
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 21 October 2021
Sec. Skin Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.651553
Background: Immune checkpoint inhibitors (ICIs) have dramatically altered the treatment landscape for patients with melanoma. However, their use also generates unique immune-related adverse effects (irAEs). We performed a systematic review and network meta‐analysis to compare the risk of pneumonitis associated with ICIs for patients with advanced or metastatic melanoma.
Methods: Phase II/III randomized clinical trials (RCTs) with ICIs were identified through comprehensive searches of multiple databases. An NMA was conducted to compare the risk of pneumonitis associated with ICIs and all‐grade (grade 1‐5) and high‐grade (grade 3‐5) immune‐related pneumonitis (IRP) were estimated by odds ratios (ORs).
Results: A total of 10 randomized clinical trials involving 5,335 patients were enrolled in this study. Conventional chemotherapy was associated with a lower risk of grade 1–5 IRP compared with ICIs monotherapy (OR, 0.14, 95% CI, 0.03 to 0.73) and dual ICIs combination (OR, 0.03, 95% CI, 0.00 to 0.19). In addition, dual ICIs combination showed a noticeably higher risk than ICI monotherapy (OR, 4.45, 95% CI, 2.14 to 9.25) of grade 1–5 IRP. No significant difference in grade 1–5 IRP was observed between cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) inhibitors. As to grade 3‐5 IRP, no statistically significant difference was found among different ICIs-based regimens.
Conclusion: These findings revealed that ICIs could increase the risk of all-grade pneumonitis for patients with advanced melanoma, compared with conventional chemotherapy. Dual ICIs combination could further increase the risk of all-grade pneumonitis than ICIs monotherapy. There was no significant difference in the risk of pneumonia between CTLA-4 and PD-1 inhibitors.
Melanoma is an aggressive type of skin cancer that arises from uncontrolled proliferation of melanocytes. The prevalence of melanoma has been rising steadily over the last several decades, with its incidence estimated to be increasing by 3–7% annually worldwide (1). While it represents < 5% of all cutaneous malignancies, melanoma is the major cause of death from skin cancer (2–4). If melanoma is diagnosed at early stage (stages I and II), resection of the lesion is associated with favorable survival rates. However, when diagnosed at late stage (stages III and IV), the 3-year survival rate of melanoma was reported to be 12.2% with dacarbazine chemotherapy from a multinational, randomized controlled trial (RCT) conducted from 2006 to 2008 (5).
Immune checkpoint targeted therapies including cytotoxic T lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors have been a major breakthrough in the cancer treatment over the past decade. Immune checkpoint inhibitors (ICIs) alone as well as in combination with chemotherapy have reshaped the landscape of treatments in melanoma. Emerging evidence has demonstrated the prolonged survival with the ICIs compared with the conventional chemotherapy in patients with advanced melanoma (5–8). However, ICIs could disrupt normal mechanisms of immune regulation and lead to immune‐related adverse events (irAEs) (9). One particularly worrisome irAE is the development of immune-related pneumonitis (IRP), which typically presents with dry cough, progressive dyspnea and hypoxia along with pulmonary infiltrates on chest imaging (10).
Awareness of the risk of IRP associated with different ICIs would aid in the appropriate utilization of ICIs in clinical practice and essential monitoring of patients with ICIs treatment. Thus, we conducted a network meta-analysis (NMA) using all available data from RCTs to determine the relative risk of IRP in regards to various regimens.
A systematic literature search of PubMed, Embase, Cochrane Central Register of Controlled Trials was performed independently by two investigators to identify eligible RCTs in which at least one treatment arm included Food and Drug Administration (FDA) approved immune checkpoint inhibitors in patients with advanced melanoma up to October 31, 2020. The language was limited to English. The comprehensive PubMed search strategy was provided in Supplementary Table S1. To identify unpublished studies, the US National Institutes of Health trials register (http://clinicaltrial.gov) and conference abstracts from the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) were also searched. The inclusion criteria were (1) head-to-head phase II/III RCTs which enrolled patient with pathologically confirmed advanced or metastatic melanoma; (2) patients received ICI treatment (at least one treatment arm); (3) reported the incidence of both 1–5 grade and grade 3–5 IRP. The exclusion criteria were (1) letters, reviews, unfinished studies, duplicate reports, or conference reports; (2) studies with insufficient data; (3) trials without a control arm; (4) RCTs in phase I.
The following information was extracted from eligible studies by two investigators independently: first author, year of publication, study name, National Clinical Trial (NCT) number, trial phase, treatment arms, the number of patients in total, number of patients per treatment arm, incidence of 1–5 grade and grade 3–5 IRP. Two investigators independently assessed the quality of the RCTs by using Cochrane risk assessment tool, and resolved the discrepancies through discussion and consult with a third one.
The primary outcome of interest was all‐grade (grade 1‐5) pneumonitis. Secondary outcome was high‐grade (grade 3‐5) pneumonitis based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (11).
