- Diagnostic and Research Institute of Pathology, Medical University of Graz, Graz, Austria
Large cell neuroendocrine carcinoma (LCNEC) together with small cell carcinoma (SCLC) and typical and atypical carcinoids form the group of pulmonary neuroendocrine tumors. LCNEC and SCLC are high-grade carcinomas. Although both can be found outside the thoracic cavity, they are most common in the lung. LCNEC differs from SCLC by morphologic pattern, and by cytological features such as nuclear size, nucleoli, chromatin pattern, but also by genetic differences. Originally thought to represent a single entity, it became evident, that three subgroups of LCNEC can be identified at the molecular level: a SCLC-like type with loss of retinoblastoma 1 gene (RB1) and TP53 mutations; a non-small cell lung carcinoma (NSCLC)-like type with wildtype RB1, TP53 mutation, and activating mutations of the phosphoinositol-3 kinase (PI3K-CA), or loss of PTEN; and a carcinoid-like type with MEN1 gene mutation. These subtypes can be identified by immunohistochemical staining for RB1, p53, and molecular analysis for PI3K and MEN1 mutations. These subtypes might also respond differently to chemotherapy. Immuno-oncologic treatment has also been applied to LCNEC, however, in addition to the evaluation of tumor cells the stroma evaluation seems to be important. Based on personal experiences with these tumors and available references this review will try to encompass our present knowledge in this rare entity and provoke new studies for better treatment of this carcinoma.
Introduction
Large cell neuroendocrine carcinoma (LCNEC) was originally created during a study of atypical carcinoids (ATC) with an unusual dismal outcome (1). The major criteria were a neuroendocrine morphology with rosettes and trabecules, the expression of neuroendocrine markers, such as chromogranin A (CGA), synaptophysin (SYN), neural cell adhesion molecule (CD56/NCAM), and others. In contrast to carcinoids, LCNEC presented with large nuclei (> 26µm), coarse chromatin, and frequently enlarged nucleoli. The mitotic rate was above 10/2mm2, and large necrotic areas are frequently seen. The prognosis for this group of carcinomas was similar to that of small cell lung carcinoma (SCLC), which is also a high-grade neuroendocrine carcinoma (2–4). The differentiation between SCLC and LCNEC is usually based on morphology: nuclei 17-23µm, absence of nucleoli, dense heterochromatin in SCLC; nuclear size >26µm, coarse chromatin, and frequently enlarged nucleoli in LCNEC. Later on, other carcinomas with large cell morphology and expression of neuroendocrine markers in > 10% of tumor cells were added to the LCNEC category (in previous WHO classifications this was based on 25% of tumor cells) – in these cases, a classical neuroendocrine morphology was not always present, but large areas of necrosis were seen. Whereas the low and intermediate-grade carcinoids arise from neuroendocrine precursor cells, and precursor lesions such as tumorlets can often be seen, the high grades carcinomas SCLC and LCNEC arise from undifferentiated probably stem cell-like precursors, but in LCNEC a transition from atypical carcinoid to LCNEC is suspected (5–8). The great majority of high-grade carcinomas are associated with cigarette smoking (9, 10). In a previous study we have shown similarities and differences in chromosomal alterations between SCLC and LCNEC. Losses of 3p, 4q, 5q, and 13q and gains of 5p were common in both entities. A gain of 3q and losses on chromosome 10 were frequently seen in SCLC but not in LCNEC. Gains of 6p occurred more frequently in LCNEC (11).
From the beginning treatment for LCNEC was discussed and handled controversially for several decades, preferring either a non-small cell lung carcinoma (NSCLC) protocol including cisplatinum or a SCLC-based protocol (12, 13).
Methods and Material
In our lung pathology archive 13412 carcinomas were collected between 1986 and 2012. Of these 163 were diagnosed as LCNEC. In addition to a neuroendocrine morphology also immunohistochemistry for the markers γγ-enolase (NSE), gastrin-releasing peptide (GRP), CGA, NCAM, SYN, calcitonin (CT), vasoactive intestinal peptide (VIP), neural peptide Y (NPY), adrenocorticotropin (ACTH), and PGP9.5, respectively, combined with low-molecular weight cytokeratin antibodies had to be applied in 53 cases to reach a definite diagnosis*. In 25 cases a mixed SCLC/LCNEC diagnosis was made. Other combinations with LCNEC restricted to few cases were squamous cell carcinoma, adenocarcinoma, and large cell carcinoma.
(*During decades markers for neuroendocrine differentiation have changed; NSE was one of the earliest markers used in this respect, whereas now CGA, NCAM, and SYN are most often used)
Morphology and Diagnosis
Clinically LCNEC presents as a tumor mass on CT scan and X-ray. There are no specific clinical symptoms. On gross examination the only feature that might point to LCNEC are large areas of necrosis, which by themselves are not specific.
