- 1Department of General Practice, Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 2Evidence-Based Medicine Center, MOE Virtual Research Center of Evidence-based Medicine at Zunyi Medical College, Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 3GCP Center, Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 4Department of Nursing, Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 5Evidence-Based Medicine Research Centre, Jiangxi University of Traditional Chinese Medicine, Nanchang, China
- 6Laboratory Research Center, Guizhou Provincial People’s Hospital, Guizhou University, Guiyang, China
- 7Department of Infection Management, Gansu Provincial People’s Hospital, Lanzhou, China
- 8Department of Oncology, Lishui People’s Hospital, Sixth Affiliated Hospital of Wenzhou Medical University, Lishui, China
Introduction: A modified and recombinant human endostatin (Rh-endostatin) is often used in the control of malignant pleural effusion (MPE) through intrapleural infusion.
Objectives: To demonstrate the clinical response, survival, and safety of Rh-endostatin plus chemical irritants, their optimal combinations, treatment threshold, and optimal usage, we performed a new systematic review and meta-analysis.
Methodology: All randomized controlled trials (RCTs) were collected from Chinese and English electronic databases (from inception until August 2020). We pooled the data using a series of meta-analyses and summarized the evidence quality following the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Results: We included 75 RCTs recruiting 4,678 patients, which reported six combinations for Rh-endostatin plus chemical irritants. Among the six combinations, only Rh-endostatin plus cisplatin (DDP) with enough trials might improve the complete response [2.29 (1.93, 2.71)] and quality of life [3.01 (2.49, 3.63)] and reduce treatment failure [0.29 (0.25, 0.33)] and progressive disease [0.27 (0.22, 0.34)]. It might not increase the risk of adverse drug reactions. For patients with lung cancer, moderate to massive effusion, initial treatment, Karnofsky Performance Status (KPS) score ≥60, or anticipated survival time ≥3 months, Rh-endostatin (30–45 mg each time, once or twice a week 3–4 times) plus DDP (30–60 mg/m2) obtained a significant improvement in clinical response and a reduction of failure and progressive disease. Most results had good robustness and moderate quality.
Conclusions: Current evidence suggests that Rh-endostatin with DDP may be an optimal combination, which may improve clinical response and reduce failure and progressive disease with good safety. Rh-endostatin (30–40 mg each time, once or twice a week 3–4 times) with DDP (30–40 mg/m2) may be an optimal usage for achieving an ideal response.
Introduction
Malignant pleural effusion (MPE) is a common clinical problem in patients with malignant tumors, with an estimated annual incidence of at least 150,000 in the USA (1). Based on postmortem records, MPE was found in 15% of patients who died with malignant tumors (2). Most patients often suffered from breathlessness and chest pain. The quality of life (QOL) was poor, and the median survival time was only 3–12 months (2, 3). Chemical pleurodesis is a first-line treatment for symptomatic patients with MPE and suspected expandable lung (4, 5) and a procedure performed to obliterate the pleural space to prevent recurrent MPE using a chemical irritant as platinum, bleomycin (BLM), tetracycline, doxycycline, or silver nitrate, among others (3–6). However, these strategies are mostly of palliative value and focus on the control of symptoms and improvement of QOL and fail to improve survivals. So, new control strategies are urgently needed.
Proangiogenic factors have been implicated as a critical cytokine in the occurrence, development, and transferring of MPE (7–10). Endostatin, a 20-kDa C-terminal fragment of type XVIII collagen, is one of the most potent inhibitors of angiogenesis (11). Endostatin and its derivatives have been reported to be more effective when combined with chemotherapy, radiotherapy, or gene transfer in the treatment of malignant tumors (12, 13). Endostar, a modified and recombinant human endostatin (Rh-endostatin), was the approved regimen in non-small-cell lung cancer (NSCLC) by the State Food and Drug Administration of China in 2005 (14). The expert consensus also recommends Rh-endostatin plus first-line chemotherapy to treat stage III/IV NSCLC (15, 16). Interestingly, eight systematic reviews (SRs)/meta-analyses had reported that intrapleural administration of Rh-endostatin with platinum (17, 18), cisplatin (DDP) (19–23), or chemotherapeutic agents (24) might improve the objective response rate [complete response (CR), partial response (PR)], disease control rate [CR + PR+ no response (NR)/stable disease (SD)], and QOL, without an increase in the incidence of adverse drug reactions (ADRs) in MPE. Three meta-analyses (25–27) had reported that Rh-endostatin with DDP also might obtain the same effects in MPE from lung cancer. Based on the above evidence, Rh-endostatin alone or plus chemical irritants was recommended in the control of MPE by expert consensus from China (28). However, strong clinical heterogeneity was found in the patient features, types, combinations, and usages of Rh-endostatin/chemical irritants. The drug usages are complex, diverse, and even inappropriate. Obviously, the current studies ignored clinical heterogeneity. Current evidence (17–27) failed to conclusively demonstrate whether Rh-endostatin plus chemical irritants improves clinical response, survival, and safety. Their optimal combinations, therapeutic threshold, and optimal usage remain unclear. In addition, no evidence revealed their thoracentesis-related adverse events (TRAEs). All these have become the new bottleneck of rational drug use decision.
Recently, many new trials (29–31) have been published. So, we performed a new SR and meta-analysis to further demonstrate the clinical response, survival, and safety of Rh-endostatin with chemical irritants, reveal their optimal combinations, therapeutic thresholds, and optimal usage for achieving a desired response, and provide evidence for developing an optimal control strategy of MPE.
Methods
According to the principle of underestimating efficacy and overestimating risk, we designed, implemented, and reported this SR and meta-analysis following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (Supplementary Material S1) (32). The retrieval, selection, assessment, data collection, statistical analysis, and summary of evidence quality were implemented by two independent evaluators. Any disagreements of implementations between evaluators were resolved by discussions, and further disagreements were resolved by a third party (ZX).
Inclusion Criteria
All subjects were patients with MPE that was diagnosed using thorax imaging, pleural fluid analysis, cytology, or pleural biopsy, without any restrictions on the tumor types. All subjects had normal heart, liver, or kidney function. The intervention used was Rh-endostatin through intrapleural administration instead of intravenous injection. Patients in the experimental group received Rh-endostatin plus chemical irritant, and the control group received chemical irritant alone, which included platinum, BLM, tetracycline, doxycycline, or silver nitrate, among others. During perfusion, all subjects did not receive hyperthermia, radiotherapy, chemotherapy, chemoradiotherapy, traditional Chinese medicine injections (TCMIs), or other biological response modifiers (BRMs). The main outcomes were clinical responses, survivals, and QOL, and the secondary outcomes were ADRs and TRAEs. The trials were randomized controlled trials (RCTs), with no restrictions on follow-up and research institutions.
Exclusion Criteria
Excluded studies included the duplicates; studies about non-MPE and non-Rh-endostatin; studies about Rh-endostatin plus hyperthermia, radiotherapy, chemotherapy, chemoradiotherapy, TCMIs, or BRMs; meeting abstracts and reviews without any specific data; non-RCTs as cohort studies, cross-sectional studies, case series, or case reports; unrelated SRs or meta-analyses; and studies without primary or secondary outcome data.
Search Strategies
Based on the principle of patients (P) plus intervention (I), we applied the MeSH and free word to build the search strategies as (“Pleural Effusion” [Mesh] OR Pleural Effusion OR Pleural Effusions OR Hydrothorax OR MPEs OR MPE) AND (“Endostatins” [Mesh] OR Endostatins OR Endostatin OR Recombinant human endostatin injection OR rhES OR Rh-endostatin OR Endostar OR Sulijia OR YH-16). Two independent evaluators (C-QW and HJ) collected all the published studies of “Rh-endostatin plus chemical irritants for MPE” from Embase, PubMed, Web of Science (ISI), China Biological Medicine Database (CBM), Wanfang Database, China National Knowledge Infrastructure Database (CNKI), Chinese Scientific Journals Full-text Database (VIP), and Cochrane Central Register of Controlled Trials (CENTRAL, Issue 8 of 12, August 2020) and ongoing trials from the Chinese clinical trial registry (Chi-CTR, http://www.chictr.org.cn), WHO International Clinical Trials Registry Platform (WHO-ICTRP, http://apps.who.int/trialsearch/), and US-clinical trials (https://clinicaltrials.gov/, up to August 2020). In addition, we critically evaluated all the SRs/meta-analyses of Rh-endostatin in MPE and selected eligible trials from the references.
Selection of Studies
Two evaluators (C-QW and MH) were asked to collect the qualified trials about Rh-endostatin plus chemical irritants for MPE according to the preestablished inclusion and exclusion criteria.
Assessment of Methodological Bias Risk
Two evaluators (X-RH and QC) were asked to assess the bias risk of methodology using the Cochrane Collaboration’s risk of bias assessment tool for RCTs (33). The bias risk was assessed as a judgment (high, low, or unclear) for individual elements of five domains (selection, performance, attrition, reporting, and other).
Indicator Definition
The clinical responses were evaluated using CR, treatment failure, and progressive disease (PD). Based on previous studies (34–37), we integrated all the criteria as follows: (i) CR, (ii) PR, (iii) NR or SD; and (iv) PD (Supplementary Material S2). Treatment failure was defined as NR/SD plus PD (38). Survival was defined as overall survival (OS) rate, progression-free survival (PFS) rate, or hazard ratio (HR) of the OS and PFS. Using the Karnofsky Performance Status (KPS) scale, if the KPS score increased ≥10 after perfusion, the QOL was improved.
The secondary outcomes were ADRs and TRAEs. According to the World Health Organization (WHO) (39) or Common Terminology Criteria for Adverse Events (CTCAE) standards (40), ADR was defined as neutropenia, thrombocytopenia, anemia, cardiotoxicity, hepatotoxicity, nephrotoxicity, gastrointestinal reactions, alopecia, peripheral neuritis, chest pain, and fever, among others. TRAE was defined as treatment-related mortality (TRM) and a series of clinical symptoms such as respiratory failure, pneumothorax, cutaneous emphysema, or catheter-related infection/chest infection, among others.
Data Collection
Two evaluators (X-TZ and T-yF) collected all the data using a predesigned data extraction form. The data included the first author, year of publication, and demographic information of patients; baseline characteristics such as primary tumors, pleural fluid volume, KPS score, treatment history (initial treatment, retreatment, or both), anticipated survival time (AST), sample size, drainage methods [indwelling pleural catheters (IPCs) or thoracocentesis]; combinations and usages of Rh-endostatin and chemical irritants; evaluation time and follow-up protocols; and outcomes including CR, treatment failure, PD, OS, PFS, QOL, ADRs, and TRAEs. Additionally, we contacted the corresponding author to obtain the available survival data. If the authors were unavailable, we adopted the Engauge Digitizer 4.1 to transform the Kaplan–Meier survival curves into available data (41, 42).
Statistical Analysis
According to the data features, the odds ratio (OR) or hazard ratio (HR) and their 95% CI were used to quantify the CR, treatment failure, PD, OS, PFS, QOL, ADRs, and TRAEs, and p < 0.05 was considered a statistical significance. Two evaluators (C-QW and X-RH) conducted a series of meta-analyses using the Review Manager 5.4.1 (as recommended by the Cochrane Collaboration). The Cochran’s χ2 test and I2 statistic were conducted to analyze the potential statistical heterogeneity. If p ≥ 0.1 and I2 ≤ 50%, a fixed-effects model (FEM) was used to pool the OR or HR and their 95% CI. Otherwise, a random-effects model (REM) was used. If the number of trials was larger than 10, a funnel plot and Egger/Begg’s test were used to examine the potential publication bias.
When at least one item was considered a high risk, the trial was defined as poor quality. When the result was statistically different and beneficial to Rh-endostatin infusion, the trial was defined as an underestimated or overestimated trial following our experiences (38, 43, 44). According to the principle of underestimating efficacy and overestimating risk, we established a sensitivity analysis model to analyze the robustness of the results before and after eliminating the trials with poor quality, underestimation, or overestimation.
Subgroup Analysis
Following the guideline (45) and our previous experiences (38, 43, 44), we established a subgroup analysis model to analyze the clinical heterogeneity and the effects of variables on CR, treatment failure, and PD and to reveal their treatment thresholds and optimal usage for achieving an ideal response. The variables included patient features, drainage methods, and combinations of Rh-endostatin plus chemical irritants and their dose, treatment frequency, and times. Finally, a univariable random-effects meta-regression was conducted to reveal the relevance between each variable and CR, treatment failure, or PD and a post-hoc multiple regression analysis adjusting for their OR under all variables.
Summary of Evidence Quality
Following the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach and integrating the results of the sensitivity analysis, we developed a quality summary model to summarize the evidence quality and classify them as “high,” “moderate,” “low,” or “very low” (38, 43, 44) (Supplementary Material S3). The quality was downgraded according to five domains as follows: (1) methodological bias risk; (2) statistical heterogeneity; (3) indirectness; (4) imprecision; and (5) publication bias. Two evaluators (X-FC and C-QW) used the GRADE profiler to summarize the quality and generate the absolute estimates for the CR, treatment failure, PD, OS rate, PFS rate, QOL, ADRs, and TRAEs (46).
Results
Search Results
A literature search conducted from inception to August 17, 2020, identified 959 studies. After duplicates were removed, 379 studies remained for a review of abstracts. After reviewing the abstracts, we identified 115 reports and 11 SRs/meta-analyses (16, 47–54). After reviewing full texts, we identified 73 qualified trials (29–31, 54–123). After reviewing the SRs/meta-analyses, we identified 39 trials (55–60, 62, 64, 66–69, 71–78, 81–92, 95, 97, 100, 101, 104, 109, 110). Excluding two ongoing trials without data (124, 125), we included one ongoing trial (ChiCTR-IPR-17011666) (126). Finally, we identified 75 trials (29–31, 54–123, 126, 127) for this SR/meta-analysis (Supplementary Material S4; Tables S1–S4; Figure 1).
