
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 12 July 2021
Sec. Radiation Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.653831
 Guanqiao Li1†
Guanqiao Li1† Jia Yao1†
Jia Yao1† Junni Chen2
Junni Chen2 Baizhen Cai2
Baizhen Cai2 Xiangying Lin2
Xiangying Lin2 Zetan Chen2
Zetan Chen2 Jiawei Chen2
Jiawei Chen2 Han Wang3*
Han Wang3* Shiping Yang2*
Shiping Yang2*Background: Peripheral lymphatic radiotherapy in patients with pT3N0M0 and pT4N0M0 breast cancer has been a matter of considerable debate among radiation oncologists. This is the first report in a non-Caucasian population.
Patients and Methods: The study included 165 pT3N0M0 and pT4N0M0 patients. Univariate, multivariate, propensity score matching (PSM), and Kaplan-Meier analyses were conducted to evaluate the survival of patients. We also review all the literature about regional lymph nodes radiation in T3-4N0M0 patients and summarize them with tables to compare with the present study.
Results: The median follow-up duration was 58.7 months. Multivariate analyses showed that advance T stage and grade were dependent poor prognostic factors for OS, DMFS, LRFS, and DFS between group A (chest wall radiation) and group B (chest wall and regional lymph nodes radiation). The overall survival (OS), disease-free survival (DFS), local relapse-free survival (LRFS), and distant metastasis-free survival (DMFS) rates were not significantly different between group A and group B. The 5-year OS rate was 92.3% vs 89.7% for group A and group B, respectively (P=0.819). The 5-year LRFS rate was 94.9% vs 94.3% for group A and group B, respectively (P=0.852). Fifty-four pairs of patients were selected after propensity score matching (PSM) analysis was conducted. There was also no significant difference between group A and group B in regard to the OS, DFS, LRFS, and DMFS rates after PSM. The patients included in previous studies were all Caucasians, and our study was focused on non-Caucasians. The cases of previous studies were 10 to 20 years ago, but our study has more recent cases. The radiotherapy techniques of previous studies were conventional, and the techniques used in our study were three-dimensional conformal radiotherapy (3DCRT) or intensity modulated radiotherapy (IMRT).
Conclusion: Both our study and previous studies suggested that regional lymph nodes radiation cannot improve the survival rate for breast cancer patients with T3-4N0M0 in non-Caucasian population. Advance T stage and grade were the dependent poor prognostic factors for T3-4N0M0 patients.
Breast cancer is one of the most prevalent cancers worldwide. If we use the seventh edition of the AJCC cancer staging, T3N0M0 is stage IIb and T4N0M0 is stage IIIa (1). A linear relationship between the tumor diameter and incidence of positive lymph nodes was indicated when using the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) data (2). This is a rare clinical scenario and the published data on breast tumors has an incidence ranging from 0.5% to 4% (3–8).
Peripheral lymphatic radiotherapy in patients with pT3N0M0 and pT4N0M0 breast cancer has also been a matter of considerable debate among radiation oncologists owning to a lack of large-scale prospective studies and data from evidence-based medicine clinic trials. Two large studies of Alphonse G. Taghian and Jennifer Goulart assess the role of postmastectomy radiotherapy (PMRT) in node-negative breast cancer patients with tumors 5 cm or larger treated by mastectomy. However, there was no comparison of patients with or without regional lymph nodes radiation. One study in 2006 analyzed the association between locoregional recurrences and peripheral lymphatic radiotherapy. However, the 156 cases in this study were collected from 1975 to 2000. Although the study suggested that there might be an association between locoregional recurrences and peripheral lymphatic radiotherapy, the numbers of the cases are limited. We felt this was worth further investigation. Another American study that analyzed the association between locoregional recurrences and peripheral lymphatic radiotherapy focused on American patients and used NCDB analysis (9).
The National Comprehensive Cancer Network guidelines indicate that patients suffering from pT3-4N0 breast cancer should be treated with RT to the chest with or without regional lymph node irradiation (RNI) (10). There are no clear criteria on using PMRT in T3-4N0M0 patients with regional lymph node. The appropriate criteria given by the American College of Radiology for postmastectomy RT is “given the conflicting prospective and retrospective data, treatment of pT3N0 patients should continue to be highly individualized”. Currently, there are no clear criteria on using PMRT in patients with pT3N0 breast cancer (11).
Do all T3-4N0M0 patients need regional lymph node irradiation? Does irradiation of regional lymph nodes improve survival rate of T3-4N0M0 patients? These questions have always puzzled oncologists. Hence, we assessed the survival rates in T3-4N0M0 patients who underwent radiotherapy of chest wall, either with or without peripheral lymphatic radiotherapy. To our knowledge, this is the first report using non-Caucasian population.