Morphologically LCNEC is defined by a neuroendocrine pattern, i.e., rosettes, trabecules, and solid cell nests (Figure 1). However, the neuroendocrine morphology is not always clearly visible, and in some cases only nesting of tumor cells is present. On low-power view, LCNEC looks organoid, similar to a carcinoid, but on higher magnification abundant mitoses are obvious. Nuclei are large polymorphic, 25-35 µm, with coarse, granular chromatin, enlarged and prominent nucleoli, landscape-like necrosis is usually present. To confirm the diagnosis staining for neuroendocrine markers (NCAM, SYN, CGA, PGP9.5) is recommended: at least 10% of cells should be positive with at least one neuroendocrine marker. LCNEC in comparison with SCLC produces and less secretes often hormones. It is also positive for low-molecular weight cytokeratin. In our cases, the vast majority presented with mitotic counts > 35/2mm2, however, there were few cases with a neuroendocrine morphology with mitotic counts from 11-18/2mm2. Based on the WHO classification (14) these cases had to be classified as LCNEC. However, we hypothesize, that likely these cases behave more like atypical carcinoids or in between ATC and LCNEC. Only one patient of our cases died within 3 years, all the others had an overall survival beyond 5 years. These cases all had metastasis in N2 lymph nodes but with low T stage (T1 or T2) and no metastasis outside the lung. Quinn et al. published a series of cases with similar findings, although mitotic counts in their cases ranged from 11-61/2mm2 (15). As these cases are rare, a multi-institutional investigation is needed to evaluate these cases and to position them into the classification of neuroendocrine tumors.
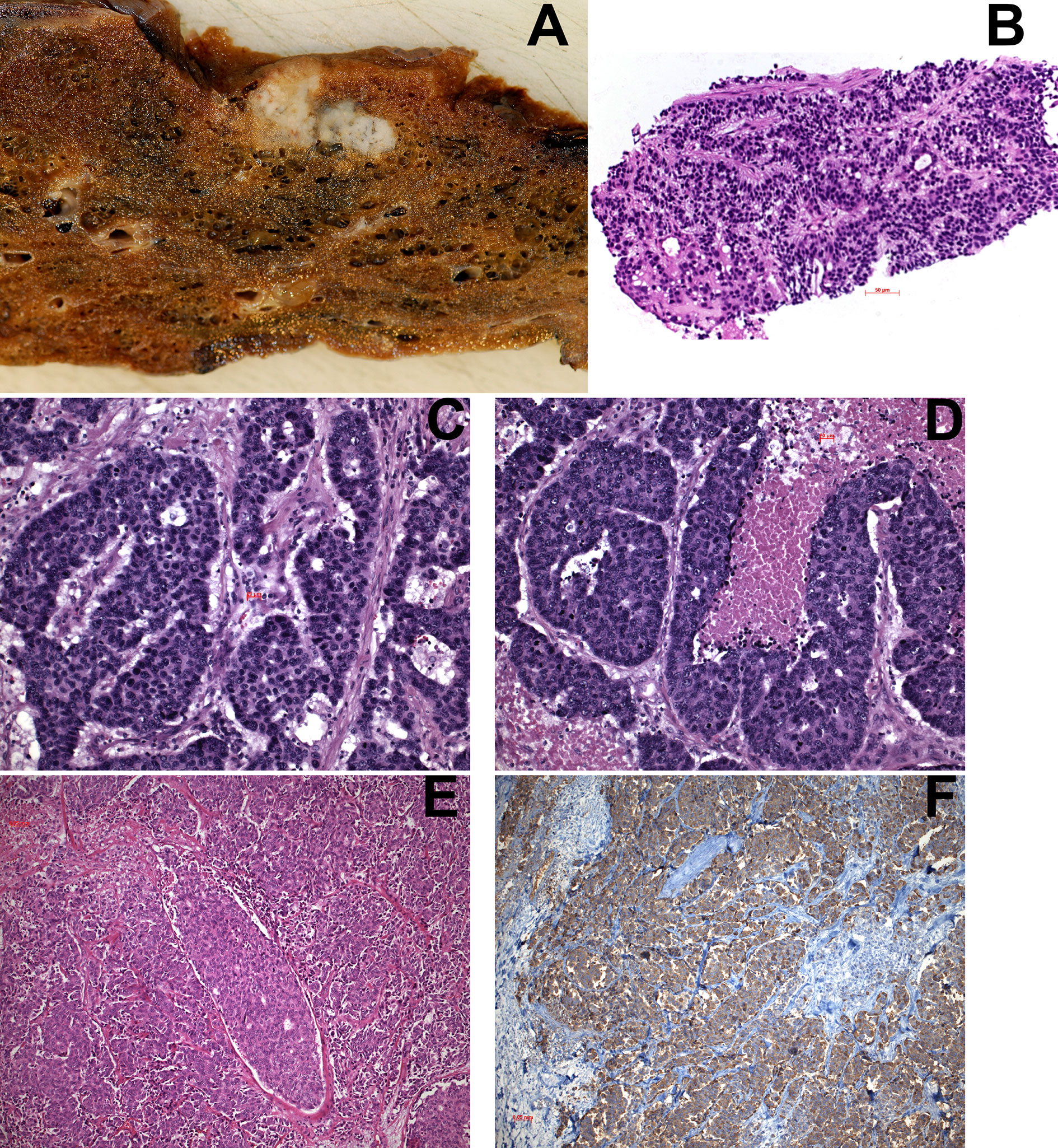
Figure 1 (A) Macroscopy of LCNEC, showing two nodular lesions separated by a small bridge of parenchyma; areas of necrosis are seen on the left side. (B) transthoracic needle biopsy of a LCNEC. (C, D) Examples of LCNEC with neuroendocrine morphology (rosettes) and areas of necrosis in (D). (E) Another LCNEC without neuroendocrine morphology. (F) Immunohistochemistry in this case showed positivity for CGA in almost 100% of tumor cells. H&E and IHC for CGA, magnifications x70, x200, and x400.
