
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 01 July 2021
Sec. Head and Neck Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.645077
 Yuliang Jiang1†
Yuliang Jiang1† Peng Zhen2†
Peng Zhen2† Jinchao Dai3
Jinchao Dai3 Yixing Li4
Yixing Li4 Shifeng Liu5
Shifeng Liu5 Junma Xu6
Junma Xu6 Yufeng Wang7
Yufeng Wang7 Suqing Tian1
Suqing Tian1 Yue Cui1
Yue Cui1 Zhe Ji1
Zhe Ji1 Fuxin Guo1
Fuxin Guo1 Bin Qiu1†
Bin Qiu1† Haitao Sun1
Haitao Sun1 Jinghong Fan1
Jinghong Fan1 Junjie Wang1*
Junjie Wang1*Purpose: To investigate the safety and efficacy of CT-guided I125 radioactive seed implantation (RSI) as a salvage therapy for recurrent head and neck squamous carcinoma (rHNSC) after external beam radiotherapy (EBRT) or surgery.
Materials and Methods: This is a multicenter retrospective study of 113 patients (83 males; median age 57 years) with rHNSC who underwent CT-guided I125 RSI between February 2003 and December 2017. Of the included patients, 107 patients previously received EBRT and 65 patients received surgery and all were ineligible or rejected for salvage surgery and/or repeat EBRT.
Results: During a median follow-up duration of 20 months (range, 3-152 months), 87 patients died. The 1-, 2-, 3-, and 5-year local control rate were 57.4%, 41.8%, 29.3%, and 15.2%, respectively. The median time to progression was 15 months [95% confidence interval (CI), 6.1-23.9 months]. The median overall survival (OS) was 20 months (95% CI, 12.4-27.6 months). The 1-, 2-, 3-, and 5-year OS rate were 63.6%, 44.6%, 29.9%, and 21.7%, respectively. Univariate and multivariate analyses revealed that KPS score and postoperative D90 were significantly associated with patients’ OS. The complications were mainly grade I/II skin and mucosal reactions: 18 cases (15.9%) of grade I/II and eight cases (7.0%) of grade III radiation dermatitis, and 14 cases (12.4%) of grade I/II and three cases (2.7%) grade III mucosal reactions. No grade IV or severer complications were found.
Conclusion: CT-guided I125 RSI may be safe as a salvage therapy for rHNSC after EBRT/surgery, yielding promising efficacy compared with historical data. KPS score and postoperative D90 may be significantly associated with OS.
Head and neck squamous carcinoma (HNSC) is the sixth most common cancer and accounts for over 600,000 new cancer cases and 350,000 deaths worldwide each year (1, 2). Despite the high local control rate of HNSC, recurrent HNSC (rHNSC) still occurs in 20%-35% of the patients after surgery/chemo-radiotherapy (3). Though long-term survival is becoming more common in HNSC, the outcome for rHNSC is still very poor (4). Salvage therapy for recurrent disease may preferentially benefit this subset; unfortunately, treatment options are limited (3).
Salvage surgery leads to a substantial improvement in outcomes for rHNSC although it only can be used in highly selected patients (5). In the context of radiation used for patients with rHNSC, the ideal technique should be able to deliver therapeutic doses to the targets with doses as low as possible to the organs at risk to improve the prognosis of rHNSC (6). Re-irradiation using external beam radiotherapy (EBRT) is promising though still challenging for managing lesions raised in previously irradiated fields. Therefore, EBRT can only be considered in well-selected patients but at the high cost of toxicity (3, 7).
Brachytherapy offers dosimetric advantages with very sharp radiation dose gradients compared with conventional external-beam techniques (8). Given the introduction of a brachytherapy-treatment planning system (BT-TPS), optimal dose distribution can be realized using radioactive seed implantation (RSI) for various cancers. Notably, I125 radioactive seed implantation (RSI) becomes an optimal tool for prostate carcinoma (9). Therefore, I125 RSI may be used as a salvage treatment for rHNSC due to its focused irradiation on the tumor and rapid dose fall-off at distance from the sources, limiting dose exposure to surrounding tissues (8). Several single-institute retrospective studies were previously published for patients with rHNSC (10–13). Here, the multicenter retrospective study reported a long-term outcome of CT-guided I125 RSI for patients with rHNSC.