In this study, we included 165 pT3N0M0 and pT4N0M0 patients who were treated with radiotherapy for invasive breast cancer. These patients were admitted to Hainan General Hospital from January 2010 and December 2018. This study was approved by the institutional review board and ethics committee of Hainan General Hospital. Of the 165 patients included in this study, 78 patients (47.3%) were treated with radiation at chest wall only, and 87 patients (52.7%) were treated with radiation at chest wall and the lymph node region areas.
Postmastectomy radiotherapy was used five times a week to the chest wall, supra/infraclavicular, and levels II, III axillary nodal region. There was no irradiation to the level I axilla or internal mammary chain. Patients had conventional fractionated radiotherapy, which consisted of postmastectomy radiotherapy at 50 Gy in 25 fractions over 5 weeks. The CW ± RNI was delivered using 3DCRT or IMRT.
Only one patient (0.6%) whose histopathology was pathologically mucinous adenocarcinoma did not receive chemotherapy, whereas all the other patients (99.4%) received chemotherapy. Because all the patients were lymph node-negative and had similar stages, the chemotherapy regimen was nearly the same [2 patients (1.2%) received chemotherapy regime of four-cycle TC, 1 patient (0.6%) received chemotherapy regime of four-cycle AC, and the others (98.2%) received chemotherapy regime of eight-cycle AC-T].
We analyzed the baseline characteristics and treatment patterns. The seventh American Joint Committee on Cancer (AJCC) staging system was used to assess the pathological tumor stage (1). The patients had a check in with the hospital every 3 months in the first two years, this changed to every six months for 3-5 years and once a year after that. The date of commencement of operation was set as the start date to measure events. We defined the end points (time to the first defining event) as follows: overall survival (OS), disease-free survival (DFS), local relapse-free survival (LRFS), and distant metastasis-free survival (DMFS).
Pearson’s chi-square test was used to compare the clinic-pathological parameters of the different groups; univariate Cox regression analysis was used to explore the risk factors of survival outcome. Using the Cox proportional hazard model, multivariate analysis of each prognostic variable was analyzed by the backward stepwise (likelihood ratio) procedure. In addition to balance of baseline characteristics between each group, propensity score matching (PSM) analysis was conducted with a ratio of 1.0. The Kaplan-Meier method was used to evaluate the survival of patients before and after matching. The difference between the two groups in survival time was investigated using log-rank test. P values less than 0.05 was considered statistically significant. Hazard ratios (HR) were presented with 95% confidence intervals. SPSS for Windows (version 22.0, SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
We identified 78 patients with chest wall radiation and 87 patients with chest wall + regional lymph nodes area radiation that were treated in our department. The clinico-pathologic characteristics of these patients are summarized in Table 1. Their average age was 48.0 years, and the median age was 47.2 years (range, 20.8–71.4 years). All the patients are female. All of them underwent surgery and chemotherapy. 87.5% of the HER2 positive patients received targeted therapy. We divided the patients into group A (chest wall radiation) and group B (chest wall + regional lymph nodes radiation) according to the RT fields. Group B had a significantly higher proportion with pre-menopausal state than group A (P=0.003). The axillary management was significantly different between the two groups. The sentinel lymph node biopsy (SLNB) was higher in group B than group A (P=0.006). There were no significant differences in T stage and histological grade between two groups. In addition, the two treatment groups were well balanced in terms of age, ER status, PR status, Her2 status, pathology, histological grade, trastuzumab, lymphovascular invasion (LVI), RT technique, and the number of negative lymph nodes.
To identify which factors affect the prognosis, univariate and multivariate Cox regression analyses were performed. Univariate Cox regression analysis in Table 2 revealed that T stage was a significant predictive factor for OS, LRFS, DMFS, and DFS. Tumor grade was a significant predictive factor for OS, DMFS, and DFS. ER was a significant predictive factor for DMFS and DFS. The OS, LRFS, DMFS, or DFS of patients with or without regional lymph nodes radiation had no significant difference.
Next, we conducted multivariate analyses to evaluate the prognostic value of T stage, histological grade, ER, and regional lymph nodes radiation (Table 3). T4 stage was an independent poor prognostic factor for OS (P=0.002), DMFS (P=0.006), LRFS (P=0.002), and DFS (P=0.013). Furthermore, advance grade were an independent poor prognostic factor for OS(P=0.021), DMFS (P=0.030), LRFS (P=0.045), and DFS (P=0.004).
