- 1Department of Thyroid and Breast Surgery, The First Affiliated Hospital of Chengdu Medical College, Chengdu, China
- 2Department of Breast Surgery, Mianyang Central Hospital, Mianyang, China
Background: To evaluate the efficacy and safety of radiofrequency ablation (RFA) of breast cancer smaller than 2 cm.
Methods: A systematic search was conducted in the PubMed and EMBASE databases to identify published studies investigating the efficacy and safety of RFA for breast cancer smaller than 2 cm. The main outcomes were technical success rate of the ablation, complete ablation rate, complications and local recurrence. Secondary considerations were mode of anesthesia, pain tolerance, mean ablation time and surgical excision after ablation.
Results: Seventeen studies involving 399 patients and 401 lesions met the inclusion criteria. Nearly 99%(95%CI=0.98-1.00) of lesions achieved good technical success rate.Notably, 83.88% of the patients received RFA under general anesthesia (333/397) whereas 15.87% received RFA under local anesthesia (63/397). Of the 63, 98.41% tolerated the pain associated with the procedure. Majority of patients (65.74%, 261/397) underwent surgical excision of the tumor after ablation whereas in a few patients (34.26%, 136/397), the tumor tissue was retained in the breast after ablation. Complete ablation was achieved in 96% of patients for a mean time of 15.8 minutes (95%CI=0.93-0.99). Overall, only 2% (95%CI=0.01-0.04) of the individuals developed complications. Skin burns (2.02%, 8/397) were the most common complications. There was no local recurrence after a median follow-up of 27.29 months, whether or not they underwent surgical resection following RFA.
Conclusion: The results show that RFA for breast cancer smaller than 2 cm is safe and effective. However, prospective studies are needed to validate this conclusion.
Introduction
Globally, breast cancer is the most prevalent malignant cancer among women (1). The need for quality life and better aesthetics has reduced the application of invasive cancer interventions. In recent years, RFA has received increasing attention because of its noninvasive nature in the treatment of breast cancer. RFA utilizes radiofrequency alternating current to ablate tissue around a needle electrode, resulting in local coagulative tumor necrosis (2). Previous studies have demonstrated that RFA is a safe and effective (63% to 96%) local treatment of breast cancer, but these findings are based on tumors with size ranging from 0.2cm to 5cm (3–5). Three meta-analyses provided preliminary evidence that RFA can effectively treat breast cancer (6–8). Of the three studies, only one was specific for RFA, and the other two combined five ablation technologies, including microwave, laser, radiofrequency, high-intensity focused ultrasound and cryoablation. Although surgical resection performed after ablation affects the complete ablation rate, occurrence of complications and local recurrence, but they did not perform subgroup analyses to determine this. Most importantly, none of the three meta-analyses limited the size of tumors, an important factor affecting safety and effectiveness of breast tumor ablation. Many studies have shown that RFA is most effective in treating small tumors (9–11). To capture evidence from recent research and eliminate the impact of heterogeneity of tumor size on the results, this meta-analysis was performed focusing on breast tumors smaller than 2 cm.
Methods
Search Strategy
A systematic literature search was performed on the PubMed and Embase databases to identify studies published up to October 1, 2020. The following key terms were used for the search: (“breast cancer, OR breast neoplasm” AND “radiofrequency ablation” OR “radiofrequency” OR “ablation”). The search was not limited to any geographical region or language.
Inclusion and Exclusion Criteria
The inclusion criteria were; 1) patients diagnosed with breast cancer; 2) patients who underwent RFA, regardless of whether there was surgical resection after ablation, immediate resection or delayed resection; 3) studies including at least one clinical outcome (rate of successful ablation, rate of complete ablation, incidences of complications or recurrence). Exclusion criteria were; 1) review articles, letters to the editor, comments, editorials and case reports; 2) Lack of clinical outcome data; 3) studies in which some tumors were larger than 2 cm.
Study Selection and Data Extraction
Data extraction was performed by researchers working independently using a standardized data extraction form. Eligible studies were carefully and systematically reviewed. The following information was obtained: author’s name, year of publication, nationality of patients, number and age of participants, the size of tumor, tumor receptor, histology, nottingham grade, lymph node metastasis, means of image guidance, mode of anesthesia, pain tolerance, mean ablation time, surgical excision, pathological evaluation, median follow-up time, technically successful ablation rate, rate of complete ablation, complications and local recurrence. Technical success rate of ablation was defined as the completion of ablation treatment in terms of technology and good cooperation from patients. Complete ablation was defined as ablation of all tumor tissues as evidenced from imaging and pathological examination, or complete necrosis of tumor tissues after ablation. Local recurrence was defined as incomplete local treatment and recurrence in the breast distant from the ablation zone.
Quality Assessment
Eight items from the non-randomized experimental research-MINORS scale established by Slim et al. were used to evaluate the quality of the selected literature (12). Study quality was scored as 0 (not reported), 1 (inadequately reported), or 2 (adequately reported). A score greater than 12 was considered high quality, 8-12 was considered medium quality, and less than 8 was considered low quality. To ensure quality results, we only retained studies with scores of 8 or more (Supplementary Table 1).
Summary Measures and Statistical Analysis
The principal outcomes were technical success rate, complete ablation rate and rate of complications. These rates were combined to perform subgroup analysis based on whether surgical resection was performed after ablation. Meta-analysis was conducted using R Project (R version 3.6.2 for Windows) with the “meta” package. Chi-squared and I2 were used to measure statistical heterogeneity among the studies. When I2>50%, the studies were considered to have significantly high heterogeneity, thus, random-effects model was used (13). Otherwise, the fixed-effects model was used. All tests were two-sided with a P < 0.05 considered statistically significant. Publication bias was evaluated using a funnel plot and Egger’s test (14). Trim and fill analyses were performed if there was publication bias.
Results
Study Selection and the Characteristics of Studies
A total of 1689 articles were identified, among which 953 were selected after removal of duplicates. After reading the title or abstract, 908 articles irrelevant to this study were also removed. Based on the inclusion and exclusion criteria, 28 more articles were excluded. In the end, 17 studies covering 399 patients and 401 lesions were included in the final analyses (10, 11, 15–29). All included studies were experimental studies. The study selection process for the meta-analysis is shown in Figure 1.
