
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 22 April 2021
Sec. Women's Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.652458
This article is part of the Research TopicFuture Perspectives of Sentinel Node Mapping in Gynecological OncologyView all 11 articles
Background: This study aimed to evaluate the clinical value of indocyanine green sentinel lymph node (SLN) mapping in patients with vulvar cancer. The conventional procedure of SLN mapping in vulvar cancer includes peritumoral injection of technetium-99m nanocolloid before surgery and intraoperative injection of a blue dye. However, these techniques harbor some limitations. Near-infrared fluorescence imaging with indocyanine green has gained popularity in SLN mapping in different types of cancer.
Methods: We analyzed retrospectively vulvar cancer patients at our institution between 2013 and 2020 undergoing indocyanine green SLN mapping by applying video telescope operating microscope system technology.
Results: 64 groins of 34 patients were analyzed. In 53 groins we used technetium-99m nanocolloid, in four patent blue, and in five both techniques, additionally to indocyanine green for SLN detection. In total, 120 SLNs were identified and removed. The SLN detection rate of indocyanine green was comparable to technetium-99m nanocolloid (p=.143) and higher than patent blue (p=.003). The best results were achieved using a combination of ICG and technetium-99m nanocolloid (detection rate of 96.9%). SLN detection rates of indocyanine green were significantly higher in patients with positive lymph nodes (p=.035) and lymphatic space invasion (p=.004) compared to technetium-99m nanocolloid.
Conclusion: Indocyanine green SLN mapping in vulvar cancer is feasible and safe, with reasonable detection rates. Due to its easy application and few side effects, it offers a sound alternative to the conventional SLN mapping techniques in vulvar cancer. In patients with lymph node metastasis, indocyanine green even outperformed technetium-99m nanocolloid in terms of detection rate.
Inguinal lymph node status represents the most significant prognostic factor for survival in vulvar cancer patients (1). Lymphadenectomy therefore plays a crucial role in both surgical treatment and staging of vulvar cancer. Complete inguinofemoral lymph node dissection leads to a short- and long-term morbidity, consisting of wound infections or dehiscence and lymph edema in up to 50% of all patients (2). However, only one third of patients with stage I or II disease have lymph node metastasis and consequently up to two thirds undergo unnecessary lymphadenectomy (3), associated with high morbidity and prolonged hospitalization (4).
The introduction of sentinel lymph node (SLN) biopsy in vulvar cancer, first described by Levenback in 1994 (5), provides a less invasive technique for staging of vulvar cancer than complete inguinofemoral lymph node dissection, with significant reduction in lymphedema, wound infection, and dehiscence without compromising groin recurrence rates or survival rates (6, 7). SLN biopsy has been shown to be oncologically safe in unifocal squamous-cell vulvar cancer up to a tumor size of 4 cm with clinically negative lymph nodes (6, 8) and with a low false negative rate of approximately 3% (9).
The conventional technique of SLN mapping in vulvar cancer involves a peritumoral injection of technetium-99m (99mTc) nanocolloid before surgery combined with an intraoperative injection of a blue dye. Preoperative 3D single photon emission tomography imaging helps detecting the SLN more precisely regarding number and anatomical localization (10). However, these techniques harbor some limitations: (a) the preoperative injection of radiotracers involves a painful procedure for the patient; (b) the transport and storage of radioactivity requires complex logistics; (c) blue dyes may lead to staining of the injection site and to allergic reactions; and (d) visualization of the blue dye is limited when the lymphatic tissue is covered by skin or fat – resulting in a lower detection rate.
In cervical and endometrial cancer, SLN mapping with near-infrared fluorescence imaging using indocyanine green (ICG) has shown better overall and bilateral detection rates as compared to a combination of blue dye and 99mTc-nanocolloid, a better safety profile than blue dyes, and an easier application than 99mTc-nanocolloid (11–14). Furthermore, ICG and near-infrared fluorescence imaging outperformed blue dye for SLN detection in skin cancer (15) and in breast cancer (16).
Until now, several studies demonstrated the technical feasibility and safety of ICG in SLN mapping in vulvar cancers, though most of them are case series characterized with methodological variations and lack of standardization. The aim of this study was to evaluate the SLN detection rate of ICG in vulvar cancer compared to the conventional technique using 99mTc-nanocolloid and blue dye in a large cohort of patients and to analyze its applicability in different risk groups.
