
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 10 January 2022
Sec. Surgical Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.648955
This article is part of the Research Topic Update of Current Evidences in Breast Cancer Surgery View all 19 articles
Purpose: We conducted a systematic literature search and pooled data from studies to compare the incidence of complications between the tumescent and non-tumescent techniques for mastectomy.
Methods: We searched PubMed, Embase, BioMed Central, Ovid, and CENTRAL databases for studies comparing the two mastectomy techniques up to November 1st, 2020. We used a random-effects model to calculate odds ratios (OR) with 95% confidence intervals (CI).
Results: Nine studies were included with one randomized controlled trial (RCT). Meta-analysis indicated no statistically significant difference in the incidence of total skin necrosis (OR 1.18 95% CI 0.71, 1.98 I2 = 82% p=0.52), major skin necrosis (OR 1.58 95% CI 0.69, 3.62 I2 = 71% p=0.28), minor skin necrosis (OR 1.11 95% CI 0.43, 2.85 I2 = 72% p=0.83), hematoma (OR 1.19 95% CI 0.80, 1.79 I2 = 4% p=0.39), and infections (OR 0.87 95% CI 0.54, 1.40 I2 = 54% p=0.56) between tumescent and non-tumescent groups. Analysis of studies using immediate alloplastic reconstruction revealed no statistically significant difference in the incidence of explantation between the two groups (OR 0.78 95% CI 0.46, 1.34 I2 = 62% p=0.37). Multivariable-adjusted ORs on total skin necrosis were available from three studies. Pooled analysis indicated no statistically significant difference between tumescent and non-tumescent groups (OR 1.72 95% CI 0.72, 4.13 I2 = 87% p=0.23).
Conclusion: Low-quality evidence derived mostly from non-randomized studies is indicative of no difference in the incidence of skin necrosis, hematoma, seroma, infection, and explantation between the tumescent and non-tumescent techniques of mastectomy. There is a need for high-quality RCTs to further strengthen the evidence.
Since the first description of the tumescent dissection mastectomy method by Worland (1) in 1996, the technique has gained popularity for both breast cancer and esthetic surgical procedures (2, 3). Tumescent dissection involves an injection of a very dilute solution of local anesthetic with epinephrine and a crystalloid into the subcutaneous tissues of the breast (4) using multiple small stab punctures. The solution is injected just before the initial incision thereby creating tension between the anatomical planes and hydro-dissecting the tissues. The space created by the solution enhances visibility and ease of dissection, and allows the surgeon to distinguish between the subcutaneous and glandular tissues (5). Dissection can be easily carried out using sharp scissors obviating the need for electrocautery near the skin flaps which might lead to soft tissue damage by the dissipating thermal energy (6). The epinephrine in the mixture causes vasoconstriction, which is further enhanced by the tamponading effect of the high volume infiltration on the sub-dermal vessels (7). Another potential advantage is the analgesic effect offered by the local anesthetic which has been confirmed by researchers (8, 9).
However, despite the technique’s benefits, the risk of skin flap necrosis with the use of the tumescent solution is disconcerting to many surgeons. Skin flap necroses after mastectomy are a serious complication leading to patient dissatisfaction and increased healthcare costs (10). More importantly, they can cause a delay in the initiation of adjuvant therapies after surgery thereby affecting patient outcomes (11). In this context, clarifying the impact of the tumescent dissection technique vis-a-vis the standard surgical technique on the incidence of postsurgical complications in patients undergoing mastectomies is important. In a systematic review and meta-analysis by Siotos et al (3), authors found that the use of the tumescent technique in mastectomies is associated with a significantly increased risk of skin necrosis. However, as pointed out in the study itself, they were only able to pool data from five studies. Thus, we conducted an updated systematic literature search and pooled data from studies to strengthen the published evidence by comparing the incidence of complications between the tumescent and non-tumescent surgical techniques for mastectomies.
Two independent reviewers carried out a comprehensive electronic search of PubMed, Embase, BioMed Central, Ovid, and CENTRAL databases without language restrictions. The search was conducted from the inception of these databases to the 1st of November, 2020. Various combinations of the following search terms were included in the database search: “breast surgery”, “mastectomy”, “hydrodissection”, “tumescent”, “lignocaine”, “epinephrine” and “local anaesthetic”. The two researchers reviewed the titles and abstracts of the articles after the database search to identify the relevant articles. They evaluated the full-text of these articles for final inclusion in the study; selection process disagreements were resolved by discussion. Finally, we also performed a manual search of the bibliography of studies meeting the inclusion criteria and of previous reviews on the topic for any missed references. We followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement guidelines during the conduct of this review (12), and we present the search strategy and results in Supplementary Table S1 accordingly.
We defined the inclusion and exclusion criteria of the review a priori based on the PICOS (Population, Intervention, Comparison, Outcome, Study type) framework as follows:
Population: Studies conducted on adult women undergoing mastectomy with or without immediate reconstruction.
Intervention: Use of tumescent dissection technique.
Comparison: Use of non-tumescent technique (defined as the standard surgical technique with electrocautery and/or harmonic scalpel).
Outcomes: Studies reporting data on complications (including skin necrosis, hematoma, infections, seroma, etc) after the relevant surgical procedures.
Study type: Randomized controlled trials (RCTs), prospective or retrospective cohort studies.
Our exclusion criteria were the following: 1) Studies on other patients (not undergoing mastectomy). 2) Non-comparative studies. 3) Studies lacking relevant outcomes. 4) Case reports and review articles.
We prepared a data extraction form beforehand to process relevant data. Information Two authors independently sourced the information, and they extracted the name of the first author, publication year, study type, study location, surgery type, non-tumescent technique used, sample size, number of breasts in the population, age of patients, proportion of smokers, proportion of diabetics, use of radiation therapy, mastectomy weight, follow-up length, and study outcomes. The outcomes of interest were the incidences of total skin necrosis, major skin necrosis, minor skin necrosis, hematoma, seroma, infections, and explantation or conversion to autologous reconstruction in cases of alloplastic reconstruction. We defined major skin necrosis as full-thickness necrosis requiring intervention in the operating room and minor skin necrosis as partial necrosis needing only local wound care. Our definition of hematoma included only those requiring surgical evacuation, and that of infections included only those requiring intravenous antibiotics with or without hospital readmission.
We assessed the quality of the studies included using the Cochrane Collaboration risk assessment tool for RCTs (13) and the risk of a bias assessment tool for non-randomized studies (RoBANS) (14). We evaluated selection of participants, confounding variables, intervention measurements, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting for each study.