Heterogeneity of LCNEC
To better define LCNEC, Rekhtman and colleagues analyzed 45 cases of LCNEC by next-generation sequencing. They identified two large and one small groups of cases, characterized by a specific genomic profile. One was a SCLC-like group with mutations of TP53 and loss of RB1, and other alterations such as MYC-L amplification. Another NSCLC-like group presented with TP53, KRAS, STK11/LKB1, and KEAP1 mutations and retained RB1, and also frequent alterations of NOTCH family genes. The third group presented with a carcinoid-like morphology and MEN1 gene mutation (16). Unfortunately, these results were not correlated with mitotic counts. These findings were also confirmed in the study by Miyoshi et al. Here 78 LCNEC samples were sequenced for the coding exons of 244 cancer-related genes. Inactivating mutations were seen for TP53 and RB1, but the mutation frequency in RB1 was lower than in SCLC. Other genetic alterations were detected in the PI3K/AKT/mTOR pathway and activating alterations were detected in KRAS, FGFR1, KIT, ERBB2, HRAS, and EGFR (17). Deleting the tumor suppressors, RB1 and PTEN, and deactivating TP53, Lazaro and colleagues created a mouse model for LCNEC, confirming again the importance of RB1 and PTEN in the development of this carcinoma (18). In two different studies, Simbolo et al. identified three different LCNEC clusters: cluster 1 with inactivation of TP53 and RB1 with an absence of MEN1 mutations; cluster 2 with mutations of TP53, MEN1, and RB1 mutations; and cluster 3 without RB1 alterations but frequent MEN1 and TP53 mutations. These findings were also evaluated and confirmed by immunohistochemistry. Patients in cluster 1 had shorter cancer-specific survival than all others (19). By performing whole-exome sequencing for 418 genes in carcinoids, LCNEC, and SCLC, the authors found MEN1 alterations almost exclusively in carcinoids, whereas TP53 and RB1 alterations were present in the high-grade carcinomas. Chromatin-remodeling genes, such as histone modifiers and members of SWI-SNF complexes, were seen at similar rates in all neuroendocrine tumors (20). In another carcinoid-study, carcinoids showed MEN1 gene alterations, resulting in failures of chromatin remodeling, while LCNEC were characterized by mutations in DNA repair genes (loss of orthopedia homeobox) and upregulation of the RET gene. These authors also reported on one group with biallelic inactivation of TP53 and RB1, and the second with biallelic inactivation of TP53 and mutations of the serine/threonine kinase 11 gene (STK11) and kelch like ECH associated protein 1 gene (KEAP1) (21).
The neuroendocrine phenotype has been attributed to the neuroendocrine master regulator ASCL1/hASH (22, 23). However, the context of cells where ASCL1 is expressed is important: in the wrong context, the expression will create different types of carcinomas expressing neuroendocrine markers, but not LCNEC or SCLC (24, 25). This mechanism might probably explain the trans-differentiation of EGFR-mutated adenocarcinomas into SCLC- or LCNEC-like carcinoma types. Here the antagonism of NOTCH/Hes1 and ASCL1 come into play: Inactivation of the NOTCH-Hes1 axis might result in overexpression of ASCL1 and pave the way to a neuroendocrine phenotype (26). In the meanwhile, other regulators of neuroendocrine differentiation have been identified in SCLC as well as LCNEC. NeuroD was identified as another neuroendocrine master gene, but less frequently in LCNEC. Interestingly patients with NeuroD expression had better survivals (27). Similar to NeuroD, ASCL1 was found to be less often expressed in LCNEC, which might explain the difficulties in staining patterns in this category of high-grade neuroendocrine carcinoma (28). Another interesting observation and probably therapy relevant was a mutation of the NTRK2 and NTRK3 genes reported by Marchetti (29). In contrast to a rearrangement seen in several malignancies, here an activating mutation prevails. If this can be treated by NTRK inhibitors needs to be proven.
Diagnosis in Small Biopsies
In small biopsies LCNEC can be diagnosed, if rosettes and trabecules are present (Figure 1B), and the nuclei are large (diameter > 26µm), the chromatin is coarse, with prominent, middle-sized nucleoli. High mitotic counts might be encountered, whereas the large necrotic areas might not be seen. Immunohistochemistry should be done using a panel of at least two neuroendocrine markers. In cytological preparations the diagnosis is more difficult, because cell adhesion is much less compared to carcinoids, which results in rarely seen rosettes. If the nuclear features are present and numerous mitoses are seen, an immunocytochemistry for neuroendocrine markers should be performed.
