- 1Thoracic and Gastrointestinal Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, United States
- 2Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, China
- 3Department of Medicine, Division of Gastroenterology and Hepatology, University of Maryland School of Medicine, Baltimore, MD, United States
- 4Sandra and Edward Meyer Cancer Center, Weill Cornell Medicine, New York, NY, United States
Background: Overall risks of hepatotoxicity with immune checkpoint inhibitors (ICIs) have yet to be compared in primary liver cancers to other solid tumors.
Methods: We reviewed data from the PubMed, Embase, and Scopus databases, and assessed the risk of hepatotoxicity associated with ICIs.
Results: A total of 117 trials were eligible for the meta‐analysis, including 7 trials with primary liver cancers. The most common hepatotoxicity was ALT elevation (incidence of all grade 5.29%, 95% CI 4.52-6.20) and AST elevation (incidence of all grade 5.88%, 95% CI 4.96-6.97). The incidence of all grade ALT and AST elevation was 6.01% and 6.84% for anti-PD‐1 (95% CI 5.04-7.18/5.69-8.25) and 3.60% and 3.72% for anti-PD-L1 (95% CI 2.72-4.76/2.82-4.94; p< 0.001/p<0.001). The incidence of ≥ grade 3 ALT and AST elevation was 1.54% and 1.48% for anti-PD‐1 (95% CI 1.19-1.58/1.07-2.04) and 1.03% and 1.08% for anti-PD-L1 (95% CI 0.71-1.51/0.80-1.45; p= 0.002/p<0.001). The incidence of all grade ALT and AST elevation was 13.3% and 14.2% in primary liver cancers (95% CI 11.1-16.0 and 9.93-20.36) vs. 4.92% and 5.38% in other solid tumors (95% CI 4.21-5.76 and 4.52-5.76 in other solid tumors; p <0.001/p<0.001).
Conclusion: Our study indicates that anti-PD-1 is associated with a higher risk of all‐ and high‐grade hepatotoxicity compared to anti-PD-L1, and primary liver cancers are associated with a higher risk of all‐ and high‐grade hepatotoxicity compared to other solid tumors.
Introduction
Liver cancers are the fourth leading cause of cancer-related mortality worldwide (1, 2) with over 800,000 new primary liver cancer cases diagnosed around the world each year (3). Hepatocellular carcinoma (HCC) is one of the most aggressive liver cancers and accounts for 75-85% of these cases (1). Biliary tract carcinoma (BTC) originates in the intra- and extrahepatic biliary ductal system and is the second most common primary liver cancer after HCC. Surgical resection or liver transplantation are potential curative options for patients with early stage disease, however, the majority of patients present with advanced stage disease and are not candidates for curative therapies.
Immunotherapy has shown promising, effective and durable antitumor responses through immune mediated mechanisms by blocking internal immunosuppressive mechanism (e.g. CTLA-4 and PD-1/PD-L1 axis) and reactivating T cell-mediated host immune surveillance. Among these, immune checkpoint inhibitors (ICIs) have shown remarkable efficacy in the therapy of multiple cancers including HCC that has led to the regulatory approval of ICI in the first and second-line setting. Unfortunately, the antitumor effects of ICIs in BTC is much more limited and is still an ongoing active research field.
Although ICIs have achieved success as cancer therapies, their use comes with risks evidenced by toxic effects (treatment-related adverse events, TRAEs), including immune-related adverse events (IRAEs) caused by stimulation of the immune system (4–7). Those IRAEs are considered a consequence of T lymphocyte hyperactivation triggered by ICI introduction to attack auto-antigens located in the normal tissue. IRAEs lead to autoimmune-like manifestations in individual organs where the activated immune surveillance machinery attacks self-antigens on normal tissues. Hepatotoxicity derived from ICIs exhibits either a hepatocellular or cholestatic injury pattern with abnormal laboratory findings (8, 9). Clinically, hepatotoxicity can present with a range findings, from mild elevation of liver enzymes to hepatobiliary disorders like autoimmune hepatitis, cholangitis, jaundice and liver failure (10, 11). Currently, there are several meta-analyses that have evaluated hepatotoxicity from ICIs and found a general incidence of 2-30% (7, 8, 11–14).
Although there is overlapped antigenicity between normal liver tissue and tumors derived from the liver, it is unknown if ICI exposure can increase the incidence or severity of hepatotoxicity in patients with primary liver cancers in comparison to other cancer types. Therefore, we conducted a meta-analysis with the aim to compare liver-specific toxicities among patients with primary liver cancers versus other solid cancers.
Methods
Search Strategy and Study Selection
This study strictly followed the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) guideline. A systematic search of the literature was conducted in PubMed, Web of Science and Embase to identify published clinical trials of ICIs that reported hepatotoxicity. The search was done using keywords anti-PD‐1, PD-1 inhibitor, anti-PD‐L1, PD-L1 inhibitor, nivolumab, pembrolizumab, avelumab, durvalumab and atezolizumab from June 1, 2008 through final research for updates on August 3, 2020. Phase I studies were excluded due to concerns about different dose ranges used. Studies eligible for this analysis must have met all of the following criteria: (1) prospective phase 2 or phase 3 human trials/cohorts in cancer therapy, (2) participants were treated with a single ICI agent to avoid cumulative hepatotoxicity from other agents, (3) reported tabulated data on any treatment-related hepatotoxicity, (4) sample size over 20, and (5) published in English. Meeting abstracts were excluded except for the phase 3 Checkmate 459 study that was included. When encountered with multiple publications from the same study population was identified, the one with the most recent, relevant and/or comprehensive hepatotoxicity data was included. The literature search, study selection, and data extraction were performed independently by 3 of the co-authors (F. J., W-Z. L and M.N.). The discrepancies were reviewed by another two investigators on the team (Y-Q. X and X.C) and resolved by consensus.
Data Collection
A standard checklist was used for all studies including name of first author, year of publication, phase of the study, registration identity at clinicaltrial.gov, targeted cancer, ICI(s) involved, number of patients enrolled, number with liver metastases of enrolled patients, and number of patients recorded with hepatotoxicity in each trial. The hepatotoxicity in this analysis are all‐grades and high‐grade (≥grade 3) liver adverse events (AEs), which can be further classified into the elevation of alanine aminotransferase (ALT), aspartate aminotransferase(AST), blood bilirubin, blood alkaline phosphatase (ALP), γ‐glutamyl transpeptidase (GGT), immune related-hepatitis (ir-hepatitis), and hepatobiliary disorders (if separated from abovementioned items, including autoimmune hepatitis, acute hepatitis, liver failure, hepatic hemorrhage, transaminitis, elevated liver enzyme, cholecystitis, cholangitis, cholelithiasis, cholestasis, hepatocellular injury, drug-induced liver injury, jaundice, and hyperbilirubinemia).
Statistical Analysis
Using the extracted data, we recorded the number of patients treated and the number of patients with hepatotoxicity reported. Meta-analysis of single incidence rates to calculate a pooled rate was performed using inverse variance method (15). Random effect models were fit using log transformation and were implemented to calculate a pooled incidence of each toxic effect and corresponding 95% confidence intervals (CIs). Continuity correction of 0.5 in studies with zero cell frequencies was applied in order to calculate individual study results with confidence limits and to conduct meta-analysis. Statistical heterogeneity was evaluated by the Cochran Q statistic and quantified with I2 statistics. A P value of less than 0.05 for Chi-square was regarded as the indicator of the presence of heterogeneity. I2 values of higher than 50% represented a high level of heterogeneity. To estimate the between-study variation in the meta-analysis, we also calculated the incidence rate of hepatotoxicity by study-level moderators, including the cancer type and drug type. Incidence differences between the different groups were compared with the Two-Proportions Z-Test.
All statistical analyses were performed by using R software (version 4.0.2, R Foundation for Statistical Computing) and the “meta” package.