We carried out our pooled analysis using “Review Manager” (RevMan, version 5.3; Nordic Cochrane Centre [Cochrane Collaboration], Copenhagen, Denmark; 2014). On account of the inherent heterogeneity of the included studies, we chose a random-effects model for the meta-analysis of all outcomes. We calculated odds ratios (ORs) with 95% confidence intervals (CIs) to compare complications between the tumescent and non-tumescent surgical techniques. We pooled the incidence of complications per breast rather than per patient. We carried out a sub-group analysis based on the use of immediate reconstruction after the mastectomies. We also extracted the multivariable-adjusted ORs of the outcomes, if available, from the included studies. We pooled variable data if reported by at least three of the studies using the generic inverse variance model. We used the I2 statistic to assess heterogeneity and classified it as low (I2 values between 25% and 50%), medium (values between 50% and 75%) or high (values higher than 75%). We avoided using funnel plots to assess publication bias because each meta-analysis was based on data from more than 10 studies.
Figure 1 shows the PRISMA flow-chart. We included nine studies in the review (15–23). Table 1 presents their characteristics. Seven studies were retrospective, one prospective, and one an RCT. Most studies were carried out in the USA. All the patients underwent immediate reconstruction in all but in two studies. In the study of Abbott et al (21), 65.7% of patients in the tumescent group and 57.8% of patients in the non-tumescent group underwent immediate reconstruction. In the trial of Lautrup et al (15), none of the patients underwent immediate reconstruction. Two studies reported the use of a harmonic scalpel in the non-tumescent group. In the study of Khavani et al (20), the use of pre-and post-surgery radiation therapy was significantly higher in the non-tumescent group than in the tumescent group. Complication data per breast were available for all studies except for that by Gipponi et al (17), which reported data per patient. Therefore, we excluded this study from the meta-analysis. In that study, the authors reported a significantly higher incidence of minor skin necrosis in the tumescent group (2/15 patients) than in the non-tumescent group (7/15 patients) (p=0.45) without major skin necroses. In addition, they found no significant differences in the incidences of hematoma or wound infection between the two groups (17).
We pooled the data on total skin necrosis from eight studies. Our meta-analysis results indicated no significant differences in the incidences of total skin necrosis between tumescent and non-tumescent groups (OR, 1.18; 95% CI, 0.71 to 1.98; I2 = 82%; p=0.52). Our subgroup analysis based on the use of immediate reconstruction, showed similar results for all sub-groups (Figure 2). The incidences of major skin necrosis (OR, 1.58; 95% CI, 0.69 to 3.62; I2 = 71%; p=0.28) and of minor skin necrosis (OR, 1.11; 95% CI, 0.43 to 2.85; I2 = 72%; p=0.83) were also similar amongst the two study groups. The results were similar for studies using immediate reconstruction and for the study by Abott et al (21) reporting on a mixed population of patients with or without immediate reconstruction (Figures 3, 4).

Figure 2 Forest plot for total skin necrosis in sub-group analysis based on immediate reconstruction. TT, tumescent group; nTT, non-tumescent group; IV, inverse variance; OR, odds ratio.
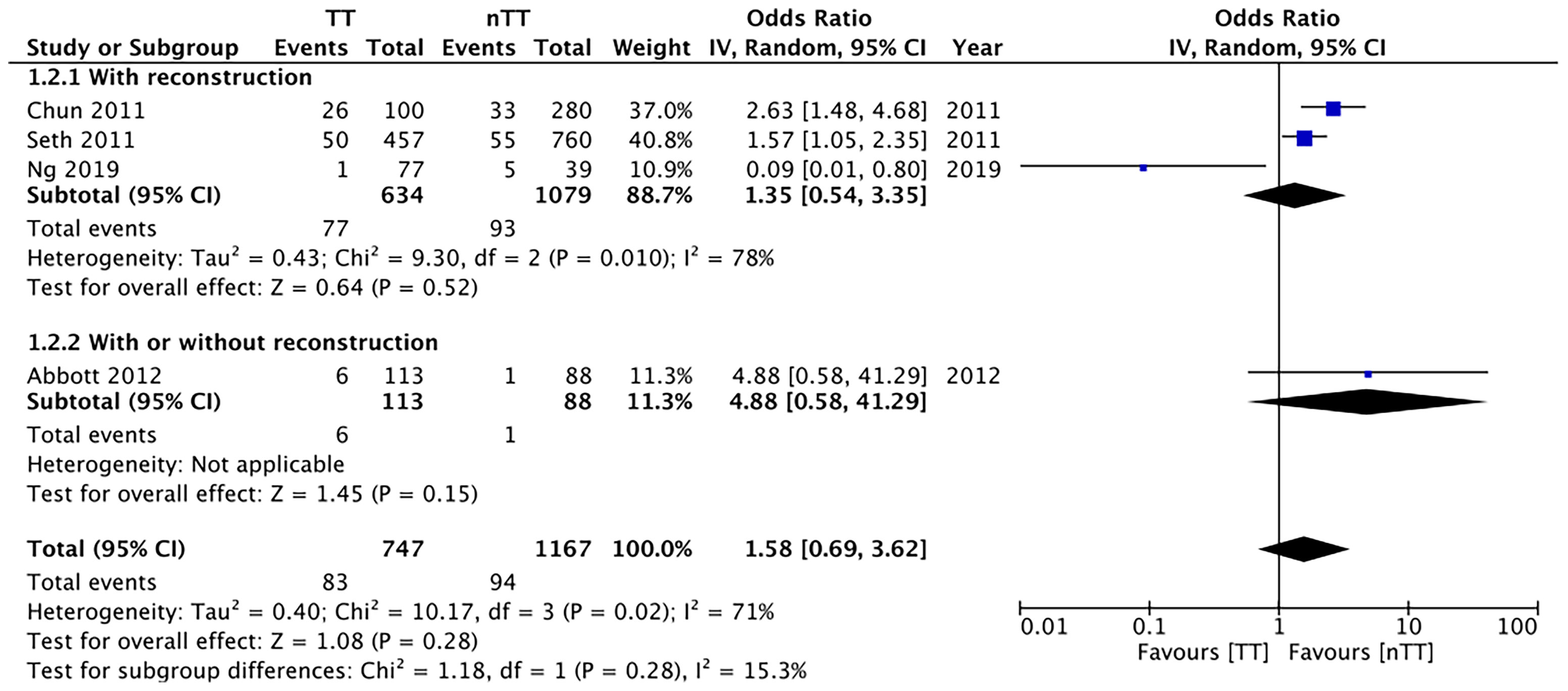
Figure 3 Forest plot for major skin necrosis in sub-group analysis based on immediate reconstruction. TT, tumescent group; nTT, non-tumescent group; IV, inverse variance; OR, odds ratio.