The median follow-up duration for the patients (range, 19.2–118.3 months) was 58.7 months. There was no significant difference between group A (chest wall radiation) and group B (chest wall and regional lymph nodes radiation) in regard to the OS, DFS, LRFS, and DMFS rates. The 5-year OS rate was 92.3% vs 89.7% for the group A and group B, respectively (P = 0.819) (Figure 1A). The 5-year LRFS rate was 94.9% vs 94.3% for the group A and group B, respectively (P = 0.852) (Figure 1B). For group A, the 5-year DMFS rate was 91.9%. For group B, it was 88.5% (P = 0.940) (Figure 1C). The 5-year DFS rates of group A was 89.7%, and group B was 86.2% (P = 0.858) (Figure 1D).
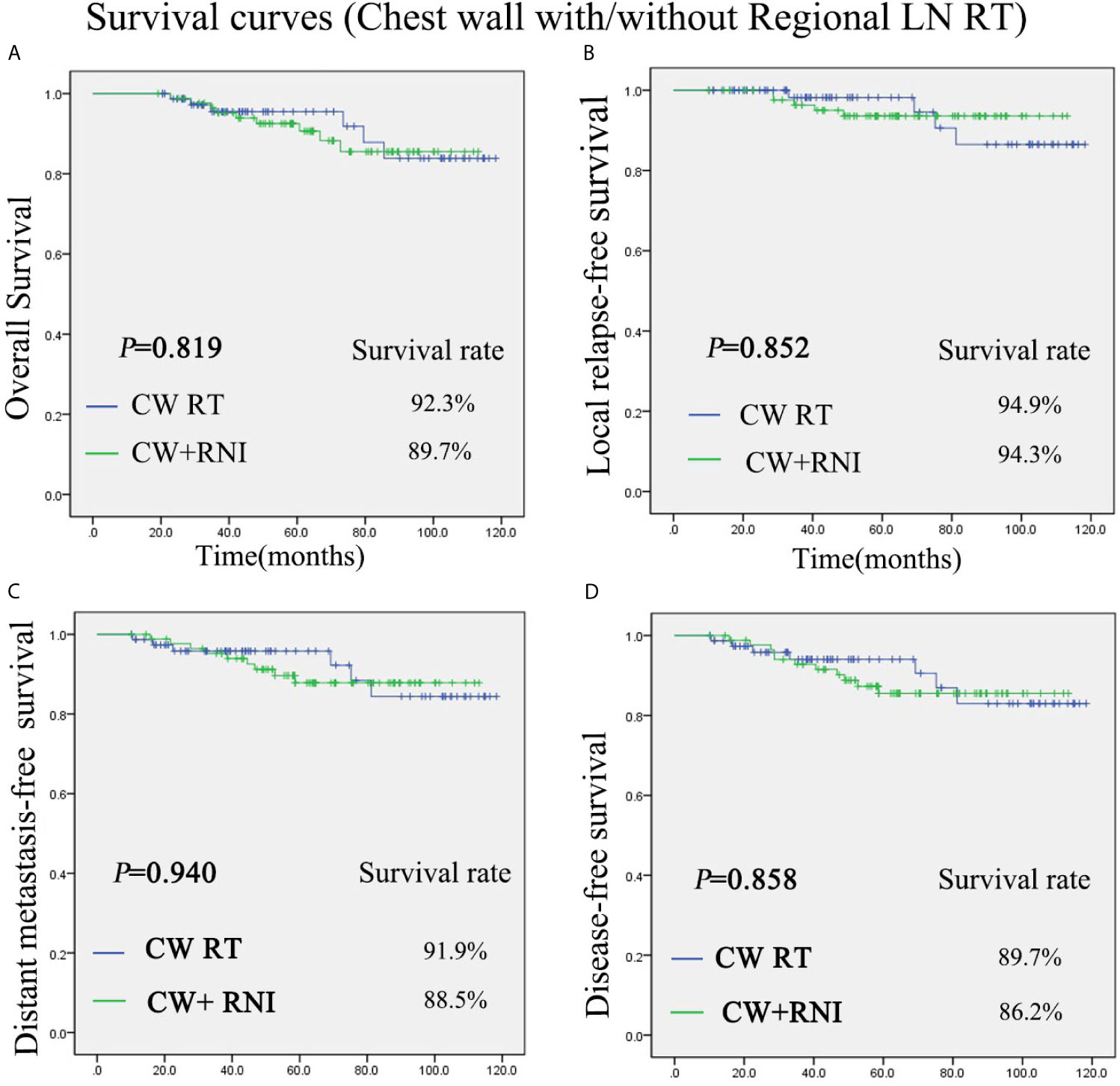
Figure 1 Kaplan-Meier survival curves for chest wall radiation group and chest wall + regional lymph nodes radiation group. Overall survival (A), disease-free survival (B), local relapse-free survival (C), and distant metastasis-free survival (D). P values were calculated with the unadjusted log-rank test. CW, chest wall; RNI, regional lymph node irradiation.
