- 1Department of Oncology, University of Turin, Turin, Italy
- 2Pathology Unit, Department of Medical Sciences, University of Turin, Turin, Italy
- 3Department of Surgical Sciences, Gynecology and Obstetrics 1, University of Turin, Turin, Italy
- 4Department of Public Health and Paediatric Sciences, University of Turin, Turin, Italy
- 5Plastic Surgery Unit, Department of General and Specialistic Surgery, Città della Salute e della Scienza Hospital, Turin, Italy
- 6Pathology Division, Candiolo Cancer Institute, FPO-IRCCS, Candiolo, Italy
Background: Tubular carcinoma (TC) is a low proliferative grade 1 (G1) breast cancer (BC). Despite its favorable outcome and allegedly lower aggressiveness, patients are treated like other luminal G1 BC, with radiotherapy (RT) and hormonal therapy (HT). We performed: (1) a retrospective study comparing a TC cohort and a control series of luminal G1 BC and (2) a systematic review and meta-analysis focused on TC outcome.
Materials and Methods: We selected a series of 572 G1 luminal BC patients [111 TC, 350 not otherwise specified (NOS), and 111 special-type (ST) BC] with follow-up and clinico-pathological data, who underwent local excision followed by RT at Città della Salute e della Scienza Hospital, Turin. Moreover, 22 and 13 studies were included in qualitative and quantitative meta-analysis, respectively.
Results: TCs were generally smaller (≤10 mm) (P < 0.001), with lower lymph node involvement (P < 0.001). TCs showed no local and/or distant recurrences, while 16 NOS and 2 ST relapsed (P = 0.036). Kaplan–Meier curves confirmed more favorable TC outcome (DFI: log-rank test P = 0.03). Meta-analysis data, including the results of our study, showed that the pooled DFI rate was 96.4 and 91.8% at 5 and 10 years, respectively. Meta-regression analyses did not show a significant influence of RT nor HT on the DFI at 10 years.
Conclusions: Compared to the other G1 BCs, TCs have an excellent outcome. The meta-analysis shows that TC recurrences are infrequent, and HT and RT have limited influence on prognosis. Hence, accurate diagnosis of TC subtype is critical to ensuring a tailored treatment approach.
Introduction
The latest 2019 WHO edition categorizes breast cancers (BCs) in numerous entities (1) based on their specific histopathological characteristics.
Although these categories are associated with distinct clinical and prognostic implications, patients' management is mostly based on the evaluation of a well-defined set of markers, such as estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki67, and the real meaning of the morphological model is often overlooked.
Tubular carcinoma (TC) is classified as “special-type” cancer and accounts for ~1–4% of all invasive BCs (2–4). TC represents a suitable example of an invasive tumor with an excellent outcome, but it is still treated like other BC with the same immune-phenotypic profile.
TC is a low proliferative grade 1 (G1) BC [according to Elston-Ellis classification (5)] belonging to the luminal A category, showing high levels of ER and PGR and lack of HER2.
Actually, several retrospective studies (6–9) reported that, among luminal G1 BCs, patients affected by TC have a significantly better prognosis, with a long-term outcome similar to that of age-matched women without BC (1).
In line with these findings, the guidelines of the Italian Association of Medical Oncology (2019) suggest avoiding systemic treatment after the diagnosis of a <1 cm TC. However, despite this recommendation, the clinical management of these lesions is often debated within the multidisciplinary tumor boards. Generally, in patients treated by conservative surgery, the management still remains radiotherapy (RT) plus 5 years of hormonal therapy (HT), like other luminal G1 BCs. Thus, a perception of an overtreatment is commonly acknowledged.
To address these issues, two types of analyses were performed: (1) a retrospective study comparing a cohort of patients affected by TC and a control series of luminal G1 BC, in order to assess possible clinico-pathological and prognostic differences and (2) a systematic review and meta-analysis focused on TC and its outcome.
Materials and Methods
Retrospective Study
Case Series
We selected 586 G1 luminal BC cases, from a retrospective series of BC patients who underwent conservative surgery at the Città della Salute e della Scienza Hospital, Turin, from January 1998 to December 2010. All patients received wide local excision followed by RT, while adjuvant systemic treatment was administrated based on patients' characteristics. The study was approved by the Research Ethics Committee for Human Biospecimen Utilization (Department of Medical Sciences—ChBU) of the University of Turin (n°9/2019). Written consent was not required considering the retrospective nature of the study. The study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). All cases were de-identified, and all clinical-pathological data were accessed anonymously.
Patients with multifocal disease who underwent mastectomy and those with luminal HER2-positive BC were excluded, due to different treatment protocols.
Data regarding age at diagnosis, tumor size, vascular invasion, lymph node involvement, ER, PR, Ki-67, and treatment information were obtained from clinical charts and pathological reports. Follow-up data, including presence of local or distant recurrence, contralateral disease, death of disease (DOD), and death for other causes (DOC) were collected.
A cutoff value was set at 1% for ER and PR positivity (10) and at 20% for Ki67 proliferation index (11).
Histological revision was performed according to WHO criteria by two of the authors (IC and JM). In particular, strict rules were applied to render the diagnosis of TC, based on (i) haphazard distribution of rounded and angulated tubules with open lumina in more than 90% of tumor tissue; (ii) a single layer of epithelial cells without significant atypia; and (iii) presence of abundant desmoplastic or fibro-elastotic stroma around the tubules (Figure 1).
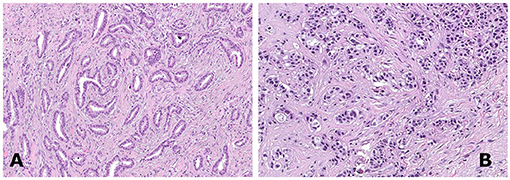
Figure 1. A case of tubular breast carcinoma composed of well-differentiated rounded to angulated tubular structures organized haphazardly [(A), hematoxylin and eosin staining, 150×]. A case of invasive ductal carcinoma not otherwise specified (NOS), grade 1, demonstrating tumor cells arranged in clusters [(B), hematoxylin and eosin staining, 150×].
Upon histological revision, we confirmed 572 cases as G1 luminal carcinomas, 111 pure TCs (19.5%), 350 (60%) non-special-type infiltrative carcinomas (NOS), and 111 (19.5%) special-type (ST) carcinomas, such as lobular, micropapillary, papillary, and cribriform BC.