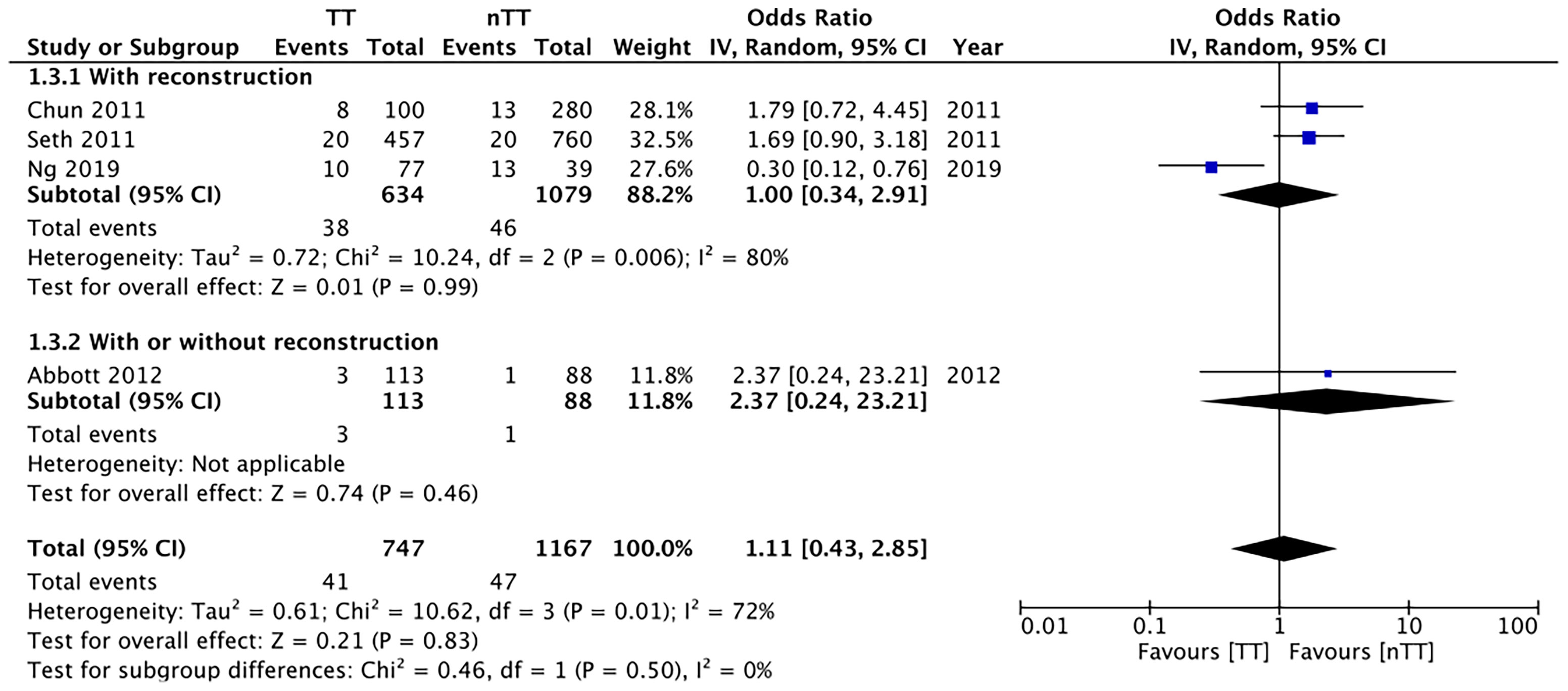
Figure 4 Forest plot for minor skin necrosis in sub-group analysis based on immediate reconstruction. TT, tumescent group; nTT, non-tumescent group; IV, inverse variance; OR, odds ratio.
We found no statistically significant differences in the incidence of hematoma requiring re-intervention between the tumescent and non-tumescent groups (OR, 1.19; 95% CI, 0.80 to 1.79; I2 = 4%; p=0.39). The results were similar on the sub-group analysis based on the use of immediate reconstruction (Figure 5). A meta-analysis of studies using immediate reconstruction with mastectomy indicated no significant differences in the incidence of seroma between two study groups (OR, 0.84; 95% CI, 0.51 to 1.38; I2 = 21%; p=0.49) (Figure 6). Similarly, we found a similar incidence of infections between the two groups (OR, 0.87; 95% CI, 0.54 to 1.40; I2 = 54%; p=0.56) (Figure 7). And, our results were similar for all studies using immediate reconstruction; only in the study by Abott et al (21) did we find the incidence of infections to be significantly lower in the tumescent group than in the non-tumescent group (OR, 0.23; 95% CI, 0.07 to 0.75; p=0.01) (Figure 7). The analysis of studies using immediate alloplastic reconstruction (tissue expander or implant) revealed no statistically significant differences in the incidences of explantation or conversion to autogenous reconstruction between the two groups (OR, 0.78; 95% CI, 0.46 to 1.34; I2 = 62%; p=0.37) (Figure 8).