Aspects for a Therapy
The prognosis of patients with LCNEC is similar to SCLC. Surgery is recommended for LCNEC in clinical stages I to IIIA. The discussion of which type of chemotherapy has to be applied remained controversial for decades (12, 13, 30–32). In recent times a chemotherapy regimen similar to SCLC is favored (31, 33). In a meta-analysis by He J. et al. overall and progression free survival was found to be superior, if LCNEC patients received a chemotherapy regimen similar to SCLC protocols (34) However, recurrence and metastasis are as high as in SCLC (35). The subtyping of LCNEC has opened possibilities for stratification in therapy: LCNEC, which have lost RB1 have been shown to respond to SCLC-like chemotherapy, whereas those retaining RB1 (Figure 2) and having either loss of PTEN, activating mutation of PI3KCA, combined with mutations of TP53, respectively, respond better to cisplatin chemotherapy (33, 36). Other therapeutic targets are being identified in LCNEC: A NTRK2/3 mutation has been reported, which might be targeted by NTRK-inhibitors (29). Furthermore, a FGFR2 mutation was detected exclusively in LCNEC (37), for which tyrosine kinase inhibitors are available. Four clinical studies targeting FGFR1 and FGFR2 are either closed or ongoing, however including so far only pulmonary squamous cell carcinoma (NCT 03762122, NCT01795768, NCT02965378, NCT01004224). Recently a new treatment was tested for SCLC. A toxin coupled to DLL3 expressed on SCLC cells showed some promises in phase 2 studies, however, failed in phase 3. DLL3 is also expressed in the majority of LCNEC, irrespective of RB1 (38). If a similar approach from SCLC might be used also in LCNEC deserves further studies (39). Resistance to chemotherapy and radiotherapy is a frequent event in both high-grade neuroendocrine carcinomas. Tumor-associated macrophages (TAMs) play an important role in this respect. The receptor tyrosine kinases Tyro3, Axl, and MerTK on macrophages are important in regulating these TAMs. These receptors help in polarizing macrophages into tumor-friendly M2 types (40). Using cell lines Ramkumar et al. inhibited Axl with a small molecule BGB324 and induced inhibition of cell proliferation and DNA damage in NSCLC and LCNEC (41). This might increase our repertoire for tumors developing resistance to radiotherapy and chemotherapy.
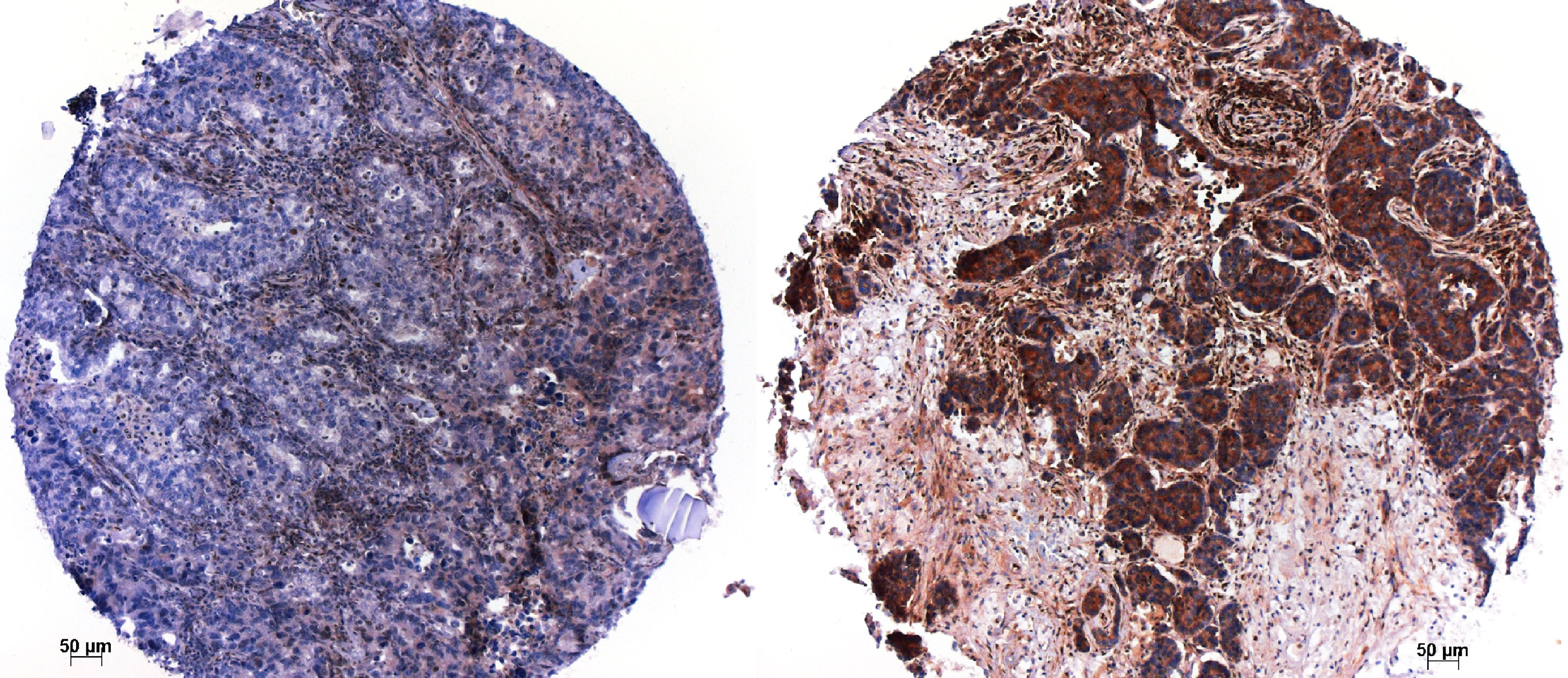
Figure 2 Immunohistochemistry for RB1; a case, which lost RB1 on the left, and another case, which retained RB1 to the right. Magnification x100.
