- 1Department of Radiation Oncology, Mayo Clinic, Rochester, MN, United States
- 2Department of Pathology, Mayo Clinic, Rochester, MN, United States
- 3Department of Statistics, Mayo Clinic, Rochester, MN, United States
- 4Department of Medical Oncology, Mayo Clinic, Rochester, MN, United States
- 5Department of Pancreas Surgery, Mayo Clinic, Rochester, MN, United States
Background: We evaluated preoperative CA 19-9 levels in patients with resected pancreatic cancer to analyze whether they were predictive of clinical outcomes and could help select patients for additional therapy. We hypothesized that elevated CA 19-9 would be associated with worse pathologic findings and oncologic outcomes.
Methods: This study assessed 509 patients with non-metastatic pancreatic adenocarcinoma who underwent resection at our institution from 1995-2011 and had preoperative CA 19-9 recorded. No patients received neoadjuvant therapy. CA 19-9 level was analyzed as a continuous and a dichotomized (> vs. ≤ 55 U/mL) variable using logistic and Cox models.
Results: Median follow-up was 7.8 years, and the median age was 66 years (33-90). 64% of patients had elevated preoperative CA 19-9 (median: 141 U/mL), that did not correlate with bilirubin level or tumor size. Most patients had ≥ T3 tumors (72%) and positive lymph nodes (62%). The rate of incomplete (R1 or R2) resection was 19%. Increasing preoperative CA 19-9 was associated with extra-pancreatic extension (p=0.0005), lymphovascular space invasion (p=0.0072), incomplete resection [HR (95% CI) 2.0 (1.2-3.5)], and lower OS [HR = 1.6 (1.3-2.0)]. Each doubling in preoperative CA 19-9 value was associated with an 8.3% increased risk of death [HR = 1.08 (1.02-1.15)] and a 10.0% increased risk of distant recurrence [HR = 1.10 (1.02-1.19)]. Patients classified as non-secretors had comparable outcomes to patients with normal CA 19-9.
Conclusions: Elevated preoperative CA 19-9 level was associated with adverse pathologic features, incomplete resection, and inferior clinical outcomes. Neither tumor size nor bilirubin confound an elevated CA 19-9 level. Preoperative CA 19-9 level may help select patients for additional therapy.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) represents the fourth leading cause of cancer death in the United States (1). The only treatment associated with potential cure is complete resection; however, only 20% of the patients have anatomically resectable disease according to standard criteria at the time of diagnosis (2). Based on adjuvant therapy trials, up to 60% of these patients will have an incomplete resection with positive microscopic (R1) or gross (R2) residual disease, negating any oncologic benefit from resection (3–5). Even for patients with complete resection, the rates of locoregional (LR) and distant relapse (DR) remain high (6). Clinical trials support the standard role of adjuvant chemotherapy though routine use of adjuvant radiation therapy remains controversial (3–5, 7–13). Several recent clinical trials show that neoadjuvant chemotherapy followed by chemoradiation may improve the rates of R0 resection, lymph node sterilization, local control, and possibly overall survival (OS) compared to upfront resection, with benefits likely being greatest for patients with borderline resectable pancreas cancer (6, 14–16). The phase III randomized PREOPANC study suggested that preoperative chemoradiotherapy may lead to tumor downstaging and a reduction in the rates of adverse pathologic features (16). The ideal selection process and factors to decide between upfront surgical versus a neoadjuvant approach are not yet optimized in patients with otherwise anatomically resectable tumors.
CA 19-9 is a Lewis blood group antigen that is measurable in up to 90% of patients, with approximately 10% of patients lacking the fucosyl-transferase necessary for expression (i.e. non-secretors). Up to two-thirds of patients have elevated CA 19-9 levels at presentation; however, these levels may also be falsely elevated due to concomitant biliary obstruction at diagnosis (17, 18). Previous studies have assessed preoperative CA 19-9 levels and suggest elevated CA 19-9 levels predict for higher tumor stage, adverse pathologic features, and tumor resectability in upfront resectable pancreas cancer patients (19–22). In addition, elevated CA 19-9 can predict worse survival outcomes (19, 23–28). An NCDB analysis of over 100,000 pancreatic cancer patients concluded that any elevation of CA 19-9 above normal was associated with a significant survival detriment, greatest in early stage resectable disease. Neoadjuvant chemotherapy was the only treatment strategy able to eliminate this survival detriment in anatomically resectable pancreatic cancer, more so even than adjuvant chemotherapy (29). Serial measures of CA 19-9 may predict tumor biology and response to therapy in locally advanced disease, and an early decrease in CA 19-9 following administration of gemcitabine is associated with improved overall survival (30). Multidisciplinary care is critical to optimizing treatment approaches for patients with pancreatic cancer, and further clarification of the predictive and prognostic value of CA 19-9 will assist healthcare teams with optimizing therapy for resectable pancreatic cancer (31, 32).
In the present study, we evaluated a large cohort of patients to assess the association of preoperative elevated CA 19-9 level with adverse pathologic features and clinical endpoints. In the era of neoadjuvant therapy for pancreas cancer, we aim to evaluate the potential of preoperative CA 19-9 level as a key tool to optimize patient selection for neoadjuvant therapy.
Methods
Patients
This retrospective study was approved by our institutional review board (IRB: 17-003122). As a minimal risk IRB, a HIPPA waiver was used, and informed consent was not required. The institutional cancer registry was queried for all patients with non-metastatic pancreatic adenocarcinoma from March 1995 to January 2011. Treatments over this timeframe allowed for a relatively homogeneous assessment in the pre-neoadjuvant era. Additionally, prolonged follow-up was available for this set of patients. 1140 patients were reviewed in total, with 631 patients excluded. Of these, 451 had no CA 19-9 available, 104 had inadequate follow-up, 50 had surgical resection at an outside institution, and 26 had neoadjuvant treatment. This resulted in the final study cohort of 509 patients available for full evaluation.
CA 19-9 Level Measurement
Serum CA 19-9 level was measured using an immunoenzymatic sandwich assay. A Bayer/Siemens ACS 180 chemistry analyzer (Siemens Healthcare Diagnostics Inc, Deerfield, IL) was utilized from 1995 to July of 2001. From July 2001 to 2005, a Bayer/Siemens Centaur (Siemens Health Siemens Healthcare Diagnostics Inc, Deerfield, IL) instrument was used. After 2005, the DXI 800 (Beckman Coulter Inc, Chaska, MN) was utilized for CA 19-9 level measurement. A threshold of 55 U/mL was used as the institutional reference for a normal CA 19-9 level during the period of patient inclusion, and elevated CA 19-9 level was defined as above this institutional reference. Patients without measurable CA 19-9 levels were considered non-secretors.