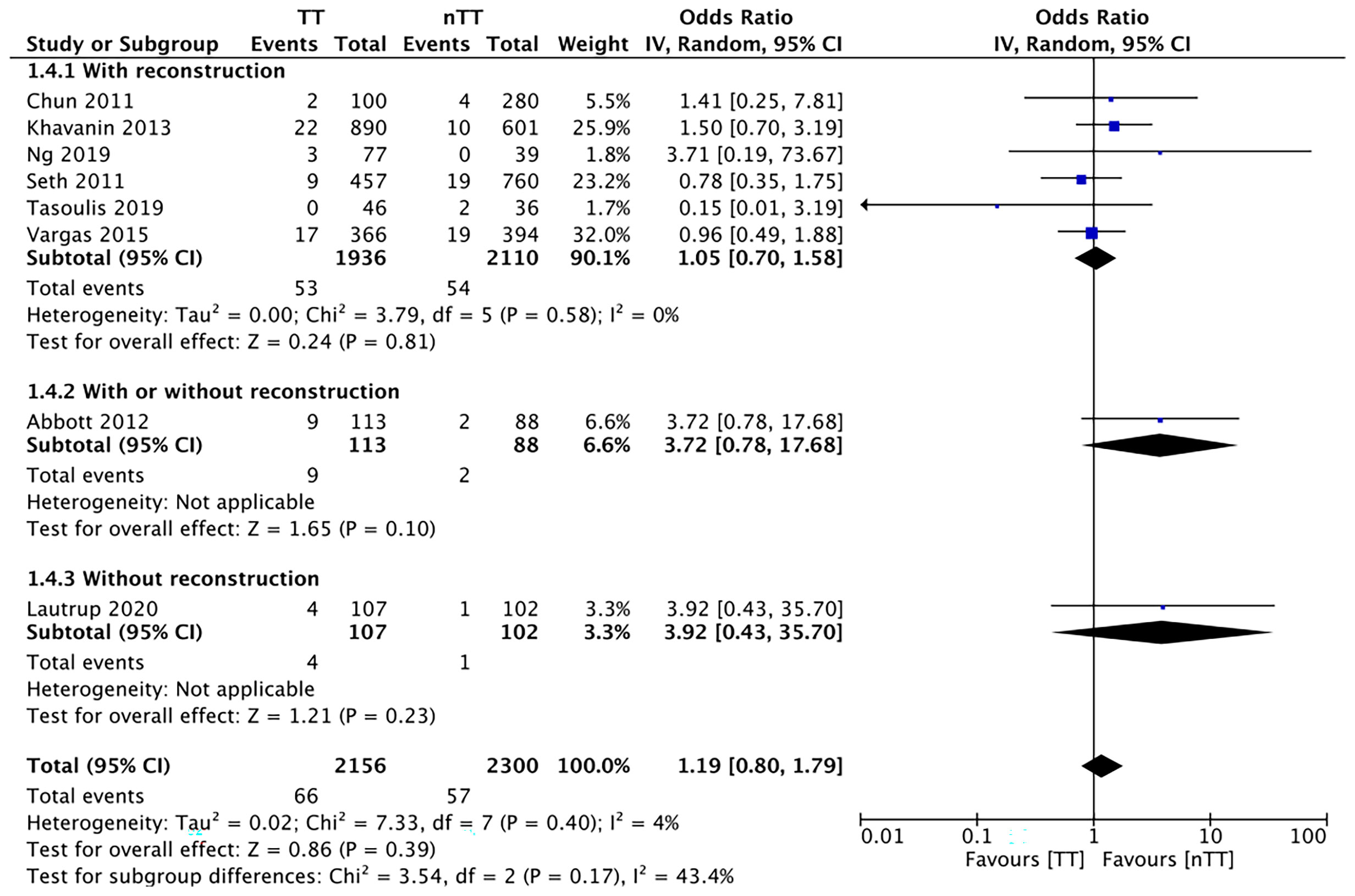
Figure 5 Forest plot for hematoma in sub-group analysis based on immediate reconstruction TT, tumescent group; nTT, non-tumescent group; IV, inverse variance; OR, odds ratio.
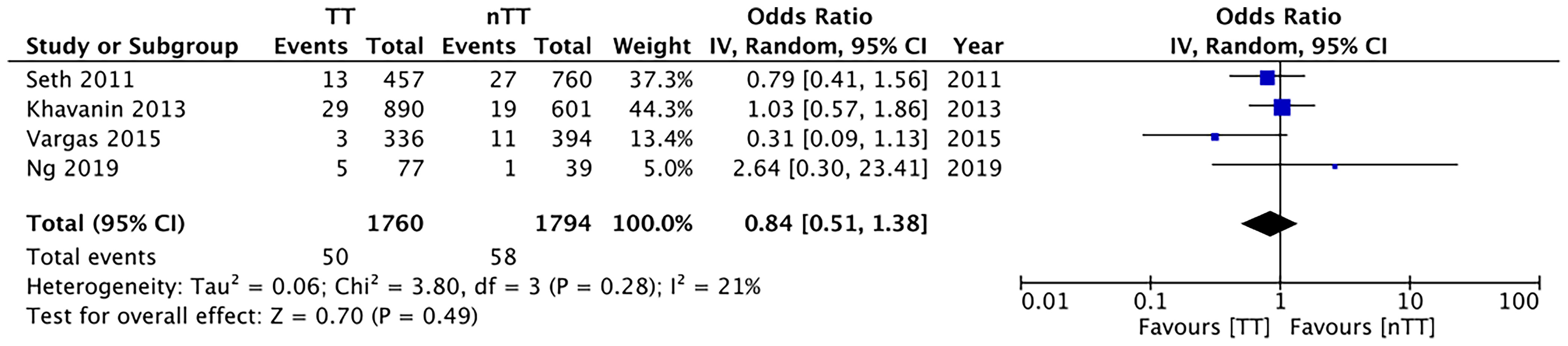
Figure 6 Forest plot for seroma with sub-group analysis based on immediate reconstruction TT, tumescent group; nTT, non-tumescent group; IV, inverse variance; OR, odds ratio.
