
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 16 March 2021
Sec. Cancer Molecular Targets and Therapeutics
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.650098
This article is part of the Research TopicTumor-Immune Microenvironment and Strategies for Translational and Clinical Cancer Treatments in Peritoneal CarcinomatosisView all 4 articles
The presence of peritoneal metastases (PM) in patients with colorectal cancer (CRC) is associated with an extremely poor prognosis. The diagnosis of PM is challenging, resulting in an underestimation of their true incidence. While surgery can be curative in a small percentage of patients, effective treatment for non-operable PM is lacking, and clinical and pre-clinical studies are relatively sparse. Here we have defined the major clinical challenges in the areas of risk assessment, detection, and treatment. Recent developments in the field include the application of organoid technology, which has generated highly relevant pre-clinical PM models, the application of diffusion-weighted MRI, which has greatly improved PM detection, and the design of small clinical proof-of-concept studies, which allows the efficient testing of new treatment strategies. Together, these developments set the stage for starting to address the clinical challenges. To help structure these efforts, a translational research framework is presented, in which clinical trial design is based on the insight gained from direct tissue analyses and pre-clinical (organoid) models derived from CRC patients with PM. This feed-forward approach, in which a thorough understanding of the disease drives innovation in its clinical management, has the potential to improve outcome in the years to come.
Colorectal cancer (CRC) is one of the most common forms of cancer and the second leading cause of cancer-related mortality in the Western world. Death from CRC is virtually always the consequence of metastatic spread to distant sites in the body such as the liver, the peritoneal cavity, and the lungs. Patients with metastases in the peritoneal cavity (peritoneal metastases, PM) have the worst prognosis (1, 2).
In general, PM are under-diagnosed as their detection with routine imaging protocols is difficult, due to their small size and limited contrast resolution in soft tissues (3, 4). The true incidence of PM is therefore unclear, although in autopsy series it was reported to be as high as 40–80% (5, 6) (Table 1). The development of PM in CRC patients is often associated with a rapidly declining performance status, involving recurrent bowel obstruction, the formation of malignant ascites, visceral pain, and malnutrition (Table 1) (7). In most cases this precludes surgery and systemic therapy, leaving only palliative care to ensure the best possible quality of life. When left untreated, the median overall survival of this patient group is ~5 months (8). The benefit of systemic chemotherapy is dramatically reduced in the subgroup of CRC patients with PM (2, 9), and their poor visualization complicates assessment of their response to treatment. In the past decade, this has resulted in the active exclusion of patients with PM from clinical trials (10). Taken together, CRC with PM is a very common and highly aggressive, but under-diagnosed and under-studied disease entity.
In this report we provide an overview of the major challenges in the field of PM from CRC (Figure 1) and describe a research framework that addresses these challenges (Figure 2). The central premise is that innovative and more effective treatment concepts can only be designed on the basis of a thorough understanding of the disease.
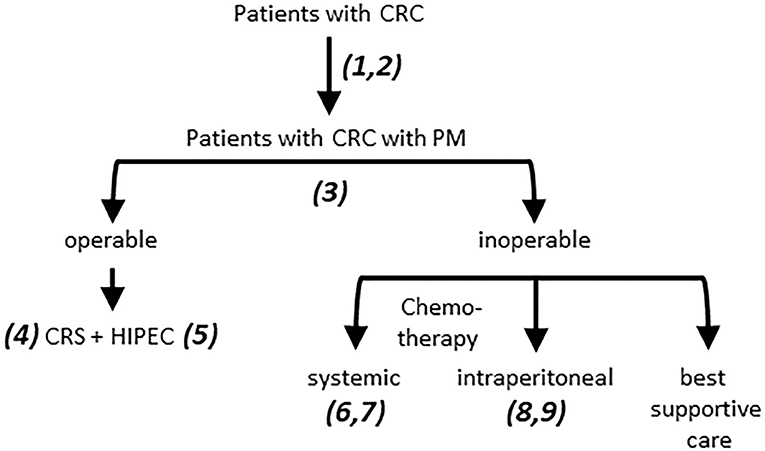
Figure 1. Challenges in the treatment of PM from CRC. 1. Patients with PM are underdiagnosed due to poor performance of routine imaging procedures. 2. Risk assessment of metachronous PM development is insufficient. This requires a comprehensive analysis of clinical parameters and (epi)genetic features of tumors and patients, informing the development of (composite) biomarkers. 3. 40–60% of the patients currently selected for CRS HIPEC experience rapid disease recurrence and are over-treated. The rate of under-treatment (patients who would have benefitted but were not selected) is unknown. Improved patient selection requires better imaging modalities for robust PCI assessment, combined with clinical and (epi-)genetic features, as in 2. 4. Incomplete resection leaves tumor residue which may initiate intra-abdominal recurrence. Novel intra-operative imaging strategies are needed to guide CRS. 5. The currently used monotherapies in HIPEC are unlikely to be effective. Novel more effective treatment strategies need to be developed. 6. Patients with PM benefit least from modern chemotherapy regimens. The factors determining PM resistance to systemic therapy need to be identified. 7. Some patients with PM do benefit from long-term chemotherapy. Biomarkers predicting such benefit are urgently needed. 8. The currently used monotherapies in i.p. chemotherapy are unlikely to be effective. Novel more effective treatment strategies need to be developed. 9. Response assessment to i.p. chemotherapy is challenging due to underperformance of CT. Improvement requires development of better imaging modalities. CRC, colorectal cancer; PM, Peritoneal metastases; CRS, cytoreductive surgery; HIPEC, hyperthermic (heated) intraperitoneal chemotherapy.
