- 1Department of Surgery, Loyola University Chicago Stritch School of Medicine, Maywood, IL, United States
- 2Department of Cancer Biology, Loyola University Chicago Stritch School of Medicine, Maywood, IL, United States
Hepatocellular carcinoma (HCC) is a highly lethal and complex malignancy strongly influenced by the surrounding tumor microenvironment. The HCC microenvironment comprises hepatic stellate cells (HSCs), tumor-associated macrophages (TAMs), stromal and endothelial cells, and the underlying extracellular matrix (ECM). Emerging evidence demonstrates that epigenetic regulation plays a crucial role in altering numerous components of the HCC tumor microenvironment. In this review, we summarize the current understanding of the mechanisms of epigenetic regulation of the microenvironment in HCC. We review recent studies demonstrating how specific epigenetic mechanisms (DNA methylation, histone regulation, and non-coding RNAs mediated regulation) in HSCs, TAMs, and ECM, and how they contribute to HCC development, so as to gain new insights into the treatment of HCC via regulating epigenetic regulation in the tumor microenvironment.
Introduction
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer death worldwide (1, 2). HCC patients' prognosis is low, with a 5-year survival of just 18% (2). For many years, the only FDA-approved systemic treatment option for advanced HCC was the multi-kinase inhibitor sorafenib, which itself provided only a modest increase in overall survival of 2.8 months compared to placebo (3). More recently, other therapeutics have garnered first-line treatment approval, including lenvatinib and atezolizumab combined with bevacizumab (4, 5). Second-line treatment options for relapsed and refractory HCC have also expanded in recent years to include regorafenib, cabozantinib, and the PD-1 checkpoint inhibitors nivolumab and pembrolizumab (6–9). While the arsenal of therapies for advanced HCC has expanded, all aforementioned agents are marked by low response rates and limited improvements in overall survival (4–9). Understanding the molecular mechanisms underlying HCC development are critical to identifying new drug candidates capable of providing enhanced clinical benefit.
In recent years, researchers have started to decipher the complicated crosstalk between the tumor and the surrounding tumor microenvironment (TME). The TME is composed of numerous different elements depending on the tumor type but generally includes surrounding vasculature, immune cells, fibroblasts, and the underlying extracellular matrix (ECM). TME has been repeatedly shown to significantly contribute to tumor initiation, progression, invasiveness, metastases formation, and angiogenesis (10–12). In the context of HCC, the TME primarily consists of hepatic stellate cells (HSCs), tumor-associated macrophages (TAMs), the ECM, mesenchymal stem/stromal cells (MSCs), myeloid-derived suppressor cells (MDSCs), and endothelial cells (ECs) with each element performing its unique role in HCC pathogenesis (13–21).
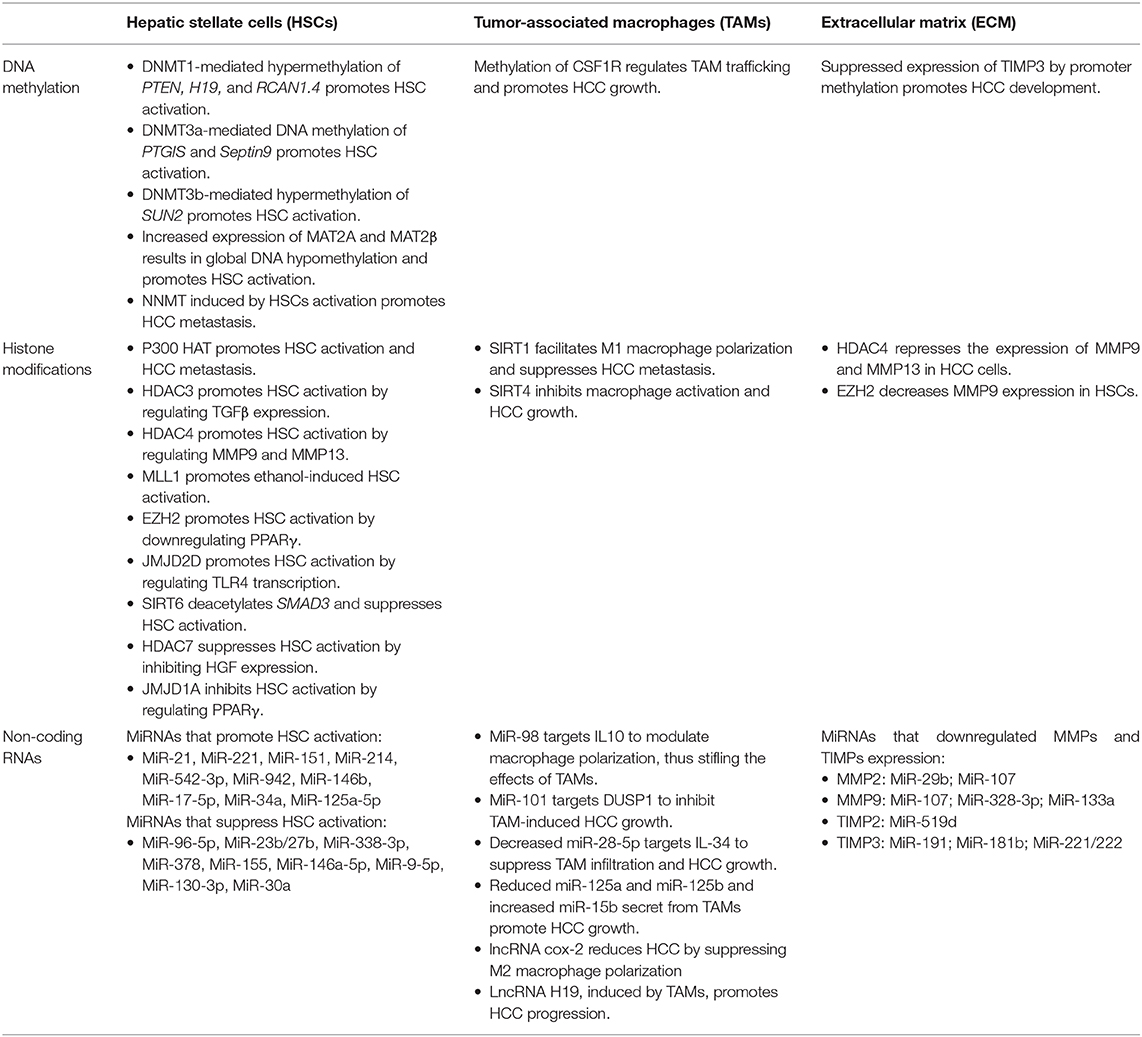
Epigenetics is the heritable modification of gene function without changes in the DNA sequence, which is mediated by a number of different factors, including but not limited to, DNA methylation, histone modifications, and non-coding RNAs (22, 23). Epigenetic regulation in tumor cells is extensively studied and plays critical roles in HCC development (24–26). Emerging evidence demonstrates that epigenetic alterations in TME also contribute to the initiation and progression of HCC (27–33). As the epigenetic regulation of MSCs, MDSCs, and ECs in HCC remain poorly defined, this review will focus on the epigenetics of HSCs, TAMs, and the ECM (Table 1).
Hepatic Stellate Cells
HSCs are resident perisinusoidal cells that contribute to diverse aspects of liver physiology, including hepatic organogenesis, regeneration, vitamin A storage, and wound healing (34). Under non-pathologic conditions, HSCs remain quiescent in the liver and are only activated in response to liver injury (35). In addition to serving as the primary source of ECM proteins, activated HSCs secrete a multitude of cytokines and growth factors that are required for fibrogenesis and promote HCC tumorigenesis (34–39). Namely, HSCs can modulate the ECM through secretion and upregulation of matrix metalloproteinases (MMPs), such as MMP2 and MMP9, both of which promote HCC tumor migration (40–43). Moreover, activated HSCs promote FAK-MMP9 signaling and invasiveness in HCC, highlighting the crosstalk between tumor cells and act ivated HSCs in the hepatic TME (44–46). The activation of HSCs is the result of extensive, but reversible, alterations in gene expression: activated HSCs can reclaim their quiescent state upon regression/resolution of liver damage in a dynamic process that is modulated extensively by epigenetic reprogramming (38, 47–49).