Results
Eligible Studies and Characteristics
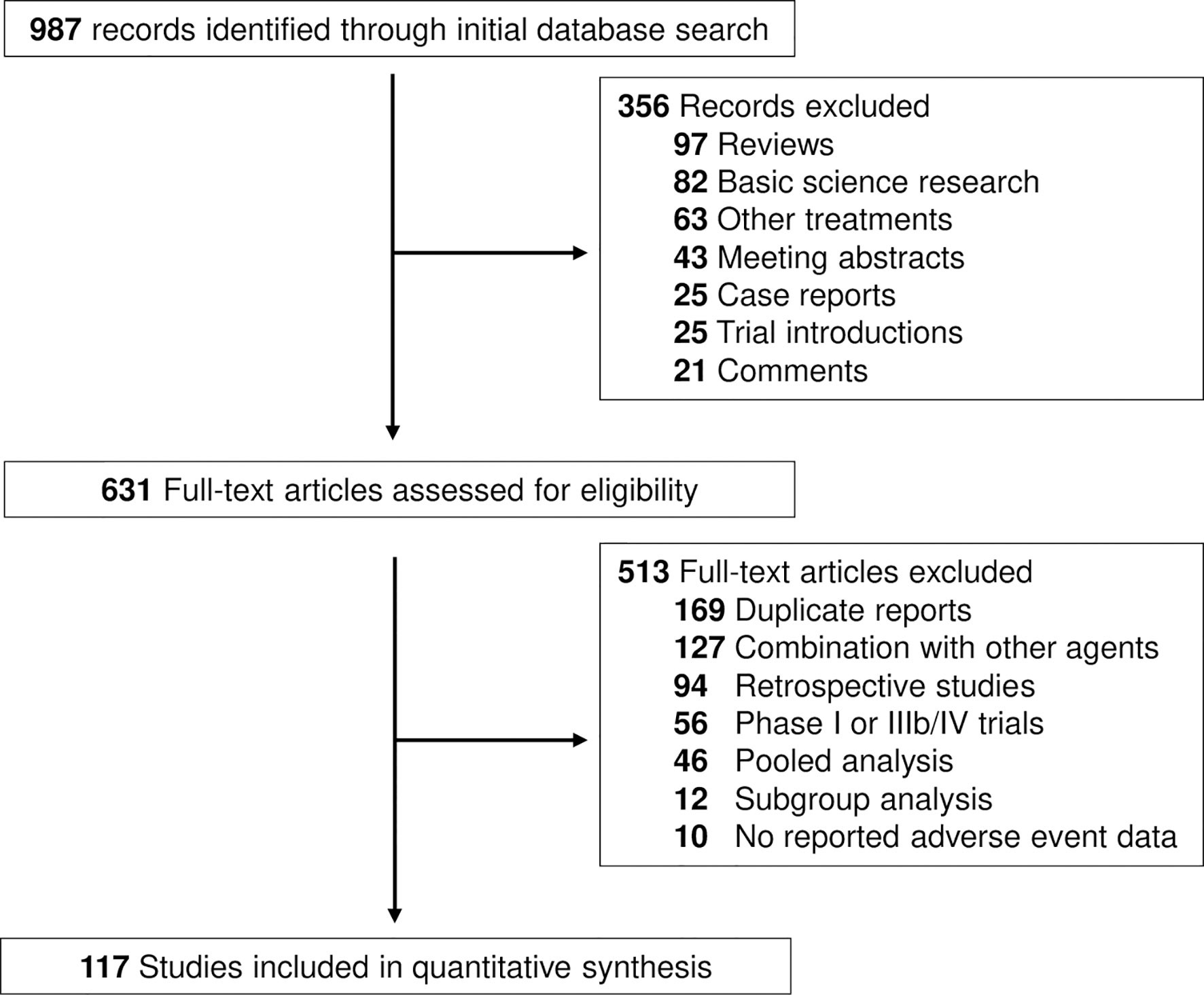
The search strategy revealed 987 potentially suitable records on ICIs from PubMed, Web of Science and Embase databases. The reasons for study exclusion are shown in Figure 1. Accordingly, a total of 117 clinical trials were considered eligible for the analysis (16–132). The trials involved the treatment of liver cancer (7 cohorts, n = 1362, 6 HCC and 1 BTC), lung cancer (27 cohorts, n = 7208), other gastrointestinal cancer (14 cohorts, n = 1798), genitourinary cancer (26 cohorts, n = 4963), melanoma (14 cohorts, n = 4169), head and neck cancer (10 cohorts, n = 1844), and other cancer types (15 cohorts, n = 994, including one cohort of thymic carcinoma, 2 cohorts of sarcoma, 2 cohorts of neuroendocrine tumor, 2 cohorts of mesothelioma, 3 cohorts of Merkle cell carcinoma and 5 cohorts of mixed solid tumors) (Supplemental Table S1). We categorized the regimens by ICI class using PD-1 inhibitor (88 cohorts; n = 16554 patients), and PD-L1 inhibitor (30 cohorts; n = 7113 patients). Specific PD-1 inhibitors included nivolumab (44 cohorts; n = 8368 patients) and pembrolizumab (44 cohorts; n = 7537 patients). Specific PD-L1 inhibitors included atezolizumab (14 cohorts, n = 4069), avelumab (5 cohorts, n = 743), and durvalumab (11 cohorts, n = 2301).
Some trials had not reported information of hepatotoxicity related to ICIs. Supplemental Figure S1 shows the proportion and pattern of missing data from all trials included in this study. The most available reported hepatoxicity parameters are elevated ALT, AST, and hepatobiliary disorders, named transaminitis, acute liver injury, hepatocellular injury, and acute liver failure. The most common missed parameter was GGT that accounted over 60% missing data (Supplemental Figure S1). This may be due to the rare incidence of GGT change with ICI exposure that was absent from reports.
Incidence of All-Grade Hepatotoxicity Across All Tumor Types
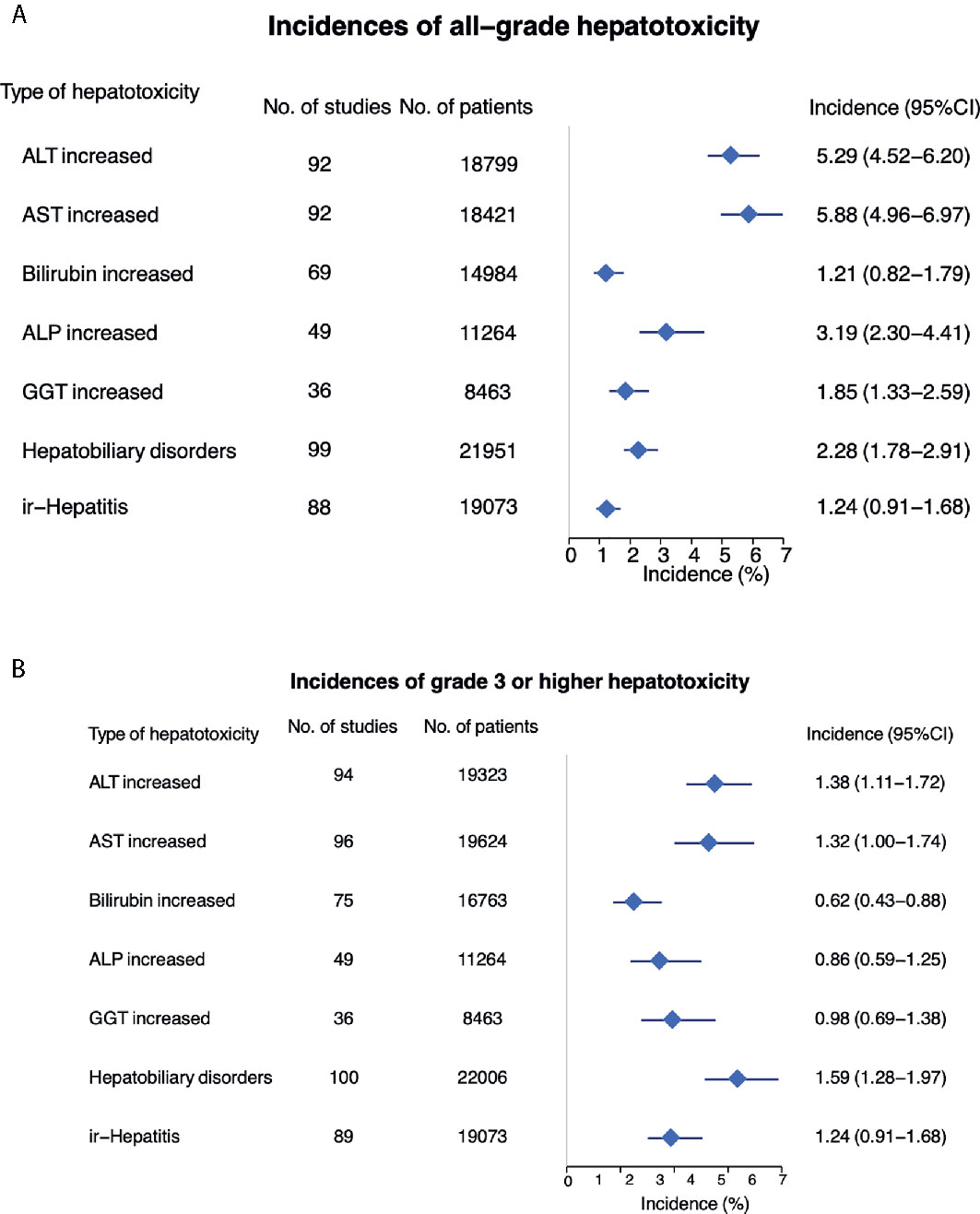
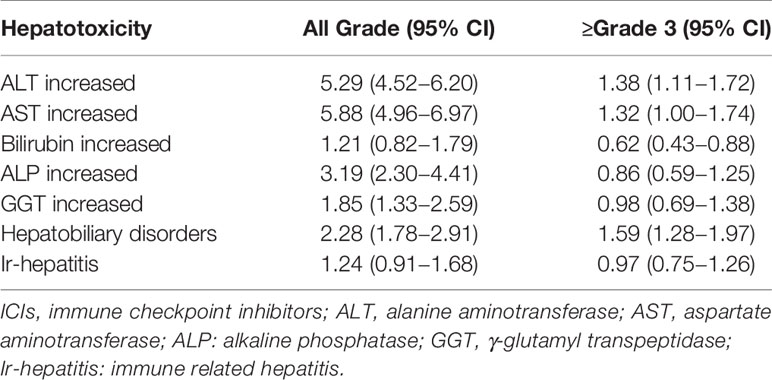
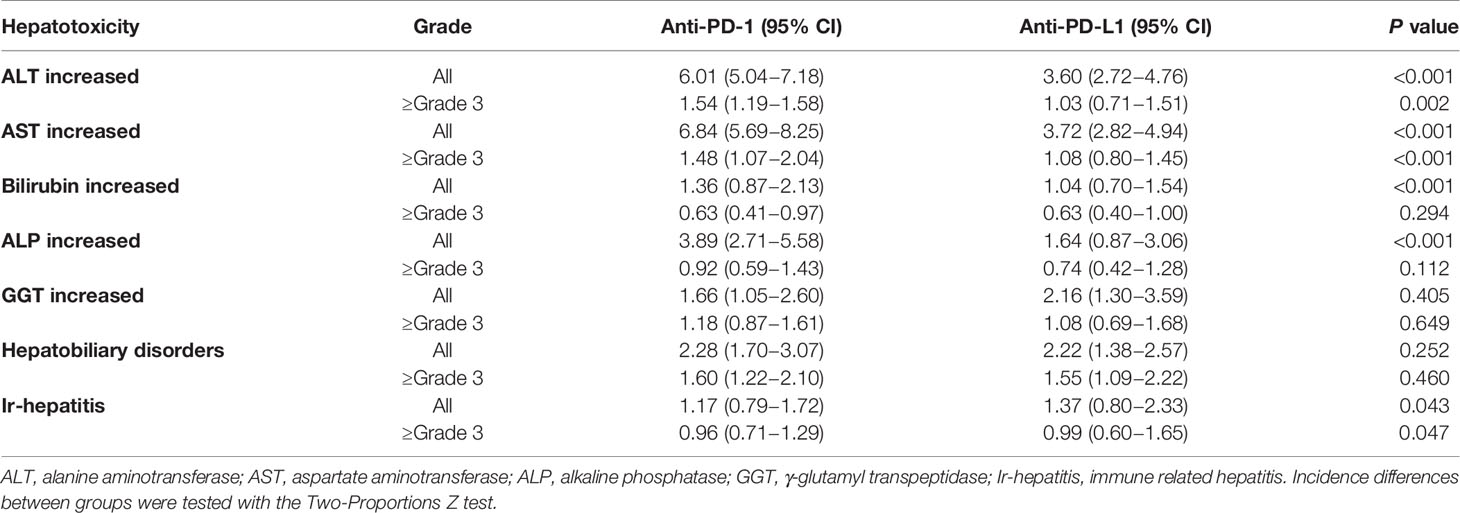