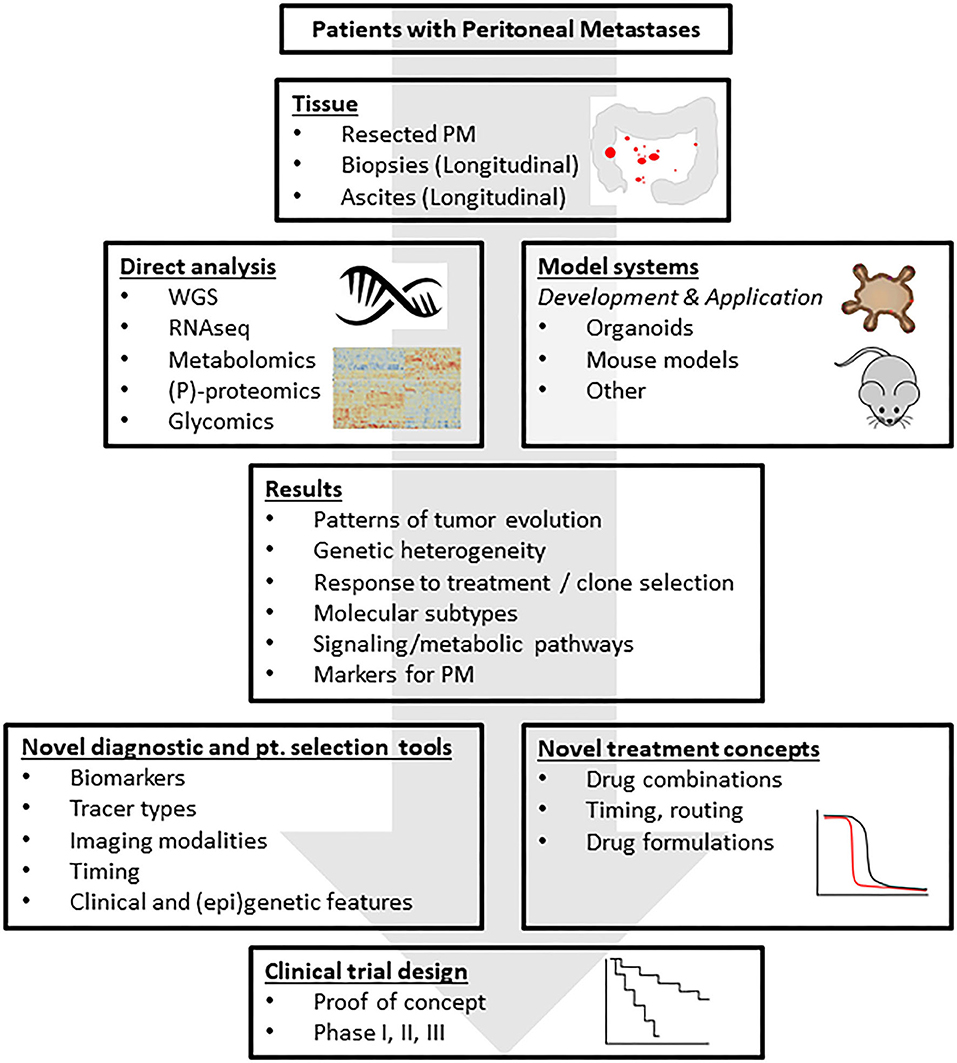
Figure 2. A research framework addressing the challenges. The standardized collection of tissue and body fluids derived from patients with peritoneal metastases from CRC generates biobanks of frozen and fixed tissues for downstream molecular analysis. Moreover, organoid technology allows the co-establishment of “living biobanks” in which individual cancer patients are represented by their tumor-derived organoids. These organoids may be used in transplantation studies generating spontaneous metastasis models in mice. The molecular tissue analyses will generate novel leads for the detection and treatment of PM, for understanding resistance mechanisms, tumor cell plasticity and intra- and inter-lesion (epi-)genetic heterogeneity. Insight into the biology of PM will lead to the formulation of novel treatment concepts that can subsequently be tested in the generated novel PM model systems. These efforts should yield a series of pre-clinically validated novel treatment strategies, possibly limited to specific, identifiable patient subgroups. These strategies can then be tested in small proof-of-concept studies to generate biological proof for the validity of the treatment concept in cancer patients, and subsequently in regular phase 1–3 clinical trials. Analysis of the tissues from such trials may subsequently identify potential resistance mechanisms and help design pre-clinical studies that are aimed at further improving the strategy. This translational feed-forward approach has the potential to impact clinical outcome in the years to come. PM, peritoneal metastases; WGS, whole genome sequencing; RNAseq, RNA sequencing; (P)-proteomics, (phospho-)proteomics.
Several approaches are used to diagnose and quantify PM from CRC (Table 2). The peritoneal cancer index (PCI; a semi-quantitative measure of intraperitoneal tumor load) is the golden standard for assessing the extent of PM, and an important tool for selecting patients for potentially curative cytoreductive surgery (CRS; see below). Exploratory laparoscopy is performed prior to CRS in case extensive PM is suspected, but this procedure leaves important intra- and extra-peritoneal regions unexamined (11). Pre-operative non-invasive imaging methods for determination of the PCI in patients with (suspected) PM are therefore urgently needed (Figure 1). Typically, the size of the individual peritoneal tumor nodules is below the detection level of conventional CT or PET. Indeed, with the current selection strategies CRS is discontinued in up to 40% of the procedures due to a high PCI score (12, 13). More recently it was shown that diffusion-weighted MR imaging (DW-MRI) outperforms other imaging techniques with regards to sensitivity and specificity (14–16). DW-MRI also detects extra-peritoneal disease such as occult liver metastases with high sensitivity. Finally, a recent study employed molecular imaging using Fibroblast Activation Protein (FAP) inhibitor (FAPI) as a PET tracer for the detection of PM and demonstrated superiority over standard FDG-PET (17). The basis for FAP-based detection of PM is presumably related to the generally high amount of FAP-positive cancer-associated fibroblasts (CAFs) in these lesions.
Prospective studies should now be designed to assess whether DW-MRI or FAPI-PET are indeed superior modalities for the diagnosis and quantification of PM and for selecting patients for CRS.
PM can be detected at first diagnosis of CRC (synchronous PM), but they can also develop in the months or years after primary tumor resection (recurrent/metachronous PM) (18, 19). Risk factors for developing recurrent PM are the presence of synchronous PM, age (<60 years), a T4 primary tumor, location of the primary tumor in the proximal colon, the presence of activating mutations in BRAF, a mucinous or signet ring cell histology, and a CMS4 molecular subtype (1, 5, 20–24). Some of these features have been used to select patients for second-look surgery (22, 23, 25–28), but validated approaches to select patients at risk for an alternative more aggressive treatment strategy are currently not available.
Novel biomarkers predicting PM development with high accuracy are urgently needed (Figure 1). These could be based on the genetic or biological trait(s) of the resected primary tumor. In addition, genetic and non-genetic variation in the patient population is also likely to determine the risk of developing PM, independently of tumor-intrinsic variables (29). Alternatively, the ultra-sensitive detection of tumor-derived biological material (e.g., cell-free tumor DNA, RNA, or protein) that is shed by microscopic tumor deposits could also serve as biomarkers predicting recurrence. For instance, the detection of circulating tumor DNA (ctDNA) in plasma identifies stage II and stage III patients at high risk of developing (any) distant metastases (30–33). However, plasma ctDNA levels are extremely low in patients with PM. Rather, PM-derived ctDNA can readily be measured in peritoneal fluid, offering an alternative source of biomarkers reporting on the potential presence of micrometastases in the peritoneum (34). Indeed, the presence of cancer-specific molecular biomarkers in peritoneal fluid predicts PM formation in gastric and pancreas cancer (35, 36).
An overview of the currently available treatment modalities for operable and inoperable PM is shown in Table 3.