Statistical Analysis
Patients' characteristics were compared using the Chi-square test for categorical variables and the T-test or ANOVA test for continuous variables, according to Bonferroni correction. The disease-free interval (DFI) was calculated from the date of surgical excision of the primary tumor to the date of the first relapse or last follow-up. Cases lost to follow-up were censored at the last visit time. Disease-specific survival (DSS) was calculated from the surgical excision date of the primary tumor to the date of breast cancer death. Survival distribution curves were plotted using the Kaplan–Meier method and compared with the log-rank test. Cox regression analyses were carried out on DFI to calculate crude and adjusted HRs and 95% CIs for the different groups. Local recurrence was defined as a tumor arising in the operated breast or in the axillary lymph nodes. The proportional hazard assumption was assessed with the Schoenfeld residuals, and this did not give reasons to suspect violation of this assumption. Cutoff values for the analyzed variables were set according to literature reports and/or the results of the log-likelihood ratio test. All statistical tests were two-sided, and p < 0.05 were considered significant. Statistical analyses were performed using Stata/SE13.0 Statistical Software (STATA, College Station, TX).
Systematic Review and Meta-Analysis
Search Strategy and Literature Selection
A systematic literature review was performed using online electronic databases (PubMed and SCOPUS) for published papers until 1st March 2018. Search was performed using the following keywords: “tubular AND (breast OR mammary) AND (carcinoma OR carcinomas OR cancer OR cancers OR tumor OR tumors OR tumors OR tumor OR neoplasia OR neoplasm) AND (prognosis OR survival OR mortality OR relapse OR relapses).”
Considering only studies on breast TC, the following criteria were applied to select articles for further examination: (i) papers written in English language, (ii) studies conducted on humans, (iii) retrospective and prospective comparative studies and randomized controlled trials, and (iv) studies considering DFI as survival outcome.
Studies lacking the above-mentioned criteria were excluded from further investigation. When results could be mathematically combined, studies were also included in the meta-analysis.
The systematic review and meta-analysis were conducted and reported in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (12).
Data Extraction
Data such as year of publication, study design, sample size, patients' characteristics including median age at diagnosis, median tumor diameter, presence of lymph node metastasis, type of surgery (mastectomy vs. conservative approach), type of adjuvant treatment (hormonal therapy, chemotherapy, radiotherapy), and 5/10-year DFI rates were independently extracted from the included studies by two investigators (AB and JM).
Statistical Analysis