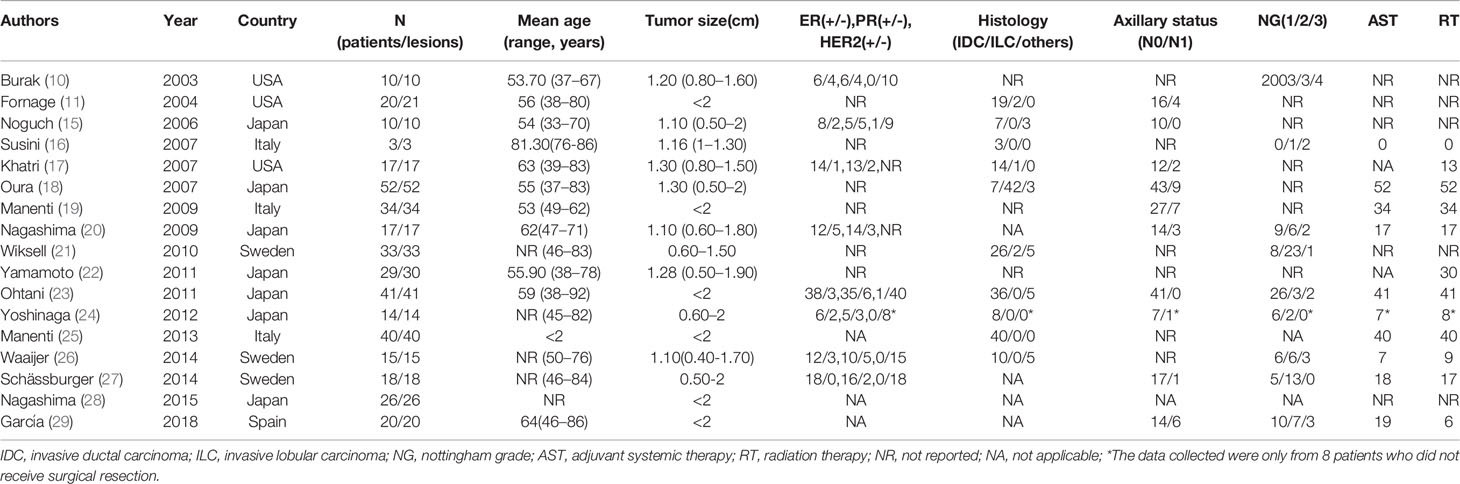
Characteristics of the included studies are shown in Table 1. The number of patients in individual studies ranged from 3 to 52, and aged between 33 to 92 years old. The size of the tumors ranged between 0.4 to 2 cm. Collectively, 399 patients and 401 lesions were analyzed as shown in Table 2.
Technical Success Rate
Technical success rate ranged from 86.67% to 100%. Of the 401 lesions, 7 (1.75%) were not successfully ablated (17, 21, 23, 26). Incomplete ablations were attributed to technical failure such as probe placement (0.75%,3/401) (21, 26), poor ultrasound imaging of the tumor (0.5%,2/401) (17, 21), uncooperative patients (0.25%,1/401) (17) and unbearable pain (0.25%,1/401) (23). Of the 7 patients who were not successfully ablated, 4 were excluded after pre-ablation assessment (17, 21), and 3 underwent ablation but did not complete the treatment (23, 26), so only 397 of the 401 lesions actually underwent ablation. Without heterogeneity (I2 = 0%, P=0.97), the combined technical success rate was 99% (95%CI=0.98-1.00). In the subgroup analysis based on whether surgical resection was performed after ablation, the success rate of ablation without surgical resection after ablation was 100% (95%CI=0.98-1.00, I2 = 0%, P=1), and the success rate of ablation with surgical operation following RFA was 99% (95%CI=0.97-1.00, I2 = 0%, P=0.83) as shown in Figure 2.
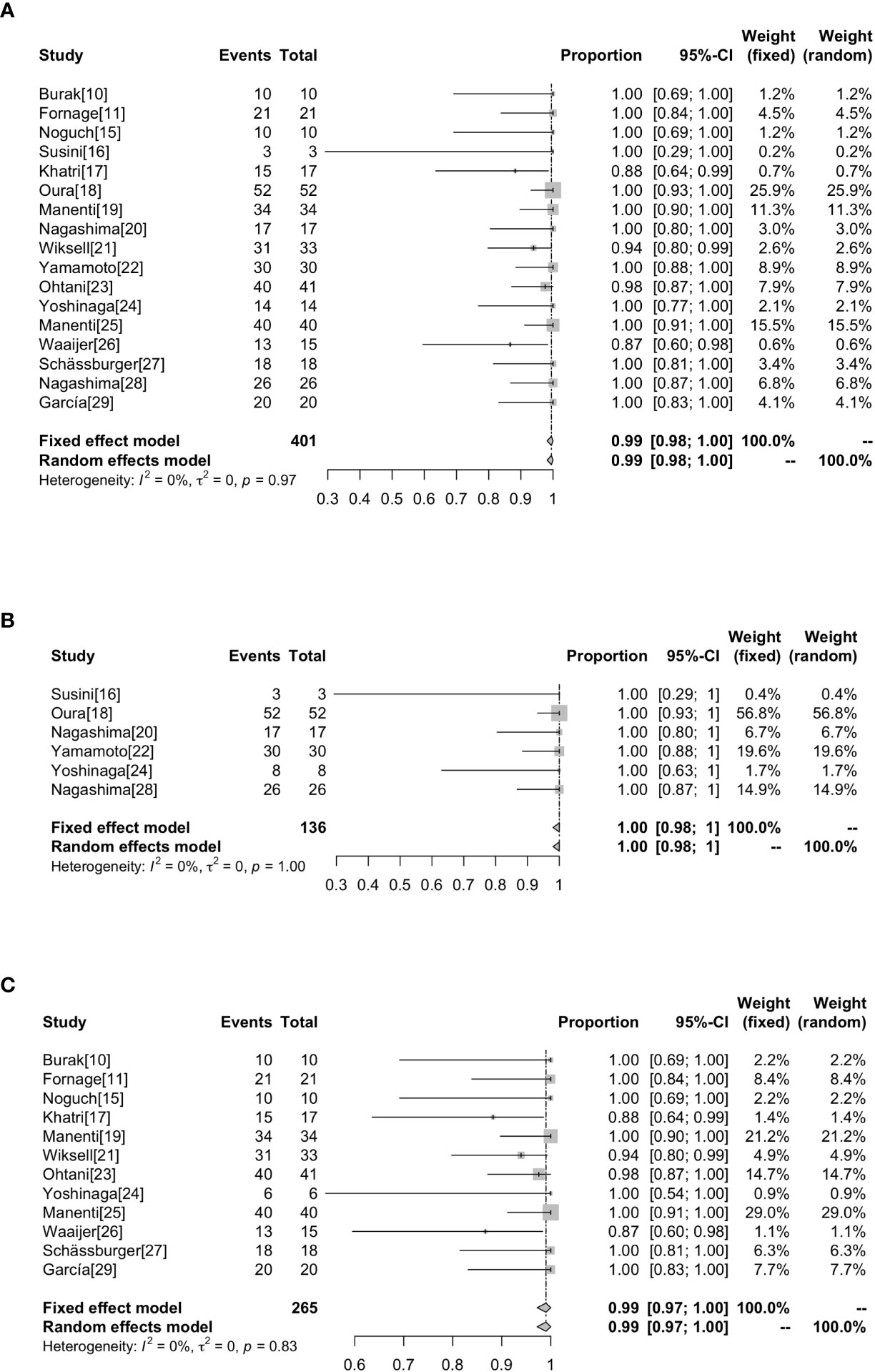
Figure 2 Forest plot showing the analysis of technically successful ablation rate in patients. The results of all the patients (A), patients without surgical resection following RFA (B), patients with surgical resection following RFA (C) are shown, respectively.