The development of biomarkers predicting PM formation after primary tumor resection calls for the design of effective adjuvant treatment strategies. Currently, the options for adjuvant therapy are limited. Adjuvant systemic chemotherapy for patients with high-risk stage II and stage III tumors reduces the risk of recurrence by only ~15%. Patients with PM are even less likely to benefit from systemic treatment, possibly due to low penetration of systemically delivered chemotherapy in the peritoneal cavity (2, 9). Therefore, the application of adjuvant intra-peritoneal chemotherapy following primary tumor resection is an attractive alternative local approach, aiming to kill any microscopic disease inside the peritoneum (27, 28). Although this adjuvant strategy recently failed to show benefit using heated oxaliplatin for 30 min (27, 28) it should be re-considered once effective intra-peritoneal chemotherapeutic regimens are identified. Such regimens are currently lacking (Figure 1). Novel PM culture systems based on organoid technology have recently become available and are now being used to design and test novel effective treatments (37, 38). Importantly, organoid technology represents the only available platform for predicting therapy response in individual patients with GI cancers (39–43).
Once PM are diagnosed, the only treatment that results in long-term survival involves radical surgery to remove all visible disease (CRS). The addition of heated intra-peritoneal chemotherapy (HIPEC), may further improve survival (44, 45). This procedure was first introduced by Paul Sugarbaker for the treatment of Pseudomyxoma Peritonei (PMP) (46) and has since been widely adapted to treat PM from multiple cancer types. However, the added value of HIPEC over CRS alone for the treatment of colorectal PM has recently been questioned in the PRODIGE7 trial (47). CRS with or without HIPEC is a high-cost procedure that is associated with considerable morbidity and mortality rates (48, 49). Therefore, careful patient selection is essential. Prognostic factors that are associated with a poor outcome following CRS include (i) presentation with an obstructed or perforated primary tumor, (ii) high PCI, and (iii) completeness of CRS. The currently used in- and exclusion criteria for surgical treatment are solely based on PCI, judgement of resectability, and the presence of distant metastases outside the peritoneum. Despite these selection criteria, approximately half of the patients experience rapid disease progression within the first year after CRS-HIPEC, which further increases to 70% after 2 years (50). Eventually, recurrence after CRS-HIPEC results in 2- and 10-year overall survival rates of ~60% and ~20% respectively (50–53).
The currently used selection criteria carry no biological information, including for instance, genetic or histological subtypes, or intra-abdominal location. Such variables may have a profound impact on tumor behavior and recurrence rates. A major challenge therefore is to identify tumor- or patient-centric biological and/or genetic variables that are associated with recurrence following CRS-HIPEC (Figure 1).
Accurate prediction of benefit from CRS will require the design of composite biomarkers in which a robust (imaging-based) PCI scoring system is combined with the relevant clinical and biological/genetic parameters. Such biomarkers may then be used to create decision tools balancing clinical benefit and morbidity in order to improve the selection of patients for surgical treatment.
The success of surgical treatment is mostly determined by the completeness of resection. A more effective approach to radically remove intraperitoneal micro-metastases would be to identify them using intra-operative imaging, which has the potential to guide surgery (54–57). Clinical feasibility of that process has now been demonstrated (56, 57). Encouragingly, systemic administration of a fluorescent anti-CEA monoclonal antibody was safe and allowed subsequent intra-operative detection of PM. Importantly, this technique not only identified superficially growing metastases, but also more deeply situated metastases that would otherwise have remained undetected, resulting in a change of the surgical strategy in about one third of the cases (56). This study demonstrates proof-of-concept that intra-operative imaging has the potential to improve the completeness of resection. Harnessing this technology for improving clinical outcome will require a further identification of relevant targets expressed on PM from CRC, and optimization of tracer backbones, imaging modalities, and routes of administration (Figures 1, 2). An important question is whether microscopic intraperitoneal target lesions have sufficient functional blood vessels to allow their detection by systemically administered tracers.
The combination of CRS and HIPEC in patients with PM from CRC can result in 5-year survival rates of over 40% (50, 51, 53). A major question is whether this is due to CRS alone, or whether HIPEC has additional value. A clear benefit of the HIPEC procedure following CRS has recently been demonstrated in patients with PM from ovarian cancer (58). However, the PRODIGE7 trial failed to prove an additional value of HIPEC in patients treated for PM from CRC (47). However a post-hoc subgroups analysis showed that HIPEC improved relapse-free and overall survival specifically in a subgroup with intermediate PCI (11–15), suggesting that future optimization of the procedure should focus on this subgroup with the potential for (near-) complete surgical PM resection. In general, one has to realize that the PRODIGE7 trial was performed using a high-dosed, short duration (30 min) oxaliplatin-based HIPEC-regimen in patients that were selected after 6 months of systemic treatment with oxaliplatin-containing regimens. The additional value of other HIPEC-regimens (including those with rationally selected drug combinations) in colorectal cancer patients has not been addressed in randomized trials yet.
Pre-clinical studies with PM-derived organoids have indicated that heated Oxalipatin or MMC monotherapies do not achieve their eradication (38). Despite their highly heterogeneous presentation, PM from CRC are mostly of the CMS4 molecular subtype (20). Importantly, CMS4 CRC do not benefit from the addition of oxaliplatin to adjuvant therapy (59, 60). Thus, while the concept of HIPEC after CRS appears to be valid and can result in a significant survival benefit in ovarian cancer (58), the currently used drugs in HIPEC for PM from CRC are unlikely to be effective as monotherapies.
Optimization of the HIPEC regimen is therefore a major challenge (Figure 1). Several approaches could be considered. First, drug combinations that are capable of killing CMS4-type micro-metastases in a very short period of time (<90 min) need to be identified. In addition, the added value of heat should be assessed and, in the case of mucinous tumors, a mucus (pre-) clearing approach could be considered. The feasibility and safety of intra-abdominal mucin dissolution by combined administration of bromelain and acetylcysteine in cancer patients with mucinous PM was recently demonstrated (61). Finally, alternative drug formulations that prolong intra-peritoneal drug exposure times (e.g., using nanoparticles, hydrogels or albumin) forms an attractive innovative approach to improve local drug efficacy (62, 63).
While systemic chemotherapy has prolonged the survival of patients with inoperable PM, this benefit is markedly higher for patients with non-PM distant metastases (2, 9). The added benefit of standard systemic peri-operative chemotherapy over CRS-HIPEC alone is currently being investigated in a large phase 3 trial using MMC-based HIPEC (CAIRO6) (64).
A third approach to target microscopic tumor residue is to improve (neo-) adjuvant treatment regimens with chemo- and/or targeted therapy. Key questions relating to this approach are (i) Do intra-peritoneal micro-metastases have sufficient functional blood vessels to allow systemically administered drugs to reach them and sort a beneficial effect?, and (ii) Does the intra-peritoneal location of PM generate specific therapeutically exploitable vulnerabilities?
Systemic therapy is widely used to treat metastatic CRC. The most effective regimens have prolonged median overall survival to more than 2 years (65). However, the benefit of systemic therapy (including regimens with novel targeted agents) is dramatically reduced in patients with PM involvement, both in terms of response and survival (2). It is currently unknown which factor(s) cause the site-dependent differential response to systemic therapy. Such factors may include site-specific differences in drug delivery and tumor penetration, tumor biology, intrinsic resistance [e.g., caused by CMS4 status (20) or mutant BRAF (1)], and drug metabolism. Insight into the factors that cause the relative resistance of PM to current systemic therapies is central to the design of more effective systemic treatment regimens (Figures 1,2).