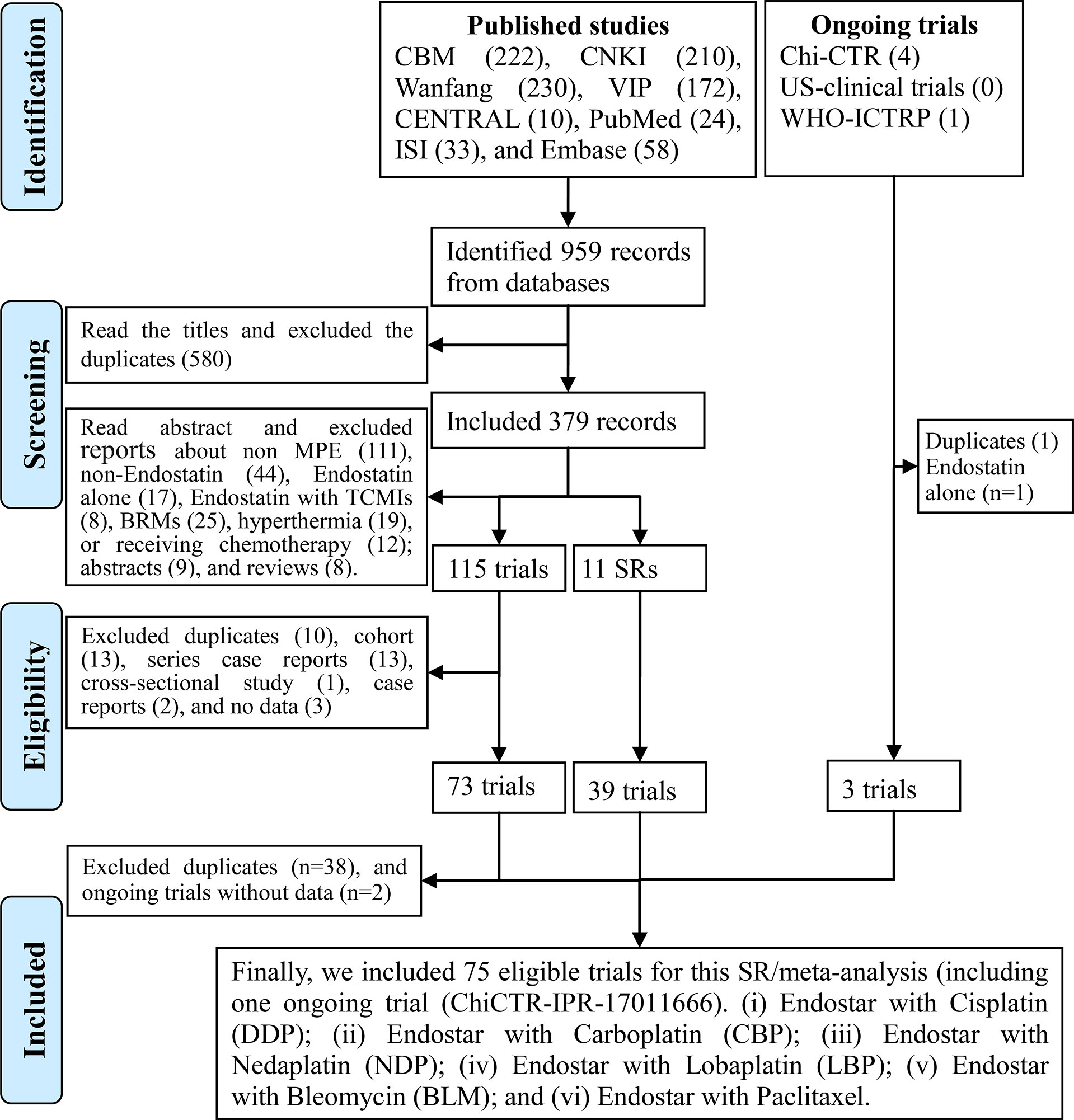
Figure 1 Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2009 flow diagram for the identification of eligible trials.
Characteristics of the Included Trials
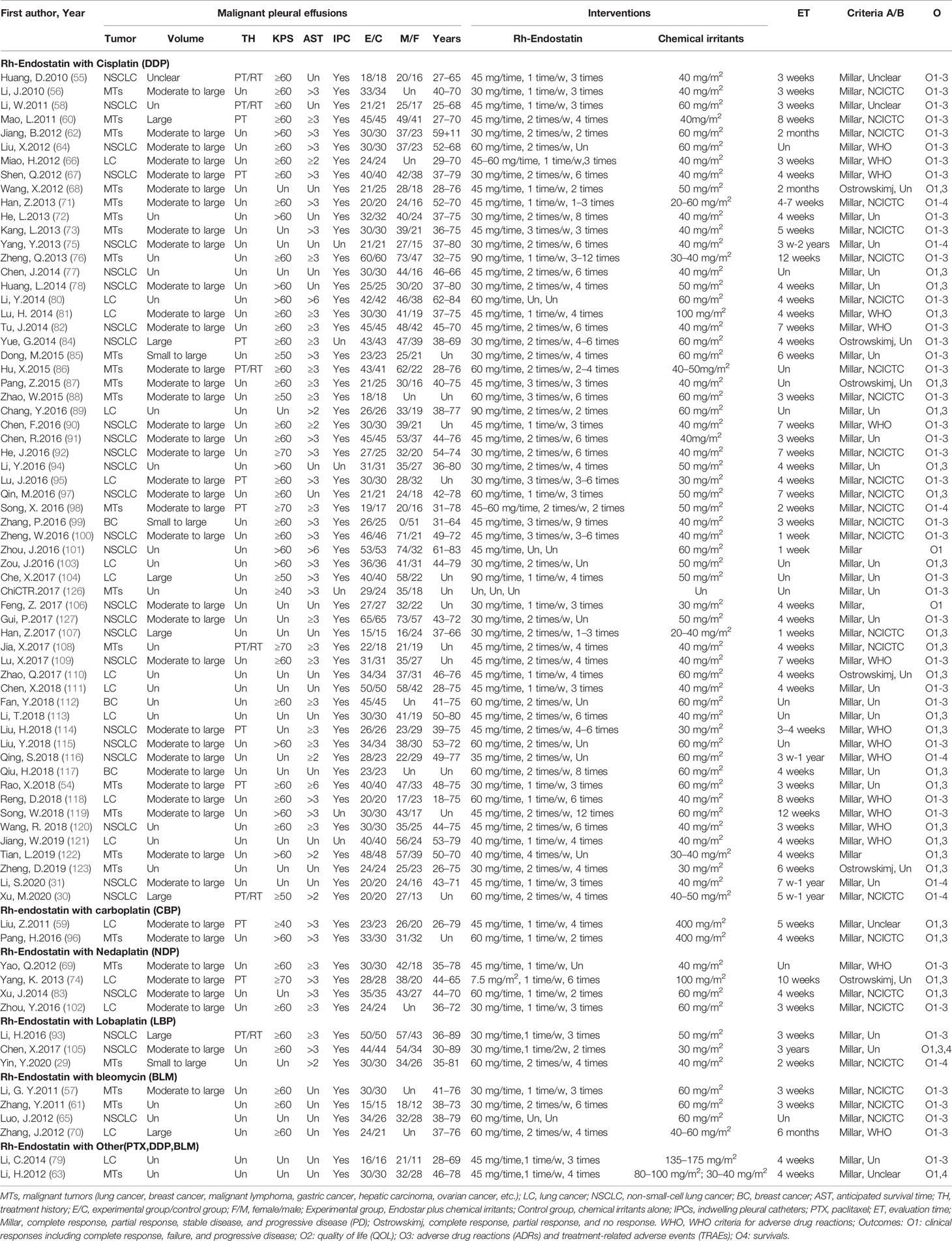
The 75 trials, published from 2010 to 2020, recruited 4,678 patients with MPE from China, which included 2,512 males and 1,738 females aged 18–89 years (Table 1). Forty-seven trials recruited patients with lung cancers (30, 31, 55, 58, 59, 64–67, 70, 74, 75, 77–84, 89–95, 97, 100–107, 109–111, 113–116, 118, 120, 121, 127), three trials recruited patients with breast cancer (99, 112, 117), and the remaining 25 trials (29, 54, 56, 57, 60–63, 68, 69, 71–73, 76, 85–88, 96, 98, 108, 119, 122, 123, 126) recruited patients with malignant tumors such as lung cancer, breast cancer, malignant lymphoma, gastric cancer, hepatic carcinoma, and ovarian cancer, among others. Trials were of varied sample sizes, from 30 to 130. The patients had small to massive effusion, KPS ≥40, and AST ≥2 months. Fifteen trials reported the treatment history as initial treatment, retreatment, or both. After the drainage of hydrothorax using an IPC or thoracocentesis, 2,352 cases accepted the intrapleural administration of Rh-endostatin plus chemical irritants, while 2,326 other cases accepted the chemical irritants alone. We found six combinations of Rh-endostatin plus DDP in 60 trials (30, 31, 54–56, 58, 60, 62, 64, 66–68, 71–73, 75–78, 80–82, 84–92, 94, 95, 97–101, 103, 104, 106–123, 126, 127), nedaplatin (NDP) (69, 74, 83, 102) and BLM in four trials (57, 61, 65, 70), lobaplatin (LBP) in three trials (29, 93, 105), carboplatin (CBP) in two trials (59, 96), paclitaxel (79) or DDP/BLM in one trial (63). Rh-endostatin (30–90 mg each time) was used once or twice a week, 1–12 times by intrapleural administration. The chemical irritant was mainly DDP and used with 20–100 mg/m2 each time. Three to 10 weeks after perfusion, the trials evaluated clinical responses using a Millar or Ostrowskimj criterion, ADRs using a WHO criterion, and QOL using a KPS scale. In addition, only nine trials reported the survivals (29–31, 63, 71, 75, 98, 105, 116) and TRAEs (56, 66, 76, 88, 95, 98, 99, 116, 127), and six trials (66, 88, 95, 98, 99, 116) reported the TRM.
Methodological Quality Assessment
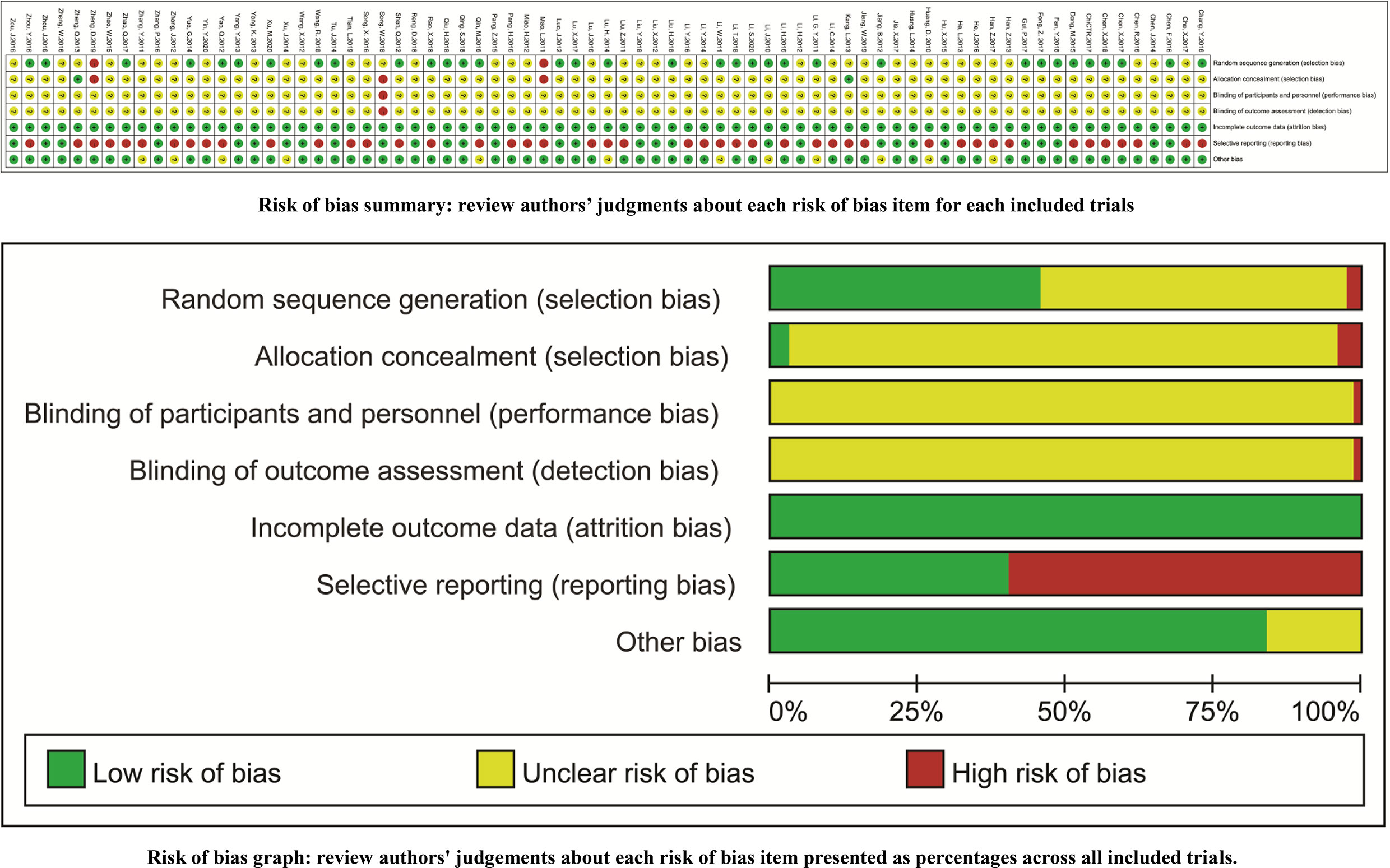
Thirty-four trials reported the random sequence generation using a random number table (a low risk of selection bias) (30, 31, 54, 56–58, 62, 65, 67, 69, 75, 81, 82, 84, 85, 89, 90, 93, 97, 101, 102, 105, 106, 109–114, 116, 117, 120, 126, 127), and two trials reported the odd or even random (a high risk of selection bias) (60, 123). Two trials reported the allocation concealment using an envelope (a low risk of selection bias) (73, 76), and two trials reported the allocation exposure (a high risk of selection bias) (60, 123). With the exception of one open RCT (119), the remaining trials failed to clearly report the blindings (an unclear risk of performance bias). All trials reported the complete outcome data (a low risk of attrition bias). Forty-four trials selectively reported the ADRs, and one trial selectively reported the CR (a high risk of reporting bias) (66). The comparability between groups (an unclear risk of other biases) was unclear in 12 trials (Figure 2).