We retrospectively investigated patients with histologically proven vulvar cancer who were operated at the certified cancer center of the Bern University Hospital, Switzerland between April 2013 and April 2020. The experimental protocols was approved by the Ethics Commission of the Canton of Bern, Switzerland (reference number: 261/2015) and meets the guidelines of the responsible governmental agency. All patients signed informed consent. Demographic, clinical, and intraoperative data were retrieved from an electronic database.
All patients underwent inguinal SLN mapping using near-infrared fluorescence imaging with ICG, applying video telescope operating microscope system technology (VITOM ICG® by Karl Storz GmbH, Germany) (Figure 1). In every case, at least one additional tracer (99mTc-nanocolloid and/or patent blue) was used. After skin incision, a gentle dissection of the fatty tissue was performed. Under near-infrared imaging, the groin was inspected for fluorescence. The groin was tested systematically for radioactivity using a handheld gamma probe and, if applicable, for blue staining by visual inspection. In accordance with international guidelines, a bilateral SLN biopsy was performed if the tumor site was located 1 cm or less from the midline. All ICG, 99mTc-nanocolloid, or patent blue-positive lymph nodes were excised and sent for frozen section. If frozen section analysis revealed lymph node metastases, a complete inguinofemoral lymphadenectomy was performed. Following the SLN extirpation procedure, tumor excision was performed, consisting of a radical local excision or a vulvectomy, in function of the size and location of the tumor. Surgeries were undertaken by a team of three experienced gynecologic oncologists. For final pathology, ultrastaging of all SLN was performed.
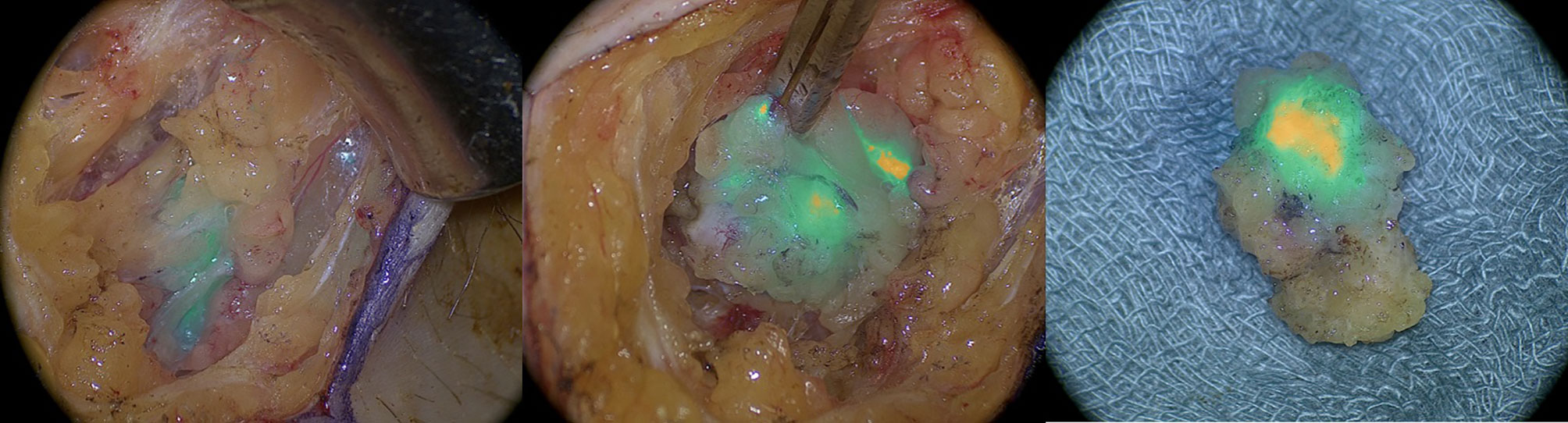
Figure 1 Intraoperative imaging of indocyanine green positive lymphatic channels and inguinal sentinel lymph node with near infrared imaging.
Injection of 99mTc-nanocolloid: A SLN scintigraphy was performed one day before surgery. Four aliquots of 15 MBq 99mTc-labeled nanocolloids (Nano-HSA®, produced by Rotop Pharmaka GmbH, Dresden, Germany, particle size ≤80 nm) were injected intradermally adjacent to the tumor. After this procedure, a single-photon emission computed tomography combined with conventional computed tomography (SPECT/CT) was carried out.