In addition, some baseline characteristics were significant difference between group A (chest wall radiation) and group B (chest wall and regional lymph nodes radiation). To balance of baseline characteristics between each group, propensity score matching (PSM) analysis was conducted. Fifty-four pairs of patients were selected for analysis. The clinico-pathologic characteristics after matching are summarized in Table 4. The baseline characteristics of two treatment groups were well balanced after matching.
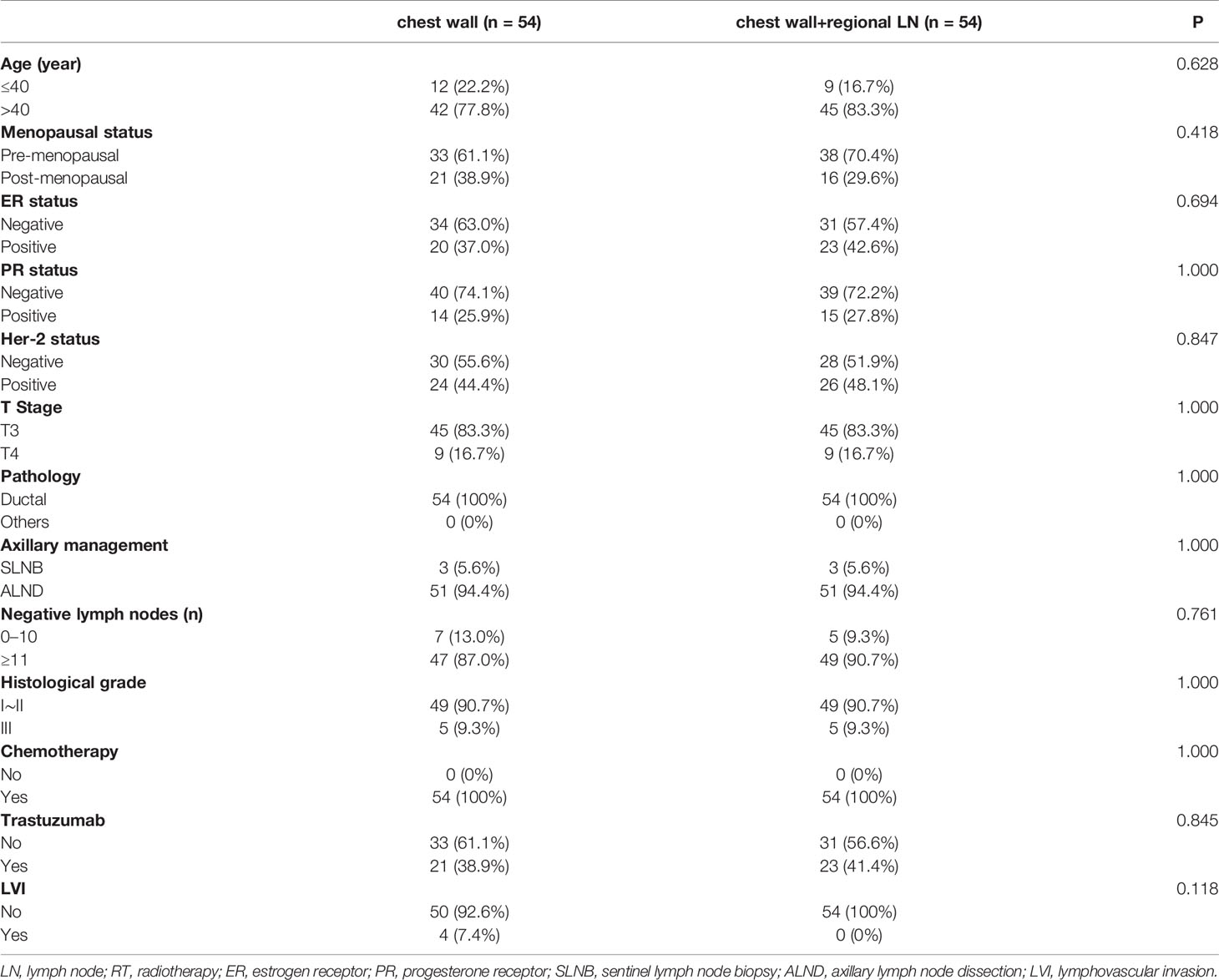
Table 4 Baseline characteristics and treatment patterns for chest wall ± regional LN RT after propensity score matching.