This is a retrospective study of 113 patients [83 males; median age 57 years (range, 26-83 years)] with rHNSC treated with CT-guided I125 RSI in six centers in China between February 2003 and December 2017 (a major center contributes 84 patients and the remaining centers contribute 1-20 cases each). Of the included patients, 107 patients previously received EBRT and 65 patients received surgery. The patient’s characteristics were listed in Table 1. The local control rate, time to progression, overall survival (OS) rate, and complications were analyzed. The evaluation of tumor response was based on the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 (14). Local control was defined as complete response, partial response, and stable disease. Time to progression was defined as the time from the RSI procedure until tumor progression. Overall survival was defined as the time from the RSI procedure until the time of death from any cause or last follow-up. Complications were determined by the Common Terminology Criteria for Adverse Events (CTCAE) v4.0 (CTCA) (15).
The indication for I125 RSI in this study was all follows: (1) Aged 18-85 year-old with KPS≥70; (2) Pathological/radiological recurrent tumor in patients with pathological diagnosed HNSC after prior EBRT/surgery, with the lesion diameter less than 7cm; (3) CT revealed a possible needles pathway capable for RSI; (4) Patients without metastasis or with stable metastasis (less than 3 lesions); (5) Expected survival time more than 3 months; and (6) Ineligibility or rejection of salvage surgery and/or repeat EBRT (Ineligibility commonly referred to uncertain R0 resection or tumor invasion of large blood vessels or poor conditions after discussion by a Multidisciplinary Team minimum consisting of a surgeon, oncologist, and radiologist). All patients had signed an informed consent form for RSI, which stated the advantages and disadvantages of RSI. Contraindication was one of the following: (1) Severe organ dysfunction, (2) Coagulation dysfunction, (3) Active infection, (4) Mental illness, or (5) Extensive necrosis/ulcer formation/skin rupture. The study was approved by the ethics committee of each center and the requirement to obtain written informed consent to the participant of the study was waived.
All patients received a blood routine test, coagulation function, and biochemistry examination before RSI to rule out contraindication. The patients were immobilized on a CT simulator couch with a custom vacuum lock bag in the supine or lateral position. Both plain and contrast CT scans were performed with 5mm thickness before 1-2 days of RSI. The image data were transmitted into BT-TPS (Beijing University of Aeronautics and Astronautics and Beijing Astro Technology Co., Ltd) for pre-plan according to planning system source data originated from the official recommendation of the American Association of Physicists in Medicine (AAPM) (16, 17). The clinical tumor volume (CTV) was formed by expanding 5-6 mm with three dimensions from gross tumor volume (GTV). The doses received by 90% of GTV (GTV D90) were supposed to be as close to the prescription dose as possible whereas the doses received by OARs were kept as low as possible. The median prescription dose was 120Gy (range, 110-160 Gy) and the activity of I125 seed (size 4.5mm×0.8mm and half-life time 59.4 days; Beijing Atom high Tech Pharmaceutical Company Inc., China) was 0.22-0.83 mCi (median 0.65mCi).
The RSI protocol was as follows: (1) patients were set up on the CT-simulator and immobilized with a vacuum pad. Local anesthesia was carried out and 2-3 reference needles were inserted 2-3 cm into the patient’s body per pre-plan; (2) CT scan was performed to determine the accurate position of the reference needles; (3) When the reference needles’ position was mismatched with pre-plan, the adjustment in real-time was made until the deviation less than 2 mm; (4) The seed needles were all inserted into the targets; (5) CT scan was performed again to confirm the position of all needle tips, similarly, if the deviation was more than 2 mm, an adjustment would be made; (6) 125I seed was delivered with applicator (Mick 200-TPV Applicator, Mick Radio-Nuclear Inc., US) in a receding manner; and (7) CT scan was performed again to confirm the distribution of I125 seeds in the targets. The CT images were transferred into the BT-TPS for post-plan doses’ evaluation. Patients would be discharged 1-2 days after RSI. All procedures followed the recommendations by the International Commission on Radiological Protection (18). The dosimetric parameters, e.g. D90, were recognized.
Patients were routinely followed with CT/MRI in 3-month intervals for the first 2 years, 6-month intervals from 3 to 5 years, and then followed up annually after RSI. The evaluation of tumor response was based on the images obtained at 3 months after RSI.
