations, none of four patients with HER2-amplified tumors responded (21). Recently, two studies on HER-mutated NSCLC patients treated with pyrotinib, a pan-HER inhibitor, showed that ORRs were 53.3 and 30%, and mPFSs were 6.4 months and 6.9 months, respectively (22, 23). An in vitro study and phase II trial demonstrated another pan-HER inhibitor poziotinib had potent clinical activity against HER2 mutations (24, 25). In breast cancer, gastric Cancer, and colorectal cancer, HER2 amplification was demonstrated to be associated with the clinical outcomes of HER2-targeted treatment (26, 27). In this study, we presented an ORR of 33% in the NSCLC patients with HER2 amplification, and this is the first time that clinical activity of afatinib in HER2-amplified NSCLC patients has been reported. These results indicate HER2-targeted treatment might be one of the choices for these patients.
Primary and acquired resistance is the main reason for progression disease when patients received TKIs treatment. Currently, we know much about the mechanisms for resistance of EGFR-targeted treatment, but researches about resistance to HER2-targerted inhibitors in NSCLC patients are lacking. Chuang et al. (13) suggested PIK3CA mutation and HER2 gene amplification may be the potential mechanisms for resistance during HER2-targeted treatment. However, the results were analyzed from four cases, which is hard to reach statistical significance. Herein, we performed NGS for nine patients when progression on afatinib treatment. Of three patients harbored secondary HER2 alterations, two carried a HER2 exon 20 insertion and another carried HER2 amplification as secondary alteration. Previous studies demonstrated that secondary ALK mutations could induce resistance of ALK inhibitors (28, 29). Whether HER2 secondary alterations resistance mechanism to afatinib need to be determined in further studies. In addition, we found TP53 was recurrently mutated (44%) in afatinib-resistant patients. Several studies reported that TP53 mutations were associated with inferior clinical effect of EGFR-targeted inhibitors (30–32). One patient harbored TP53 and RB1 co-mutations, which were associated with an increasing risk for small cell transformation and resistance to TKIs treatment (33–36).
This study still has several limitations. Firstly, we cannot completely avoid the reporting bias because of this work’s retrospective nature. Secondly, due to a lack of control arm, comparison with other therapies was not feasible. Thirdly, only nine patients were performed NGS when progression, so these data cannot fully reflect the whole cohort and no statistical significance can be reached about resistance of afatinib. Despite these limitations, this study provides deep insights into clinical activity of afatinib in NSCLC with HER2 alterations.
Conclusion
Our results suggest that afatinib has a potential efficacy in these patients, especially in the patients with HER2 amplification or other pathologic mutations in exons except exon 20. Further studies, especially prospective studies, are warranted to investigate the clinical activity of afatinib and the mechanism of resistance to HER2-targeted therapy.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Zhejiang Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication. ZS and QL contributed to the study design. All authors were responsible for interpretation of the results. ZS and SC contributed to statistical analysis. ZS and SC prepared the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
Authors SC and GW were employed by company 3D Medicines Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.657283/full#supplementary-material
Supplementary Figure 1 | Next-generation sequencing result showed a HER2 Y772_A775dup mutation. The BAM file was viewed using integrative genomics viewer.
Supplementary Figure 2 | Kaplan-Meier estimates of progression-free survival and overall survival according to according to HER2 mutations. (A) progression-free survival; (B) overall survival.