All-grade hepatotoxicity parameters were reported as following (Figure 2A and Table 1): elevated ALT was reported in 92 studies (incidence 5.29%; 95% CI 4.52 - 6.20); elevated AST in 92 studies (incidence 5.88%; 95% CI 4.96 – 6.97); elevated bilirubin in 69 studies (incidence of 1.21%; 95% CI 0.82 – 1.79); elevated ALP in 49 studies (incidence 3.19%; 95% CI 2.30 – 4.41); elevated GGT in 36 studies (incidence of 1.85%; 95% CI 1.33 – 2.59); hepatobiliary disorder in 99 studies (incidence of 2.28%; 95% CI 1.78 – 2.91); and ir-hepatitis in 88 studies (incidence of 1.24%; 95% CI 0.91 – 1.68). Across all studies, the anti-PD‐1 subgroup showed statistically higher incidence of all-grade elevated ALT, AST, bilirubin and ALP, and ir-hepatitis in comparison with the anti-PD-L1 subgroup (p<0.05, Supplemental Figure S2A and Table 2), but not the incidence of elevated GGT and hepatobiliary disorders.
Incidence of High-Grade Hepatotoxicity Across All Tumor Types
The incidence of ≥ grade 3 hepatotoxicity was reported as follows (Figure 2B and Table 1): elevated ALT in 94 studies with an incidence of 1.38% (95% CI 1.11 – 1.72); elevated AST in 96 studies with an incidence of 1.32% (95% CI 1.00 – 1.74); elevated bilirubin in 75 studies with an incidence of 0.62% (95% CI 0.43 – 0.88); elevated ALP in 49 studies with an incidence of 0.86% (95% CI 0.59 – 1.25); elevated GGT in 36 studies with an incidence of 0.98% (95% CI 0.69 – 1.38); hepatobiliary disorders in 100 studies with an incidence of 1.59% (95% CI 1.28 – 1.97); and ir-hepatitis in 89 studies with an incidence of 0.97% (95% CI 0.75 – 1.26). Across all studies, the anti-PD‐1 subgroup showed statistically higher incidence of ≥ grade 3 elevation of ALT/AST and ir-hepatitis in comparison with the anti-PD-L1 subgroup (p<0.05, Supplemental Figure S2B and Table 2).
Individual All-Grade Hepatoxicity in Primary Liver Cancers Compared to Other Cancer Types
Overall incidence of all grade elevated ALT from anti-PD-1 or anti-PD-L1 in patients with primary liver cancer was 14.22% (95% CI 9.93 – 20.36); elevated AST 13.30% (95% CI 11.10 – 16.00); elevated bilirubin 7.95% (95% CI 2.96 – 21.33); elevated ALP 2.30% (95% CI 1.10 – 4.83); elevated GGT 1.62% (95% CI 0.75 – 3.50), non-specific liver toxicity was 11.7% (95% CI 9.74 – 14.00); and ir-hepatitis 2.02 (95% CI 1.05 – 3.88) (Figure 3A and Supplemental Figure S3). Interestingly, the incidence of all-grade elevated ALT, AST and bilirubin, and hepatobiliary disorders was statistically higher in primary liver cancer versus other cancer types (p<0.001, Table 3), but not the elevation of ALP/GGT and ir-hepatitis.