A Chi-square test was used for the analysis of tumor response. Survival was estimated using the Kaplan-Meier method and univariate analysis was conducted using the log-rank test, and multifactor analysis was conducted using Cox regression. P<0.05 was considered significant.
Of the 113 patients included, 87 patients died and 26 survived (20 patients were lost to follow-up since August 2019 and were counted as truncated numbers) during a median follow-up duration of 20 months (range, 3-152 months).
There were 20 cases of complete response, 74 cases of partial response, 13 cases of stable disease, and six cases of progressive disease, Figure 1. The 1-, 2-, 3-, and 5-year local control rate were 57.4%, 41.8%, 29.3%, and 15.2%, respectively. The median time to progression was 15 months [95% confidence interval (CI), 6.1-23.9 months], Table 2. Univariate analysis showed that age, sex, previous surgery, radiotherapy, chemotherapy, and site of recurrence were not associated with local control (p = 0.311, 0.079, 0.582, 0.511, 0.697, and 0.738, respectively). The local control of patients with D90 > 90Gy was significantly better than that of patients with D90 ≤ 90Gy (p=0.000). The local control rate of the group with good performance (KPS score ≥ 80) was better than that of the group with poor performance (KPS score < 80) (p = 0.003), Table 3 and Figure 2.
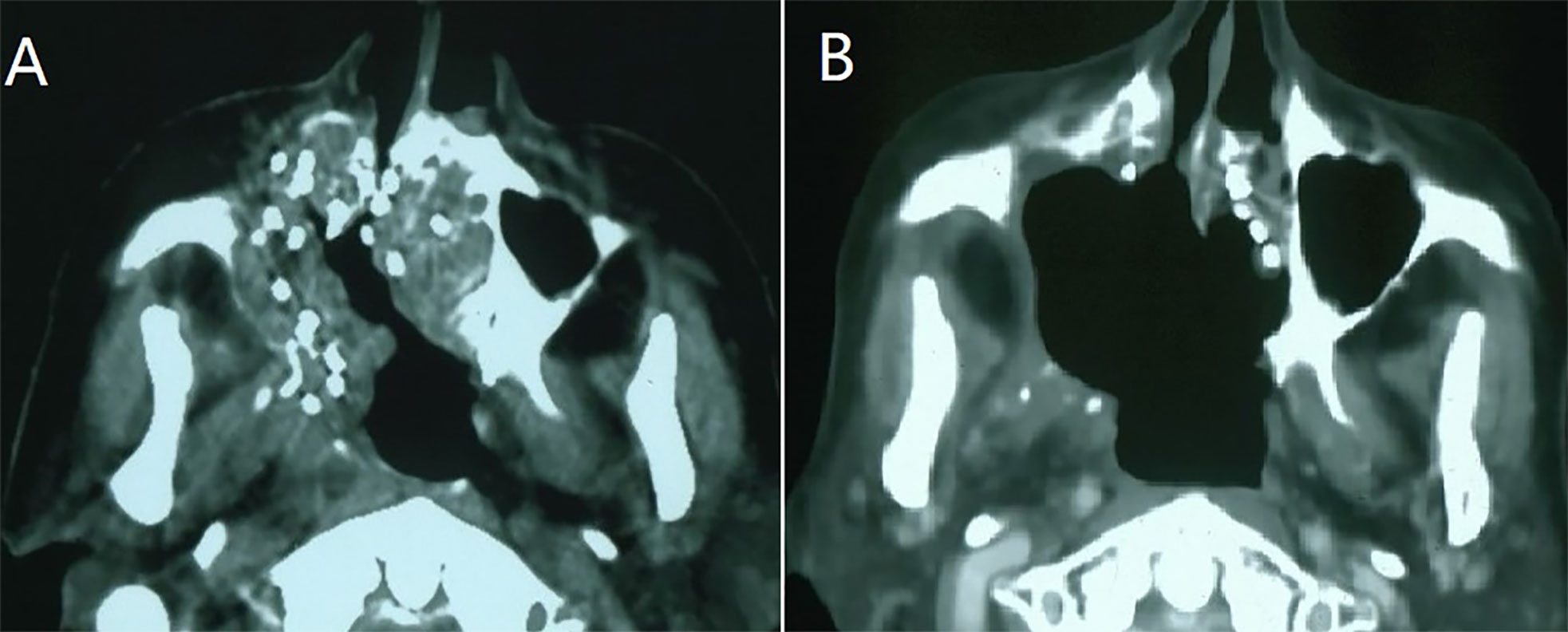
Figure 1 A case of recurrent maxillary sinus squamous carcinoma following surgery and adjuvant radiotherapy. (A) CT-guided I125 seeds were implanted as a salvage treatment; (B): Three months after the seed implantation, partial response was observed.