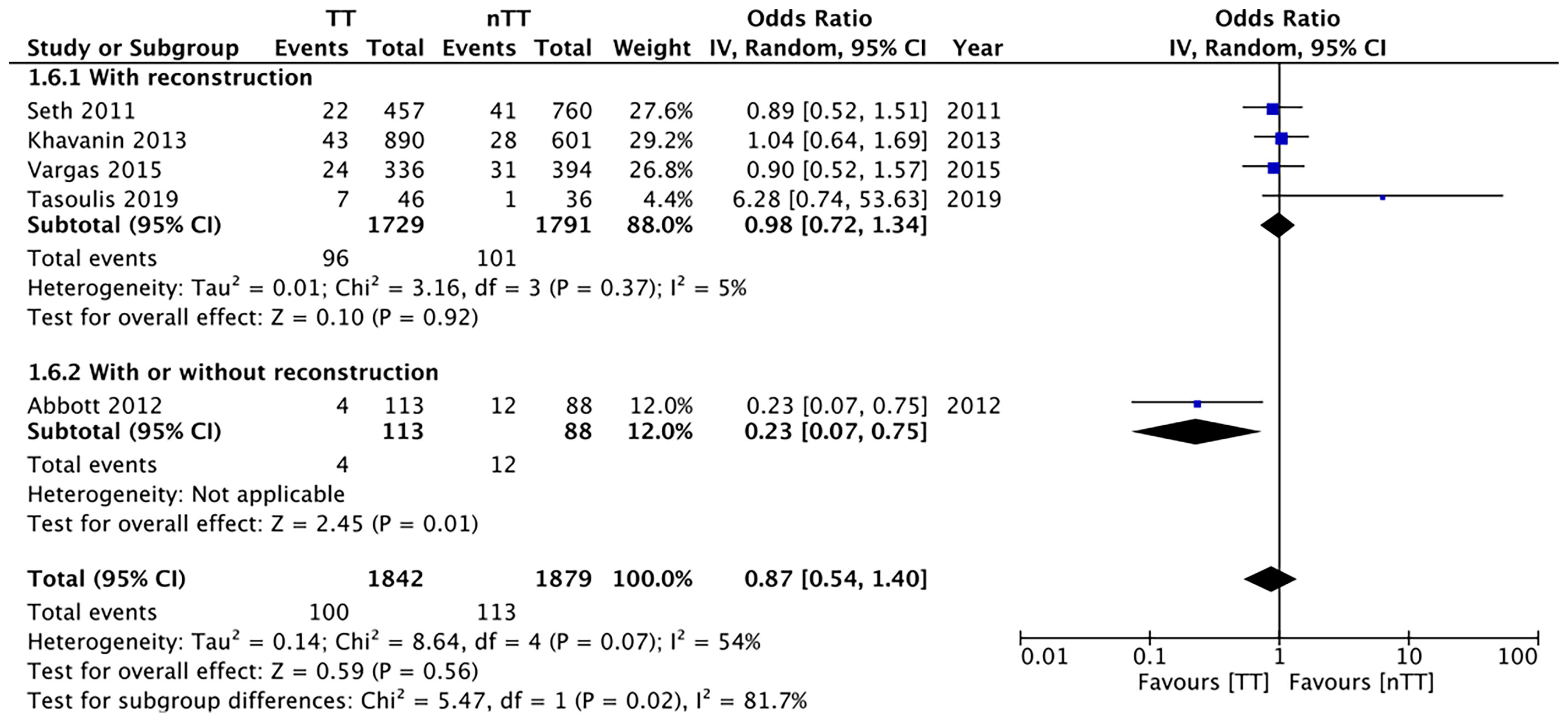
Figure 7 Forest plot for infections in sub-group analysis based on immediate reconstruction TT, tumescent group; nTT, non-tumescent group; IV, inverse variance; OR, odds ratio.
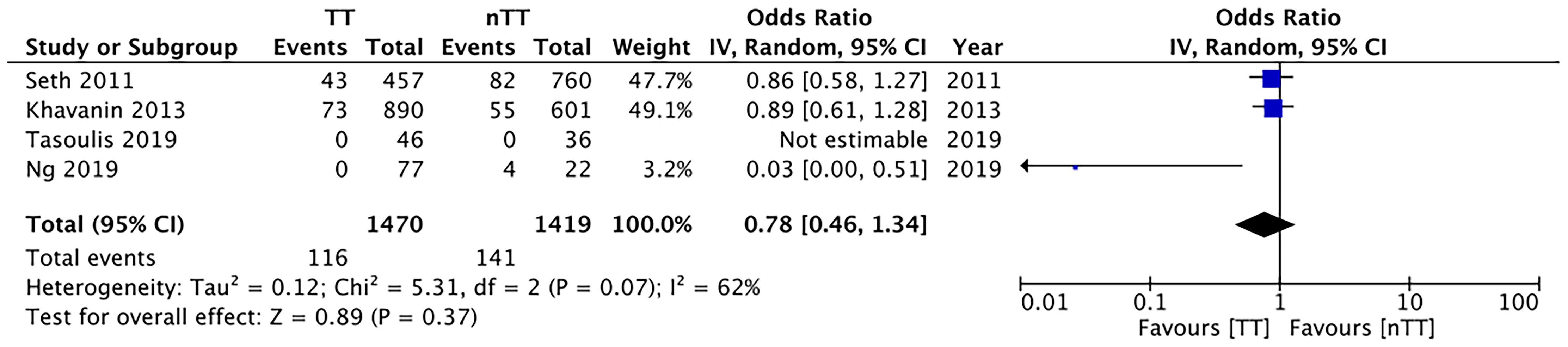
Figure 8 Forest plot for explantation or conversion to autologous reconstruction in patients undergoing immediate alloplastic reconstruction. TT, tumescent group; nTT, non-tumescent group; IV, inverse variance; OR, odds ratio.
Multivariable-adjusted ORs on total skin necrosis were available from three studies. Our pooled analysis indicated similarities between the tumescent and non-tumescent groups (OR, 1.72; 95% CI, 0.72 to 4.13; I2 = 87%; p=0.23) (Figure 9). Due to lack of data we could not complete this type of analysis for other outcomes.
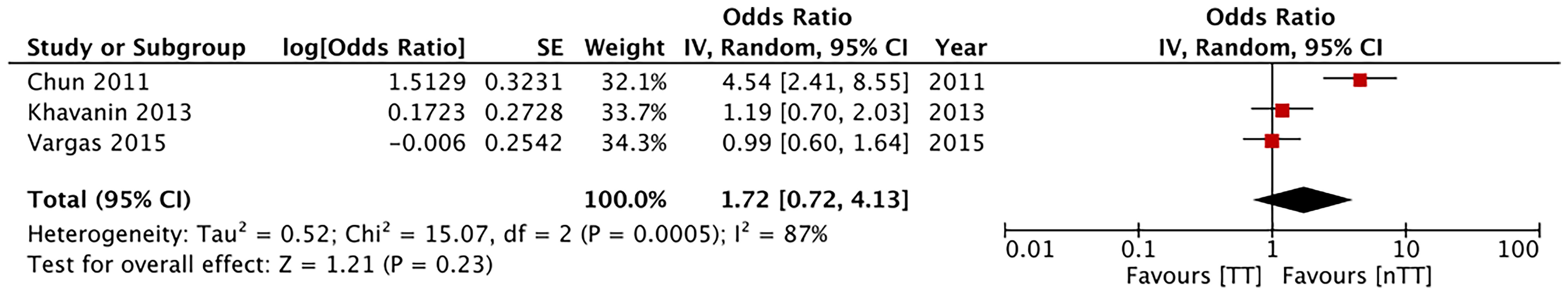
Figure 9 Forest plot of multivariable adjusted odds ratios for total complications and total skin necrosis. TT, tumescent group; nTT, non-tumescent group; IV, inverse variance; OR, odds ratio.
