
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 10 March 2021
Sec. Gastrointestinal Cancers
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.650937
This article is part of the Research TopicAdvanced Molecular Targets in the Diagnosis and Treatment of Gastrointestinal CancersView all 55 articles
 Meijuan Wu1†
Meijuan Wu1† Mengxi Huang1†
Mengxi Huang1† Chenglong He2†
Chenglong He2† Cheng Chen1,2
Cheng Chen1,2 Huiyu Li1
Huiyu Li1 Jing Wang1
Jing Wang1 Mengyan Liu1
Mengyan Liu1 Gongbo Fu1,2*
Gongbo Fu1,2* Zengjie Lei1,2*
Zengjie Lei1,2* Xiaoyuan Chu1,2*
Xiaoyuan Chu1,2*Background: Previous studies have revealed an increased risk of second primary malignancies (SPMs) after colorectal cancer (CRC); however, no previous investigation has quantified differences in the risk of SPMs based on the histological subtypes of first primary CRC.
Methods: Patients diagnosed with first primary CRC between 2000 and 2011 were identified from the Surveillance, Epidemiology, and End Results cancer registries. The patients were divided into three cohorts: classical adenocarcinoma (CA), mucinous adenocarcinoma (MA), and signet-ring cell carcinoma (SRCC). Standardized incidence ratios were calculated to assess the risk of SPMs among the patients.
Results: Overall risk of SPMs was significantly higher among patients with three histological subtypes of CRC than in the general population. The risk of esophagus cancer was significantly increased in SRCC. The risk of small intestine, colon and rectum, and corpus uteri cancers was high in three histological subtypes, with the highest risk observed in SRCC, followed by MA. Increased risks of second stomach, uterus, urinary bladder, kidney, and thyroid cancers were only observed in CA patients, while increased risk of second renal pelvis cancer was limited to MA patients. Furthermore, the high overall risk of SPMs in CA patients persisted regardless of clinicopathological factors. After surgery combined with chemotherapy treatment, CA patients were more prone to developing second small intestine, colon and rectum cancers than those treated with surgery only. A lower second prostate cancer risk was observed in rectal CA patients treated with surgery combined with radiotherapy than in patients treated with surgery only.
Conclusion: The present study revealed that the risk of developing SPMs after CRC varied based on the histological subtypes of the first primary CRC. Although the mechanisms underlying the observed patterns of SPM risk remain unknown, the study provided insights into future cancer surveillance based on the histological subtypes of CRC.
Colorectal cancer (CRC) is the third most widespread cause of cancer-related deaths in both men and women in the United States, and ranks second when men and women are combined (1). According to Survivorship Statistics released by the American Cancer Society, it was estimated that more than 1.5 million survivors in the United States were living with a previous CRC diagnosis in 2019 (2). Advances in early detection and treatment of CRC have contributed to enhancements in CRC prognosis, which could be the reason for the increase in the population of CRC survivors (2). Therefore, a long life exposes these survivors to long-term health concerns, including the development of second primary malignancies (SPMs). Several population-based studies have demonstrated an increased risk of developing SPMs after a previous diagnosis of CRC compared with the general population (3–7). Although the underlying mechanisms remain unknown, the increased risk could be associated with shared genetic or environmental risk factors for different malignancies or a side effect of previous treatment for CRC.
Most CRCs are adenocarcinomas, including three key comprehensively studied subtypes: classical adenocarcinoma (CA), mucinous adenocarcinoma (MA), and signet-ring cell carcinoma (SRCC) (8). MA and SRCC are rare subtypes of CRC and exist distinct characteristics from CA, including a younger age of onset, more advanced stage, and increased possibility of lymphatic invasion and perineural infiltration (8–12). Therefore, it is plausible that the risk and distribution of SPMs in different histological subtypes of CRC could differ.
In addition, the treatment of CRC varies with the stage at diagnosis; however, the most widespread treatment administered is surgery, with additional therapy including systemic chemotherapy and radiation therapy (radiation therapy is used more often in rectal cancer than in colon cancer cases) administered either in the neoadjuvant or adjuvant setting (13). The treatment patterns could influence the risk of SPMs as chemotherapy and radiotherapy are carcinogenic and have been demonstrated to increase the risk of SPMs at various sites, including lung, stomach, colorectal sites and the bladder (14–16).
To the best of our knowledge, this is the first systematic retrospective study focusing on risk and distribution of SPMs after CRC based on the histological subtypes, with a large sample of CRC patients obtained from the Surveillance, Epidemiology, and End Results (SEER) database. Understanding the patterns could provide further insights into the epidemiology of CRC and guide clinical decisions regarding surveillance and adjuvant treatment after CRC.
Data used in the present study were extracted from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program database, which contained information from population-based cancer registries on patient demographics, cancer incidence, treatment, and outcomes (https://seer.cancer.gov). The database we selected was SEER 18 regs, excluding AK Custom Data (with additional treatment fields), which was submitted in November 2018 (2000–2016). To distinguish second primary malignancies from recurrences and metastases, SEER registrars follow a series of coding rules considering site, histology, timing and whether anastomotic lesions have mucosal involvement.
Patients aged 20–79 years, who had been initially diagnosed with CRC presenting malignant behavior between January 2000 and December 2011 were included in the study. Patients were followed-up for at least 5 years to determine the risk of developing SPMs. Patients with reports presented in the form of death certificates or autopsy only were not enrolled, as were those without pathologically confirmed diagnoses. We further selected patients diagnosed with three histological subtypes of CRC: CA (Codes: 8140–8147, 8210–8213, 8220–8221, 8255, 8260–8263, 8310-8323), MA (Codes: 8480–8481), and SRCC (Codes: 8490) based on ICD-O-3 codes. Patients diagnosed as other histological subtypes were excluded. Detailed corresponding descriptions of the three histological subtypes of CRC are presented in Supplementary Table 1. To enhance the validity and authenticity of the present study, patients with missing values on crucial covariates such as race, grade and SEER summary stage were excluded. Additional exclusion criteria included patients with performance of surgery noted death certificate/autopsy or unknown operation. To identify the location of tumors, tumor sites such as appendix, overlapping lesions of colon or colon not otherwise specified (NOS) were excluded. Finally, patients who were followed <6 months or were diagnosed with SPMs within the first 6 months after initial CRC were not enrolled to exclude synchronous primary malignancies. Overall, the enrolled CRC patients were divided into three cohorts: classical adenocarcinoma (CA), mucinous adenocarcinoma (MA), and signet-ring cell carcinoma (SRCC) cohorts. A flowchart of the selection criteria of patients is presented in Supplementary Figure 1.