Clinical Responses
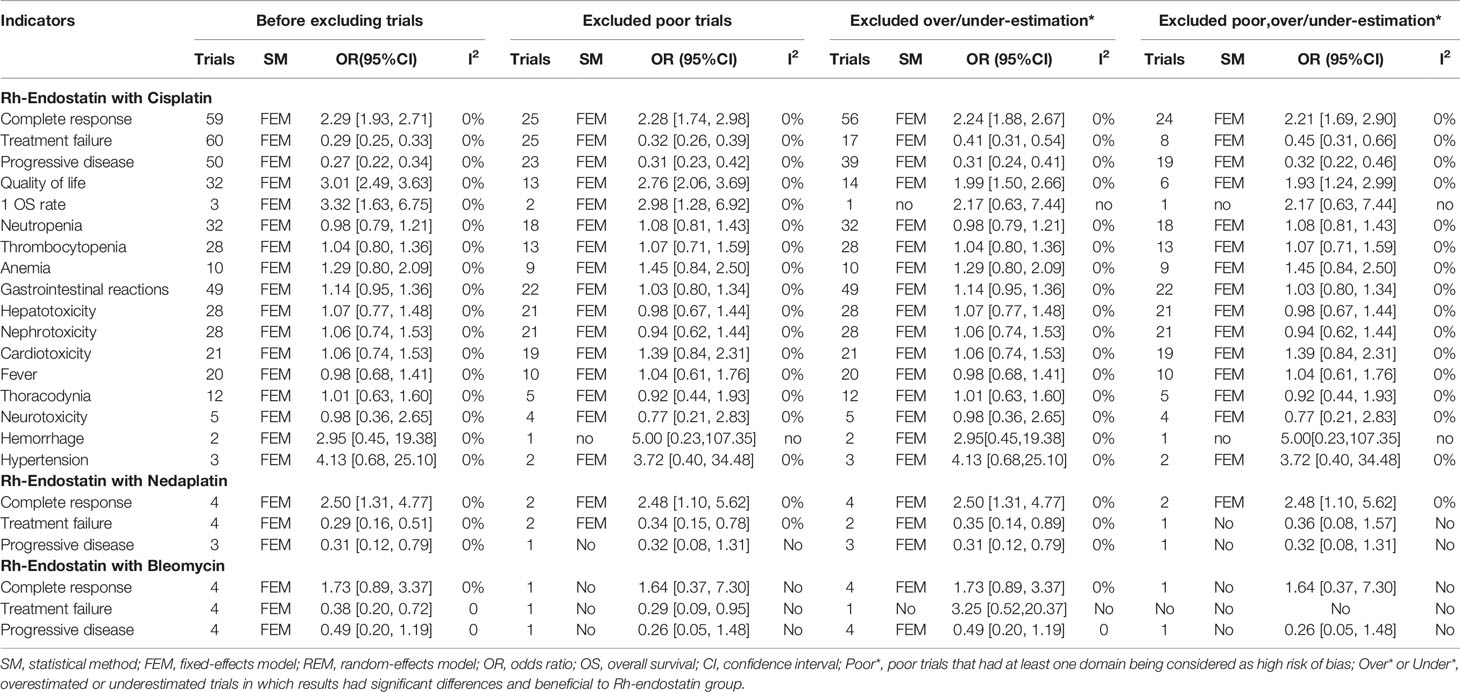
Seventy-five trials reported the clinical responses of six combinations of Rh-endostatin with DDP (30, 31, 54–56, 58, 60, 62, 64, 66–68, 71–73, 75–78, 80–82, 84–92, 94, 95, 97–101, 103, 104, 106–123, 126, 127), NDP (69, 74, 83, 102), BLM (57, 61, 65, 70), LBP (29, 93, 105), CBP (59, 96), paclitaxel (79), or DDP/BLM (63). The statistical heterogeneity was not found using Cochran’s χ2 test and I2 statistic (I2 = 0%). So, the data were pooled using an FEM. The ORs of fixed effects were 2.29 (95% CI 1.93–2.71, p < 0.00001), 2.50 (95% CI 1.31–4.77, p = 0.005), 2.71 (95% CI 1.37–5.35, p = 0.004), which showed that the CR of Rh-endostatin with DDP, NDP, or LBP was significantly higher than that of irritants alone (Figure 3A). The treatment failure of Rh-endostatin with DDP, CBP, NDP, LBP, or BLM was significantly lower than that of irritants alone. The ORs were 0.29 (95% CI 0.25–0.33, p < 0.00001), 0.28 (95% CI 0.12–0.64, p = 0.003), 0.29 (95% CI 0.16–0.51, p < 0.0001), 0.25 (95% CI 0.15–0.44, p < 0.00001), and 0.25 (95% CI 0.13–0.50, p < 0.0001), respectively (Figure 3B). The PD of Rh-endostatin with DDP, CBP, NDP, LBP, or BLM was significantly lower than that of irritants alone. The ORs were 0.27 (95% CI 0.22–0.34, p < 0.00001), 0.25 (95% CI 0.09–0.67, p = 0.006), 0.31 (95% CI 0.12–0.79, p = 0.01), 0.32 (95% CI 0.12–0.86, p = 0.02), and 0.31 (95% CI 0.12–0.80, p = 0.02), respectively (Figure 3C).

Figure 3 The analysis of clinical responses between the two groups. (A) The forest plot of complete response. (B) The forest plot of treatment failure. (C) The forest plot of progressive disease.
Overall Survival
Nine trials reported the survivals (29–31, 63, 71, 75, 98, 105, 116). Only five trials reported the OS time and PFS of Rh-endostatin with DDP (30, 71, 75, 98) or LBP (29), but without the available data. Five trials reported the OS rates, and three reported the 1-year OS rate of Rh-endostatin with DDP (31, 75, 116). The statistical heterogeneity between trials was not found using Cochran’s χ2 test and I2 statistic (I2 = 0%). So, we pooled the data using an FEM. The 1-year OS rate of Rh-endostatin with DDP was significantly higher than that of DDP alone. The OR was 3.32 (95% CI 1.63–6.75, p = 0.0009) (Figure 4). The remaining OS rates were reported in only one trial, and the data were analyzed descriptively using forest plots. Statistical analysis showed that the 0.5-year OS rate of Rh-endostatin with DDP (116), 1-year OS rate of DDP/BLM (63), 2-year OS rate of DDP (75), and 3-year OS rate of LBP (105) were significantly higher than that of irritants alone. The ORs were 5.36 (95% CI 1.24–23.10, p = 0.02), 5.21 (95% CI 1.28–21.24, p = 0.02), 10.00 (95% CI 2.05–90.59, p = 0.04), and 3.60 (95% CI 1.46–8.89, p = 0.005), respectively.
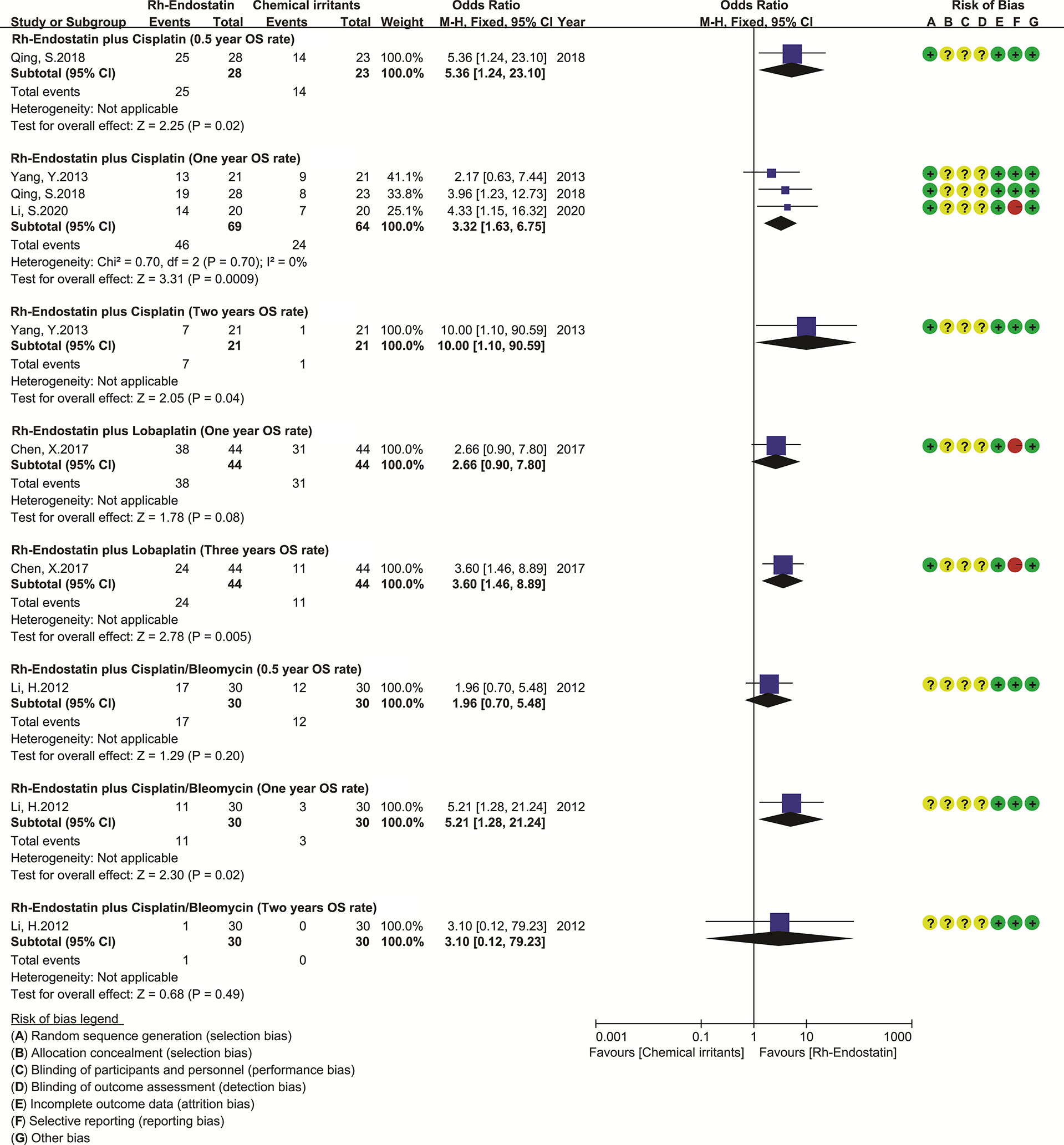
Figure 4 The forest plot of overall survival. DDP, cisplatin; LBP, lobaplatin; BLM, bleomycin; OS, overall survival.
Quality of Life
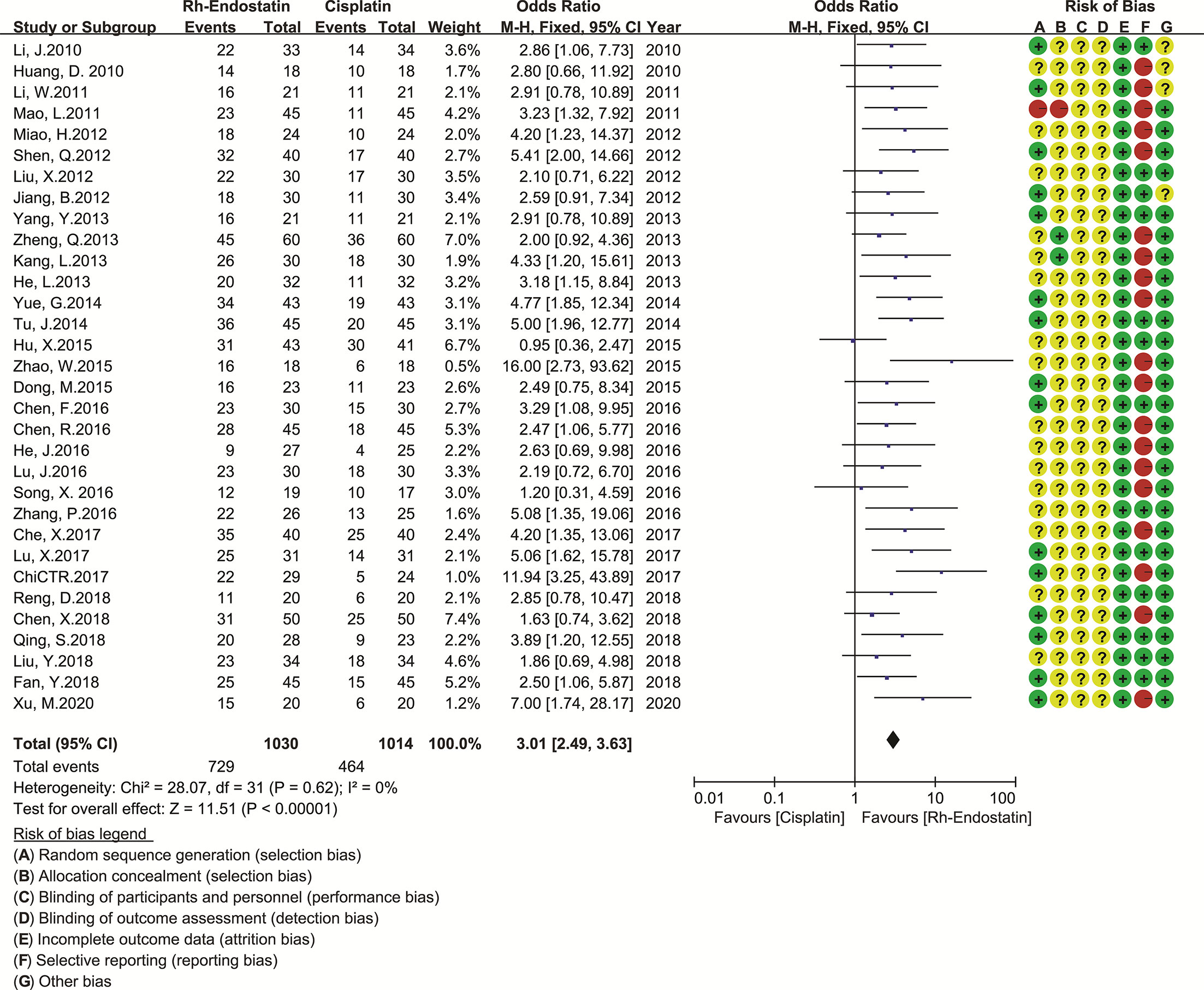
Given the limited trials for Rh-endostatin with CBP, NDP, LBP, or BLM, we only evaluated the QOL of Rh-endostatin with DDP (30, 55, 56, 58, 60, 62, 64, 66, 67, 72, 73, 75, 76, 82, 84–86, 88, 90–92, 95, 98, 99, 104, 109, 111, 112, 115, 116, 118, 126) (Figure 5). The statistical heterogeneity between trials was not found using Cochran’s χ2 test and I2 statistic (I2 = 0%). So, the data were pooled using an FEM. The OR was 3.01 (95% CI 2.49–3.63, p < 0.00001), which indicated that the QOL was significantly higher than that of DDP alone.