Supplementary Figure 3 | Kaplan-Meier estimates of progression-free survival and overall survival according to lines of treatment. (A) progression-free survival; (B) overall survival.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
2. Herbst RS, Morgensztern D, Boshoff C. The Biology and Management of non-Small Cell Lung Cancer. Nature (2018) 553(7689):446–54. doi: 10.1038/nature25183
3. Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr., Wu YL, et al. Lung Cancer: Current Therapies and New Targeted Treatments. Lancet (2017) 389(10066):299–311. doi: 10.1016/S0140-6736(16)30958-8
4. Thomas A, Liu SV, Subramaniam DS, Giaccone G. Refining the Treatment of NSCLC According to Histological and Molecular Subtypes. Nat Rev Clin Oncol (2015) 12(9):511–26. doi: 10.1038/nrclinonc.2015.90
5. Mazieres J, Peters S, Lepage B, Cortot AB, Barlesi F, Beau-Faller M, et al. Lung Cancer That Harbors an HER2 Mutation: Epidemiologic Characteristics and Therapeutic Perspectives. J Clin Oncol (2013) 31(16):1997–2003. doi: 10.1200/JCO.2012.45.6095
6. Song Z, Yu X, Shi Z, Zhao J, Zhang Y. HER2 Mutations in Chinese Patients With non-Small Cell Lung Cancer. Oncotarget (2016) 7(47):78152–8. doi: 10.18632/oncotarget.11313
7. Li BT, Ross DS, Aisner DL, Chaft JE, Hsu M, Kako SL, et al. Her2 Amplification and HER2 Mutation Are Distinct Molecular Targets in Lung Cancers. J Thorac Oncol (2016) 11(3):414–9. doi: 10.1016/j.jtho.2015.10.025
8. Kim EK, Kim KA, Lee CY, Shim HS. The frequency and Clinical Impact of HER2 Alterations in Lung Adenocarcinoma. PloS One (2017) 12(2):e0171280. doi: 10.1371/journal.pone.0171280
9. Lee J, Franovic A, Shiotsu Y, Kim ST, Kim KM, Banks KC, et al. Detection of ERBB2 (Her2) Gene Amplification Events inand Response to Anti-HER2 Agents in a Large Asian Cancer Patient Cohort. Front Oncol (2019) 9:212. doi: 10.3389/fonc.2019.00212
10. Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado-Trastuzumab Emtansine for Patients With HER2-Mutant Lung Cancers: Results From a Phase Ii Basket Trial. J Clin Oncol (2018) 36(24):2532–7. doi: 10.1200/JCO.2018.77.9777
11. Dziadziuszko R, Smit EF, Dafni U, Wolf J, Wasag B, Biernat W, et al. Afatinib in NSCLC With HER2 Mutations: Results of the Prospective, Open-Label Phase Ii NICHE Trial of European Thoracic Oncology Platform (Etop). J Thorac Oncol (2019) 14(6):1086–94. doi: 10.1016/j.jtho.2019.02.017
12. Mazieres J, Barlesi F, Filleron T, Besse B, Monnet I, Beau-Faller M, et al. Lung Cancer Patients With HER2 Mutations Treated With Chemotherapy and HER2-targeted Drugs: Results From the European EUHER2 Cohort. Ann Oncol (2016) 27(2):281–6. doi: 10.1093/annonc/mdv573
13. Chuang JC, Stehr H, Liang Y, Das M, Huang J, Diehn M, et al. Erbb2-Mutated Metastatic non-Small Cell Lung Cancer: Response and Resistance to Targeted Therapies. J Thorac Oncol (2017) 12(5):833–42. doi: 10.1016/j.jtho.2017.01.023
14. Liu Z, Wu L, Cao J, Yang Z, Zhou C, Cao L, et al. Clinical Characterization of ERBB2 Exon 20 Insertions and Heterogeneity of Outcomes Responding to Afatinib in Chinese Lung Cancer Patients. Onco Targets Ther (2018) 11:7323–31. doi: 10.2147/OTT.S173391