Patient Treatment
All patients underwent curative intent resection in an upfront surgical approach, without any preoperative therapy. Resection types included pancreaticoduodenectomy, distal pancreatectomy, and total pancreatectomy. Pathologic features evaluated include TNM stage, the ratio of positive lymph nodes to total dissected (LNR), resection margin status, perineural and lymphovascular space invasion (LVSI), tumor grade, and tumor size. Staging was assessed using the AJCC 7th edition. For this reason, comparisons were made between T1 or T2 tumors, compared with T3 or T4 tumors (those demonstrating extra-pancreatic extension). In this study, an R0 resection was defined as a resection achieving negative gross and microscopic margins within 1 mm of the inked margin. After surgery, patients received adjuvant therapy per the discretion of the multidisciplinary treatment team. Adjuvant therapy consisted of chemotherapy alone, chemoradiation or combination chemotherapy and chemoradiation. Radiation was delivered with concurrent chemotherapy, most frequently 5-flourouracil or capecitabine.
Assessment of Relapse
LR was defined as local (tumor bed or remnant pancreas) or regional (regional lymph node) disease progression based on imaging. DR was defined as distant organ or distant lymph node spread consistent with metastatic disease. When available, CA 19-9 level, biopsy, and surgical results were used to corroborate radiographic findings of relapse. The presence of LR or DR was assessed until the time of death. OS was defined from the surgical date until patient death or last follow up. To minimize selection bias, patients with less than 90 days of survival after surgery, those with R2 resection, or patients who did not receive adjuvant therapy were excluded from the survival and oncology outcome analysis.
Statistics
Logistic regression and Spearman’s rank correlation were used to assess the relationship between CA 19-9 levels and pathologic outcomes. The Spearman rank test was used rather than the Pearson test due to the wide range and right-skew of the data. The Kaplan Meier method was used to estimate survival free of DR and OS from the time of surgery to recurrence or death. For LR, the cumulative incidence was estimated considering death as a competing risk. Univariate Cox proportional hazard models were used to evaluate associations between CA 19-9 level and adverse pathology features with OS and DR. For LR, the Fine and Gray extension of the Cox model was used. The backward selection process was used to identify variables for inclusion in the multivariable model. Patients classified as non-secretors were only included in survival analysis. A p-value < 0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 509 patients were evaluated and included in the study. With a median follow up of 7.8 years (range, 0.1-16 years), 21% of patients remained alive at the time of last follow-up. Patient and tumor characteristics are reported in Table 1. A total of 324 patients (64%) had elevated preoperative CA 19-9; 138 patients (27%) had normal CA 19-9; and 47 (9%) were found to be non-secretors. The median preoperative CA 19-9 value was 140.5 U/mL (range, 4 to 335,300 U/mL). For all patients, the median OS was 2.0 years and the 5-year OS was 21.6% (95% CI 18%-26%) (Figure 1A). Gemcitabine was the most frequently administered adjuvant chemotherapy, with a median 6 of cycles given. The median radiation dose was 50.4 Gy in 28 fractions (range 45-55 Gy).
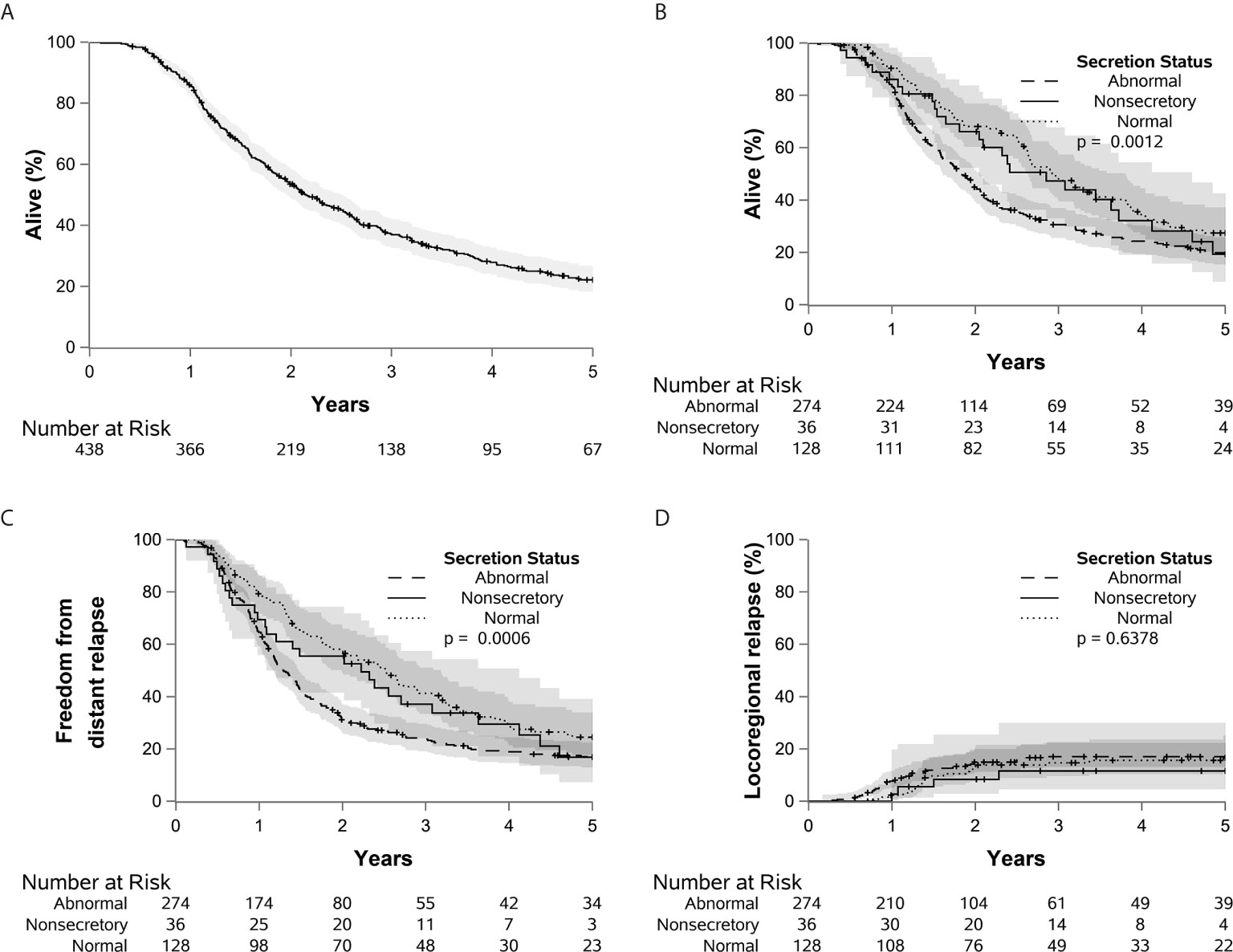
Figure 1 Kaplan-Meier curves are shown demonstrating the overall survival of the entire cohort (A), as well as overall survival (B), distant relapse (C), and locoregional relapse (D) stratified by CA 19-9 status.