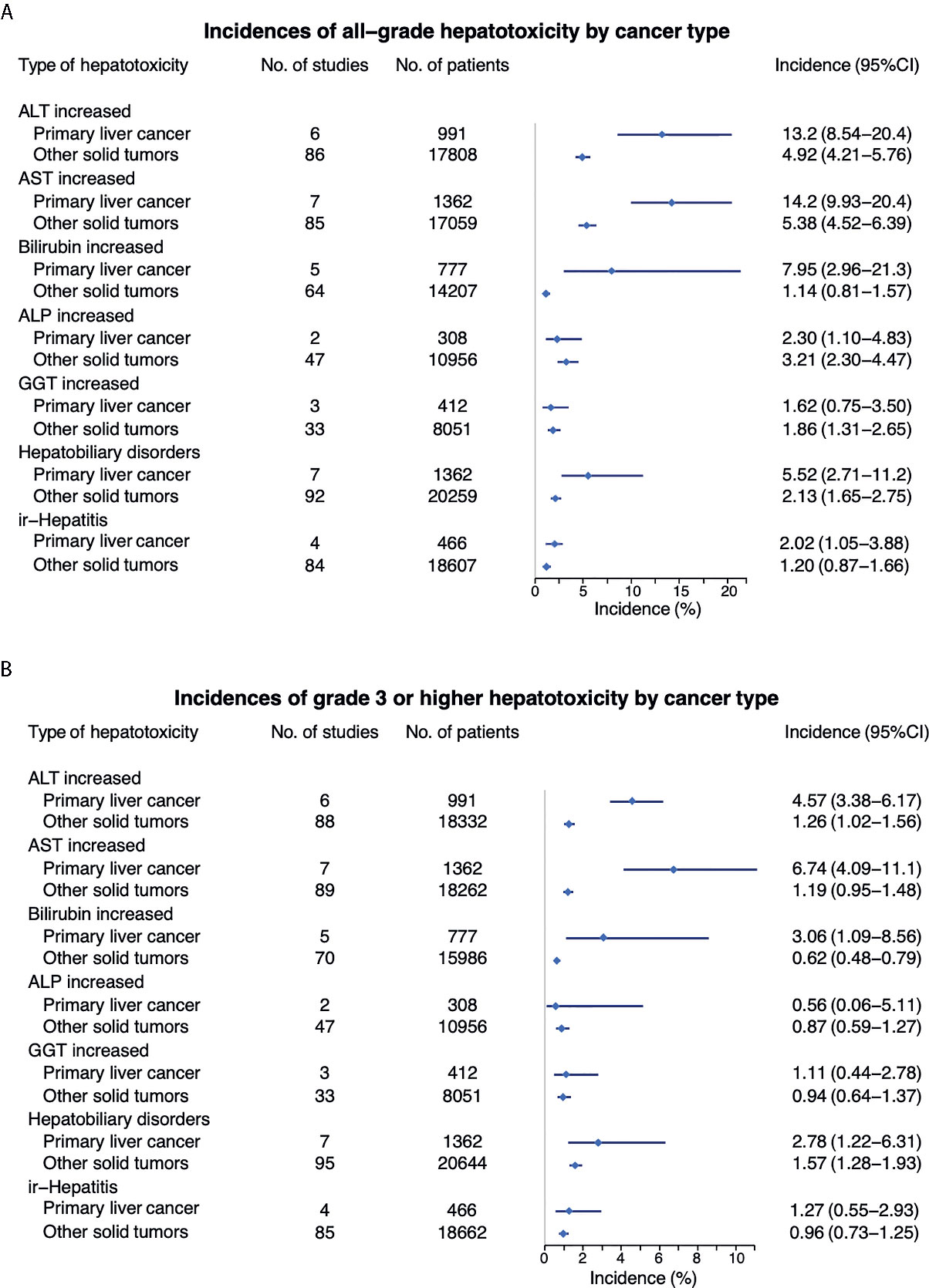
Figure 3 Comparison of incidence of all-grade (A) and high grade (B) hepatotoxicity between primary liver cancer vs other tumor types.
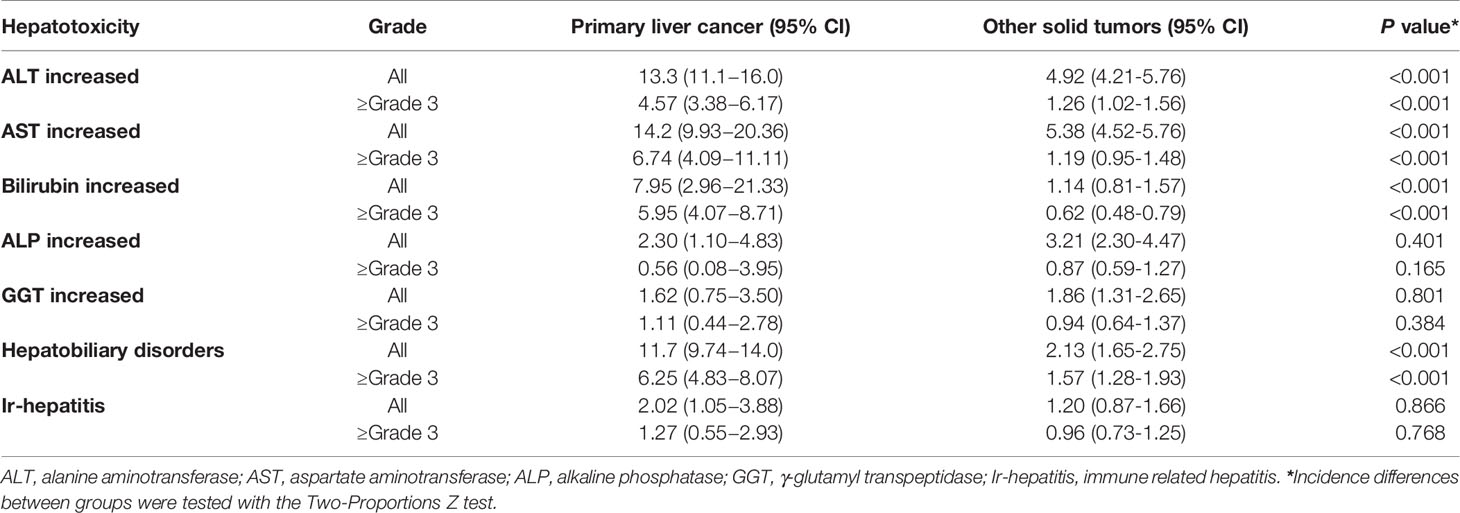
Table 3 Incidence of hepatotoxicity in primary liver cancers and other solid tumors treated with anti-PD-1/PD-L1.
Individual High-Grade Hepatoxicity in Primary Liver Cancers Compared to Other Cancer Types
The incidence of ≥ grade 3 hepatotoxicity from anti-PD-1 or anti-PD-L1 in patients with primary liver cancers was as follows: elevated ALT 6.74% (95% CI 4.09 – 11.11); elevated AST 4.57% (95% CI 3.38 – 6.17); elevated bilirubin 5.95% (95% CI 4.07 – 8.71); elevated ALP 0.56% (95% CI 0.08 – 3.95); elevated GGT 1.11% (95% CI 0.44 – 2.78), hepatobiliary disorder 11.7% (95% CI 9.74 – 14.00); and ir-hepatitis 1.27 (95% CI 0.55 – 2.93) (Figure 3B and Supplemental Figure S4). Furthermore, the incidence of ≥ grade 3 elevated ALT, AST, bilirubin, and hepatobiliary disorders were significantly higher in primary liver cancers versus other cancer types (p<0.001, Table 3).
Discussion
In the present meta-analysis of all published phase 2 and 3 clinical trials of anti-PD-1 and anti-PD-L1 in patients with solid tumors, we reported the overall incidence of individual hepatotoxicity parameters secondary to treatment. There was an increased risk of all-grade and ≥ grade 3 ALT/AST elevation and ir-hepatitis when patients were treated with anti-PD-1 compared to anti-PD-L1. The overall incidence of all-grade and ≥ grade 3 elevated ALT, AST, bilirubin, and hepatobiliary disorders was increased in patients with primary liver cancer compared with other cancer types, treated with anti-PD-1/PD-L1, though incidence of reported ir-hepatitis was not significantly increased.
Hepatotoxicity triggered by ICI has been recognized as an important cause of morbidity and mortality and considered to be contributed by immune related mechanisms of action of ICIs. The elevation of ALT and AST is the earliest sign of liver injury. Hepatotoxicity can emerge days after ICI administration or be delayed by several months with a median onset ranging from 3 to 9 weeks (133). It commonly presents with isolated elevations of liver transaminases that can often subside after treatment discontinuation (133). Some severe cases are associated with other manifestations of liver dysfunction, e.g. coagulopathy, or even life-threatening liver failure. Previous meta-analyses have showed the incidence of all-grade liver toxicity induced by single agent of ICIs ranges from 2-37% (8, 12, 134–136) and this incidence increases when ICIs are administrated in combination with anti-CTLA-4 monoclonal antibodies or traditional chemotherapy (8, 136). In this study, we found that ALT and AST elevation were the most common presentation of all-grade treatment related hepatotoxicity with an incidence rate of 5.29% and 5.88%, respectively. There was a 1.24% incidence of reported all grade ir-hepatitis with high grade 0.97%. Other less common laboratory findings of liver toxicities included ALP elevation, hepatobiliary disorders, elevated bilirubin and GGT. The differences of reported incidence of hepatotoxicity from ICIs between this current study and previous results may be due to the substantial variations of terms used to report hepatotoxicity.
In addition, the anti-PD-1 treated patient population had higher incidence of all grade and ≥ grade 3 elevated ALT/AST and ir-hepatitis compared to anti-PD-L1. The causative mechanism is unclear. It has been proposed that the PD-L2 ligand may stimulate additional checkpoint signaling when anti-PD-L1 only targets PD-L1 versus anti-PD- blocks both PD-L1 and PD-L2 (135). Moreover, the anti-PD-1 executes an unselected T-Cell hyperactivation given generalized PD-1 expression on T cell membrane, that leads to potentially higher and more severe IRAEs than anti-PD-L1 (137). Furthermore, PD-1 expression is upregulated upon T cell activation and prevents the self-reactive and pathognomonic T cell activation (138). Therefore, the blockade of PD-1 by anti-PD-1 on dendric cells and other antigen-presenting cells could be relevant to worsen side effects than anti-PD-L1 (139). There are no head-to-head randomized clinical trials available to compare hepatotoxicity between anti-PD-1 and anti-PD-L1 inhibitors. Nevertheless, baseline liver dysfunction and primary or metastatic liver tumor burden in the trials may also contribute to these differences between trials using anti-PD-1 or anti-PD-L1.