Meta-analyses were performed to estimate the pooled 5/10-year DFI rates among women with TC. Meta-regression analyses were then carried out to perform a pooled analysis of factors affecting DFI rates, such as RT and HT. The Cochran Q and I2 were used to evaluate heterogeneity between the studies. To tackle potential sources of heterogeneity, the random-effect model was used to combine studies if heterogeneity was identified (Cochran Q p < 0.10 and I2 > 50%). The probability of publication bias was evaluated through the Egger's regression test and expressed by Funnel plots. Statistical analyses were performed using Comprehensive Meta-Analysis (CMA) software, version 3, Biostat Inc., Englewood, NJ, USA.
Results
Retrospective Study
The clinical-pathological characteristics of our case series are reported in Table 1.
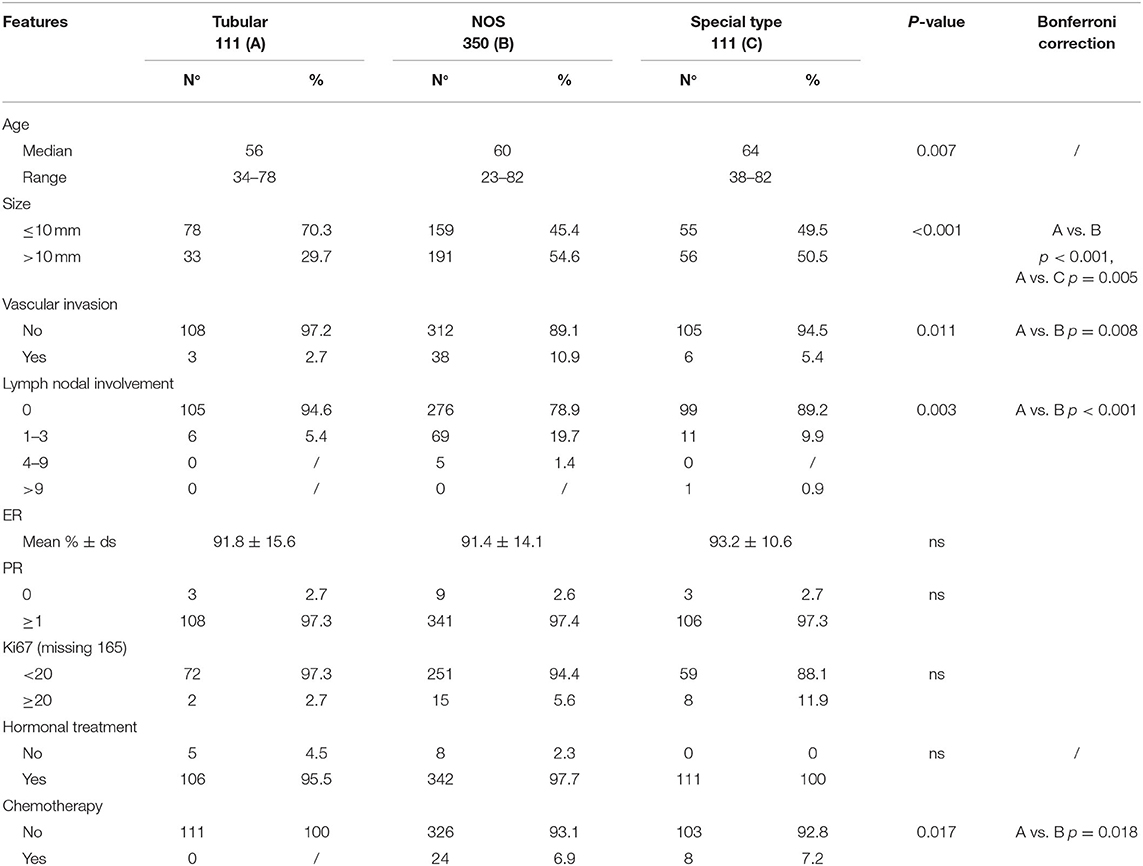
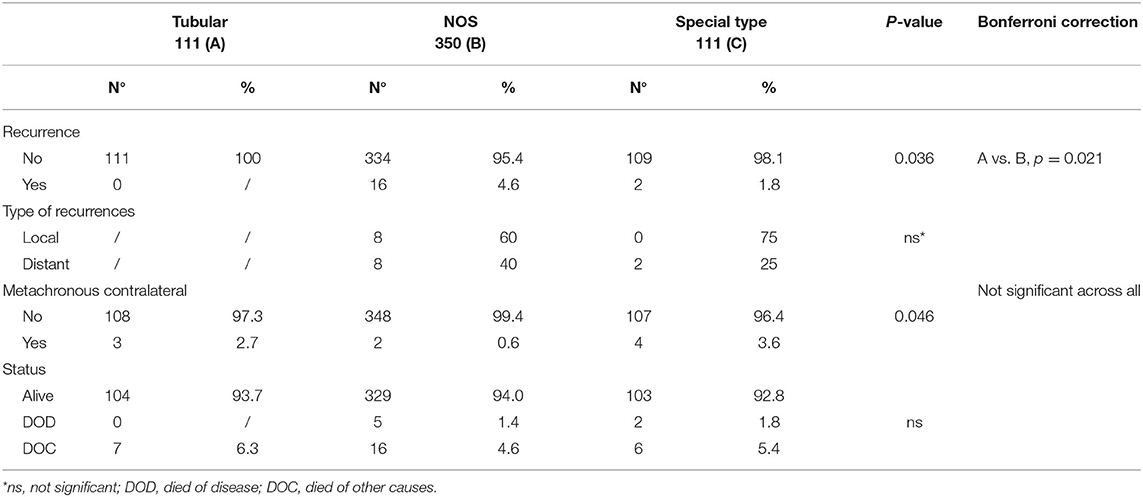
Table 1. Clinical and pathological characteristics and outcome of the whole case series composed by G1 breast cancer patients, stratified according to histotype.
Tumor size was the main observed difference between TC and other G1 histotypes. Specifically, 70% of TC vs. 45 and 49.5% of G1-NOS and G1-ST, respectively, measured 10 mm or less (P < 0.001). Moreover, only 5% of TC cases showed lymph node involvement against 21% of G1-NOS and 11% of G1-ST (P < 0.001). In line with this, the presence of vascular invasion resulted to be different between TCs (3/111), G1-NOS (38/350), and G1-ST (6/111) (P = 0.011). Furthermore, the treatment approach varied between the groups: 7% of both G1-NOS and G1-ST patients received chemotherapy differently from TC cases which were treated with HT alone.
After a median follow-up of 9.3 years (7.2–11.1 years), we did not observe any recurrences (either local or distant) within the TCs, while we registered 16 events (eight local and eight distant recurrences) in G1 NOS BCs and 2 distant recurrences in G1 ST tumors (P = 0.036) (Table 2).
In three patients with TC (measuring 15, 11, and 9 mm, respectively) contralateral BCs (two NOS and one lobular invasive carcinoma) were diagnosed. At follow-up, no patients with TC died of disease, while five patients with G1-NOS and two with G1-ST carcinomas were registered in the DOD category (Table 2).
Kaplan–Meier curves (Figure 2) demonstrate that TC is associated with longer DFI (log-rank test P = 0.03) compared to the other histotypes. However, no statistical differences were observed regarding DSS analyses (log-rank test P = 0.38) (Figure 3). Univariate logistic regression analysis of DFI and DSS comparing TC and the other G1 histotypes did not reach statistical significance due to the low number of events (data not shown).
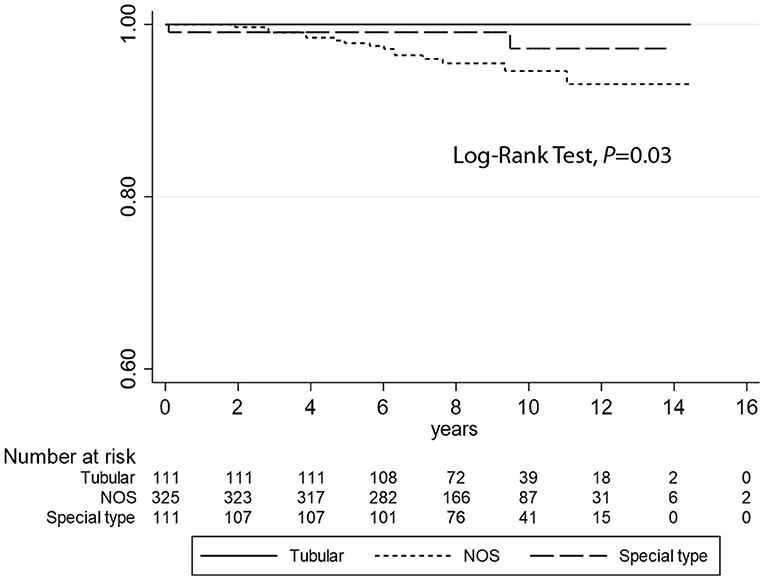
Figure 2. Kaplan–Meier estimates of DFI (log-rank test, P = 0.03) comparing tubular carcinomas with the other histotypes (not otherwise specified and special type breast cancers).
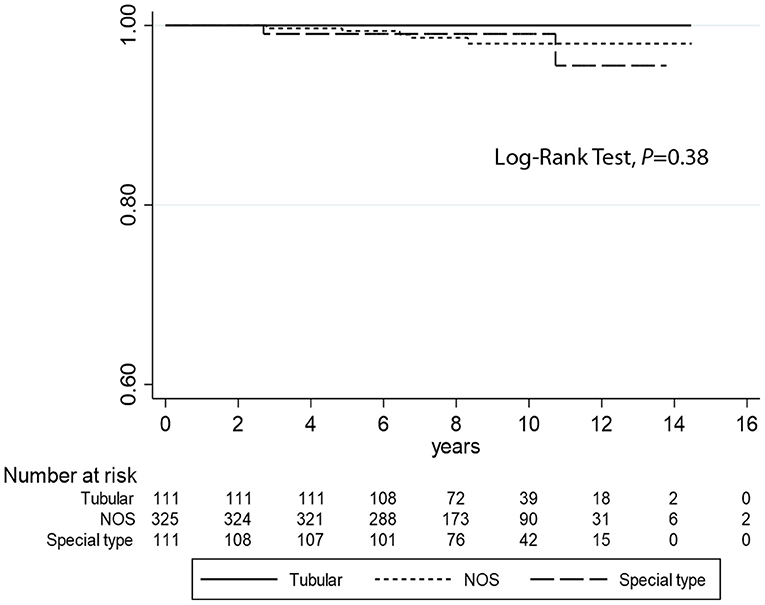
Figure 3. Kaplan–Meier estimates of DSS (log-rank test, P = 0.38) comparing tubular carcinomas with the other histotypes (not otherwise specified and special type breast cancers).
