- Department of General Surgery, Jiangdu People' s Hospital Affiliated to Yangzhou University Medical School, Yangzhou, China
Purpose: The prognostic significance of ypN0 rectal cancer with comparison to pN0 disease still remains poorly defined. This study aimed to compare the prognosis of ypN0 and pN0 rectal cancer.
Methods: Eligible patients were identified from the SEER18 registries research database (the latest data up to date was on April 15, 2019). Propensity score (PS) matching was usually performed to reduce the imbalance and potential confounding that were introduced by inherent differences between the groups. The cause-specific survival (CSS) was analyzed to evaluate the prognostic prediction of ypN0 and pN0 groups using the Kaplan–Meier method with the log-rank test. Cox proportional hazard model was also used to identify independent prognostic variables.
Results: In total, 26,832 patients diagnosed with pN0 or ypN0 rectal cancer were confirmed as the final cohort, including 7,237 (27.0%) patients with radiation and 19,595 (73.0%) patients without radiation prior to surgery. The median follow-up time was up to 81 months. After adjusting for other prognostic factors, neoadjuvant radiotherapy was not an independent prognostic variable of CSS (HR = 1.100, 95%CI = 0.957–1.265, P = 0.180, using pN0 group as the reference).
Conclusions: ypN0 rectal cancer was strongly associated with worse pathological diagnoses compared with pN0 rectal cancer, contributing to worse oncologic outcomes. However, the receipt of neoadjuvant chemoradiotherapy was not an independent prognostic factor of worse prognosis in pathological node-negative patients. Our study could give guidance to the treatment of ypN0 rectal cancer.
Introduction
Colorectal cancer was one of the most frequently diagnosed malignances around the world (1, 2). Due to the different anatomical location characteristics of the rectum from colon, the treatment of rectal cancer is more complex.
Currently, neoadjuvant chemoradiotherapy followed by total mesorectal excision (TME) has been widely accepted as the standard treatment for locally advanced rectal cancer (3, 4). And the histopathological evaluation of TME resection specimens played a vital role in evaluating the prognosis of rectal cancer after neoadjuvant chemoradiotherapy, which was highly dependent on the accurate assessment of postoperative lymph node status (5).
Previous studies had shown that lymph node-negative rectal cancer after neoadjuvant chemoradiation therapy (ypN0) was associated with an excellent prognosis, and the 5-year disease-free survival ranged from 79.8 to 87% (6–8). Later in 2014, with a retrospective analysis of a total of 473 patients diagnosed with rectal cancer, Erlenbach-Wünsch et al. (9) found that ypN0 rectal cancer could achieve similar oncologic results compared with pN0 disease, which suggested that adjuvant chemotherapy for ypN0 might result in overtreatment. However, this study had just a small sample size and needs to be validated in other studies, and the prognostic significance of ypN0 rectal cancer with comparison to pN0 disease still remains poorly defined (9). Here, therefore, using the newly released large population-based cancer database, we conducted this propensity score (PS) matched study to compare the prognosis of ypN0 and pN0 rectal cancer.
Methods
Ethics
The present study complied with the Declaration of Helsinki. All authors reviewed and approved the final edition of this manuscript. The US Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute (NCI) was an open public database, and the release of data from the SEER database did not require informed patient consent because cancer was a reportable disease in every state of the USA.
Patients
As a population-based cancer registration system, the US SEER database of the NCI provides different datasets on cancer demographic information and survival, covering approximately 28% of US populations. Using the SEER* Stat 8.3.5 software, we identified patients from the SEER18 registries research database (the latest data up to date was on April 15, 2019). The SEER18 database contained data from the SEER9 registries, the SEER13 registries (SEER 9 plus Los Angeles, San Jose-Monterey, Rural Georgia, and the Alaska Native Tumor Registry), and the registries of Greater California, Kentucky, Louisiana, New Jersey, and Greater Georgia (10). Patients' characteristics including No. of LNs dissected, American Joint Committee on Cancer T-stage (T1, T2, T3, and T4), age at diagnosis (years), race (white, black, and other), gender (male and female), year of diagnosis (2004–2011), tumor site (rectosigmoid primary and rectal primary), grade (well/Moderate, poor/anaplastic, and unknown), chemotherapy, serum carcinoembryonic antigen (CEA) level (negative, positive, and unknown), tumor size (≤5, >5 cm, and unknown) and perineural invasion (no, yes, and unknown) were obtained from the SEER database.