Another option for the treatment of LCNEC is immunotherapy (Table 1). PD-L1 expression in LCNEC was associated with poor survival, while PD-L1 expression in the tumor microenvironment seemed to have a beneficial effect (42). Other studies on the expression of PD-L1 in both SCLC and LCNEC were performed, and several studies analyzed not only the expression of PDL1 on tumor cells but also on cells of the tumor stroma. The frequency of PD-L1 expression on tumor cells was in the range of 15-35% (43–47). However, more important than the expression on tumor cells was the expression on stromal cells and the association with infiltrating cytotoxic lymphocytes (CD8+) (43). Furthermore, analyzing lymphocytes for additional markers, Ohtaki et al. showed a favorable prognosis for cases with FoxP3 expressing tumor-associated lymphocytes, whereas the presence of CD4+ helper lymphocytes conferred an unfavorable prognosis (48). This was confirmed by the study of Shirasawa et al, who also included lymphocyte density into their study (49). Combining immunotherapy with chemo- and radiotherapy was shown to improve survival (50). Trials on immunotherapy are still ongoing (Table 2). Recently the stimulator of interferon genes (STING) has been shown as a probably new target to stimulate the patients’ immune system towards cancer. Normally STING is activated, if DNA or RNA is detected within the cytoplasm of cells. Treatment with cisplatin increases fragmented DNA and stimulates the STING pathway in STK11 and TP53 co-mutated NSCLC and LCNEC (51). In these cases, a PD-L1 therapy could be successfully applied.
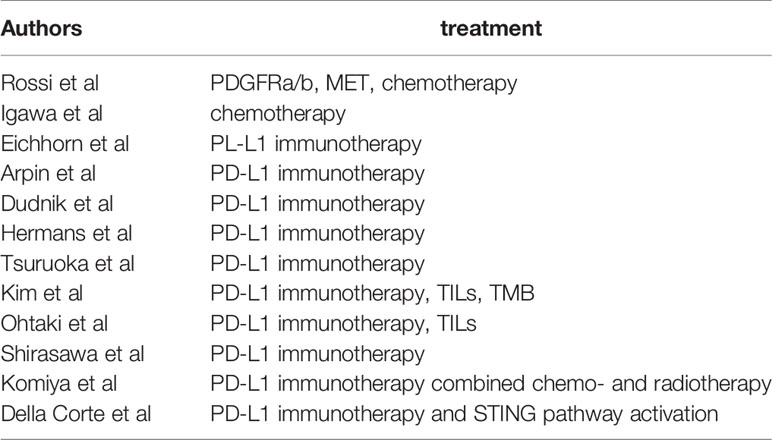
Table 1 Studies reporting on treatment modalities for LCNEC; TILs, tumor associated lymphocytes; TMB, tumor mutational burden.

Table 2 Ongoing clinical trials focusing on immunotherapy (chemotherapy trials are not included here).
Some of the above-mentioned biomarkers, like RB1 and p53 expression, can easily be evaluated by immunohistochemistry (Figure 2). As next-generation sequencing is regularly done in many laboratories for NSCLC with non-squamous histology, it should not be a problem to establish a mutational profile (including the above-mentioned genes) for LCNEC cases as well.
In conclusion, immunohistochemistry and molecular profiling will complement histology for better diagnostic definition and prognostic stratification of lung neuroendocrine tumors, and especially LCNEC, and will open new avenues for treatment. The molecular characterization of LCNEC should be included in the routine pathology practice.
Author Contributions
HP designed the review. LB and HP worked on the manuscript and finalized it. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Travis WD, Linnoila RI, Tsokos MG, Hitchcock CL, Cutler GB, Nieman L, et al. Neuroendocrine Tumors of the Lung With Proposed Criteria for Large-Cell Neuroendocrine Carcinoma. An Ultrastructural, Immunohistochemical, and Flow Cytometric Study of 35 Cases. Am J Surg Pathol (1991) 15:529–53. doi: 10.1097/00000478-199106000-00003
2. Onuki N, Wistuba II, Travis WD, Virmani AK, Yashima K, Brambilla E, et al. Survival Analysis of 200 Pulmonary Neuroendocrine Tumors With Clarification of Criteria for Atypical Carcinoid and its Separation From Typical Carcinoid. Cancer (1999) 85:600–7. doi: 10.1002/(sici)1097-0142(19990201)85:3<600::aid-cncr10>3.0.co;2-w
3. Asamura H, Kameya T, Matsuno Y, Noguchi M, Tada H, Ishikawa Y, et al. Neuroendocrine Neoplasms of the Lung: A Prognostic Spectrum. J Clin Oncol (2006) 24:70–6. doi: 10.1200/JCO.2005.04.1202
4. Filosso PL, Ruffini E, Di Gangi S, Guerrera F, Bora G, Ciccone G, et al. Prognostic Factors in Neuroendocrine Tumours of the Lung: A Single-Centre Experience. Eur J Cardiothorac Surg (2014) 45:521–6; discussion 526. doi: 10.1093/ejcts/ezt442
5. Yazawa T. Recent Advances in Histogenesis Research of Lung Neuroendocrine Cancers: Evidence Obtained From Functional Analyses of Primitive Neural/Neuroendocrine Cell-Specific Transcription Factors. Pathol Int (2015) 65:277–85. doi: 10.1111/pin.12267
6. Swarts DR, Ramaekers FC, Speel EJ. Molecular and Cellular Biology of Neuroendocrine Lung Tumors: Evidence for Separate Biological Entities. Biochim Biophys Acta (2012) 1826:255–71. doi: 10.1016/j.bbcan.2012.05.001