Odds ratios (ORs) and their 95% confidence intervals (CIs) were used as summary statistics to estimate treatment effects. The heterogeneity among studies was assessed using the chi-squared (χ2) and I-squared (I2) tests. We assessed the magnitude of the heterogeneity between the included publications by constructing a visual forest plot. A p value greater than 0.10 or an I2 value greater than 50% indicated substantial heterogeneity, and a random-effects model was used; otherwise, a fixed-effects model was used.
Inconsistency appraisal was achieved via two steps. First, we made a general comparison between the consistency model and the inconsistency model, calculating for inconsistency factors (IF), standard error of inconsistency factors (seIF) and p value (p). If the 95% CI of IF contained ‘0’ and the p value was greater than ‘0.05’, it was considered that direct evidence and indirect evidence were completely consistent, and there was no inconsistency. Second, node-splitting models were adopted to identify any inconsistencies. Each node of the network meta-analysis was analyzed by comparing the difference between direct comparison and indirect comparison. Significant inconsistency was defined as a p value less than 0.05. Overall, if there were no inconsistencies in the evidence, a consistency model was used to assess the relative effect of the included treatments; otherwise, an inconsistency model would be used.
The probability of treatment ranking was based on the surface under the cumulative ranking curve (SUCRA), and a higher SUCRA score was associated with a higher risk of IRP. A ‘comparison-adjusted’ funnel plot was used to assess publication bias within a network of interventions.
All statistical tests were two sided and used a significance level of p < 0.05. And we used STATA (version 15.1) for all statistical analyses.
A total of 4,776 records were initially retrieved from PubMed, Embase, Cochrane Library up to October 31, 2020. Then, 4,056 records remained after removal of the duplicates. Screening of the titles and abstracts resulted in the exclusion of 3,862 records. After full-text reading, 182 records were excluded: unable to access full text (n=19); systematic reviews, meta-analysis or pooled analysis (n=56); trials phase I/Ib (n=34); single-arm trials (n=26); sufficient outcomes unavailable (n=36); not meeting the inclusion criteria (n=11); not meeting the standard dose of ipilimumab (n=2). In total, 10 eligible randomized trials (6, 7, 12–19) were included in this study. The PRISMA flow diagram of study selection is shown as follows (Figure 1).
The Cochrane tool for risk of bias was used to measure the quality of each study. The detailed assessment results were shown in Supplementary Figure S1. No obvious publication bias was observed in this NMA; the funnel plots were roughly symmetrical and near the zero line (Supplementary Figure S2–S5).
There were 6 phase III trials, 4 phase II trials (Table 1). Among the 10 eligible RCTs, 8 studies were two-arm trials and 2 studies were three-arm trials. There were 22 treatment arms in total and the most common treatment arm was ICIs monotherapy (n=13, 59.1%), followed by dual ICIs combination (n=4, 18.2%), chemotherapy (n=3, 13.6%) and placebo (n=2, 9.1%). The ICIs used for the melanoma treatment included ipilimumab (CTLA-4), nivolumab (PD-1) and pembrolizumab (PD-1) while chemotherapy regimens included carboplatin, dacarbazine, and/or paclitaxel. A total of 5,335 patients were included in this study and the sample size ranged from 60 to 1,011.
The rate of IRP in different treatment regimens was compared among four groups including chemotherapy, ICIs monotherapy, dual ICIs combination and placebo. Furthermore, we subdivided the four treatment groups into six subgroups based on the different types of ICIs: chemotherapy, ipilimumab, nivolumab, nivolumab+ipilimumab, pembrolizumab and placebo.
According to the established NMA based on the consistency model (Figure 2 and Table 2), chemotherapy had a lower risk of grade 1–5 IRP compared with dual ICIs combination (OR, 0.03, 95% CI, 0.00 to 0.19) and ICI monotherapy (OR, 0.14, 95% CI, 0.03 to 0.73). In addition, dual ICIs combination showed a noticeably higher risk than ICI monotherapy (OR, 4.45, 95% CI, 2.14 to 9.25). Compared with placebo, dual ICIs combination (OR, 23.94, 95% CI, 6.56 to 87.38) and ICI monotherapy (OR, 5.38, 95% CI, 1.72 to 16.80) were associated with a considerable higher risk of grade 1–5 IRP. As to high‐grade (grade 3‐5) IRP, no statistically significant difference was found among the four treatment groups (Table 3).