Preoperative CA 19-9 and Adverse Pathologic Features
Preoperative CA 19-9 level was analyzed as a continuous and a dichotomized value (elevated vs. normal). For patients with multiple recorded preoperative CA 19-9 levels, the last value drawn before surgery was used. For these analyses, CA 19-9 non-secretors were excluded to prevent skewing of the resulting analysis. We found that serum CA 19-9 levels did not correlate with either serum bilirubin or tumor size (Figure 2). Using a rank sum test of continuous CA 19-9 value, higher CA 19-9 levels were associated with T stage ≥ 3 vs. < 3 (p=0.0005, median 172 vs. 94), LVSI present vs. absent (p=0.0072, median 224 vs. 130), and LNR > 7% vs. ≤ 7% (p=0.048, median 167 vs. 115) (Table 2). Increasing levels of CA 19-9 were correlated with increases in the number of positive nodes (p=0.004, correlation coefficient 0.14), LNR (p=0.0005, correlation coefficient 0.16), and tumor size (p=0.002, correlation coefficient 0.15) when considered as a continuous variable.
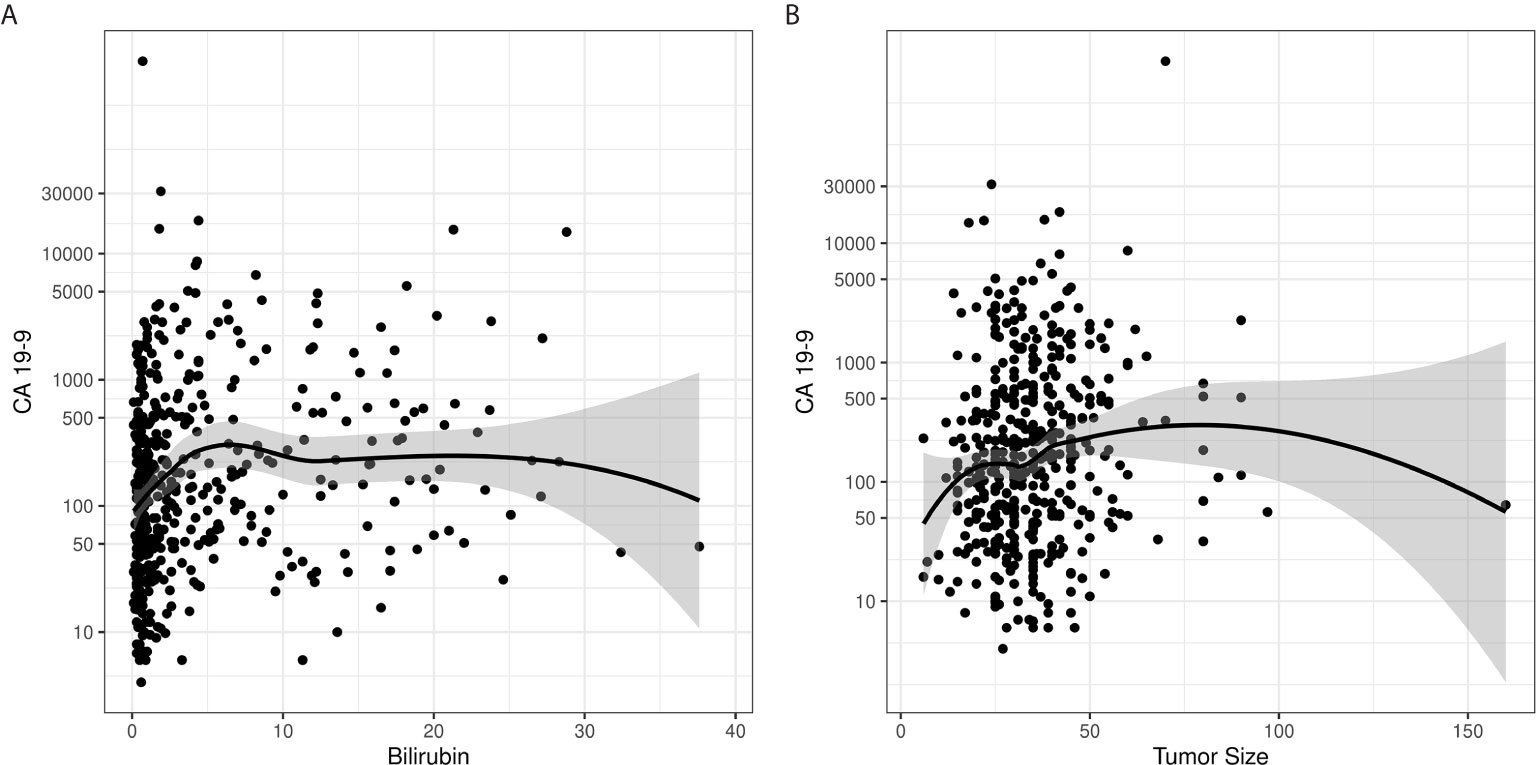
Figure 2 Loess curves demonstrate a lack of correlation between CA 19-9 levels and (A) bilirubin and (B) tumor size.
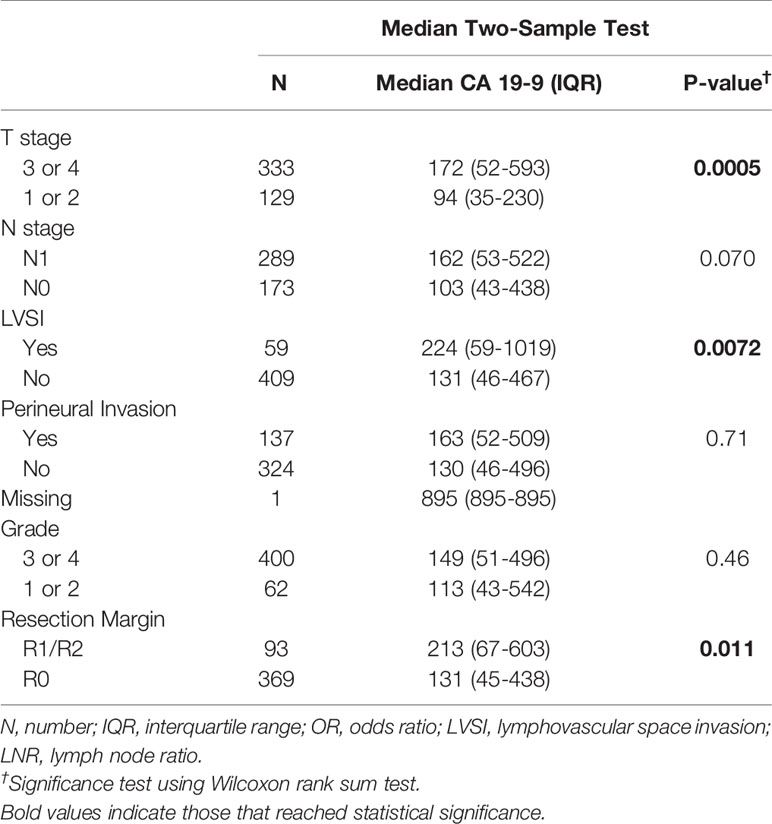
Table 2 The association of CA 19-9 and adverse pathologic features among patients with normal and elevated CA 19-9 is demonstrated.