DNA Methylation in HSC Activation
DNA methylation influences HSC activation and activity. Activation of HSCs in vitro results in significant changes in DNA methylation and treatment with the DNA methylation inhibitor 5-aza-2'-deoxycytidine (5-azadC) block can block HSC transdifferentiation (50, 51). DNA methyltransferase 1 (DNMT1)-mediated hypermethylation of the Phosphatase and tensin homolog (PTEN) promoter leads to a loss of PTEN expression, subsequent activation of the PI3K/AKT and ERK pathways, and HSC activation (52). DNMT1 expression is increased in activated HSCs and rat liver fibrosis tissue, which leads to enhanced methylation of the lncRNA H19 promoter and elevates H19 expression and ERK activation. Treatment with 5'-aza-2'-deoxycytidine in activated HSCs model reduced fibrosis-associated gene expression as well as DNMT1 expression while simultaneously enhancing H19 expression and attenuated HSCs activation. Consistently, sennoside A can prevent liver fibrosis by binding DNMT1 and suppress DNMT1-mediated PTEN hypermethylation in HSC activation and proliferation (53).
Inhibition of DNA methyltransferase 3a (DNMT3a) causes activated HSCs to lose the fibrogenic phenotype in a carbon tetrachloride (CCl4)-induced liver fibrosis model (54). In addition to PTEN, DNA methylation of prostacyclin synthase (PTGIS) enhances HSC activation and liver fibrogenesis, with its methylation being induced by DNMT1 as well as DNMT3b, as determined by chromatin immunoprecipitation (ChIP) (55). DNMT3a has also been reported to regulate the methylation of Septin9 to promote hepatic stellate cell activation and liver fibrogenesis (56).
SAD1/UNC84 domain protein-2 (SUN2) gene hypermethylation at CpG sites has also been identified during liver fibrogenesis in mice with CCl4-induced hepatic fibrosis, accompanied by low expression of SUN2 (57). In vivo overexpression of SUN2 following adeno-associated virus-9 (AAV9) administration inhibited CCl4-induced liver injury and reduced fibrogenesis marker expression. Mechanistically, DNMT3b is the principal regulator of SUN2 expression, and inhibition of AKT phosphorylation may be a crucial pathway for SUN2-mediated HSC activation. S-adenosylmethionine suppresses the expression of Smad3/4 in activated human hepatic stellate cells via Rac1 promoter methylation (58). Methylation of RCAN1.4 is mediated by DNMT1 and DNMT3b and enhances HSC activation and liver fibrogenesis through Calcineurin/NFAT3 signaling (59).
Methionine adenosyltransferases (MATs) catalyze the biosynthesis of S-adenosylmethionine (SAMe), the principal methyl donor in DNA methylation. Methionine adenosyltransferase 2A and 2B (MAT2A and MAT2B), the sole regulators of the SAMe homeostasis in HSCs, are induced during in vitro and in vivo HSC activation. MATII enzyme activity and intracellular SAMe levels decline during HSC activation, accompanied by a global decrease in DNA methylation. MAT2A and MAT2B are induced during HSC activation and are essential for this process. The SAMe level falls, resulting in global DNA hypomethylation (60). Elevated nicotinamide N-methyltransferase (NNMT) expression induced by hepatic stellate cells promotes HCC metastasis by altering the histone H3 methylation and transcriptionally activating CD44 (61).
Histone Modification in HSC Activation
Histone modification also plays a critical in HSC activation. Acetylation and methylation are the two most extensively characterized histone modifications. Histone acetylation and methylation are carried out by two families of enzymes: histone acetyltransferases (HATs) and histone methyltransferases (HMTs). In opposition to these enzymes, histones are deacetylated and demethylated by histone deacetylases (HDACs) and histone demethylases (HDMTs), respectively. Both epigenetic marks can change quickly in response to intracellular and environmental cues and confer immense control of cellular responses (62). The p300 HAT is involved in stiffness-mediated HSC activation to promote liver tumor metastasis (63). Mechanistically, stiffness induces p300 nuclear accumulation, which, in turn, promotes the p300-dependent transcription of critical fibrogenic genes and several secreted profibrogenic and tumor-promoting factors. Furthermore, Sirtuin 6 (SIRT6), a NAD-dependent histone deacetylase which is a critical epigenetic regulator in alcoholic liver disease (64), deacetylates Smad family member 3 (Smad3) and attenuates its expression induced by transforming growth factor β (TGF-β) in the activated HSCs (65).
HSC activation and transdifferentiation are accompanied by gene expression changes in numerous HDACs. Notably, HDAC1, HDAC2, HDAC9, and HDAC10 were reported to be downregulated during HSC activation, while the expression of HDAC4, HDAC5, HDAC6, and HDAC8 is increased (66). MC1568, a class II selective HDAC4 and HDAC5 inhibitor, abrogates HSC activation and proliferation in vitro and in vivo (67). Inhibition of HDAC1, HDAC2, and HDAC4 by nilotinib is also known to induce HSC cell death (68). The mechanisms by which several of these HDACs regulate HSC activation and activity have been characterized in detail. For example, HDAC4 accumulates during HSC activation, and forced overexpression of HDAC4 in quiescent HSCs suppresses expression of the endopeptidases MMP9 and MMP13 resulting in ECM accumulation (66, 67, 69). Conversely, genetic knockdown of HDAC4 in activated HSCs promotes the expression of MMP9 and MMP13, thereby promoting ECM degradation. HDAC7 is also known to contribute to HSC activation by regulating the expression of hepatocyte growth factor (HGF), inhibiting HSC activation and liver fibrosis (70). The expression of HGF is reduced during the activation of HSCs, and knockdown of HDAC7 restores HGF levels to quiescent HSC levels (70). HDAC3 is required to activate HSCs by regulating TGF-β, which plays a crucial role in ECM formation and remodeling (71). Consistently, HDAC inhibitors such as SAHA suppress HSC activation and liver fibrosis by attenuating the TGF-β signaling pathway (72).
Besides HDACs, HMTs regulate gene expression during HSC activation. Ethanol exposure promotes HSC activation by inducing the expression of mixed lineage leukemia protein-1 (MLL1), a histone 3 lysine 4 (H3K4) methyltransferase. Following increased expression, MLL1 is recruited to the elastin gene promoter, where it is associated with increased H3K4me3 levels, increased ECM deposition, and HSC transdifferentiation (73). During HSC activation, several histone methyltransferases, such as ASH2, WDR5, and SET1, are recruited to the promoters of pro-fibrogenic genes in response to TGF-β treatment by myocardin-related transcription factor A (MRTF-A) (74). Enhancer of zeste homolog 2 (EZH2), the catalytic methyltransferase subunit of the polycomb repressive complex 2 (PCR2), is another HMT that regulates HSC activation and hepatic fibrosis. EZH2 expression is induced in HSCs upon TGF-β treatment in both in vitro and CCl4-treated mouse livers (75). Upregulation of EZH2 downregulates expression of peroxisome proliferator-activated receptor-gamma (PPARγ), a nuclear receptor essential for HSC activation (76, 77).
Histone demethylation by HDMTs is also a process critical to HSC activation. Jumonji domain-containing protein 1A (JMJD1A) is one of these HDMTs. JMJD1A knockdown in HSCs increases H3K9me2 levels on the PPARγ gene promoter and represses the expression of PPARγ (78). JMJD2D, a histone H3 demethylase, also contributes to HSC activation by demethylating H3K9 residues. The result of this demethylation is increased TLR4 transcription and activation of the TLR4/MyD88/NF-kB signaling pathway, which has a well-established role in liver fibrosis (79). JMJD2D expression is markedly increased in activated HSCs, and AAV9 shRNA-mediated knockdown of JMJD2D suppresses hepatic fibrosis in the CCl4 model of liver fibrosis (80).