Injection of ICG: One vial of 25mg ICG powder (Verdye®, produced by Diagnostic Green GmbH, Germany) was suspended in 10 ml of sterile water and injected intradermally directly before surgery at four injection sites around the tumor (Figure 2).
Injection of patent blue: A peritumoral intradermal injection of a total amount of 4ml patent blue (Patentblau V Guerbet® 25mg/ml, produced by Guerbet AG, Zurich, Switzerland) was performed immediately before surgery.
A false negative SLN was defined as a SLN with negative tumor involvement detected with one SLN mapping technique in combination with a metastatic SLN detected with another SLN mapping technique or a metastatic non-SLN. The SLN detection rate was calculated for each SLN mapping technique, defined as the number of procedures in which at least one SLN was identified divided by the total number of procedures performed. Detection rates among the different subgroups were compared using the chi-square test. Statistical calculations were performed using the Statistical Package for Social Sciences (IBM SPSS Statistic Version 25.0).
In accordance with the journal’s guidelines, we will provide our data for the reproducibility of this study in other centers if this is requested.
Between April 2013 and April 2020, 34 patients were analyzed retrospectively for this study. Patient demographics and operative data are presented in Table 1. The majority of the patients had FIGO stage IB disease with a median age of 71.0 years and a median body mass index (BMI) of 27.85 kg/m2. The median tumor size in final pathology was 2.50 cm with a median depth of infiltration of 5.50 mm. Final histology was a squamous epithelial carcinoma in all of the cases. Adjuvant treatment was performed in eight patients. Groin recurrence rate was 2.9% with a mean follow up time of 29.9 months.
Of the 34 patients, SLN mapping was performed in 64 groins with 30 patients having bilateral SLN biopsy and four patients having only unilateral. The mean amount of ICG injected was 8.4 ml (range 5 to 10 ml). No intra- or postoperative complications occurred due to the administration of ICG. In 51 groins (79.7%), a SLN biopsy alone was performed while in 13 groins (20.3%) an additional complete inguinofemoral lymphadenectomy was performed. The mean number of SLNs per groin removed was 1.88. In addition to using ICG for SLN mapping, in 53 groins we used 99mTc-nanocolloid, in four groins patent blue, and in five groins both methods. In total, 120 SLNs were identified and removed, of which 103 (85.8%) were positive for ICG. In 10 groins (15.6%), we found lymph node metastases; in eight of these a SLN was detected. In seven groins no further positive lymph nodes were identified at final pathology in addition to the SLN, while in one groin two positive non-SLNs were detected in the final pathology of the complete lymph node dissection. No additional SLN was found to have metastatic disease using ultrastaging in the final pathology. In one patient (both groins affected), SLN mapping was unsuccessful using each of the three techniques. This patient therefore underwent bilateral complete inguinofemoral lymph node dissection. No false negative sentinel lymph nodes were recorded with ICG in the 13 patients who underwent complete lymphadenectomy.
The SLN detection rate of ICG (87.5%) was comparable to 99mTc-nanocolloid (89.7%, p=.143) and significantly higher than patent blue (77.8%, p=0.003) (Table 2). The best detection rates were achieved using a combination of ICG and 99mTc-nanocolloid (96.9%).
In 19 groins the tumors showed lymph vascular space invasion and in 10 groins showed positive lymph nodes. In these cases, the SLN detection rate of ICG was significantly higher than that of 99mTc-nanocolloid (p values of .004 and .035 respectively) (Table 3). Furthermore, we observed a higher detection rate of ICG compared to 99mTc-nanocolloid in obese patients (BMI > 30 kg/m2), although statistically not significant (p=.707).
Accurate SLN mapping is a crucial part of vulvar cancer staging, and enables avoiding unnecessary inguinofemoral lymphadenectomies. The current standard SLN procedure consists of a combination of a radioactive tracer and a blue dye. The SLN detection rates reported in the literature are 63-82%, 88-96%, and 91-98% for blue dye, 99mTc-nanocolloid, and the combination of both, respectively (17–19). Although these techniques show reasonable results in terms of detection and false negative rates, they have some shortcomings, including painful injections, complex logistics, and allergic reactions. SLN mapping with near-infrared fluorescence imaging has recently gained popularity in gynecological cancers (11, 13). Advantages include easier application, absence of radioactivity, and fewer side effects.