Standardized incidence ratios (SIRs) were calculated using multiple primary-standardized incidence ratio (MP-SIR) sessions of SEER*Stat version 8.3.8 (SEER Program, National Cancer Institute). The SIR, also known as relative risk, represents the ratio of the observed number of second cancers to the expected number of cancers based on the US general population, with adjustment for race, sex, age and calendar year by the specific stratified person-time variable accrued from the CRC cohort. Data on cancer incidence among the general population were retrieved from the SEER 18 regs, excluding AK Custom Data (with additional treatment fields), which was submitted in November 2018. More detailed information regarding the SEER*Stat software and the methods to calculate the SIRs is available on the SEER-registry website (https://seer.cancer.gov/resources/). We compared SIRs between CA, MA, and SRCC cases for each multiple primary cancer site using poisson regression. SIRs for subgroup analyses were further stratified by sex, race, age at diagnosis, year at diagnosis, grade, tumor site, and SEER summary stage of the first primary CRC. We compared SIRs between different treatments received by patients (surgery only vs. surgery combined with chemotherapy, and surgery only vs. surgery combined with radiotherapy) in CA, MA, and SRCC cases using poisson regression. Demographic and clinical features were analyzed using a Chi-square test. R statistical software version 3.5.0 (Bell Laboratories, Murray Hill, New Jersey, United States) was used to perform Chi-square test and poisson regression. Evaluation of the confidence intervals (CIs) of SIRs was used to determine any overlap. A two-sided p < 0.05 was considered statistically significant for all analyses.
Patient demographics grouped based on the histological subtypes of CRC are summarized in Table 1. White patients (79.56%) and colon cancers (69.56%) accounted for most of the cases in the present study. Patients with SRCC were slightly younger and more likely to be diagnosed with poorly differentiated or undifferentiated cancers, and a distant stage than the other subtypes. Most patients underwent surgical treatment for CRC: 95.51, 98.32, and 93.33% for patients with CA, MA, and SRCC, respectively. Patients with SRCC presented a higher ratio of receiving radiotherapy (16.41 vs. 14.87 vs. 21.39%, CA vs. MA vs. SRCC, respectively) and chemotherapy (44.74 vs. 51.07 vs. 67.81%, CA vs. MA vs. SRCC, respectively) than the other subtypes.
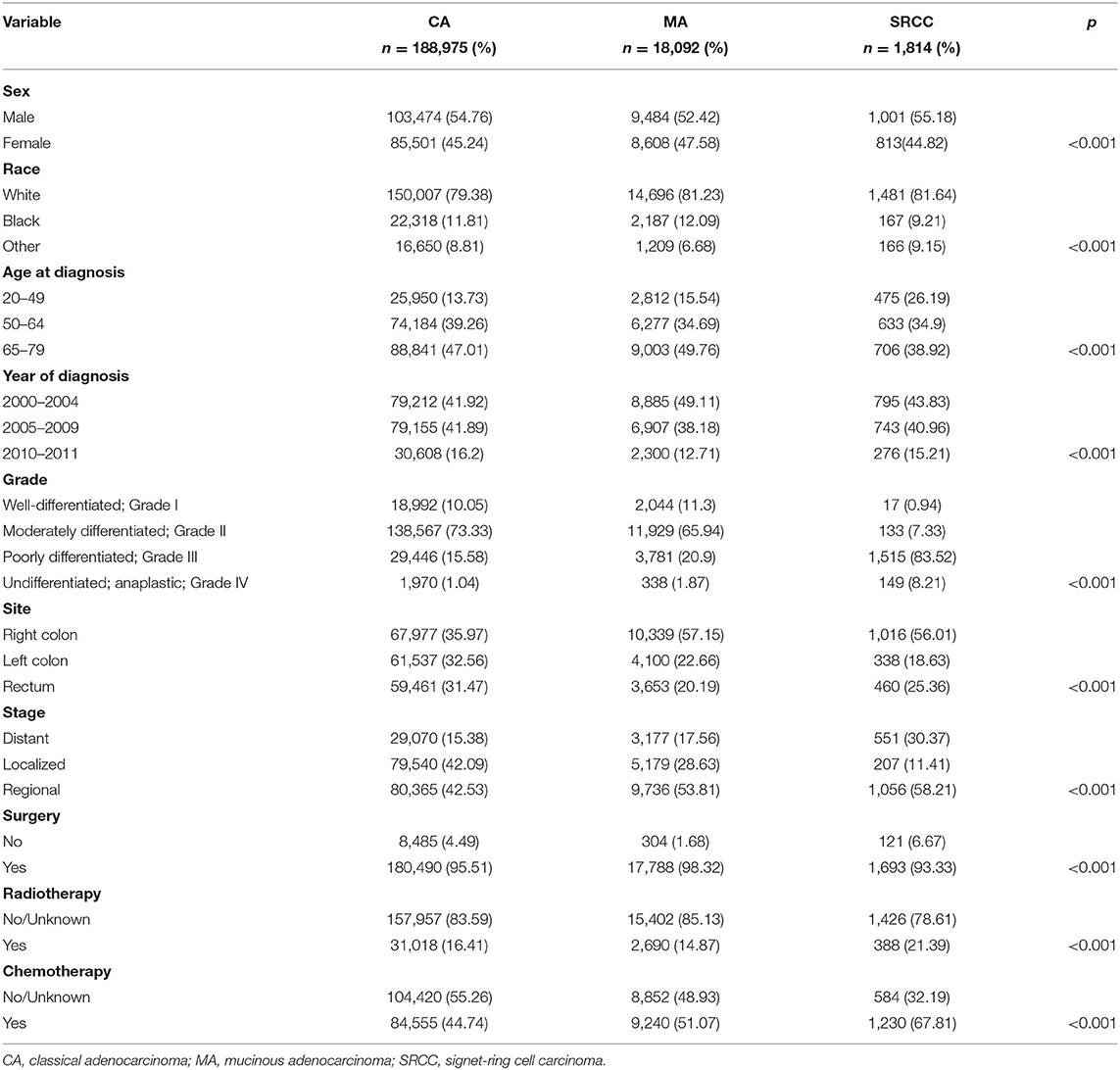
Table 1. Clinicopathological characteristics of patients with colorectal cancer stratified by histological subtypes.