Adverse Drug Reactions and Treatment-Related Adverse Events
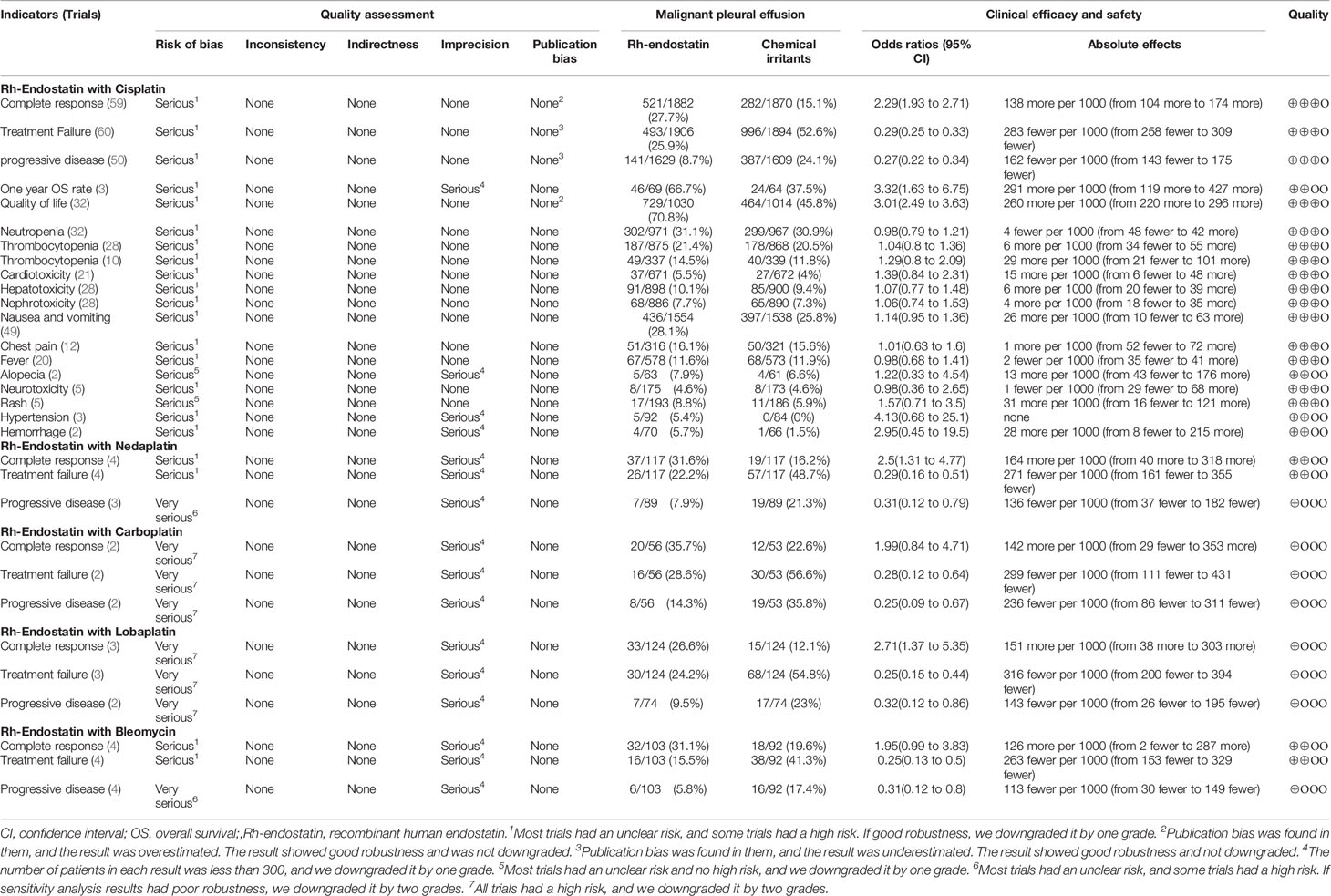
Given the limited trials for Rh-endostatin with CBP, NDP, LBP, or BLM, we only evaluated the ADRs and TRAEs in Rh-endostatin with DDP. Fifty-eight trials observed hematotoxicity (neutropenia, thrombocytopenia, and anemia), cardiotoxicity (arrhythmia), hepatotoxicity, nephrotoxicity, gastrointestinal reaction, alopecia, neurotoxicity, rash, hypertension, hemorrhage, chest pain, and fever (30, 31, 54–56, 58, 60, 62, 64, 66–68, 71–73, 75–78, 80–82, 84–92, 94, 95, 97–100, 103, 104, 107–123, 126, 127) (Table 2). Cochran’s χ2 test and I2 statistic showed no heterogeneity in all of the ADRs (I2 = 0%). So, the data were pooled using an FEM. Rh-endostatin with DDP had a similar risk of ADRs in DDP alone. The ORs showed no significant difference between the two groups. In addition, nine trials (56, 66, 76, 88, 95, 98, 99, 116, 127) reported no risk of TRAEs, and six trials (66, 88, 95, 98, 99, 116) reported no risk of TRM.
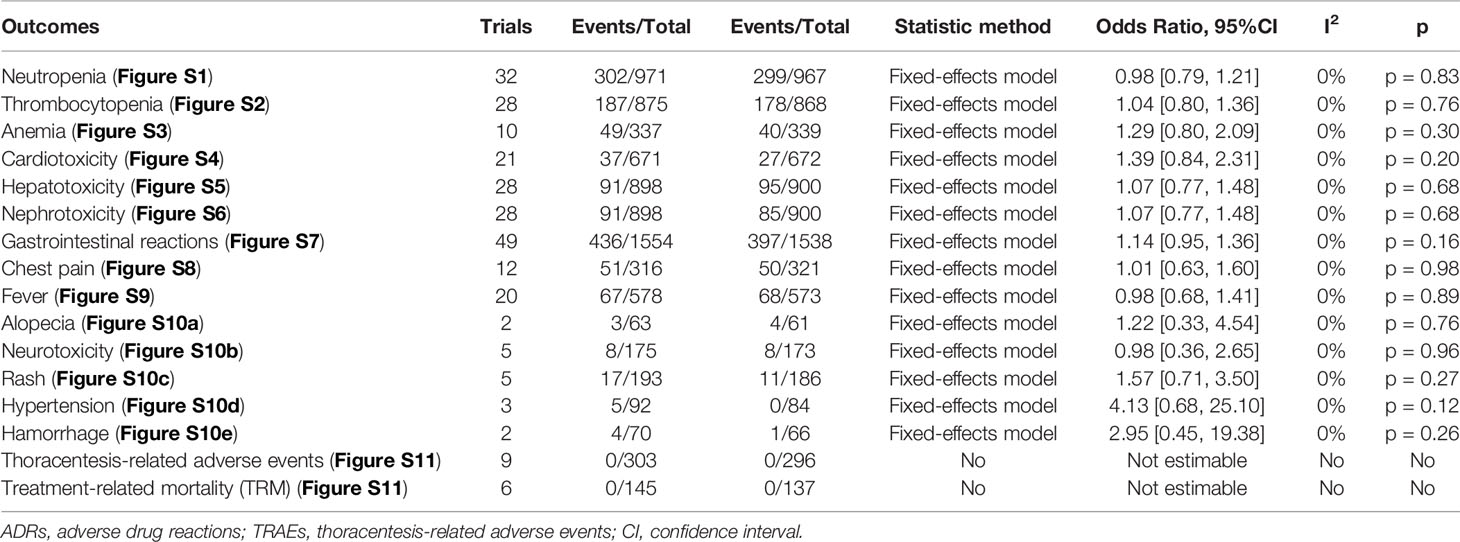
Table 2 Meta-analysis results of ADRs and TRAEs (Figures S1–S11).
Subgroup Analysis of Clinical Responses
The patient feature was defined as primary tumor, pleural fluid volume, treatment history, KPS score, and AST. First, the primary tumor was classified as lung cancer, breast cancer, or malignant tumors. In patients with lung cancer/malignant tumors, Rh-endostatin with DDP obtained a significant increase of CR and a reduction of failure and PD. In breast cancer, it only obtained a reduction of failure and PD (Table 3A and Figures S12, S14, S16). The pleural fluid was classified as small to large, moderate to large, or large (Table 3B and Figures S18, S20, S22); treatment history was initial treatment, retreatment, or both (Table 3C and Figures S24, S26, S28); KPS score was <50, ≥50, or ≥60 (Table 3D and Figures S30, S32, S34); and the AST was ≥2 months or ≥3 months (Table 3E and Figures S36, S38, S40). In patients with moderate to massive effusion, initial treatment, KPS score (≥60), or AST (≥3 months), the Rh-endostatin with DDP groups obtained a significant increase of CR and a reduction of failure and PD.
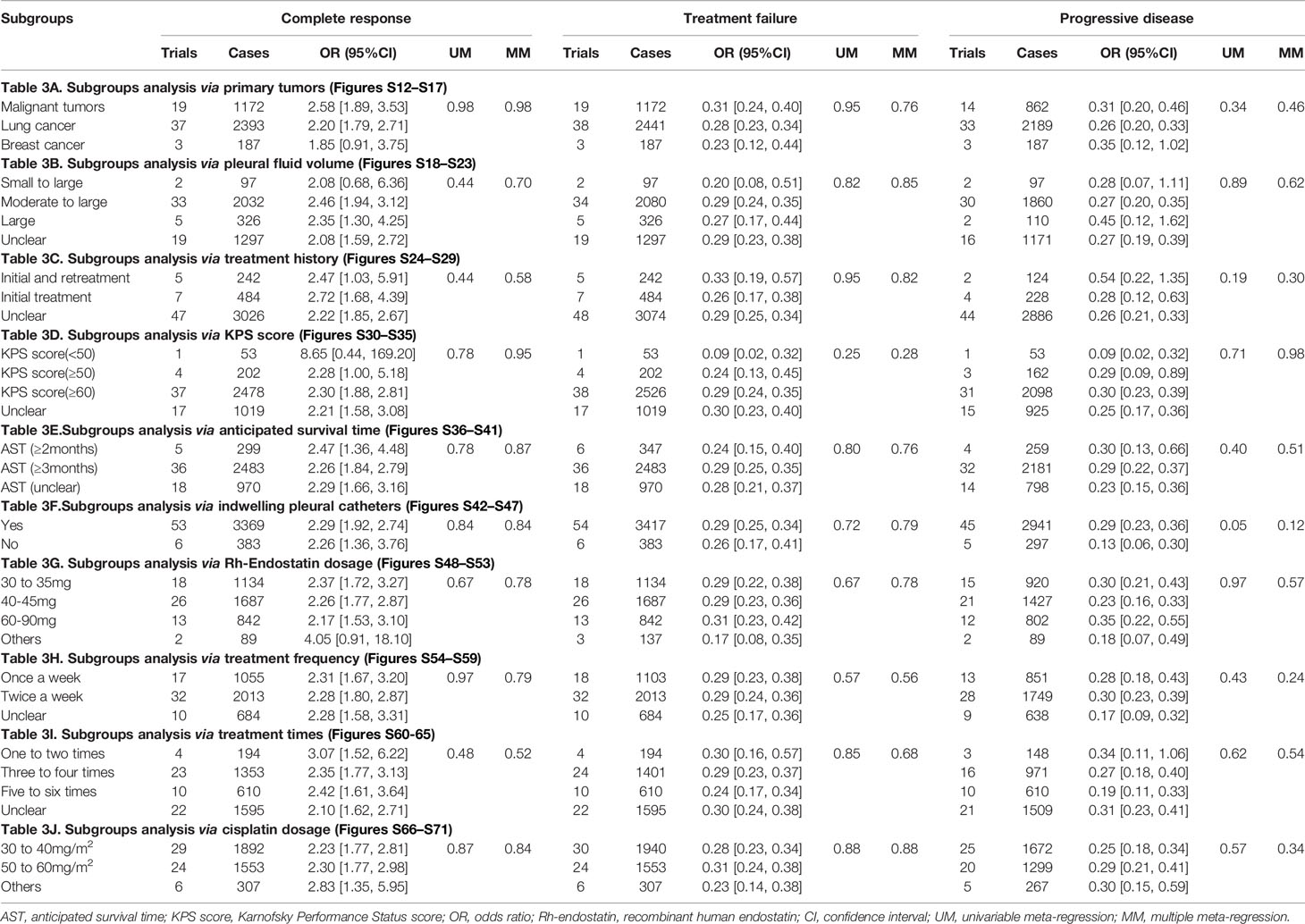
Table 3 Subgroup analysis results (Supplementary Materials 6A, B).
The majority of patients mainly received the IPCs (Table 3F and Figures S42, S44, S46). Subgroup analyses found that whether IPC is used or not had no effect on the clinical responses. Rh-endostatin was used with 30–90 mg each time, once or twice a week 1–12 times (Tables 3G–I and Figures S48–S64). DDP was used with 30–40 mg/m2 or 50–60 mg/m2 each time (Table 3J and Figures S66, S68, S70). Rh-endostatin (30–35 mg or 40–45 mg each time, once or twice a week 3–4 times) with DDP (30–40 mg/m2 or 50–60 mg/m2) obtained a significant increase of response and a reduction of failure and PD in MPE. However, univariate regression analysis did not discover a positive or negative correlation between CR, treatment failure, and PD and each variable (Table 3 and Figures S13–S71). Multiple meta-regression analysis also did not discover a positive or negative correlation (Table 3).