In our study, SLN mapping with ICG and near-infrared fluorescence imaging in vulvar cancer was feasible and safe. SLN detection with near-infrared fluorescence imaging performed equally well as 99mTc-nanocolloid (87.5% vs 89.7%, p=0.143) and significantly better than patent blue alone (87.5% vs 77.8%, p= 0.003); the best results were achieved using a combination of ICG and 99mTc-nanocolloid (96.9%). In patients with lymph node metastases or lymph vascular space invasion, ICG alone outperformed 99mTc-nanocolloid, with a significantly higher detection rate. For the conventional SLN mapping techniques, a compromised detection rate in lymph node positive patients is described in the literature, as a result of the complete replacement of true SNL by tumor cells and a redirection of the lymphatic vessels to other nodes (17, 20). However, particularly in these patients, a reliable SLN mapping is of utmost importance. 2019 Frumovitz et al. described a superior detection rate of ICG compared to blue dye in case of metastatic sentinel lymph nodes in endometrial and cervical cancer patients (21). Furthermore, several studies describe a restricted application for ICG in obese patients due to its limited tissue penetration, with an increased BMI identified as a potential risk factor for failure in SLN mapping (22–25). Results obtained from our cohort do not support this assumption, as the detection rates of ICG and 99mTc-nanocolloid did not differ significantly in obese patients, even with a slight tendency towards a higher detection rate with ICG in obese patients (94.7 vs 88.2%, p=0.707). Over all groin recurrence rate was 2.9%, which is consistent with the literature (6). The only patient with recurrence was successfully mapped with ICG and 99mTc-nanocolloid revealing two negative SLNs.
Up to now, several studies reported reasonable results in ICG SLN mapping for vulvar cancer patients (Table 4). The largest cohort was described by Broach et al. with ICG SLN mapping in 85 patients with different histological subtypes of vulvar cancer, including melanomas and less frequent tumors (26). The further studies are mainly case series of fewer than 20 patients (22, 24, 27–30), with methodological variations. For instance, ICG was administered in different formats: either absorbed in human serum albumin (23, 27) or as the hybrid tracer ICG-99mTc-nanocolloid (28, 30). Different near-infrared fluorescence imaging devices were applied, some of which were custom made (22, 29) and others commercially available [VITOM® II ICG exoscope (24, 31), the Mini-FLARE™ imaging system (23, 27, 28), Photodynamic Eye (30)]. In one exploratory study, the imaging device was changed during the course of the study from SPY® to PinPoint® (32). In addition, few case reports have been published on feasibility and safety of robot-assisted SLN mapping with ICG in vulvar cancer patients (33, 34). This minimally invasive approach might be a valid option to further reduce short- and long-term morbidity in these patients. However, follow-up data on a larger cohort of patients are needed. Several studies focused on the sensitivity of ICG compared to 99mTc-nanocolloid (23, 27, 30, 31). After injection, ICG travels via lymphatic vessels to the SLN as well as to echelon and second-echelon lymph nodes, potentially leading to the removal of additional, non-SLNs. Therefore, the number of lymph nodes removed is less important: the crucial point is removing the right lymph nodes. In cervical and endometrial cancer, a retrospective analysis demonstrated that a higher SLN count did not seem to increase the accuracy of SLN mapping (35). In our opinion, the SLN detection rate is the more reliable variable to investigate. Another important test characteristic is the false negative rate. As the majority of our patients did not undergo complete lymphadenectomy, we are not able to establish a false negative rate with our data.
To our knowledge, this study contains one of the largest cohort of ICG SLN mapping in squamous cell vulvar cancer patients to date. Beside its relatively large sample size, its major strengths include the risk group analysis of patients. This research adds to a growing body of literature supporting the use of ICG in SLN mapping in vulvar cancer patients. One of its most interesting aspects is the improvement of the SLN detection rate using a combination of 99mTc-nanocolloid with ICG. Based on our findings, a combination of ICG and 99mTc-nanocolloid offers a reasonable alternative to the conventional SLN mapping techniques in vulvar cancer. However, the major limitation of our study is the inability to determine if ICG alone improves the SLN detection rate; specifically the value of the additional information of the SPECT/CT performed preoperatively cannot be defined in this setting.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Commission of the Canton of Bern, Switzerland. The patients/participants provided their written informed consent to participate in this study.