The RFA and Surgical Excision
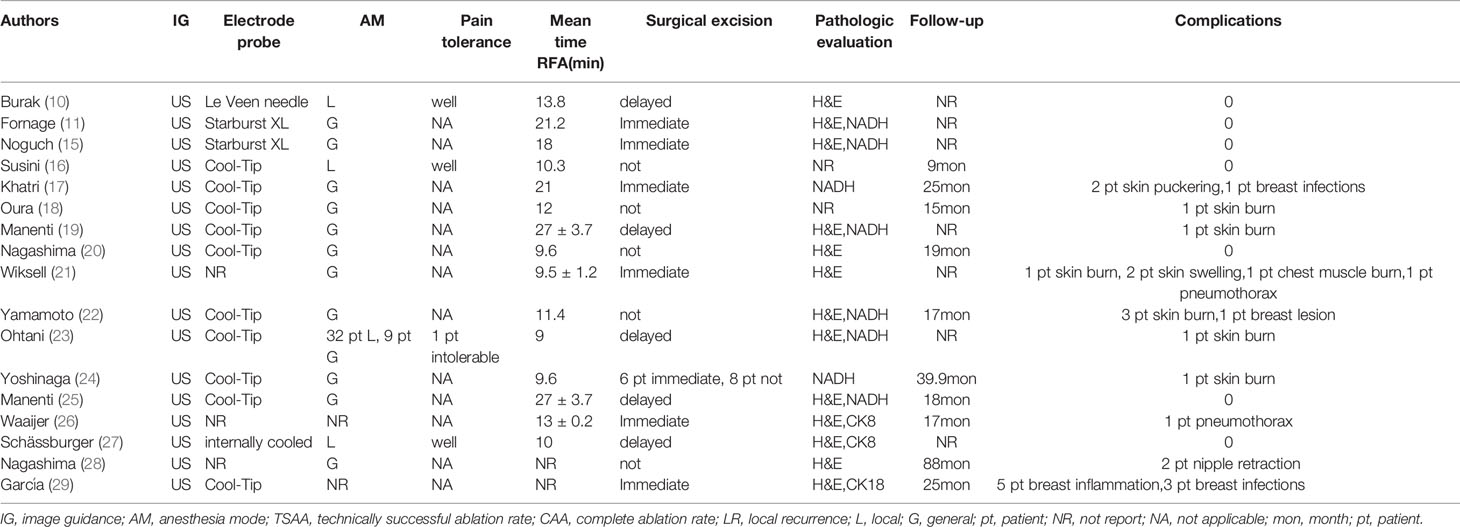
Ablation time ranged between 9 (23) to 30 minutes (19, 25) with an average of 15.8 minutes. Majority of patients, 333/397 (83.88%), received RFA under general anesthesia, whereas the rest, 63/397 (15.87%), received local anesthesia. Of the 63 patients who received local anesthesia, 62 reported tolerable pain whereas only 1 (1.59%) reported unbearable pain to the extent of not completing the ablation (23). These statistics are summarized in Table 2.
Majority of patients underwent surgical tumor excision after ablation (65.74%, 261/397). Of these, 118/261 (45.21%) received immediate excision whereas 143/261 (54.79%) received delayed excision. Of the 261, 241 (92.34%) underwent tumor resection (10, 11, 15, 17, 19, 21, 23–26, 29), whereas 20 (7.67%) underwent total mastectomy (10, 11, 15, 17, 27). Of the total patients analyzed in this study, 136 (34.26%) did not undergo surgical resection after tumor ablation (16, 18, 20, 22, 24, 28) (Table 2). Among the 136 patients who only received RFA, 133 patients received postoperative adjuvant radiotherapy and received systemic adjuvant therapy as needed according to the St. Gallen consensus except 3 patients who did not receive radiotherapy or systemic adjuvant therapy due to their poor age and general condition (16). Patients who received surgical resection after ablation also received radiotherapy or systemic adjuvant therapy as needed.
Complete Ablation Rate
Whether the tumor is completely ablated is evaluated by imaging and pathological examination. At present, there is no uniform standard for the staining technology to evaluate the survival rate of tumor cells after RFA. The most widely used methods in our research are hemathoxylineosin (H & E) and NADH (nicotinamide adenine dinucleotide in its reduced form) diaphorase (Table 2). The highest complete ablation rate was 100% in 8 studies and the lowest complete ablation rate was 66.7% (26). Overall, the complete ablation rate was 98% (95%CI=0.97-1.00) without heterogeneity (I2 = 50%, P<0.01). Subgroup analysis showed that the rate of complete ablation without surgical resection after ablation was 100% (95%CI=0.97-1.00, I2 = 32%, P=0.20), whereas the rate of complete ablation with surgical resection after ablation was 94% (95%CI=0.90-0.98, I2 = 51%, P=0.02) (Figure 3).