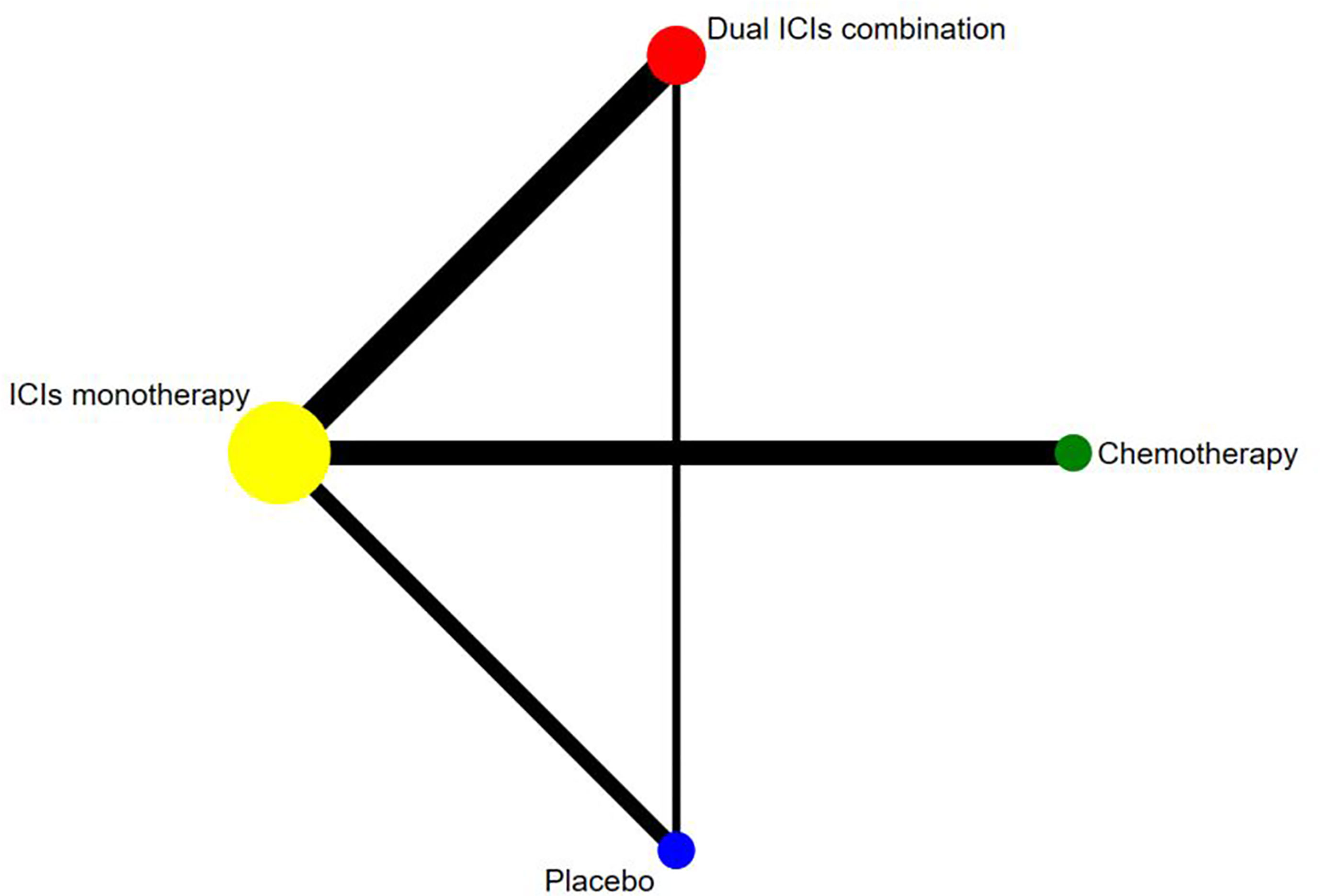
Figure 2 Network established for comparisons based on four treatment groups. Circular nodes indicate treatment regimens. The node size corresponds with the total number of patients randomized to receive the treatment. Each line represents a type of head-to-head comparison. The width of the lines is proportional to the number of trials comparing the connected treatments.
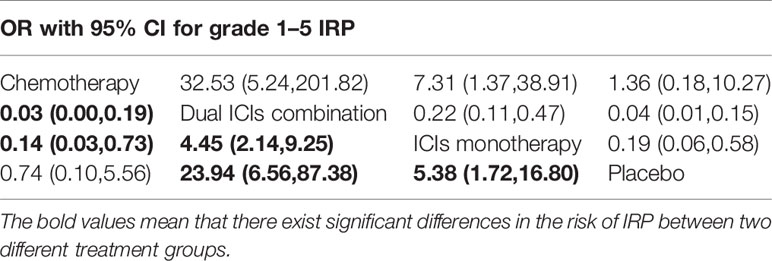
Table 2 Multiple treatment comparison for IRP based on network consistency model (for grade 1–5 IRP).
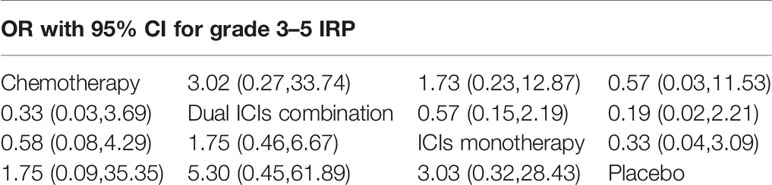
Table 3 Multiple treatment comparison for IRP based on network consistency model (for grade 3–5 IRP).