There was also no significant difference between group A (chest wall radiation) and group B (chest wall and regional lymph nodes radiation) in regard to the OS, DFS, LRFS, and DMFS rates after PSM. The 5-year OS rate was 90.7% vs 88.9% for the group A and group B, respectively (P = 0.949) (Figure 2A). The 5-year LRFS rate was 96.3% vs 90.7% for the group A and group B, respectively (P = 0.423) (Figure 2B). For group A, the 5-year DMFS rate was 88.9%. For group B, it was 87.0% (P = 0.885) (Figure 2C). The 5-year DFS rates of group A was 87.0%, and group B was 83.3% (P = 0.739) (Figure 2D).
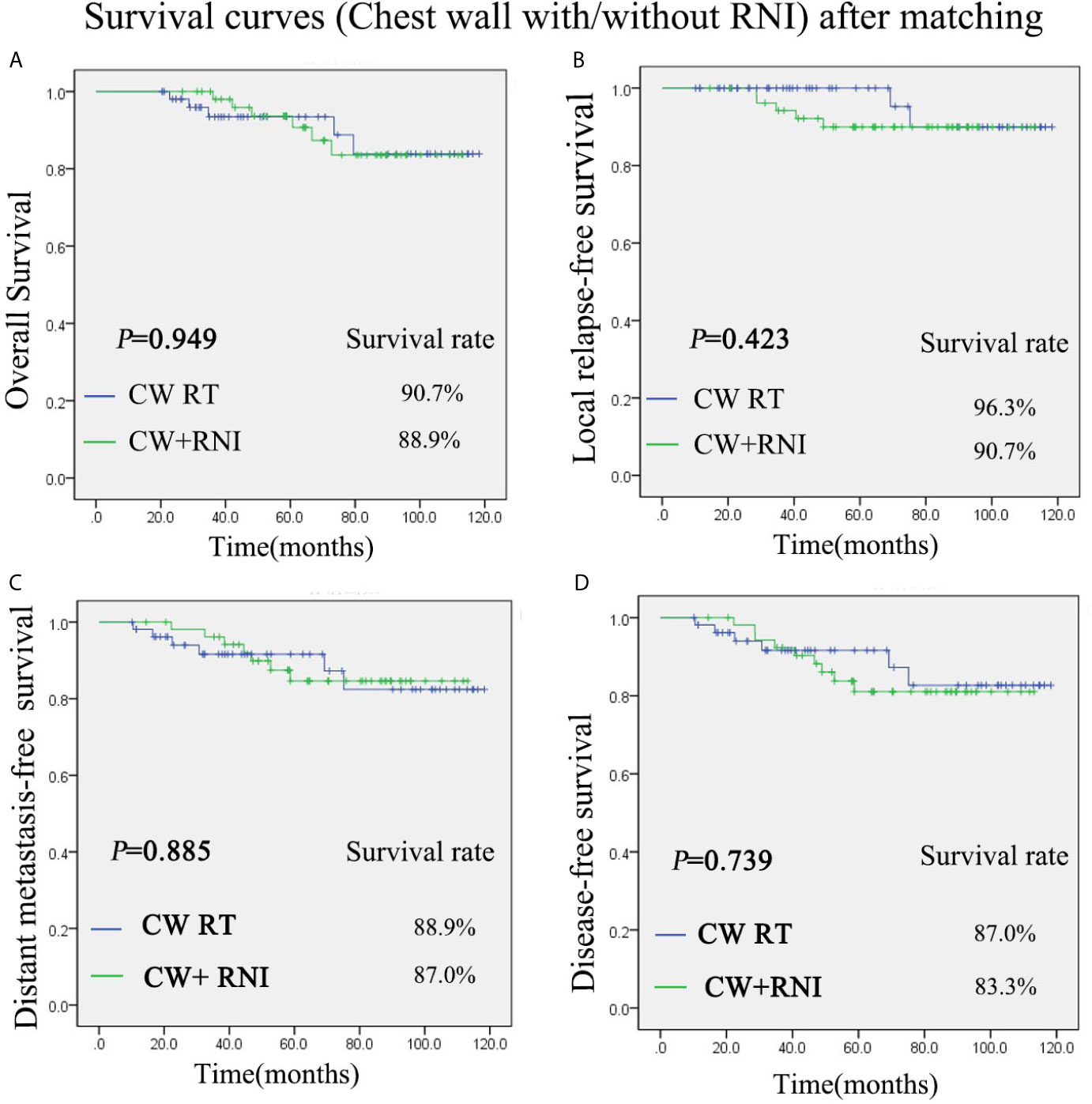
Figure 2 Kaplan-Meier survival curves after propensity score matching (PMS) for group of chest wall radiation and group of chest wall + regional lymph nodes radiation. Overall survival (A), disease-free survival (B), local relapse-free survival (C), and distant metastasis-free survival (D). P values were calculated with the unadjusted log-rank test. CW, chest wall; RNI, regional lymph node irradiation.