CA 19-9 was analyzed as a dichotomous variable in two separate analyses, one in which the non-secretor groups was combined with the patients with normal CA 19-9 levels, and one in which the non-secretors, normal CA 19-9 level patients, and elevated CA 19-9 level patients were separated into 3 distinct groups. Elevated CA 19-9 (> 55 U/mL) was then analyzed as a predictor of T stage 3 or 4, node positivity, LVSI, perineural invasion, grade 3 or 4, tumor size ≥ 3.5 cm, and incomplete resection. Elevated CA 19-9 as a dichotomized variable only reached statistical significance for incomplete resection in the two and three group settings (p=0.007 and p=0.0047, respectively).
Preoperative CA 19-9 and Resection Margins
The overall rate of R1/R2 resection was 19%. The median CA 19-9 values for patients with R0, R1 and R2 resections were 108 U/mL (interquartile range (IQR), 28-361), 154 U/mL (IQR, 53-543), and 642 U/mL (IQR, 185-2135). Using a Kruskal Wallis test, CA 19-9 level was significantly different for patients with R0, R1, and R2 resections (p=0.003). The Wilcoxon rank sum test showed statistically higher levels of CA 19-9 was associated with R1 resections, compared to R0 (p=0.0009). Patients with a normal CA 19-9 level had a rate of incomplete resection (R1 or R2) of 12.4% vs. 22% for patients with elevated CA 19-9. Similarly, logistic regression analysis showed an association between abnormal CA 19-9 level and incomplete resection [p=0.01, HR=2.0 (1.2-3.5)].
Preoperative CA 19-9 and Relapse and Survival
At the time of evaluation, there were a total of 404 patient deaths. This included 33 patients (70%) in the non-secretor group, 101 patients (73%) in the normal CA 19-9 group, and 270 patients (83%) in the elevated CA 19-9 group. Prior to further analysis of the outcomes, 55 patients without adjuvant therapy, 16 with R2 resection, and one with death within 90 days of surgery were excluded. The 5-year OS was 19% (95% CI, 9%-43%), 27% (95% CI, 20%-37%), and 20% (95% CI, 15%-26%) for the non-secretors, normal and elevated CA 19-9 groups respectively (p=.0012) (Figures 1B–D). Relative to patients with normal CA 19-9, elevated CA19-9 was associated with a statistically significant decrease in OS [p=0.0015, HR=1.49 (1.17-1.90)] and an increased risk of DR [p=0.0121, HR=1.45 (1.09-1.93)]. Elevated CA19-9 was also associated with decreased freedom from death or DR [p<0.001, HR=1.57 (1.24-1.99)] (Supplementary Figure 1). These values are reported using the parsimonious model, retaining only variables where p < 0.05 using backward selection. Elevated CA 19-9 was not associated with risk of LR [p=0.69, HR=1.11 (0.66-1.87)]. There was no significant difference between the normal CA 19-9 group and the non-secretor group in regards to OS, DR, or LR.
When analyzed as a continuous variable using linear association with log base 2 of the preoperative CA 19-9 value, each doubling in CA 19-9 value was associated with an 8.3% increased risk of death [p<0.0001, HR=1.08 (1.02-1.15)], a 10.0% increased risk of DR [p=0.006, HR=1.10 (1.02-1.19)]. For this analysis, patients classified as non-secretors were excluded. Adverse pathologic features that were associated with elevated CA 19-9 were also found to have a negative impact on patient survival (Figure 3). This analysis was conducted with univariate and multivariate Cox analyses (Table 3). Adjuvant chemotherapy along (without radiotherapy) was also associated with decreased overall survival [p=0.0019, HR=1.60 (1.19-2.16)] and LR [p<0.0001, HR=3.07 (1.84-5.12)] in the overall cohort.
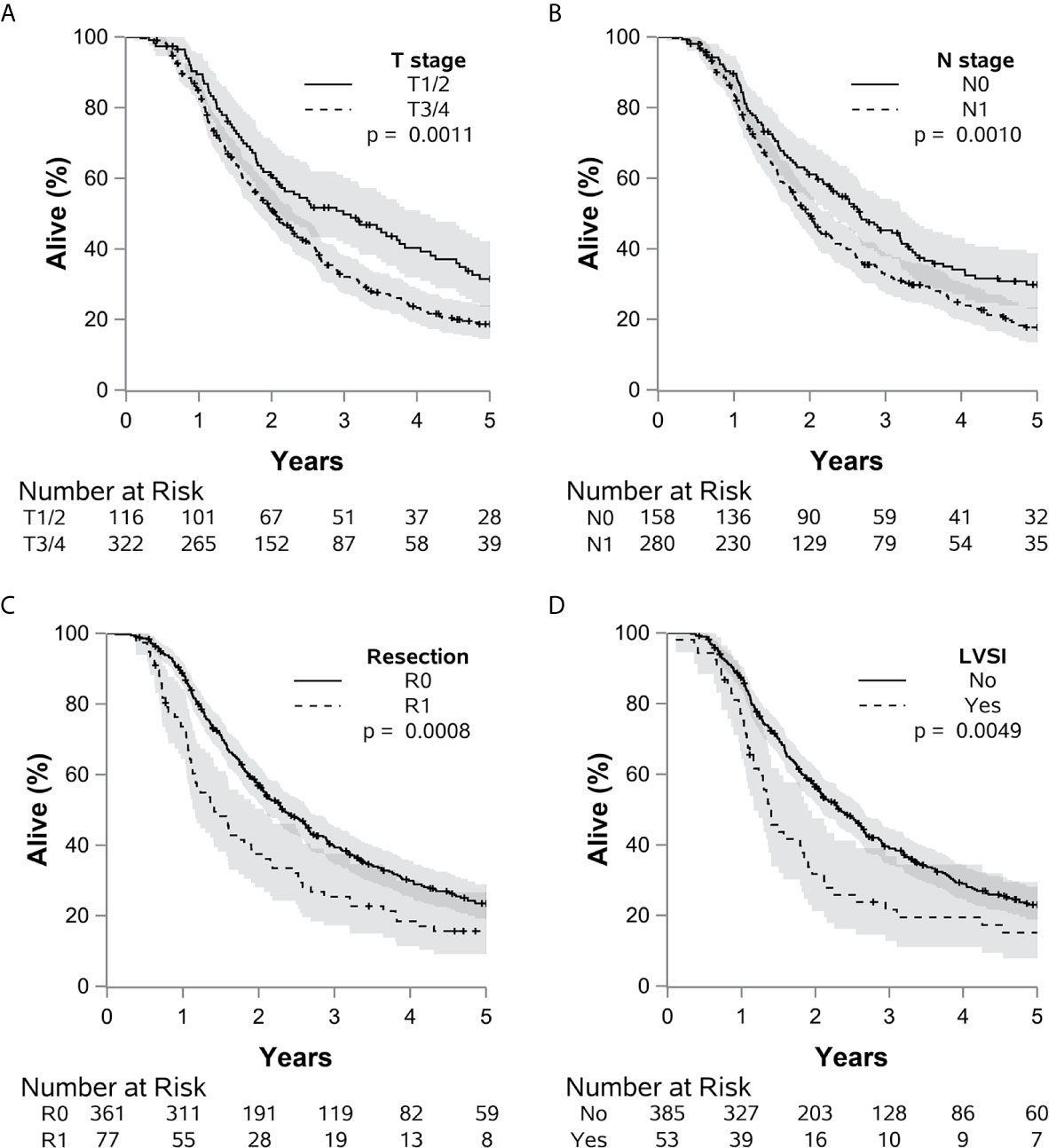
Figure 3 The impact of many adverse pathologic factors on survival is portrayed, with (A) T 3 or 4 vs. 1 or 2 (p=0.0011), (B) N1 vs. N0 (p=0.0010), (C) R1 vs. R0 (p=0.0009), and (D) LVSI present vs. absent (p=0.0049) all reaching statistical significance. Grade 4 or 3 vs. 1 or 2 (p=0.061) demonstrated a potential statistical trend.