The overall risk of SPMs was higher among CRC patients than in the general population for the three CRC subtypes, with higher risks observed in MA and SRCC (CA: Obs = 24276, SIR = 1.14, 95% CI, 1.12-1.15; MA: Obs = 2461, SIR = 1.25, 95% CI, 1.2-1.3; SRCC: Obs = 161, SIR = 1.48, 95% CI, 1.26–1.73; p < 0.001; Figure 1). Notably, the risk patterns differed substantially among the three CRC subtypes. The risk of esophagus cancer was significantly increased in SRCC (SIR: CA = 1.16, MA = 1.17, SRCC = 4.12; p = 0.004), while the risk of lung and bronchus cancers was significantly increased in CA and MA, but not in SRCC. Risks of small intestine, colon and rectum, and corpus uteri cancers were increased in the three CRC subtypes, with the highest risk observed in SRCC, followed by MA (SIR for small intestine cancer: CA = 3.50, MA = 4.40, SRCC = 11.74; SIR for colon and rectum cancer: CA = 2.15, MA = 2.46, SRCC = 4.01; SIR for corpus uteri cancer: CA = 1.47, MA = 1.70, SRCC = 3.28; p < 0.001). Increased risk of renal pelvis cancer (SIR = 2.94) and reduced risk of liver cancer (SIR = 0.59) were limited to MA patients. In addition to the previously-mentioned cancers, the observed numbers of other second primary malignancies did not differ from expectation after MA and SRCC. However, CA patients were more likely to develop stomach (SIR = 1.26), uterus (SIR = 1.64), urinary bladder (SIR = 1.09), kidney (SIR = 1.09), and thyroid (SIR = 1.67) cancers, whereas less likely to develop melanoma of the skin (SIR = 0.88), breast (SIR = 0.95), ovary (SIR = 0.70), prostate (SIR = 0.86), eye and obit (SIR = 0.49), lymphoma (SIR = 0.87), and myeloma (SIR = 0.88) cancers.
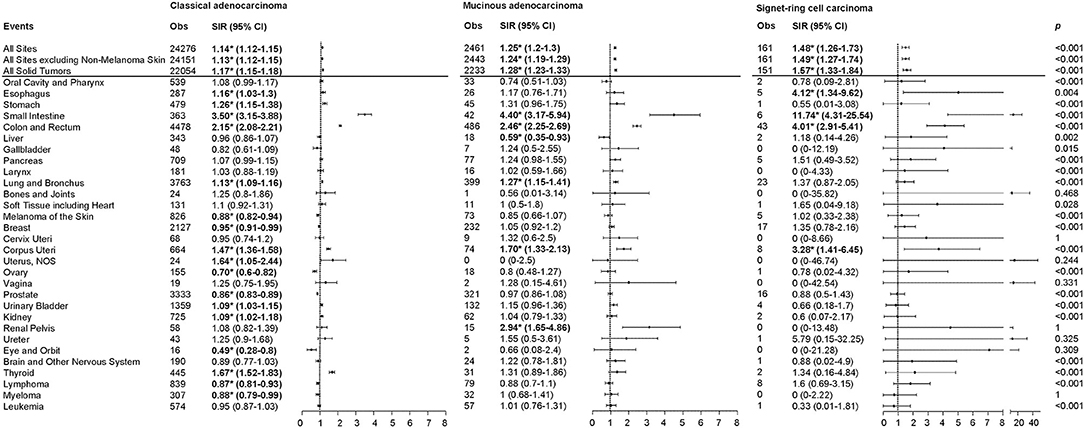
Figure 1. SIRs for SPMs at various anatomical sites based on the histological subtypes of the first primary CRC. Obs, observed events; SIR, standard incidence ratio; CI, confidence interval. *P < 0.05 (compared with general population). P-values comparing SIR differences among colorectal cancer subtypes were calculated using Poisson regression.
Furthermore, we conducted subgroup analyses of the overall risk of SPMs based on clinicopathological characteristics. The overall SIRs of CA patients remained high when stratified by different variables (Figure 2, Table 2). The overall SIRs of MA patients remained high in all subgroups other than that of rectum cancer (Table 2). Moreover, the overall SIRs of SRCC patients remained high when stratified by sex and race, but did not alter in several other subgroups (Table 2). Notably, among the three CRC subtypes, patients aged 20–49 years were more likely to develop SPMs than the older patients (Figure 2, Table 2).
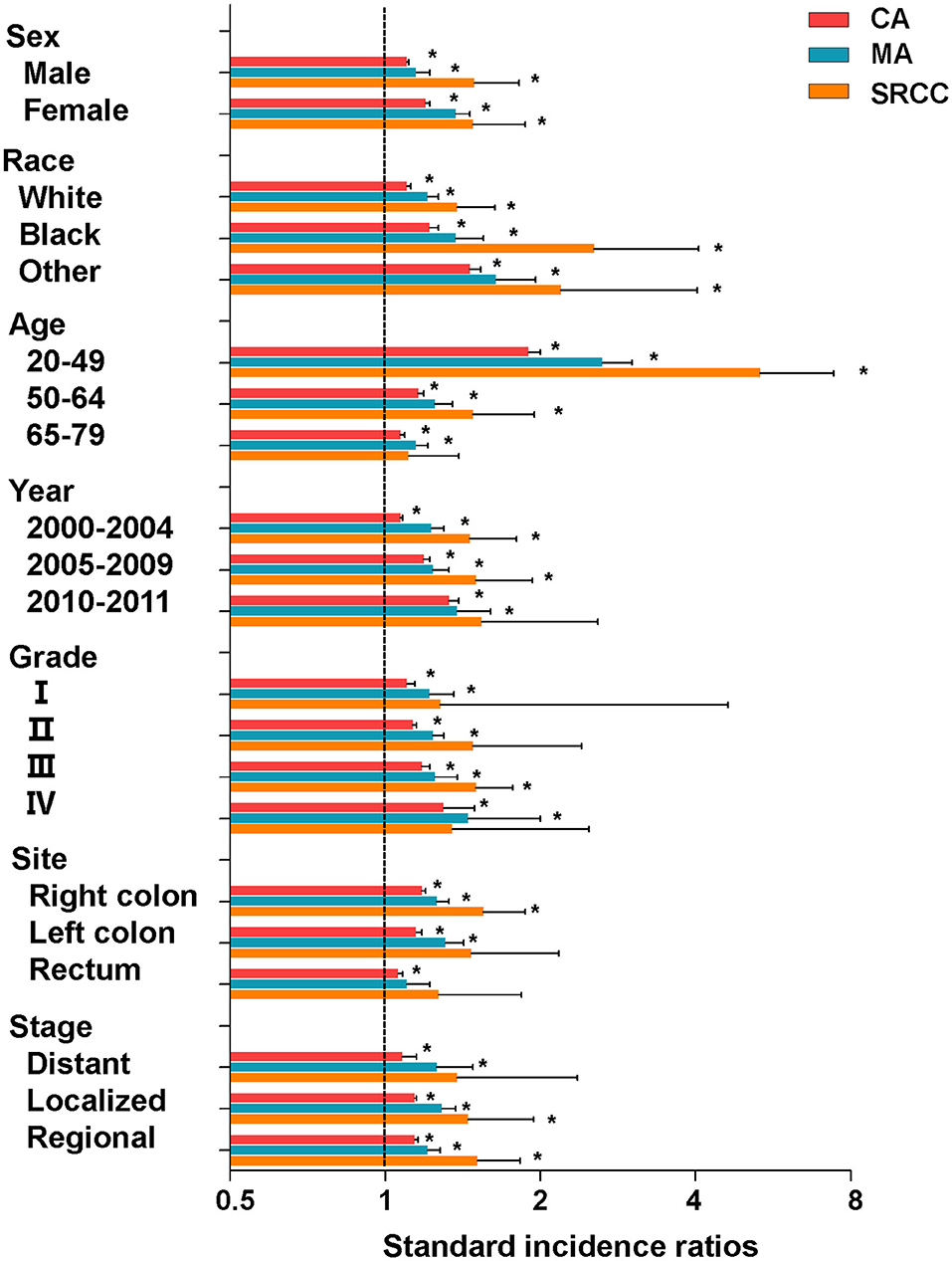
Figure 2. SIRs for SPMs at all anatomical sites combined among CRC survivors stratified by sex, race, age, year, grade, site, stage, and histological subtype. CA, classical adenocarcinoma; MA, mucinous adenocarcinoma; SRCC, signet-ring cell carcinoma. *P < 0.05 (compared with general population).