The T3-4N0M0 breast cancer is relatively rare, so it is difficult to carry out a phase III clinical trial study. We repeatedly reviewed the extensive literature on this topic by PubMed. We searched PubMed with the Title/Abstract of “Breast cancer, T3N0M0 (or T4N0M0), radiotherapy” published from 1990/01/01 to 2020/10/01. There were only four relevant publications. We summarized and compared the related literature of the previous four studies and our current research, and the results are shown in Tables 5, 6. As shown in Table 5, the collection time of the four previous studies (5, 9, 12, 13) is relatively long (10 to 20 years ago), and the cases in our study all within the last 10 years. The patients of previous studies were all Caucasians, and we conducted this study using non-Caucasian patients. Besides, the number of cases in the other three retrospective studies is relatively small and most are postoperative radiotherapy cases, except the Cassidy RJ’s study (9), which was an NCDB analysis from the United States. Two of them (5, 12) only listed the number of regional lymph nodes radiation cases but did not carry out survival analysis of patients, whereas the other carried out OS comparative analysis in the subgroup analysis of NCDB study in the United States. The advantage of NCDB study is the large numbers of patients. However, data information of NCDB is often incomplete. 16% of the cases in Cassidy RJ’s research (9) lack ER and PR status information, and 75% of the cases lack HER2 status. Moreover, detailed information on chemotherapy and hormone therapy were unavailable. The retrospective study of G.Aksu (13) collected the information of patients from 1975 to 2000 and lacked the status information of ER/PR or HER2. The radiation technology used in the study could now be considered outdated. The chest wall and/or peripheral lymphatics were administered with two daily fractions by Co60-6MV photon beam and electron beams after mastectomy. In our study, the information of each patient is relatively complete, and the radiation technology is based on modern radiotherapy techniques (3DCRT or IMRT). IMRT technology reduced the contralateral breast, ipsilateral lung, skin toxicity, and maintained reasonable target homogeneity when compared with conventional tangential techniques (14–16). The radiotherapy techniques of the previous study were conventional, and the techniques used in our study were 3DCRT or IMRT. Our results suggested that increasing regional lymph node irradiation did not improve the overall survival rate, which is consistent with the results of Cassidy RJ (9) and G.Aksu (13), whereas the Cassidy RJ study did not analyze the regional lymph node irradiation on recurrence rate (Table 6). Our study also analyzed the local recurrence rate. The results suggested that increasing regional lymph node radiation did not reduce the local recurrence rate.

Table 5 Characteristics on radiotherapy to chest wall with or without regional lymph node in previous three reports and the present study.
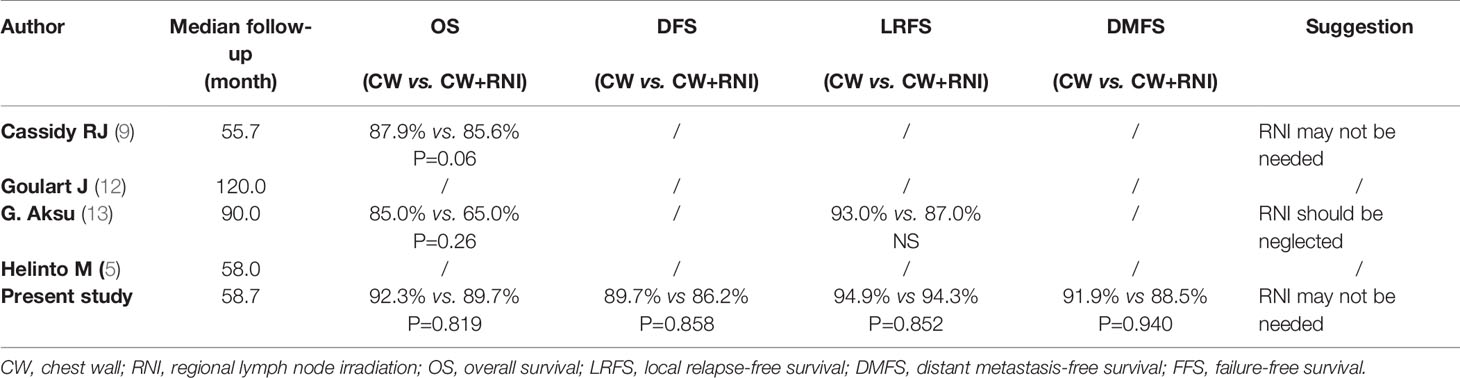
Table 6 Survival radiotherapy to chest wall with or without regional lymph node in previous three reports and the present study.