The enclosed nature of the peritoneal space offers the unique but underexplored possibility to design and test novel intra-peritoneal treatment strategies. The limited systemic uptake of intraperitoneally delivered drugs further allows the use of much higher drug concentrations than could ever be administered systemically. Furthermore, PM are directly exposed to the drugs, which could help target even poorly vascularized tumors.
Pressurized intraperitoneal aerosolized chemotherapy (PIPAC) is used to achieve a homogenous spread of chemotherapy throughout the peritoneal cavity. The procedure is safe and allows repeated cycles of treatment (66). Thus far, PIPAC has been based on oxaliplatin monotherapy. However, oxaliplatin monotherapy has never been demonstrated to be effective in the systemic treatment of distant metastases. Moreover, as outlined above, both experimental and clinical evidence indicate that it is unlikely that oxaliplatin monotherapy will be an effective treatment against PM from CRC given the intrinsic resistance that is associated with their CMS4 status (38). In order to fully exploit the potential benefits of the procedure more effective drug combinations need to be identified and tested. Small proof-of-concept PIPAC studies involving tens of patients are ideally suited to test such novel treatment combinations–e.g., identified on the PM organoid platform–in a relatively short period of time.
An alternative procedure involves repeated infusion of drugs into the abdominal cavity via a peritoneal access port. In the INTERACT study this approach is investigated using irinotecan as monotherapy (67). Irinotecan monotherapy has demonstrated value in the systemic treatment of distant metastases (68). However, differences in the mode of administration, the timing of treatment, and biological differences between PM and metastases at other sites may all have an impact on how metastases respond to irinotecan monotherapy.
The PIPAC and INTERACT designs and the enclosed nature of the peritoneal cavity offer the exciting possibility to rapidly test existing and novel drugs, alone and in combination, for their anti-PM activity in relatively small patient groups. Realistically, it must be possible to identify local PM-targeting regimens that are more effective than the currently used monotherapies (next section, Figure 2).
The evolutionary history of metastases is a topic of intense research in many types of cancer (29, 69, 70). Models of tumor evolution describe the processes that lead to the generation of metastasis-competent clones in primary tumors, the timing of their dissemination, and the factors determining successful outgrowth at distant sites. Primary tumors that have breached the colon wall have immediate access to the peritoneal cavity. Therefore, PM formation may not require intra- and extravasation steps. Furthermore, the PM-seeding entities are clusters of tumor cells that bud off from the primary tumor (71). Cancer-associated fibroblasts within the peritoneal micro-environment may play an important role in the process of peritoneal seeding of cancer cells (72). For a detailed description of the role of the microenvironment in PM formation, we refer the reader to an excellent recent review on this topic (73). Apparently, a single cell stage is also not required during this mode of dissemination. Interestingly, tumors that have not completely breached the colon wall (i.e., T1-T3) frequently also give rise to PM (74). This may be due to sampling bias that is intrinsic to histology-based TNM staging. Alternatively, cancer cells may exfoliate from the primary tumor during surgery and subsequently seed the peritoneum. Nevertheless, it is also conceivable that alternative biological routes to PM formation exist that are currently unknown. Determination of the evolutionary relationships between multiple primary tumor regions, PM and metastases at other sites will provide insight into this matter.
Pseudomyxoma Peritonei (PMP) represents a unique form of PM with unique clinical challenges and underlying biology. We refer the reader to a focused recent review on PMP discussing the biology underlying PM formation and the consequences for treatment (75).
The micro-environments in distant organs are highly distinct. Therefore, the phenotypic and genotypic traits that are selected for during metastasis formation are likely to be site-dependent. However, whole genome sequencing has demonstrated that the driver mutations in distant non-PM metastases are highly homogeneous within individual patients, presumably because they were all derived from a specific sub-clone within the primary tumor (76–78). Despite these overall similarities, site-specific genetic differences affecting distinct biological processes exist between CRC liver and brain metastases, illustrating the principle of site-specific adaptation (79, 80). Very little is known about the evolutionary processes (mutations, copy number alterations, epigenetic modifications, etc.) that specifically shape the PM-competent tumor genome. Nevertheless, specific histological subtypes–in particular mucinous adenocarcinoma and signet ring cell carcinoma–show a remarkable preference for metastasizing to the peritoneum. In addition, tumors with activating mutations in BRAF, and those of the CMS4 molecular subtype are also prone to form PM. Understanding why these specific histological and genetic tumor subtypes are associated with PM formation will provide leads to the design of novel therapies and diagnostic tools.
Even within the peritoneum the requirements for successful site-specific adaptation may be different depending on the site within the peritoneum, such as omental fat, diaphragm, and the surface of intra-abdominal organs. Processes governing site-specific adaptation (through whatever evolutionary mechanism) and (epi)genetic diversity within and between PM are likely to be highly relevant for the potential success of intraperitoneal therapies. Metabolic adaptation of PM to the fatty acid-rich microenvironment of the abdomen may create a targetable, generic, PM-specific vulnerability (81).
Finally, while the vast majority of studies on metastasis formation focus on tumor-centric parameters, the process is also likely to be influenced by genetic and epigenetic variation in the human population (29, 82). Therefore, a full understanding of the biology of PM will require (epi-)genetic analyses of both the tumor and the patient.
An in-depth insight into the biology of PM will greatly help in meeting the clinical challenges listed in Figure 1. We envision a research framework that is built on the acquisition of tumor tissue, ascites, blood, and normal tissues of patients with PM from CRC (Figure 2). Whenever and wherever possible tissues and ascites should be collected in a longitudinal fashion, allowing the in-patient analysis of tumor progression and response to treatment over time. Direct cross-omics analysis of multiple lesions and paired primary tumor samples (derived, for instance, from CRS procedures) will give unprecedented insight into many aspects of PM biology, including their evolutionary history, the genetic and epigenetic intra- and inter-tumor heterogeneity, the association with specific (epi-) genetic features, expression of PM-specific (cell surface) markers, activation of specific signaling and metabolic pathways, molecular subtypes, etc. These data will form a solid basis for designing effective PM-specific detection and treatment strategies. For example, the finding that PM are largely of the CMS4 molecular subtype and frequently carry activating mutations in the BRAF oncogene have already provided two new leads for developing and testing alternative therapeutic approaches. For instance, TGFb is a central player in aggressive CRC (83) and orchestrates tumor-associated fibrosis and fibroblast-cancer cell crosstalk, which is a hallmark of CMS4 CRC (83–85). Multiple TGFb-targeting agents are under development for the treatment of CRC (trial numbers: NCT03470350; NCT03436563) and may be especially effective in targeting CMS4 CRC, including PM.