Interestingly, in our study, there was a significantly higher incidence of elevated ALT/AST/bilirubin and hepatobiliary disorders in patients with primary liver cancers in comparison with other solid tumors. Although the majority of participants in the trials may or may not have abnormal liver function test results in the beginning with the background of liver disease, e.g. chronic viral hepatitis and cirrhosis, it is important to note that these underlying liver diseases may exacerbate/magnify the presentation of damaged liver functions after the administration of ICIs. Anti-PD-1/PD-L1 blocks the PD-1/PD-L1 pathway and activates T cells to target tumor antigens and kill tumors, but T cell activation may also be reactive against an antigen shared between normal tissue and tumors, that leads to normal organ injury (140). However, there is no consensus for the definition of drug induced ir-hepatitis related to ICI treatment in cancer. In our study, there was a significant percentage of missing information regarding ir-hepatitis. However, surrogate markers like elevated ALT/AST may be indication of ir-hepatitis and the incidence may be underestimated. This likely contributes to no difference seen between primary liver cancers and other solid tumors in incidence of ir-hepatitis.
The results of this analysis should be interpreted cautiously as there were several limitations to the study. First, there is publication bias given that the data was collected from published studies and only prevalent AEs were reported. For example, the incidence of > 5- 10% AEs were reported in the studies, dependent on the sample size of the trials. Since hepatotoxicity is not a common AE from ICIs, the information may not always be available for review. As a result, missing information can generate an overestimation of the incidence of hepatoxicity from ICIs. Second, potential co-founders were not reported in all publications. For instance, case numbers of liver metastasis for other solid tumors were missing. Metastatic liver lesions can result in hypoperfusion of normal liver tissues causing liver injury, which can be exacerbated by treatment caused by immune cell infiltration. Therefore, the incidence of liver toxicity directly contributed by ICIs in patients with solid tumors other than primary liver cancers could be inflated. Nevertheless, the hepatotoxicity derived from non-immune related factors is not able to be distinguished. Thirdly, the type and terms of reported hepatotoxicity were highly variable between trials, although every trial followed similar grading systems. Some studies reported hepatotoxicity as a whole labeled as one TRAEs or AE of interest, whereas others presented various laboratory liver function changes or clinical diagnoses like transaminitis and acute liver failure, or pathologic diagnoses like hepatocellular injury and liver injury. Moreover, there was significant variation or heterogeneity in the definition of hepatotoxicity across trials. Fourthly, the data was analyzed from published aggregated data and not from individual patients which is a common pitfall of meta-analysis data collection. It would be challenging to establish additional potential risk factors associated with the development of liver toxicity. Lastly, variations in the trial design, including eligibility criteria and phase of trial, can lead to selection bias. However, such a bias should not be significant because evaluation for hepatotoxicity was done with objective laboratory tests, rather than subjective AEs.
Conclusion
This analysis found that primary liver cancers are associated with a higher risk of all‐ and high‐grade hepatotoxicity compared to other solid cancer types. Additionally, we found that anti-PD‐1 therapy results in a higher risk of hepatotoxicity compared to anti-PD-L1.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Conceptualization Y-QX, CX. Methodology JF, W-ZL, Y-QX, CX. Software JF, W-ZL. Validation Y-QX, CX. Formal analysis JF, W-ZL, CX. Investigation JF, WZ-L, NM, Y-QX, CX. Resources JF, WL, NM, CX. Data curation JF, W-ZL, NM, CX. Writing—original draft preparation JF, NM, CX. Writing—review and editing JF, W-ZL, NM, GB, CL, Y-QX, CX. Visualization Y-QX, CX. Supervision Y-QX, CX. Project administration NM, CX. Funding acquisition Y-QX, CX. All authors contributed to the article and approved the submitted version.
Funding
Y-QX is sponsored by National Natural Science Foundation of China (Program Grants 81572665). CX is supported by NIH/NCI/CCR Physician-Scientist Early Investigator Program (ZIA BC 011888).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.650292/full#supplementary-material
References
1. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PloS Med (2006) 3:e442. doi: 10.1371/journal.pmed.0030442
2. Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, et al. Global Burden of 5 Major Types Of Gastrointestinal Cancer. Gastroenterology (2020) 159:335–49. doi: 10.1053/j.gastro.2020.02.068
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
4. Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, et al. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol (2018) 4:173–82. doi: 10.1001/jamaoncol.2017.3064
5. Ma K, Lu Y, Jiang S, et al. The Relative Risk and Incidence of Immune Checkpoint Inhibitors Related Pneumonitis in Patients With Advanced Cancer: A Meta-Analysis. Front Pharmacol (2018) 9:1430. doi: 10.3389/fphar.2018.01430
6. Wang DY, Salem J-E, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol (2018) 4:1721–8. doi: 10.1001/jamaoncol.2018.3923
7. Wang P-F, Chen Y, Song S-Y, Wang T-J, Ji W-J, Li S-W, et al. Immune-Related Adverse Events Associated with Anti-PD-1/PD-L1 Treatment for Malignancies: A Meta-Analysis. Front Pharmacol (2017) 8:730. doi: 10.3389/fphar.2017.00730
8. Jennings JJ, Mandaliya R, Nakshabandi A, Lewis JH. Hepatotoxicity induced by immune checkpoint inhibitors: a comprehensive review including current and alternative management strategies. Expert Opin Drug Metab Toxicol (2019) 15:231–44. doi: 10.1080/17425255.2019.1574744
9. Parlati L, Vallet-Pichard A, Batista R, Hernvann A, Sogni P, Pol S, et al. Incidence of grade 3-4 liver injury under immune checkpoints inhibitors: A retrospective study. J Hepatol (2018) 69:1396–7. doi: 10.1016/j.jhep.2018.08.014
10. Alessandrino F, Tirumani SH, Krajewski KM, Shinagare AB, Jagannathan JP, Ramaiya NH, et al. Imaging of hepatic toxicity of systemic therapy in a tertiary cancer centre: chemotherapy, haematopoietic stem cell transplantation, molecular targeted therapies, and immune checkpoint inhibitors. Clin Radiol (2017) 72:521–33. doi: 10.1016/j.crad.2017.04.003
11. Chascsa DM, Rakela J. Knowns and Unknowns: the Safety and Efficacy of Cancer Immunotherapy in Chronic Liver Disease. Curr Hepatol Rep (2018) 17:153–5. doi: 10.1007/s11901-018-0408-8
12. Wang W, Lie P, Guo M, He J. Risk of hepatotoxicity in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis of published data. Int J Cancer (2017) 141:1018–28. doi: 10.1002/ijc.30678
13. Peeraphatdit T, Wang J, Odenwald MA, Hu S, Hart J, Charlton MR. Hepatotoxicity From Immune Checkpoint Inhibitors: A Systematic Review and Management Recommendation. Hepatology (2020) 72:315–29. doi: 10.1002/hep.31227
14. Miller ED, Abu-Sbeih H, Styskel B, Nogueras Gonzalez GM, Blechacz B, Naing A, et al. Clinical Characteristics and Adverse Impact of Hepatotoxicity due to Immune Checkpoint Inhibitors. Off J Am Coll Gastroenterol | ACG (2020) 115:251–61. doi: 10.14309/ajg.0000000000000398
15. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods (2010) 1:97–111. doi: 10.1002/jrsm.12
16. Adams S, Loi S, Toppmeyer D, Cescon DW, De Laurentiis M, Nanda R, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol (2019) 30:405–11. doi: 10.1093/annonc/mdy518
17. Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol (2019) 30:397–404. doi: 10.1093/annonc/mdy517
18. Antonia SJ, Balmanoukian A, Brahmer J, Ou SI, Hellmann MD, Kim SW, et al. Clinical Activity, Tolerability, and Long-Term Follow-Up of Durvalumab in Patients With Advanced NSCLC. J Thorac Oncol (2019) 14:1794–806. doi: 10.1016/j.jtho.2019.06.010
19. Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol (2016) 17:883–95. doi: 10.1016/S1470-2045(16)30098-5
20. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med (2017) 377:1919–29. doi: 10.1056/NEJMoa1709937
21. Azad NS, Gray RJ, Overman MJ, Schoenfeld JD, Mitchell EP, Zwiebel JA, et al. Nivolumab Is Effective in Mismatch Repair-Deficient Noncolorectal Cancers: Results From Arm Z1D-A Subprotocol of the NCI-MATCH (EAY131) Study. J Clin Oncol (2020) 38:214–22. doi: 10.1200/JCO.19.00818
22. Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet (2017) 389:67–76. doi: 10.1016/S0140-6736(16)32455-2
23. Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol (2017) 18:1483–92. doi: 10.1016/S1470-2045(17)30616-2
24. Bang YJ, Kang YK, Catenacci DV, Muro K, Fuchs CS, Geva R, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer (2019) 22:828–37. doi: 10.1007/s10120-018-00909-5
25. Bang YJ, Van Cutsem E, Fuchs CS, Ohtsu A, Tabernero J, Ilson DH, et al. Phase III KEYNOTE-585 study of chemotherapy (Chemo) + pembrolizumab (Pembro) vs chemo + placebo as neoadjuvant/adjuvant treatment for patients (Pts) with gastric or gastroesophageal junction (G/GEJ) cancer. Ann Oncol (2018) 29:viii268. doi: 10.1093/annonc/mdy282.167
26. Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol (2018) 19:1468–79. doi: 10.1016/S1470-2045(18)30673-9
27. Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: Results from a single-arm, phase II study. J Clin Oncol (2017) 35:1542–9. doi: 10.1200/JCO.2016.70.1524
28. Bauml JM, Mick R, Ciunci C, Aggarwal C, Davis C, Evans T, et al. Pembrolizumab after Completion of Locally Ablative Therapy for Oligometastatic Non-Small Cell Lung Cancer: A Phase 2 Trial. JAMA Oncol (2019) 5:1283–90. doi: 10.1001/jamaoncol.2019.1449
29. Bellmunt J, De Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med (2017) 376:1015–26. doi: 10.1056/NEJMoa1613683
30. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
31. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
32. Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G Jr, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet (2019) 394:1915–28. doi: 10.1016/S0140-6736(19)32591-7
33. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med (2017) 376:2415–26. doi: 10.1056/NEJMoa1613493
34. Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol (2019) 37:1470–8. doi: 10.1200/JCO.18.01265
35. Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet (2019) 393:156–67.