7. Pelosi G, Bianchi F, Dama E, Simbolo M, Mafficini A, Sonzogni A, et al. Most High-Grade Neuroendocrine Tumours of the Lung Are Likely to Secondarily Develop From Pre-Existing Carcinoids: Innovative Findings Skipping the Current Pathogenesis Paradigm. Virchows Arch (2018) 4:567–77. doi: 10.1007/s00428-018-2307-3
8. Alcala N, Leblay N, Gabriel AAG, Mangiante L, Hervas D, Giffon T, et al. Integrative and Comparative Genomic Analyses Identify Clinically Relevant Pulmonary Carcinoid Groups and Unveil the Supra-Carcinoids. Nat Commun (2019) 10:3407. doi: 10.1038/s41467-019-11276-9
9. Petzmann S, Ullmann R, Klemen H, Renner H, Popper HH. Loss of Heterozygosity on Chromosome Arm 11q in Lung Carcinoids. Hum Pathol (2001) 32:333–8. doi: 10.1053/hupa.2001.22762
10. Ullmann R, Petzmann S, Klemen H, Fraire AE, Hasleton P, Popper HH. The Position of Pulmonary Carcinoids Within the Spectrum of Neuroendocrine Tumors of the Lung and Other Tissues. Genes Chromosomes Cancer (2002) 34:78–85. doi: 10.1002/gcc.10049
11. Ullmann R, Petzmann S, Sharma A, Cagle PT, Popper HH. Chromosomal Aberrations in a Series of Large-Cell Neuroendocrine Carcinomas: Unexpected Divergence From Small-Cell Carcinoma of the Lung. Hum Pathol (2001) 32:1059–63. doi: 10.1053/hupa.2001.28248
12. Rossi G, Cavazza A, Marchioni A, Longo L, Migaldi M, Sartori G, et al. Role of Chemotherapy and the Receptor Tyrosine Kinases KIT, PDGFRalpha, PDGFRbeta, and Met in Large-Cell Neuroendocrine Carcinoma of the Lung. J Clin Oncol (2005) 23:8774–85. doi: 10.1200/JCO.2005.02.8233
13. Igawa S, Watanabe R, Ito I, Murakami H, Takahashi T, Nakamura Y, et al. Comparison of Chemotherapy for Unresectable Pulmonary High-Grade non-Small Cell Neuroendocrine Carcinoma and Small-Cell Lung Cancer. Lung Cancer (2010) 68:438–45. doi: 10.1016/j.lungcan.2009.07.003
14. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of Tumours of the Lung, Pleura, Thymus and Hear. Geneva: IARC, WHO Press (2015).
15. Quinn AM, Chaturvedi A, Nonaka D. High-Grade Neuroendocrine Carcinoma of the Lung With Carcinoid Morphology: A Study of 12 Case. Am J Surg Pathol (2017) 41:263–70. doi: 10.1097/PAS.0000000000000767
16. Rekhtman N, Pietanza MC, Hellmann MD, Naidoo J, Arora A, Won H, et al. Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma-Like and Non-Small Cell Carcinoma-Like Subset. Clin Cancer Res (2016) 22:3618–29. doi: 10.1158/1078-0432.CCR-15-2946
17. Miyoshi T, Umemura S, Matsumura Y, Mimaki S, Tada S, Makinoshima H, et al. Genomic Profiling of Large-Cell Neuroendocrine Carcinoma of the Lun. Clin Cancer Res (2017) 23:757–65. doi: 10.1158/1078-0432.CCR-16-0355
18. Lázaro S, Pérez-Crespo M, Lorz C, Bernardini A, Oteo M, Enguita AB, et al. Differential Development of Large-Cell Neuroendocrine or Small-Cell Lung Carcinoma Upon Inactivation of 4 Tumor Suppressor Genes. Proc Natl Acad Sci USA (2019) 116:22300–6. doi: 10.1073/pnas.1821745116
19. Simbolo M, Barbi S, Fassan M, Mafficini A, Ali G, Vicentini C, et al. Gene Expression Profiling of Lung Atypical Carcinoids and Large Cell Neuroendocrine Carcinomas Identifies Three Transcriptomic Subtypes With Specific Genomic Alteration. J Thorac Oncol (2019) 14:1651–61. doi: 10.1016/j.jtho.2019.05.003
20. Simbolo M, Mafficini A, Sikora KO, Fassan M, Barbi S, Corbo V, et al. Lung Neuroendocrine Tumours: Deep Sequencing of the Four World Health Organization Histotypes Reveals Chromatin-Remodelling Genes as Major Players and a Prognostic Role for TERT, RB1, MEN1 and KMT2. J Pathol (2017) 241:488–500. doi: 10.1002/path.4853
21. Derks JL, Leblay N, Lantuejoul S, Dingemans AC, Speel EM, Fernandez-Cuesta L. New Insights Into the Molecular Characteristics of Pulmonary Carcinoids and Large Cell Neuroendocrine Carcinomas, and the Impact on Their Clinical Managemen. J Thorac Oncol (2018) 13:752–66. doi: 10.1016/j.jtho.2018.02.002
22. Borges M, Linnoila RI, van de Velde HJ, Chen H, Nelkin BD, Mabry M, et al. An Achaete-Scute Homologue Essential for Neuroendocrine Differentiation in the Lung. Nature (1997) 386:852–5. doi: 10.1038/386852a0
23. Linnoila RI, Zhao B, DeMayo JL, Nelkin BD, Baylin SB, DeMayo FJ, et al. Constitutive Achaete-Scute Homologue-1 Promotes Airway Dysplasia and Lung Neuroendocrine Tumors in Transgenic Mice. Cancer Res (2000) 60:4005–9.