Pre-clinical evaluation of the potential value of any new treatment strategy requires the availability of clinically relevant model systems. The pre-clinical model systems that have been used to date are based on traditional CRC cell lines in vitro or injected into the peritoneal cavity of mice or rats. Unfortunately, this approach does not take into account that PM are a highly specific disease entity with features that are not necessarily recapitulated in traditional cell line models. Moreover, the culturing technique that is used to establish traditional cell lines causes profound genomic rearrangements, resulting in the outgrowth of highly selected sub-clones poorly resembling the original tumors. Therefore, the validity of these models in studying PM biology is questionable.
As an alternative, we propose that the tissues and ascites samples that are derived from patients with PM can be used to create representative PM model systems, particularly based on organoid technology (37, 38). Organoid technology is now widely considered to be the most relevant culturing method for many types of cancer and normal tissues as it preserves the genetic and phenotypic characteristics of the original (tumor) tissue and allows limitless expansion of the tissue in culture (39). Organoid cultures capture (at least to some extent) the functional and genetic heterogeneity that is characteristic of CRC (86, 87). Furthermore, organoids can also be used to generate spontaneous metastasis models by making use of microsurgical transplantation techniques (88). Organoid technology has already proven to be of clinical relevance in primary and metastatic CRC as the treatment responses observed in organoids closely resemble those observed in cancer patients, in multiple independent studies (40–43). However, PM have so far not been included in these studies. We and others have recently shown that organoid technology can be used to create novel PM models and that these models have the potential to improve the treatment of PM either through a rational approach of drug selection and testing or, via unbiased drug screens (37, 38). Indeed, drug screens on PM-derived organoids have the potential to identify alternative personalized treatment options for patients in whom standard of care treatment failed (37).
Ideally, all histological, molecular and genetic subtypes are represented in a PM organoid biobank. Organoid generation from all PM subtypes and from all-intra-peritoneal locations may require further optimization of culturing conditions. The results obtained from direct analysis of the PM tumor tissue samples may inform such adaptation of culture conditions. For instance, specific niche factors and/or stromal cell populations (e.g., adipocytes, fibroblasts, mesothelial cells, factors present in ascites, etc.) may be tested for their influence on organoid growth and behavior and response to therapy. Ultimately, the value of PM-derived organoid models in predicting therapy response should be tested in clinical trials, similar to those performed in patients with non-PM primary and metastatic CRC (40–43). Clearly, this will also require optimization of the detection of intraperitoneal drug responses. DW-MRI, or molecular imaging based on expression of FAP, may have sufficient specificity and selectivity to design such trials (14, 15). In addition, PM-specific markers or metabolic pathways may be identified based on PM tissue analysis. These could serve as targets for developing alternative methods to detect and treat PM.
Organoid-based models (in vitro and in animals) are also ideally suited to study novel drug combinations, guided by the results from direct PM analysis (see above). The efficacy of any drug on any cell type is directly correlated to the time of exposure. Drug exposure times during the currently used HIPEC procedures is very short (30–90 min). Novel nanoparticle-, hydrogel- or albumin-based drug formulations may be used to improve local treatment efficacy simply by prolonging the time of drug exposure following a similarly short procedure (62). In particular, hydrogels with self-healing properties hold great promise in this area and should be tested in in vitro and in vivo pre-clinical models using PM-derived organoids.
Another issue that needs resolving is the added value of heat. From the first clinical trials exploring the benefit of HIPEC, heat has always been an integral part of the treatment strategy (51, 52, 89) but the added value of heat over drug treatment alone has never been assessed. Of note, heat may compromise drug activity and this should be evaluated for any candidate drug to be included in the HIPEC procedure. The PM-specific model systems can now be used to directly address this question without having to design clinical studies. The natural cellular defense response against heat involves activation of the ATR DNA damage checkpoint pathway (90), for which many drug are available. Indeed, ATR inhibition showed great synergy in killing PM-derived organoids in combination with the standard-of-care drug MMC at 42°C (38). To maximize the anti-tumor effects of heat application during HIPEC this concept–and others–must now be further tested on the organoid platform, and in pre-clinical animal models with organoid-initiated PM.
We foresee that the recent developments in the field that are described here, in particular the application of organoid technology, the use of DW-MRI and molecular imaging for PM detection, the further improvement of intra-peritoneal drug delivery, and the design of novel clinical proof-of-concept studies, will drive innovation in the clinical management of PM from CRC. An extensive integration of research and care will continue to be required to gain in-depth insight into the biology of PM, and this will form the basis for designing and testing better PM detection and treatment strategies. We hope that the research framework described here will help structure the efforts toward reaching that goal.
All authors contributed to the conceptualization and writing of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT, et al. Research in cancers of the digestive system: prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the analysis and research in cancers of the digestive system (ARCAD) database. Lancet Oncol. (2016) 17:1709–19. doi: 10.1016/S144444470-2045(16)30500-9
2. Franko J. Therapeutic efficacy of systemic therapy for colorectal peritoneal carcinomatosis: surgeon's perspective. Pleura Peritoneum. (2018) 3:20180102. doi: 10.1515/pp-2018-0102