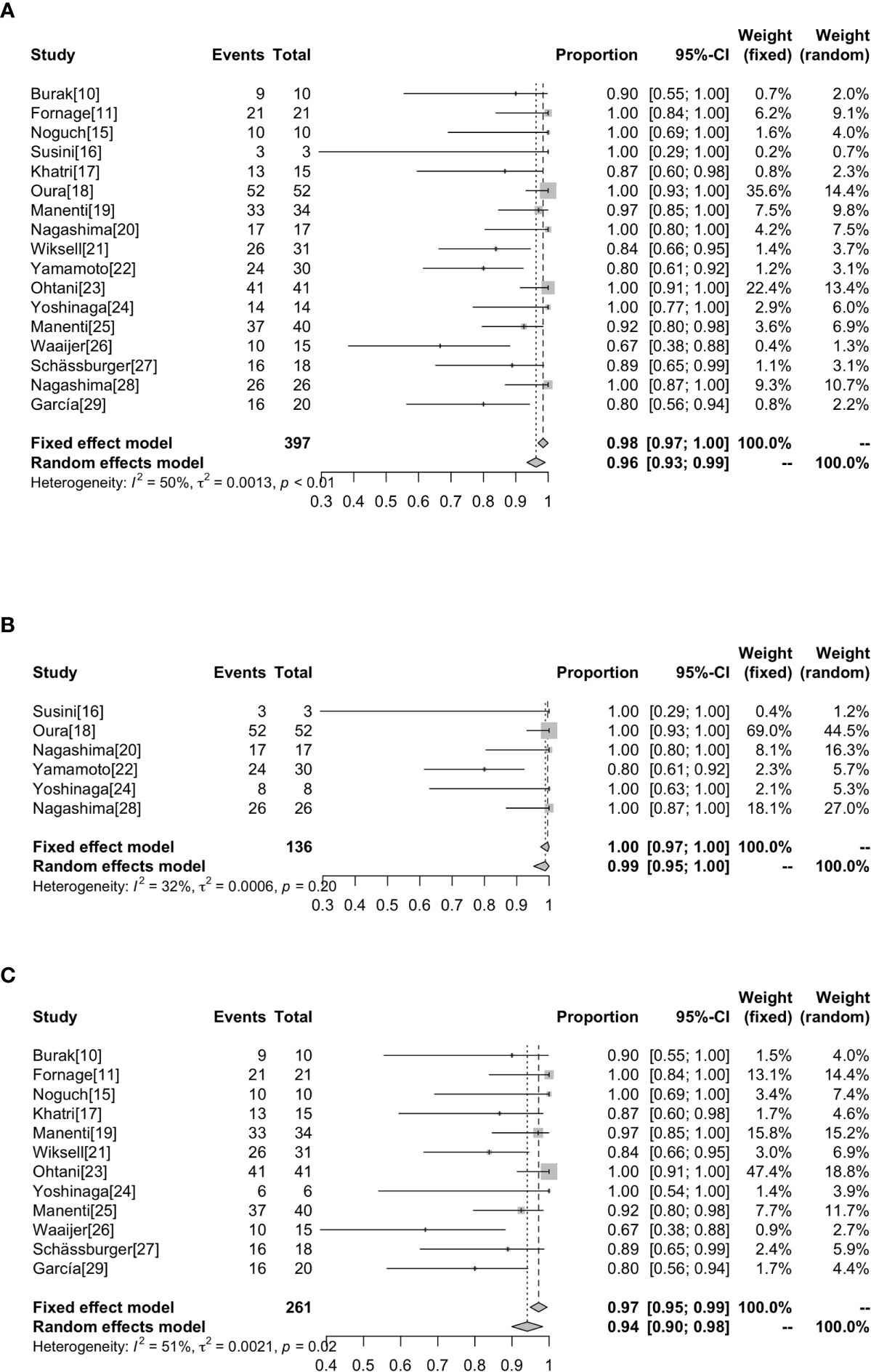
Figure 3 Forest plot showing the analysis of complete ablation rate in patients receiving RFA. The results of all the patients (A), patients without surgical resection following RFA (B), patients with surgical resection following RFA (C) are shown, respectively.
Complications
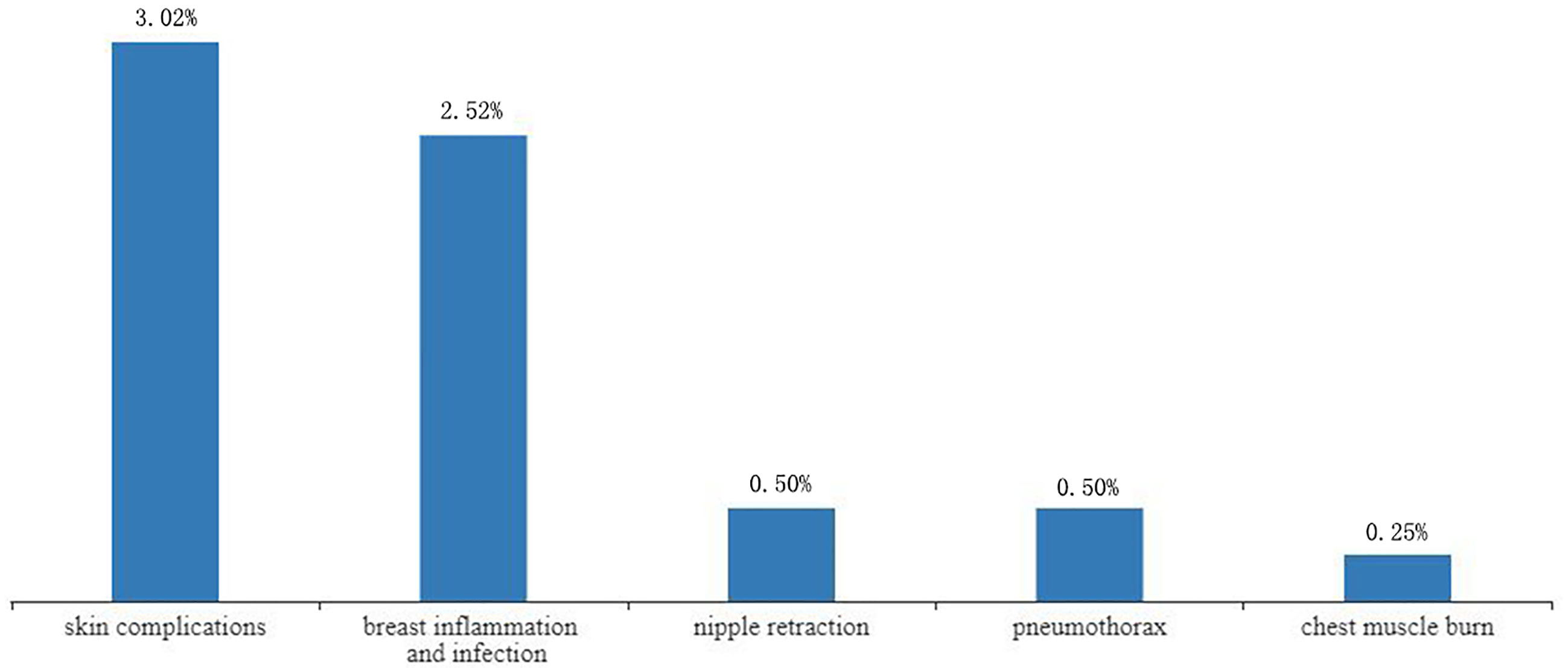
Complications were reported in 17 studies. Of the 401 lesions included in the study, 397 received RFA, of which 27 developed complications (6.80%, 27/397) (17–19, 21–24, 26, 28, 29) (Figure 4). The most common complications was skin burn (2.02%, 8/397) (18, 19, 21–24), skin puckering (0.50%, 2/397) (17) and skin swelling (0.50%, 2/397) (21), some other complications are related to breast inflammation (1.51%, 6/397) (21, 29) and infections (1.01%, 4/397) (17, 29). Other complications included nipple retraction (0.50%, 2/397) (28), pneumothorax (0.50%, 2/397) (21, 26) and chest muscle burn (0.25%, 1/397) (21). Overall results revealed that the complications rate was 2% (95%CI=0.01-0.04) without heterogeneity (I2 = 42%, P=0.03). The results of subgroup analysis showed that the incidence of complications without surgical resection after ablation was 3% (95%CI=0.00-0.06, I2 = 4%, P=0.39), and the incidence of complications with surgical resection after ablation was 3% (95%CI=0.00-0.07, I2 = 51%, P=0.02) (Figure 5).