Micro-RNA Involvement in HSC Activation
miRNAs are small, non-coding RNAs (20–25 nucleotides in length) that regulate gene expression by binding to target mRNA transcripts and subsequently facilitating the transcript's cleavage, destabilization, or inhibiting its translation (81). MiRNAs can also control the activation of HSCs by several different mechanisms (38). Namely, HCC cells secrete extracellular vesicles (EV) with oncogenic miRNAs (oncomiRs), specifically miR-21, miR-221, and miR-151, which can activate HSCs. This activation promotes HCC cell invasion, epithelial to mesenchymal transition (EMT), and activation of the AKT/ERK signaling pathway (82). miR-214 promotes HSC activation by inhibiting the expression of suppressor-of-fused homolog (Sufu), a negative regulator of Hedgehog signaling pathway (83). miR-542-3p is also capable of stimulating HSC activation and fibrosis by targeting bone morphogenetic protein 7 (BMP7), a potent surpressor of TGF-β signaling (84). miR-942, which is induced by both TGF-β and LPS, assists HSC activation through downregulation of BMP and activin membrane-bound inhibitor homolog (BAMBI) (85). miR-146b boosts HSC activation by targeting of Kruppel-like factor 4 (KLF4) (86). miR-17-5p targets Smad7 thus stimulating the activation of HSCs (87). Likewise, miR-34a facilitates HSC activation through decreasing the expression of acyl-CoA synthetase-1 (ACSL1) (88). miR-125a-5p advances activation of HSCs by targeting a negative regulator of hypoxia-inducible factor 1 (HIF1), the aptly named factor inhibiting HIF-1 or FIH1 (89).
Conversely, several miRNAs have been shown to suppress HSC activation. miR-96-5p suppresses the activation of HSCs by inhibiting ATG7 and autophagy (90). The miR-23b/27b cluster bind to 3′-UTR of gremlin 1, contributing to the reduction of TGF-β expression and HSC inactivation (91). Additionally, miR-338-3p inhibits HSC activation and proliferation through targeting cyclin-dependent kinase 4 (CDK4) (92). mi-378 represses HSC activation by targeting Gli3 and reducing the expression of Gli3 (93). miR-155 inhibits STAT5 and FOXO3a expression, thus silencing HSC activation (94). miR-146a-5p abrogates HSC activation by inhibiting TGF-β1/Smad and LPS/NF-κB signaling pathways (95, 96). Similarly, both miR-9-5p and miR-130-3p attenuate the activation of HSCs by targeting TGFβR1 and TGFβR2 (97). miR-30a impedes HSC activation through the inhibition of EMT via directly targeting SNAI1 (98).
Tumor-Associated Macrophages
Macrophages are essential innate immune cells in the tumor microenvironment and are also closely associated with cancer occurrence, metastases, and disease progression (99). Tumor-associated macrophages (TAMs) that reside in the surrounding TME are crucial components in the HCC immune microenvironment for the formation and development of HCC (100, 101). TAMs promote HCC growth, angiogenesis, invasion, and migration while simultaneously suppressing the antitumor immune response (20). The epigenetic regulation of TAMs has been shown to be critical to their differentiation and functional programming (102–105).
DNA Methylation and Histone Modification in TAMs
DNA methylation and histone modification are critical regulatory mechanisms in TAMs for promoting tumor growth and progression (106–109). However, the regulation of DNA methylation and histone modification of TAMs in HCC remains poorly understood. A recent study showed that colony-stimulating factor 1 receptor (CSF1R), which plays an essential role in forming TAMs (108, 110), is regulated by DNA methylation in HCC (111). DNA methylation leads to decreased expression of CSF1R in HCC tissues and associates with poor clinicopathological characteristics of HCC (111). SIRT1, a NAD-dependent histone deacetylase, suppresses HCC metastasis by facilitating M1 macrophage polarization via the stimulation of the NF-κB pathway (112). Downregulation of SIRT4, another sirtuin protein, in TAMs promotes macrophages activation and HCC growth via the FAO-PPARδ-STAT3 axis (113).
miRNAs and Long Non-coding RNAs in TAMs
The epigenetic remodeling by miRNA regulates macrophages' activation and functional programming (114–117). For example, the down-regulation of miR-98 was accompanied by up-regulation of IL-10 in TAMs of HCC (118). miR-98 modulates macrophage polarization from M2 to M1 in HCC by targeting IL-10, thus stifling the effects of TAMs on advancing EMT and HCC metastasis (118, 119). miR-101 targets dual-specificity phosphatase 1 (DUSP1) to inhibit TAM-induced HCC growth through TGF-β secretion regulation (120). Decreased expression of miR-28-5p suppresses HCC growth and metastasis by directly targeting interleukin-34 (IL-34) and affecting IL-34-mediated TAM infiltration (121). Reduced expression of miR-125a and miR-125b secreted from TAMs promote HCC proliferation and cancer stem cell properties directly targeting CD90 (122). miR-15b secreted from TAMs promotes the aggressiveness of HCC by impeding the LATS1-mediated hippo pathway (123).
Long non-coding RNAs (lncRNAs) are a class of RNAs >200 nucleotides in length, which lack protein-coding capabilities and have gained increased attention in recent years due to their ability to function guides, signals, and decoys in a highly tissue-specific manner (124). Several lncRNAs have been shown to participate in tumorigenesis and cancer progression, a number of which do so by modulating elements of the TME (125, 126). In the context of HCC, lncRNA cox-2 reduces HCC immune evasion and metastasis formation by suppressing M2 macrophage polarization (127). lncRNA H19, induced by TAMs, promotes the progression of HCC via regulating the miR-193b/MAPK1 axis (128).
Epigenetics in the Extracellular Matrix
The ECM plays a critical role in physiological and pathological processes in HCC (129). MMPs, which digest the ECM, are key players in ECM remodeling and are associated with tumor growth and invasion through collagen and matrix degradation (130, 131). Metallopeptidase inhibitor proteins (TIMPs) are natural inhibitors of MMPs (132). Epigenetic modification is a critical mechanism for regulating MMP and TIMP expression, thus playing a pivotal role in HCC development (133).
DNA Methylation and Histone Modification in MMPs and TIMPs
Overexpression of MMPs, such as MMP2 and MMP9, is frequently observed in HCC patients and associated with cancer invasive potential (41, 42). While some studies have shown that DNA methylation can regulate the expression of MMPs in other cancers, it remains unknown if DNA methylation directly regulates the expression of MMPs in HCC (134). Emerging evidence, however, indicates that DNA methylation promotes the dysregulation of TIMPs in HCC. Specifically, TIMP3 suppresses HCC cell proliferation and survival, and TIMP3 expression is suppressed by promoter methylation in HCC cells (135, 136). Histone deacetylation by HDAC4 represses MMP promoter activities and the expression of MMP9 and MMP13 in HSCs (66, 137). Sodium butyrate, a histone deacetylase inhibitor, decreases MMP1 mRNA expression in human liver cancer cells (138). Further, overexpression of histone methyltransferase EZH2 decreases MMP9 expression in HSCs (139).
miRNAs in MMPs and TIMPs
miRNAs are also involved in the Regulation of MMP and TIMP expression (140). For instance, miR-29b directly targets MMP2 and suppresses HCC growth (141). The blocking of MMP2, either by neutralizing antibody or RNA interference, phenocopies the anti-invasion and antiangiogenesis effects of miR-29b, whereas, the introduction of MMP2 antagonizes the function of miR-29b in HCC (141). miR-107 has also been shown to downregulate MMP2 and MMP9 in HCC cells (142). miR-328-3p and miR-133a modulate the expression of MMP9 to inhibit HCC cell proliferation (143, 144). miR-191 promotes EMT and HCC tumor growth by directly targeting TIMP3 and inhibiting TIMP3 expression (145). MiR-181b and miR-221/222 can also target TIMP3 to promote HCC growth (146, 147). Moreover, miR-519d targets TIMP2 and promotes HCC cell proliferation and metastasis (148).