3. Koh JL, Yan TD, Glenn D, Morris DL. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol. (2009) 16:327–33. doi: 10.1245/s10434-008-0234-2
4. Rivard JD, Temple WJ, McConnell YJ, Sultan H, Mack LA. Preoperative computed tomography does not predict resectability in peritoneal carcinomatosis. Am J Surg. (2014) 207:760–4. doi: 10.1016/j.amjsurg.2013.12.024
5. Hugen N, van de Velde CJ, de Wilt JH, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. (2014) 25:651–7. doi: 10.1093/annonc/mdt591
6. Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. (2006) 243:212–22. doi: 10.1097/01.sla.0000197702.46394.16
7. Franke J, Iqbal A, Starr JS, Nair RM, George TJ Jr. Management of malignant bowel obstruction associated with GI cancers. J Oncol Pract. (2017) 13:426–34. doi: 10.1200/JOP.2017.022210
8. Sadeghi Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. (2000) 88:358–63. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o
9. Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. (2012) 30:263–7. doi: 10.1200/JCO.2011.37.1039
10. Tseng J, Bryan DS, Poli E, Sharma M, Polite BN, Turaga KK. Under-representation of peritoneal metastases in published clinical trials of metastatic colorectal cancer. Lancet Oncol. (2017) 18:711–2. doi: 10.1016/S1470-2045(17)30336-4
11. Jayakrishnan TT, Zacharias AJ, Sharma A, Pappas SG, Gamblin TC, Turaga KK. Role of laparoscopy in patients with peritoneal metastases considered for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). World J Surg Oncol. (2014) 12:270. doi: 10.1186/1477-7819-12-270
12. van Oudheusden TR, Braam HJ, Luyer MD, Wiezer MJ, van Ramshorst B, Nienhuijs SW, et al. Peritoneal cancer patients not suitable for cytoreductive surgery and HIPEC during explorative surgery: risk factors, treatment options, and prognosis. Ann Surg Oncol. (2015) 22:1236–42. doi: 10.1245/s10434-014-4148-x
13. Goere D, Souadka A, Faron M, Cloutier AS, Viana B, Honore C, et al. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol. (2015) 22:2958–64. doi: 10.1245/s10434-015-4387-5
14. Dresen RC, De Vuysere S, De Keyzer F, Van Cutsem E, Prenen H, Vanslembrouck R, et al. Whole-body diffusion-weighted MRI for operability assessment in patients with colorectal cancer and peritoneal metastases. Cancer Imaging. (2019) 19:1. doi: 10.1186/s40644-018-0187-z
15. van 't Sant I, van Eden WJ, Engbersen MP, Kok NFM, Woensdregt K, Lambregts DMJ, et al. Diffusion-weighted MRI assessment of the peritoneal cancer index before cytoreductive surgery. Br J Surg. (2019) 106:491–8. doi: 10.1002/bjs.10989
16. van 't Sant I, Engbersen MP, Bhairosing PA, Lambregts DMJ, Beets-Tan RGH, van Driel WJ, et al. Diagnostic performance of imaging for the detection of peritoneal metastases: a meta-analysis. Eur Radiol. (2020) 30:3101–12. doi: 10.1007/s00330-019-06524-x
17. Zhao L, Pang Y, Luo Z, Fu K, Yang T, Zhao L, et al. Role of [(68)Ga]Ga-DOTA-FAPI-04 PET/CT in the evaluation of peritoneal carcinomatosis and comparison with [(18)F]-FDG PET/CT. Eur J Nucl Med Mol Imaging. (2021). doi: 10.1007/s00259-020-05146-6
18. van Gestel YR, de Hingh IH, van Herk-Sukel MP, van Erning FN, Beerepoot LV, Wijsman JH, et al. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol. (2014) 38:448–54. doi: 10.1016/j.canep.2014.04.004
19. van Gestel YR, Thomassen I, Lemmens VE, Pruijt JF, van Herk-Sukel MP, Rutten HJ, et al. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur J Surg Oncol. (2014) 40:963–9. doi: 10.1016/j.ejso.2013.10.001
20. Ubink I, van Eden WJ, Snaebjornsson P, Kok NFM, van Kuik J, van Grevenstein WMU, et al. Histopathological and molecular classification of colorectal cancer and corresponding peritoneal metastases. Br J Surg. (2018) 105:e204–11. doi: 10.1002/bjs.10788
21. Honore C, Gelli M, Francoual J, Benhaim L, Elias DD. Goere: ninety percent of the adverse outcomes occur in 10% of patients: can we identify the populations at high risk of developing peritoneal metastases after curative surgery for colorectal cancer? Int J Hyperthermia. (2017) 33:505–10. doi: 10.1080/02656736.2017.1306119
22. Segelman Akre O, Gustafsson UO, Bottai M, Martling A. Individualized prediction of risk of metachronous peritoneal carcinomatosis from colorectal cancer. Colorectal Dis. (2014) 16:359–67. doi: 10.1111/codi.12552
23. Segelman Akre O, Gustafsson UO, Bottai M, Martling A. External validation of models predicting the individual risk of metachronous peritoneal carcinomatosis from colon and rectal cancer. Colorectal Dis. (2016) 18:378–85. doi: 10.1111/codi.13219
24. Lurvink RJ, Bakkers C, Rijken A, van Erning FN, Nienhuijs SW, Burger JW, et al. Increase in the incidence of synchronous and metachronous peritoneal metastases in patients with colorectal cancer: a nationwide study. Eur J Surg Oncol. (2020). S0748-7983:31031–3. doi: 10.1016/j.ejso.2020.11.135
25. Sugarbaker PH. Second-look surgery for colorectal cancer: revised selection factors and new treatment options for greater success. Int J Surg Oncol. (2011) 2011:915078. doi: 10.1155/2011/915078
26. Elias D, Honore C, Dumont F, Ducreux M, Boige V, Malka D, et al. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg. (2011) 254:289–93. doi: 10.1097/SLA.0b013e31822638f6
27. Klaver CEL, Wisselink DD, Punt CJA, Snaebjornsson P, Crezee J, Aalbers AGJ, et al. c. group: adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol. (2019) 4:761–70. doi: 10.1016/S2468-1253(19)30239-0
28. Goere D, Glehen O, Quenet F, Ducreux M, Guilloit J-M, Texier M, et al. Results of a randomized phase 3 study evaluating the potential benefit of a second-look surgery plus HIPEC in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP- NTC01226394). J Clin Oncol. (2018) 36:3531. doi: 10.1200/JCO.2018.36.15_suppl.3531
29. Hunter W, Amin R, Deasy S, Ha NH, Wakefield L. Genetic insights into the morass of metastatic heterogeneity. Nat Rev Cancer. (2018) 18:211–23. doi: 10.1038/nrc.2017.126
30. Wang Y, Li L, Cohen JD, Kinde I, Ptak J, Popoli M, et al. Prognostic potential of circulating tumor DNA measurement in postoperative surveillance of nonmetastatic colorectal cancer. JAMA Oncol. (2019) 95:1118–23. doi: 10.1001/jamaoncol.2019.0512
31. Tie Wang Y, Tomasetti C, Li L, Springer S, Kinde I, Silliman N, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. (2016) 8:346ra92. doi: 10.1126/scitranslmed.aaf6219
32. Tie J, Cohen JD, Wang Y, Christie M, Simons K, Lee M, et al. Circulating tumor DNA analyses as markers of recurrence risk and benefit of adjuvant therapy for stage III colon cancer. JAMA Oncol. (2019) 5:1710–7. doi: 10.1001/jamaoncol.2019.3616