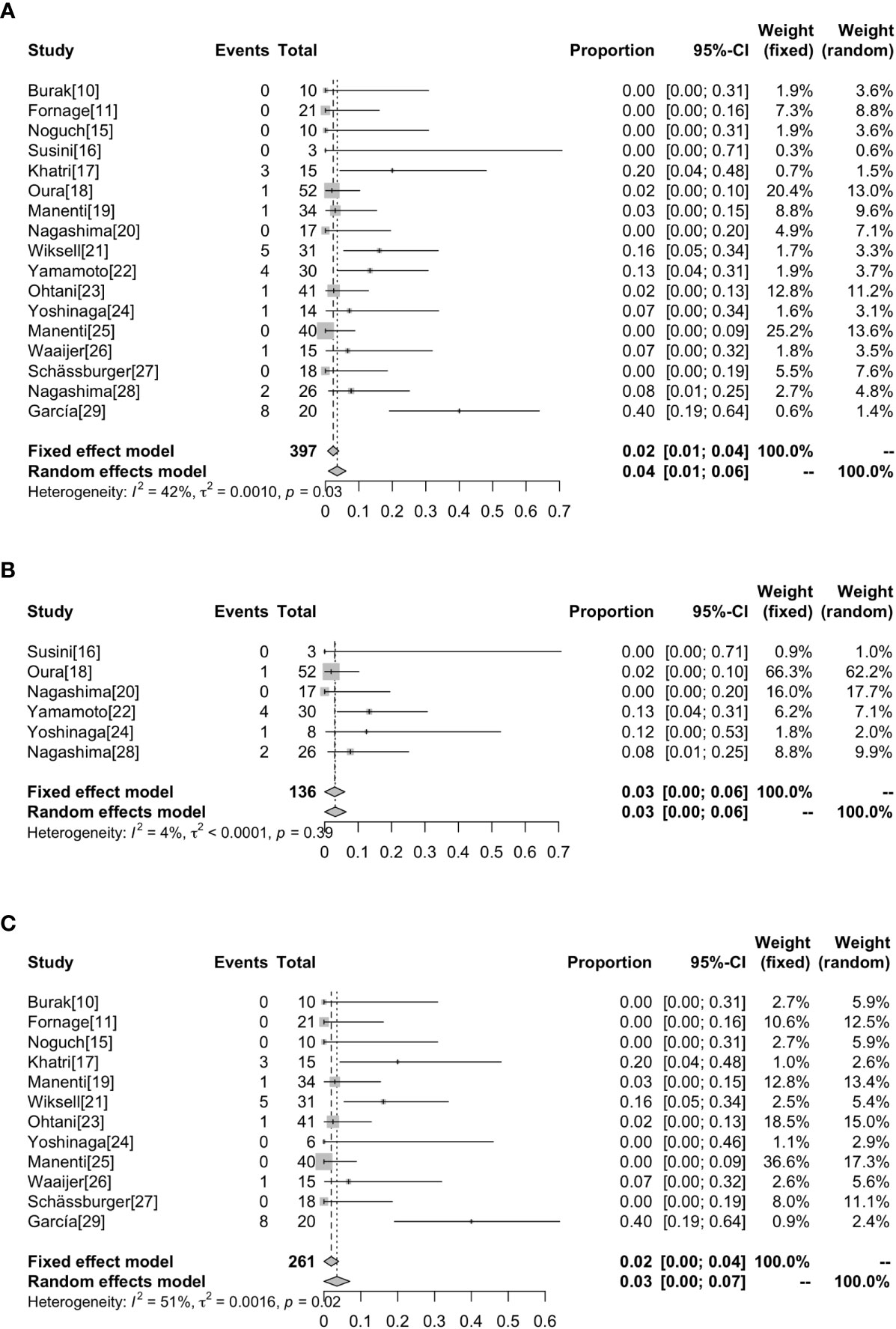
Figure 5 Forest plot showing the analysis of complications rate in patients receiving RFA. The results of all the patients (A), patients without surgical resection following RFA (B), patients with surgical resection following RFA (C) are shown, respectively.
Local Recurrence
Local recurrence after RFA was reported in 10 out of the 17 studies (16–18, 20, 22, 24–26, 28, 29). The median follow-up time ranged from 9 and 88 months. Local recurrence was evaluated according to clinical and imaging results. A total of 232 cases were reported whether they had local recurrence after RFA, including 136 cases who received RFA alone and 96 cases who underwent surgical resection after RFA. The median follow-up after ablation was 29 months for those who did not undergo surgical resection and 23 months for those who underwent surgical resection. There was no local recurrence after a median follow-up of 27.29 months in 232 patients, whether or not they underwent surgical resection following RFA.
Using funnel plots and Egger ‘s test, we analyzed publication bias on technical success rate, complete ablation rate and complication rate and the results are shown in Figure 6. All P-values were <0.05, indicating that there was potential publication bias (Supplementary Table 2). The impact of publication bias was assessed by trim and fill method. In the analysis of publication bias for the technical success rate, we found that there was no new study added, indicating that the conclusion was reliable and the effect of publication bias on the results was small. After addition of the estimated studies to the funnel plot of the complete ablation rate and complication rate, funnel plots become symmetrical, and the combined effect was still statistically significant (P <0.05) (Supplementary Figure 1).