SUCRA provided a ranking of the four treatment groups according to the incidence of IRP (Figure 3). For grade 1–5 IRP, dual ICIs combination was with the highest ranking (1.00), followed by ICI monotherapy (0.66), placebo (0.21) and chemotherapy (0.13). The ranking of grade 3–5 IRP was consistent with grade 1–5 IRP, with dual ICIs combination (0.84) ranking the highest, followed by ICI monotherapy (0.58), chemotherapy (0.39) and placebo (0.19).
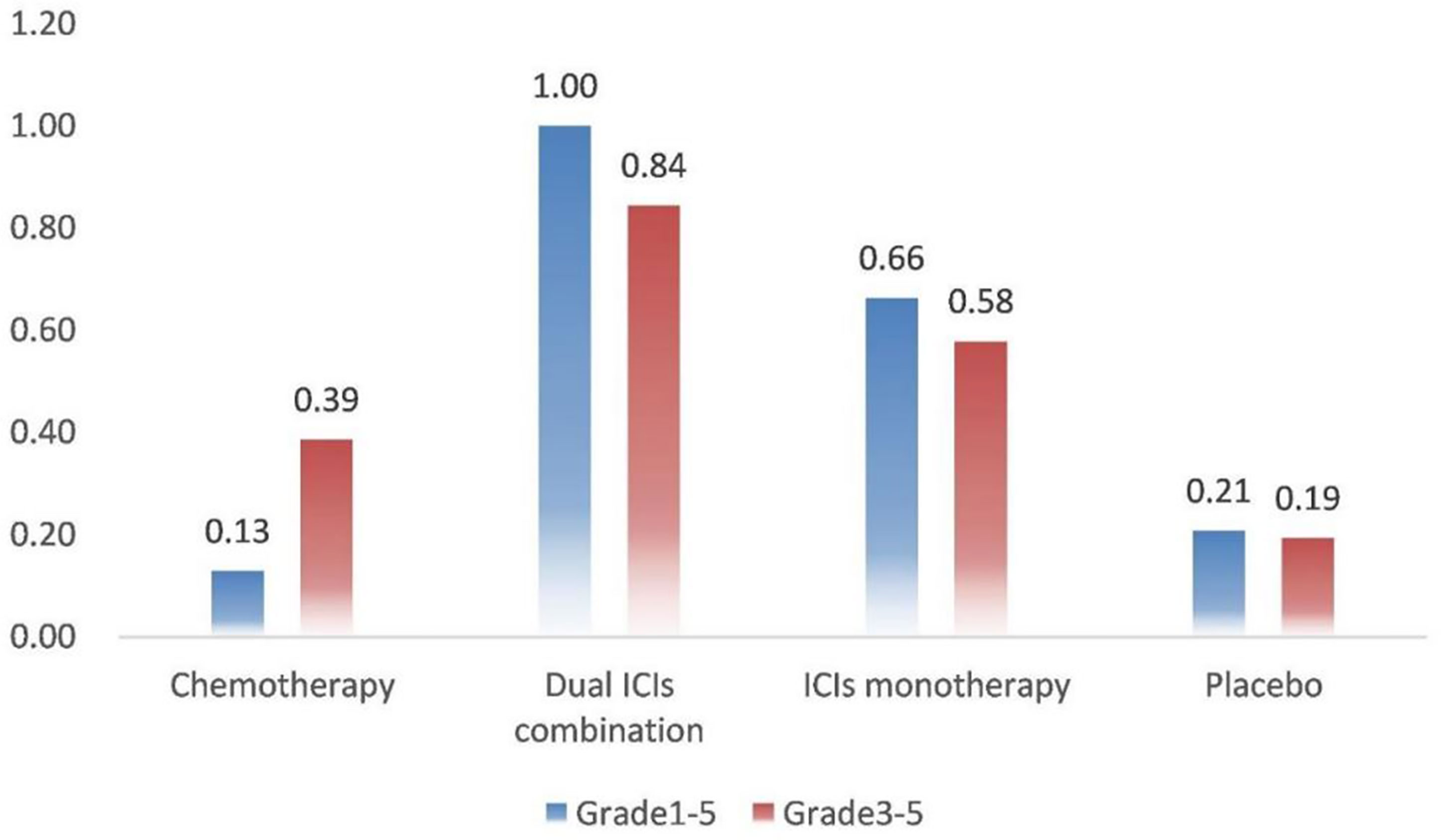
Figure 3 Rank probabilities with SUCRA value for IRP in four treatment groups based on the network consistency model. Higher SUCRA scores are correlated with higher risk of IRP. SUCRA, surface under the cumulative ranking curve.