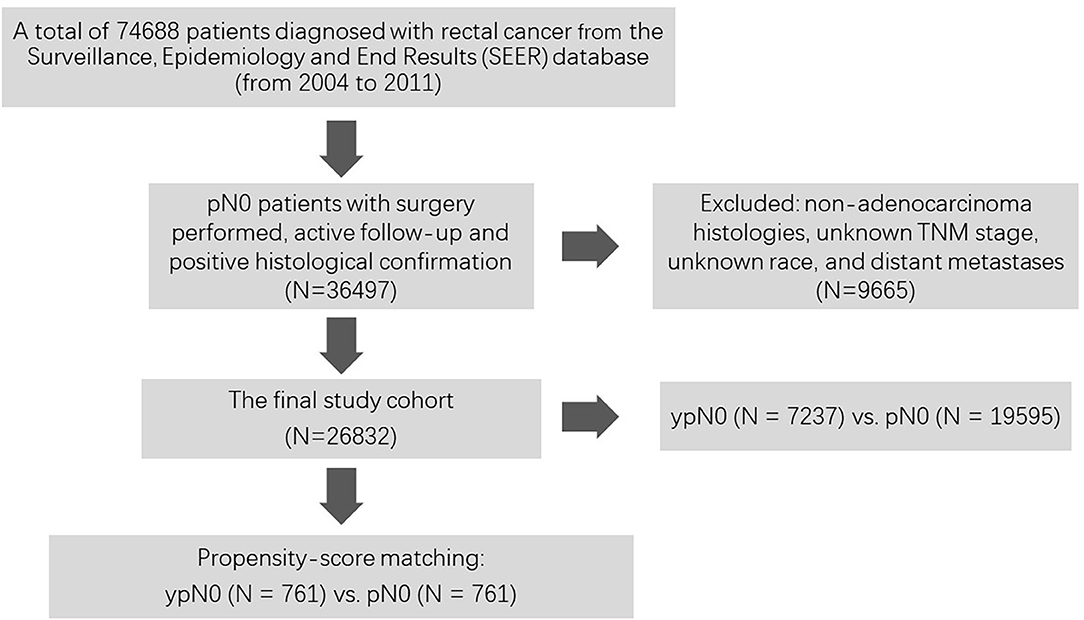
As shown in Figure 1, at first, a total of 74,688 patients diagnosed with rectal cancer between 2004 and 2011 were identified from the Surveillance, Epidemiology, and End Results (SEER) database. Then, patients with surgery performed, active follow-up, positive histological confirmation, and pathological N0 status were included into our analyses. Those with non-adenocarcinoma histologies, unknown TNM stage, unknown race, and distant metastases were excluded from the present study. Among them, patients with (n = 7,237) or without (n = 19,595) radiation prior to surgery were confirmed as the final cohort.
Propensity-Score Matching
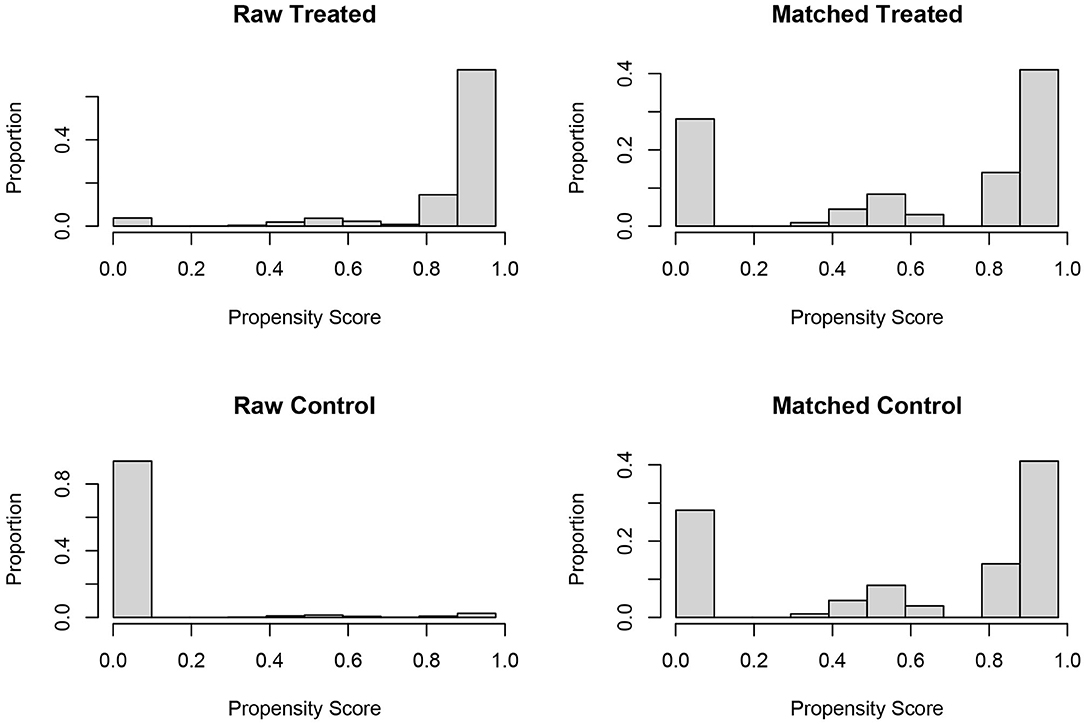
In the analyses of retrospective cohort without randomization, propensity score (PS) matching was usually performed to reduce the imbalance and potential confounding that were introduced by inherent differences between the groups (11). In the present study, one to one PS matching was also used to reduce selection bias in patient characteristics between ypN0 and pN0 groups based on the following covariates: No. of LNs dissected, American Joint Committee on Cancer T stage (T1, T2, T3, and T4), age at diagnosis (years), race (white, black, and other), gender (male and female), year of diagnosis (2004–2011), tumor site (rectosigmoid primary, and rectal primary), grade (well/Moderate, poor/anaplastic, and unknown), chemotherapy, serum carcinoembryonic antigen (CEA) level (negative, positive, and unknown), tumor size (≤5, >5 cm, and unknown) and perineural invasion (no, yes, and unknown). PS matching was performed based on nearest-neighbor matching, propensity scores reflected the probability that patients would be in ypN0 and pN0 groups based on their baseline characteristics. Once the propensity scores were estimated, patients in the pN0 group were matched to patients with radiation prior to the surgery. The histograms of propensity score before and after PS matching were shown in Figure 2. Finally, 761 matched pairs (761 patients in ypN0 group and 761 patients in pN0 group) were selected from the whole cohort (n = 26,832).
Statistical Analyses
The differences in the baseline characteristics between the ypN0 and pN0 groups were analyzed using the Pearson's chi-square test. The causes of death in the present study were categorized as rectal cancer specific or non–rectal cancer related. Rectal cancer cause-specific survival (CSS) was calculated from the date of diagnosis to the date of death due to rectal cancer. However, patients who died of other causes were censored at the date of death.