Systematic Review
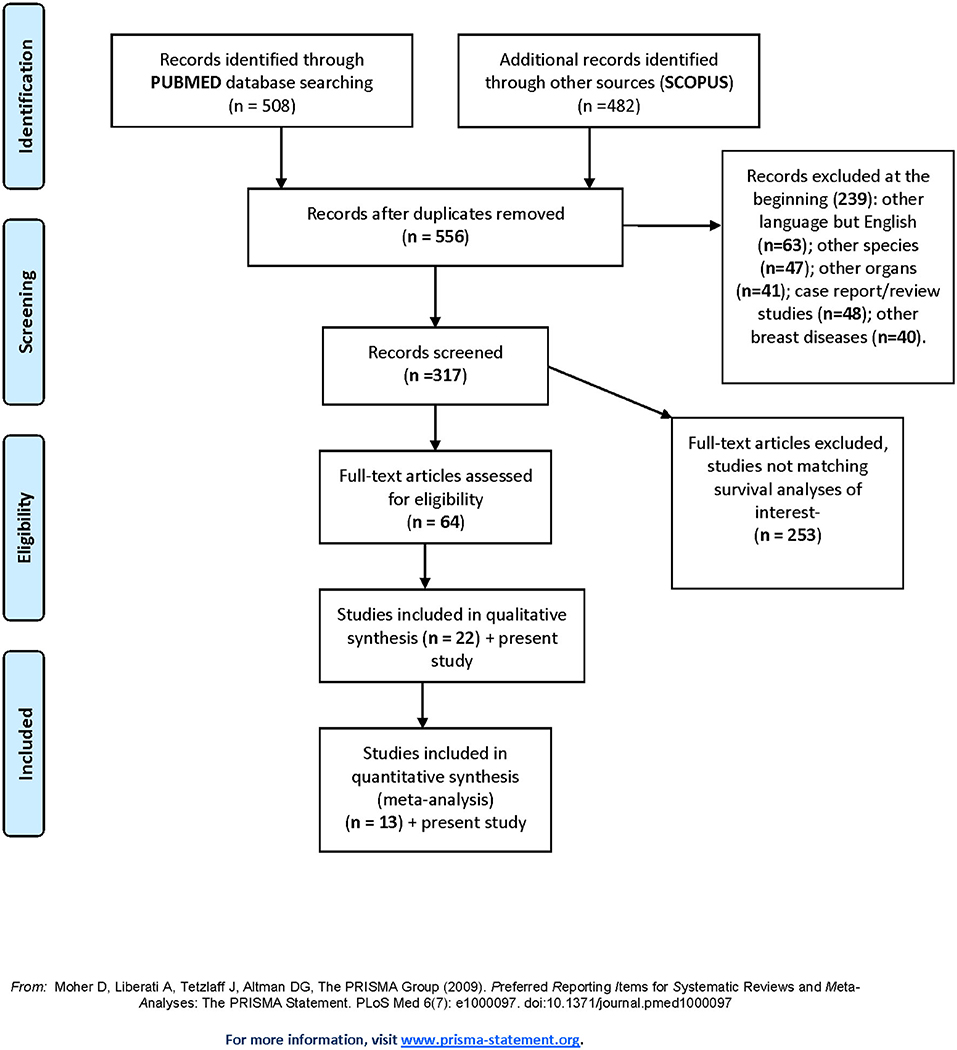
The results of the literature search and study selection process are summarized in PRISMA flow diagram (Figure 4).
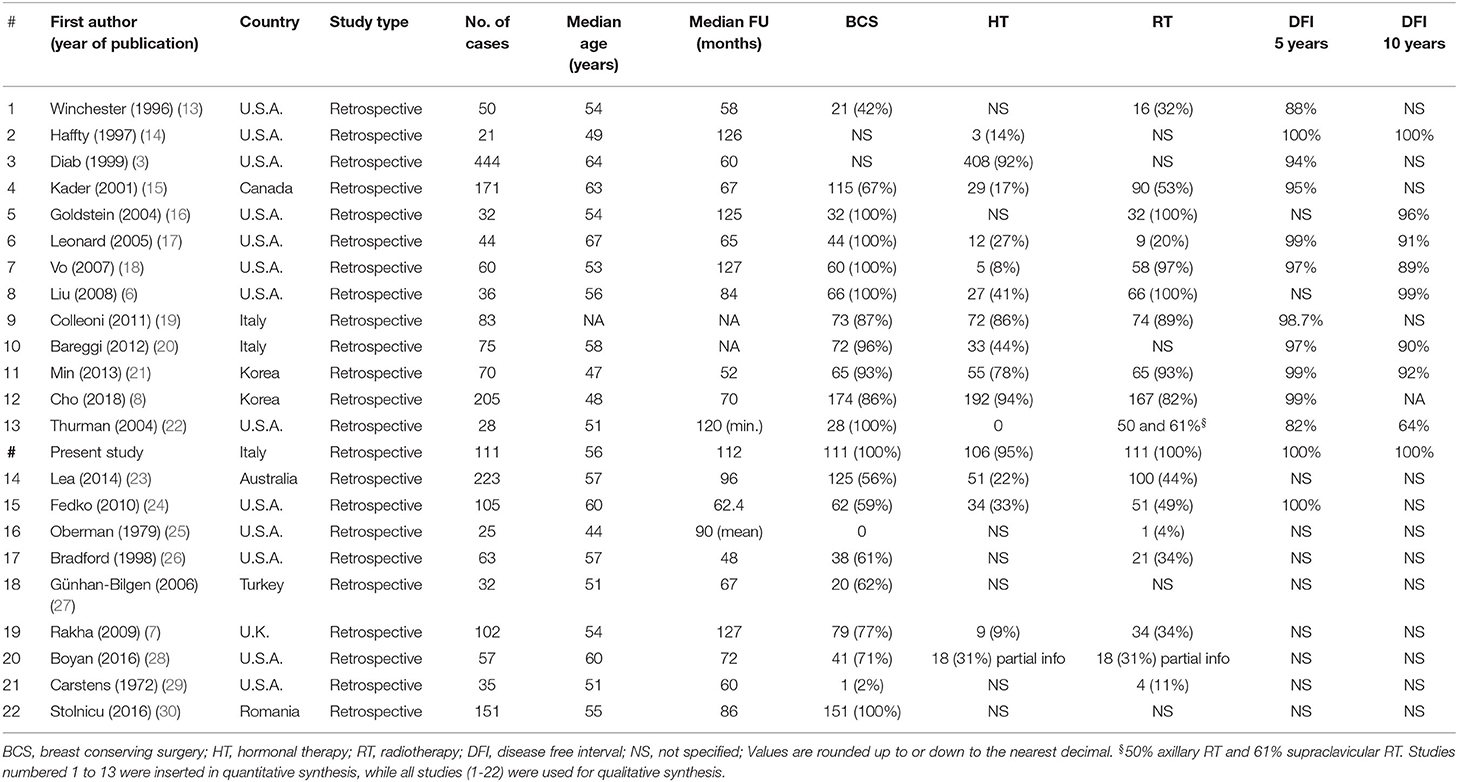
A total of 556 titles and abstracts were obtained by electronic searches. Of these, 239 articles did not match the inclusion criteria described in the research strategy. Furthermore, 253 studies did not report the required survival analyses. Finally, 64 full-text articles were considered relevant and examined in detail. Following this comprehensive review, 22 studies were included in qualitative and 13 in quantitative synthesis for a total of 1,430 patients with TC (including our retrospective study). All the selected studies were retrospectively conducted. Most of the patients (1,378/1,430, 96.3%) were treated with conservative surgery, and ~70% of them also received adjuvant RT. The major features of the selected studies are summarized in Table 3. The present retrospective study was also included in the quantitative and qualitative analyses (Figure 4, Table 3).
Meta-Analyses