Conclusion and Future Perspectives
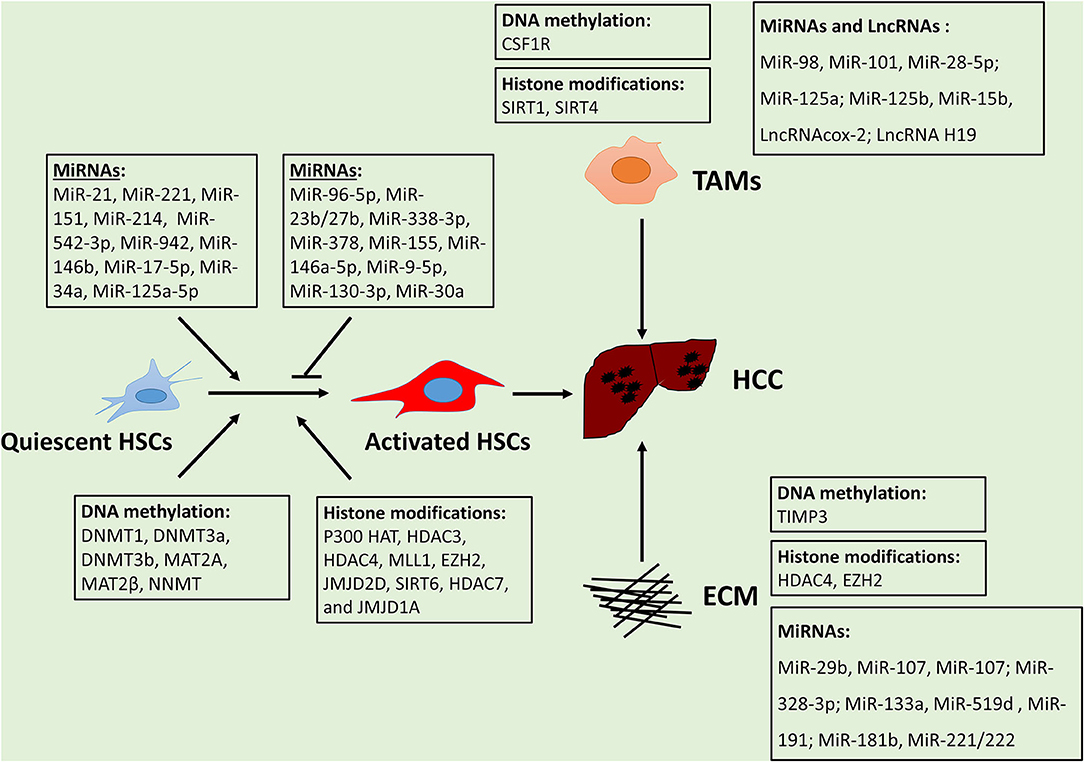
The tumor microenvironment plays a critical role in HCC initiation and progression. Epigenetic regulation is a crucial mechanism for the alteration of TMEs in HCC. Overall, epigenetic reprogramming extensively modulates gene expression alterations in HSCs, TAMs, and ECMs, thus promoting HCC development (Figure 1). The effect of metabolic factors (e.g., SAMe) on epigenetic alterations of TMEs in HCC is evident, suggesting dysregulation metabolites establish can affect the epigenome and subsequently impact HCC development. The mechanisms of epigenetic regulation in MSCs, MDSCs, and ECs remain poorly investigated, thus necessitating future study. Understanding the interactions between tumors and TMEs will further enhance our understanding of critical drivers or suppressors for tumor progression. Despite substantial advances in understanding the mechanisms of TMEs, developing therapeutics using this knowledge remains a challenge. Further examination and deciphering of the complex epigenetic landscape of the TME in HCC will help identify new targets and strategies for the treatment of HCC.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This project was supported by RSG-18-107 (WQ) from the American Cancer Society and R01CA197128 (WQ) from the National Cancer Institute. The content is solely the authors' responsibility and does not necessarily represent the official views of the American Cancer Society or the National Institutes of Health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Jacques F, Isabelle S, Rajesh D, Sultan E, Colin M, Marise R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136:E359–86. doi: 10.1002/ijc.29210
2. Villanueva. Hepatocellular Carcinoma. N Engl J Med. (2019) 380:1450–62. doi: 10.1056/NEJMra1713263
3. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc F-J, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. (2008) 359:378–90. doi: 10.1056/NEJMoa0708857
4. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. The Lancet. (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
5. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
6. Bruix J, Qin S, Merle P, Granito A, Huang Y-H, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. (2017) 389:56–66. doi: 10.1016/S0140-6736(16)32453-9
7. Abou-Alfa GK, Meyer T, Cheng A-L, El-Khoueiry AB, Rimassa L, Ryoo B-Y, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. (2018) 379:54–63. doi: 10.1056/NEJMoa1717002
8. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. The Lancet. (2017) 389:2492–502. doi: 10.1016/S0140-6736(17)31046-2
9. Finn RS, Ryoo B-Y, Merle P, Kudo M, Bouattour M, Lim H-Y, et al. Investigators, Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol. (2019) 37:4004. doi: 10.1200/JCO.2019.37.15_suppl.4004
10. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
11. Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. (2013) 144:512–27. doi: 10.1053/j.gastro.2013.01.002
12. Zhou BY, Gong JH, Cai XY, Wang JX, Luo F, Jiang N, et al. An imbalance between stellate cells and gamma delta T cells contributes to hepatocellular carcinoma aggressiveness and recurrence. Hepatol Int. (2019) 13:631–40. doi: 10.1007/s12072-019-09969-w
13. Fu JL, Xu DP, Liu ZW, Shi M, Zhao P, Fu BY, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. (2007) 132:2328–39. doi: 10.1053/j.gastro.2007.03.102
14. Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. (2008) 135:234–43. doi: 10.1053/j.gastro.2008.03.020
15. Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. J Leukocyte Biol. (2006) 80:1197–213. doi: 10.1189/jlb.0506297
16. Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. (2006) 10:99–111. doi: 10.1016/j.ccr.2006.06.016
17. Jeong WI, Park O, Suh YG, Byun JS, Park SY, Choi E, et al. Suppression of innate immunity (natural killer cell/interferon-gamma) in the advanced stages of liver fibrosis in mice. Hepatology. (2011) 53:1342–51. doi: 10.1002/hep.24190
18. Seo HR. Roles of tumor microenvironment in hepatocelluar carcinoma. Curr Cancer Ther Rev. (2015) 11:82–93. doi: 10.2174/1573394711666151022203313
19. Sevic I, Spinelli FM, Cantero MJ, Reszegi A, Kovalszky I, Garcia MG, et al. Chapter 2: The role of the tumor microenvironment in the development and progression of hepatocellular carcinoma. In: Tirnitz-Parker JEE, editor. Hepatocellular Carcinoma. Brisbane, QLD: Codon Publications (2019). doi: 10.15586/hepatocellularcarcinoma.2019.ch2
20. Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, et al. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. (2013) 2013:187204. doi: 10.1155/2013/187204
21. Bresnahan E, Lindblad KE, Ruiz de Galarreta M, Lujambio A. Mouse models of oncoimmunology in hepatocellular carcinoma. Clin Cancer Res. (2020) 26:5276–86. doi: 10.1158/1078-0432.CCR-19-2923
22. Hardy T, Mann DA. Epigenetics in liver disease: from biology to therapeutics. Gut. (2016) 65:1895–905. doi: 10.1136/gutjnl-2015-311292
23. Hanover JA, Krause MW, Love DC. Bittersweet memories: linking metabolism to epigenetics through O-GlcNAcylation. Nat Rev Mol Cell Biol. (2012) 13:312–21. doi: 10.1038/nrm3334
24. Santhakumar C, Gane EJ, Liu K, McCaughan GW. Current perspectives on the tumor microenvironment in hepatocellular carcinoma. Hepatol Int. (2020) 14:947–57. doi: 10.1007/s12072-020-10104-3
25. Wang G, Wang Q, Liang N, Xue H, Yang T, Chen X, et al. Oncogenic driver genes and tumor microenvironment determine the type of liver cancer. Cell Death Dis. (2020) 11:313. doi: 10.1038/s41419-020-2509-x
26. Thomson JP, Ottaviano R, Unterberger EB, Lempiainen H, Muller A, Terranova R, et al. Loss of tet1-associated 5-hydroxymethylcytosine is concomitant with aberrant promoter hypermethylation in liver cancer. Cancer Res. (2016) 76:3097–108. doi: 10.1158/0008-5472.CAN-15-1910
27. Wachowska M, Gabrysiak M, Golab J. Epigenetic remodeling combined with photodynamic therapy elicits anticancer immune responses. Oncoimmunology. (2014) 3:e28837. doi: 10.4161/onci.28837
28. Setiadi AF, David MD, Seipp RP, Hartikainen JA, Gopaul R, Jefferies WA. Epigenetic control of the immune escape mechanisms in malignant carcinomas. Mol Cell Biol. (2007) 27:7886–94. doi: 10.1128/MCB.01547-07
29. Liu M, Jiang L, Guan XY. The genetic and epigenetic alterations in human hepatocellular carcinoma: a recent update. Protein Cell. (2014) 5:673–91. doi: 10.1007/s13238-014-0065-9