15. Lai WV, Lebas L, Barnes TA, Milia J, Ni A, Gautschi O, et al. Afatinib in Patients With Metastatic or Recurrent HER2-mutant Lung Cancers: A Retrospective International Multicentre Study. Eur J Cancer (2019) 109:28–35. doi: 10.1016/j.ejca.2018.11.030
16. Ou SI, Schrock AB, Bocharov EV, Klempner SJ, Haddad CK, Steinecker G, et al. Her2 Transmembrane Domain (Tmd) Mutations (V659/G660) That Stabilize Homo- and Heterodimerization are Rare Oncogenic Drivers in Lung Adenocarcinoma That Respond to Afatinib. J Thorac Oncol (2017) 12(3):446–57. doi: 10.1016/j.jtho.2016.11.2224
17. Yang N, Li Y, Liu Z, Qin H, Du D, Cao X, et al. The Characteristics of ctDNA Reveal the High Complexity in Matching the Corresponding Tumor Tissues. BMC Cancer (2018) 18(1):319. doi: 10.1186/s12885-018-4199-7
18. Peters S, Curioni-Fontecedro A, Nechushtan H, Shih JY, Liao WY, Gautschi O, et al. Activity of Afatinib in Heavily Pretreated Patients With ERBB2 Mutation-Positive Advanced Nsclc: Findings From a Global Named Patient Use Program. J Thorac Oncol (2018) 13(12):1897–905. doi: 10.1016/j.jtho.2018.07.093
19. Bocharov EV, Lesovoy DM, Pavlov KV, Pustovalova YE, Bocharova OV, Arseniev AS. Alternative Packing of EGFR Transmembrane Domain Suggests That Protein-Lipid Interactions Underlie Signal Conduction Across Membrane. Biochim Biophys Acta (2016) 1858(6):1254–61. doi: 10.1016/j.bbamem.2016.02.023
20. Lin L, Ge H, Yan Z, Wang G, Wu X, Lv D. Response to Afatinib in a Patient With Non-Small Cell Lung Cancer Harboring Her2 R896G Mutation: A Case Report. Onco Targets Ther (2019) 12:10897–902. doi: 10.2147/OTT.S228726
21. Kris MG, Camidge DR, Giaccone G, Hida T, Li BT, O’Connell J, et al. Targeting HER2 Aberrations as Actionable Drivers in Lung Cancers: Phase II Trial of the pan-HER Tyrosine Kinase Inhibitor Dacomitinib in Patients With HER2-mutant or Amplified Tumors. Ann Oncol (2015) 26(7):1421–7. doi: 10.1093/annonc/mdv186
22. Wang Y, Jiang T, Qin Z, Jiang J, Wang Q, Yang S, et al. HER2 Exon 20 Insertions in non-Small-Cell Lung Cancer are Sensitive to the Irreversible pan-HER Receptor Tyrosine Kinase Inhibitor Pyrotinib. Ann Oncol (2019) 30(3):447–55. doi: 10.1093/annonc/mdy542
23. Zhou C, Li X, Wang Q, Gao G, Zhang Y, Chen J, et al. Pyrotinib in HER2-Mutant Advanced Lung Adenocarcinoma After Platinum-Based Chemotherapy: A Multicenter, Open-Label, Single-Arm, Phase Ii Study. J Clin Oncol (2020) 38(24):2753–61. doi: 10.1200/JCO.20.00297
24. Koga T, Kobayashi Y, Tomizawa K, Suda K, Kosaka T, Sesumi Y, et al. Activity of a Novel HER2 Inhibitor, Poziotinib, for HER2 Exon 20 Mutations in Lung Cancer and Mechanism of Acquired Resistance: An In Vitro Study. Lung Cancer (2018) 126:72–9. doi: 10.1016/j.lungcan.2018.10.019
25. Robichaux JP, Elamin YY, Vijayan RSK, Nilsson MB, Hu L, He J, et al. Pan-Cancer Landscape and Analysis of ERBB2 Mutations Identifies Poziotinib as a Clinically Active Inhibitor and Enhancer of T-DM1 Activity. Cancer Cell (2019) 36(4):444–57.e7. doi: 10.1016/j.ccell.2019.09.001
26. Oh DY, Bang YJ. HER2-Targeted Therapies - a Role Beyond Breast Cancer. Nat Rev Clin Oncol (2020) 17(1):33–48. doi: 10.1038/s41571-019-0268-3