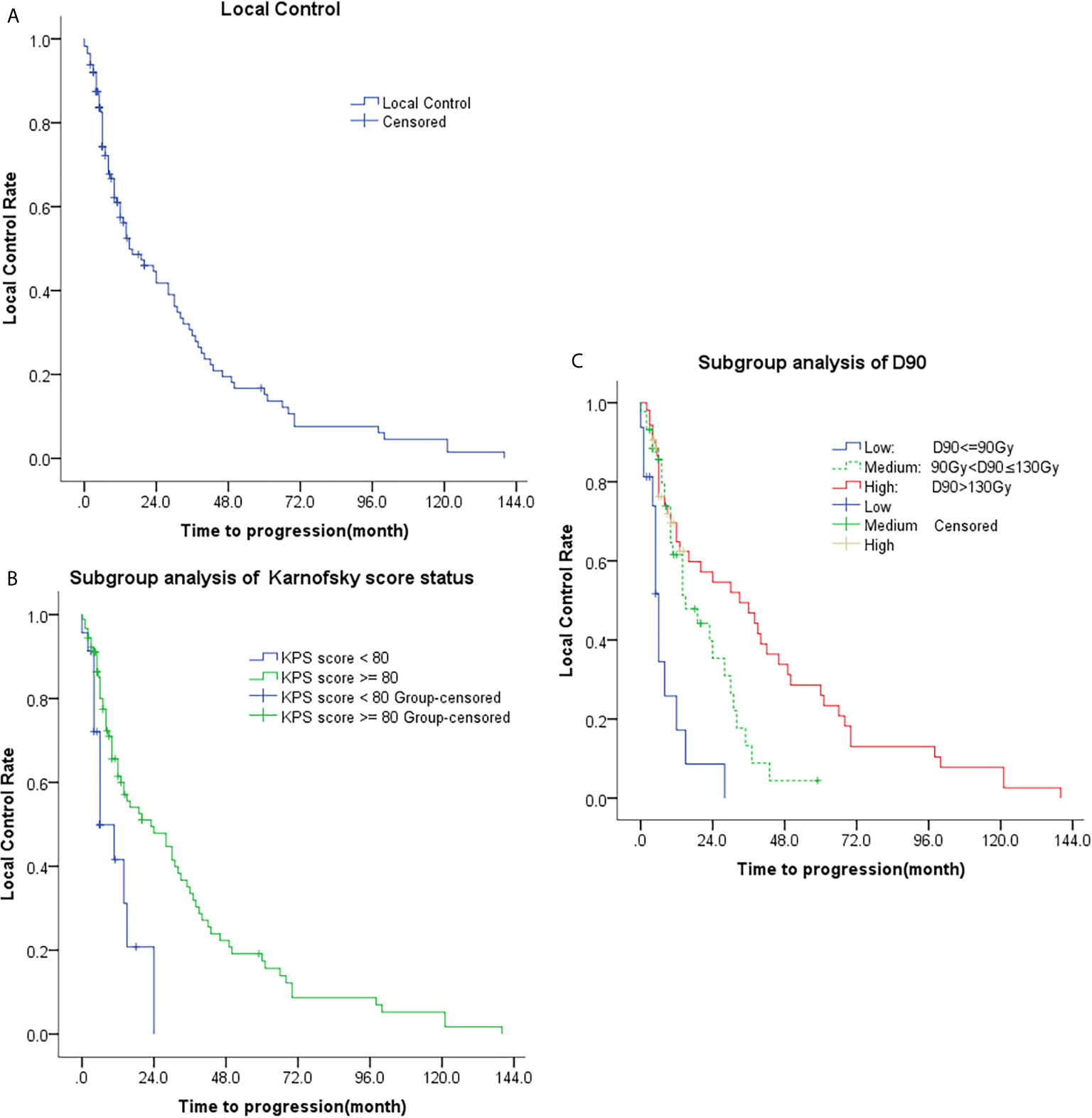
Figure 2 Kaplan-Meier plots of time to progression; (A) Analysis of all patients; (B) Subgroup analysis of KPS (KPS < 80 and KPS ≥ 80); (C) Subgroup analysis of D90 (D90 ≤ 90Gy, 90Gy < D90 ≤ 130Gy, and D90 > 130Gy).