In our analyses, the CSS was analyzed to evaluate the prognostic prediction of ypN0 and pN0 groups using the Kaplan–Meier method with the log-rank test. The prognostic variables were entered in the multivariable analyses using the Cox proportional hazard model to identify independent prognostic variables. All the hazard ratios (HRs) were shown with 95% confidence intervals (CI). All tests were two sided, and two-sided P-values <0.05 were considered to be statistically significant in our analyses. Statistical analyses were mainly performed using SPSS version 23 (IBM, Armonk, NY, USA).
Results
Patient Characteristics Before PS Matching
In total, 26,832 patients diagnosed with pN0 or ypN0 rectal cancer were confirmed as the final cohort, including 7,237 (27.0%) patients with radiation and 19,595 (73.0%) patients without radiation prior to surgery. 8,177 (30.5%) patients received chemotherapy and 18,655 (69.5%) patients did not. The median follow-up time was up to 81 months, which was more than 5 years. At the end of follow-up time, 3,453 (12.9%) patients died of rectal cancer. The 5-year CSS rate of the whole cohort was 89.8%. The median ages of ypN0 group and pN0 group were 68 and 62 years old, respectively.
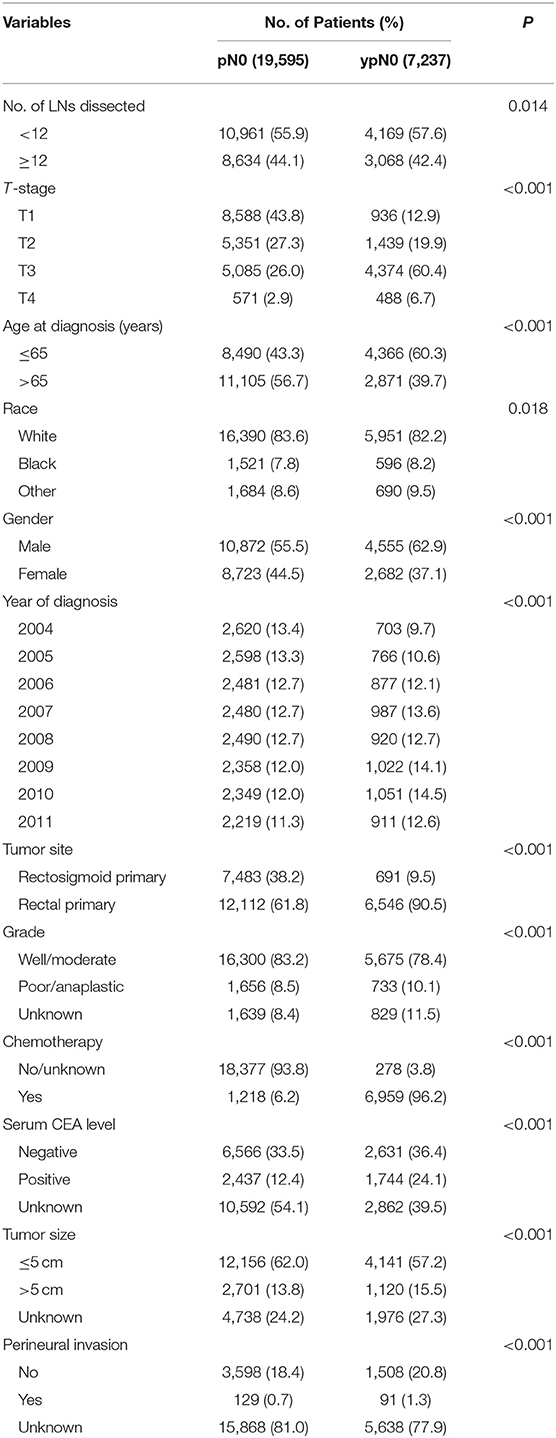
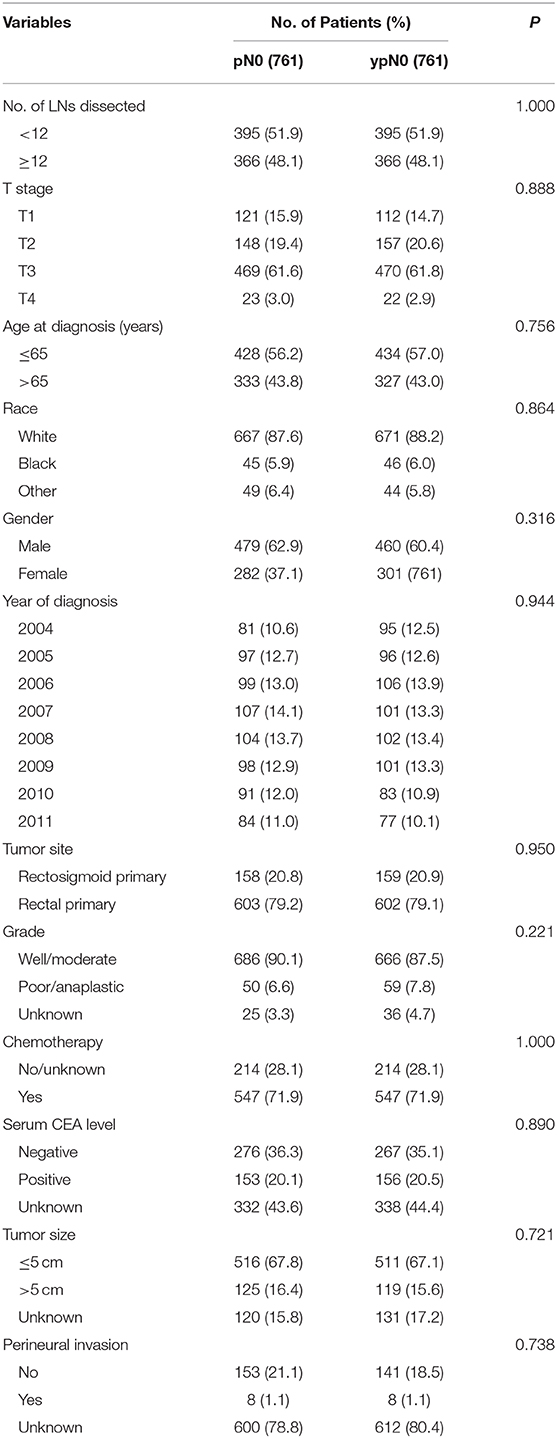
Shown as Table 1, patient demographics and pathological features between ypN0 and pN0 groups were summarized. For the number of lymph nodes dissected in total, patients in the ypN0 group were more likely to be associated with <12 lymph nodes dissected than patients in the pN0 group (P = 0.014); for American Joint Committee on Cancer (AJCC) T-stage, patients in the ypN0 group were more likely to be associated with higher T-stage than patients in the pN0 group (P < 0.001); for postoperative tumor grade, patients in the ypN0 group were more likely to be associated with higher postoperative tumor grade than patients in the pN0 group (P < 0.001). The above findings showed that ypN0 was strongly associated with advanced postoperative clinicopathological characteristics.