Table 2 presents our quality assessment for the included studies. Blinding was not possible in the RCT due to the nature of the intervention. For all non-RCTs, we found a high risk of bias due to unadjusted confounding factors.
The results of our updated systematic review and meta-analysis based mostly on non-RCTs indicate that the complication rates (skin necrosis, hematoma, seroma, and infections) may be similar for both the tumescent and non-tumescent dissection mastectomy techniques. The incidences of explantation or conversion to autogenous reconstruction were similar between the two dissection techniques in patients undergoing alloplastic reconstruction.
The optimal separation of the subcutaneous tissues containing the sub-dermal plexus from the gland parenchyma is essential to maximize flap survival during mastectomies. This dissection is also important from an oncological point of view; thick flaps may result in recurrence of malignancy in the remnant breast tissue, but thin flaps may lead to skin necrosis (18, 24). Electrocautery has been widely used as a conventional dissection technique in institutions worldwide. When compared to scalpel dissection, electrocautery is associated with less blood loss and a lack of cosmetic outcome or patient satisfaction score differences (25). However, the high temperatures needed for the electrocautery can cause significant ischemic lesions on the skin and subcutaneous tissues leading to wide areas of necrosis (17, 26, 27). The tumescent technique is thought to reduce the thermo-dispersion of the electrocautery thereby improving flap survival. However, concerns about the incidence of skin necrosis with the tumescent technique itself have also been raised. The vasoconstrictor effect of epinephrine and the compressive effect of the solution, which both reduce the blood loss are thought to contribute to reduced skin flap survival (23). In this context, our review presents important findings on the complications of these two techniques for surgeons carrying out mastectomies.
Our analysis revealed similar incidences for minor and major skin necrosis with both techniques. Skin necroses after mastectomies can be influenced by different confounding factors such as age, obesity, smoking, diabetes mellitus, and prior radiation therapy (11, 28, 29) that can be controlled for only in well-conducted RCTs to provide high-quality evidence. Unfortunately, only one RCT was available for inclusion in our review. For the remaining studies, the allocations were not randomized, and known and unknown patient characteristics differed between the study groups. Therefore, our results need to be interpreted with caution as the non-significant difference between the two techniques may not necessarily be due to intervention equivalence, but could have been caused by systematic differences between the groups themselves (30). While all non-RCTs in our analysis reported minimal differences between the two groups, none of them carried out propensity-score matching to adjust for baseline factors. Only three studies reported results of multivariable regression analysis for total skin necrosis. Our pooled analysis of such data indicated a similar incidence of total skin necrosis for both techniques. The use of immediate reconstruction, especially alloplastic reconstruction, is an important variable affecting immediate local complications (31). While most of the studies included used reconstruction in all the patients, two studies did not. Hence, we conducted a sub-group analysis including a single study in each group and obtained similar results. We further analyzed the incidence of explantation or conversion to autologous grafts in patients undergoing alloplastic reconstruction and the results of our meta-analysis demonstrated no adverse effect of the tumescent technique on the risk of explantation. However, it needs to be mentioned that the outcomes with tumescent technique can depend on many factors related to breast reconstruction namely the position of implant (pre-pectoral or retro-pectoral) and the use of mesh (synthetic or acellular). Traditionally, there has been a strong correlation between the use of tumescent technique and pre-pectoral implant placement and use of mesh due to advantages like shorter surgical time, reduced bleeding and easier dissection (32). More recently, tumescent technique has been used for retro-pectoral implant placement as well. Shimuzu et al (33) in a retrospective review of 35 patients undergoing awake breast augmentation with intercostal nerve blocks and tumescent technique have reported good outcomes with both pre-pectoral and retro-pectoral implant placement. Depending upon the position of implant, the tumescent solution needs to be injected either between the mammary gland and the pectoralis muscle or beneath the muscle in case of retro-pectoral implant placement. An important difference between the two is the amount of tumescent solution needed. In case of pre-pectoral implant placement, researchers have reported use of 400 to 700 ml of solution per breast while around 740 ml was needed for retro-pectoral implant placement (34). In our review, the included studies used various modalities of autologous and alloplastic reconstruction with differences in the use mesh and position of implant. Since outcomes were not reported separately for each modality of breast reconstruction, we were unable to discern evidence on the efficacy of tumescent technique for different methods of breast reconstruction. Additionally, it also needs to be pointed out that in recent times video-assisted and robot-assisted surgery is slowly gaining attention, especially for nipple-sparing mastectomy (35, 36). These minimally invasive techniques have proven to be safe, with low conversion rate to open surgery and acceptable complication rates. Lai et al (35) in a consensus statement on robotic-nipple sparing mastectomy have recommended the use of tumescent technique for development of the skin flap. Our review was, however, unable to assess the efficacy of tumescent technique for robotic surgeries due to lack of comparative data.
In the case of the tumescent technique, once the effect of the vasoconstrictor disappears, rebound bleeding and hematoma formation can ensue (15). Hematoma requiring re-intervention is a serious complication, we assessed its incidence in our review. Our meta-analysis revealed similar hematoma incidences with the use of the tumescent technique. We obtained similar results for seroma and infectious complications. As mentioned earlier, these outcomes could have been caused by several confounding factors like co-morbidities, axillary dissection, surgical technique, use of antibiotics, and local wound care, variables which were not controlled in retrospective studies (37, 38).
Our results differed from those in the previously published meta-analysis. Siotos et al (3) reported a significant increase in the risk of total skin necrosis (OR, 1.56; 95% CI, 1.04 to 2.35; I2 = 71%; p=0.03), major skin necrosis (OR, 2.01; 95% CI, 1.29 to 3.14; I2 = 29%; p=0.002), and minor skin necrosis (OR, 1.75; 95% CI, 1.06 to 2.90; I2 = 0%; p=0.03) with the use of the tumescent technique. However, we found no such difference after our analysis and review. This can be attributed to the inclusion of three more studies in our analysis that provided a significant update. Furthermore, our review was strengthened by the additional analyses on the incidences of seroma and explantation, which had not been carried out in the previous study. To account for confounding factors and provide a comprehensive review, we pooled the data on multivariable-adjusted ORs.