The median OS was 20 months (95% CI, 12.4-27.6 months). The 1-, 2-, 3-, and 5-year OS rate were 63.6%, 44.6%, 29.9%, and 21.7%, respectively, Table 2. Univariate analysis showed that age, sex, previous surgery/radiotherapy/chemotherapy, and site of recurrence were not associated with OS (p = 0.422, 0.793, 0.994, 0.328, 0.614, and 0.708, respectively). The OS of patients with D90 > 90Gy was significantly better than that of patients with D90 ≤ 90Gy (p<0.001) and the OS of patients with good performance (KPS score ≥80) was also better than that of patients with poor performance (KPS score < 80) (p<0.001). Multivariate analyses also revealed that only KPS score and postoperative D90 were significantly associated with patients’ OS (both p<0.001), Table 4 and Figure 3.
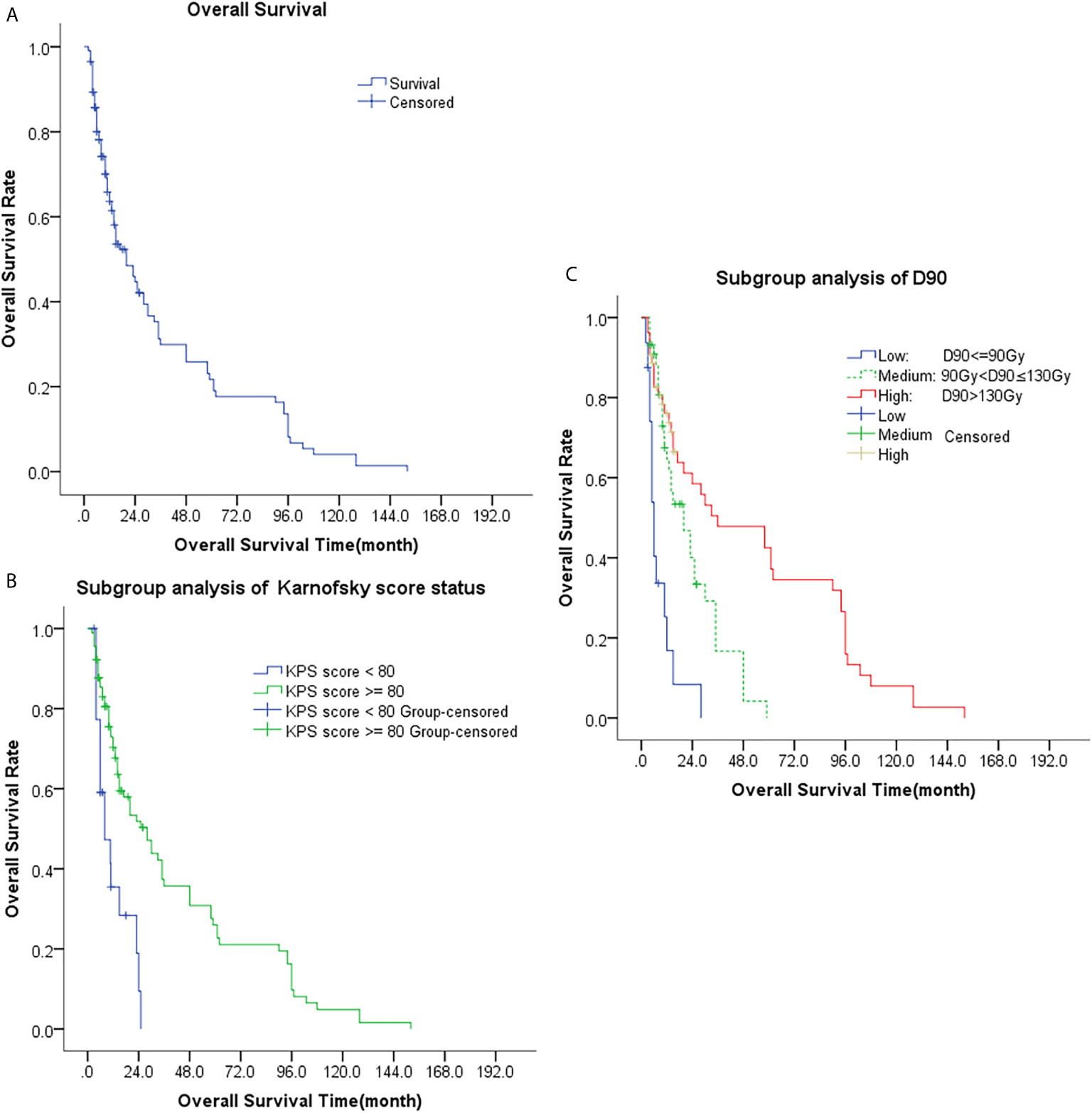
Figure 3 Kaplan-Meier plots of overall survival curves; (A) Analysis of all patients; (B) Subgroup analysis of KPS (KPS < 80 and KPS ≥ 80); (C) Subgroup analysis of D90 (D90 ≤ 90Gy, 90Gy < D90 ≤ 130Gy, and D90 > 130Gy).