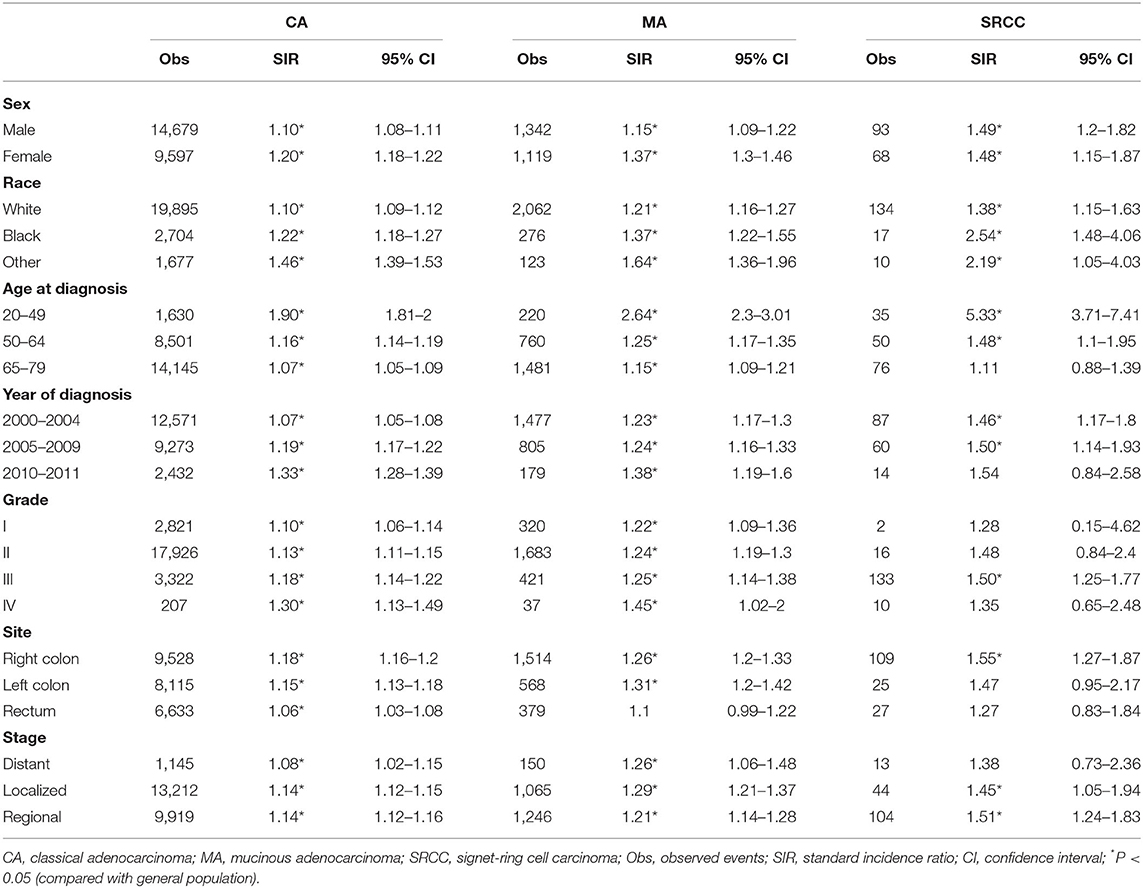
Table 2. SIRs for SPMs at all anatomical sites combined among CRC survivors stratified by sex, race, age, year, grade, site, stage, and histological subtype.
We further analyzed the risks of SPMs among the three histological subtypes of CRC after administration of different treatments. For CRC patients, we compared SIRs between surgery only and surgery combined with chemotherapy, since most patients received the two treatments. No statistically significant difference was observed in the overall SIRs of SPMs between the two treatment groups for the three CRC subtypes (CA, p = 0.445; MA, p = 0.421; SRCC, p = 0.209; Table 3, Supplementary Tables 2, 3). However, surgery combined with chemotherapy appeared to increase the risk of small intestine (surgery only group: SIR = 2.91; surgery combined with chemotherapy group: SIR = 5.62; p = 0.011), colon and rectum cancers (surgery only group: SIR = 2.07; surgery combined with chemotherapy group: SIR = 2.45; p = 0.009) in CA patients (Table 3).