27. Siena S, Sartore-Bianchi A, Marsoni S, Hurwitz HI, McCall SJ, Penault-Llorca F, et al. Targeting the Human Epidermal Growth Factor Receptor 2 (HER2) Oncogene in Colorectal Cancer. Ann Oncol (2018) 29(5):1108–19. doi: 10.1093/annonc/mdy100
28. Gainor JF, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R, et al. Molecular Mechanisms of Resistance to First- and Second-Generation Alk Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discovery (2016) 6(10):1118–33. doi: 10.1158/2159-8290.CD-16-0596
29. Katayama R. Drug Resistance in Anaplastic Lymphoma Kinase-Rearranged Lung Cancer. Cancer Sci (2018) 109(3):572–80. doi: 10.1111/cas.13504
30. Labbe C, Cabanero M, Korpanty GJ, Tomasini P, Doherty MK, Mascaux C, et al. Prognostic and Predictive Effects of TP53 Co-Mutation in Patients With EGFR-mutated non-Small Cell Lung Cancer (NSCLC). Lung Cancer (2017) 111:23–9. doi: 10.1016/j.lungcan.2017.06.014
31. Canale M, Petracci E, Delmonte A, Chiadini E, Dazzi C, Papi M, et al. Impact of TP53 Mutations on Outcome in EGFR-Mutated Patients Treated With First-Line Tyrosine Kinase Inhibitors. Clin Cancer Res (2017) 23(9):2195–202. doi: 10.1158/1078-0432.CCR-16-0966
32. Yu HA, Suzawa K, Jordan E, Zehir A, Ni A, Kim R, et al. Concurrent Alterations in EGFR-Mutant Lung Cancers Associated With Resistance to EGFR Kinase Inhibitors and Characterization of MTOR as a Mediator of Resistance. Clin Cancer Res (2018) 24(13):3108–18. doi: 10.1158/1078-0432.CCR-17-2961
33. George J, Lim JS, Jang SJ, Cun Y, Ozretic L, Kong G, et al. Comprehensive Genomic Profiles of Small Cell Lung Cancer. Nature (2015) 524(7563):47–53. doi: 10.1038/nature14664
34. Niederst MJ, Sequist LV, Poirier JT, Mermel CH, Lockerman EL, Garcia AR, et al. RB Loss in Resistant EGFR Mutant Lung Adenocarcinomas That Transform to Small-Cell Lung Cancer. Nat Commun (2015) 6:6377. doi: 10.1038/ncomms7377
35. Offin M, Chan JM, Tenet M, Rizvi HA, Shen R, Riely GJ, et al. Concurrent RB1 and TP53 Alterations Define a Subset of EGFR-Mutant Lung Cancers At Risk for Histologic Transformation and Inferior Clinical Outcomes. J Thorac Oncol (2019) 14(10):1784–93. doi: 10.1016/j.jtho.2019.06.002
Keywords: non-small cell lung cancer (NSCLC), HER2, afatinib, efficacy, resistance
Citation: Song Z, Lv D, Chen S, Huang J, Wang L, Xu S, Chen H, Wang G and Lin Q (2021) Efficacy and Resistance of Afatinib in Chinese Non-Small Cell Lung Cancer Patients With HER2 Alterations: A Multicenter Retrospective Study. Front. Oncol. 11:657283. doi: 10.3389/fonc.2021.657283
Received: 22 January 2021; Accepted: 06 April 2021;
Published: 07 May 2021.
Edited by:
Hideharu Kimura, Kanazawa University, JapanReviewed by:
Sagun Parakh, University of Melbourne, AustraliaValerio Gristina, University of Palermo, Italy
Copyright © 2021 Song, Lv, Chen, Huang, Wang, Xu, Chen, Wang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quan Lin, bHF1YW4wMDdAMTYzLmNvbQ==; Zhengbo Song, c29uZ3poZW5nYm84M0AxNjMuY29t
 Zhengbo Song
Zhengbo Song Dongqing Lv3†
Dongqing Lv3† Shiqing Chen
Shiqing Chen Quan Lin
Quan Lin