Local pain at the implantation site was found in five cases (6.0%) during RSI. During follow-up, the complications were mainly grade I/II skin and mucosal reactions: 18 cases (15.9%) of grade I/II and eight cases (7.0%) of grade III radiation dermatitis, and 14 cases (12.4%) of grade I/II and three cases (2.7%) of grade III mucosal reactions. No grade IV or severer complication were found, Table 2.
The multicenter retrospective study reported a favorable long-term result of CT-guided I125 RSI as a salvage therapy for rHNSC. During a median follow-up duration of 20 months, patients experienced a relatively high rate of local control rate and OS rate. KPS score and postoperative D90 were significantly associated with local control and patients’ OS. The complications were mainly minor skin and mucosal reactions.
With the development of modern radiation techniques, such as 3-dimensional conformal radiotherapy (3D-CRT) or intensify modulated radiotherapy (IMRT), reirradiation is applied for rHNSC after EBRT. Reirradiation using EBRT with or without chemotherapy improved the local control rates in half of the patients and long-term survival in selected patients (19, 20). EBRT as a salvage option is promising for rHNSC in the previously irradiated area although it still poses a great challenge as it is hard to deliver sufficient doses to the targets while sparing the normal tissues, especially for tumors previously treated with full doses. The treatment-related toxicity was as high as 20-30% (20, 21). I125 RSI may have the following advantages: (1) Radioactive seeds once implanted were continuously irradiating the tumors without interval compared with EBRT; (2) The radiation dose of the target boosted high enough to achieve an ablative effect while sparing the normal tissue; and (3) The therapy was minimally invasive and resulted in a short hospital stay for the patients. However, a direct comparison was not available.
In the largest multi-institutional phase II trial of Radiation Therapy Oncology Group for unresectable rHNSC managed with repeat EBRT (reirradiation) and chemotherapy (RTOG-9610) (22), the 2-year and 5-year OS were only 15.2% and 3.8%. The worst acute toxicity was grade 4 in 17.7% and grade 5 in 7.6%. Grade 3 and 4 late toxicities were found in 19.4% and 3.0%, respectively. In a randomized phase III trial (GORTEC 98-03) comparing repeat EBRT (reirradiation) plus chemotherapy versus methotrexate in 57 patients with rHNSC (23), 1-year OS was only 23% versus 22%. Sixteen patients (28%) experienced clinical grade ≧̸3 late toxicities. This was a retrospective study of 327 patients with recurrent and metastatic (RM) HNSC (24). All patients received at least one line of active treatment (eg, surgery, concurrent chemoradiotherapy, or radiotherapy/chemotherapy alone/with surgery) and those receiving only the best supportive care were excluded. The median OS was only 14 months with a favorable group (46%) and 10 months with an unfavorable group (54%), which seems inferior to that reported here (i.e. 20 months). Compared with these historical data of irradiation, the current study reveals CT-guided I125 RSI may be promising as a salvage therapy for rHNSC after EBRT/surgery.