30. Sceusi EL, Loose DS, Wray CJ. Clinical implications of DNA methylation in hepatocellular carcinoma. HPB (Oxford). (2011) 13:369–76. doi: 10.1111/j.1477-2574.2011.00303.x
31. Nakamura M, Chiba T, Kanayama K, Kanzaki H, Saito T, Kusakabe Y, et al. Epigenetic dysregulation in hepatocellular carcinoma: an up-to-date review. Hepatol Res. (2019) 49:3–13. doi: 10.1111/hepr.13250
32. Toh TB, Lim JJ, Chow EK. Epigenetics of hepatocellular carcinoma. Clin Transl Med. (2019) 8:13. doi: 10.1186/s40169-019-0230-0
33. Fernandez-Barrena MG, Arechederra M, Colyn L, Berasain C, Avila MA. Epigenetics in hepatocellular carcinoma development and therapy: the tip of the iceberg. JHEP Rep. (2020) 2:100167. doi: 10.1016/j.jhepr.2020.100167
34. Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. (2008) 88:125–72. doi: 10.1152/physrev.00013.2007
35. Barry AE, Baldeosingh R, Lamm R, Patel K, Zhang K, Dominguez DA, et al. Hepatic stellate cells and hepatocarcinogenesis. Front Cell Dev Biol. (2020) 8:709. doi: 10.3389/fcell.2020.00709
36. Amann T, Bataille F, Spruss T, Muhlbauer M, Gabele E, Scholmerich J, et al. Activated hepatic stellate cells promote tumorigenicity of hepatocellular carcinoma. Cancer Sci. (2009) 100:646–53. doi: 10.1111/j.1349-7006.2009.01087.x
37. Santamato A, Fransvea E, Dituri F, Caligiuri A, Quaranta M, Niimi T, et al. Hepatic stellate cells stimulate HCC cell migration via laminin-5 production. Clin Sci (Lond). (2011) 121:159–68. doi: 10.1042/CS20110002
38. Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastro Hepat. (2017) 14:397–411. doi: 10.1038/nrgastro.2017.38
39. Coulouarn C, Clement B. Stellate cells and the development of liver cancer: therapeutic potential of targeting the stroma. J Hepatol. (2014) 60:1306–9. doi: 10.1016/j.jhep.2014.02.003
40. Lachowski D, Cortes E, Rice A, Pinato D, Rombouts K, Hernandez AD. Matrix stiffness modulates the activity of MMP-9 and TIMP-1 in hepatic stellate cells to perpetuate fibrosis. Sci Rep-Uk. (2019) 9:7299: doi: 10.1038/s41598-019-43759-6
41. Giannelli G, Bergamini C, Marinosci F, Fransvea E, Quaranta M, Lupo L, et al. Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular carcinoma. Int J Cancer. (2002) 97:425–31. doi: 10.1002/ijc.1635
42. Arii S, Mise M, Harada T, Furutani M, Ishigami S, Niwano M, et al. Overexpression of matrix metalloproteinase 9 gene in hepatocellular carcinoma with invasive potential. Hepatology. (1996) 24:316–22. doi: 10.1002/hep.510240206
43. Scheau C, Badarau IA, Costache R, Caruntu C, Mihai GL, Didilescu AC, et al. The role of matrix metalloproteinases in the epithelial-mesenchymal transition of hepatocellular carcinoma. Anal Cell Pathol. (2019) 2019:9423907. doi: 10.1155/2019/9423907
44. Chen JS, Huang XH, Wang Q, Chen XL, Fu XH, Tan HX, et al. FAK is involved in invasion and metastasis of hepatocellular carcinoma. Clin Exp Metastas. (2010) 27:71–82. doi: 10.1007/s10585-010-9306-3
45. Jia YL, Shi L, Zhou JN, Fu CJ, Chen L, Yuan HF, et al. Epimorphin promotes human hepatocellular carcinoma invasion and metastasis through activation of focal adhesion kinase/extracellular signal-regulated kinase/matrix metalloproteinase-9 axis. Hepatology. (2011) 54:1808–18. doi: 10.1002/hep.24562
46. Han SS, Han L, Yao YM, Sun H, Zan XF, Liu QG. Activated hepatic stellate cells promote hepatocellular carcinoma cell migration and invasion via the activation of FAK-MMP9 signaling. Oncol Rep. (2014) 31:641–8. doi: 10.3892/or.2013.2872
47. Yin C, Evason KJ, Asahina K, Stainier DY. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Investig. (2013) 123:1902–10. doi: 10.1172/JCI66369
48. Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. (2012) 109:9448–53. doi: 10.1073/pnas.1201840109
49. Troeger JS, Mederacke I, Gwak GY, Dapito DH, Mu X, Hsu CC, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. (2012) 143:1073–83 e22. doi: 10.1053/j.gastro.2012.06.036
50. Mann J, Oakley F, Akiboye F, Elsharkawy A, Thorne AW, Mann DA. Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: implications for wound healing and fibrogenesis. Cell Death Differ. (2007) 14:275–85. doi: 10.1038/sj.cdd.4401979
51. Gotze S, Schumacher EC, Kordes C, Haussinger D. epigenetic changes during hepatic stellate cell activation. PLoS ONE. (2015) 10:e0128745. doi: 10.1371/journal.pone.0128745
52. Bian EB, Huang C, Ma TT, Tao H, Zhang H, Cheng C, et al. DNMT1-mediated PTEN hypermethylation confers hepatic stellate cell activation and liver fibrogenesis in rats. Toxicol Appl Pharm. (2012) 264:13–22. doi: 10.1016/j.taap.2012.06.022
53. Zhu H, He C, Zhao H, Jiang W, Xu S, Li J, et al. Sennoside A prevents liver fibrosis by binding DNMT1 and suppressing DNMT1-mediated PTEN hypermethylation in HSC activation and proliferation. FASEB J. (2020) 34:14558–71. doi: 10.1096/fj.202000494RR
54. Page A, Paoli P, Salvador EM, White S, French J, Mann J. Hepatic stellate cell transdifferentiation involves genome-wide remodeling of the DNA methylation landscape. J Hepatol. (2016) 64:661–73. doi: 10.1016/j.jhep.2015.11.024
55. Pan XY, Yang Y, Meng HW, Li HD, Chen X, Huang HM, et al. DNA methylation of PTGIS enhances hepatic stellate cells activation and liver fibrogenesis. Fron Pharmacol. (2018) 9:553. doi: 10.3389/fphar.2018.00553
56. Wu Y, Bu F, Yu H, Li W, Huang C, Meng X, et al. Methylation of Septin9 mediated by DNMT3a enhances hepatic stellate cells activation and liver fibrogenesis. Toxicol Appl Pharmacol. (2017) 315:35–49. doi: 10.1016/j.taap.2016.12.002
57. Chen X, Li WX, Chen Y, Li XF, Li HD, Huang HM, et al. Suppression of SUN2 by DNA methylation is associated with HSCs activation and hepatic fibrosis. Cell Death Dis. (2018) 9:1021. doi: 10.1038/s41419-018-1032-9
58. Bian K, Zhang F, Wang T, Zou X, Duan X, Chen G, et al. S-Adenosylmethionine suppresses the expression of Smad3/4 in activated human hepatic stellate cells via Rac1 promoter methylation. Mol Med Rep. (2016) 13:3867–73. doi: 10.3892/mmr.2016.4997
59. Pan X-Y, You HM, Wang L, Bi YH, Yang Y, Meng HW, et al. Methylation of RCAN1.4 mediated by DNMT1 and DNMT3b enhances hepatic stellate cell activation and liver fibrogenesis through Calcineurin/NFAT3 signaling. Theranostics. (2019) 9:4308–23. doi: 10.7150/thno.32710
60. Ramani K, Yang H, Kuhlenkamp J, Tomasi L, Tsukamoto H, Mato JM, et al. Changes in the expression of methionine adenosyltransferase genes and S-adenosylmethionine homeostasis during hepatic stellate cell activation. Hepatology. (2010) 51:986–95. doi: 10.1002/hep.23411