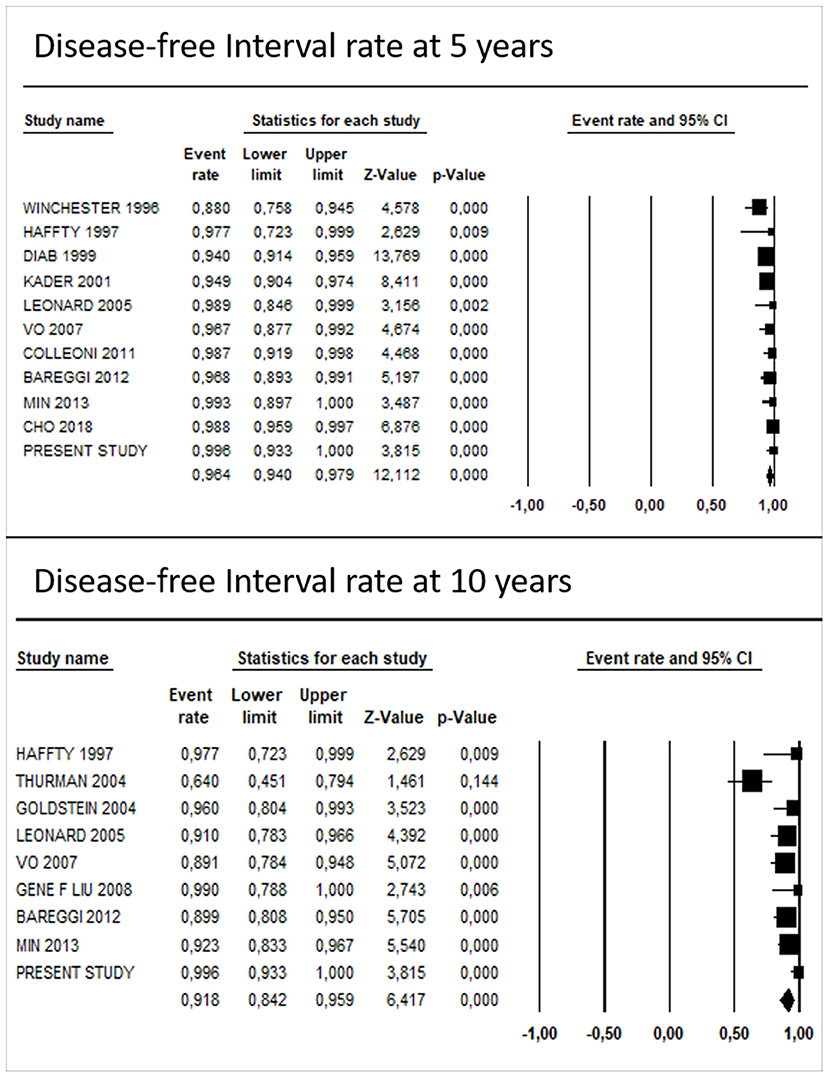
The pooled DFI rate of TC was 96.1% (95% CI: 93.6–97.6%) at 5 years and 90.1% (95% CI: 82.1–94.8%) at 10 years. Egger's regression test showed no significant evidence for funnel plot asymmetry at 10 years (P = 0.11), while at 5 years it showed a potential publication bias (P = 0.03).
After including the results of our study in the analyses, the pooled survival rate was 96.4% (95% CI: 94.0–97.9%) and 91.8% (95% CI: 84.2–95.9%) at 5 and 10 years, respectively (Figure 5). The results of Egger's regression test showed a potential publication bias, both at 5 years (P = 0.01) and 10 years (P = 0.04).
Meta-Regression Analyses
Meta-regression analyses did not show a significant influence of RT on the DFI rates of TC at 10 years, after adjusting for median age and presence of lymph node metastasis, [1.78 (95% CI: −2.16; 5.72) and 2.00, (95% CI: −1.79; 5.79)] before and after including results of the present study, respectively. Also, HT did not show a significant association with the DFI rates [0.81 (95% CI: −0.63; 2.26) and 0.94 (95% CI: −0.48; 2.37)] (Table 4).