SI, AP and MM contributed to conception and design of the study. SM and LK organized the database. FS and SI performed the statistical analysis. FS wrote the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Lisa Cuthbertson for the illustration of the injection of indocyanine green (Figure 2).
1. Burger MPM, Krans M, Bouma J, Hollema H, Emanuels AG, Pras E. The Importance of the Groin Node Status for the Survival of T1 and T2 Vulval Carcinoma Patients. Gynecol Oncol (1995) 57(3):327–34. doi: 10.1006/gyno.1995.1151
2. Hinten F, Van Den Einden LCG, Hendriks JCM, Van Der Zee AGJ, Bulten J, Massuger LFAG, et al. Risk Factors for Short-and Long-Term Complications After Groin Surgery in Vulvar Cancer. Br J Cancer (2011) 105(9):1279–87. doi: 10.1038/bjc.2011.407
3. de Hullu JA, van der Zee AGJ. Surgery and Radiotherapy in Vulvar Cancer. Crit Rev Oncol Hematol (2006) 60(1):38–58. doi: 10.1016/j.critrevonc.2006.02.008
4. Johann S, Klaeser B, Krause T, Mueller MD. Comparison of Outcome and Recurrence-Free Survival After Sentinel Lymph Node Biopsy and Lymphadenectomy in Vulvar Cancer. Gynecol Oncol (2008) 110(3):324–8. doi: 10.1016/j.ygyno.2008.04.004
5. Levenback C, Burke TW, Gershenson DM, Morris M, Malpica A, Ross MI. Intraoperative Lymphatic Mapping for Vulvar Cancer. Obstet Gynecol (1994) 84(2):163–7. doi: 10.1006/gyno.1995.0011
6. Van Der Zee AGJ, Oonk MH, De Hullu JA, Ansink AC, Vergote I, Verheijen RH, et al. Sentinel Node Dissection is Safe in the Treatment of Early-Stage Vulvar Cancer. J Clin Oncol (2008) 26(6):884–9. doi: 10.1200/JCO.2007.14.0566
7. Te Grootenhuis NC, Van Der Zee AGJ, Van Doorn HC, Van der Velden J, Vergote I, Zanagnolo V, et al. Sentinel Nodes in Vulvar Cancer: Long-Term Follow-Up of the Groningen International Study on Sentinel Nodes in Vulvar Cancer (GROINSS-V) I. Gynecol Oncol (2016) 140(1):8–14. doi: 10.1016/j.ygyno.2015.09.077
8. Brincat MR, Baron YM. Sentinel Lymph Node Biopsy in the Management of Vulvar Carcinoma: An Evidence-Based Insight. Int J Gynecol Cancer (2017) 27(8):1769–73. doi: 10.1097/IGC.0000000000001075
9. Levenback CF, Ali S, Coleman RL, Gold MA, Fowler JM, Judson PL, et al. Lymphatic Mapping and Sentinel Lymph Node Biopsy in Women With Squamous Cell Carcinoma of the Vulva: A Gynecologic Oncology Group Study. J Clin Oncol (2012) 30(31):3786–91. doi: 10.1200/JCO.2011.41.2528
10. Beneder C, Fuechsel FG, Krause T, Kuhn A, Mueller MD. The Role of 3D Fusion Imaging in Sentinel Lymphadenectomy for Vulvar Cancer. Gynecol Oncol (2008) 109(1):76–80. doi: 10.1016/j.ygyno.2007.11.045
11. Cibula D, Oonk MHM, Abu-Rustum NR. Sentinel Lymph Node Biopsy in the Management of Gynecologic Cancer. Curr Opin Obstet Gynecol (2015) 27(1):66–72. doi: 10.1097/GCO.0000000000000133
12. Darin MC, Gómez-Hidalgo NR, Westin SN, Soliman PT, Escobar PF, Frumovitz M, et al. Role of Indocyanine Green in Sentinel Node Mapping in Gynecologic Cancer: is Fluorescence Imaging the New Standard? J Minim Invasive Gynecol (2016) 23(2):186–93. doi: 10.1016/j.jmig.2015.10.011
13. Papadia A, Imboden S, Siegenthaler F, Gasparri ML, Mohr S, Lanz S, et al. Laparoscopic Indocyanine Green Sentinel Lymph Node Mapping in Endometrial Cancer. Ann Surg Oncol (2016) 23(7):2206–11. doi: 10.1245/s10434-016-5090-x