To further compare the difference of IRP among various ICIs, including ipilimumab, nivolumab and pembrolizumab, another NMA for IRP by different ICIs based on six treatment groups was built (Supplementary Figure S6 and Table S2). Chemotherapy was with a lower risk of grade 1–5 IRP, compared with nivolumab (OR, 0.17, 95% CI, 0.03 to 0.97), nivolumab+ ipilimumab (OR, 0.04, 95% CI, 0.01 to 0.22) and pembrolizumab (OR, 0.09, 95% CI, 0.01 to 0.64). Both ipilimumab (OR, 0.22, 95% CI, 0.10 to 0.49) and nivolumab (OR, 0.21, 95% CI, 0.09 to 0.47) were associated with a lower risk, when compared with their combination (nivolumab+ ipilimumab). The risk of 1-5 IRP was comparable among nivolumab, pembrolizumab and ipilimumab. Therefore, it could be inferred that there was no significant difference between CTLA-4 and PD-1 inhibitors. In terms of grade 3‐5 IRP, no significant difference was seen among the six treatment groups (Supplementary Table S3).
The ranking of grade 1–5 IRP from high to low was: nivolumab+ipilimumab (0.98), pembrolizumab (0.72), chemotherapy (0.70), nivolumab (0.51), placebo (0.18) and ipilimumab (0.00). As to grade 3‐5 IRP, the ranking was pembrolizumab (0.85), nivolumab+ipilimumab (0.68), chemotherapy (0.42), ipilimumab (0.38), nivolumab (0.38) and placebo (0.29) (Supplementary Figure S7).
Pairwise comparisons with heterogeneity estimates are presented in Supplementary Figures S8–S11. The results showed low heterogeneity in relation to the NMA results. In addition, the results of the inconsistency evaluation are presented in Supplementary Tables S4–S7. No significant inconsistency was observed between direct and indirect studies. The model’s overall fit was satisfactory.
Immune checkpoint inhibitors have now become the first-line treatment options for advanced melanoma in the US according to the National Comprehensive Cancer Network guidelines (20). ICIs are increasingly used in cancer therapy and a key challenge we have to face is the uncontrolled collateral effects on the immune system (21). Immune-related adverse events are autoimmune-toxic effects associated with ICIs used for the treatment of advanced solid tumors (22). The differences in the risk of irAEs may attribute to the different mechanisms of each agent and the combined use of ICIs (23). Ipilimumab is a cytotoxic T-lymphocyte–associated protein 4 inhibitor that increases T cell function and antitumor responses in patients with advanced melanoma, through suppressing T cell effector function following initial activation by costimulatory signals (24). Pembrolizumab and nivolumab are both PD-1 inhibitors, that reinvigorate tumor-specific exhausted T cells and promote immune-mediated elimination of tumor cells (25, 26). Theoretically, CTLA-4 inhibitors may induce a greater magnitude of T cell proliferation or reduced regulatory T cell-mediated immunosuppression, while PD-1 inhibitors may activate a relatively smaller number of T cells (27, 28). Thus, PD-1 inhibitors are often considered to be better tolerated than CTLA-4 inhibitor (29). However, in reality, the types of irAEs related to monotherapy targeting the CTLA-4 or PD-1 differ (30). One previous study revealed that rash, colitis and hypophysitis were more common with CTLA-4 inhibitors; pneumonitis, arthralgia and hypothyroidism were more frequently seen with PD-1 inhibitors (31). The biomedical explanations for the differences in irAE localization with different ICIs have not yet been fully elucidated.
In this study, we found that ICIs were associated with a higher risk of grade 1–5 IRP compared with conventional chemotherapy in melanoma patients. In addition, dual ICIs combination showed a higher risk than ICI monotherapy. It was implied that ICIs could increase the risk of IRP in melanoma patients compared with conventional chemotherapy, which was consistent with previous studies that conducted in lung cancer (32) and other solid tumors (33). When comparing the risk of IRP among three kinds of ICI monotherapy (ipilimumab, nivolumab and pembrolizumab), no significant difference was observed in this study. It could be inferred that the risk of IRP was comparable among the three kinds of ICI monotherapy and there was no significant difference between CTLA-4 and PD-1 inhibitors. It was inconsistent with the aforementioned conclusion that IRP was more common with PD-1 inhibitors (31). It is worth mentioning that there were only ten eligible randomized clinical trials enrolled in this study and most assessments of IRP risk of ICIs come from comparisons between ICI monotherapy and dual ICIs combination or chemotherapy. There were no randomized clinical trials that evaluated IRP risk involving ICI in combination with chemotherapy. It is implausible to see the higher ranking of placebo versus chemotherapy in terms of grade 1‐5 IRP, although we have demonstrated there were no statistically significant differences between placebo and chemotherapy. It was probably due to the limited number of eligible RCTs and relatively small sample size. In addition, no statistically significant differences were seen between the four treatment groups in the risk of high‐grade (grade 3‐5) IRP. It could mainly attribute to the low prevalence of high-grade IRP. Despite our current study provides insight in indirect comparisons, head‐to‐head comparisons among different ICI-based regimens are still lacking. More well-constructed, adequately powered randomized clinical trials should be conducted to assess the safety of the combination of ICIs and chemotherapy to enrich the evidence.