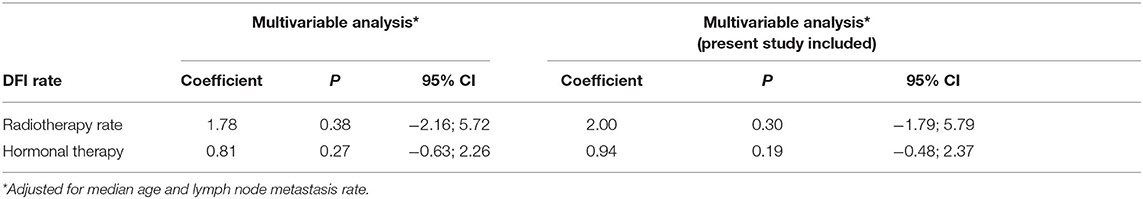
Table 4. Multivariable meta-regression analysis: potential influence of radiotherapy and hormonal therapy on disease-free survival rates of tubular carcinoma at 10 years.
Discussion
TC is special-type invasive BC, characterized by a well-differentiated histology and an excellent outcome. The diagnosis of TC is rendered only in case of tubule formation being demonstrated in 90% of the tumor. To date, generally, patients with TC undergo both HT and RT, like other luminal BCs. Despite a perception of an overtreatment, the proposed de-escalation of TC management is still to be translated to the daily practice. This is mainly due to different clinical experience and presence of heterogeneous literature data, including small case series, different clinical approaches, different follow-up lengths, and lack of strict histological review.
To tackle this open issue, we performed a retrospective analysis of our large institutional series of G1 BC focusing on TC outcome and we comprehensively analyzed the available data through a systematic review of literature and meta-analysis.
Our results confirmed the small size (generally <10 mm) of TC and their excellent outcome, in line with literature data (3, 31). Moreover, in our case series no local/distant recurrences or DOD were observed.
Actually, compared to invasive ductal carcinoma, TC is more likely to be detected on mammographic screening (7), mainly due to its typical dense fibro-elastotic stroma that is promptly visualized on mammography (32).
Considering that TC has limited impact on outcome (3, 8, 18, 33–36), several groups hypothesized that its detection could represent an example of overdiagnosis (37). However, Aulmann et al. (38) provided the molecular evidence for a direct clonal relationship of flat epithelial atypia and low-grade ductal carcinoma in situ with TC, indicating their precursor role. Furthermore, it is important to note that many NOS BC have a tubular component suggesting that TCs could de-differentiate into more aggressive cancers if left unresected. Moreover, almost 5% of TC patients show metastatic disease in axillary lymph nodes (24, 39) as also demonstrated in our study; thus, they seem to harbor an intrinsic malignant potential All these data confirm the importance of surgical treatment (37). On the other hand, even in the presence of lymph node involvement, TC showed no consequences on outcome, suggesting that omission of axillary surgery, even after metastatic sentinel lymph node, may be a valid choice (40, 41).
We would like to emphasize the importance of distinguishing TC from other similar BC histotypes, following strict morphological criteria. In fact, despite their histopathological similarity with G1 NOS BC, TCs differed in terms of size, lymph nodal status, and angioinvasion, thus supporting their true biological peculiarity.
Concerning adjuvant treatments, despite the more common use of chemotherapy, patients with both G1-NOS and ST BCs recurred in ~5 and 2% of cases, respectively, unlike TC which had a more favorable outcome in terms of DFI (log rank test P = 0.03).
These results are in agreement with the comprehensive systematic review and meta-analysis here performed which included 22 studies and data of 1,430 patients. The pooled 5- and 10-year DFI rates (96.4 and 92%, respectively) support the extremely favorable outcome of TC. Moreover, our multivariable meta-regression results suggest that neither HT (P = 0.27) nor RT (P = 0.38) has a significant association with DFI, after adjusting for median age and presence of lymph node metastases.
In line with our data (3, 7, 42), some studies demonstrated that the survival of patients with TCs after conserving surgery and RT is similar to the general population, suggesting that these patients may be safely spared the side effects and costs of HT (43, 44).
On the other hand, TC is associated with an increased number of contralateral disease (7, 8, 13, 27). In our series, we found 3/111 cases with metachronous contralateral tumor; all of them measured 10 mm or more.
These data suggest that HT, as proposed by official guidelines [AIOM (45) and NCCN (46)], may still have a role in TC with larger diameter, although it may be omitted in TC sized <1 cm.
Regarding RT, although a de-escalation has been proposed in low-risk BC patients (47), its employment in TC is a matter of debate. A recent work by Wu et al. (48) proposed omitting RT in patients aged ≥65 years, while in another study by Chen et al. (49) the authors suggested a potential benefit of RT in patients aged ≤ 50 years. In our series, since all the patients were treated with RT, it was not possible to evaluate its effect on prognosis. However, taking into consideration the complete absence of local relapses and that RT showed no significant impact on outcome by meta-regression analysis, its omission could be a possible choice, to be investigated in future studies.
In conclusion, our study confirms that patients with TC have an excellent outcome, superior to other G1 BCs. Moreover, to the best of our knowledge, this is the first meta-analysis aimed at specifically evaluating the characteristics and outcome of TC, showing that recurrences are infrequent and that both HT and RT have limited influence on prognosis. Thus, accurate histopathological diagnosis of the TC subtype is crucial to providing correct prognostic information, paving the way for treatment personalization.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
IC contributed to conception and design of the study. Data acquisition was performed by JM, AB, FB, and PF. JM and AB organized the database. Data analyses were performed by SO-A, MG, EO, and GS. Interpretation of the data was executed by IC, JM, SO-A, LB, MG, EO, and RS. Project supervision was offered by IC, PC, and AS. The first draft of the manuscript was written by IC, JM, SO-A, MG, and EO. All authors contributed to manuscript revision and read and approved the submitted version.
Funding
This study was supported in part by Rete Oncologica del Piemonte e della Valle d'Aosta and by Fondazione Ricerca Molinette onlus.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all members of Breast Unit of Città della Salute e della Scienza Hospital (Turin).
References
1. WHO Classification of Tumors Editorial Board. WHO Classification of the Tumors- 5th ed- Breast tumors. Lyon: International Agency for Research on Cancer (2019).
2. Anderson WF, Chu KC, Chang S, Sherman M. Comparison of age-specific incidence rate patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev. (2004) 13:1128–35.
3. Diab SG, Clark GM, Osborne CK, Libby A, Allred DC, Elledge RM. Tumor characteristics and clinical outcome of tubular and mucinous breast carcinomas. J Clin Oncol. (1999) 17:1442–8. doi: 10.1200/JCO.1999.17.5.1442
4. McBoyle MF, Razek HA, Carter JL, Helmer SD. Tubular carcinoma of the breast: an institutional review. Am Surg. (1997) 63:639–45.
5. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. (1991) 19:403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x
6. Liu GFF, Yang Q, Haffty BG, Motan MS. Clinical-pathologic features and long-term outcomes of tubular carcinoma of the breast compared with invasive ductal carcinoma treated with breast conservation therapy. Int J Radiat Oncol Biol Phys. (2009) 75:1304–8. doi: 10.1016/j.ijrobp.2008.12.070
7. Rakha EA, Lee AH, Evans AJ, Menon S, Assad NY, Hodi Z, et al. Tubular carcinoma of the breast: Further evidence to support its excellent prognosis. J Clin Oncol. (2010) 28:99–104. doi: 10.1200/JCO.2009.23.5051
8. ChoWK Choi DH, Lee J, Park W, Kim YB, Suh CO, et al. Comparison of failure patterns between tubular breast carcinoma and invasive ductal carcinoma (KROG 14–25). Breast. (2018) 38:165–70. doi: 10.1016/j.breast.2018.01.004
9. Romano AM, Wages NA, Smolkin M, Fortune KL, Atkins K, Dillon PM. Tubular carcinoma of the breast: institutional and SEER database analysis supporting a unique classification. Breast Dis. (2015) 35:103–11. doi: 10.3233/BD-140396
10. Burstein HJ, Curigliano G, Loibl S, Dubsky P, Gnant M, Poortmans P, et al. Estimating the benefits of therapy for early-stage breast cancer: The St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. (2019) 30:1541–57. doi: 10.1093/annonc/mdz235
11. Bustreo S, Osella-Abate S, Cassoni P, Donadio M, Airoldi M, Pedani F, et al. Optimal Ki67 cut-off for luminal breast cancer prognostic evaluation: a large case series study with a long-term follow-up. Breast Cancer Res Treat. (2016) 157:363–71. doi: 10.1007/s10549-016-3817-9
12. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
13. Winchester DJ, Sahin AA, Tucker SL, Singletary SE. Tubular carcinoma of the breast predicting axillary nodal metastases and recurrence. Ann Surg. (1996) 223:342–7. doi: 10.1097/00000658-199603000-00015
14. Haffty BG, Perrotta PL, Ward B, Moran M, Beinfielg M, McKhann C, et al. Conservatively treated breast cancer: outcome by histologyc subtype. Breast J. (1997) 3:7–14. doi: 10.1111/j.1524-4741.1997.tb00134.x
15. Kader HA, Jackons J, Mates D, Andersen S, Hayes M, Olivotto IA. Tubular carcinoma of the breast: a population-based study of nodal metastases at presentation and of patterns of relapse. Breast J. (2001) 7:8–13. doi: 10.1046/j.1524-4741.2001.007001008.x
16. Goldstein NS, Kestin LL, Vicini FA. Refined morphologic criteria for tubular carcinoma to retain its favorable outcome status in contemporary breast carcinoma patients. Am J Clin Pathol. (2004) 122:728–39. doi: 10.1309/9FEP8U8AUGQNGY3V
17. Leonard CE, Howell K, Shapiro H, Ponce J, Kercher J. Excision only for tubular carcinoma of the breast. Breast J. (2005) 11:129–33. doi: 10.1111/j.1075-122X.2005.21549.x
18. Vo T, Xing Y, Meric-Bernstam F, Mirza N, Vlastos G, Symmans WF, et al. Long-term outcomes in patients with mucinous, medullary, tubular, and invasive ductal carcinomas after lumpectomy. Am J Surg. (2007) 194:527–31. doi: 10.1016/j.amjsurg.2007.06.012
19. Colleoni M, Rotmensz N, Maisonneuve P, Mastropasqua MG, Luini A, Veronesi P, et al. Outcome of special types of luminal breast cancer. Ann Oncol. (2012) 23:1428–36. doi: 10.1093/annonc/mdr461
20. Bareggi CMR, Consonni D, Galassi B, Gambini D, Locatelli E, Visintin R, et al. Uncommon breast malignancies: presentation pattern, prognostic issue and treatment outcome in an Italian single institution experience. Tumori. (2013) 99:39–44. doi: 10.1177/030089161309900107
21. Min Y, Bae SY, Lee H, Lee JH, Kim M, Kim J, et al. Tubular carcinoma of the breast: clinicopathologic features and survival outcome compared with ductal carcinoma in situ. J Breast Cancer. (2013) 16:404–9. doi: 10.4048/jbc.2013.16.4.404
22. Thurman SA, Schnitt SJ, Connolly JL, Gelman R, Silver B, Harris JR, et al. Outcome after breast-conserving therapy for patients with stage I or II mucinous, medullary, or tubular breast carcinoma. Int J Radiat Oncol Biol Phys. (2004) 59:152–9. doi: 10.1016/j.ijrobp.2003.10.029
23. Lea V, Gluch L, Kennedy CW, Carlmalt H, Gillet D. Tubular carcinoma of the breast: axillary involvement and prognostic factors. ANZ J Surg. (2015) 85:448–51. doi: 10.1111/ans.12791
24. Fedko MG, Scow JS, Shash SS, Reynolds C, Degnim AC, Jakub JW, et al. Pure tubular carcinoma and axillary nodal metastases. Ann Surg Oncol. (2010) 17:338–42. doi: 10.1245/s10434-010-1254-2
25. Oberman HA, Fidler WJ. Tubular carcinoma of the breast. Am J Surg Pathol. (1979) 3:387–95. doi: 10.1097/00000478-197910000-00001
26. Bradford WZ, Christensen WN, Fraser H, Cloninger T. Treatment of pure tubular carcinoma of the breast. Breast J. (1998) 4:437–40. doi: 10.1046/j.1524-4741.1998.460437.x
27. Günhan-Bilgen I, Oktay A. Tubular carcinoma of the breast: mammographic, sonographic, clinical and pathologic findings. Eur J Radiol. (2007) 61:158–62. doi: 10.1016/j.ejrad.2006.08.021
28. Boyan W, Shea B, Farr M, Kohli M, Ginalis E. Tubular carcinoma of the breast: a single institution's experience of a favorable prognosis. Am Surg. (2016) 82:505–9. doi: 10.1177/000313481608200610