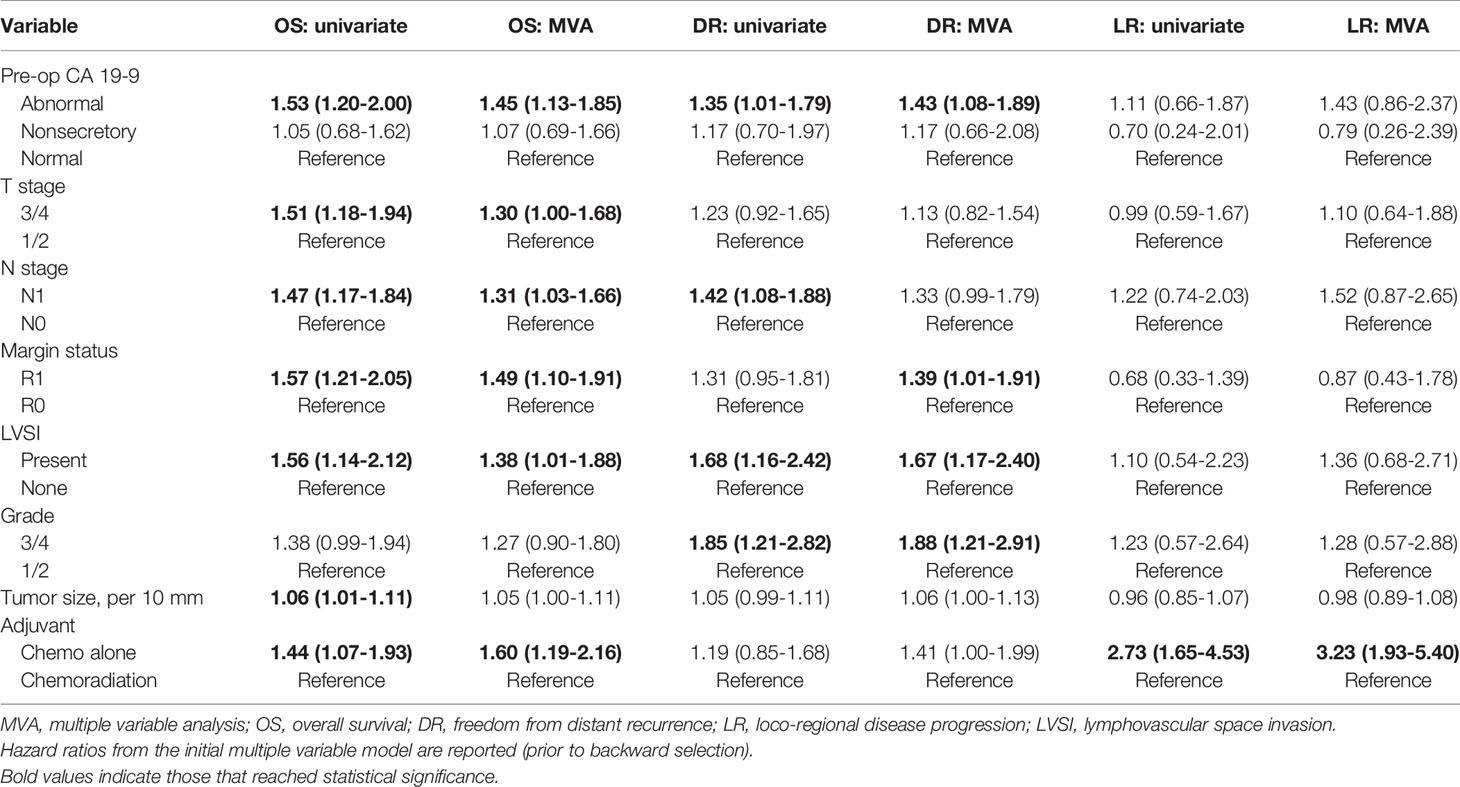
Table 3 Multiple variable (MVA) and univariate Cox analyses for overall survival (OS), freedom from distant recurrence (DR), and loco-regional disease progression (LR) are shown.
Discussion
This large, single institutional study with long-term follow-up of patients with localized pancreas cancer treated with an upfront surgical approach demonstrates the utility of pre-operative CA 19-9 level in predicting adverse pathologic features, resection margin outcomes. In agreement with previously reported series, elevated pre-operative CA 19-9 level was associated with higher risk pathologic features, including T stage, multiple lymph node involvement, and LVSI (19, 21, 33, 34). We show these adverse pathologic features associated with CA 19-9 elevation are also predictive of patient survival, further reinforcing the link between elevated CA 19-9, advanced cancer stage, and subsequently worse patient outcomes (21, 28, 35–40). Additionally, there was no association between CA 19-9 levels and serum bilirubin levels or tumor size. This analysis constitutes the first robust demonstration that these potential confounders do not significantly influence CA 19-9 level; therefore, any CA 19-9 elevation may be biologically relevant, despite the presence of potential confounders.
The association between elevated CA 19-9 level and increased rates of R1 and R2 resections is a critical finding because achieving R0 resection is required to potentially cure pancreas cancer (41, 42). In the present study, patients with elevated CA 19-9 levels were twice as likely to have positive margins as patients with normal CA 19-9 levels. CA 19-9 level was predictive of increased rates of incomplete resection both as a continuous variable and as a dichotomized value with a threshold of 55 U/mL, the reference value for normal during the period of this study. Other studies have posited that higher CA 19-9 values might predict for unresectable disease, but they have generally been smaller analyses (20). Hartwig et al. reported findings from their analysis of 1,626 consecutive patients who underwent upfront surgical resection for primary pancreatic adenocarcinoma, and they noted R0 rates as low as 15% in patients with CA 19-9 levels ≥ 1000 U/mL (43). This study importantly also found a continuous decrease in the R0 resection rate and subsequent survival with increased values of CA 19-9, and multivariate analysis found the CA 19-9 level to be the most valuable independent predictor for resectability.
The standard reference of “normal CA 19-9” has changed over the years dependent on specific institutional assays, making direct comparisons between specific upper limits of CA 19-9 difficult. Although multiple centers have published different CA 19-9 cutoffs, none of these thresholds are clinically utilized to decide between upfront surgery or neoadjuvant therapy (22, 35, 40). Coupled with the loss of significance of CA 19-9’s predictive power for the many pathologic factors when analyzed as a dichotomized variable, as opposed to as a continuous variable, these findings suggest that a simple threshold is an insufficient way to assess CA 19-9 level.