33. Tie J, Cohen JD, Wang Y, Li L, Christie M, Simons K, et al. Serial circulating tumour DNA analysis during multimodality treatment of locally advanced rectal cancer: a prospective biomarker study. Gut. (2019) 68:663–71. doi: 10.1136/gutjnl-2017-315852
34. van t Erve I. Detection of tumor-derived cell-free DNA from colorectal cancer peritoneal metastases in plasma and peritoneal fluid. J Pathol. in press (2021)
35. Kelly J, Wong J, Gladdy R, Moore-Dalal K, Woo Y, Gonen M, et al. Prognostic impact of RT-PCR-based detection of peritoneal micrometastases in patients with pancreatic cancer undergoing curative resection. Ann Surg Oncol. (2009) 16:3333–9. doi: 10.1245/s10434-009-0683-2
36. Yu L, Lv P, Han J, Zhu X, Hong LL, Zhu WY, et al. Methylated TIMP-3 DNA in body fluids is an independent prognostic factor for gastric cancer. Arch Pathol Lab Med. (2014) 138:1466–73. doi: 10.5858/arpa.2013-0285-OA
37. Narasimhan V, Wright JA, Churchill M, Wang T, Rosati R, Lannagan TR, et al. Medium-throughput drug screening of patient-derived organoids from colorectal peritoneal metastases to direct personalized therapy. Clin Cancer Res. (2020). 26:3662–70. doi: 10.1158/1078-0432.CCR-20-0073
38. Ubink Bolhaqueiro ACF, Elias SG, Raats DAE, Constantinides A, Peters NA, Wassenaar ECE, et al. Organoids from colorectal peritoneal metastases as a platform for improving hyperthermic intraperitoneal chemotherapy. Br J Surg. (2019) 106:1404–14. doi: 10.1002/bjs.11206
39. Lau HCH, Kranenburg O, Xiao H, Yu J. Organoid models of gastrointestinal cancers in basic and translational research. Nat Rev Gastroenterol Hepatol. (2020) 17:203–22. doi: 10.1038/s41575-019-0255-2
40. Ganesh Wu C, O'Rourke KP, Szeglin BC, Zheng Y, Sauve CG, Adileh M, et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat Med. (2019) 25:1607–14. doi: 10.1038/s41591-019-0584-2
41. Ooft SN, Weeber F, Dijkstra KK, McLean CM, Kaing S, van Werkhoven E, et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci Transl Med. (2019) 11:eaay2574. doi: 10.1126/scitranslmed.aay2574
42. Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernandez-Mateos J, Khan K, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. (2018) 359:920–6. doi: 10.1126/science.aao2774
43. Yao Y, Xu X, Yang L, Zhu J, Wan J, Shen L, et al. Patient-Derived Organoids Predict Chemoradiation Responses of Locally Advanced Rectal Cancer. Cell Stem Cell. (2020) 26:17–26e6. doi: 10.1016/j.stem.2019.10.010
44. Hall B, Padussis J, Foster JM. Cytoreduction and hyperthermic intraperitoneal chemotherapy in the management of colorectal peritoneal metastasis. Surg Clin North Am. (2017) 97:671–82. doi: 10.1016/j.suc.2017.01.013
45. Razenberg G, Lemmens VE, Verwaal VJ, Punt CJ, Tanis PJ, Creemers GJ, et al. Challenging the dogma of colorectal peritoneal metastases as an untreatable condition: results of a population-based study. Eur J Cancer. (2016) 65:113–20. doi: 10.1016/j.ejca.2016.07.002
46. Sugarbaker PH, Zhu BW, Sese GB, Shmookler B. Peritoneal carcinomatosis from appendiceal cancer: results in 69 patients treated by cytoreductive surgery and intraperitoneal chemotherapy. Dis Colon Rectum. (1993) 36:323–9. doi: 10.1007/BF02053933
47. Quenet F, Elias D, Roca L, Goere D, Ghouti L, Pocard M, et al. Group: cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:256–66. doi: 10.1016/S1470-2045(20)30599-4
48. Verwaal VJ, van Tinteren H, Ruth SV, Zoetmulder FA. Toxicity of cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. J Surg Oncol. (2004) 85:61–7. doi: 10.1002/jso.20013
49. Hallam S, Tyler R, Price M, Beggs A, Youssef H. Meta-analysis of prognostic factors for patients with colorectal peritoneal metastasis undergoing cytoreductive surgery and heated intraperitoneal chemotherapy. BJS Open. (2019) 3:585–94. doi: 10.1002/bjs5.50179
50. Kuijpers M, Mirck B, Aalbers AG, Nienhuijs SW, de Hingh IH, Wiezer MJ, et al. Cytoreduction and HIPEC in the Netherlands: nationwide long-term outcome following the Dutch protocol. Ann Surg Oncol. (2013) 20:4224–30. doi: 10.1245/s10434-013-3145-9
51. Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. (2008) 15:2426–32. doi: 10.1245/s10434-008-9966-2
52. Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. (2003) 21:3737–43. doi: 10.1200/JCO.2003.04.187
53. Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, et al. French surgical: toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. (2010) 116:5608–18. doi: 10.1002/cncr.25356
54. van Dam GM, Themelis G, Crane LM, Harlaar NJ, Pleijhuis RG, Kelder W, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. (2011) 17:1315–9. doi: 10.1038/nm.2472
55. Alam S, Steinberg I, Vermesh ON, van den Berg S, Rosenthal EL, van Dam GM, et al. Emerging Intraoperative Imaging Modalities to Improve Surgical Precision. Mol Imaging Biol. (2018) 20:705–15. doi: 10.1007/s11307-018-1227-6
56. Boogerd SF, Hoogstins CES, Schaap DP, Kusters M, Handgraaf HJM, van der Valk MJM, et al. Safety and effectiveness of SGM-101, a fluorescent antibody targeting carcinoembryonic antigen, for intraoperative detection of colorectal cancer: a dose-escalation pilot study. Lancet Gastroenterol Hepatol. (2018) 3:181–91. doi: 10.1016/S2468-1253(17)30395-3
57. Harlaar J, Koller M, de Jongh SJ, van Leeuwen BL, Hemmer PH, Kruijff S, et al. Molecular fluorescence-guided surgery of peritoneal carcinomatosis of colorectal origin: a single-centre feasibility study. Lancet Gastroenterol Hepatol. (2016) 1:283–90. doi: 10.1016/S2468-1253(16)30082-6
58. van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans R, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. (2018) 378:230–40. doi: 10.1056/NEJMoa1708618
59. Song Pogue-Geile KL, Gavin PG, Yothers G, Kim SR, Johnson NL, Lipchik C, et al. Clinical outcome from oxaliplatin treatment in stage II/III colon cancer according to intrinsic subtypes: secondary analysis of NSABP C-07/NRG oncology randomized clinical trial. JAMA Oncol. (2016) 2:1162–9. doi: 10.1001/jamaoncol.2016.2314
60. Trumpi Ubink I, Trinh A, Djafarihamedani M, Jongen JM, Govaert KM, Elias SG, et al. Neoadjuvant chemotherapy affects molecular classification of colorectal tumors. Oncogenesis. (2017) 6:e357. doi: 10.1038/oncsis.2017.48
61. Valle SJ, Akhter J, Mekkawy AH, Lodh S, Pillai K, Badar S, et al. A novel treatment of bromelain and acetylcysteine (BromAc) in patients with peritoneal mucinous tumours: a phase I first in man study. Eur J Surg Oncol. (2021) 47:115–22. doi: 10.1016/j.ejso.2019.10.033
62. Van Oudheusden TR, Grull H, Dankers PY, De Hingh IH. Targeting the peritoneum with novel drug delivery systems in peritoneal carcinomatosis: a review of the literature. Anticancer Res. (2015) 35:627–34.