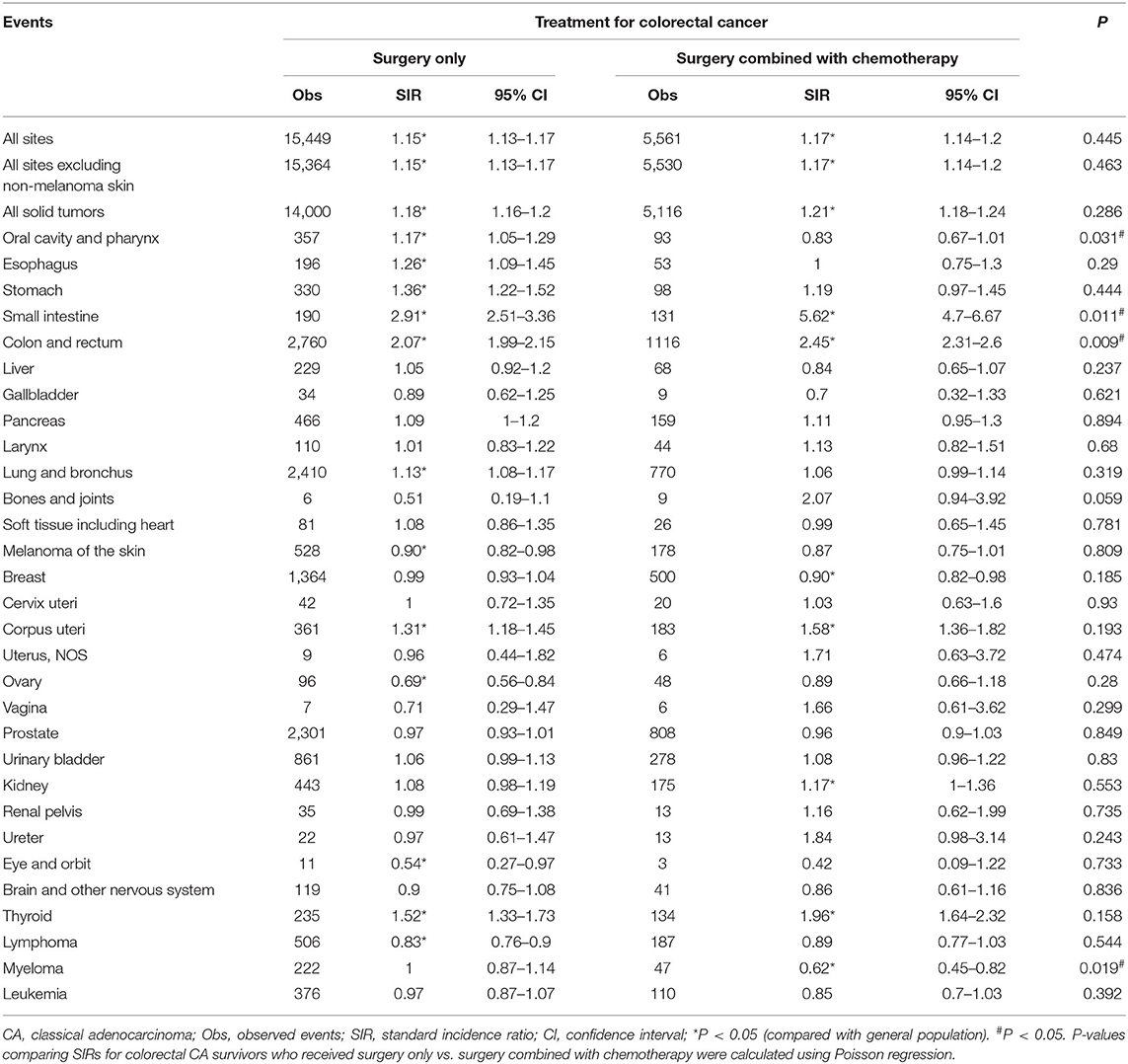
Table 3. SPM risks at various anatomical sites based on previous surgery and chemotherapy treatments in colorectal CA patients.
In addition, we compared SIRs between surgery only and surgery combined with radiotherapy treatments in rectal cancer patients. Rectal SRCC patients were not included in this analysis due to the limited number of observed events of SPMs. No significant differences in the overall risk of SPMs in rectal CA and MA patients were observed between the two treatments (CA, p = 0.782; MA, p = 0.099; Table 4, Supplementary Table 4). However, a lower risk of second prostate cancer was observed in rectal CA patients of the surgery combined with radiotherapy group (surgery only group, SIR = 0.86, surgery combined with radiotherapy group, SIR = 0.26, p < 0.001; Table 4).
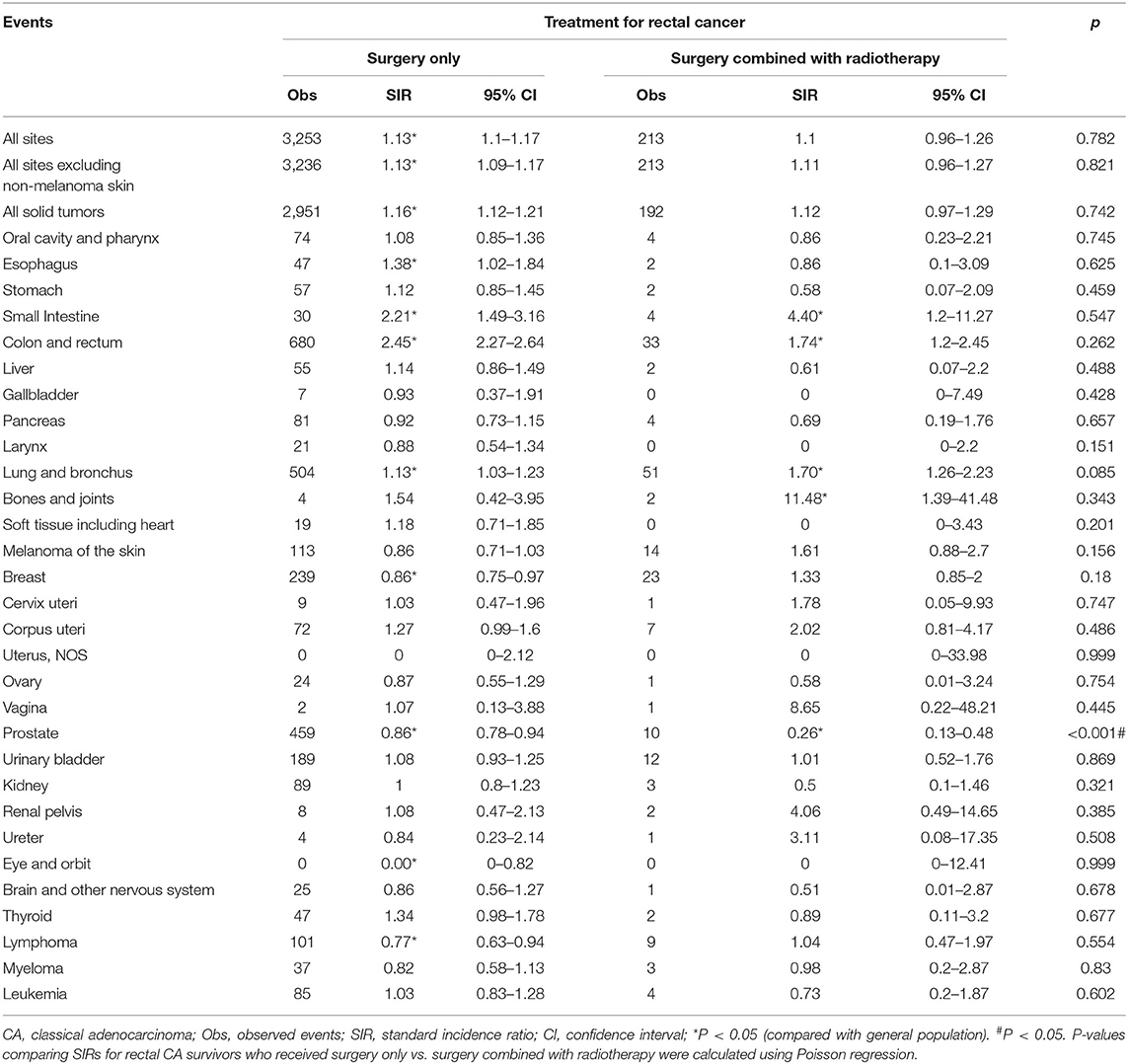
Table 4. SPM risks at various anatomical sites based on previous surgery and radiotherapy treatments in rectal CA patients.