In the present study, we show an 8.3% increased risk of death and a 10.0% increased risk of DR with each doubling of CA 19-9 level. This association between elevated preoperative CA 19-9 level and decreased OS is consistent with the literature, and our result reinforces the prognostic value of this test (43, 44). These results correlate with the findings of Mattiuci et al., who reported a graded decrease in 5-year OS using 4 separate CA 19-9 cutoffs (45).
Coupled with our findings in this study, these results suggest that any elevation of CA 19-9 above reference level should be considered an adverse risk feature, with higher elevations portending decreased rates of resectability and subsequent survival with an upfront, surgery-first approach. Thus, patients presenting with any CA 19-9 level elevation should be strongly considered for neoadjuvant therapy, regardless of anatomic resectability. The association of CA 19-9 level with numerous adverse pathologic features and decreased survival supports the idea that these patients may benefit from neoadjuvant therapy. Additional justification includes the fact that patients had decreased OS (HR=1.60 [1.12-2.16]) with adjuvant chemotherapy alone, compared to chemoradiation. Administration of neoadjuvant chemotherapy and chemoradiation presents the opportunity to (1) downstage unresectable or borderline resectable patients, (2) detect occult metastatic disease in patients likely to develop regional or distant progression in the immediate post-operative period, and (3) enhance the tolerance of and ability to complete all intended therapies that may otherwise be in jeopardy due to post-operative complications or performance status changes.
There is growing evidence showing the benefit of neoadjuvant therapy prior to resection in the management of pancreas cancer. For example, the R0 resection rates with neoadjuvant chemoradiotherapy were 71% vs. 40% for upfront surgery in patients enrolled on the PREOPANC clinical trial, inclusive of both resectable and borderline-resectable pancreas cancer (16). Further, adverse pathologic features such as pathologic lymph nodes and LVSI were less common in patients who received neoadjuvant chemoradiation. In addition to assessing a patient’s response to chemoradiotherapy, the association of preoperative CA 19-9 level with adverse pathologic features and subtotal resection may aid in selecting the optimal timing of resection (23, 46, 47). Future studies are needed to better understand the kinetics of CA 19-9 level changes with neoadjuvant therapy, and additional biomarkers would help risk stratify patient who are CA 19-9 non-secretors (48). Lastly, it should be noted that other critical factors, such as radiographic findings of vascular involvement, assessments of a patient’s performance status, and recommendations from multidisciplinary tumor boards, should also be carefully considered. We suggest that the best practice recommendation is for all patients with elevated CA 19-9 level to be considered for neoadjuvant or other additional therapy.
The chief change over the time period of this study (1995 to 2011) involved the different technology used to detect CA 19-9. The impact of the resulting variance would likely be quite small, especially because CA 19-9 was also analyzed as a continuous variable. There was also evidence of changes in practice patterns, chiefly consisting of a decline of adjuvant chemoradiation in favor of chemotherapy followed by chemoradiation. Patients generally had similar rates of adjuvant treatment, overall. This cohort also excluded patients who were found to be unresectable at the time of surgery because emphasis was placed on assessment of patients who underwent resection. It is likely that these patients had generally higher CA 19-9 levels and, therefore, could be candidates for inclusion in future studies. Finally, the retrospective nature of this analysis carries inherent biases, which somewhat limit the scope of the conclusions.
Conclusions
Elevated and increasing preoperative CA19-9 levels were associated with adverse pathologic features, incomplete resection, and inferior clinical outcomes and not associated with potential confounders, like tumor size or serum bilirubin. Preoperative CA 19-9 level may aid in patient selection for neoadjuvant therapy, but further investigation and validation is warranted.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Mayo Clinic Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
RK: writing and methodology. SL: writing. WH: formal analysis and methodology. MT: methodology. KM: conceptualization, data curation, and writing. All authors: review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
RM is the Editor in Chief of ASTRO Advances in Radiation Oncology.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.651119/full#supplementary-material
Supplementary Figure 1 | The association between death or distant relapse and CA 19-9 is shown. Abnormal CA 19-9 was associated with decreased freedom from death or distant relapse (p<0.001).
References
1. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal Cancer Statistics, 2020. CA: Cancer J Clin (2020) 70:145–64. doi: 10.3322/caac.21601
2. Howard TJ, Krug JE, Yu J, Zyromski NJ, Schmidt CM, Jacobson LE, et al. A Margin-Negative R0 Resection Accomplished With Minimal Postoperative Complications is the Surgeon’s Contribution to Long-Term Survival in Pancreatic Cancer. J Gastrointest Surg (2006) 10:1338–46. doi: 10.1016/j.gassur.2006.09.008
3. Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A Randomized Trial of Chemoradiotherapy and Chemotherapy After Resection of Pancreatic Cancer. New Engl J Med (2004) 350:1200–10. doi: 10.1056/NEJMoa032295
4. Regine WF, Winter KA, Abrams R, Safran H, Hoffman JP, Konski A, et al. Fluorouracil-Based Chemoradiation With Either Gemcitabine or Fluorouracil Chemotherapy After Resection of Pancreatic Adenocarcinoma: 5-Year Analysis of the Us Intergroup/Rtog 9704 Phase Iii Trial. Ann Surg Oncol (2011) 18:1319–26. doi: 10.1245/s10434-011-1630-6