Publication Bias Analysis
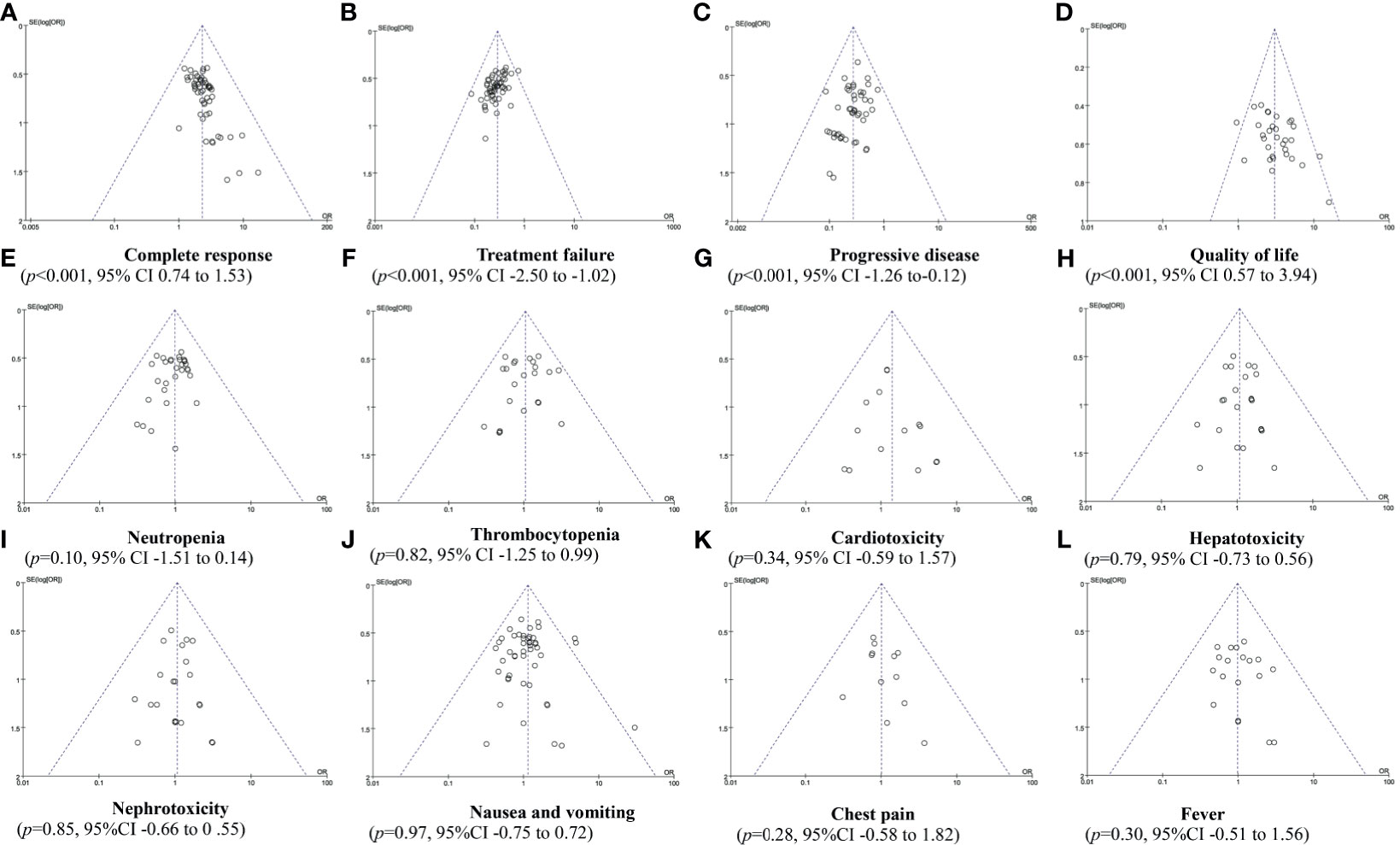
In Rh-endostatin with DDP, more than 10 trials were included for the CR, treatment failure, PD, QOL, and ADRs. So, funnel plot and Egger/Begg’s tests were used to analyze their potential bias of publication. The analysis found a publication bias in CR (p < 0.001, 95% CI 0.74–1.53), treatment failure (p < 0.001, 95% CI -2.50 to -1.02), PD (p < 0.001, 95% CI -1.26 to -0.12), and QOL (p < 0.001, 95% CI 0.57–3.94) (Figures 6A–D). The trials overestimated the CR and QOL and underestimated the treatment failure and PD. The analysis did not find a bias in neutropenia (p = 0.10, 95% CI -1.51 to 0.14), thrombocytopenia (p = 0.82, 95% CI -1.25 to 0.99), cardiotoxicity (p = 0.34, 95% CI -59 to 1.57), hepatotoxicity (p = 0.79, 95% CI -0.73 to 0.56), nephrotoxicity (p = 0.85, 95% CI -0.66 to 0.55), gastrointestinal reactions (p = 0.97, 95% CI -0.75 to 0.72), chest pain (p = 0.28, 95% CI -0.58 to 1.82), and fever (p = 0.30, 95% CI -0.51 to 0.14) (Figures 6E–L). The trials objectively reported the ADRs.
Sensitivity Analysis
In Rh-endostatin with DDP, the poor trials involved clinical response, 1-year OS rate, QOL, and ADRs. Some trials overestimated the CR, 1-year OS rate, and QOL and underestimated the treatment failure and PD. According to the underestimating efficacy and overestimating risk, we evaluated the robustness through removing the poor trials, overestimation/underestimation, and both. Before and after removing the poor trials, the results demonstrated a good robustness of all outcomes. Before and after removing the overestimation and both, the OR of 1-year OS rate was poor robust, and other indicators were robust. In addition, the OR of CR was robust in Rh-endostatin with NDP, and the OR of CR and PD was robust in Rh-endostatin with BLM (Table 4).
Quality of Evidence
In methodology, 46 poor trials were included for this analysis. Sensitivity analysis demonstrated that the OR of 1-year OS rate was poor robustness in Rh-endostatin with DDP, the CR and PD were poor in Rh-endostatin with NDP, and the CR was poor in Rh-endostatin with BLM. Therefore, we downgraded their quality by two grades. Other results had good robustness, and we downgraded their quality by one grade. No heterogeneity was found in all of the indicators; all indicators were not downgraded. In Rh-endostatin with DDP, the sample size of 1-year OS rate, alopecia, hypertension, and hemorrhage was lower than 300 subjects. In Rh-endostatin with NDP, CBP, LBP, or BLM, the CR, treatment failure, and PD were lower than 300. So, we downgraded their quality by one grade. In addition, the funnel plot and Egger’s test showed a publication bias of CR, treatment failure, PD, and QOL in Rh-endostatin with DDP. The sensitivity analysis results were good robust, and we did not downgrade their quality. So, we summarized a low quality for 1-year OS rate, alopecia, hypertension, and hemorrhage and a moderate quality for other results of Rh-endostatin with DDP; a low quality for CR and treatment failure in Rh-endostatin with NDP or BLM; and a very low quality for the remaining indicators (Table 5).
Discussion
Intrapleural administration of Rh-endostatin alone or plus chemical irritants is recommended for the control of MPE by expert consensus from China (28). To demonstrate the optimal combinations of Rh-endostatin with chemical irritants and their clinical efficacy and safety, we further included 75 trials for analysis (29–31, 54–123, 126, 127). In this study, we found six combinations such as Rh-endostatin with DDP, CBP, NDP, LBP, BLM, or paclitaxel. The results of meta-analysis demonstrated that the Rh-endostatin with DDP might improve the response and reduce the failure and PD, with “moderate” quality. We further found that this combination might also improve the QOL, without increasing the risk of hematotoxicity, cardiotoxicity, hepatotoxicity, nephrotoxicity, gastrointestinal reaction, chest pain, and fever, with “moderate” quality. In addition, there were limited reports on the combinations of Rh-endostatin with CBP, NDP, LBP, BLM, or paclitaxel. Only the combinations Rh-endostatin with NDP and LBP might increase the response and reduce the failure and PD, but with “low to very low” quality. A few trials reported the survival; only Rh-endostatin with DDP or LBP might improve 1- to 2-year OS rate, with “low to very low” quality. And most trials failed to report the TRAEs and TRM. Evidently, these outcomes are not fully evaluated and need to be further confirmed.
Eight previous evaluations had reported that the intrathoracic infusion with Rh-endostatin combined with platinum (17, 18), DDP (19–23), or chemotherapeutic agents (24) might improve the objective response rate, disease control rate, and QOL without an increase in the incidence of ADRs in MPEs from malignant tumors. Rh-endostatin with DDP might also obtain the same effects in MPEs from lung cancer (25–27). In this evaluation, we redefined the clinical efficacy as CR, treatment failure, PD, and survival and added the TRAEs and TRM as security indexes, further integrated previous studies (17–27), and added 36 trials with 2,209 patients for analysis. This evaluation found that all six combinations, especially Rh-endostatin with DDP, might show an improvement of clinical response and a reduction of failure and PD, without an increase of the ADRs. The result indicates that a significant synergistic effect exists between Rh-endostatin and DDP. In clinical practice, the BRMs (38, 128) and TCMIs (129–131) were also used in the control of MPEs through intrathoracic infusion. Previous studies (129–131) had reported that chemical irritants plus TCMIs might increase the clinical benefit rate and decrease the ADRs. Chemical irritants plus BRMs (38, 128) also obtain the same benefit. But compared with TCMIs and BRMs, Rh-endostatin did not reduce the risk of ADRs, which may limit its clinical application. All in all, the results indicate that intrapleural administration of TCMIs, BRMs, or Rh-endostatin might be an important pathway to perform pleurodesis and control the hydrothorax (Figure 7).
Figure 7 Intrapleural infusion with Rh-endostatin for MPE. AST, anticipated survival time, QOL, quality of life; KPS, Karnofsky Performance Status; MPE, malignant pleural effusion.
In a previous analysis (38), we found that moderate to large pleural fluid, KPS scores ≥50, or AST ≥3 months might be the treatment thresholds for lentinan with DDP. So, we performed a series of subgroup analyses to reveal the therapeutic thresholds and optimal usage of Rh-endostatin with DDP for achieving a desired response and security. Our analyses found that MPE patients with lung cancer, moderate to massive effusion, initial treatment, KPS score ≥60, or AST ≥3 months might be more suitable for Rh-endostatin with DDP infusion than patients with other conditions. The infusion conditions, the volume of pleural effusion, treatment history, and AST are the same as that of lentinan with DDP infusion. But Rh-endostatin infusion requires a higher KPS (≥60) than lentinan infusion, which suggests that Rh-endostatin infusion seems to have a higher threshold than lentinan. Yoon et al. (132) had reported that poor performance status [Eastern Cooperative Oncology Group (ECOG) 3 or 4] was an independent risk factor of poor survival after video-assisted thoracic surgery (VATS) talc pleurodesis. Compared with VATS talc pleurodesis, endostatin infusion seems to have a lower threshold. In all, the results indicate that endostatin seems to have a special threshold for infusion. The moderate to massive effusion, KPS score ≥60, or AST ≥3 months may be a treatment threshold for Rh-endostatin with DDP, which may be more suitable for MPE with initial treatment or for lung cancer. So, the objective assessment of patients’ baseline should be considered when choosing Rh-endostatin with DDP. In expert consensus (28), Rh-endostatin (45 mg each time) with DDP (40 mg/m2) is recommended to control MPEs. Further subgroup analysis revealed that Rh-endostatin (30–35 mg or 40–45 mg each time, once or twice a week 3–4 times) with DDP (30–40 mg/m2 or 50–60 mg/m2) obtained a significant increase of clinical response and a reduction of failure and PD. Based on the low dose and cost matching, we believe that Rh-endostatin (30–40 mg each time, once or twice a week 3–4 times) with DDP (30–40 mg/m2) may be a possible strategy for achieving an ideal response and a low failure and deterioration (Figure 7). The dose of Rh-endostatin and DDP may be lower than the recommended dose (28). All these findings demonstrate a possible treatment threshold and optimum strategy of intrapleural administration of Rh-endostatin with DDP for MPEs, which is of important clinical significance for further improving scientific decision-making of drug rational application. But the meta-regressions did not further confirm the positive or negative correlation. In addition, whether endostatin with DDP infusion is suitable for drug-resistant, refractory, retreatment, or recurrent MPEs and MPEs from other tumors remains unclear. For Rh-endostatin with CBP, NDP, or LBP/BLM, the treatment threshold and optimal strategy remain unclear. So, these questions need to be further answered.
All kinds of potential limitations should be taken into consideration. First, in this study, only Chinese and English databases were searched, which might result in potential retrieval biases. Second, a considerable number of trials did not clearly describe the baseline features such as the volume of hydrothorax, KPS score, AST, initial treatment, retreatment, drug-resistant, refractory, or recurrent. Third, only 34 studies described the generation of random sequence, and 44 studies selectively reported the CR, ADRs, or TRAEs. Fourth, there was lack of a unified standard for clinical efficacy of chemical pleurodesis in MPEs, and the majority of trials did not clearly report the survivals, TRAEs, and TRM. Fifth, due to limited trials for Rh-endostatin with CBP, NDP, LBP/BLM, the treatment thresholds and optimal strategy remain unclear. Sixth, the univariate or multivariate regression analysis did not find any positive or negative correlation between clinical responses and all variables.
Conclusion
The evidence indicates that among all six combinations, only Rh-endostatin with DDP may be an optimal combination, which may improve the clinical response and QOL and reduce the failure and PD without increasing the ADRs in MPEs. For Rh-endostatin with DDP infusion, the treatment threshold may be moderate to massive effusion, KPS score ≥ 60, or AST ≥3 months. The combination may be more suitable for MPE with initial treatment or for lung cancer. Rh-endostatin (30–40 mg each time, once or twice a week 3–4 times) with DDP (30–40 mg/m2) may be a possible strategy for achieving an ideal response. The pooled results from limited trials reveal that Rh-endostatin with DDP/LBP might increase the 0.5–2-year OS rate. But the evidence fails to support that Rh-endostatin plus chemical irritants also does for MPE what it does for non-lung cancer, refractory/recurrent, or drug-resistant patients. Their ADRs and potential TRAEs remain unclear. In addition, whether Rh-endostatin with CBP, NDP, or LBP/BLM improves the clinical response and their treatment thresholds and optimal strategy also remains unclear. All of these questions need further new trials to demonstrate. Finally, these findings provide valuable references for an optimal control strategy based on Rh-endostatin in MPE.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material further inquiries can be directed to the corresponding authors.