In the present large population-based study, we demonstrated for the first time that the risk of developing SPMs among CRC patients varied with the histological subtypes of CRC. Compared with the general population, the overall risk of SPMs was higher among CRC patients, which is consistent with previous study (3–7). However, when stratified by histological subtypes, we established that the overall risk of SPMs was slightly higher in MA and SRCC patients than in CA patients, while increased risk of SPMs in specific anatomical sites was observed more in CA patients. In addition, a significant increase in SIRs was only persistent in CA patients when stratified by different variables. The mechanism underlying the pattern is unclear, but it could be associated with the biological variations between the different subtypes of CRC. Indeed, CRC exhibits notable differences in incidence, location of tumor, pathogenesis, molecular pathways, and outcome based on the histological subtypes (17–19). Critical confounders such as lifestyle factors, genetic susceptibility, and detailed treatment data could also considerably influence the development of SPMs after CRC.
Patterns of the risk of SPMs after CRC indicate the existence of several overlapping mechanisms, including shared aetiological factors with the primary cancer, genetic predisposition and late side effects of cancer treatment (14, 15). Analysis of specific SPMs based on histological subtypes allows us to further explore the potential mechanisms facilitating the development of SPMs after CRC. For example, the present study revealed that the risk of second esophagus cancer was increased in CA and SRCC, and the risk of second lung and bronchus cancer was increased in CA and MA. This is likely associated with the well-established link between tobacco smoking and increased risk of CRC (20, 21). Similarly, increased risk of second kidney cancer in CA and second corpus uteri cancer in the three subtypes of CRC could partially be associated with obesity (22, 23).
Lynch syndrome is caused by germline mutations and is associated with an increased risk of colorectum, stomach, small intestine, and pancreatic cancers, as well as other genitourinary cancers (24–27). Similarly, hereditary non-polyposis CRC, which is attributed to mutations in the DNA mismatch repair genes, increases the risk of developing multiple primary CRCs and tumors at extracolonic sites, including the endometrium, ovary, small intestine, biliary tract, urinary tract, stomach, kidney, thyroid, and nervous system (16, 28, 29). In the present study, we established that specific risks of SPMs varied with the histological subtypes of CRC. For example, the risk of second stomach, uterus, urinary bladder, kidney, and thyroid cancers was only high in CA, while the risk of second renal pelvis cancer was specifically high in MA. However, the risk of second small intestine, colon and rectum, and corpus uteri cancers was high in the three subtypes of CRC. The increased risk of SPMs of specific anatomical sites in different histological subtypes of CRC necessitates the evaluation of family history and clinical screening for hereditary CRC.
Previous studies have revealed that young patients were more likely to develop SPMs (4, 30). Liang et al. demonstrated that young patients in Taiwan (aged <50 years) had a higher risk of developing SPMs than the general population (30). Furthermore, He et al. established that young CRC survivors exhibited a considerably high risk of developing SPMs in relation to the general population (4). In the present study, the significantly high risk of SPMs was observed in young patients aged 20–49 years in the three subtypes of CRC, which is consistent with the previous studies (4, 30). Although the mechanisms responsible for increased risk of SPMs among the younger population are poorly understood, a comprehensive understanding of the risk faced by young CRC patients and regular surveillance could help to determine appropriate prevention strategies.
Surgery is a standard therapy for curable CRC, and radiotherapy and chemotherapy are used as auxiliary therapies to a variable degree. MA patients appeared to be less responsive to chemotherapy, which could be partially explained by genetic differences such as high rates of microsatellite instability (31) and distinct patterns of tumor spread including peritoneal dissemination (32). Other studies have also revealed that MA patients exhibited a worse prognosis than non-MA patients despite of the different chemotherapy regimens being used (33–36). Conversely, Hugen et al. demonstrated that there was no significant interaction between SRCC and adjuvant chemotherapy when compared with CA, suggesting a comparable benefit from adjuvant chemotherapy in CA and SRCC (32). However, SRCC remained a poor prognostic factor when compared with CA, which could be due to its higher invasive potential (8, 10, 11, 32). Moreover, radiation and chemotherapy agents could contribute to the observed patterns of SPM risk, as a result of carcinogenic and immunosuppressive effects (14–16). Several studies have revealed an increased risk of CRC with chemotherapy, although the underlying mechanisms remain unknown (16, 37). In the present study, no significant difference in the overall risk of SPMs was observed between patients received surgery only and those received surgery combined with chemotherapy for the three subtypes of CRC. However, for CA patients, a higher risk of second small intestine, colon and rectum cancers was observed in the surgery combined with chemotherapy group than in the surgery only group. As mentioned above, a better overall survival in CA patients, which possessed them adequate time to develop SPMs, as well as the carcinogenic effects of chemotherapy, could play a role in the development of SPMs. Nevertheless, further investigation is required to understand the role of SPM risk caused by chemotherapy.
There is conflicting data on whether rectal cancer survivors are at high risk of developing SPMs due to radiotherapy. A few studies have reported an increased risk of SPMs after radiotherapy while others reported no increase or low risk (38–41). In the present study, no alteration of the overall risk of SPMs was observed after radiotherapy, but a reduced risk of second prostate cancer was observed in irradiated CA patients. The role of radiotherapy in CA patients in the present study is consistent with the findings of Martling et al., who reported that no increased risk of SPMs was observed in irradiated vs. non-irradiated patients; however, a reduced risk of prostate cancer was observed in irradiated patients (40). Moreover, a recent analysis of the Netherlands population-based cancer registry revealed that radiotherapy seemed to exhibit a protective effect against the development of other second pelvic tumors, predominantly prostate cancer, which is consistent with the results of the present study (41). By contrast, the Uppsala and Swedish Rectal Cancer Trials suggested an increased risk of SPMs in rectal cancer patients treated with radiotherapy in combination with surgery, which is contrary to the results of the present study (38). The contrasting observations could be explained by a few probable reasons. For example, most studies including the present study did not consider the irradiated volume and radiation dose received by patients, as well as the impact of preoperative or postoperative adjuvant radiotherapy. Furthermore, the studies did not investigate the impact of radiation based on the different histological subtypes and the sample sizes of most studies were small. In addition, the lower risk of prostate cancer after radiotherapy in CA patients observed in the present study could be partially attributed to “incidental” radiation of the prostate when treating the rectum. Hormonal changes caused by scattered radiation to the testicles during radiotherapy of the rectum could also impede the development of prostate cancer (42). Finally, the number of rectal cancer patients who developed SPMs after radiotherapy was small, which suggested that some observed associations could have occurred by chance. Therefore, we strongly recommend the use of a larger patient population with a considerable follow-up to draw firm conclusions with regard to the impact of radiation on rectal cancer patients based on the histological subtypes.