The limitations of our review include the large number of retrospective studies in the analysis with their inherent bias and the fact that many studies had small sample sizes, which may have skewed our results. In addition, the studies included presented different types of surgical procedures and of reconstructions during the standard non-tumescent technique, and different follow-up durations creating methodological heterogeneity among them. Moreover, Seth et al (22) and Khavanin et al (20) reported data from the same institution with an overlap of four years. Also, complications with mastectomies can be influenced by the surgical skill and experience of the surgeons, and the impact of this factor on our results is difficult to assess. Lastly, we were unable to analyse oncological outcomes between tumescent and standard surgical techniques due to lack of data from include studies. Future studies should also report oncological outcomes in order to better assess the outcomes associated with the use of tumescent technique.
To conclude, the available evidence was of low-quality and derived mostly from non-randomized studies, but our analysis results suggest that the incidences of skin necrosis, hematoma, seroma, infection, and explantation between the tumescent and non-tumescent mastectomy techniques are similar. High-quality RCTs assessing the role of the tumescent technique with different reconstruction methods are needed to strengthen the evidence.
Publicly available datasets were analyzed in this study. This data can be found here: PubMed, Embase, BioMed Central, Ovid, and CENTRAL databases.
YY designed the project. JZ, XQ, and JF were involved in data collection and data analysis. YY prepared the manuscript. FS edit the manuscript. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.648955/full#supplementary-material
1. Worland RG. Expanded Utilization of the Tumescent Technique for Mastectomy. Plast Reconstr Surg (1996) 98:1321. doi: 10.1097/00006534-199612000-00048
2. Hardwicke JT, Jordan RW, Skillman JM. Infiltration of Epinephrine in Reduction Mammaplasty: A Systematic Review of the Literature. Plast Reconstr Surg (2012) 130:773–8. doi: 10.1097/PRS.0b013e318262f085
3. Siotos C, Aston JW, Euhus DM, Seal SM, Manahan MA, Rosson GD. The Use of Tumescent Technique in Mastectomy and Related Complications: A Meta-Analysis. Plast Reconstructive Surg (Lippincott Williams Wilkins) (2019) 143(1):39–48. doi: 10.1097/PRS.0000000000005100
4. Khater A, Mazy A, Gad M, Eldayem OTA, Hegazy M. Tumescent Mastectomy: The Current Indications and Operative Tips and Tricks. Breast Cancer Targets Ther (2017) 9:237–43. doi: 10.2147/BCTT.S131398
5. Carlson GW. Total Mastectomy Under Local Anesthesia: The Tumescent Technique. Breast J (2005) 11:100–2. doi: 10.1111/j.1075-122X.2005.21536.x
6. Dent BL, Small K, Swistel A, Talmor M. Nipple-Areolar Complex Ischemia After Nipple-Sparing Mastectomy With Immediate Implant-Based Reconstruction: Risk Factors and the Success of Conservative Treatment. Aesthetic Surg J (2014) 34:560–70. doi: 10.1177/1090820X14528352
7. Paige KT, Bostwick J, Bried JT. TRAM Flap Breast Reconstruction: Tumescent Technique Reduces Blood Loss and Transfusion Requirement. Plast Reconstr Surg (2004) 113:1645–9. doi: 10.1097/01.PRS.0000117195.00724.27
8. Gümüş N. Tumescent Infiltration of Lidocaine and Adrenaline for Burn Surgery(2011). (Accessed November 28, 2020).
9. Salgaonkar SV, Jain NM, Pawar SP. Total Intravenous Anaesthesia With Tumescent Infiltration Anaesthesia Without Definitive Airway for Early Excision and Skin Grafting in a Major Burn - A Prospective Observational Study. Indian J Anaesth (2020) 64:611–7. doi: 10.4103/ija.IJA_975_19
10. Reintgen C, Leavitt A, Pace E, Molas-Pierson J, Mast BA. Risk Factor Analysis for Mastectomy Skin Flap Necrosis: Implications for Intraoperative Vascular Analysis. Ann Plast Surg (2016) 76:S336–9. doi: 10.1097/SAP.0000000000000740
11. Robertson SA, Jeevaratnam JA, Agrawal A, Cutress RI. Mastectomy Skin Flap Necrosis: Challenges and Solutions. Breast Cancer Targets Ther (2017) 9:141–52. doi: 10.2147/BCTT.S81712
12. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
13. Higgins J, Altman D, Sterne J. Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing Risk of Bias in Included Studies. In: Cochrane Handbook for Systemic Reviews of Interventions (The Cochrane Collaboration). Available at: www.training.cochrane.org/ (accessed January 1, 2021).
14. Kim SY, Park JE, Lee YJ, Seo H-J, Sheen S-S, Hahn S, et al. Testing a Tool for Assessing the Risk of Bias for Nonrandomized Studies Showed Moderate Reliability and Promising Validity. J Clin Epidemiol (2013) 66:408–14. doi: 10.1016/j.jclinepi.2012.09.016
15. Lautrup MD, Thomsen JB, Christensen RD, Kjaer C. Tumescent Technique Versus Electrocautery Mastectomy: A Randomized Controlled Trial. Surg Oncol (2020) 34:276–82. doi: 10.1016/j.suronc.2020.05.003
16. Ng T, Knowles S, Brackstone M, Doherty C. Mastectomy Flap Necrosis After Nipple-Sparing Mastectomy and Immediate Implant-Based Reconstruction: An Evaluation of Tumescence and Sharp Dissection Technique on Surgical Outcomes. Breast J (2019) 25:1079–83. doi: 10.1111/tbj.13442
17. Gipponi M, Baldelli I, Atzori G, Fregatti P, Murelli F, Pesce M, et al. Tumescent Anesthesia in Skin- and Nipple-Sparing Mastectomy: Results of a Prospective Clinical Study. Anticancer Res (2017) 37:349–52. doi: 10.21873/anticanres.11328