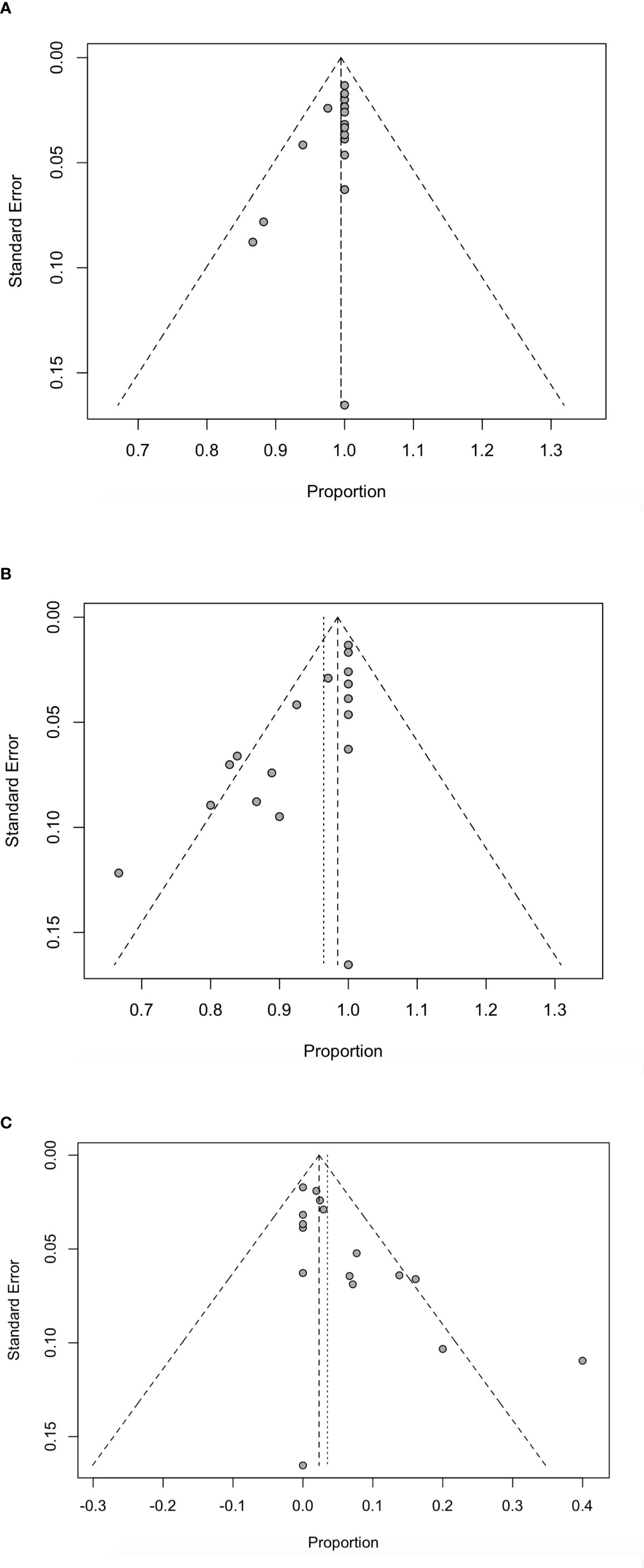
Figure 6 Funnel plot for technically successful ablation rate (A), complete ablation rate (B) and complications rate (C) in patients receiving RFA.
Discussion
RFA is a minimally invasive treatment technique for tumors. However, tumors larger than 3cm may not be successfully ablated by this procedure (30). In this research, 17 studies investigating RFA ablation for small breast tumors were analyzed. Overall, the technical success rate was 99% whereas the complete ablation rate was achieved in 96% of the cases. Complication rate was 2% of the cases. There was no local recurrence after a median follow-up of 27.29 months, whether or not the patients underwent surgical resection following RFA.
Accurate ultrasonic imaging and correct placement of the probe are the most important factors for a successful RFA (31). In RFA ablation, radiographic assistance is required to determine the location of the radiofrequency electrode needle inserted into the tumor as well as in monitoring the ablation effect in real time. In our analysis, 7 patients were not successfully ablated using RFA, partly attributed to poor tumor imaging and probe placement. Studies show that MRI is a promising guide for monitoring the electrode needle in RFA (32, 33). MRI can be used when a tumor cannot be clearly imaged by ultrasound. Our analysis excluded tumors larger than 2 cm, and found a higher technically successful ablation rate than that found by Mauri et al. (99% vs. 96%) (8).
Pathological examination of tumor tissue combined with imaging can assess the ablation status of a tumor. Since H & E cannot be used for immediate viability assessment after RFA (34, 35), evaluating NADH or cytokeratine 8 (CK8) has been preferred. H & E staining is most commonly used to assess the level of necrosis whereas NADH diaphorase or CK8 assays are used to assess tissue activity. In most patients (65.74%, 261/397), sample tissues were obtained by immediate or delayed local resection or total mastectomy after RFA. For those who retained the tumor tissues after ablation, samples were obtained by core needle biopsy (34.26%, 136/397). The complete ablation rate obtained in this study was higher than that reported by Chen et al. (96% vs. 89%) (7), indicating that the complete ablation rate of tumors less than 2 cm is higher than that of large tumors. We hypothesize that ablation time may influence the ability of RFA to inactivate tumor tissues, because thermal cell death is time-dependent (36, 37). In addition, diffuse echo-blocking of a tumor (fog effect) may occur during ablation, hence affect the success of tumor inactivation (38). Larger tumors may be more susceptible to the fog effect, thus reducing the complete ablation rate.
In this study, the incidence of complications was low. Chen et al. also reported complications after ablation, but only summarized the incidence of skin burns at 4%, and did not report other complications in the study (7). Our study, however, detailed all the complications and their possible cause. Accordingly, skin burn was the most common, with less than 3% incidence rate. This may be due to the small size of the breast, coupled with the proximity of the thin subcutaneous fat layer or tumor tissue to the skin. This also suggests that ablation of tumors smaller than 2cm has fewer complications and is safer than tumors larger than 2cm.Other complications such as skin puckering, skin swelling and chest muscle burn associated with mild heat damage were also observed. Nipple retraction also occurred in some individuals, which may be caused by the proximity of the nipple to the tumor. Of note, one study recorded 40% incidence of complications (26). Here, inflammation of the breast was the main complication (25%), but the inflammations were thought to be “clinically insignificant” by the authors. In this study, breast infections were largely associated with local radiotherapy. In view of treatment related complications, better approaches are undoubtedly needed (39).
Currently, research on RFA in the treatment of breast cancer is in the initial stage, and most experimental designs include surgical resection after ablation, thus findings on RFA alone are very scarce (136 cases). Surgical resection performed after RFA may affect the local recurrence rate because surgical resection after RFA may increase local control rate compared with RFA only. However, in this study, there was no difference in local recurrence rate between the two groups. Only 10 of 17 studies reported whether local recurrence occurred after ablation, and none of the 232 patients developed local recurrence, whether they underwent surgical resection following RFA or not. This may be related to postoperative treatment. Of the 136 patients who only received RFA, 133 patients received postoperative radiotherapy and systemic adjuvant treatment. In addition, most of the patients who only received RFA were hormone receptor positive, HER-2 negative and lymph node negative. Therefore, the lack of local recurrence in patients who did not undergo surgical resection after ablation may not be attributed to the success of RFA alone. Nevertheless, this shows that for small tumors, RFA combined with postoperative radiotherapy and systemic adjuvant treatment may achieve a good local control rate similar to that of surgical operation after RFA. Elsewhere, recurrence was observed in 9 of 564 (6) and 5 of 404 patients that had received RFA (7). The local recurrence of tumors after ablation is related to the rate of tumor cell inactivation after ablation. The complete ablation rate of tumors smaller than 2 cm is higher than that of tumors larger than 2 cm, which also means that the local recurrence rate of tumor less than 2cm is lower when there is no difference in other characteristics of tumor.