36. D’Angelo SP, Mahoney MR, Van Tine BA, Atkins J, Milhem MM, Jahagirdar BN, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol (2018) 19:416–26. doi: 10.1016/S1470-2045(18)30006-8
37. Davis KL, Fox E, Merchant MS, Reid JM, Kudgus RA, Liu X, et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol (2020) 21:541–50. doi: 10.1016/S1470-2045(20)30023-1
38. Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med (2018) 378:1789–801. doi: 10.1056/NEJMoa1802357
39. Eng C, Kim TW, Bendell J, Argiles G, Tebbutt NC, Di Bartolomeo M, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol (2019) 20:849–61. doi: 10.1016/S1470-2045(19)30027-0
40. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet (2016) 387:1837–46. doi: 10.1016/S0140-6736(16)00587-0
41. Fehrenbacher L, von Pawel J, Park K, Rittmeyer A, Gandara DR, Ponce Aix S, et al. Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezolizumab versus Docetaxel in Patients with Previously Treated Advanced Non–Small Cell Lung Cancer. J Thorac Oncol (2018) 13:1156–70. doi: 10.1016/j.jtho.2018.04.039
42. Felip E, Ardizzoni A, Ciuleanu T, Cobo M, Laktionov K, Szilasi M, et al. CheckMate 171: A phase 2 trial of nivolumab in patients with previously treated advanced squamous non-small cell lung cancer, including ECOG PS 2 and elderly populations. Eur J Cancer (2020) 127:160–72. doi: 10.1016/j.ejca.2019.11.019
43. Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med (2016) 375:1856–67. doi: 10.1056/NEJMoa1602252
44. Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol (2020) 31:942–50. doi: 10.1016/j.annonc.2020.04.001
45. Feun LG, Li YY, Wu C, Wangpaichitr M, Jones PD, Richman SP, et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer (2019) 125:3603–14. doi: 10.1002/cncr.32339
46. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol (2020) 38:193–202. doi: 10.1200/JCO.19.01307
47. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol (2018) 4:e180013. doi: 10.1001/jamaoncol.2018.0013
48. Gadgeel SM, Pennell NA, Fidler MJ, Halmos B, Bonomi P, Stevenson J, et al. Phase II Study of Maintenance Pembrolizumab in Patients with Extensive-Stage Small Cell Lung Cancer (SCLC). J Thorac Oncol (2018) 13:1393–9. doi: 10.1016/j.jtho.2018.05.002
49. Galsky MD, Mortazavi A, Milowsky MI, George S, Gupta S, Fleming MT, et al. Randomized Double-Blind Phase II Study of Maintenance Pembrolizumab Versus Placebo After First-Line Chemotherapy in Patients With Metastatic Urothelial Cancer. J Clin Oncol (2020) 38:1797–806. doi: 10.1200/JCO.19.03091
50. Garassino MC, Cho BC, Kim JH, Mazieres J, Vansteenkiste J, Lena H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol (2018) 19:521–36. doi: 10.1016/S1470-2045(18)30144-X
51. Geoerger B, Kang HJ, Yalon-Oren M, Marshall LV, Vezina C, Pappo A, et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1–2 trial. Lancet Oncol (2020) 21:121–33. doi: 10.1016/S1470-2045(19)30671-0
52. Geoerger B, Zwaan CM, Marshall LV, Michon J, Bourdeaut F, Casanova M, et al. Atezolizumab for children and young adults with previously treated solid tumours, non-Hodgkin lymphoma, and Hodgkin lymphoma (iMATRIX): a multicentre phase 1-2 study. Lancet Oncol (2020) 21:134–44. doi: 10.1016/S1470-2045(19)30693-X
53. Giaccone G, Kim C, Thompson J, McGuire C, Kallakury B, Chahine JJ, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol (2018) 19:347–55. doi: 10.1016/S1470-2045(18)30062-7
54. Goldberg SB, Schalper KA, Gettinger SN, Mahajan A, Herbst RS, Chiang AC, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol (2020) 21:655–63. doi: 10.1016/S1470-2045(20)30111-X
55. Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of Anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol (2015) 33:4015–22. doi: 10.1200/JCO.2015.62.3397
56. Hamid O, Puzanov I, Dummer R, Schachter J, Daud A, Schadendorf D, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer (2017) 86:37–45. doi: 10.1016/j.ejca.2017.07.022
57. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med (2018) 378:2093–104. doi: 10.1056/NEJMoa1801946
58. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (2016) 387:1540–50. doi: 10.1016/S0140-6736(15)01281-7
59. Hida T, Nishio M, Nogami N, Ohe Y, Nokihara H, Sakai H, et al. Efficacy and safety of nivolumab in Japanese patients with advanced or recurrent squamous non-small cell lung cancer. Cancer Sci (2017) 108:1000–6. doi: 10.1111/cas.13225
60. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2017) 390:2461–71. doi: 10.1016/S0140-6736(17)31827-5
61. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20:1506–17. doi: 10.1016/S1470-2045(19)30626-6
62. Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D'Angelo SP, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol (2016) 17:1374–85. doi: 10.1016/S1470-2045(16)30364-3
63. Kelly RJ, Lee J, Bang YJ, Almhanna K, Blum-Murphy M, Catenacci DVT, et al. Safety and efficacy of durvalumab and tremelimumab alone or in combination in patients with advanced gastric and gastroesophageal junction adenocarcinoma. Clin Cancer Res (2020) 26:846–54. doi: 10.1158/1078-0432.CCR-19-2443
64. Kim JH, Kim SY, Baek JY, Cha YJ, Ahn JB, Kim HS, et al. A Phase II Study of Avelumab Monotherapy in Patients with Mismatch Repair-Deficient/Microsatellite Instability-High or POLE-Mutated Metastatic or Unresectable Colorectal Cancer. Cancer Res Treat (2020) 52:1135–44. doi: 10.4143/crt.2020.218
65. Kim RD, Chung V, Alese OB, El-Rayes BF, Li D, Al-Toubah TE, et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer. JAMA Oncol (2020) 6:888–94. doi: 10.1001/jamaoncol.2020.0930
66. Kluger HM, Chiang V, Mahajan A, Zito CR, Sznol M, Tran T, et al. Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase II trial. J Clin Oncol (2019) 37:52–60. doi: 10.1200/JCO.18.00204
67. Konstantinopoulos PA, Luo W, Liu JF, Gulhan DC, Krasner C, Ishizuka JJ, et al. Phase II study of avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent/persistent endometrial cancer. J Clin Oncol (2019) 37:2786–94. doi: 10.1200/JCO.19.01021
68. Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol (2017) 18:631–9. doi: 10.1016/S1470-2045(17)30181-X
69. Larkin J, Minor D, D’Angelo S, Neyns B, Smylie M, Miller WH Jr., et al. Overall Survival in Patients With Advanced Melanoma Who Received Nivolumab Versus Investigator’s Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. J Clin Oncol (2018) 36:383–+. doi: 10.1200/JCO.2016.71.8023
70. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med (2015) 372:2509–20. doi: 10.1056/NEJMoa1500596
71. Lee JS, Lee KH, Cho EK, Kim DW, Kim SW, Kim JH, et al. Nivolumab in advanced non-small-cell lung cancer patients who failed prior platinum-based chemotherapy. Lung Cancer (2018) 122:234–42. doi: 10.1016/j.lungcan.2018.05.023
72. Levy BP, Giaccone G, Besse B, Felip E, Garassino MC, Domine Gomez M, et al. Randomised phase 2 study of pembrolizumab plus CC-486 versus pembrolizumab plus placebo in patients with previously treated advanced non-small cell lung cancer. Eur J Cancer (2019) 108:120–8. doi: 10.1016/j.ejca.2018.11.028
73. Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol (2018) 19:672–81. doi: 10.1016/S1470-2045(18)30139-6
74. Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol (2019) 20:1083–97. doi: 10.1016/S1470-2045(19)30274-8