5. Sergeant G, Ectors N, Van Steenbergen W, Aerts R, Topal B. Patterns of Recurrence After Curative Resection of Pancreatic Ductal Adenocarcinoma. Eur J Surg Oncol (EJSO) (2009) 35:600–4. doi: 10.1016/j.ejso.2008.12.006
6. Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, et al. Long-Term Survival After Multidisciplinary Management of Resected Pancreatic Adenocarcinoma. Ann Surg Oncol (2009) 16:836. doi: 10.1245/s10434-008-0295-2
7. Corsini MM, Miller RC, Haddock MG, Donohue JH, Farnell MB, Nagorney DM, et al. Adjuvant Radiotherapy and Chemotherapy for Pancreatic Carcinoma: The Mayo Clinic Experience (1975-2005). J Clin Oncol (2008) 26:3511–6. doi: 10.1200/JCO.2007.15.8782
8. Doi R, Imamura M, Hosotani R, Imaizumi T, Hatori T, Takasaki K, et al. Surgery Versus Radiochemotherapy for Resectable Locally Invasive Pancreatic Cancer: Final Results of a Randomized Multi-Institutional Trial. Surg Today (2008) 38:1021–8. doi: 10.1007/s00595-007-3745-8
9. Group GTS. Further Evidence of Effective Adjuvant Combined Radiation and Chemotherapy Following Curative Resection of Pancreatic Cancer. Cancer (1987) 59:2006–10. doi: 10.1002/1097-0142(19870615)59:12<2006::AID-CNCR2820591206>3.0.CO;2-B
10. Kaiser MH, Ellenberg SS. Pancreatic Cancer: Adjuvant Combined Radiation and Chemotherapy Following Curative Resection. Arch Surg (1985) 120:899–903. doi: 10.1001/archsurg.1985.01390320023003
11. Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, et al. Adjuvant Radiotherapy and 5-Fluorouracil After Curative Resection of Cancer of the Pancreas and Periampullary Region: Phase Iii Trial of the Eortc Gastrointestinal Tract Cancer Cooperative Group. Ann Surg (1999) 230:776. doi: 10.1097/00000658-199912000-00006
12. Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant Chemotherapy With Gemcitabine vs Observation in Patients Undergoing Curative-Intent Resection of Pancreatic Cancer: A Randomized Controlled Trial. Jama (2007) 297:267–77. doi: 10.1001/jama.297.3.267
13. Van Laethem J-L, Hammel P, Mornex F, Azria D, Van Tienhoven G, Vergauwe P, et al. Adjuvant Gemcitabine Alone Versus Gemcitabine-Based Chemoradiotherapy After Curative Resection for Pancreatic Cancer: A Randomized eortc-40013-22012/ffcd-9203/gercor Phase Ii Study. J Clin Oncol (2010) 28:4450. doi: 10.1200/JCO.2010.30.3446
14. Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative Gemcitabine-Based Chemoradiation for Patients With Resectable Adenocarcinoma of the Pancreatic Head. J Clin Oncol (2008) 26:3496–502. doi: 10.1200/JCO.2007.15.8634
15. Han S, Choi SH, Choi DW, Heo JS, Han IW, Park D-J, et al. Neoadjuvant Therapy Versus Upfront Surgery for Borderline-Resectable Pancreatic Cancer. Minerva Chirurgica (2019) 75:15–24. doi: 10.23736/S0026-4733.19.07958-6
16. Versteijne E, Suker M, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase Iii Preopanc Trial. J Clin Oncol (2020) 38:1763–73. doi: 10.1200/JCO.19.02274
17. Katsanos KH, Kitsanou M, Christodoulou DK, Tsianos EV. High Ca 19-9 Levels in Benign Biliary Tract Diseases: Report of Four Cases and Review of the Literature. Eur J Internal Med (2002) 13:132–5. doi: 10.1016/S0953-6205(02)00002-X
18. Mann D, Edwards R, Ho S, Lau W, Glazer G. Elevated Tumour Marker Ca19-9: Clinical Interpretation and Influence of Obstructive Jaundice. Eur J Surg Oncol (2000) 26:474–9. doi: 10.1053/ejso.1999.0925
19. Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-delCastillo C, Warshaw AL. Perioperative ca19-9 Levels can Predict Stage and Survival in Patients With Resectable Pancreatic Adenocarcinoma. J Clin Oncol (2006) 24:2897–902. doi: 10.1200/JCO.2005.05.3934
20. Forsmark CE, Lambiase L, Vogel SB. Diagnosis of Pancreatic Cancer and Prediction of Unresectability Using the Tumor-Associated Antigen Ca19-9. Pancreas (1994) 9:731–4. doi: 10.1097/00006676-199411000-00010
21. Hallemeier CL, Botros M, Corsini MM, Haddock MG, Gunderson LL, Miller RC. Preoperative Ca 19-9 Level is an Important Prognostic Factor in Patients With Pancreatic Adenocarcinoma Treated With Surgical Resection and Adjuvant Concurrent Chemoradiotherapy. Am J Clin Oncol (2011) 34:567–72. doi: 10.1097/COC.0b013e3181f946fc
22. Hata S, Sakamoto Y, Yamamoto Y, Nara S, Esaki M, Shimada K, et al. Prognostic Impact of Postoperative Serum Ca 19-9 Levels in Patients With Resectable Pancreatic Cancer. Ann Surg Oncol (2012) 19:636–41. doi: 10.1245/s10434-011-2020-9
23. Berger AC, Garcia M Jr, Hoffman JP, Regine WF, Abrams RA, Safran H, et al. Postresection Ca 19-9 Predicts Overall Survival in Patients With Pancreatic Cancer Treated With Adjuvant Chemoradiation: A Prospective Validation by Rtog 9704. J Clin Oncol (2008) 26:5918–22. doi: 10.1200/JCO.2008.18.6288
24. Berger AC, Meszoely IM, Ross EA, Watson JC, Hoffman JP. Undetectable Preoperative Levels of Serum Ca 19-9 Correlate With Improved Survival for Patients With Resectable Pancreatic Adenocarcinoma. Ann Surg Oncol (2004) 11:644–9. doi: 10.1245/ASO.2004.11.025
25. Berger AC, Winter K, Hoffman JP, Regine WF, Abrams RA, Safran H, et al. Five Year Results of Us Intergroup/Rtog 9704 With Postoperative Ca 19-9 <= 90 U/Ml and Comparison to the conko-001 Trial. Int J Radiat Oncol (2012) 84:E291–7. doi: 10.1016/j.ijrobp.2012.04.035
26. Katz A, Hanlon A, Lanciano R, Hoffman J, Coia L. Prognostic Value of Ca 19-9 Levels in Patients With Carcinoma of the Pancreas Treated With Radiotherapy. Int J Radiat Oncol Biol Phys (1998) 41:393–6. doi: 10.1016/S0360-3016(98)00058-3
27. Kinsella TJ, Seo Y, Willis J, Stellato TA, Siegel CT, Harpp D, et al. The Impact of Resection Margin Status and Postoperative ca19-9 Levels on Survival and Patterns of Recurrence After Postoperative High-Dose Radiotherapy With 5-Fu-Based Concurrent Chemotherapy for Resectable Pancreatic Cancer. Am J Clin Oncol (2008) 31:446–53. doi: 10.1097/COC.0b013e318168f6c4
28. Lundin J, Roberts PJ, Kuusela P, Haglund C. The Prognostic Value of Preoperative Serum Levels of Ca 19-9 and Cea in Patients With Pancreatic Cancer. Br J Cancer (1994) 69:515–9. doi: 10.1038/bjc.1994.93