Previous evidence of RSI for rHNSC were all single-institute retrospective studies. The largest study by Ji et al. (10) reported 101 patients treated with CT-guided I125 RSI for recurrent head and neck cancer (rHNC) after EBRT. The median previous cumulative external radiation dose was 66 Gy, and the median D90 after RSI was 117 Gy. The 5-year local control rate was 26.6%, the median survival time was 15 months, and the 5-year OS rate was 15.5%. The 5- year local control rate was 11.5% (2-year) when D90 < 120 Gy and was 44.2% when D90 = 120 Gy (p=0.001). There were 26 (25.7%) cases of skin/mucosa ulceration among them; 15.8% were grade I to II, 7.9% were grade III, and only 2% were grade IV. Fourteen cases suffered from pain (13.9%) and two cases with dry mouth (2%). The report was the only published study for I125 RSI with over 100 cases and the outcomes were similar to the present study. This was a study by Jiang et al. (13). involving 64 patients, with 81 rHNC in total, treated with permanent I125 RSI under ultrasound guidance. The median follow-up period was 14 months. The total response rate was 80% (27% complete response and 53% partial remission), which is similar to that of our study. The 1-, 3-, and 5-year tumor control rates were 75.2%, 73.0%, and 69.1%, respectively, which is better than that reported here; this may be attributed to the difference of pathological pattern and recurrent sites between the two studies. This is because the results for cervical lymph node recurrence were better than those for primary rHNC reported in her study, with 5-year local control rates of 72.7% and 39.9%, respectively. The 1-, 3-, and 5-year overall survival rates were 57.4%, 31%, and 26.6%, respectively, with a median survival of 20 months. D90 was also found to be an independent prognostic factor of the efficacy. Grade I/II skin reactions were seen in 11 patients (17%) who had received external beam radiotherapy before. Other severe complications were absent. These survival and complication outcomes were all similar to the present study, while Grade IV skin ulceration was seen in two patients in their study. In the present study, the rate and severity of toxicity were relatively low and minor. The potential reasons may include: (1) RSI was conducted with a 0.5-1 cm safety margin away from the organ at risk under CT-guidance; (2) The activity of I125 seed was kept as 0.4-0.5 mCI when the tumor invaded the organ at risk; (3) Patients with tumor-infiltrated skin or mucosa were excluded, so skin or mucosa ulceration may be avoided; and (4) The indication for I125 RSI was strict with an appropriate diameter of the tumor or needle pathway. Within the above screening criteria, CT-guided RSI may be a feasible and safe treatment for patients with rHNSC after EBRT; further study is warranted.
There are several limitations to this study. Firstly, as a multicenter study, the majority (74%, 84/113) of the patients were included from a major center, which may result in potential bias. Secondly, the study is a retrospective study with a single arm and 20 patients were lost to follow-up since August 2019, which may also lead to a certain bias. Thirdly, RSI is used as salvage therapy for the patients, so there was no control group with the current standard treatment of salvage surgery or repeat EBRT. However, the study was the first multicenter study with over 100 cases investigating the long-term safety and efficacy of RSI for rHNSC obtaining favorable outcomes, therefore, prospective studies with a control group are needed in the future.
CT-guided I125 RSI may be safe as a salvage therapy for rHNSC after EBRT/surgery, yielding promising efficacy compared with historical data. KPS score and postoperative D90 may be significantly associated with patients’ outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Peking university Third Hospital Medical Science Rearch Ethics Committee. The ethics committee waived the requirement of written informed consent for participation.