14. Ruscito I, Gasparri ML, Braicu EI, Bellati F, Raio L, Sehouli J, et al. Sentinel Node Mapping in Cervical and Endometrial Cancer: Indocyanine Green Versus Other Conventional Dyes—A Meta-Analysis. Ann Surg Oncol (2016) 23(11):3749–56. doi: 10.1245/s10434-016-5236-x
15. Burnier P, Niddam J, Bosc R, Hersant B, Meningaud JP. Indocyanine Green Applications in Plastic Surgery: A Review of the Literature. J Plast Reconstr Aesthet Surg (2017) 70(6):814–27. doi: 10.1016/j.bjps.2017.01.020
16. Hutteman M, Mieog JSD, Van Der Vorst JR, Liefers GJ, Putter H, Löwik CWGM, et al. Randomized, Double-Blind Comparison of Indocyanine Green With or Without Albumin Premixing for Near-Infrared Fluorescence Imaging of Sentinel Lymph Nodes in Breast Cancer Patients. Breast Cancer Res Treat (2011) 127(1):163–70. doi: 10.1007/s10549-011-1419-0
17. Hassanzade M, Attaran M, Treglia G, Yousefi Z, Sadeghi R. Lymphatic Mapping and Sentinel Node Biopsy in Squamous Cell Carcinoma of the Vulva: Systematic Review and Meta-Analysis of the Literature. Gynecol Oncol (2013) 130(1):237–45. doi: 10.1016/j.ygyno.2013.04.023
18. Meads C, Sutton AJ, Rosenthal AN, Malysiak S, Kowalska M, Zapalska A, et al. Sentinel Lymph Node Biopsy in Vulval Cancer: Systematic Review and Meta-Analysis. Br J Cancer (2014) 110(12):2837–46. doi: 10.1038/bjc.2014.205
19. Lawrie TA, Patel A, Martin-Hirsch PPL, Bryant A, Ratnavelu NDG, Naik R, et al. Sentinel Node Assessment for Diagnosis of Groin Lymph Node Involvement in Vulval Cancer (Review). Cochrane Database Syst Rev (2014) 2014(6):CD010409. doi: 10.1002/14651858
20. De Hullu JA, Oonk MHM, Ansink AC, Hollema H, Jager PL, Van Der Zee AGJ. Pitfalls in the Sentinel Lymph Node Procedure in Vulvar Cancer. Gynecol Oncol (2004) 94(1):10–5. doi: 10.1016/j.ygyno.2004.02.031
21. Frumovitz PM, Plante PM, Lee PS, Sandadi S, Lilja JF, Escobar PF, et al. A Randomized Phase III Multicenter Study Assessing Near Infrared Fluorescence in the Detection of Sentinel Lymph Nodes in Women With Cervical and Uterine Cancers: The FILM Trial. Lancet Oncol (2019) 19(10):1394–403. doi: 10.1016/S1470-2045(18)30448-0.A
22. Crane LMA, Themelis G, Arts HJG, Buddingh KT, Brouwers AH, Ntziachristos V, et al. Intraoperative Near-Infrared Fluorescence Imaging for Sentinel Lymph Node Detection in Vulvar Cancer: First Clinical Results. Gynecol Oncol (2011) 120(2):291–5. doi: 10.1016/j.ygyno.2010.10.009
23. Schaafsma BE, Verbeek FPR, Peters AAW, Van der Vorst JR, de Kroon CD, van Poelgeest MIE, et al. Near-Infrared Fluorescence Sentinel Lymph Node Biopsy in Vulvar Cancer: A Randomised Comparison of Lymphatic Tracers. BJOG (2013) 120(6):758–64. doi: 10.1111/1471-0528.12173
24. Buda A, Dell’Anna T, Vecchione F, Verri D, Di Martino G, Milani R. Near-Infrared Sentinel Lymph Node Mapping With Indocyanine Green Using the VITOM II ICG Exoscope for Open Surgery for Gynecologic Malignancies. J Minim Invasive Gynecol (2016) 23(4):628–32. doi: 10.1016/j.jmig.2016.02.015
25. Vahrmeijer AL, Hutteman M, Van Der Vorst JR, Van De Velde CJH, Frangioni JV. Image-Guided Cancer Surgery Using Near-Infrared Fluorescence. Nat Rev Clin Oncol (2013) 10(9):507–18. doi: 10.1038/nrclinonc.2013.123