In addition, postoperative lymph node negative patients who were aged <65 years old, black, male, diagnosed in later years, rectal primary and received chemotherapy correlated with higher probability to have received neoadjuvant treatment.
Prognosis of ypN0 and pN0 Groups Before PS Matching
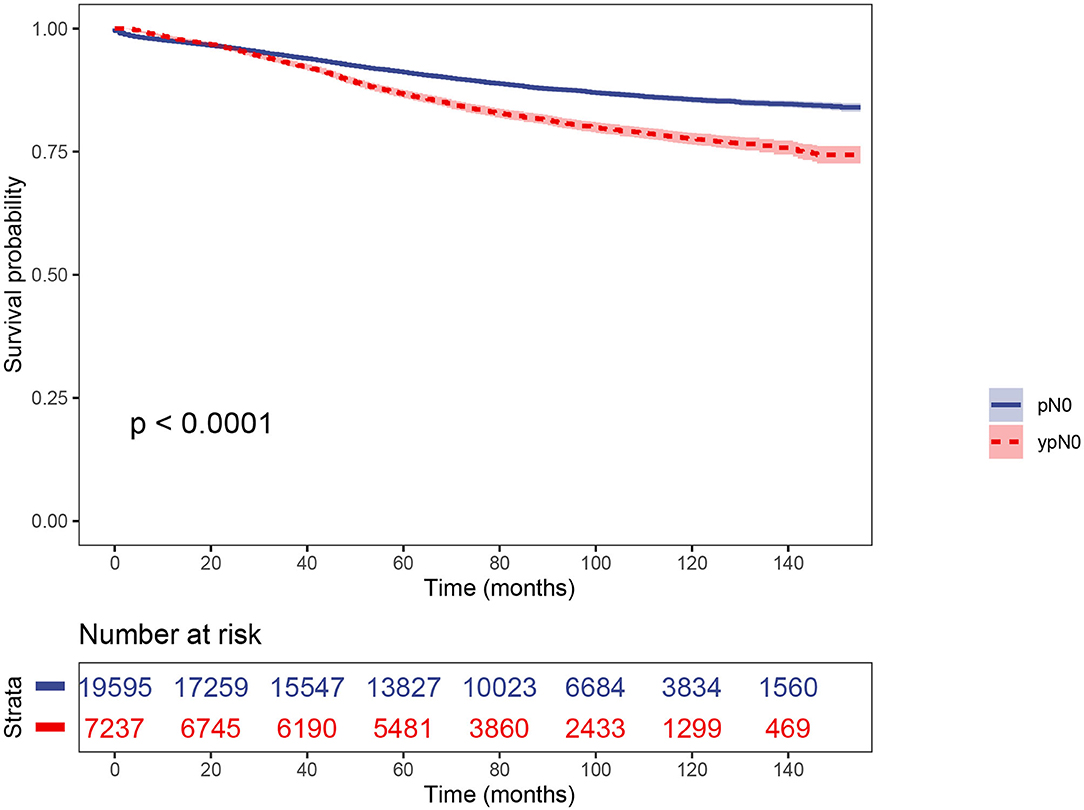
Using Kaplan–Meier estimates, we analyzed the CSS between ypN0 and pN0 groups. Patients in the ypN0 group had significantly worse survival compared with patients in the pN0 group: the 5-year CSS rate of ypN0 and pN0 were 86.6 and 91.1%, respectively, (P < 0.001, Figure 3). Then, results of multivariable analyses using the Cox proportional hazard were summarized in Table 2. No. of LNs dissected <12 (HR =0.700, 95%CI = 0.650–0.753, P < 0.001 for No. of LNs dissected ≥ 12, using No. of LNs dissected <12 as the reference), higher T-stage (HR = 1.518, 95%CI = 1.359–1.695, P < 0.001 for T2 stage; HR = 2.439, 95%CI = 2.194–2.712, P < 0.001 for T3 stage; HR = 5.353, 95%CI = 4.619–6.204, P < 0.001 for T4 stage; using T1 stage as the reference), aged than 65 years old (HR = 1.697, 95%CI = 1.582–1.820 for age at diagnosis > 65, P < 0.001, using age at diagnosis ≤ 65 as the reference), black (HR = 1.457, 95%CI = 1.305–1.626, P < 0.001 for black race, using white race as the reference), rectal primary (HR = 1.159, 95%CI = 1.068–1.257, P < 0.001 for rectal primary, using rectosigmoid primary as the reference), and higher tumor grade (HR = 1.360, 95%CI = 1.228–1.506, P < 0.001 for poor/anaplastic grade, using well/moderate grade as the reference) were independently associated with significantly worse CSS. With regards to neoadjuvant radiotherapy, however, after adjusting for other prognostic factors, it was not an independent prognostic variable of CSS (HR = 1.095, 95%CI = 0.952–1.260, P = 0.205, using pN0 group as the reference).