Author Contributions
Conception and design by ZX, XX, and X-FC. Development of methodology by ZX, C-QW, and X-FC. Literature search by C-QW and HJ. Article selection by C-QW and MH. Assessment of methodological bias risk by X-RH and QC. Data extraction by X-TZ and T-yF. Statistical analysis by C-QW and X-RH. GRADE assessment by X-FC and C-QW. Preparing the manuscript draft by ZX, XX, and X-FC. Review and revision of the manuscript by XX, X-FC, LZ, JL, and J-HF. Study supervision by ZX. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by special funds for academic seedlings training and innovation at Zunyi Medical College [Qian Kehe Pingtai Rencai No. (2017) 5733-034], special funds for science and technology research into traditional Chinese and national medicine in Guizhou (No. QZYY 2017-084), and a high-level innovative talent program in Guizhou (No. fzc 120171001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.649999/full#supplementary-material
Abbreviations
ADRs, adverse drug reactions; AST, anticipated survival time; BLM, bleomycin; CBM, China Biological Medicine Database; CBP, carboplatin; CENTRAL, Cochrane Central Register of Controlled Trials; Chi-CTR, Chinese clinical trial registry; CNKI, China National Knowledge Infrastructure Database; CR, complete response; CTCAE, Common Terminology Criteria for Adverse Events standards; DDP, cisplatin; FEM, fixed-effects model; GRADE approach, Grading of Recommendations Assessment, Development and Evaluation approach; HR, hazard ratio; IPC, indwelling pleural catheter; ISI, Web of Science; KPS, Karnofsky Performance Status; LBP, lobaplatin; MPE, malignant pleural effusion; NDP, nedaplatin; NSCLC, non-small-cell lung cancer; NR, no response; OS, overall survival; OR, odds ratio; PD, progressive disease; PFS, progression-free survival; PR, partial response; PRISMA guidelines, Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines; QOL, quality of life; RCTs, randomized controlled trials; REM, random-effects model; Rh-endostatin, recombinant human endostatin; SD, stable disease; SRs, systematic reviews; TCMIs, traditional Chinese medicine injections; TRAEs, treatment-related adverse events; TRM, treatment-related mortality; VATS, video-assisted thoracic surgery; VIP, Chinese Scientific Journals Full-text Database; WHO-ICTRP, WHO International Clinical Trials Registry Platform; WHO, World Health Organization.
References
1. Society AT. Management of Malignant Pleural Effusions. Am J Respir Crit Care Med (2000) 162(5):1987–2001. doi: 10.1164/ajrccm.162.5.ats8-00
2. Rodrîguez-Panadero F, Borderas Naranjo F, López Mejîas J. Pleural Metastatic Tumours and Effusions. Frequency and Pathogenic Mechanisms in a Post-Mortem Series. Eur Respir J (1989) 2(4):366–9.
3. Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ. Management of a Malignant Pleural Effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax (2010) 65(Suppl 2):ii32–40. doi: 10.1136/thx.2010.136994
4. Feller-Kopman DJ, Reddy CB, DeCamp MM, Diekemper RL, Gould MK, Henry T, et al. Management of Malignant Pleural Effusions. An Official ATS/STS/STR Clinical Practice Guideline. Am J Respir Crit Care Med (2018) 198(7):839–49. doi: 10.1164/rccm.201807-1415ST
5. Shafiq M, Feller-Kopman D. Management of Malignant Pleural Effusions. Clin Chest Med (2020) 41(2):259–67. doi: 10.1016/j.ccm.2020.02.009[doi
6. Lombardi G, Zustovich F, Nicoletto MO, Donach M, Artioli G, Pastorelli D. Diagnosis and Treatment of Malignant Pleural Effusion: A Systematic Literature Review and New Approaches. Am J Clin Oncol (2010) 33(4):420–3. doi: 10.1097/COC.0b013e3181aacbbf
7. Marech I, Leporini C, Ammendola M, Porcelli M, Gadaleta CD, Russo E, et al. Classical and Non-Classical Proangiogenic Factors as a Target of Antiangiogenic Therapy in Tumor Microenvironment. Cancer Lett (2016) 380(1):216–26. doi: 10.1016/j.canlet.2015.07.028
8. Lieser EA, Croghan GA, Nevala WK, Bradshaw MJ, Markovic SN, Mansfield AS. Up-Regulation of Pro-Angiogenic Factors and Establishment of Tolerance in Malignant Pleural Effusions. Lung Cancer (2013) 82(1):63–8. doi: 10.1016/j.lungcan.2013.07.007
9. Zebrowski BK, Yano S, Liu W, Shaheen RM, Hicklin DJ, Putnam JB Jr., et al. Vascular Endothelial Growth Factor Levels and Induction of Permeability in Malignant Pleural Effusions. Clin Cancer Res (1999) 5(11):3364–8.
10. Bradshaw M, Mansfield A, Peikert T. The Role of Vascular Endothelial Growth Factor in the Pathogenesis, Diagnosis and Treatment of Malignant Pleural Effusion. Curr Oncol Rep (2013) 15(3):207–16. doi: 10.1007/s11912-013-0315-7
11. Walia A, Yang JF, Huang YH, Rosenblatt MI, Chang JH, Azar DT. Endostatin’s Emerging Roles in Angiogenesis, Lymphangiogenesis, Disease, and Clinical Applications. Biochim Biophys Acta (2015) 1850(12):2422–38. doi: 10.1016/j.bbagen.2015.09.007
12. Li XQ, Shang BY, Wang DC, Zhang SH, Wu SY, Zhen YS. Endostar, a Modified Recombinant Human Endostatin, Exhibits Synergistic Effects With Dexamethasone on Angiogenesis and Hepatoma Growth. Cancer Lett (2011) 301(2):212–20. doi: 10.1016/j.canlet.2010.12.004
13. Zhang Y, Qu ZH, Cui M, Guo C, Zhang XM, Ma CH, et al. Combined Endostatin and TRAIL Gene Transfer Suppresses Human Hepatocellular Carcinoma Growth and Angiogenesis in Nude Mice. Cancer Biol Ther (2009) 8(5):466–73. doi: 10.4161/cbt.8.5.7687
14. Folkman J. Antiangiogenesis in Cancer Therapy–Endostatin and its Mechanisms of Action. Exp Cell Res (2006) 312(5):594–607. doi: 10.1016/j.yexcr.2005.11.015
15. s. o. r. d. Lung cancer group, Chinese Medical Association, C. l. c. p. a. t. alliance. Expert Consensus on Antiangiogenic Drugs for Advanced Non-Small Cell Lung Cancer in China (2016 Edition). Chin J Tuberc Respir Dis (2016) 39(11):839–49. doi: 10.3760/cma.j.issn.1001-0939.2016.11.004
16. Shi Y, Sun Y, Yu J, Ding C, Wang Z, Wang C, et al. Expert Consensus on Diagnosis and Treatment of Advanced Primary Lung Cancer in China (2016 Edition). Chin J Lung Cancer (2016) 19(1):1–15. doi: 10.3779/j.issn.1009-3419.2016.01.01
17. Liang R, Xie H-Y, Lin Y, Li Q, Yuan C-L, Liu Z-H, et al. Intraperitoneal Perfusion Therapy of Endostar Combined With Platinum Chemotherapy for Malignant Serous Effusions: A Meta-Analysis. Asian Pac J Cancer Prev (2015) 16(18):8637–44. doi: 10.7314/apjcp.2015.16.18.8637
18. Liu M, Yan P, Chang G, Li K, Ouyang X. Efficacy and Safety of Injecting Endostar Combined With Platinum Complexes Into Pleural Cavity for Treatment of Malignant Pleural Effusion: A Meta-Analysis Review. J Chin Physician (2017) 19(2):257–62. doi: 10.3760/cma.j.issn.1008-1372.2017.02.024
19. Yang M, He W, Wang F, Wu M, Fan Q. Recombinant Human Endostatin Combined With Cisplatin Perfusion Chemotherapy for Malignant Pleural Effusions:a Meta-Analysis. Chin Clin Oncol (2015) 20(12):1117–23.
20. Jiang J, Xie J, Zhang L, Wang Y, Quan X. Efficacy and Safety of Recombinant Human Endostatin Combined With Cisplatin for Malignant Pleural Effusion:a Meta-Analysis. Pract J Cancer (2016) 31(3):411–6. doi: 10.3969/j.issn.1001-5930.2016.03.019
21. Sun Y, Yu Z. Endostar Combined With Cisplatin in the Treatment of Malignant Pleural Effusion: A Meta-Analysis. Mod Bus Trade Ind (2016) 37(33):166–9. doi: 10.19311/j.cnki.1672-3198.2016.33.079
22. Yang H, Chen T, Ying M. Efficacy and Safety of Combination of Recombinant Human Endostatin and Cisplatin in Treatment of Malignant Pleural Effusion: A Meta-Analysis. J Chin Oncol (2016) 22(5):410–6. doi: 10.11735/j.issn.1671-170X.2016.05.B015
23. Lv C, Wang S, Yuan F, Yao J, Gong X, Liang D, et al. Short-Term Therapeutic Effect of Recombinant Human Endostatin Combined With Cis-Platinum on Malignant Pleural Effusions: A Meta-Analysis. Herald Med (2017) 36(05):558–63.
24. Biaoxue R, Xiguang C, Hua L, Wenlong G. And Shuanying: Thoracic Perfusion of Recombinant Human Endostatin (Endostar) Combined With Chemotherapeutic Agents Versus Chemotherapeutic Agents Alone for Treating Malignant Pleural Effusions: A Systematic Evaluation and Meta-Analysis. BMC Cancer (2016) 16(1):888. doi: 10.1186/s12885-016-2935-4
25. Liu ZL, Huang LN, Wang B, Liu JZ, Liu WW. Efficacy and Safety of Endostar Combined With Cisplatin in Treatment of Non-Small Cell Lung Cancer With Malignant Pleural Effusion: A Meta-Analysis. Chin J Evid-based Med (2016) 16(5):557–63. doi: 10.7507/1672-2531.20160086
26. Zhao Z, Yang Y, Wang X, Zhou W, Wang Z. A Meta-Analysis of the Efficacy and Safety of Recombinant Human Vascular Endostatin Combined With Cisplatin in the Treatment of Non-Small Cell Lung Cancer With Malignant Pleural Effusion. Asian Case Rep Oncol (2018) 7(03):17–25. doi: 10.12677/acrpo.2018.73003
27. Zhou Y, Guo Y, Yang X. Intrapleural Injection of Endostar Combined With Cisplatin for Lung Cancer With Malignant Pleural Effusion:a Meta-Analysis. J Pract Oncol (2018) 33(6):553–9. doi: 10.13267/j.cnki.syzlzz.2018.06.012
28. E. C. o. s. m. o. a. d. o. C. s. o. C. Oncology, E. C. o. v. t. t. o. C. s. o. C. Oncology. Expert Consensus on Clinical Application of Recombinant Human Endostatin in the Treatment of Malignant Serous Effusion. Chin Clin Oncol (2020) 25(9):849–56.
29. Yin Y, Zhou H, Yang J, Yang H, Shao Y, Xu Q, et al. Clinical Efficacy of Recombinant Human Endostatin Combined With Lobaplatin in the Treatment of Malignant Thoracic Fluid and Prognosis Analysis. Eval Anal Drug-Use Hosp China (2020) 20(07):804–807+811. doi: 10.14009/j.issn.1672-2124.2020.07.010
30. Xu M, Chen Y, Hu J. Clinical Study of Intrathoracic Perfusion of Endostar Combined With Cisplatin in the Treatment of Non-Small Cell Lung Cancer Complicated With Massive Malignant Pleural Effusion. J Guangdong Med Coll (2020) 38(2):178–80. doi: 10.3969/j.issn.1005-4057.2020.02.013
31. Li S. Effects of Recombinant Human Endostatin Combined With Intraleural Injection of Cisplatin on Patients With Non-Small Cell Lung Cancer Complicated With Blood Pleural Effusion. Chin J Prac Med (2020) 47(3):102–4. doi: 10.3760/cma.j.issn.1674-4756.2020.03.027
32. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Int J Surg (2010) 8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007[doi
33. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
34. Kessinger A, Wigton RS. Intracavitary Bleomycin and Tetracycline in the Management of Malignant Pleural Effusions: A Randomized Study. J Surg Oncol (1987) 36(2):81–3. doi: 10.1002/jso.2930360202[doi
35. Paladine W, Cunningham TJ, Sponzo R, Donavan M, Olson K, Horton J. Intracavitary Bleomycin in the Management of Malignant Effusions. Cancer (1976) 38(5):1903–8. doi: 10.1002/1097-0142(197611)38:5<1903::aid-cncr2820380506>3.0.co;2-a
36. Jie Wang X, Miao K, Luo Y, Li R, Shou T, Wang P, et al. Randomized Controlled Trial of Endostar Combined With Cisplatin/Pemetrexed Chemotherapy for Elderly Patients With Advanced Malignant Pleural Effusion of Lung Adenocarcinoma. J buon (2018) 23(1):92–7.