29. Carstens PH, Huvos AG, Foote F, Ashikari R. Tubular carcinoma of the breast: a clinicopathologic study of 35 cases. Am J Clin Pathol. (1972) 58:231–8. doi: 10.1093/ajcp/58.3.231
30. Stolnicu S, Moldovan C, Resetkova E. Even small pure tubular carcinoma of the breast (stage T1a and T1b) can be associated with lymph node metastases – The U T MD Anderson Cancer Center experience. Eur J Sur Oncol. (2016) 42:911–2. doi: 10.1016/j.ejso.2016.01.025
31. McDivitt RW, Boyce W, Gersell D. Tubular carcinoma of the breast. clinical and pathological observations concerning 135 cases. Am J Surg Pathol. (1982) 6:401–11. doi: 10.1097/00000478-198207000-00002
32. Porter PL, El-Bastawissi AY, Mandelson MT, Lin MG, Khalid N, Watney EA, et al. Breast tumor characteristics as predictors of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. (1999) 91:2020–8. doi: 10.1093/jnci/91.23.2020
33. Louwman MWJ, Vriezen M, Van Beek MW, Nolthenius-Puylaert MC, van der Sangen MJ, Roumen RM, et al. Uncommon breast tumors in perspective: incidence, treatment and survival in the Netherlands. Int J Cancer. (2007) 121:127–35. doi: 10.1002/ijc.22625
34. Li CI, Moe RE, Daling JR. Risk of mortality by histologic type of breast cancer among women aged 50 to 79 years. Arch Intern Med. (2003) 163:2149–53. doi: 10.1001/archinte.163.18.2149
35. Berg JW, Hutter RV. Breast cancer. Cancer. (1995) 75(Suppl. 1):257–269. doi: 10.1002/1097-0142(19950101)75:1+<257::AID-CNCR2820751311>3.0.CO;2-Y
36. Javid SH, Smith BL, Mayer E, Bellon J, Murphy CD, Lipsitz S, et al. Tubular carcinoma of the breast: results of a large contemporary series. Am J Surg. (2009) 197:674–7. doi: 10.1016/j.amjsurg.2008.05.005
37. Evans A, Vinnicombe S. Overdiagnosis in breast imaging. Breast. (2017) 31:270–3. doi: 10.1016/j.breast.2016.10.011
38. Aulmann S, Elsawaf Z, Penzel R, Schirmacher P, Sinn HP. Invasive tubular carcinoma of the breast frequently is clonally related to flat epithelial atypia and low-grade ductal carcinoma in situ. Am J Surg Pathol. (2009) 33:1646–53. doi: 10.1097/PAS.0b013e3181adfdcf
39. Chen SL, Zhang WW, Wang J, Sun JY, Wu SG, He ZY. The role of axillary lymph node dissection in tubular carcinoma of the breast: a population database study. Med Sci Monit. (2019) 25(Suppl. 3):880–7. doi: 10.12659/MSM.913077
40. Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis a randomized clinical trial. JAMA. (2011) 305:569–75. doi: 10.1001/jama.2011.90
41. Giuliano AE, Ballman K, McCall L, Beitsch P, Whitworth PW, Blumencranz P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American college of surgeons oncology group (Alliance) ACOSOG Z0011 randomized trial. Ann Surg. (2016) 264:413–9. doi: 10.1097/SLA.0000000000001863
42. Fritz P, Brendat K, Sonnenberg M, Trautmann C, Ott G, Heidemann E, et al. Tubular breast cancer. A retrospective study. Anticancer Res. (2014) 34:3647–56.
43. Mitchell NJ, Porter DJ. Does endocrine therapy in mucinous and tubular breast cancer improve outcome? J Clin Oncol. (2014) 32:566. doi: 10.1200/jco.2014.32.15_suppl.566
44. Ruhstaller T, Giobbie-Hurder A, Colleoni M, Jensen MB, Ejlertsen B, de Azambuja E, et al. Adjuvant letrozole and tamoxifen alone or sequentially for postmenopausal women with hormone receptor-positive breast cancer: long-term follow-up of the BiG 1-98 trial. J Clin Oncol. (2019) 37:105–14. doi: 10.1200/JCO.18.00440
47. Franco P, Iorio GC, Bartoncini S, Airoldi M, De Sanctis C, Castellano I, et al. De-escalation of breast radiotherapy after conserving surgery in low-risk early breast cancer patients. Med Oncol. (2018) 35:62. doi: 10.1007/s12032-018-1121-8
48. Wu SG, Zhang WW, Sun JY, Li FY, Chen YX, He ZY. Omission of postoperative radiotherapy in women aged 65 years or older with tubular carcinoma of the breast after breast-conserving surgery. Front Oncol. (2018) 8:190. doi: 10.3389/fonc.2018.00190
Keywords: tubular carcinoma, meta-analysis, prognosis, hormone treatment, radiotherapy
Citation: Metovic J, Bragoni A, Osella-Abate S, Borella F, Benedetto C, Gualano MR, Olivero E, Scaioli G, Siliquini R, Ferrando PM, Bertero L, Sapino A, Cassoni P and Castellano I (2021) Clinical Relevance of Tubular Breast Carcinoma: Large Retrospective Study and Meta-Analysis. Front. Oncol. 11:653388. doi: 10.3389/fonc.2021.653388
Received: 14 January 2021; Accepted: 19 February 2021;
Published: 29 April 2021.
Edited by:
John Vincent Kiluk, Moffitt Cancer Center, United StatesReviewed by:
Mauro Giuseppe Mastropasqua, University of Bari Medical School, ItalyPatrick Dillon, University of Virginia, United States
Copyright © 2021 Metovic, Bragoni, Osella-Abate, Borella, Benedetto, Gualano, Olivero, Scaioli, Siliquini, Ferrando, Bertero, Sapino, Cassoni and Castellano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabella Castellano, aXNhYmVsbGEuY2FzdGVsbGFub0B1bml0by5pdA==
 Jasna Metovic
Jasna Metovic Alberto Bragoni2
Alberto Bragoni2 Simona Osella-Abate
Simona Osella-Abate Fulvio Borella
Fulvio Borella Maria Rosaria Gualano
Maria Rosaria Gualano Elena Olivero
Elena Olivero Roberta Siliquini
Roberta Siliquini Luca Bertero
Luca Bertero Anna Sapino
Anna Sapino Paola Cassoni
Paola Cassoni Isabella Castellano
Isabella Castellano