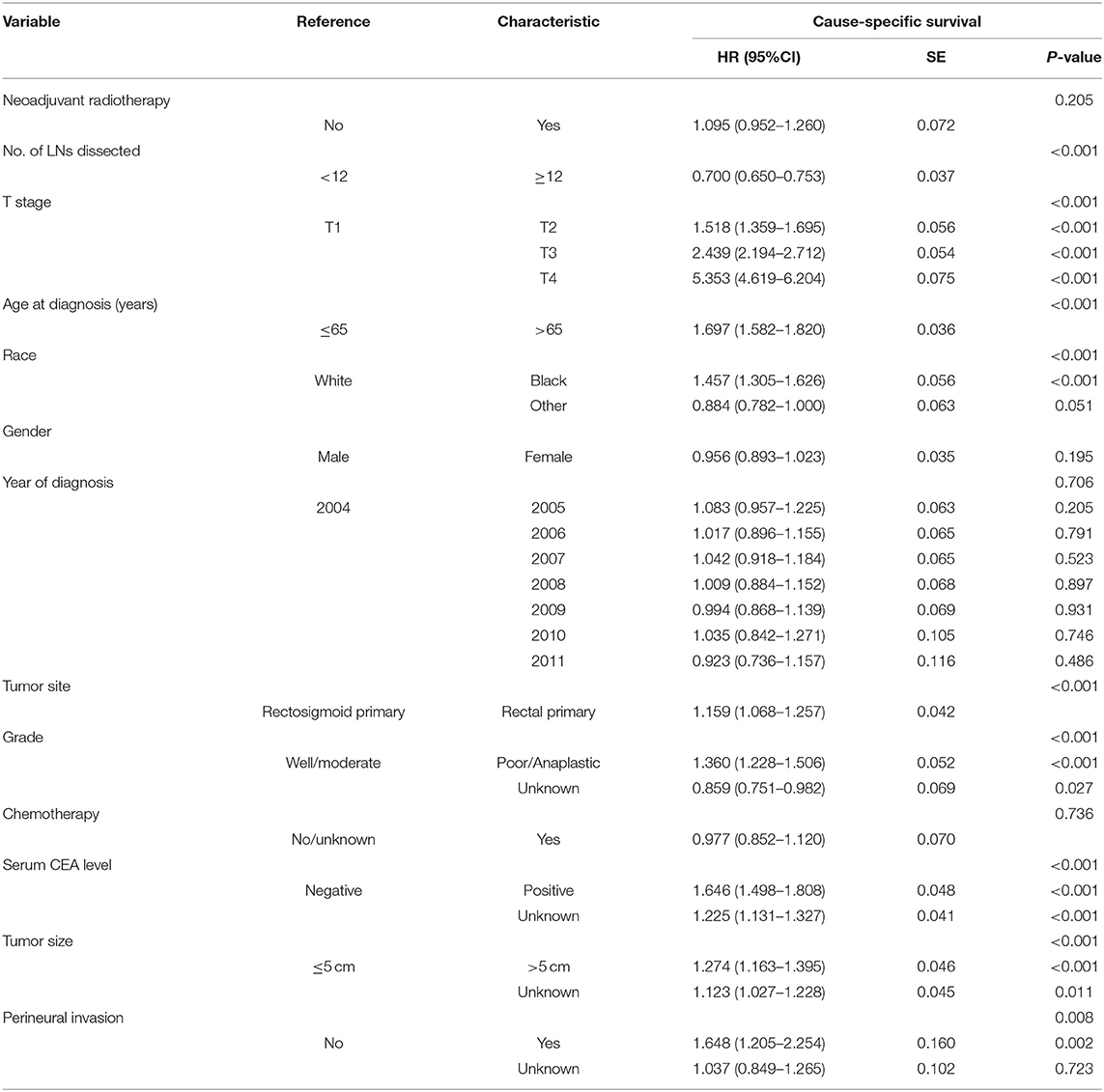
Table 2. Multivariate Cox regression analyses of the clinicopathological characteristics concerning CSS.