With the increasing application of ICIs in cancer treatment, there is undoubtedly a rise in the absolute burden and mortality of pneumonitis. It is important to define a tailored treatment strategy to maximize the treatment benefits and minimize immune‐related adverse events, especially serious or fatal adverse events. In the present study, we found that the combination of two ICIs was associated with a higher risk of irAEs compared with the monotherapy alone. Previous studies suggested that the incidence of pneumonitis with combination therapy may be higher and the time to onset is sooner. The median time to onset of pneumonitis was 2.7 months in patients with dual ICIs combination and 4.6 months in patients with ICI monotherapy (34). Furthermore, some research groups found that the patients who experienced irAEs had significantly better clinical outcomes (35, 36). There have been other groups that reported the safety of resuming immunotherapy after immune-related toxicity (37, 38). The Society for Immunotherapy of Cancer recommended that resuming ICI therapy remained to be an available treatment option in patients in patients with grade 2-3 pneumonitis, which has resolved completely (39). Our findings may provide important implications for better clinical practice guidance on ICI use in terms of irAEs (40, 41). For patients with preexisting autoimmune diseases, prior radiotherapy and heavy smoking history, ICI monotherapy at low dose initially is recommended to avoid irAEs, instead of ICI combination. In addition, intensive monitoring is essential for early identification and intervention.
To the best of our knowledge, this is the first systematic review and network meta-analysis which provides the most updated and comprehensive evidence of IRP for ICIs related therapeutic regimens in melanoma patients. This study had several limitations that should be acknowledged. First, no consensus diagnostic criteria of IRP are available and the identification of IRP in different studies may not be completely homogeneous. In addition, some low-grade IRP related symptoms such as cough, malaise and mild fever are not specific and likely ignored by clinicians. Therefore, the identification of IRP might not be accurate and complete, which would lead to bias for the assessment of IRP. Second, 30% of the enrolled trials in this study were open-label and may bring unconscious bias. Third, the reported incidence of IRP would increase gradually with longer follow-up and more patients receiving ICIs. IRP is an uncommon but potentially fatal toxicity that results in a high rate of treatment discontinuation and mortality in patients. It implies that IRP requires clinicians to pay close attention, and formulate corresponding prevention. Fourth, commonly accepted risk factors for IRP such as prior radiotherapy and smoking history were potential confounders for the evaluation of risk of IRP. Last, patients may receive corticosteroids when pneumonitis occurred. As is known to all, corticosteroids could suppress the immune system, reduce inflammation and relieve the development of IRP. However, how immune checkpoint inhibitor agents and corticosteroids suppress and regulate the immune system has not been clarified. There has been preliminary evidence that the use of corticosteroids for IRP did not influence the effectiveness of ICIs (42). More relevant works including fundamental researches and clinical trials are in urgent need to address this important clinical issue.
In summary, our network meta-analysis has demonstrated that ICI-based therapy is associated with a higher risk of IRP than chemotherapy in melanoma patients. Dual ICIs combination therapy has a higher risk of IRP than ICI monotherapy. There was no significant difference between CTLA-4 and PD-1 inhibitors in terms of risk of IRP. These findings may provide important implication for making individualized treatment therapy for melanoma patients.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Y-MS and WL contributed equally to the work. Y-MS and WL finished the initial design and conception of the research. Y-MS, WL, and Z-YC contributed to the acquisition of data, analysis and interpretation. YW participated in drafting and revising the article. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.651553/full#supplementary-material
ICIs, immune checkpoint inhibitors; irAEs, immune-related adverse effects; RCT, randomized clinical trial; NMA, network meta-analysis; IRP, immune‐related pneumonitis; ORs, odds ratios; CTLA-4, cytotoxic T lymphocyte-associated protein 4; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; FDA, Food and Drug Administration; ASCO, American Society of Clinical Oncology; ESMO, European Society of Medical Oncology; NCT, National Clinical Trial; CIs, confidence intervals; IF, inconsistency factors; seIF, standard error of inconsistency factors; SUCRA, surface under the cumulative ranking curve.
1. Johnston KM, McPherson E, Osenenko K, Vergidis J, Levy AR, Peacock S. Cost-Effectiveness of Therapies for Melanoma. Expert Rev Pharmacoecon Outcomes Res (2015) 15:229–42. doi: 10.1586/14737167.2015.1017563
2. Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing Burden of Melanoma in the United States. J Invest Dermatol (2009) 129:1666–74. doi: 10.1038/jid.2008.423
3. Surveillance, Epidemiology, and End Results (SEER). Program Cancer Statistics Review, 1975–2013. Bethesda, Maryland: National Cancer Institute (2015). Available at: http://seer.cancer.gov/csr/1975_2013/.