61. Li J, You S, Zhang S, Hu Q, Wang F, Chi X, et al. Elevated N-methyltransferase expression induced by hepatic stellate cells contributes to the metastasis of hepatocellular carcinoma via regulation of the CD44v3 isoform. Mol Oncol. (2019) 13:1993–2009. doi: 10.1002/1878-0261.12544
62. Zhang T, Cooper S, Brockdorff N. The interplay of histone modifications - writers that read. EMBO Rep. (2015) 16:1467–81. doi: 10.15252/embr.201540945
63. Dou C, Liu Z, Tu K, Zhang H, Chen C, Yaqoob U, et al. P300 acetyltransferase mediates stiffness-induced activation of hepatic stellate cells into tumor-promoting myofibroblasts. Gastroenterology. (2018) 154:2209–21 e14. doi: 10.1053/j.gastro.2018.02.015
64. Kim HG, Huang M, Xin Y, Zhang Y, Zhang X, Wang G, et al. The epigenetic regulator SIRT6 protects the liver from alcohol-induced tissue injury by reducing oxidative stress in mice. J Hepatol. (2019) 71:960–9. doi: 10.1016/j.jhep.2019.06.019
65. Zhong X, Huang M, Kim HG, Zhang Y, Chowdhury K, Cai W, et al. SIRT6 protects against liver fibrosis by deacetylation and suppression of SMAD3 in hepatic stellate cells. Cell Mol Gastroenterol Hepatol. (2020) 10:341–64. doi: 10.1016/j.jcmgh.2020.04.005
66. Qin L, Han YP. Epigenetic repression of matrix metalloproteinases in myofibroblastic hepatic stellate cells through histone deacetylases 4: implication in tissue fibrosis. Am J Pathol. (2010) 177:1915–28. doi: 10.2353/ajpath.2010.100011
67. Mannaerts I, Eysackers N, Onyema OO, Van Beneden K, Valente S, Mai A, et al. Class II HDAC inhibition hampers hepatic stellate cell activation by induction of microRNA-29. PloS ONE. (2013) 8:e55786. doi: 10.1371/journal.pone.0055786
68. Shaker ME, Ghani A, Shiha GE, Ibrahim TM, Mehal WZ. Nilotinib induces apoptosis and autophagic cell death of activated hepatic stellate cells via inhibition of histone deacetylases. Biochim Biophysica Acta. (2013) 1833:1992–2003. doi: 10.1016/j.bbamcr.2013.02.033
69. Duarte S, Baber J, Fujii T, Coito AJ. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. (2015) 44–46:147–56. doi: 10.1016/j.matbio.2015.01.004
70. Pannem RR, Dorn C, Hellerbrand C, Massoumi R. Cylindromatosis gene CYLD regulates hepatocyte growth factor expression in hepatic stellate cells through interaction with histone deacetylase 7. Hepatology. (2014) 60:1066–81. doi: 10.1002/hep.27209
71. Barter MJ, Pybus L, Litherland GJ, Rowan AD, Clark IM, Edwards DR, et al. HDAC-mediated control of ERK- and PI3K-dependent TGF-beta-induced extracellular matrix-regulating genes. Matrix Biol. (2010) 29:602–12. doi: 10.1016/j.matbio.2010.05.002
72. Wang Y, Zhao L, Jiao FZ, Zhang WB, Chen Q, Gong ZJ. Histone deacetylase inhibitor suberoylanilide hydroxamic acid alleviates liver fibrosis by suppressing the transforming growth factor-beta1 signal pathway. Hepatobiliary Pancreat Dis Int. (2018) 17:423–9. doi: 10.1016/j.hbpd.2018.09.013
73. Page A, Paoli PP, Hill SJ, Howarth R, Wu R, Kweon SM, et al. Alcohol directly stimulates epigenetic modifications in hepatic stellate cells. J Hepatol. (2015) 62:388–97. doi: 10.1016/j.jhep.2014.09.033
74. Tian W, Fan Z, Li J, Hao C, Li M, Xu H, et al. Myocardin-related transcription factor A (MRTF-A) plays an essential role in hepatic stellate cell activation by epigenetically modulating TGF-beta signaling. Int J Biochem Cell Biol. (2016) 71:35–43. doi: 10.1016/j.biocel.2015.12.005
75. Martin-Mateos R, De Assuncao TM, Arab JP, Jalan-Sakrikar N, Yaqoob U, Greuter T, et al. Enhancer of zeste homologue 2 inhibition attenuates TGF-beta dependent hepatic stellate cell activation and liver fibrosis. Cell Mol Gastroenterol Hepatol. (2019) 7:197–209. doi: 10.1016/j.jcmgh.2018.09.005
76. Mann J, Chu DC, Maxwell A, Oakley F, Zhu NL, Tsukamoto H, et al. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology. (2010) 138:705–14. doi: 10.1053/j.gastro.2009.10.002
77. Panebianco C, Oben JA, Vinciguerra M, Pazienza V. Senescence in hepatic stellate cells as a mechanism of liver fibrosis reversal: a putative synergy between retinoic acid and PPAR-gamma signalings. Clin Exp Med. (2017) 17:269–80. doi: 10.1007/s10238-016-0438-x
78. Jiang Y, Wang S, Zhao Y, Lin C, Zhong F, Jin L, et al. Histone H3K9 demethylase JMJD1A modulates hepatic stellate cells activation and liver fibrosis by epigenetically regulating peroxisome proliferator-activated receptor gamma. FASEB J. (2015) 29:1830–41. doi: 10.1096/fj.14-251751
79. Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. (2007) 13:1324–32. doi: 10.1038/nm1663
80. Dong F, Jiang S, Li J, Wang Y, Zhu L, Huang Y, et al. The histone demethylase KDM4D promotes hepatic fibrogenesis by modulating Toll-like receptor 4 signaling pathway. EBioMedicine. (2019) 39:472–83. doi: 10.1016/j.ebiom.2018.11.055
81. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. (2004) 116:281–97. doi: 10.1016/S0092-8674(04)00045-5
82. Li J, Yan Y, Ang L, Li X, Liu C, Sun B, et al. Extracellular vesicles-derived OncomiRs mediate communication between cancer cells and cancer-associated hepatic stellate cells in hepatocellular carcinoma microenvironment. Carcinogenesis. (2020) 41:223–34. doi: 10.1093/carcin/bgz096
83. Ma L, Yang X, Wei R, Ye T, Zhou JK, Wen M, et al. MicroRNA-214 promotes hepatic stellate cell activation and liver fibrosis by suppressing Sufu expression. Cell Death Dis. (2018) 9:718. doi: 10.1038/s41419-018-0752-1
84. Ji F, Wang K, Zhang Y, Mao XL, Huang Q, Wang J, et al. MiR-542-3p controls hepatic stellate cell activation and fibrosis via targeting BMP-7. J Cell Biochem. (2019) 120:4573–81. doi: 10.1002/jcb.27746
85. Tao L, Xue D, Shen D, Ma W, Zhang J, Wang X, et al. MicroRNA-942 mediates hepatic stellate cell activation by regulating BAMBI expression in human liver fibrosis. Arch Toxicol. (2018) 92:2935–46 doi: 10.1007/s00204-018-2278-9
86. Ge S, Zhang L, Xie J, Liu F, He J, He J, et al. MicroRNA-146b regulates hepatic stellate cell activation via targeting of KLF4. Ann Hepatol. (2016) 15:918–28. doi: 10.5604/16652681.1222111
87. Yu F, Guo Y, Chen B, Dong P, Zheng J. MicroRNA-17-5p activates hepatic stellate cells through targeting of Smad7. Lab Invest. (2015) 95:781–9. doi: 10.1038/labinvest.2015.58
88. Yan G, Li B, Xin X, Xu M, Ji G, Yu H. MicroRNA-34a promotes hepatic stellate cell activation via targeting ACSL1. Med Sci Monit. (2015) 21:3008–15. doi: 10.12659/MSM.894000
89. Li G, Li J, Li C, Qi H, Dong P, Zheng J, et al. MicroRNA-125a-5p contributes to hepatic stellate cell activation through targeting FIH1. Cell Physiol Biochem. (2016) 38:1544–52. doi: 10.1159/000443095