Patient Characteristics and Prognosis of ypN0 and pN0 Groups After PS Matching
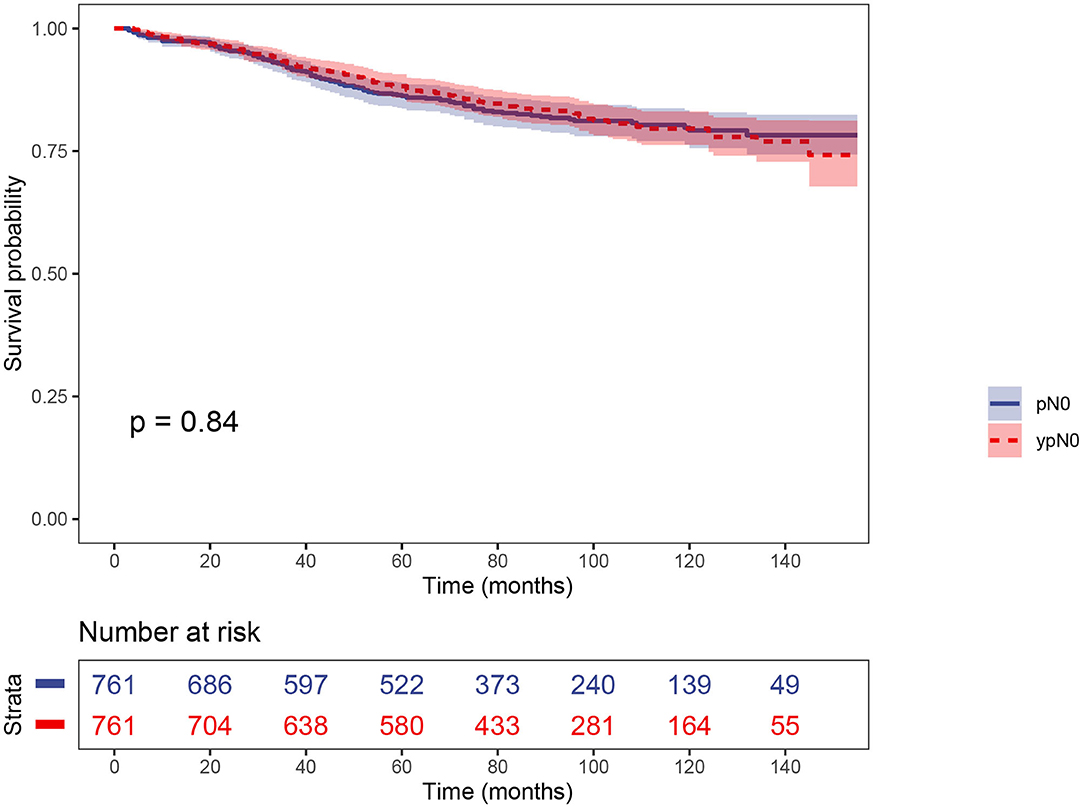
PS matching created 761 matched pairs, including 761 patients in the ypN0 group and 761 patients in the pN0 group. The comparison of baseline characteristics between the two groups were summarized in Table 3. All the tumor and patient characteristics except year of diagnosis showed no statistically significant differences between ypN0 and pN0 groups (P > 0.05, Table 3). Then, we also conducted CSS analyses using the Kaplan–Meier method, which indicated that there was no statistically significant CSS difference between the two groups, the 5-year CSS rates of the ypN0 and pN0 groups were 88.2 and 86.2%, respectively, (P = 0.84; Figure 4).
Discussion
The use of neoadjuvant chemoradiotherapy in advanced rectal cancer could result in pathologic response of the primary tumor, and many studies demonstrated that tumor response of neoadjuvant treatment was significantly associated with the prognosis of rectal cancer (12–16). According to the clinical guidelines of National Comprehensive Cancer Network (NCCN), patients who had received neoadjuvant chemoradiotherapy followed by surgery were recommended to receive adjuvant chemotherapy (17). However, the use of adjuvant chemotherapy in ypN0 rectal cancer was still controversial and some researchers questioned the clinical value of adjuvant chemotherapy in ypN0 patients (6, 7, 17). As early as in 2006, the study Fietkau et al. reported that disease-free survival (36 months) for rectal cancer without lymph node metastases (ypN0) was excellent, independent of whether they had received postoperative chemotherapy (6). Then in 2010, after identifying randomized studies exploring adjuvant chemotherapy against observation in patients with rectal cancer previously treated with preoperative radio(chemo)therapy, Bujko et al. (18). concluded that delivery of adjuvant chemotherapy in patients undergoing preoperative radio(chemo)therapy was not evidence based. Later, after comparing the prognosis of ypN0 patients who had received adjuvant chemotherapy and those who had not, Kiran et al. (7) found that ypN0 rectal cancer, whether or not the patient had received adjuvant chemotherapy, showed similar local recurrence, disease-free survival, and overall survival after prolonged follow-up. The famous EORTC 22921 trial's long-term results also showed that adjuvant fluorouracil-based chemotherapy after preoperative radiotherapy (with or without chemotherapy) does not affect either 10-year overall survival or disease-free survival of rectal cancer (19). Therefore, it was quite necessary to examine the long-term oncologic results of ypN0 disease.
To the best of our knowledge, the present population-based study was the largest study to compare the oncologic outcomes of ypN0 and pN0 rectal cancer. In the present study, at first, shown as the results of Kaplan–Meier estimates, patients in the ypN0 group had significantly worse survival compared with patients in the pN0 group. However, after adjusting for other known prognostic factors, the results of multivariate analyses showed that the prognostic difference between ypN0 and pN0 groups was not statistically significant. More importantly, PS matching was also used to validate our results and we found that there was no statistically significant CSS difference between the two groups after PS matching. Therefore, we held the belief that ypN0 status could achieve similarly good oncologic outcomes compared with pN0 disease. Therefore, we strongly believed that having received neoadjuvant chemoradiotherapy should not be the reason for adjuvant chemotherapy in pathological node-negative patients.
Although the nature of the retrospective design and small sample size were considered to be potential limitations, two previous studies questioned the routine use of adjuvant chemotherapy for ypN0 rectal cancer patients who had undergone curative surgery following neoadjuvant chemoradiation (6, 7). What is more, a recent analysis of the SEER database found that rectal cancer patients with ypTis-2N0 did not benefit from adjuvant chemotherapy after neoadjuvant treatment followed by radical surgery (20). Therefore, our research could add new evidence supporting the above findings.