Taken together, our results are consistent with previous results. We also analyzed the distant metastasis and disease-free survival rate. The results showed that there was no significant difference in distant metastasis and disease-free survival rate between chest wall + regional lymph node irradiation and chest wall radiation only. Therefore, for T3-4N0M0 patients, we do not recommend radiation of regional lymph nodes. Our research also indicates that advance T stage and grade were poor prognostic factors for OS, DMFS, LRFS, and DFS. Will advance T stage and grade profit from radiation of regional lymph nodes? Because of the small sample size in this study, we did not carry out further subgroup analysis.
The limitations of the study are that it is a retrospective study and the follow-up time was not long enough. In addition, the scale of cases is relatively small because T3-4N0M0 is a rare case, making large-scale case research is difficult to achieve.
Both our study and previous studies suggested that regional lymph nodes radiation cannot improve the survival rate for breast cancer patients with T3-4N0M0 in a non-Caucasian population. We suggest that regional lymph nodes radiation might be neglected. Advance T stage and grade were the dependent poor prognostic factors for T3-4N0M0 patients.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Hainan General Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
SY designed and edited the manuscript of the study. LG and SY collected and interpreted the data, and drafted the manuscript. JY and HW carried out statistical analysis and critically revised the manuscript. JNC, BC, JWC, and XL were involved in study design, statistical analysis, data interpretation. All authors contributed to the article and approved the submitted version.
This work was supported by Natural Science Foundation of Hainan Province, China (819QN345) and (818QN314).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are very grateful to Shiling Moody, who helped us in improving the English writing.
LN, lymph node; RT, radiotherapy; ER, estrogen receptor; PR, progesterone receptor; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; LVI, lymphovascular invasion; OS, overall survival; LRFS, local relapse-free survival; DMFS, distant metastasis-free survival; DFS, disease-free survival; PSM, propensity score matching.
1. Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol (2010) 17(6):1471–4. doi: 10.1245/s10434-010-0985-4
2. Carter CL, Allen C, Henson DE. Relation of Tumor Size, Lymph Node Status, and Survival in 24,740 Breast Cancer Cases. Cancer (1989) 63:181–7. doi: 10.1002/1097-0142(19890101)63:1<181::AID-CNCR2820630129>3.0.CO;2-H
3. Taghian AG, Jeong J-H, Mamounas EP, Parda DS, Deutsch M, Costantino JP, et al. Low Locoregional Recurrence Rate Among Node-Negative Breast Cancer Patients With Tumors 5 Cm or Larger Treated by Mastectomy, With or Without Adjuvant Systemic Therapy and Without Radiotherapy: Results From Five National Surgical Adjuvant Breast and Bowel Project Randomized Clinical Trials. J Clin Oncol (2006) 24:3927–32. doi: 10.1200/JCO.2006.06.9054
4. Floyd SR, Buchholz TA, Haffty BG, Goldberg S, Niemierko A, Raad RA, et al. Low Local Recurrence Rate Without Postmastectomy Radiation in Node-Negative Breast Cancer Patients With Tumors 5 Cm and Larger. Int J Radiat Oncol Biol Phys (2006) 66:358–64. doi: 10.1016/j.ijrobp.2006.05.001
5. Helintö M, Blomqvist C, Heikkilä P, Joensuu H. Post-Mastectomy Radiotherapy in pT3N0M0 Breast Cancer: Is it Needed? Radiother Oncol (1999) 52:213–7. doi: 10.1016/S0167-8140(99)00099-7