18. Vargas CR, Koolen PGL, Ho OA, Ricci JA, Tobias AM, Lin SJ, et al. Tumescent Mastectomy Technique in Autologous Breast Reconstruction. J Surg Res (2015) 198:525–9. doi: 10.1016/j.jss.2015.03.050
19. Tasoulis MK, Agusti A, Karakatsanis A, Montgomery C, Marshall C, Gui G. The Use of Hydrodissection in Nipple- A Nd Skin-Sparing Mastectomy: A Retrospective Cohort Study. Plast Reconstr Surg - Glob Open (2019) 7:e2495. doi: 10.1097/GOX.0000000000002495
20. Khavanin N, Fine NA, Bethke KP, Mlodinow AS, Khan SA, Jeruss JS, et al. Tumescent Technique Does Not Increase the Risk of Complication Following Mastectomy With Immediate Reconstruction. Ann Surg Oncol (2014) 21:384–8. doi: 10.1245/s10434-013-3311-0
21. Abbott AM, Miller BT, Tuttle TM. Outcomes After Tumescence Technique Versus Electrocautery Mastectomy. Ann Surg Oncol (2012) 19:2607–11. doi: 10.1245/s10434-012-2304-8
22. Seth AK, Hirsch EM, Fine NA, Dumanian GA, Mustoe TA, Galiano RD, et al. Additive Risk of Tumescent Technique in Patients Undergoing Mastectomy With Immediate Reconstruction. Ann Surg Oncol (Ann Surg Oncol) (2011) 18(11):3041–6. doi: 10.1245/s10434-011-1913-y
23. Chun YS, Verma K, Rosen H, Lipsitz SR, Breuing K, Guo L, et al. Use of Tumescent Mastectomy Technique as a Risk Factor for Native Breast Skin Flap Necrosis Following Immediate Breast Reconstruction. Am J Surg (2011) 201:160–5. doi: 10.1016/j.amjsurg.2009.12.011
24. Samper A, Blanch A. Improved Subcutaneous Mastectomy With Hydrodissection of the Subcutaneous Space [7]. Plast Reconstr Surg (2003) 112:694–5. doi: 10.1097/01.PRS.0000072293.47341.F6
25. Chau JKM, Dzigielewski P, Mlynarek A, Cote DW, Allen H, Harris JR, et al. Steel Scalpel Versus Electrocautery Blade: Comparison of Cosmetic and Patient Satisfaction Outcomes of Different Incision Methods. J Otolaryngol - Head Neck Surg (2009) 38:427–33. doi: 10.2310/7070.2009.080080
26. Türkan A, Akkurt G, Yalaza M, Deǧirmencioǧlu G, Kafadar MT, Yenidünya S, et al. Effect of LigaSure™, Monopolar Cautery, and Bipolar Cautery on Surgical Margins in Breast-Conserving Surgery. Breast Care (2019) 14:194–9. doi: 10.1159/000493985
27. Družijanić N, Pogorelić Z, Perko Z, Mrklić I, Tomić S. Comparison of Lateral Thermal Damage of the Human Peritoneum Using Monopolar Diathermy, Harmonic Scalpel and LigaSure. Can J Surg (2012) 55:317–21. doi: 10.1503/cjs.000711
28. Mlodinow AS, Fine NA, Khavanin N, Kim JYS. Risk Factors for Mastectomy Flap Necrosis Following Immediate Tissue Expander Breast Reconstruction. J Plast Surg Handb Surg (2014) 48:322–6. doi: 10.3109/2000656X.2014.884973
29. Matsen CB, Mehrara B, Eaton A, Capko D, Berg A, Stempel M, et al. Skin Flap Necrosis After Mastectomy With Reconstruction: A Prospective Study. Ann Surg Oncol (2016) 23:257–64. doi: 10.1245/s10434-015-4709-7
30. Kuss O, Blettner M, Börgermann J. Propensity Score - Eine Alternative Methode Zur Analyse Von Therapieeffekten - Teil 23 Der Serie Zur Bewertung Wissenschaftlicher Publikationen. Dtsch Arztebl Int (2016) 113:597–603. doi: 10.3238/arztebl.2016.0597
31. Lee KT, Pyon JK, Bang SI, Lee JE, Nam SJ, Mun GH. Does the Reconstruction Method Influence Development of Mastectomy Flap Complications in Nipple-Sparing Mastectomy? J Plast Reconstr Aesthetic Surg (2013) 66:1543–50. doi: 10.1016/j.bjps.2013.06.032
32. Rusciani A, Pietramaggiori G, Troccola A, Santoprete S, Rotondo A, Curinga G. The Outcome of Primary Subglandular Breast Augmentation Using Tumescent Local Anesthesia. Ann Plast Surg (2016) 76:13–7. doi: 10.1097/SAP.0000000000000215
33. Shimizu Y, Nagasao T, Taneda H, Sakamoto Y, Asou T, Imanishi N, et al. Combined Usage of Intercostal Nerve Block and Tumescent Anaesthesia: An Effective Anaesthesia Technique for Breast Augmentation. J Plast Surg Handb Surg (2014) 48:51–5. doi: 10.3109/2000656X.2013.800529
34. Bolletta A, Dessy LA, Fiorot L, Tronci A, Rusciani A, Ciudad P, et al. Sub-Muscular Breast Augmentation Using Tumescent Local Anesthesia. Aesthetic Plast Surg (2019) 43:7–13. doi: 10.1007/s00266-018-1181-3
35. Lai HW, Toesca A, Sarfati B, Park HS, Houvenaeghel G, Selber JC, et al. Consensus Statement on Robotic Mastectomy - Expert Panel From International Endoscopic and Robotic Breast Surgery Symposium (IERBS) 2019. Ann Surg (2020) 271:1005–12. doi: 10.1097/SLA.0000000000003789
36. Lai H-W, Chen S-T, Mok CW, Lin S-L, Tai C-M, Chen D-R, et al. Single-Port 3-Dimensional Videoscope-Assisted Endoscopic Nipple-Sparing Mastectomy in the Management of Breast Cancer. Plast Reconstr Surg Glob Open (2019) 7:e2367. doi: 10.1097/GOX.0000000000002367
37. Chavan RN, Chikkala B, Mondal P, Sarkar DK. Comparison Study Between Scalpel and Electrocautery, in Causation of Seroma After Modified Radical Mastectomy. Indian J Surg (2017) 79:423–6. doi: 10.1007/s12262-016-1501-2
Keywords: breast cancer, mastectomy, epinephrine, lignocaine, complications, skin necrosis
Citation: Yang Y, Zhu J, Qian X, Feng J and Sun F (2022) Complication Differences Between the Tumescent and Non-Tumescent Dissection Techniques for Mastectomy: A Meta-Analysis. Front. Oncol. 11:648955. doi: 10.3389/fonc.2021.648955
Received: 02 February 2021; Accepted: 20 December 2021;
Published: 10 January 2022.
Edited by:
Gianluca Franceschini, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Gilles Houvenaeghel, Institut Paoli-Calmettes (IPC), FranceCopyright © 2022 Yang, Zhu, Qian, Feng and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fukun Sun, ZnVrdW5zdW5AMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.