This meta-analysis had a few limitations. First, publication bias was found in the studies, possibly because we only included published studies. However, the influence of publication bias was small and the conclusions were reliable as determined by the trim and fill method. Second, the use of minimally invasive treatments is at the foundational stage and the quality of the findings may be weakened by the small sample size (401 cases). Finally, in the analysis of local recurrence rate, RFA alone was performed in 136 cases and the median follow-up time was 29 months. This implies that the conclusion regarding local recurrence rate in early breast cancer following treatment with RFA being 0 should be interpreted with caution. These results illustrate the safety and efficacy of RFA. It can be used for patients unfit for surgery or those unwilling to receive surgical resection of the tumor.
Conclusion
This meta-analysis shows that the RFA is safe and effective for the treatment of breast cancer with small tumor size with few complications. However, standard adjuvant therapy is needed after operation. Future prospective studies investigating the use of ablation alone in small breast cancer should be conducted to support our findings.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Protocol/project development: L-YX and Q-LH. Data acquisition and interpretation of data: L-YX and W-YX. Statistics analysis of data: L-YX and W-YX. Manuscript drafting: L-YX and W-YX. Manuscript revision and accountable for all aspects of the study: L-YX and Q-LH. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.651646/full#supplementary-material
Supplementary Figure 1 | Trim and fill plot of publication bias for complete ablation rate (A) and complications rate (B).
Abbreviations
RFA, radiofrequency ablation; H & E, hematoxylineosin; NADH, nicotinamide adenine dinucleotide in its reduced form; CK8, cytokeratine 8.
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin (2017) 67:7–30. doi: 10.3322/caac.21387
2. Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor Ablation With Radio-Frequency Energy. Radiology (2000) 217(3):633–46. doi: 10.1148/radiology.217.3.r00dc26633
3. Izzo F, Thomas R, Delrio P, Rinaldo M, Vallone P, DeChiara A, et al. Radiofrequency Ablation in Patients With Primary Breast Carcinoma: A Pilot Study in 26 Patients. Cancer (2001) 92(8):2036–44. doi: 10.1002/1097-0142(20011015)92:8<2036::aid-cncr1542>3.0.co;2-w
4. Kinoshita T, Iwamoto E, Tsuda H, Seki K. Radiofrequency Ablation as Local Therapy for Early Breast Carcinomas. Breast Cancer (2011) 18:10–7. doi: 10.1007/s12282-009-0186-9
5. Tsuda H, Seki K, Hasebe T, Sasajima Y, Shibata T, Iwamoto E, et al. A Histopathological Study for Evaluation of Therapeutic Effects of Radiofrequency Ablation in Patients With Breast Cancer. Breast Cancer (2011) 18(1):24–32. doi: 10.1007/s12282-010-0222-9
6. Peek MC, Ahmed M, Napoli A, Usiskin S, Baker R, Douek M. Minimally Invasive Ablative Techniques in the Treatment of Breast Cancer: A Systematic Review and Meta-Analysis. Int J Hyperthermia (2017) 33(2):191–202. doi: 10.1080/02656736.2016.1230232
7. Chen JY, Zhang C, Li F, Xu LP, Zhu HC, Wang S, et al. A Meta-Analysis of Clinical Trials Assessing the Effect of Radiofrequency Ablation for Breast Cancer. OncoTargets Terapy (2016) 9:1759–66. doi: 10.2147/OTT.S97828
8. Mauri G, Sconfienza LM, Pescatori LC, Fedeli MP, Ali M, Leo GD, et al. Technical Success, Technique Efficacy and Complications of Minimally-Invasive Imaging-Guided Percutaneous Ablation Procedures of Breast Cancer: A Systematic Review and Meta-Analysis. Eur Radiol (2017) 27(8):3199–210. doi: 10.1007/s00330-016-4668-9
9. Ito T, Oura S, Nagamine S, Takahashi M, Yamamoto N, Yamamichi N, et al. Radiofrequency Ablation of Breast Cancer: A Retrospective Study. Clin Breast Cancer (2018) 18(4):e495–500. doi: 10.1016/j.clbc.2017.09.007
10. Burak WE Jr, Agnese DM, Povoski SP, Yanssens TL, Bloom KJ, Wakely PE, et al. Radiofrequency Ablation of Invasive Breast Carcinoma Followed by Delayed Surgical Excision. Cancer (2003) 98(7):1369–76. doi: 10.1002/cncr.11642
11. Fornage BD, Sneige N, Ross MI, Mirza AN, Kuerer AN, Edeiken BS, et al. Small (or 2-Cm) Breast Cancer Treated With US-guided Radiofrequency Ablation: Feasibility Study. Radiology (2004) 231(1):215–24. doi: 10.1148/radiol.2311030651
12. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J, et al. Methodological Index for non-Randomized Studies (MINORS): Development and Validation of a New Instrument. ANZ J Surg (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
13. Armitage P, Berry G, Matthews J. Analysing Means and Proportions. In: Statistical Methods in Medical Research. Oxford: Blackwell Science (2002). p. 83–146. doi: 10.1136/bmj.315.7109.629
14. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
15. Noguchi M, Earashi M, Fujii H, Yokoyama K, Harada K, Tsuneyama K. Radiofrequency Ablation of Small Breast Cancer Followed by Surgical Resection. J Surg Oncol (2006) 93(2):120–8. doi: 10.1002/jso.20398
16. Susini T, Nori J, Olivieri S, Livi L, Bianchi S, Mangialavori G, et al. Radiofrequency Ablation for Minimally Invasive Treatment of Breast Carcinoma. A Pilot Study in Elderly Inoperable Patients. Gynecol Oncol (2007) 104:304–10. doi: 10.1016/j.ygyno.2006.08.049
17. Khatri VP, McGahan JP, Ramsamooj R, Griffey S, Brock J, Cronan M, et al. A Phase II Trial of Image-Guided Radiofrequency Ablation of Small Invasive Breast Carcinomas: Use of Saline-Cooled Tip Electrode. Ann Surg Oncol (2007) 14:1644–52. doi: 10.1245/s10434-006-9315-2
18. Oura S, Tamaki T, Hirai I, Yoshimasu T, Ohta F, Nakamura R, et al. Radiofrequency Ablation Therapy in Patients With Breast Cancers Two Centimeters or Less in Size. Breast Cancer (2007) 14(1):48–54. doi: 10.2325/jbcs.14.48