37. Keeratichananont W, Limthon T, Keeratichananont S. Efficacy and Safety Profile of Autologous Blood Versus Tetracycline Pleurodesis for Malignant Pleural Effusion. Ther Adv Respir Dis (2015) 9(2):42–8. doi: 10.1177/1753465815570307[doi
38. Xiao Z, Jiang Y, Chen XF, Wang CQ, Zheng XT, Xu WH, et al. Intrathoracic Infusion Therapy With Lentinan and Chemical Irritants for Malignant Pleural Effusion: A Systematic Review and Meta-Analysis of 65 Randomized Controlled Trials. Phytomedicine (2020) 76:153260. doi: 10.1016/j.phymed.2020.153260
39. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting Results of Cancer Treatment. Cancer (1981) 47(1):207–14. doi: 10.1002/1097-0142(19810101)47:1<207::AID-CNCR2820470134>3.0.CO;2-6
40. Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE V3.0: Development of a Comprehensive Grading System for the Adverse Effects of Cancer Treatment. Semin Radiat Oncol (2003) 13(3):176–81. doi: 10.1016/S1053-4296(03)00031-6[doi
41. Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced Secondary Analysis of Survival Data: Reconstructing the Data From Published Kaplan-Meier Survival Curves. BMC Med Res Methodol (2012) 12:9. doi: 10.1186/1471-2288-12-9[doi
42. Xiao Z, Wang CQ, Zhou MH, Li NN, Liu SY, He YJ, et al. Clinical Efficacy and Safety of CIK Plus Radiotherapy for Lung Cancer: A Meta-Analysis of 16 Randomized Controlled Trials. Int Immunopharmacol (2018) 61:363–75. doi: 10.1016/j.intimp.2018.06.012
43. Xiao Z, Jiang Y, Wang CQ, Hu SS, Huang XR, Chen XF, et al. Clinical Efficacy and Safety of Aidi Injection Combination With Vinorelbine and Cisplatin for Advanced Non-Small-Cell Lung Carcinoma: A Systematic Review and Meta-Analysis of 54 Randomized Controlled Trials. Pharmacol Res (2020) 153:104637. doi: 10.1016/j.phrs.2020.104637
44. Xiao Z, Jiang Y, Chen XF, Wang CQ, Xu WH, Liu Y, et al. The Hepatorenal Toxicity and Tumor Response of Chemotherapy With or Without Aidi Injection in Advanced Lung Cancer: A Meta-Analysis of 80 Randomized Controlled Trials. Clin Ther (2020) 42(3):515–543 e31. doi: 10.1016/j.clinthera.2020.01.011
45. Sun X, Briel M, Walter SD, Guyatt GH. Is a Subgroup Effect Believable? Updating Criteria to Evaluate the Credibility of Subgroup Analyses. Bmj (2010) 340:c117. doi: 10.1136/bmj.c117
46. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. Bmj (2008) 336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD
47. Aydin Y, Turkyilmaz A, Intepe YS, Eroglu A. Malignant Pleural Effusions: Appropriate Treatment Approaches. Eurasian J Med (2009) 41(3):186–93.
48. Yang J, Ding M. Eddie Combined With Gemcitabine and Cisplatin for Advanced non-Small Cell Lung Cancer: Meta-Analysis. Chin Gen Pract (2012) 15(24):2794–8. doi: 10.3969/j.issn.1007-9572.2012.08.100
49. Tian J, Zhao Y, Li J, Ge L, Yang K. Network Meta-Analysis of 12 Chinese Herb Injections Combined With Gemcitabine and Cisplatin for non-Small Cell Lung Cancer. Chin J Drug Eval (2014) 31(6):350–5. doi: 10.3969/j.issn.2095-3593.2014.06.010
50. Xiao Z, Wang C, Sun Y, Li N, Li J, Chen L, et al. Can Aidi Injection Restore Cellular Immunity and Improve Clinical Efficacy in non-Small-Cell Lung Cancer Patients Treated With Platinum-Based Chemotherapy? A Meta-Analysis of 17 Randomized Controlled Trials Following the PRISMA Guidelines. Med (Baltimore) (2016) 95(44):e5210. doi: 10.1097/MD.0000000000005210[doi]00005792-201611010-00029[pii
51. Xiao Z, Wang C, Chen L, Tang X, Li L, Li N, et al. Has Aidi Injection the Attenuation and Synergistic Efficacy to Gemcitabine and Cisplatin in Non-Small Cell Lung Cancer? A Meta-Analysis of 36 Randomized Controlled Trials. Oncotarget (2017) 8(1):1329–42. doi: 10.18632/oncotarget.13617[doi
52. Wu J, Wang Q, Lin L, Sun L. Clinical Efficacy of Aidi Injection Combined With First-Line Chemotherapy in the Treatment of Non-Small Cell Lung Cancer: A Meta-Analysis. Chin Tradit Patent Med (2017) 39(6):1323–8. doi: 10.3969/j.issn.1001-1528.2017.06.051
53. Wang J, Li G, Yu L, Mo T, Wu Q, Zhou Z. Aidi Injection Plus Platinum-Based Chemotherapy for Stage IIIB/IV Non-Small Cell Lung Cancer: A Meta-Analysis of 42 RCTs Following the PRISMA Guidelines. J Ethnopharmacol (2018) 221:137–50. doi: 10.1016/j.jep.2018.04.013
54. Rao X, Long J, Zha G, Mei L, Zheng Q, Li Y. Efficacy and Safety of Endostar Combined With Cisplatin in the Treatment of Malignant Pleural Effusion by Intrapleural Injection. Contemp Med (2018) 24(20):60–2. doi: 10.3969/j.issn.1009-4393.2018.20.025
55. Huang D. Clinical Observation of Endostar Combined With Cisplatin in the Treatment of Malignant Pleural Effusion. J Clin Med Pract (2010) 14(13):63–4. doi: 10.3969/j.issn.1672-2353.2010.13.023
56. Li J, Gong K, Zhu Z, Yang Q, Ha M. Recombinant Human Endostatin Combined With Cisplatin for Malignant Pleural Effusion:A Clinical Study. Chin Pharm (2010) 21(14):1308–10.
57. Li GY, Zhao JD, Li YY, Di J, Jiang J, Zhao JH. Application of Endostar Combined With Bleomycin in the Treatment of Malignant Pleural Effusion. Chin J New Drugs (2011) 20(16):1544–7.
58. Li W. Clinical Study of Endothoracic Perfusion in the Treatment of Malignant Pleural Effusion. J Med Forum (2011) 32(7):170–1.
59. Liu Z, Cui E, Wang B, Zhang S. Clinical Study of Recombinant Human Endostatin Combined With Carboplatin in the Treatment of Malignant Pleural Effusion. Zhejiang JITCWM (2011) 21(11):784–5. doi: 10.3969/j.issn.1005-4561.2011.11.017
60. Mao L, Liao G, Wang H, Liu P, Xie G. Observation of Clinical Efficacy of Endostar Combined With DDP on Malignant Bloody Pleural Effusion. Med J NDFSC (2011) 21(7):723–5. doi: 10.3969/j.issn.1004-0188.2011.07.008
61. Zhang Y, Liu J, Long L, Xiang H. Clinical Research on Recombinant Human Endostatin Combined With Bleomycin in the Treatment of Malignant Pleural Effusion. Anti-tumor Pharm (2011) 01(6):541–3. doi: 10.3969/j.issn.2095-1264.2011.06.021
62. Jiang B. Recombinant Human Endostatin Combined With Cisplatin in the Treatment of Malignant Pleural Effusion: A Clinical Study. In: Internal Medicine. Xiling: Qinghai University (2012).
63. Li H, Ji F, Ma J, Lin M, Wei W, Zhao J. Recombinant Human Endostatin Injection Combined With Cisplatin and Bleomycin in the Treatment of Malignant Pleural Effusion a Clinical Study of 30 Cases. Chin J Gerontol (2012) 32(18):3998–9. doi: 10.3969/j.issn.1005-9202.2012.18.063
64. Liu X, Wang L. Clinical Observation of Intracavitary Chemotherapy of Endostar Combined With Chemotherapy in the Treatment of Non-Small Cell Lung Cancer With Malignant Pleural Effusion. J Basic Clinl Oncol (2012) 25(3):233–5. doi: 10.3969/j.issn.1673-5412.2012.03.016
65. Luo J, Hua F, Wang B. Effect of Recombinant Human Endostatin Injection Combined With Bleomycin Injection in the Treatment of Thoracic Metastasis of Lung Cancer. Chin J Postgrad Med (2012) 35(4):31–2. doi: 10.3760/cma.j.issn.1673-4904.2012.04.012
66. Miao H, Kong F. Radiofrequency Hyperthermia Combined With Endostar and Cisplatin in the Treatment of Malignant Pleural Effusion. Chin Commun Doctors (2012) 14(22):86–7. doi: 10.3969/j.issn.1007-614x.2012.22.082
67. Shen Q, Gu A, Wu J, Jin F, Zhu J, Yao X, et al. Therapeutic Observation of Endostar Combined With Cisdiammi Dichloride Platinum on non-Small Cell Lung Cancer With Malignant Pleural Effusion. J Clin Med Pract (2012) 16(5):29–31. doi: 10.3969/j.issn.1672-2353.2012.05.010
68. Wang X, Chen C. Study of New Recombinant Endostatin Combined With Cisplatin Injection for Treatment of Malignant Pleural Effusion. Mod Prev Med (2012) 39(1):200–1.
69. Yao Q, Lin Q, Liu S, Shen D, Lin F, Mao W. Efficacy of Intrapleural Infusion of Endostar Combined With Nedaplatin in the Treatment of Malignant Pleural Effusion. J Basic Clinl Oncol (2012) 25(6):492–4.
70. Zhang J, Liu H, Li Q. Clinical Observation on the Treatment of Endoststin Combined With Bleomycin in Patients With Malignant Pleurisy Effusions Caused by Lung Cancer. J Mod Oncol (2012) 20(5):975–7. doi: 10.3969/j.issn.1672-4992.2012.05.32
71. Han Z, Wang F, Liu J. The Effect of Rhendostatin Combined With Dichlorodiamineplatinum on Patients With Malignant Pleural Effusion. Chin J Clin Pharmacol (2013) 29(8):587–9. doi: 10.13699/j.cnki.1001-6821.2013.08.013
72. He L, Shi Y, Hua G. Recombinant Human Endostatin Combined With Cisplatin in the Treatment of Malignant Pleural Effusion: A Clinical Study of 32 Cases. China Pharm (2013) 22(12):49–50. doi: 10.3969/j.issn.1006-4931.2013.12.033
73. Kang L, Gao L, Cao J, Fu Z, Xu H, Zheng L. Clinical Observation on Pleural Perfusion of Recombinant Human Endostain Combined With Cisplatin on Malignant Pleural Effusion. Clin Focus (2013) 28(12):1371–3. doi: 10.3969/j.issn.1004-583X.2013.12.016
74. Yang K. Clinical Study of Recombinant Human Endostatin Combined With Nadaplatin for Treatment of Malignant Pleural Effusion. Acta Acad Med Xuzhou (2013) 33(12):891–3.
75. Yang Y, Lin R, Cao G. Short-Term and Long-Term Efficacy of Endostar Combined With Cis-Diaminedichloroplatinum in Treating Malignant Pleural Effusion of Non-Small Cell Lung Cancer. China Pharm (2013) 22(19):21–2. doi: 10.3969/j.issn.1006-4931.2013.19.011
76. Zheng Q, Hu W, Liao X, Zheng X, Zhou Z, Wu S, et al. A Comparision Between Intrapleural Injection of Cisplatin Combined With Endostar and Cisplatin Alone in the Treatment for Malignant Pleural Effusion. J Chin Oncol (2013) 19(5):386–9.
77. Chen J, Gou S, Luan W. Study on the Efficacy of Endostar Combined With Cisplatin in Treatment of Non-Small Cell Lung Cancer With Malignant Pleural Effusion and Influence on Tumor Markers VEGF and HIF-1α. J Clin Exp Med (2014) 13(21):1778–80. doi: 10.3969/j.issn.1671-4695.2014.21.012
78. Huang L. Clinical Study of Endostar Combined With Cisplatin in the Treatment of Malignant Pleural Effusion of Non-Small Cell Lung Cancer. Jilin Med J (2014) 35(19):4308–9.
79. Li C, Zhao Y, Zhang C. Clinical Study of Recombinant Endostatin Injection Combined With Paclitaxel Injection in the Treatment of Malignant Pleural Effusion. PJCCPVD (2014) 22(03):123–4.
80. Li Y, Zhao J, Luo Y, Li G, Li J. Effect of Endostatin on Vascular Endothelial Growth Factor and Epidermal Growth Factor Receptor in Pleural Effusion of Elderly Patients With Malignant Pleural Effusion. Chin J Gerontol (2014) 34(23):6611–6612,6613. doi: 10.3969/j.issn.1005-9202.2014.23.032
81. Lu H. Clinical Observation of Recombinant Human Endostatin Combined With Chemotherapy in the Treatment of Pleural Effusion. Yiayao Qianyan (2014) 4(14):396–6. doi: 10.3969/j.issn.2095-1752.2014.14.433
82. Tu J, Huang S, Wang M. Clinical Efficacy of Pleural Perfusion With Recombinant Human Endostatin Combined With Cisdiammi Dichloride Platinum for Advanced Non-Small Cell Lung Cancer Patients With Malignant Pleural Effusion. Pract J Cancer (2014) 29(12):1592–4. doi: 10.3969/j.issn.1001-5930.2014.12.026
83. Xu J, Qi D, Li X, Wang R. Efficacy of Recombinant Human Endostatin (Endostar) Combined With Chemotherapy for Malignant Pleural Effusion in Non-Small Cell Lung Cancer Patients. Chin J Clin Oncol (2014) 41(24):1573–6. doi: 10.3969/j.issn.1000-8179.20141512
84. Yue G, Bo Y, Ma H, Li N, Bing W, Shi L, et al. Analysis of Efficacy and Security Cisplatin Endostar in the Treatment of non-Small Cell Lung Cancer Bloody Pleural Effusion. Mod Diag Treat (2014) 25(11):2478–80. doi: 10.3969/j.issn.1001-8174.2014.11.049
85. Dong M. Comparison of Combination of Endostar and Cisplatin on the Curative Effect and Immune Function in Malignant Pleural Effusion Patients. In: Medical Oncology. Nanchang: Nanchang University (2015). doi: 10.7666/d.D692128
86. Hu X, Wang H, Zhang C, Liu P, Wang Y, Li J, et al. Clinical Study on Intra-Thoracic Chemotherapy With Recombinant Human Endostain Combined With Cisplatin in Treatment of Patients With Malignant Pleural Effusion. Clin Med J (2015) 13(3):23–7. doi: 10.3969/j.issn.1672-3384.2015.03.006
87. Pang Z, Sun X. The Clinical Observation and Safety of Intrapleural Injection of Endostar Combined With Cisplatin in Treatment of Malignant Pleural Effusion. China Med Pharm (2015) 5(4):176–8.