The key strength of the present study is an evaluation of SPM risks based on the histological subtypes of CRC. In addition, the use of large-scale population-based registry data enabled us to investigate the risk of developing SPMs among survivors of three specific histological subtypes of CRC. However, the present study had a few limitations. First, detailed information on lifestyle characteristics, family history, genetic factors, as well as specific treatment regiments could not be obtained from the database. Second, a diagnostic bias in CRC survivors could have existed, because the patients likely underwent more examinations and surveillance than the general population. Finally, despite of the large number of CRC survivors in the SEER database, cases used for SIR calculations were limited to less common SPM sites, particularly when stratified by histological subtypes of CRC.
The present study revealed substantial differences in the risk of developing SPMs among specific CRC subtypes. Further studies with detailed patient medical history, treatment regiments, and laboratory data should be conducted to validate the results of the present study. Overall, the findings suggest that strategies for cancer surveillance after previous CRC could be personalized based on the histological subtype of previous CRC.
Publicly available datasets were analyzed in this study. This data can be found here: Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/).
XC, ZL, and GF: study concept and design. MW: acquisition of data. MH and CH: analysis and interpretation of data. CC, HL, JW, and ML: critical revision of manuscript. All the authors were involved in approval of the final version.
This work was supported by the National Natural Science Foundation of China [82072725 and 81872042 to XC, 81702442 to ZL, 81972332 to Yitian Chen, 82002583 to MH, 82002591 to GF], the Natural Science Foundation of Jiangsu province [BK20170623 to ZL, BK20200273 to GF], the China Postdoctoral Science Foundation [2020M670090ZX to ZL], and the Postdoctoral Science Found of Jiangsu province [2018K090B to ZL].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank the SEER program for providing open access to the database.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.650937/full#supplementary-material
Supplementary Figure 1. A flow chart of the selection criteria of patients enrolled in the Surveillance, Epidemiology, and End Results (SEER) research database.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
2. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. (2019) 69:363–85. doi: 10.3322/caac.21565
3. Phipps AI, Chan AT, Ogino S. Anatomic subsite of primary colorectal cancer and subsequent risk and distribution of second cancers. Cancer. (2013) 119:3140–7. doi: 10.1002/cncr.28076
4. He X, Wu W, Ding Y, Li Y, Si J, Sun L. Excessive risk of second primary cancers in young-onset colorectal cancer survivors. Cancer Med. (2018) 7:1201–10. doi: 10.1002/cam4.1437
5. Jia H, Li Q, Yuan J, Sun X, Wu Z. Second primary malignancies in patients with colorectal cancer: a population-based analysis. Oncologist. (2020) 25: e644–50. doi: 10.1634/theoncologist.2019-0266
6. Ahmed F, Goodman MT, Kosary C, Ruiz B, Wu XC, Chen VW, et al. Excess risk of subsequent primary cancers among colorectal carcinoma survivors, 1975–2001. Cancer. (2006) 107:1162–71. doi: 10.1002/cncr.22013
7. Yang J, Li S, Lv M, Wu Y, Chen Z, Shen Y, et al. Risk of subsequent primary malignancies among patients with prior colorectal cancer: a population-based cohort study. Onco Targets Ther. (2017) 10:1535–48. doi: 10.2147/OTT.S129220
8. Nitsche U, Zimmermann A, Spath C, Muller T, Maak M, Schuster T, et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg. (2013) 258:775–82. doi: 10.1097/SLA.0b013e3182a69f7e
9. Liang Z, Yan D, Li G, Cheng H. Clinical analysis of primary colorectal signet-ring cell carcinoma. Clin Colorectal Cancer. (2018) 17: e39–44. doi: 10.1016/j.clcc.2017.06.010
10. Hugen N, van de Velde CJH, de Wilt JHW, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. (2014) 25:651–7. doi: 10.1093/annonc/mdt591
11. Hyngstrom JR, Hu CY, Xing Y, You YN, Feig BW, Skibber JM, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol. (2012) 19:2814–21. doi: 10.1245/s10434-012-2321-7
12. Wu X, Lin H, Li S. Prognoses of different pathological subtypes of colorectal cancer at different stages: a population-based retrospective cohort study. BMC Gastroenterol. (2019) 19:164. doi: 10.1186/s12876-019-1083-0
13. El-Shami K, Oeffinger KC, Erb NL, Willis A, Bretsch JK, Pratt-Chapman ML, et al. American cancer society colorectal cancer survivorship care guidelines. CA Cancer J Clin. (2015) 65:428–55. doi: 10.3322/caac.21286
14. Travis LB, Demark Wahnefried W, Allan JM, Wood ME, Ng AK. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol. (2013) 10:289–301. doi: 10.1038/nrclinonc.2013.41
15. Allan JM, Travis LB. Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer. (2005) 5:943–55. doi: 10.1038/nrc1749
16. Hemminki K, Li X, Dong C. Second primary cancers after sporadic and familial colorectal cancer. Cancer Epidemiol Biomarkers Prev. (2001) 10:793–8.
17. Remo A, Pancione M, Zanella C, Vendraminelli R. Molecular pathology of colorectal carcinoma. A systematic review centred on the new role of the pathologist. Pathologica. (2012) 104:432–41.