Why did the Kaplan–Meier survival analyses, before adjusting for other prognostic variables or PS matching, show worse survival of ypN0 disease? In our analyses of differences in the baseline characteristics between the ypN0 and pN0 groups, we could easily find that, compared with pN0 rectal cancer, ypN0 status was strongly associated with poorer postoperative pathological diagnoses: ypN0 was more likely to be associated with <12 lymph nodes dissected, higher T stage and higher postoperative tumor grade. Before adjusting for other prognostic factors, therefore, it was normal to find that ypN0 disease was more likely to be associated with worse oncologic outcomes compared with pN0 rectal cancer.
In 2014, Erlenbach-Wünsch et al. (9) retrospectively analyzed the prognosis of 132 rectal cancer patients who underwent standard TME surgery after neoadjuvant chemoradiotherapy (ypN0) and those of 341 patients diagnosed with pN0 rectal disease without neoadjuvant chemoradiotherapy, showing a similar oncologic outcome between the two groups, which was consistent with our analyses. However, the sample size of this study was still too small for any general recommendation. Maybe limited to the sample size, they did not find that ypN0 status was strongly associated with poorer postoperative pathological diagnoses (less lymph nodes dissected, higher T stage and higher postoperative tumor grade) compared with pN0 rectal cancer, which contributed to the phenomenon that ypN0 disease was more likely to be associated with worse oncologic outcomes than pN0 rectal cancer before adjusting for other prognostic factors.
Although previous research had showed that the histological lymph node status after chemoradiotherapy seemed to be the only significant prognostic parameter of oncologic outcomes, to our knowledge, few studies were reported to study on the prognostic value of ypN0 status (6). In 2007, with the analyses of 35 patients who underwent neoadjuvant chemoradiotherapy followed by excisional surgery with TEM for rectal cancer, Caricato et al. (21) reported the effect of preoperative chemoradiotherapy on postoperative lymph node status, though the prognostic assessment was not performed due to the low case number.
Lindebjerg et al. (22) reported that rectal cancer patients with a major tumor response and no lymph node metastases after treatment had a survival rate of 100% compared to 60% in the group of patients with major response but lymph node metastases after surgery. Like ypCR patients, ypN0 patients were reported to achieve significantly better oncologic outcomes compared with lymph node-positive patients (17). Sprenger et al. (23) shared the similar view that residual nodal status was the most important predictor of individual outcome after analyzing the effect of preoperative and pathological nodal status on disease-free and overall survival in 496 patients with rectal adenocarcinoma identified from a prospective database.
Our research, therefore, as the largest one focused on the comparison of prognosis between ypN0 and pN0 rectal cancer, could add strong evidence that the receipt of neoadjuvant chemoradiotherapy was not an independent prognostic factor in rectal cancer patients with negative pathological nodal status. However, ypN0 status was strongly associated with worse postoperative pathological diagnoses compared with pN0 rectal cancer: ypN0 was more likely to be associated with <12 lymph nodes dissected, higher T stage higher postoperative tumor grade, contributing to the phenomenon that ypN0 disease was more likely to be associated with worse oncologic outcomes than pN0 rectal cancer before adjusting for other prognostic factors.
However, this study was only a retrospective one, we hope further randomized prospective study could be conducted to provide higher grade evidence to guide the treatment of rectal cancer with negative pathological nodal status who had received neoadjuvant chemoradiotherapy followed by total mesorectal excision (TME). Moreover, regimens used in the present study were not available in SEER database, which was also a limitation of our research.
In summary, our study showed that ypN0 rectal cancer was strongly associated with worse postoperative pathological diagnoses compared with pN0 rectal cancer, contributing to worse oncologic outcomes. After adjusting for other known prognostic factors, however, the prognostic difference between ypN0 and pN0 groups was not statistically significant, which could give guidance to the treatment of ypN0 rectal cancer.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: seer.cancer.gov.
Author Contributions
YH and GF: conceptualization. YH and WW: data curation and writing—original draft. YH, WW, and ZW: data analysis. TL and ST: visualization. GF: writing—review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Shiba S, Okamoto M, Kiyohara H, Ohno T, Kaminuma T, Asao T, et al. Prospective observational study of high-dose carbon-ion radiotherapy for pelvic recurrence of rectal cancer (GUNMA 0801). Front Oncol. (2019) 9:702. doi: 10.3389/fonc.2019.00702
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018 GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
3. Tominaga T, Akiyoshi T, Yamamoto N, Oba K, Nagasaki T, Yamaguchi T, et al. Prognostic value of metastatic lymph node regression grade after neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer. Surgery. (2019) 166:1061–7. doi: 10.1016/j.surg.2019.06.009