29. Bergquist JR, Puig CA, Shubert CR, Groeschl RT, Habermann EB, Kendrick ML, et al. Carbohydrate Antigen 19-9 Elevation in Anatomically Resectable, Early Stage Pancreatic Cancer is Independently Associated With Decreased Overall Survival and an Indication for Neoadjuvant Therapy: A National Cancer Database Study. J Am Coll Surgeons (2016) 223:52–65. doi: 10.1016/j.jamcollsurg.2016.02.009
30. Nakai Y, Kawabe T, Isayama H, Sasaki T, Yagioka H, Yashima Y, et al. Ca 19-9 Response as an Early Indicator of the Effectiveness of Gemcitabine in Patients With Advanced Pancreatic Cancer. Oncology (2008) 75:120–6. doi: 10.1159/000155213
31. Silvestris N, Brunetti O, Vasile E, Cellini F, Cataldo I, Pusceddu V, et al. Multimodal Treatment of Resectable Pancreatic Ductal Adenocarcinoma. Crit Rev Oncol/Hematol (2017) 111:152–65. doi: 10.1016/j.critrevonc.2017.01.015
32. Silvestris N, Longo V, Cellini F, Reni M, Bittoni A, Cataldo I, et al. Neoadjuvant Multimodal Treatment of Pancreatic Ductal Adenocarcinoma. Crit Rev Oncol/Hematol (2016) 98:309–24. doi: 10.1016/j.critrevonc.2015.11.016
33. Piagnerelli R, Marrelli D, Roviello G, Ferrara F, Di Mare G, Voglino C, et al. Clinical Value and Impact on Prognosis of Peri-Operative Ca 19-9 Serum Levels in Stage I and Ii Adenocarcinoma of the Pancreas. Tumor Biol (2016) 37:1959–66. doi: 10.1007/s13277-015-3986-x
34. Regine W, Winter K, Chen I, Fugazzi J, Donnelly E, DiPetrillo T, et al. Ca19-9 and Surgical Margin Status (Sms) Associations With Local-Regional (Lrf) and Distant Failure (Df) in Patients (Pts) With Pancreatic Cancer: Rtog 9704 Secondary Analysis. J Clin Oncol (2015) 33(3_suppl):347–347. doi: 10.1200/jco.2015.33.3_suppl.347
35. Ballehaninna UK, Chamberlain RS. The Clinical Utility of Serum Ca 19-9 in the Diagnosis, Prognosis and Management of Pancreatic Adenocarcinoma: An Evidence Based Appraisal. J Gastrointest Oncol (2012) 3:105–19. doi: 10.3978/j.issn.2078-6891.2011.021
36. Barton JG, Bois JP, Sarr MG, Wood CM, Qin R, Thomsen KM, et al. Predictive and Prognostic Value of Ca 19-9 in Resected Pancreatic Adenocarcinoma. J Gastrointest Surg (2009) 13:2050–8. doi: 10.1007/s11605-009-0849-z
37. Boeck S, Stieber P, Holdenrieder S, Wilkowski R, Heinemann V. Prognostic and Therapeutic Significance of Carbohydrate Antigen 19-9 as Tumor Marker in Patients With Pancreatic Cancer. Oncology (2006) 70:255–64. doi: 10.1159/000094888
38. Montgomery RC, Hoffman JP, Riley LB, Rogatko A, Ridge JA, Eisenberg BL. Prediction of Recurrence and Survival by Post-Resection Ca 19-9 Values in Patients With Adenocarcinoma of the Pancreas. Ann Surg Oncol (1997) 4:551–6. doi: 10.1007/BF02305535
39. Sperti C, Pasquali C, Catalini S, Cappellazzo F, Bonadimani B, Behboo R, et al. Ca 19-9 as a Prognostic Index After Resection for Pancreatic Cancer. J Surg Oncol (1993) 52:137–41. doi: 10.1002/jso.2930520302
40. Yasue M, Sakamoto J, Teramukai S, Morimoto T, Yasui K, Kuno N, et al. Prognostic Values of Preoperative and Postoperative Cea and ca19.9 Levels in Pancreatic Cancer. Pancreas (1994) 9:735–40. doi: 10.1097/00006676-199411000-00011
41. Merrell KW, Haddock MG, Quevedo JF, Harmsen WS, Kendrick ML, Miller RC, et al. Predictors of Locoregional Failure and Impact on Overall Survival in Patients With Resected Exocrine Pancreatic Cancer. Int J Radiat Oncol Biol Phys (2016) 94:561–70. doi: 10.1016/j.ijrobp.2015.11.003
42. Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of Adjuvant Gemcitabine and Capecitabine With Gemcitabine Monotherapy in Patients With Resected Pancreatic Cancer (Espac-4): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet (2017) 389:1011–24. doi: 10.1016/S0140-6736(16)32409-6
43. Hartwig W, Strobel O, Hinz U, Fritz S, Hackert T, Roth C, et al. Ca19-9 in Potentially Resectable Pancreatic Cancer: Perspective to Adjust Surgical and Perioperative Therapy. Ann Surg Oncol (2013) 20:2188–96. doi: 10.1245/s10434-012-2809-1
44. Park JK, Paik WH, Ryu JK, Kim Y-T, Kim YJ, Kim J, et al. Clinical Significance and Revisiting the Meaning of Ca 19-9 Blood Level Before and After the Treatment of Pancreatic Ductal Adenocarcinoma: Analysis of 1,446 Patients From the Pancreatic Cancer Cohort in a Single Institution. PloS One (2013) 8:e78977. doi: 10.1371/journal.pone.0078977
45. Mattiucci GC, Morganti AG, Cellini F, Buwenge M, Casadei R, Farioli A, et al. Prognostic Impact of Presurgical ca19-9 Level in Pancreatic Adenocarcinoma: A Pooled Analysis. Trans Oncol (2019) 12:1–7. doi: 10.1016/j.tranon.2018.08.017
46. Okusaka T, Okada S, Sato T, Wakasugi H, Saisho H, Furuse J, et al. Tumor Markers in Evaluating the Response to Radiotherapy in Unresectable Pancreatic Cancer. Hepato-gastroenterology (1998) 45:867–72.
47. Willett CG, Daly WJ, Warshaw AL. Ca 19-9 is an Index of Response to Neoadjunctive Chemoradiation Therapy in Pancreatic Cancer. Am J Surg (1996) 172:350–2. doi: 10.1016/S0002-9610(97)89547-5
Keywords: pancreatic cancer, CA19-9, prognostic factors, neoadjuvant, resection
Citation: Kowalchuk RO, Lester SC, Graham RP, Harmsen WS, Zhang L, Halfdanarson TR, Smoot RL, Gits HC, Ma WW, Owen D, Mahipal A, Miller RC, Wittich MAN, Cleary SP, McWilliams RR, Haddock MG, Hallemeier CL, Truty MJ and Merrell KW (2021) Predicting Adverse Pathologic Features and Clinical Outcomes of Resectable Pancreas Cancer With Preoperative CA 19-9. Front. Oncol. 11:651119. doi: 10.3389/fonc.2021.651119
Received: 08 January 2021; Accepted: 20 April 2021;
Published: 11 May 2021.
Edited by:
Oronzo Brunetti, Istituto Nazionale dei Tumori (IRCCS), ItalyReviewed by:
Ming Cui, Peking Union Medical College Hospital (CAMS), ChinaNicola Silvestris, University of Bari Aldo Moro, Italy
Copyright © 2021 Kowalchuk, Lester, Graham, Harmsen, Zhang, Halfdanarson, Smoot, Gits, Ma, Owen, Mahipal, Miller, Wittich, Cleary, McWilliams, Haddock, Hallemeier, Truty and Merrell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kenneth W. Merrell, bWVycmVsbC5rZW5uZXRoQG1heW8uZWR1
 Roman O. Kowalchuk
Roman O. Kowalchuk Scott C. Lester1
Scott C. Lester1 Rondell P. Graham
Rondell P. Graham Lizhi Zhang
Lizhi Zhang Thorvardur R. Halfdanarson
Thorvardur R. Halfdanarson Sean P. Cleary
Sean P. Cleary Christopher L. Hallemeier
Christopher L. Hallemeier Mark J. Truty
Mark J. Truty Kenneth W. Merrell
Kenneth W. Merrell