24. McFadden DG, Papagiannakopoulos T, Taylor-Weiner A, Stewart C, Carter SL, Cibulskis K, et al. Genetic and Clonal Dissection of Murine Small Cell Lung Carcinoma Progression by Genome Sequencing. Cell (2014) 156:1298–311. doi: 10.1016/j.cell.2014.02.031
25. Gazdar AF, Savage TK, Johnson JE, Berns A, Sage J, Linnoila RI, et al. The Comparative Pathology of Genetically Engineered Mouse Models for Neuroendocrine Carcinomas of the Lung. J Thorac Oncol (2015) 10:553–64. doi: 10.1097/JTO.0000000000000459
26. Jia S, Wildner H, Birchmeier C. Insm1 Controls the Differentiation of Pulmonary Neuroendocrine Cells by Repressing Hes1. Dev Biol (2015) 408:90–8. doi: 10.1016/j.ydbio.2015.10.009
27. Hiroshima K, Iyoda A, Shida T, Shibuya K, Iizasa T, Kishi H, et al. Distinction of Pulmonary Large Cell Neuroendocrine Carcinoma From Small Cell Lung Carcinoma: A Morphological, Immunohistochemical, and Molecular Analysis. Mod Pathol (2006) 19:1358–68. doi: 10.1038/modpathol.3800659
28. Nasgashio R, Sato Y, Matsumoto T, Kageyama T, Hattori M, Iyoda A, et al. The Balance Between the Expressions of Hash1 and HES1 Differs Between Large Cell Neuroendocrine Carcinoma and Small Cell Carcinoma of the Lung. Lung Cancer (2011) 74:405–10. doi: 10.1016/j.lungcan.2011.04.012
29. Marchetti A, Felicioni L, Pelosi G, Del Grammastro M, Fumagalli C, Sciarrotta M, et al. Frequent Mutations in the Neurotrophic Tyrosine Receptor Kinase Gene Family in Large Cell Neuroendocrine Carcinoma of the Lung. Hum Mutat (2008) 29:609–16. doi: 10.1002/humu.20707
30. Glisson BS, Moran CA. Large-Cell Neuroendocrine Carcinoma: Controversies in Diagnosis and Treatment. J Natl Compr Canc Netw (2011) 9:1122–9. doi: 10.6004/jnccn.2011.0093
31. Lo Russo G, Pusceddu S, Proto C, Macerelli M, Signorelli D, Vitali M, et al. Treatment of Lung Large Cell Neuroendocrine Carcinoma. Tumour Biol (2016) 37:7047–57. doi: 10.1007/s13277-016-5003-4
32. Derks JL, Hendriks LE, Buikhuisen WA, Groen HJ, Thunnissen E, van Suylen RJ, et al. Clinical Features of Large Cell Neuroendocrine Carcinoma: A Population-Based Overview. Eur Respir J (2016) 47:615–24. doi: 10.1183/13993003.00618-2015
33. Derks JL, Leblay N, Thunnissen E, van Suylen RJ, den Bakker M, Groen HJM, et al. Molecular Subtypes of Pulmonary Large-Cell Neuroendocrine Carcinoma Predict Chemotherapy Treatment Outcom. Clin Cancer Res (2018) 24:33–42. doi: 10.1158/1078-0432.CCR-17-1921
34. He J, Li S, He J. What Regimen Should Be Chosen for Pulmonary Large Cell Neuroendocrine Carcinoma? A Systematic Review and Meta-Analysi. J Thoracic Oncol (2021) 16:S180. doi: 10.1016/j.jtho.2021.01.262
35. Iyoda A, Hiroshima K, Moriya Y, Iwadate Y, Takiguchi Y, Uno T, et al. Postoperative Recurrence and the Role of Adjuvant Chemotherapy in Patients With Pulmonary Large-Cell Neuroendocrine Carcinoma. J Thorac Cardiovasc Surg (2009) 138:446–53. doi: 10.1016/j.jtcvs.2008.12.037
36. George J, Walter V, Peifer M, Alexandrov LB, Seidel D, Leenders F, et al. Integrative Genomic Profiling of Large-Cell Neuroendocrine Carcinomas Reveals Distinct Subtypes of High-Grade Neuroendocrine Lung Tumors. Nat Commun (2018) 9:1048. doi: 10.1038/s41467-018-03099-x
37. Vollbrecht C, Werner R, Walter RF, Christoph DC, Heukamp LC, Peifer M, et al. Mutational Analysis of Pulmonary Tumours With Neuroendocrine Features Using Targeted Massive Parallel Sequencing: A Comparison of a Neglected Tumour Group. Br J Cancer (2015) 113:1704–11. doi: 10.1038/bjc.2015.397
38. Brcic L, Kuchler C, Eidenhammer S, Pabst D, Quehenberger F, Gazdar AF, et al. Comparison of Four DLL3 Antibodies Performance in High Grade Neuroendocrine Lung Tumor Samples and Cell Cultures. Diagn Pathol (2019) 14:47. doi: 10.1186/s13000-019-0827-z