4. GLOBOCAN 2012 V1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. (2013). Available at: http://globocan.iarc.fr.
5. Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab Plus Dacarbazine for Previously Untreated Metastatic Melanoma. N Engl J Med (2011) 364:2517–26. doi: 10.1056/NEJMoa1104621
6. Hamid O, Puzanov I, Dummer R, Schachter J, Daud A, Schadendorf D, et al. Final Analysis of a Randomised Trial Comparing Pembrolizumab Versus Investigator-Choice Chemotherapy for Ipilimumab-Refractory Advanced Melanoma. Eur J Cancer (2017) 86:37–45. doi: 10.1016/j.ejca.2017.07.022
7. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in Previously Untreated Melanoma Without BRAF Mutation. N Engl J Med (2015) 372:320–30. doi: 10.1056/NEJMoa1412082
8. Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab Versus Chemotherapy in Patients With Advanced Melanoma Who Progressed After Anti-CTLA-4 Treatment (CheckMate 037): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol (2015) 16:375–84. doi: 10.1016/S1470-2045(15)70076-8
9. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-Related Adverse Events With Immune Checkpoint Blockade: A Comprehensive Review. Eur J Cancer (2016) 54:139–48. doi: 10.1016/j.ejca.2015.11.016
10. Delaunay M, Prevot G, Collot S, Guilleminault L, Didier A, Mazieres J. Management of Pulmonary Toxicity Associated With Immune Checkpoint Inhibitors. Eur Respir Rev (2019) 28. doi: 10.1183/16000617.0012-2019
11. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 (2010). Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40.
12. Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and Ipilimumab Versus Ipilimumab in Untreated Melanoma. N Engl J Med (2015) 372:2006–17. doi: 10.1056/NEJMoa1414428
13. Larkin J, Minor D, D’Angelo S, Neyns B, Smylie M, Miller WH Jr., et al. Overall Survival in Patients With Advanced Melanoma Who Received Nivolumab Versus Investigator’s Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. J Clin Oncol (2018) 36:383–90. doi: 10.1200/JCO.2016.71.8023
14. Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, et al. Combination Nivolumab and Ipilimumab or Nivolumab Alone in Melanoma Brain Metastases: A Multicentre Randomised Phase 2 Study. Lancet Oncol (2018) 19:672–81. doi: 10.1016/S1470-2045(18)30139-6
15. Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab Versus Ipilimumab in Advanced Melanoma (KEYNOTE-006): Post-Hoc 5-Year Results From an Open-Label, Multicentre, Randomised, Controlled, Phase 3 Study. Lancet Oncol (2019) 20:1239–51. doi: 10.1016/S1470-2045(19)30388-2
16. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-Year Survival With Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2019) 381:1535–46. doi: 10.1056/NEJMoa1910836
17. Eggermont AMM, Kicinski M, Blank CU, Mandala M, Long GV, Atkinson V, et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol (2020) 6:519–27. doi: 10.1001/jamaoncol.2019.5570
18. Zimmer L, Livingstone E, Hassel JC, Fluck M, Eigentler T, Loquai C, et al. Adjuvant Nivolumab Plus Ipilimumab or Nivolumab Monotherapy Versus Placebo in Patients With Resected Stage IV Melanoma With No Evidence of Disease (IMMUNED): A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet (2020) 395:1558–68. doi: 10.1016/S0140-6736(20)30417-7
19. Ascierto PA, Del Vecchio M, Mandalá M, Gogas H, Arance AM, Dalle S, et al. Adjuvant Nivolumab Versus Ipilimumab in Resected Stage IIIB-C and Stage IV Melanoma (CheckMate 238): 4-Year Results From a Multicentre, Double-Blind, Randomised, Controlled, Phase 3 Trial. Lancet Oncol (2020) 21:1465–77. doi: 10.1016/S1470-2045(20)30494-0
20. National Comprehensive Cancer Network. Cutaneous Melanoma (Version 4.2020). Updated September 1, 2020 (Accessed November 15, 2020).
21. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chavez A, Keegan N, Khamashta MA, et al. Immune-Related Adverse Events of Checkpoint Inhibitors. Nat Rev Dis Primers (2020) 6:38. doi: 10.1038/s41572-020-0160-6
22. Petrelli F, Grizzi G, Ghidini M, Ghidini A, Ratti M, Panni S, et al. Immune-Related Adverse Events and Survival in Solid Tumors Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J Immunother (2020) 43:1–7. doi: 10.1097/CJI.0000000000000300
23. Khan Z, Hammer C, Guardino E, Chandler GS, Albert ML. Mechanisms of Immune-Related Adverse Events Associated With Immune Checkpoint Blockade: Using Germline Genetics to Develop a Personalized Approach. Genome Med (2019) 11:39. doi: 10.1186/s13073-019-0652-8
24. Buchbinder E, Hodi FS. Cytotoxic T Lymphocyte Antigen-4 and Immune Checkpoint Blockade. J Clin Invest (2015) 125:3377–83. doi: 10.1172/JCI80012
25. Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-Inhibitory Receptors With Specialized Functions in Immune Regulation. Immunity (2016) 44:989–1004. doi: 10.1016/j.immuni.2016.05.001
26. Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, et al. TIGIT and PD-1 Impair Tumor Antigen-Specific CD8(+) T Cells in Melanoma Patients. J Clin Invest (2015) 125:2046–58. doi: 10.1172/JCI80445
27. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front Oncol (2018) 8:86. doi: 10.3389/fonc.2018.00086
28. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall Survival With Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2017) 377:1345–56. doi: 10.1056/NEJMoa1709684
29. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med (2015) 373:23–34. doi: 10.1056/NEJMoa1504030
30. Pauken KE, Dougan M, Rose NR, Lichtman AH, Sharpe AH. Adverse Events Following Cancer Immunotherapy: Obstacles and Opportunities. Trends Immunol (2019) 40:511–23. doi: 10.1016/j.it.2019.04.002
31. Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and Class-Specific Patterns of Immune-Related Adverse Events of Immune Checkpoint Inhibitors: A Systematic Review. Ann Oncol (2017) 28:2377–85. doi: 10.1093/annonc/mdx286
32. Chen X, Zhang Z, Hou X, Zhang Y, Zhou T, Liu J, et al. Et Al: Immune-Related Pneumonitis Associated With Immune Checkpoint Inhibitors in Lung Cancer: A Network Meta-Analysis. J Immunother Cancer (2020) 8. doi: 10.1136/jitc-2020-001170
33. Su Q, Zhu EC, Wu JB, Li T, Hou YL, Wang DY, et al. Risk of Pneumonitis and Pneumonia Associated With Immune Checkpoint Inhibitors for Solid Tumors: A Systematic Review and Meta-Analysis. Front Immunol (2019) 10:108. doi: 10.3389/fimmu.2019.00108
34. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol (2017) 35:709–17. doi: 10.1200/JCO.2016.68.2005
35. Fujii T, Colen RR, Bilen MA, Hess KR, Hajjar J, Suarez-Almazor ME, et al. Incidence of Immune-Related Adverse Events and Its Association With Treatment Outcomes: The MD Anderson Cancer Center Experience. Invest New Drugs (2018) 36:638–46. doi: 10.1007/s10637-017-0534-0
36. Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, et al. Autoimmunity Correlates With Tumor Regression in Patients With Metastatic Melanoma Treated With Anti-Cytotoxic T-Lymphocyte Antigen-4. J Clin Oncol (2005) 23:6043–53. doi: 10.1200/JCO.2005.06.205
37. Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M, et al. Safety and Efficacy of Re-Treating With Immunotherapy After Immune-Related Adverse Events in Patients With NSCLC. Cancer Immunol Res (2018) 6:1093–9. doi: 10.1158/2326-6066.CIR-17-0755
38. Pollack MH, Betof A, Dearden H, Rapazzo K, Valentine I, Brohl AS, et al. Safety of Resuming Anti-PD-1 in Patients With Immune-Related Adverse Events (irAEs) During Combined Anti-CTLA-4 and Anti-PD1 in Metastatic Melanoma. Ann Oncol (2018) 29:250–5. doi: 10.1093/annonc/mdx642
39. Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, et al. Managing Toxicities Associated With Immune Checkpoint Inhibitors: Consensus Recommendations From the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer (2017) 5:95. doi: 10.1186/s40425-017-0300-z
40. Johnson DB, Sullivan RJ, Ott PA, Carlino MS, Khushalani NI, Ye F, et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol (2016) 2:234–40. doi: 10.1001/jamaoncol.2015.4368
41. Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, et al. Anti-PD-1 Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders or Major Toxicity With Ipilimumab. Ann Oncol (2017) 28:368–76. doi: 10.1093/annonc/mdw443
42. Gaucher L, Adda L, Sejourne A, Joachim C, Chaby G, Poulet C, et al. Impact of the Corticosteroid Indication and Administration Route on Overall Survival and the Tumor Response After Immune Checkpoint Inhibitor Initiation. Ther Adv Med Oncol (2021) 13:1758835921996656. doi: 10.1177/1758835921996656
Keywords: immune checkpoint inhibitors, network meta-analysis, melanoma, immune-related pneumonitis, systematic review
Citation: Sun Y-M, Li W, Chen Z-Y and Wang Y (2021) Risk of Pneumonitis Associated With Immune Checkpoint Inhibitors in Melanoma: A Systematic Review and Network Meta-Analysis. Front. Oncol. 11:651553. doi: 10.3389/fonc.2021.651553
Received: 25 January 2021; Accepted: 06 October 2021;
Published: 21 October 2021.
Edited by:
Marcus O. Butler, University Health Network, CanadaReviewed by:
Jinfeng Wu, Fudan University, ChinaCopyright © 2021 Sun, Li, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Wang, MTE4NzAzNkB6anUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.