18. Pancione M, Giordano G, Remo A, Febbraro A, Sabatino L, Manfrin E, et al. Immune escape mechanisms in colorectal cancer pathogenesis and liver metastasis. J Immunol Res. (2014) 2014:686879. doi: 10.1155/2014/686879
19. Remo A, Fassan M, Vanoli A, Bonetti LR, Barresi V, Tatangelo F, et al. Morphology and molecular features of rare colorectal carcinoma histotypes. Cancers. (2019) 11:71036. doi: 10.3390/cancers11071036
20. Hamada T, Nowak JA, Masugi Y, Drew DA, Song M, Cao Y, et al. Smoking and risk of colorectal cancer sub-classified by tumor-infiltrating T cells. J Natl Cancer Inst. (2019) 111:42–51. doi: 10.1093/jnci/djy137
21. Gram IT, Park SY, Wilkens LR, Haiman CA, Le Marchand L. Smoking-related risks of colorectal cancer by anatomical subsite and sex. Am J Epidemiol. (2020) 189:543–53. doi: 10.1093/aje/kwaa005
22. Gibson TM, Park Y, Robien K, Shiels MS, Black A, Sampson JN, et al. Body mass index and risk of second obesity-associated cancers after colorectal cancer: a pooled analysis of prospective cohort studies. J Clin Oncol. (2014) 32:4004–11. doi: 10.1200/JCO.2014.56.8444
23. Dignam JJ, Polite BN, Yothers G, Raich P, Colangelo L, O'Connell MJ, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. (2006) 98:1647–54. doi: 10.1093/jnci/djj442
24. Stoffel E, Mukherjee B, Raymond VM, Tayob N, Kastrinos F, Sparr J, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. (2009) 137:1621–7. doi: 10.1053/j.gastro.2009.07.039
25. Bonadona V, Bonaiti B, Olschwang S, Grandjouan S, Huiart L, Longy M, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. (2011) 305:2304–10. doi: 10.1001/jama.2011.743
26. Win AK, Lindor NM, Young JP, Macrae FA, Young GP, Williamson E, et al. Risks of primary extracolonic cancers following colorectal cancer in lynch syndrome. J Natl Cancer Inst. (2012) 104:1363–72.
27. Moller P, Seppala T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut. (2017) 66:464–72. doi: 10.1136/gutjnl-2015-309675
28. Stoffel EM, Mangu PB, Gruber SB, Hamilton SR, Kalady MF, Lau MW, et al. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J Clin Oncol. (2015) 33:209–17. doi: 10.1200/JCO.2014.58.1322
29. Casellas-Cabrera N, Diaz-Algorri Y, Carlo-Chevere VJ, Gonzalez-Pons M, Rodriguez-Manon N, Perez-Mayoral J, et al. Risk of thyroid cancer among Caribbean Hispanic patients with familial adenomatous polyposis. Fam Cancer. (2016) 15:267–74. doi: 10.1007/s10689-015-9862-4
30. Liang YH, Shao YY, Chen HM, Lai CL, Lin ZZ, Kuo RN, et al. Young patients with colorectal cancer have increased risk of second primary cancers. Jpn J Clin Oncol. (2015) 45:1029–35. doi: 10.1093/jjco/hyv137
31. Benedix F, Kuester D, Meyer F, Lippert H. Influence of mucinous and signet-ring cell differentiation on epidemiological, histological, molecular biological features, and outcome in patients with colorectal carcinoma. Zentralbl Chir. (2013) 138:427–33. doi: 10.1055/s-0031-1283870
32. Hugen N, Verhoeven RH, Lemmens VE, van Aart CJ, Elferink MA, Radema SA, et al. Colorectal signet-ring cell carcinoma: benefit from adjuvant chemotherapy but a poor prognostic factor. Int J Cancer. (2015) 136:333–9. doi: 10.1002/ijc.28981
33. Catalano V, Loupakis F, Graziano F, Torresi U, Bisonni R, Mari D, et al. Mucinous histology predicts for poor response rate and overall survival of patients with colorectal cancer and treated with first-line oxaliplatin- and/or irinotecan-based chemotherapy. Br J Cancer. (2009) 100:881–7. doi: 10.1038/sj.bjc.6604955
34. Negri FV, Wotherspoon A, Cunningham D, Norman AR, Chong G, Ross PJ. Mucinous histology predicts for reduced fluorouracil responsiveness and survival in advanced colorectal cancer. Ann Oncol. (2005) 16:1305–10. doi: 10.1093/annonc/mdi244
35. Mekenkamp LJ, Heesterbeek KJ, Koopman M, Tol J, Teerenstra S, Venderbosch S, et al. Mucinous adenocarcinomas: poor prognosis in metastatic colorectal cancer. Eur J Cancer. (2012) 48:501–9. doi: 10.1016/j.ejca.2011.12.004
36. Park JS, Huh JW, Park YA, Cho YB, Yun SH, Kim HC, et al. Prognostic comparison between mucinous and non-mucinous adenocarcinoma in colorectal cancer. Medicine. (2015) 94:e658. doi: 10.1097/MD.0000000000000658
37. Henderson TO, Oeffinger KC, Whitton J, Leisenring W, Neglia J, Meadows A, et al. Secondary gastrointestinal cancer in childhood cancer survivors: a cohort study. Ann Intern Med. (2012) 156:757–66. doi: 10.7326/0003-4819-156-11-201206050-00002
38. Birgisson H, Pahlman L, Gunnarsson U, Glimelius B. Occurrence of second cancers in patients treated with radiotherapy for rectal cancer. J Clin Oncol. (2005) 23:6126–31. doi: 10.1200/JCO.2005.02.543
39. Wiltink LM, Nout RA, Fiocco M, Meershoek-Klein Kranenbarg E, Jurgenliemk-Schulz IM, Jobsen JJ, et al. No increased risk of second cancer after radiotherapy in patients treated for rectal or endometrial cancer in the randomized TME, PORTEC-1, and PORTEC-2 trials. J Clin Oncol. (2015) 33:1640–6. doi: 10.1200/JCO.2014.58.6693
40. Martling A, Smedby KE, Birgisson H, Olsson H, Granath F, Ekbom A, et al. Risk of second primary cancer in patients treated with radiotherapy for rectal cancer. Br J Surg. (2017) 104:278–87. doi: 10.1002/bjs.10327
41. Rombouts AJM, Hugen N, Elferink MAG, Feuth T, Poortmans PMP, Nagtegaal ID, et al. Incidence of second tumors after treatment with or without radiation for rectal cancer. Ann Oncol. (2017) 28:535–40. doi: 10.1093/annonc/mdw661
Keywords: colorectal cancer, second primary malignancies, histological subtypes, classical adenocarcinoma, mucinous adenocarcinoma, signet-ring cell carcinoma, SEER database
Citation: Wu M, Huang M, He C, Chen C, Li H, Wang J, Liu M, Fu G, Lei Z and Chu X (2021) Risk of Second Primary Malignancies Based on the Histological Subtypes of Colorectal Cancer. Front. Oncol. 11:650937. doi: 10.3389/fonc.2021.650937
Received: 08 January 2021; Accepted: 15 February 2021;
Published: 10 March 2021.
Edited by:
Zsolt Kovács, George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureş, RomaniaReviewed by:
Savio George Barreto, Medanta the Medicity, IndiaCopyright © 2021 Wu, Huang, He, Chen, Li, Wang, Liu, Fu, Lei and Chu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyuan Chu, Y2h1eGlhb3l1YW4wMDBAMTYzLmNvbQ==; Zengjie Lei, bGVpemVuZ2ppZUAxNjMuY29t; Gongbo Fu, bWhrc2ZnYkAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.