26. Broach V, Abu- NR, Sonoda Y, Brown CL, Jewell E, Gardner G, et al. Evolution and Outcomes of Sentinel Lymph Node Mapping in Vulvar Cancer. Int J Gynecol Cancer (2020) 30(3):383–6. doi: 10.1136/ijgc-2019-000936
27. Hutteman M, Van Der Vorst JR, Gaarenstroom KN, Peters AAW, Mieog JSD, Schaafsma BE, et al. Optimization of Near-Infrared Fluorescent Sentinel Lymph Node Mapping for Vulvar Cancer. Am J Obstet Gynecol (2012) 206(1):89.e1–5. doi: 10.1016/j.ajog.2011.07.039
28. Verbeek FPR, Tummers QRJG, Rietbergen DDD, Peters AAW, Schaafsma BE, van de Velde CJH, et al. Sentinel Lymph Node Biopsy in Vulvar Cancer Using Combined Radioactive and Fluorescence Guidance. Int J Gynecol Cancer (2015) 25(6):1086–93. doi: 10.1097/IGC.0000000000000419
29. Laios A, Volpi D, Tullis IDC, Woodward M, Kennedy S, Pathiraja S, et al. A Prospective Pilot Study of Detection of Sentinel Lymph Nodes in Gynaecological Cancers Using a Novel Near Infrared Fluorescence Imaging System Medical Imaging. BMC Res Notes (2015) 8(1):1–9. doi: 10.1186/s13104-015-1576-z
30. Mathéron HM, Van Den Berg NS, Brouwer OR, Kleinjan GH, van Driel WJ, Trum JW, et al. Multimodal Surgical Guidance Towards the Sentinel Node in Vulvar Cancer. Gynecol Oncol (2013) 131(3):720–5. doi: 10.1016/j.ygyno.2013.09.007
31. Soergel P, Hertel H, Nacke AK, Klapdor R, Derlin T, Hillemanns P. Sentinel Lymphadenectomy in Vulvar Cancer Using Near-Infrared Fluorescence From Indocyanine Green Compared With Technetium 99m Nanocolloid. Int J Gynecol Cancer (2017) 27(4):805–12. doi: 10.1097/IGC.0000000000000996
32. Prader S, Philipp B, Elisabeth H, Schneider S, Baert T, Ataseven B. Sentinel Lymph Node Mapping With Fluorescent and Radioactive Tracers in Vulvar Cancer Patients. Arch Gynecol Obstet (2020) 301(3):729–36. doi: 10.1007/s00404-019-05415-2
33. Mohammad A, Hunter M. Robot-Assisted Sentinel Lymph Node Mapping and Inguinal Lymph Node Dissection, Using Near-Infrared Fluorescence, in Vulvar Cancer. J Minim Invasive Gynecol (2019) 26(5):968–72. doi: 10.1016/j.jmig.2019.04.002
34. Naldini A, Vizzielli G, Perrone E, Gallotta V, Scambia G. Robotic Video Endoscopic Inguinal Lymphadenectomy (R-VEIL) for Vulvar Cancer With Sentinel Node Mapping Using Indocyanine Green and Near-Infrared Fluorescence Imaging Technology. Gynecol Oncol (2018) 150(1):203–4. doi: 10.1016/j.ygyno.2018.04.568
Keywords: vulvar cancer, sentinel lymph node, indocyanine green, near-infrared imaging, Technetium-99m
Citation: Siegenthaler F, Imboden S, Knabben L, Mohr S, Papadia A and Mueller MD (2021) Exploratory Study of the Clinical Value of Near-Infrared Sentinel Lymph Node Mapping With Indocyanine Green in Vulvar Cancer Patients. Front. Oncol. 11:652458. doi: 10.3389/fonc.2021.652458
Received: 12 January 2021; Accepted: 30 March 2021;
Published: 22 April 2021.
Edited by:
Sarah M. Temkin, Anne Arundel Medical Center, United StatesReviewed by:
Edward James Tanner, Northwestern University, United StatesCopyright © 2021 Siegenthaler, Imboden, Knabben, Mohr, Papadia and Mueller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Franziska Siegenthaler, ZnJhbnppc2thLnNpZWdlbnRoYWxlckBpbnNlbC5jaA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.