6. Katz A, Strom EA, Buchholz TA, Thames HD, Smith CD, Jhingran A, et al. Locoregional Recurrence Patterns After Mastectomy and Doxorubicin-Based Chemotherapy: Implications for Postoperative Irradiation. J Clin Oncol (2000) 18:2817–27. doi: 10.1200/JCO.2000.18.15.2817
7. Wallgren A, Bonetti M, Gelber RD, Goldhirsch A, Castiglione-Gertsch M, Holmberg SB, et al. Risk Factors for Locoregional Recurrence Among Breast Cancer Patients: Results From International Breast Cancer Study Group Trials I Through VII. J Clin Oncol (2003) 21:1205–13. doi: 10.1200/JCO.2003.03.130
8. Trudeau ME, Pritchard KI, Chapman J-AW, Hanna WM, Kahn HJ, Murray D, et al. Prognostic Factors Affecting the Natural History of Node-Negative Breast Cancer. Breast Cancer Res Treat (2005) 89:35–45. doi: 10.1007/s10549-004-1368-y
9. Cassidy RJ, Liu Y, Kahn ST, Jegadeesh NK, Liu X, Subhedar PD, et al. The Role of Postmastectomy Radiotherapy in Women With Pathologic T3N0M0 Breast Cancer. Cancer (2017) 123(15):2829–39. doi: 10.1002/cncr.30675
10. Goetz MP, Gradishar WJ, Anderson BO, Abraham J, Aft R, Allison KH, et al. NCCN Guidelines Insights: Breast Cancer, Version 3.2018. J Natl Compr Canc Netw (2019) 17(2):118–26. doi: 10.6004/jnccn.2019.0102
11. Taylor ME, Haffty BG, Rabinovitch R, Arthur DW, Halberg FE, Strom EA, et al. ACR Appropriateness Criteria on Postmastectomy Radiotherapy Expert Panel on Radiation Oncology-Breast. Int J Radiat Oncol Biol Phys (2009) 73:997–1002. doi: 10.1016/j.ijrobp.2008.10.080
12. Goulart J, Truong P, Woods R, Speers CH, Kennecke H, Nichol A. Outcomes of Node-Negative Breast Cancer 5 Centimeters and Larger Treated With and Without Postmastectomy Radiotherapy. Int J Radiat Oncol Biol Phys (2011) 80(3):758–64. doi: 10.1016/j.ijrobp.2010.02.014
13. Aksu G, Kucucuk S, Fayda M, Saynak M, Baskaya S, Saip P, et al. The Role of Postoperative Radiotherapy in Node Negative Breast Cancer Patients With pT3-T4 Disease. Eur J Surg Oncol (2007) 33(3):285–93. doi: 10.1016/j.ejso.2006.10.037
14. Bhatnagar AK, Brandner E, Sonnik D, Wu A, Kalnicki S, Deutsch M, et al. Intensity-Modulated Radiation Therapy (IMRT) Reduces the Dose to the Contralateral Breast When Compared to Conventional Tangential Fields for Primary Breast Irradiation: Initial Report. Cancer J (2004) 10(6):381–5. doi: 10.1097/00130404-200411000-00008
15. Bhatnagar AK, Brandner E, Sonnik D, Wu A, Kalnicki S, Deutsch M, et al. Intensity Modulated Radiation Therapy (IMRT) Reduces the Dose to the Contralateral Breast When Compared to Conventional Tangential Fields for Primary Breast Irradiation. Breast Cancer Res Treat (2006) 96(1):41–6. doi: 10.1007/s10549-005-9032-8
16. Choi KH, Ahn SJ, Jeong JU, Yu M, Kim JH, Jeong BK, et al. Postoperative Radiotherapy With Intensity-Modulated Radiation Therapy Versus 3-Dimensional Conformal Radiotherapy in Early Breast Cancer: A Randomized Clinical Trial of KROG 15-03. Radiother Oncol (2021) 154:179–86. doi: 10.1016/j.radonc.2020.09.043
Keywords: breast cancer, T3~4N0M0, regional lymph node, radiotherapy, survival
Citation: Li G, Yao J, Chen J, Cai B, Lin X, Chen Z, Chen J, Wang H and Yang S (2021) The Survival Effect of Chest Wall With or Without Regional Lymphatic Radiotherapy for Breast Cancer Patients With T3~4N0M0. Front. Oncol. 11:653831. doi: 10.3389/fonc.2021.653831
Received: 15 January 2021; Accepted: 27 May 2021;
Published: 12 July 2021.
Edited by:
Ira Ida Skvortsova, Innsbruck Medical University, AustriaReviewed by:
Wan Xiangbo, The Sixth Affiliated Hospital of Sun Yat-Sen University, ChinaCopyright © 2021 Li, Yao, Chen, Cai, Lin, Chen, Chen, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiping Yang, c2hpcGluZ3lhbmcxOTgyQHNpbmEuY29t; Han Wang, d2FuZ2hhbjI4MDFAc29odS5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.