75. Ma BBY, Lim WT, Goh BC, Hui EP, Lo KW, Pettinger A, et al. Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742). J Clin Oncol (2018) 36:1412–8. doi: 10.1200/JCO.2017.77.0388
76. Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol (2019) 30:1080–7. doi: 10.1093/annonc/mdz135
77. McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med (2018) 24:749–57. doi: 10.1038/s41591-018-0053-3
78. Middleton G, Brock K, Savage J, Mant R, Summers Y, Connibear J, et al. Pembrolizumab in patients with non-small-cell lung cancer of performance status 2 (PePS2): a single arm, phase 2 trial. Lancet Respir Med (2020) 8:895–904. doi: 10.1016/S2213-2600(20)30033-3
79. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (2019) 393:1819–30. doi: 10.1016/S0140-6736(18)32409-7
80. Morris VK, Salem ME, Nimeiri H, Iqbal S, Singh P, Ciombor K, et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study. Lancet Oncol (2017) 18:446–53. doi: 10.1016/S1470-2045(17)30104-3
81. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med (2015) 373:1803–13. doi: 10.1056/NEJMoa1510665
82. Motzer RJ, Rini BI, McDermott DF, Aren Frontera O, Hammers HJ, Carducci MA, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol (2019) 20:1370–85. doi: 10.1016/S1470-2045(19)30413-9
83. Naing A, Meric-Bernstam F, Stephen B, Karp DD, Hajjar J, Rodon Ahnert J, et al. Phase 2 study of pembrolizumab in patients with advanced rare cancers. J Immunother Cancer (2020) 8(1):e000347. doi: 10.1136/jitc-2019-000347corr1
84. Nathan P, Ascierto PA, Haanen J, Espinosa E, Demidov L, Garbe C, et al. Safety and efficacy of nivolumab in patients with rare melanoma subtypes who progressed on or after ipilimumab treatment: a single-arm, open-label, phase II study (CheckMate 172). Eur J Cancer (2019) 119:168–78. doi: 10.1016/j.ejca.2019.07.010
85. Nghiem P, Bhatia S, Lipson EJ, Sharfman WH, Kudchadkar RR, Brohl AS, et al. Durable tumor regression and overall survival in patients with advanced Merkel cell carcinoma receiving pembrolizumab as first-line therapy. J Clin Oncol (2019) 37:693–702. doi: 10.1200/JCO.18.01896
86. Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 blockade with pembrolizumab in advanced merkel-cell carcinoma. N Engl J Med (2016) 374:2542–52. doi: 10.1056/NEJMoa1603702
87. Nishio M, Hida T, Atagi S, Sakai H, Nakagawa K, Takahashi T, et al. Multicentre phase II study of nivolumab in Japanese patients with advanced or recurrent non-squamous non-small cell lung cancer. Esmo Open (2017) 2(4):e000108. doi: 10.1136/esmoopen-2016-000108
88. Nomura M, Oze I, Masuishi T, Yokota T, Satake H, Iwasawa S, et al. Multicenter prospective phase II trial of nivolumab in patients with unresectable or metastatic mucosal melanoma. Int J Clin Oncol (2020) 25:972–7. doi: 10.1007/s10147-020-01618-9
89. O’Reilly EM, Oh DY, Dhani N, Renouf DJ, Lee MA, Sun W, et al. Durvalumab with or Without Tremelimumab for Patients with Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol (2019) 5:1431–8. doi: 10.1001/jamaoncol.2019.1588
90. Okada M, Kijima T, Aoe K, Kato T, Fujimoto N, Nakagawa K, et al. Clinical efficacy and safety of nivolumab: Results of a multicenter, open-label, single-arm, Japanese phase II study in malignant pleural mesothelioma (MERIT). Clin Cancer Res (2019) 25:5485–92. doi: 10.1158/1078-0432.CCR-19-0103
91. Ott PA, Bang YJ, Berton-Rigaud D, Elez E, Pishvaian MJ, Rugo HS, et al. Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1-Positive Endometrial Cancer: Results From the KEYNOTE-028 Study. Obstet Gynecol Surv (2018) 73:26–7. doi: 10.1097/01.ogx.0000527579.58363.20
92. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol (2017) 18:1182–91. doi: 10.1016/S1470-2045(17)30422-9
93. Pal SK, Hoffman-Censits J, Zheng HZ, Kaiser C, Tayama D, Bellmunt J. Atezolizumab in Platinum-treated Locally Advanced or Metastatic Urothelial Carcinoma: Clinical Experience from an Expanded Access Study in the United States. Eur Urol (2018) 73:800–6. doi: 10.1016/j.eururo.2018.02.010
94. Peters S, Gettinger S, Johnson ML, Janne PA, Garassino MC, Christoph D, et al. Phase II Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1-Selected Advanced Non-Small-Cell Lung Cancer (BIRCH). J Clin Oncol (2017) 35:2781–+. doi: 10.1200/JCO.2016.71.9476
95. Planchard D, Reinmuth N, Orlov S, Fischer JR, Sugawara S, Mandziuk S, et al. ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann Oncol (2020) 31:609–18. doi: 10.1016/j.annonc.2020.02.006
96. Powles T, Duran I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet (2018) 391:748–57. doi: 10.1016/S0140-6736(17)33297-X
97. Powles T, Kockx M, Rodriguez-Vida A, Duran I, Crabb SJ, Van Der Heijden MS, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med (2019) 25:1706–14. doi: 10.1038/s41591-019-0628-7
98. Pujol JL, Greillier L, Audigier Valette C, Moro-Sibilot D, Uwer L, Hureaux J, et al. A randomized non-comparative phase II study of anti-PD-L1 ATEZOLIZUMAB or chemotherapy as second-line therapy in patients with small cell lung cancer: Results from the IFCT-1603 trial. Ann Oncol (2018) 29:viii596. doi: 10.1016/j.jtho.2019.01.008
99. Raj N, Zheng Y, Kelly V, Katz SS, Chou J, Do RKG, et al. PD-1 blockade in advanced adrenocortical carcinoma. J Clin Oncol (2020) 38:71–80. doi: 10.1200/JCO.19.01586
100. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non–small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol (2019) 37:537–46. doi: 10.1200/JCO.18.00149
101. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, et al. Durvalumab with or without Tremelimumab vs Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol (2020), E1–E14. doi: 10.1001/jamaoncol.2020.0237
102. Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol (2015) 16:257–65. doi: 10.1016/S1470-2045(15)70054-9
103. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med (2015) 372:320–30. doi: 10.1056/NEJMoa1412082
104. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med (2015) 372:2521–32. doi: 10.1056/NEJMoa1503093
105. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet (2016) 387:1909–20. doi: 10.1016/S0140-6736(16)00561-4
106. Scherpereel A, Mazieres J, Greillier L, Lantuejoul S, Do P, Bylicki O, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol (2019) 20:239–53. doi: 10.1016/S1470-2045(18)30765-4
107. Segal NH, Ou SHI, Balmanoukian A, Fury MG, Massarelli E, Brahmer JR, et al. Safety and efficacy of durvalumab in patients with head and neck squamous cell carcinoma: results from a phase I/II expansion cohort. Eur J Cancer (2019) 109:154–61. doi: 10.1016/j.ejca.2018.12.029
108. Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, et al. Efficacy and Safety of Pembrolizumab for Heavily Pretreated Patients with Advanced, Metastatic Adenocarcinoma or Squamous Cell Carcinoma of the Esophagus: The Phase 2 KEYNOTE-180 Study. JAMA Oncol (2019) 5:546–50. doi: 10.1001/jamaoncol.2018.5441
109. Sharma P, Callahan MK, Bono P, Kim J, Spiliopoulou P, Calvo E, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol (2016) 17:1590–8. doi: 10.1016/S1470-2045(16)30496-X
110. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol (2017) 18:312–22. doi: 10.1016/S1470-2045(17)30065-7
111. Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, et al. KEYNOTE-061: Phase 3 study of pembrolizumab vs paclitaxel for previously treated advanced gastric or gastroesophageal junction (G/GEJ) cancer. Ann Oncol (2018) 29:v123. doi: 10.1093/annonc/mdy208.004
112. Siu LL, Even C, Mesia R, Remenar E, Daste A, Delord JP, et al. Safety and Efficacy of Durvalumab With or Without Tremelimumab in Patients With PD-L1-Low/Negative Recurrent or Metastatic HNSCC: The Phase 2 CONDOR Randomized Clinical Trial. JAMA Oncol (2019) 5:195–203. doi: 10.1001/jamaoncol.2018.4628
113. Spigel DR, Chaft JE, Gettinger S, Chao BH, Dirix L, Schmid P, et al. FIR: Efficacy, Safety, and Biomarker Analysis of a Phase II Open-Label Study of Atezolizumab in PD-L1–Selected Patients With NSCLC. J Thorac Oncol (2018) 13:1733–42. doi: 10.1016/j.jtho.2018.05.004
114. Sternberg CN, Loriot Y, James N, Choy E, Castellano D, Lopez-Rios F, et al. Primary Results from SAUL, a Multinational Single-arm Safety Study of Atezolizumab Therapy for Locally Advanced or Metastatic Urothelial or Nonurothelial Carcinoma of the Urinary Tract. Eur Urol (2019) 76:73–81. doi: 10.1016/j.eururo.2019.03.015
115. Strosberg JR, Mizuno N, Doi T, Grande E, Delord JP, Shapira-Frommer R, et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase 2 KEYNOTE-158 Study. Clin Cancer Res (2020) 26:2124–30. doi: 10.1158/1078-0432.CCR-19-3014
116. Tamura K, Hasegawa K, Katsumata N, Matsumoto K, Mukai H, Takahashi S, et al. Efficacy and safety of nivolumab in Japanese patients with uterine cervical cancer, uterine corpus cancer, or soft tissue sarcoma: Multicenter, open-label phase 2 trial. Cancer Science (2019) 110:2894–904. doi: 10.1111/cas.14148
117. Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol (2017) 18:1493–501. doi: 10.1016/S1470-2045(17)30624-1
118. Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts J, et al. Effect of Pembrolizumab after Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients with Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol (2019) 5:1276–82. doi: 10.1001/jamaoncol.2019.1478
119. Varga A, Piha-Paul S, Ott PA, Mehnert JM, Berton-Rigaud D, Morosky A, et al. Pembrolizumab in patients with programmed death ligand 1–positive advanced ovarian cancer: Analysis of KEYNOTE-028. Gynecol Oncol (2019) 152:243–50. doi: 10.1016/j.ygyno.2018.11.017
120. Vijayvergia N, Dasari A, Deng M, Litwin S, Al-Toubah T, Alpaugh RK, et al. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: joint analysis of two prospective, non-randomised trials. Br J Cancer (2020) 122:1309–14. doi: 10.1038/s41416-020-0775-0
121. Voorwerk L, Slagter M, Horlings HM, Sikorska K, van de Vijver KK, de Maaker M, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med (2019) 25:920–8. doi: 10.1038/s41591-019-0432-4
122. Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med (2017) 377:1824–35. doi: 10.1056/NEJMoa1709030
123. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2017) 377:1345–56. doi: 10.1056/NEJMoa1709684
124. Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J Thorac Oncol (2019) 14:867–75. doi: 10.1016/j.jtho.2019.01.006
125. Yamazaki N, Kiyohara Y, Uhara H, Iizuka H, Uehara J, Otsuka F, et al. Cytokine biomarkers to predict antitumor responses to nivolumab suggested in a phase 2 study for advanced melanoma. Cancer Science (2017) 108:1022–31. doi: 10.1111/cas.13226
126. Yamazaki N, Kiyohara Y, Uhara H, Uehara J, Fujisawa Y, Takenouchi T, et al. Long-term follow up of nivolumab in previously untreated Japanese patients with advanced or recurrent malignant melanoma. Cancer Science (2019) 110:1995–2003. doi: 10.1111/cas.14015
127. Zamarin D, Burger RA, Sill MW, Powell DJ Jr., Lankes HA, Feldman MD, et al. Randomized Phase II Trial of Nivolumab Versus Nivolumab and Ipilimumab for Recurrent or Persistent Ovarian Cancer: An NRG Oncology Study. J Clin Oncol (2020). 38:1814–23. doi: 10.1200/JCO.19.02059
128. Zandberg DP, Algazi AP, Jimeno A, Good JS, Fayette J, Bouganim N, et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: Results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer (2019) 107:142–52. doi: 10.1016/j.ejca.2018.11.015
129. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with previously treated advanced hepatocellular carcinoma: Phase 2 KEYNOTE-224 study. Ann Oncol (2016) 27:vi242. doi: 10.1016/S1470-2045(18)30351-6
130. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Nivolumab (NIVO) plus ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040. J Clin Oncol (2019) 37(15_suppl):4012–2. doi: 10.1200/JCO.2019.37.15_suppl.4012
131. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol (2019) 30(Supple_5):V874–5. doi: 10.1093/annonc/mdz394.029
132. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (2017) 389:2492–502. doi: 10.1016/S0140-6736(17)31046-2
133. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol (2019) 16:563–80. doi: 10.1038/s41571-019-0218-0
134. Shah N, Puthiamadathil J, Serzan MT, Belouali A, Kelly WJ, Ma B, et al. 1222P - Clinical outcome of immune related hepatitis (IrHep) in patients with advanced melanoma (AM) treated with single agent or combination immune checkpoint inhibitors (ICIs). Ann Oncol (2018) 29:viii433–4. doi: 10.1093/annonc/mdy288.093
135. Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol (2019) 5:1008–19. doi: 10.1001/jamaoncol.2019.0393
136. Da L, Teng Y, Wang N, Zaguirre K, Liu Y, Qi Y, et al. Organ-Specific Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitor Monotherapy Versus Combination Therapy in Cancer: A Meta-Analysis of Randomized Controlled Trials. Front Pharmacol (2019) 10:1671. doi: 10.3389/fphar.2019.01671
137. Weinmann SC, Pisetsky DS. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology (Oxford) (2019) 58:vii59–67. doi: 10.1093/rheumatology/kez308
138. Rosskopf S, Jahn-Schmid B, Schmetterer KG, Zlabinger GJ, Steinberger P. PD-1 has a unique capacity to inhibit allergen-specific human CD4+ T cell responses. Sci Rep (2018) 8:13543. doi: 10.1038/s41598-018-31757-z
139. Brown JA, Dorfman DM, Ma F-R, Sullivan EL, Munoz O, Wood CR, et al. Blockade of Programmed Death-1 Ligands on Dendritic Cells Enhances T Cell Activation and Cytokine Production. J Immunol (2003) 170:1257–66. doi: 10.4049/jimmunol.170.3.1257
Keywords: immune checkpoint inhibitor, hepatotoxicity, primary liver cancer, PD-1, immune-related hepatitis
Citation: Fu J, Li W-Z, McGrath NA, Lai CW, Brar G, Xiang Y-Q and Xie C (2021) Immune Checkpoint Inhibitor Associated Hepatotoxicity in Primary Liver Cancer Versus Other Cancers: A Systematic Review and Meta‐Analysis. Front. Oncol. 11:650292. doi: 10.3389/fonc.2021.650292
Received: 06 January 2021; Accepted: 29 March 2021;
Published: 21 April 2021.
Edited by:
Bastian Schilling, University Hospital Würzburg, GermanyReviewed by:
Othmar Schoeb, Clinic Hirslanden Zurich, SwitzerlandJifeng Zhang, University of Michigan, United States
Copyright © 2021 Fu, Li, McGrath, Lai, Brar, Xiang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changqing Xie, Y2hhbmdxaW5nLnhpZUBuaWguZ292
†These authors have contributed equally to this work
 Jianyang Fu
Jianyang Fu Wang-Zhong Li
Wang-Zhong Li Nicole A. McGrath
Nicole A. McGrath Chunwei Walter Lai
Chunwei Walter Lai Gagandeep Brar
Gagandeep Brar Yan-Qun Xiang2
Yan-Qun Xiang2 Changqing Xie
Changqing Xie