39. Hermans BCM, Derks JL, Thunnissen E, van Suylen RJ, den Bakker MA, Groen HJM, et al. DLL3 Expression in Large Cell Neuroendocrine Carcinoma (LCNEC) and Association With Molecular Subtypes and Neuroendocrine Profile. Lung Cancer (2019) 138:102–8. doi: 10.1016/j.lungcan.2019.10.010
40. Myers KV, Amend SR, Pienta KJ. Targeting Tyro3, Axl and MerTK (TAM Receptors): Implications for Macrophages in the Tumor Microenvironment. Mol Cancer (2019) 18:94. doi: 10.1186/s12943-019-1022-2
41. Ramkumar K, Stewart CA, Cargill KR, Della Corte CM, Wang Q, Shen L, et al. AXL Inhibition Induces DNA Damage and Replication Stress in Non-Small Cell Lung Cancer Cells and Promotes Sensitivity to ATR Inhibitor. Mol Cancer Res (2021) 19:485–97. doi: 10.1158/1541-7786.MCR-20-0414
42. Eichhorn F, Harms A, Warth A, Muley T, Winter H, Eichhorn ME. PD-L1 Expression in Large Cell Neuroendocrine Carcinoma of the Lung. Lung Cancer (2018) 118:76–82. doi: 10.1016/j.lungcan.2018.02.003
43. Arpin D, Charpentier MC, Bernardi M, Monnet I, Boni A, Watkin E, et al. PD-L1-Expression Patterns in Large-Cell Neuroendocrine Carcinoma of the Lung: Potential Implications for Use of Immunotherapy in These Patients: The GFPC 03-2017 “EPNE” Study. Ther Adv Med Oncol (2020) 12:1758835920937972. doi: 10.1177/1758835920937972
44. Hermans BCM, Derks JL, Thunnissen E, van Suylen RJ, den Bakker MA, Groen HJM, et al. Prevalence and Prognostic Value of PD-L1 Expression in Molecular Subtypes of Metastatic Large Cell Neuroendocrine Carcinoma (LCNE). Lung Cancer (2019) 130:179–86. doi: 10.1016/j.lungcan.2019.02.022
45. Tsuruoka K, Horinouchi H, Goto Y, Kanda S, Fujiwara Y, Nokihara H, et al. PD-L1 Expression in Neuroendocrine Tumors of the Lung. Lung Cancer (2017) 108:115–20. doi: 10.1016/j.lungcan.2017.03.006
46. Kim HS, Lee JH, Nam SJ, Ock CY, Moon JW, Yoo CW, et al. Association of PD-L1 Expression With Tumor-Infiltrating Immune Cells and Mutation Burden in High-Grade Neuroendocrine Carcinoma of the Lun. J Thorac Oncol (2018) 13:636–48. doi: 10.1016/j.jtho.2018.01.008
47. Dudnik E, Kareff S, Moskovitz M, Kim C, Liu SV, Lobachov A, et al. Real-World Survival Outcomes With Immune Checkpoint Inhibitors in Large-Cell Neuroendocrine Tumors of Lung. J Immunother Cancer (2021) 9:e001999. doi: 10.1136/jitc-2020-001999
48. Ohtaki Y, Kaira K, Atsumi J, Nagashima T, Kawashima O, Ibe T, et al. Prognostic Significance of PD-L1 Expression and Tumor Infiltrating Lymphocytes in Large Cell Neuroendocrine Carcinoma of Lung. Am J Transl Res (2018) 10:3243–53.
49. Shirasawa M, Yoshida T, Takayanagi D, Shiraishi K, Yagishita S, Sekine K, et al. Activity and Immune Correlates of Programmed Death-1 Blockade Therapy in Patients With Advanced Large Cell Neuroendocrine Carcinom. Clin Lung Cancer (2021) 8:S15258–7304. doi: 10.1016/j.cllc.2021.02.003
50. Komiya T, Ravindra N, Powell E. Role of Immunotherapy in Stage IV Large Cell Neuroendocrine Carcinoma of the Lun. Asian Pac J Cancer Prev (2021) 22:365–70. doi: 10.31557/APJCP.2021.22.2.365
Keywords: pulmonary neuroendocrine tumors, SCLC, LCNEC, mutations, subtyping
Citation: Popper H and Brcic L (2021) Diagnosis and Molecular Profiles of Large Cell Neuroendocrine Carcinoma With Potential Targets for Therapy. Front. Oncol. 11:655752. doi: 10.3389/fonc.2021.655752
Received: 19 January 2021; Accepted: 22 June 2021;
Published: 07 July 2021.
Edited by:
Takefumi Komiya, Parkview Health, United StatesReviewed by:
Diego Signorelli, Niguarda Ca ‘Granda Hospital, ItalyArnold Manfred Herskovic, Rush University, United States
Copyright © 2021 Popper and Brcic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helmut Popper, aGVsbXV0LnBvcHBlckBtZWR1bmlncmF6LmF0
 Helmut Popper
Helmut Popper Luka Brcic
Luka Brcic