4. Liu Q, Luo D, Cai S, Li Q, Li X. Circumferential resection margin as a prognostic factor after rectal cancer surgery: a large population-based retrospective study. Cancer Med. (2018) 7:3673–81. doi: 10.1002/cam4.1662
5. Madariaga ML, Berger DL. The quandary of N0 disease after neoadjuvant therapy for rectal cancer. J Gastrointest Oncol. (2012) 3:299–300. doi: 10.3978/j.issn.2078-6891.2012.049
6. Fietkau R, Barten M, Klautke G, Klar E, Ludwig K, Thomas H, et al. Postoperative chemotherapy may not be necessary for patients with ypN0-category after neoadjuvant chemoradiotherapy of rectal cancer. Dis Colon Rectum. (2006) 49:1284–92. doi: 10.1007/s10350-006-0570-x
7. Kiran RP, Burgess AN, Nisar PJ, Kalady MF, Lavery IC. Is adjuvant chemotherapy really needed after curative surgery for rectal cancer patients who are node-negative after neoadjuvant chemoradiotherapy? Ann Surg Oncol. (2012) 19:1206–12. doi: 10.1245/s10434-011-2044-1
8. Tae Hyun K, Hee Jin C, Dae Yong K, Kyung Hae J, Sang HY, Young KS, et al. Pathologic nodal classification is the most discriminating prognostic factor for disease-free survival in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Int J Radiat Oncol Biol Phys. (2010) 77:1158–65. doi: 10.1016/j.ijrobp.2009.06.019
9. Erlenbach-Wünsch K, Semrau S, Fietkau R, Weber K, Hohenberger W, Rau T, et al. ypN0 nodal status after neoadjuvant chemoradiotherapy for rectal carcinoma is not associated with adverse prognosis as compared with pN0 after primary surgery. Int J Colorectal Dis. (2014) 29:231–7. doi: 10.1007/s00384-013-1790-x
10. Giannakeas V, Sopik V, Narod SA. A comparison of two models for breast cancer mortality for women with ductal carcinoma in situ: an SEER-based analysis. Breast Cancer Res Treat. (2018) 169:1–8. doi: 10.1007/s10549-018-4716-z
11. Plum PS, Holscher AH, Pacheco Godoy K, Schmidt H, Berlth F, Chon SH, et al. Prognosis of patients with superficial T1 esophageal cancer who underwent endoscopic resection before esophagectomy-A propensity score-matched comparison. Surg Endosc. (2018) 32:3972–80. doi: 10.1007/s00464-018-6139-7
12. Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. (2005) 23:8688–96. doi: 10.1200/JCO.2005.02.1329
13. Beddy D, Hyland JM, Winter DC, Lim C, White A, Moriarty M, et al. A simplified tumor regression grade correlates with survival in locally advanced rectal carcinoma treated with neoadjuvant chemoradiotherapy. Ann Surg Oncol. (2008) 15:3471–7. doi: 10.1245/s10434-008-0149-y
14. Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. (2014) 32:1554–62. doi: 10.1200/JCO.2013.54.3769
15. Habr-Gama A, Perez RO, Nadalin W, Nahas SC, Ribeiro U Jr, Campos FG, et al. Long-term results of preoperative chemoradiation for distal rectal cancer correlation between final stage and survival. J Gastrointest Surg. (2005) 9:90–9; discussion 99–101. doi: 10.1016/j.gassur.2004.10.010
16. De Stefano A, Moretto R, Bucci L, Pepe S, Romano FJ, Cella AC, et al. Adjuvant treatment for locally advanced rectal cancer patients after preoperative chemoradiotherapy: when, and for whom? Clin Colorectal Cancer. (2014) 13:185–91. doi: 10.1016/j.clcc.2014.05.004
17. Lee KH, Kim JC, Kim JY, Kim JS. Oncologic results and prognostic predictors of patients with locally advanced rectal cancer showing ypN0 after radical surgery following neoadjuvant chemoradiotherapy. Int J Colorectal Dis. (2015) 30:1041–50. doi: 10.1007/s00384-015-2261-3
18. Bujko K, Glynnejones R, Bujko M. Does adjuvant fluoropyrimidine-based chemotherapy provide a benefit for patients with resected rectal cancer who have already received neoadjuvant radiochemotherapy? A systematic review of randomised trials. Ann Oncol. (2010) 21:1743–750. doi: 10.1093/annonc/mdq054
19. Jean-Fran?Ois B, Gilles C, Laurent M, Philippe M, Suzana SR, René-Jean B, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. (2014) 15:184–90. doi: 10.1016/S1470-2045(13)70599-0
20. Hu X, Li YQ, Li QG, Ma YL, Peng JJ, Cai SJ. Adjuvant chemotherapy seemed not to have survival benefit in rectal cancer patients with ypTis-2N0 after preoperative radiotherapy and surgery from a population-based propensity score analysis. Oncologist. (2019) 24:803–11. doi: 10.1634/theoncologist.2017-0600
21. Caricato M, Ausania F, Dominicis E, De Vincenzi B, Rabitti C, Tonini G, et al. Tumor regression in mesorectal lymphnodes after neoadjuvant chemoradiation for rectal cancer. Eur J Surg Oncol. (2007) 33:724–8. doi: 10.1016/j.ejso.2007.01.023
22. Lindebjerg J, Spindler KJ, Jakobsen A. The prognostic value of lymph node metastases and tumour regression grade in rectal cancer patients treated with long-course preoperative chemoradiotherapy. Colorectal Dis. (2010) 11:264–9. doi: 10.1111/j.1463-1318.2008.01599.x
Keywords: neoadjuvant chemoradiotherapy, rectal cancer, ypN0, pN0, propensity score matching
Citation: Huang Y, Wei W, Wang Z, Liang T, Tian S and Fu G (2021) Neoadjuvant Chemoradiotherapy Does Not Contribute to Worse Survival in Pathological Node-Negative Rectal Cancer. Front. Oncol. 11:649313. doi: 10.3389/fonc.2021.649313
Received: 04 January 2021; Accepted: 11 February 2021;
Published: 08 March 2021.
Edited by:
Chang-In Choi, Pusan National University Hospital, South KoreaReviewed by:
Nazri Mustaffa, Universiti Sains Malaysia (USM), MalaysiaGiovanni Rosti, Fondazione Ospedale San Matteo (IRCCS), Italy
Paul Willemsen, Hospital Network Antwerp (ZNA), Belgium
Copyright © 2021 Huang, Wei, Wang, Liang, Tian and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangshun Fu, ZnV4aWFvZGVqaWFAc2luYS5jb20=
†These authors have contributed equally to this work
 Yong Huang†
Yong Huang† Wei Wei
Wei Wei Guangshun Fu
Guangshun Fu