90. Yu K, Li N, Cheng Q, Zheng J, Zhu M, Bao S, et al. miR-96-5p prevents hepatic stellate cell activation by inhibiting autophagy via ATG7. J Mol Med (Berl). (2018) 96:65–74. doi: 10.1007/s00109-017-1593-6
91. Zeng XY, Zhang YQ, He XM, Wan LY, Wang H, Ni YR, et al. Suppression of hepatic stellate cell activation through downregulation of gremlin1 expression by the miR-23b/27b cluster. Oncotarget. (2016) 7:86198–210. doi: 10.18632/oncotarget.13365
92. Duan B, Hu J, Zhang T, Luo X, Zhou Y, Liu S, et al. miRNA-338-3p/CDK4 signaling pathway suppressed hepatic stellate cell activation and proliferation. BMC Gastroenterol. (2017) 17:12. doi: 10.1186/s12876-017-0571-3
93. Hyun J, Wang S, Kim J, Rao KM, Park SY, Chung I, et al. MicroRNA-378 limits activation of hepatic stellate cells and liver fibrosis by suppressing Gli3 expression. Nat Commun. (2016) 7:10993. doi: 10.1038/ncomms10993
94. Zhu D, Yang C, Shen P, Chen L, Chen J, Sun X, et al. rSjP40 suppresses hepatic stellate cell activation by promoting microRNA-155 expression and inhibiting STAT5 and FOXO3a expression. J Cell Mol Med. (2018) 22:5486–93. doi: 10.1111/jcmm.13819
95. Zou Y, Cai Y, Lu D, Zhou Y, Yao Q, Zhang S. MicroRNA-146a-5p attenuates liver fibrosis by suppressing profibrogenic effects of TGFbeta1 and lipopolysaccharide. Cell Signal. (2017) 39:1–8. doi: 10.1016/j.cellsig.2017.07.016
96. Chen Y, Wu Z, Yuan B, Dong Y, Zhang L, Zeng Z. MicroRNA-146a-5p attenuates irradiation-induced and LPS-induced hepatic stellate cell activation and hepatocyte apoptosis through inhibition of TLR4 pathway. Cell Death Dis. (2018) 9:22. doi: 10.1038/s41419-017-0038-z
97. Yu F, Chen B, Fan X, Li G, Dong P, Zheng J. Epigenetically-regulated MicroRNA-9-5p suppresses the activation of hepatic stellate cells via TGFBR1 and TGFBR2. Cell Physiol Biochem. (2017) 43:2242–52. doi: 10.1159/000484303
98. Zheng J, Wang W, Yu F, Dong P, Chen B, Zhou MT. MicroRNA-30a Suppresses the activation of hepatic stellate cells by inhibiting epithelial-to-mesenchymal transition. Cell Physiol Biochem. (2018) 46:82–92. doi: 10.1159/000488411
99. Larionova E, Kazakova E, Patysheva M, Kzhyshkowska J. Transcriptional, epigenetic and metabolic programming of tumor-associated macrophages. Cancers (Basel). (2020) 12:1411: doi: 10.3390/cancers12061411
100. Singh S, Mehta N, Lilan J, Budhthoki MB, Chao F, Yong L. Initiative action of tumor-associated macrophage during tumor metastasis. Biochim Open. (2017) 4:8–18. doi: 10.1016/j.biopen.2016.11.002
101. Zhou D, Luan J, Huang C, Li J. Tumor-associated macrophages in hepatocellular carcinoma: friend or foe? Gut Liver. (2020). doi: 10.5009/gnl20223
102. Hoeksema MA, de Winther MP. Epigenetic regulation of monocyte and macrophage function. Antioxid Redox Signal. (2016) 25:758–74. doi: 10.1089/ars.2016.6695
103. Jain N, Shahal T, Gabrieli T, Gilat N, Torchinsky D, Michaeli Y, et al. Global modulation in DNA epigenetics during pro-inflammatory macrophage activation. Epigenetics. (2019) 14:1183–93. doi: 10.1080/15592294.2019.1638700
104. Nakamura K, Smyth MJ. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol Immunol. (2020) 17:1–12. doi: 10.1038/s41423-019-0306-1
105. Van den Bossche J, Neele AE, Hoeksema MA, de Winther MP. Macrophage polarization: the epigenetic point of view. Curr Opin Lipidol. (2014) 25:367–73. doi: 10.1097/MOL.0000000000000109
106. Dekkers KF, Neele AE, Jukema JW, Heijmans BTM, de Winther PJ. Human monocyte-to-macrophage differentiation involves highly localized gain and loss of DNA methylation at transcription factor binding sites. Epigenetics Chromatin. (2019) 12:34. doi: 10.1186/s13072-019-0279-4
107. Wang X, Cao Q, Yu L, Shi H, Xue B, Shi H. Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity. JCI Insight. (2016) 1:e87748. doi: 10.1172/jci.insight.87748
108. Ishii M, Wen H, Corsa CA, Liu T, Coelho AL, Allen RM, et al. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. (2009) 114:3244–54. doi: 10.1182/blood-2009-04-217620
109. de Groot AE, Pienta KJ. Epigenetic control of macrophage polarization: implications for targeting tumor-associated macrophages. Oncotarget. (2018) 9:20908–27. doi: 10.18632/oncotarget.24556
110. Zhu Y, Yang J, Xu D, Gao XM, Zhang Z, Hsu JL, et al. Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut. (2019) 68:1653–66. doi: 10.1136/gutjnl-2019-318419
111. Cui B, Fan XX, Zhou DZ, He LF, Li YR, Li DD, et al. CSF1R methylation is a key regulatory mechanism of tumor-associated macrophages in hepatocellular carcinoma. Oncol Lett. (2020) 20:1835–45. doi: 10.3892/ol.2020.11726
112. Zhou B, Yang Y, Li C. SIRT1 inhibits hepatocellular carcinoma metastasis by promoting M1 macrophage polarization via NF-kappaB pathway. OncoTargets Ther. (2019) 12:2519–29. doi: 10.2147/OTT.S195234
113. Li Z, Li H, Zhao ZB, Zhu W, Feng PP, Zhu XW, et al. SIRT4 silencing in tumor-associated macrophages promotes HCC development via PPARdelta signalling-mediated alternative activation of macrophages. J Exp Clin Cancer Res. (2019) 38:469. doi: 10.1186/s13046-019-1456-9
114. Zhang Y, Zhang M, Zhong M, Suo Q, Lv K. Expression profiles of miRNAs in polarized macrophages. Int J Mol Med. (2013) 31:797–802. doi: 10.3892/ijmm.2013.1260
115. Liu G, Abraham E. MicroRNAs in immune response and macrophage polarization. Arterioscler Thromb Vasc Biol. (2013) 33:170–7. doi: 10.1161/ATVBAHA.112.300068
116. Cai X, Yin Y, Li N, Zhu D, Zhang J, Zhang CY, et al. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J Mol Cell Biol. (2012) 4:341–3. doi: 10.1093/jmcb/mjs044
117. Liu M, Zhou J, Chen Z, Cheng AS. Understanding the epigenetic regulation of tumours and their microenvironments: opportunities and problems for epigenetic therapy. J Pathol. (2017) 241:10–24. doi: 10.1002/path.4832
118. Li L, Sun P, Zhang C, Li Z, Zhou W. MiR-98 suppresses the effects of tumor-associated macrophages on promoting migration and invasion of hepatocellular carcinoma cells by regulating IL-10. Biochimie. (2018) 150:23–30. doi: 10.1016/j.biochi.2018.04.016
119. Li L, Sun P, Zhang C, Li Z, Cui K, Zhou W. MiR-98 modulates macrophage polarization and suppresses the effects of tumor-associated macrophages on promoting invasion and epithelial-mesenchymal transition of hepatocellular carcinoma. Cancer Cell Int. (2018) 18:95. doi: 10.1186/s12935-018-0590-3
120. Wei X, Tang C, Lu X, Liu R, Zhou M, He D, et al. MiR-101 targets DUSP1 to regulate the TGF-beta secretion in sorafenib inhibits macrophage-induced growth of hepatocarcinoma. Oncotarget. (2015) 6:18389–405. doi: 10.18632/oncotarget.4089