88. Zhao W, Ma C, Zhao W, Zhang L, Wang S, Wei H. Clinical Study of Endostar on Malignant Cavity Effusion. J Taishan Med Coll (2015) 36(10):1089–92. doi: 10.3969/j.issn.1004-7115.2015.10.003
89. Chang Y. Clinical Analysis of Cisplatin Combined With Endostar in the Treatment of Malignant Pleural Effusion of Lung Cancer. Chin J Mod Drug Appl (2016) 10(10):155–6. doi: 10.14164/j.cnki.cn11-5581/r.2016.10.115
90. Chen F, Li Q, Jin G, Zhang H. Efficacy of Recombinant Human Endostatin Combined With Cisplatin in the Treatment of Malignant Pleural Effusion of Non-Small Cell Lung Cancer. Chin J Oncol Prev Treat (2016) 8(4):246–9. doi: 10.3969/j.issn.1674-5671.2016.04.10
91. Chen R, Zhang C, Wu H, Yang S. Clinical Effect of Pleural Perfusion of Human Recombinant Endostatin Injection Combined With Cisplatin Injection on Advanced Non-Small Cell Lung Cancer Complicated With Malignant Pleural Effusion. PJCCPVD (2016) 24(5):118–20. doi: 10.3969/j.issn.1008-5971.2016.05.033
92. He J, Hou J, Zhai M, Zheng X. Evaluation of Curative Effect of Endostar Combined With Cisplatin Intrapleural Administration in Treatment of Malignant Pleural Effusion Induced by non-Small Cell Lung Cancer. Int J Resp (2016) 36(15):1127–30. doi: 10.3760/cma.j.issn.1673-436X.2016.15.002
93. Li H, Cai S, Zhao G, Guo A. Observation of the Clinical Curative Effect of Recombinant Human Endostatin Injection Combined With Lobaplatin in the Treatment of Malignant Pleural Effusion. Chin J Front Med Sci (2016) 8(12):42–5. doi: 10.12037/yxqy.2016.12-09
94. Li Y. Efficacy and Adverse Reactions of Endostar Combined With Cisplatin in the Treatment of Malignant Pleural Effusion of Non-Small Cell Lung Cancer. China Med Dev (2016) 31(S1):223–3.
95. Lu J, Xie Q, Chen Q, Sun W, Zhong A, Shi Q, et al. Clinical Study of Intrapleural Injection of Recombinant Human Endostatin Combined With Cisplatin in the Treat-Ment of Lung Adenocarcinoma With Malignant Pleural Effusion. J Clin Pulm Med (2016) 21(9):1664–7. doi: 10.3969/j.issn.1009-6663.2016.09.032
96. Pang H, Nie F, Zhang E. Clinical Observation of Endostar Combined With Carboplatin in the Treatment of Malignant Pleural Effusion. China Med Eng (2016) 24(3):64–5. doi: 10.19338/j.issn.1672-2019.2016.03.030
97. Qin M. Clinical Study of Cisplatin Combined With Endostar in the Treatment of Malignant Pleural Effusion of Advanced Non-Small Cell Lung Cancer. China Prac Med (2016) 11(26):228–9. doi: 10.14163/j.cnki.11-5547/r.2016.26.147
98. Song X. Comparison of VEGF Changes in Malignant Pleural Effusion and the Efficacy of Endostar Combined With Cisplatin. In: Oncology. Nanchang: Nanchang University (2016). doi: 10.7666/d.D01016609
99. Zhang P, Gong C, Zhang Q, Xiong H. Clinical Effect of Intrapleural Infusion With Recombinant Human Endostatin Combined With Cisplatin on Malignant Pleura Effusion in Breast Cancer Patients. Chin J Hemorh (2016) 26(1):32–5,49. doi: 10.3969/j.issn.1009-881X.2016.01.008
100. Zheng W, Kang J, Wen J, Wang J. Influence of Recombinant Human Endostatin Combined With Cisplatinum on VEGF, EGFR and Tumor Markers in Patients With Malignant Pleural Effusion. J Clin Med Pract (2016) 20(23):32–5. doi: 10.7619/jcmp.201623010
101. Zhou J, Du Y. Effect of Recombinant Human Endostatin Combined With Cisplatin on Inflammatory Factors and Immune Function in Elderly Patients With Malignant Pleural Effusion. Chin J Med (2016) 51(4):95–7. doi: 10.3969/j.issn.1008-1070.2016.04.029
102. Zhou Y, Peng D, He L. Endostar Combined With Nedaplatin in the Treatment of Malignant Pleural Effusion Caused by Lung Cancer. Hainan Med J (2016) 27(21):3550–1. doi: 10.3969/j.issn.1003-6350.2016.21.036
103. Zou J, Hu Z, Chen Y. Clinical Efficacy and Adverse Reactions of Cisplatin Combined With Endostar Thoracic Perfusion in the Treatment of Malignant Pleural Effusion of Lung Cancer. China Mod Med (2016) 23(24):22–4.
104. Che X. Study of Endostain Combined With Cisplation in Malignant Pleural Effusion of Lung Cancer. Chin J Biochem Pharm (2017) 37(2):157–61. doi: 10.3969/j.isn.1005-1678.2017.02.047
105. Chen X, Chen J. Clinical Study of Lobaplatin Combined With Endostar in the Treatment of Lung Cancer Complicated With Malignant Pleural Effusion. In: Annual Meeting of Digital Chinese Medicine Branch of International Digital Medical Association & the Second Academic Exchange Meeting of Digital Chinese Medicine. Guangzhou, China (2017).
106. Feng Z. Effects of Endostar Combined With Cisplatin on Platelet Parameters, VEGF and HIF-1 α Levels in Patients With Non-Small Cell Lung Cancer Complicated With Malignant Pleural Effusion. Henan Med Res (2017) 26(24):4454–5. doi: 10.3969/j.issn.1004-437X.2017.24.015
107. Han Z, Ma L, Liu J, Cui Z. Recombinant Human Endostatin Combined With Cisplatin in the Treatment of Non-Small Cell Lung Cancer Complicated With Malignant Pleural Effusion: A Clinical Study of 30 Cases. Chongqing Med (2017) 46(A03):96–7.
108. Jia X, Wen Z. Clinical Observation of Endostar Combined With Cisplatin in Treatment of Patients With Malignant Pleural Effusion. J Dis Monit Control (2017) 11(8):627–8.
109. Lu X, Zhang T. Clinical Efficacy of Pleural Perfusion With Recombinant Human Endostatin and Cisplatin in Advanced Non-Small Cell Lung Cancer Patients With Malignant Pleural Effusion. Jiangsu Med J (2017) 43(14):1023–5. doi: 10.19460/j.cnki.0253-3685.2017.14.013
110. Zhao Q, Ouyang X, Li K. Observe the Curative Effect in the Treatment of Malignant Pleural Effusion Intrapleural Injection of Endostar. J Clin Med Lit (2017) 4(1):14–5. doi: 10.3877/cma.j.issn.2095-8242.2017.01.009
111. Chen X. Clinical Observation of Recombinant Human Endostatin Combined With Cisplatin in the Treatment of Pleural Effusion of Lung Cancer. Chin J Prim Med Pharm (2018) 25(7):902–5. doi: 10.3760/cma.j.issn.1008-6706.2018.07.023
112. Fan Y, Zou Q, Li X, Qi X, Dong J, Liu J, et al. Analysis of the Efficacy of Endostar Thoracic Perfusion and DDP Intravenous Chemotherapy for Malignant Pleural Effusion of Breast Cancer. Pract J Cancer (2018) 33(7):1175–7. doi: 10.3969/j.issn.1001-5930.2018.07.038
113. Li T. Clinical Efficacy of Recombinant Human Endostatin Combined With Cisplatin in the Treatment of Pleural Effusion of Lung Cancer. Yiyao Qianyan (2018) 8(2):216–7. doi: 10.3969/j.issn.2095-1752.2018.02.187
114. Liu H, Tan W. Recombinant Vascular Endostatin Therapy for Malignant Pleural Effusion. Acta Acad Med Weifang (2018) 40(3):217–9. doi: 10.16846/j.issn.1004-3101.2018.03.017
115. Liu Y, Huang M, Yao W. Clinical Analysis of Endostatin Combined With Cisplatin in the Treatment of Non-Small Cell Lung Cancer With Malignant Pleural Effusion. J Hunan Univ Chin Med (2018) 38(A01):159–60.
116. Qing S, Wei M, Gong D, He D. Efficacy of Intrapleural Injection of Recombinant Human Endostatin Injection Combined With Cisplatin on Treatment of Non-Small Cell Lung Cancer With Bloody Pleural Effusion. J Chengdu Med Coll (2018) 13(4):487–489,492. doi: 10.3969/j.issn.1674-2257.2018.04.025
117. Qiu H, Li L, Yang Q, Chen G, Zhang J. Efficacy Observation of Recombinant Human Endostatin Combined With Cisplatin in the Treatment of Malignant Pleural Effusion in Breast Cancer. China Med Pharm (2018) 8(9):170–3. doi: 10.3969/j.issn.2095-0616.2018.09.051
118. Reng D. Clinical Study of YH-16 Combined With Cisplatin in the Treatment of Malignant Pleural Effusion of Lung Adenocarcinoma. In: Oncology. Luoyang: Henan University of Science and Technology (2018). doi: 10.7666/d.D01493120
119. Song W, Bao Y, Qian J, Zhu L, Cao S, Tao Z. Clinical Trial of Recombinant Human Endostatin Injection Combined With Cisplatin Injection in the Treatment of Malignant Pleural Effusion. Chin J Clin Pharmacol (2018) 34(7):802–4. doi: 10.13699/j.cnki.1001-6821.2018.07.020
120. Wang R. Clinical Efficacy of Recombinant Human Endostatin Combined With Cisplatin in the Treatment of Malignant Pleural Effusion of Non-Small Cell Lung Cancer. China Prac Med (2018) 13(8):96–7. doi: 10.14163/j.cnki.11-5547/r.2018.08.053
121. Jiang W, Dai X, Huang C, Li K, Leng L. Analysis of Expression Level Influence and Clinical Efficacy of Endostar Combined With Cisplatin on Malignant Pleural Effusion of Lung Cancer Rrm1. Drug Eval Res (2019) 42(5):944–8. doi: 10.7501/j.issn.1674-6376.2019.05.026
122. Tian L, Wu G, Yu H. Clinical Effect of Cisplatin Combined With Recombinant Human Vascular Endostatin Intrapleural Perfusion in the Treatment of Non-Small Cell Lung Cancer Complicated by Malignant Pleural Effusion. Trauma Crit Care Med (2019) 7(1):20–2. doi: 10.16048/j.issn.2095-5561.2019.01.06
123. Zheng D, Wang L. Clinical Study of Endostar Combined With Cisplatin in the Treatment of Malignant Pleural Effusion. Guide China Med (2019) 17(17):104–5. doi: 10.15912/j.cnki.gocm.2019.17.083
124. ChiCtr. A Multicenter and Randomized Controlled Clinical Study on the Treatment of Malignant Pleural Effusion With Endostar and/or Nedaplatin (2018). Available at: http://www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1800016259.
125. Chi CI. The Randomized Controlled Study of Intrathoracic Application of Recombinant Human Endostatin Single or Combined With Cisplatin for the Treatment of Malignant Pleural Effusion (2016). Available at: http://www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-IPR-16008803.
126. Chi CI. Effect of Recombinant Human Endostatin on the Clinical Treatment of Malignant Cavity Effusion (2017). Available at: http://www.chictr.org.cn/showproj.aspx?proj=19870.
127. Gui P, Song Y, Guo G. Effect of Endostar Combined With Cisplatin Cavity Chemotherapy on Peripheral Blood sPD-L1 and CEA Levels in Treatment of Middle-Late Stage Non-Small Cell Lung Cancer. J Clin Pulm Med (2017) 22(7):1218–21. doi: 10.3969/j.issn.1009-6663.2017.07.016
128. Liu Y, Zhang X, Huang L. Meta -Analysis of the Treatment Efficacy of Staphylococcin Plus Cisplatin Versus Cisplatin Monotherapy in Malignant Pleural Effusion and Malignant Intraperitoneal Effusion. J Modern Oncol (2016) 24(21):3479–84. doi: 10.3969/j.issn.1672-4992.2016.21.037
129. Fuhong D, Xiang G, Haiying L, Jiangye W, Xueming G, Wenxiao C. Evaluation of Efficacy and Safety for Brucea Javanica Oil Emulsion in the Control of the Malignant Pleural Effusions via Thoracic Perfusion. BMC Cancer (2018) 18(1):411. doi: 10.1186/s12885-018-4328-3
130. Chen J, Chen YJ, Wu MD. Herbal Extract Elemene Intrathoracic Injection in the Treatment of Lung Cancer Patients With Malignant Pleural Effusion: A Meta-Anaylsis. J Cancer Res Ther (2014) 10(Suppl 1):56–9. doi: 10.4103/0973-1482.139761
131. Xu C, Hu Z, Xie M, Liu Y. Meta-Analysis of Intrapleural Injection of Compound Kushen Injection Combined With Chemotherapy in Treating Malignant Pleural Effusion. China Pharm (2015) 24(18):31–3.
Keywords: endostatin, recombinant human endostatin (Rh-endostatin), chemical irritants, cisplatin, optimal adjuvant strategy, meta-analysis
Citation: Wang C-Q, Huang X-R, He M, Zheng X-T, Jiang H, Chen Q, Fan T-Y, Zhan L, Ling J, Feng J-H, Xiao X, Chen X-F and Xiao Z (2021) Intrapleural Administration With Rh-Endostatin and Chemical Irritants in the Control of Malignant Pleural Effusion: A Systematic Review and Meta-Analysis. Front. Oncol. 11:649999. doi: 10.3389/fonc.2021.649999
Received: 15 March 2021; Accepted: 15 July 2021;
Published: 03 August 2021.
Edited by:
Luciano Mutti, Temple University, United StatesReviewed by:
Janaki Deepak, University of Maryland, Baltimore, United StatesRiccardo Giovannetti, Azienda Ospedaliera Integrata Verona, Italy
Copyright © 2021 Wang, Huang, He, Zheng, Jiang, Chen, Fan, Zhan, Ling, Feng, Xiao, Chen and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Xiao, enk0MjZmQDE2My5jb20=; Xue Xiao, eHhlbGxlbkAxNjMuY29t; Xiao-Fan Chen, eGlhb2ZhbmNpMTIyMzA2QDE2My5jb20=
†These authors have contributed equally to this work
 Cheng-Qiong Wang1,2†
Cheng-Qiong Wang1,2† Xue Xiao
Xue Xiao Zheng Xiao
Zheng Xiao