19. Manenti G, Bolacchi F, Perretta T, Lossu E, Pistolese CA, Buonomo OC, et al. Small Breast Cancers: In Vivo Percutaneous US-guided Radiofrequency Ablation With Dedicated Cool-Tip Radiofrequency System. Radiology (2009) 251:339–46. doi: 10.1148/radiol.2512080905
20. Nagashima T, Sakakibara M, Sangai T, Kazama T, Fujimoto H, Miyaaki M. Surrounding Rim Formation and Reduction in Size After Radiofrequency Ablation for Primary Breast Cancer. Jpn J Radiol (2009) 27:197–204. doi: 10.1007/s11604-009-0322-7
21. Wiksell H, Löfgren L, Schässburger KU, Grundstom H, Janicijevic M, Lagerstedt U, et al. Feasibility Study on the Treatment of Small Breast Carcinoma Using Percutaneous US-guided Preferential Radiofrequency Ablation (PRFA). Breast (2010) 19:219–25. doi: 10.1016/j.breast.2010.01.016
22. Yamamoto N, Fujimoto H, Nakamura R, Arai M, Yoshii A, Kaji S, et al. Pilot Study of Radiofrequency Ablation Therapy Without Surgical Excision for T1 Breast Cancer: Evaluation With MRI and Vacuum-Assisted Core Needle Biopsy and Safety Management. Breast Cancer (2011) 18(1):3–9. doi: 10.1007/s12282-010-0197-6
23. Ohtani S, Kochi M, Ito M, Higaki K, Takada S, Matsuura H, et al. Radiofrequency Ablation of Early Breast Cancer Followed by Delayed Surgical Resection – a Promising Alternative to Breast-Conserving Surgery. Breast (2011) 20(5):431–6. doi: 10.1016/j.breast.2011.04.007
24. Yoshinaga Y, Enomoto Y, Fujimitsu R, Shimakura M, Nabeshi K, Iwasaki A, et al. Image and Pathological Changes After Radiofrequency Ablation of Invasive Breast Cancer: A Pilot Study of Nonsurgical Therapy of Early Breast Cancer. World J Surg (2013) 37:356–63. doi: 10.1007/s00268-012-1820-9
25. Manenti G, Scarano AL, Pistolese CA, Perretta T, Bonanno E, Orlandi A, et al. Subclinical Breast Cancer: Minimally Invasive Approaches. Our Experience With Percutaneous Radiofrequency Ablation vs Cryotherapy. Breast Care (Basel) (2013) 8:356–60. doi: 10.1159/000355707
26. Waaijer L, Kreb DL, Fernandez Gallardo MA, Rossum PSN, Postma EL, Koelemij R, et al. Radiofrequency Ablation of Small Breast Tumours: Evaluation of a Novel Bipolar Cool-Tip Application. Eur J Surg Oncol (2014) 40:1222–9. doi: 10.1016/j.ejso.2014.07.031
27. Schässburger KU, Löfgren L, Lagerstedt U, Leiflfland K, Thorneman K, Sandstedt B, et al. Minimallyinvasive Treatment of Early Stage Breast Cancer: A Feasibility Study Using Radiofrequency Ablation Under Local Anesthesia. Breast (2014) 23:152–8. doi: 10.1016/j.breast.2013.12.007
28. Nagashima T, Sakakibara M, Sangai T, Sakakibara J, Iwase T, Hayama S, et al. Long-Term Outcome of Radiofrequency Ablation Therapy for Breast Cancer. Gan To Kagaku Ryoho (2015) 42(12):1788–90.
29. García TA, Guma A, Soler T, Valdivieso A, Petit A, Contreras N. Radiofrequency Ablation Followed by Surgical Excision Versus Lumpectomy for Early Stage Breast Cancer: A Randomized Phase Ii Clinical Trial. Radiology (2018) 289(2):317–24. doi: 10.1148/radiol.2018180235
30. Jeffrey SS, Birdwell RL, Ikeda DM, Daniel BL, Nowels KW, Dirbas FM, et al. Radiofrequency Ablation of Breast Cancer: First Report of an Emerging Technology. Arch Surg (1999) 134(10):1064–8. doi: 10.1001/archsurg.134.10.1064
31. Singletary SE. Feasibility of Radiofrequency Ablation for Primary Breast Cancer. Breast Cancer (2003) 10:4–9. doi: 10.1007/BF02967618
32. Van den Bosch M, Daniel B, Rieke V, Butts-Pauly K, Kermit E, Jeffrey S, et al. MRI-Guided Radiofrequency Ablation of Breast Cancer: Preliminary Clinical Experience. J Magn Reson Imaging (2008) 27:204–8. doi: 10.1002/jmri.21190
33. Postma EL, van HR, Daniel BL, Merckel LG, Verkooijen HM, van den Bosch MAAJ. MRI-Guided Ablation of Breast Cancer: Where do We Stand Today? J Magn Reson Imaging (2011) 34(2):254–61. doi: 10.1002/jmri.22599
34. Kreb DL, Bosscha K, Ernst MF, Rutten MJ, Jager GJ, van Diest PJ, et al. Use of Cytokeratin 8 Immuno-Histochemistry for Assessing Cell Death After Radiofrequency Ablation of Breast Cancers. Biotech Histochem (2011) 86:404–12. doi: 10.3109/10520295.2010.517473
35. Motoyoshi A, Noguchi M, Earashi M, Zen Y, Fujii H. Histopathological and Immunohistochemical Evaluations of Breast Cancer Treated With Radiofrequency Ablation. J Surg Oncol (2010) 102(5):385.e91. doi: 10.1002/jso.21429
36. Tannock IF, Bristow RG, Hill RP. The Basic Science of Oncology. New York: Pergamon Press (1987) p. 337–57.
37. Haines DE, Watson DD, Halperin C. Characteristics of Heat Transfer and Determination of Temperature Gradient and Viability Threshold During Radiofrequency Fulguration of Isolated Perfused Canine Right Ventricle. Circulation (1987) 76:278–82.
38. Fornage BD, Hunt KK. Image-Guided Percutaneous Ablation of Small Breast Cancer: Which Technique is Leading the Pack? Technol Cancer Res Treat (2015) 14(2):209–11. doi: 10.7785/tcrt.2012.500395
Keywords: breast cancer, radiofrequency ablation, effective, safe, meta-analysis
Citation: Xia L-Y, Hu Q-L and Xu W-Y (2021) Efficacy and Safety of Radiofrequency Ablation for Breast Cancer Smaller Than 2 cm: A Systematic Review and Meta-Analysis. Front. Oncol. 11:651646. doi: 10.3389/fonc.2021.651646
Received: 10 January 2021; Accepted: 08 April 2021;
Published: 03 May 2021.
Edited by:
Ira Ida Skvortsova, Innsbruck Medical University, AustriaReviewed by:
Salomao Faintuch, Harvard Medical Faculty Physicians, United StatesBogdan Ungureanu, University of Medicine and Pharmacy of Craiova, Romania
Catalina Falo, Catalan Institute of Oncology, Spain
Copyright © 2021 Xia, Hu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin-Yu Xia, bHlsYzEwMjNAMTYzLmNvbQ==
 Lin-Yu Xia
Lin-Yu Xia Qing-Lin Hu1
Qing-Lin Hu1