121. Zhou SL, Hu ZQ, Zhou ZJ, Dai Z, Wang Z, Cao Y, et al. miR-28-5p-IL-34-macrophage feedback loop modulates hepatocellular carcinoma metastasis. Hepatology. (2016) 63:1560–75. doi: 10.1002/hep.28445
122. Wang Y, Wang B, Xiao S, Li Y, Chen Q. miR-125a/b inhibits tumor-associated macrophages mediated in cancer stem cells of hepatocellular carcinoma by targeting CD90. J Cell Biochem. (2019) 120:3046–55. doi: 10.1002/jcb.27436
123. Li J, Xue J, Ling M, Sun J, Xiao T, Dai X, et al. MicroRNA-15b in extracellular vesicles from arsenite-treated macrophages promotes the progression of hepatocellular carcinomas by blocking the LATS1-mediated Hippo pathway. Cancer Lett. (2021) 497:137–53. doi: 10.1016/j.canlet.2020.10.023
124. Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. (2011) 43:904–14. doi: 10.1016/j.molcel.2011.08.018
125. Han D, Fang Y, Guo Y, Hong W, Tu J, Wei W. The emerging role of long non-coding RNAs in tumor-associated macrophages. J Cancer. (2019) 10:6738–46. doi: 10.7150/jca.35770
126. Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. (2015) 47:199–208. doi: 10.1038/ng.3192
127. Ye Y, Xu Y, Lai Y, He W, Li Y, Wang R, et al. Long non-coding RNA cox-2 prevents immune evasion and metastasis of hepatocellular carcinoma by altering M1/M2 macrophage polarization. J Cell Biochem. (2018) 119:2951–63. doi: 10.1002/jcb.26509
128. Ye Y, Guo J, Xiao P, Ning J, Zhang R, Liu P, et al. Macrophages-induced long noncoding RNA H19 up-regulation triggers and activates the miR-193b/MAPK1 axis and promotes cell aggressiveness in hepatocellular carcinoma. Cancer Lett. (2020) 469:310–22. doi: 10.1016/j.canlet.2019.11.001
129. Carloni V, Luong TV, Rombouts K. Hepatic stellate cells and extracellular matrix in hepatocellular carcinoma: more complicated than ever. Liver Int. (2014) 34:834–43. doi: 10.1111/liv.12465
130. Hadler-Olsen E, Fadnes B, Sylte I, Uhlin-Hansen L, Winberg JO. Regulation of matrix metalloproteinase activity in health and disease. FEBS J. (2011) 278:28–45. doi: 10.1111/j.1742-4658.2010.07920.x
131. Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. (2000) 10:415–33. doi: 10.1006/scbi.2000.0379
132. Jackson HW, Defamie V, Waterhouse P, Khokha R. TIMPs: versatile extracellular regulators in cancer. Nat Rev Cancer. (2017) 17:38–53. doi: 10.1038/nrc.2016.115
133. Han TS, Ban HS, Hur K, Cho HS. The epigenetic regulation of HCC metastasis. Int J Mol Sci. (2018) 19:3978: doi: 10.3390/ijms19123978
134. Chernov AV, Strongin AY. Epigenetic regulation of matrix metalloproteinases and their collagen substrates in cancer. Biomol Concepts. (2011) 2:135–147. doi: 10.1515/bmc.2011.017
135. Shen B, Jiang Y, Chen YR, Zheng HC, Zeng W, Li YY, et al. Expression and inhibitory role of TIMP-3 in hepatocellular carcinoma. Oncol Rep. (2016) 36:494–502. doi: 10.3892/or.2016.4818
136. Yuan Y, Wang J, Li J, Wang L, Li M, Yang Z, et al. Frequent epigenetic inactivation of spleen tyrosine kinase gene in human hepatocellular carcinoma. Clin Cancer Res. (2006) 12:6687–95. doi: 10.1158/1078-0432.CCR-06-0921
137. Yang Z, Liu Y, Qin L, Wu P, Xia Z, Luo M, et al. Cathepsin H-mediated degradation of HDAC4 for matrix metalloproteinase expression in hepatic stellate cells: implications of epigenetic suppression of matrix metalloproteinases in fibrosis through stabilization of class IIa histone deacetylases. Am J Pathol. (2017) 187:781–97. doi: 10.1016/j.ajpath.2016.12.001
138. Kaneko F, Saito H, Saito Y, Wakabayashi K, Nakamoto N, Tada S, et al. Down-regulation of matrix-invasive potential of human liver cancer cells by type I interferon and a histone deacetylase inhibitor sodium butyrate. Int J Oncol. (2004) 24:837–45. doi: 10.3892/ijo.24.4.837
139. Atta H, El-Rehany M, Hammam O, Abdel-Ghany H, Ramzy M, Roderfeld M, et al. Mutant MMP-9 and HGF gene transfer enhance resolution of CCl4-induced liver fibrosis in rats: role of ASH1 and EZH2 methyltransferases repression. PloS ONE. (2014) 9:e112384. doi: 10.1371/journal.pone.0112384
140. Wentz-Hunter KK, Potashkin JA. The role of miRNAs as key regulators in the neoplastic microenvironment. Mol Biol Int. (2011) 2011:839872. doi: 10.4061/2011/839872
141. Fang JH, Zhou HC, Zeng C, Yang J, Liu Y, Huang X, et al. MicroRNA-29b suppresses tumor angiogenesis, invasion, and metastasis by regulating matrix metalloproteinase 2 expression. Hepatology. (2011) 54:1729–40. doi: 10.1002/hep.24577
142. Xiao D, Gao HX. Mechanism of miR-107-targeting of regulator of G-protein signaling 4 in hepatocellular carcinoma. Oncol Lett. (2019) 18:5145–54. doi: 10.3892/ol.2019.10857
143. Li JZ, Li J, Liu BZ. MicroRNA-328-3p inhibits malignant progression of hepatocellular carcinoma by regulating MMP-9 level. Eur Rev Med Pharmacol Sci. (2019) 23:9331–40. doi: 10.26355/eurrev_201911_19426
144. Chen X, Bo L, Zhao X, Chen Q. MicroRNA-133a inhibits cell proliferation, colony formation ability, migration and invasion by targeting matrix metallopeptidase 9 in hepatocellular carcinoma. Mol Med Rep. (2015) 11:3900–7. doi: 10.3892/mmr.2015.3232
145. He Y, Cui Y, Wang W, Gu J, Guo S, Ma K, et al. Hypomethylation of the hsa-miR-191 locus causes high expression of hsa-mir-191 and promotes the epithelial-to-mesenchymal transition in hepatocellular carcinoma. Neoplasia. (2011) 13:841–53. doi: 10.1593/neo.11698
146. Wang B, Hsu SH, Majumder S, Kutay H, Huang W, Jacob ST, et al. TGFbeta-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene. (2010) 29:1787–97. doi: 10.1038/onc.2009.468
147. Garofalo M, Di Leva G, Romano G, Nuovo G, Suh SS, Ngankeu A, et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell. (2009) 16:498–509. doi: 10.1016/j.ccr.2009.10.014
Keywords: tumor microenvironment, DNA methylation, histone methyltransferases, histone deacetylases, microRNAs, hepatic stellate cells, tumor-associated macrophages, extracellular matrix
Citation: Wang F, Malnassy G and Qiu W (2021) The Epigenetic Regulation of Microenvironment in Hepatocellular Carcinoma. Front. Oncol. 11:653037. doi: 10.3389/fonc.2021.653037
Received: 13 January 2021; Accepted: 22 February 2021;
Published: 15 March 2021.
Edited by:
Dongshi Chen, University of Pittsburgh, United StatesReviewed by:
Chiung-Kuei Huang, Indiana University School of Medicine-Lafayette, United StatesAlessandro Carrer, Veneto Institute of Molecular Medicine (VIMM), Italy
Copyright © 2021 Wang, Malnassy and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Qiu, d3FpdUBsdWMuZWR1
 Fang Wang
Fang Wang Greg Malnassy
Greg Malnassy Wei Qiu
Wei Qiu