63. Van de Sande Cosyns S, Willaert W, Ceelen W. Albumin-based cancer therapeutics for intraperitoneal drug delivery: a review. Drug Deliv. (2020) 27:40–53. doi: 10.1080/10717544.2019.1704945
64. Rovers P, Bakkers C, Simkens G, Burger JWA, Nienhuijs SW, Creemers GM, et al. Dutch Peritoneal Oncology and G. Dutch Colorectal Cancer: Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: protocol of a multicentre, open-label, parallel-group, phase II-III, randomised, superiority study (CAIRO6). BMC Cancer. (2019) 19:390. doi: 10.1186/s12885-019-5545-0
65. Punt CJ, Koopman M, Vermeulen L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat Rev Clin Oncol. (2017) 14:235–46. doi: 10.1038/nrclinonc.2016.171
66. Alyami Hubner M, Grass F, Bakrin N, Villeneuve L, Laplace N, Passot G, et al. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol. (2019) 20:e368–77. doi: 10.1016/S1470-2045(19)30318-3
67. de Boer L, Brandt-Kerkhof ARM, Madsen EVE, Diepeveen M, van Meerten E, van Eerden RAG, et al. Dutch Peritoneal Oncology and G. Dutch Colorectal Cancer: Concomitant intraperitoneal and systemic chemotherapy for extensive peritoneal metastases of colorectal origin: protocol of the multicentre, open-label, phase I, dose-escalation INTERACT trial. BMJ Open. (2019) 9:e034508. doi: 10.1136/bmjopen-2019-034508
68. Oostendorp J, Stalmeier PF, Pasker-de Jong PC, Van der Graaf WT, Ottevanger PB. Systematic review of benefits and risks of second-line irinotecan monotherapy for advanced colorectal cancer. Anticancer Drugs. (2010) 21:749–58. doi: 10.1097/CAD.0b013e32833c57cf
69. Turajlic S, Sottoriva A, Graham T, Swanton C. Resolving genetic heterogeneity in cancer. Nat Rev Genet. (2019) 20:404–16. doi: 10.1038/s41576-019-0114-6
70. Turajlic S, Swanton C. Metastasis as an evolutionary process. Science. (2016) 352:169–75. doi: 10.1126/science.aaf2784
71. Zajac Raingeaud J, Libanje F, Lefebvre C, Sabino D, Martins I, Roy P, et al. Tumour spheres with inverted polarity drive the formation of peritoneal metastases in patients with hypermethylated colorectal carcinomas. Nat Cell Biol. (2018) 20:296–306. doi: 10.1038/s41556-017-0027-6
72. Demuytere Ceelen W, Van Dorpe J, Hoorens A. The role of the peritoneal microenvironment in the pathogenesis of colorectal peritoneal carcinomatosis. Exp Mol Pathol. (2020) 115:104442. doi: 10.1016/j.yexmp.2020.104442
73. Ceelen W, Ramsay RG, Narasimhan V, Heriot AG, De Wever O. Targeting the tumor microenvironment in colorectal peritoneal metastases. Trends Cancer. (2020) 6:236–46. doi: 10.1016/j.trecan.2019.12.008
74. Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JW, de Hingh IH. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer. (2011) 128:2717–25. doi: 10.1002/ijc.25596
75. Calabro L, Lazzari N, Rigotto G, Tonello M, Sommariva A. Role of epithelial-mesenchymal plasticity in pseudomyxoma peritonei: implications for locoregional treatments. Int J Mol Sci. (2020) 21:9120. doi: 10.3390/ijms21239120
76. Brannon R, Vakiani E, Sylvester BE, Scott SN, McDermott G, Shah RH, et al. Comparative sequencing analysis reveals high genomic concordance between matched primary and metastatic colorectal cancer lesions. Genome Biol. (2014) 15:454. doi: 10.1186/s13059-014-0454-7
77. Yaeger R, Chatila WK, Lipsyc MD, Hechtman JF, Cercek A, Sanchez-Vega F, et al. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell. (2018) 33:125–36e3. doi: 10.1016/j.ccell.2017.12.004
78. Priestley Baber J, Lolkema MP, Steeghs N, de Bruijn E, Shale C, Duyvesteyn K, et al. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature. (2019) 575:210–6. doi: 10.1038/s41586-019-1689-y
79. Ishaque N, Abba ML, Hauser C, Patil N, Paramasivam N, Huebschmann D, et al. Whole genome sequencing puts forward hypotheses on metastasis evolution and therapy in colorectal cancer. Nat Commun. (2018) 9:4782. doi: 10.1038/s41467-018-07041-z
80. Sun J, Wang C, Zhang Y, Xu L, Fang W, Zhu Y, et al. Genomic signatures reveal DNA damage response deficiency in colorectal cancer brain metastases. Nat Commun. (2019) 10:3190. doi: 10.1038/s41467-019-10987-3
81. Ladanyi Mukherjee A, Kenny HA, Johnson A, Mitra AK, Sundaresan S, Nieman KM, et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene. (2018) 37:2285–301. doi: 10.1038/s41388-017-0093-z
82. Gartner K. Commentary: random variability of quantitative characteristics, an intangible epigenomic product, supporting adaptation. Int J Epidemiol. (2012) 41:342–6. doi: 10.1093/ije/dyr221
83. Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Cespedes MV, et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell. (2012) 22:571–84. doi: 10.1016/j.ccr.2012.08.013
84. Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet. (2015) 47:320–9. doi: 10.1038/ng.3225
85. Isella C, Terrasi A, Bellomo SE, Petti C, Galatola G, Muratore A, et al. Stromal contribution to the colorectal cancer transcriptome. Nat Genet. (2015). 47:312–9. doi: 10.1158/1538-7445.AM2015-4760
86. Roerink SF, Sasaki N, Lee-Six H, Young MD, Alexandrov LB, Behjati S, et al. Intra-tumour diversification in colorectal cancer at the single-cell level. Nature. (2018) 556:457–62. doi: 10.1038/s41586-018-0024-3
87. Weeber F, van de Wetering M, Hoogstraat M, Dijkstra KK, Krijgsman O, Kuilman T, et al. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc Natl Acad Sci U S A. (2015) 112:13308–1. doi: 10.1073/pnas.1516689112
88. Fumagalli A, Suijkerbuijk SJE, Begthel H, Beerling E, Oost KC, Snippert HJ, et al. A surgical orthotopic organoid transplantation approach in mice to visualize and study colorectal cancer progression. Nat Protoc. (2018) 13:235–47. doi: 10.1038/nprot.2017.137
89. Kok HP, Cressman ENK, Ceelen W, Brace CL, Ivkov R, Grull H, et al. Heating technology for malignant tumors: a review. Int J Hyperthermia. (2020) 37:711–41. doi: 10.1080/02656736.2020.1779357
Keywords: colorectal, CMS4, peritoneal, imaging, organoid
Citation: Kranenburg O, Speeten Kvd and Hingh Id (2021) Peritoneal Metastases From Colorectal Cancer: Defining and Addressing the Challenges. Front. Oncol. 11:650098. doi: 10.3389/fonc.2021.650098
Received: 06 January 2021; Accepted: 15 February 2021;
Published: 16 March 2021.
Edited by:
Eduardo Huarte, Incyte, United StatesReviewed by:
Robert Ramsay, Peter MacCallum Cancer Centre, AustraliaCopyright © 2021 Kranenburg, Speeten and Hingh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Onno Kranenburg, by5rcmFuZW5idXJnQHVtY3V0cmVjaHQubmw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.