YJ, PZ, and JW conceived and designed the study. YJ, PZ, JD, YL, SL, JX, YW, ST, YC, ZJ, FG, BQ, HS, JF, and JW performed the study and data collection. YJ is responsible for statistical analysis. YJ, BQ, and JW wrote the paper. JW reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
National Key Research and Development Plan of China (Grant No. 2019YFB1311300) to JW supports the implementation (e.g., labor cost and data collection) and publication of the project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Santuray RT, Johnson DE, Grandis JR. New Therapies in Head and Neck Cancer. Trends Cancer (2018) 4(5):385–96. doi: 10.1016/j.trecan.2018.03.006
2. Global Burden of Disease Cancer C, Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol (2019) 5(12):1749–68. doi: 10.1001/jamaoncol.2019.2996
3. Baliga S, Kabarriti R, Ohri N, Haynes-Lewis H, Yaparpalvi R, Kalnicki S, et al. Stereotactic Body Radiotherapy for Recurrent Head and Neck Cancer: A Critical Review. Head Neck (2017) 39(3):595–601. doi: 10.1002/hed.24633
4. Cohen EE, LaMonte SJ, Erb NL, Beckman KL, Sadeghi N, Hutcheson KA, et al. American Cancer Society Head, and Neck Cancer Survivorship Care Guideline. CA Cancer J Clin (2016) 66(3):203–39. doi: 10.3322/caac.21343
5. Patil VM, Noronha V, Thiagarajan S, Joshi A, Chandrasekharan A, Talreja V, et al. Salvage Surgery in Head and Neck Cancer: Does It Improve Outcomes? Eur J Surg Oncol (2020) 46(6):1052–8. doi: 10.1016/j.ejso.2020.01.019
6. Chargari C, Magne N, Guy JB, Rancoule C, Levy A, Goodman KA, et al. Optimize and Refine Therapeutic Index in Radiation Therapy: Overview of a Century. Cancer Treat Rev (2016) 45:58–67. doi: 10.1016/j.ctrv.2016.03.001
7. Awan MJ, Nedzi L, Wang D, Tumati V, Sumer B, Xie XJ, et al. Final Results of a Multi-Institutional Phase II Trial of Reirradiation With Concurrent Weekly Cisplatin and Cetuximab for Recurrent or Second Primary Squamous Cell Carcinoma of the Head and Neck. Ann Oncol (2018) 29(4):998–1003. doi: 10.1093/annonc/mdy018
8. Chargari C, Deutsch E, Blanchard P, Gouy S, Martelli H, Guerin F, et al. Brachytherapy: An Overview for Clinicians. CA Cancer J Clin (2019) 69(5):386–401. doi: 10.3322/caac.21578
9. Ragde H, Grado GL, Nadir B, Elgamal AA. Modern Prostate Brachytherapy. CA Cancer J Clin (2000) 50(6):380–93. doi: 10.3322/canjclin.50.6.380
10. Ji Z, Jiang Y, Tian S, Guo F, Peng R, Xu F, et al. The Effectiveness and Prognostic Factors of CT-Guided Radioactive I-125 Seed Implantation for the Treatment of Recurrent Head and Neck Cancer After External Beam Radiation Therapy. Int J Radiat Oncol Biol Phys (2019) 103(3):638–45. doi: 10.1016/j.ijrobp.2018.10.034
11. Jiang Y, Ji Z, Guo F, Peng R, Sun H, Fan J, et al. Side Effects of CT-Guided Implantation of (125)I Seeds for Recurrent Malignant Tumors of the Head and Neck Assisted by 3D Printing non Co-Planar Template. Radiat Oncol (2018) 13(1):18. doi: 10.1186/s13014-018-0959-4
12. Chen Y, Jiang Y, Ji Z, Jiang P, Xu F, Zhang Y, et al. Efficacy and Safety of CT-Guided (125)I Seed Implantation as a Salvage Treatment for Locally Recurrent Head and Neck Soft Tissue Sarcoma After Surgery and External Beam Radiotherapy: A 12-Year Study at a Single Institution. Brachytherapy (2020) 19(1):81–9. doi: 10.1016/j.brachy.2019.09.006
13. Jiang P, Wang J, Ran W, Jiang Y, Tian S, Sun H. Five-Year Outcome of Ultrasound-Guided Interstitial Permanent (125)I Seeds Implantation for Local Head and Neck Recurrent Tumors: A Single Center Retrospective Study. J Contemp Brachyther (2019) 11(1):28–34. doi: 10.5114/jcb.2019.83336
14. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
15. Chen AP, Setser A, Anadkat MJ, Cotliar J, Olsen EA, Garden BC, et al. Grading Dermatologic Adverse Events of Cancer Treatments: The Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol (2012) 67(5):1025–39. doi: 10.1016/j.jaad.2012.02.010
16. Fraass B, Doppke K, Hunt M, Kutcher G, Starkschall G, Stern R, et al. American Association of Physicists in Medicine Radiation Therapy Committee Task Group 53: Quality Assurance for Clinical Radiotherapy Treatment Planning. Med Phys (1998) 25(10):1773–829. doi: 10.1118/1.598373
17. Nath R, Anderson LL, Meli JA, Olch AJ, Stitt JA, Williamson JF. Code of Practice for Brachytherapy Physics: Report of the AAPM Radiation Therapy Committee Task Group No. 56. Am Assoc Phys Med Med Phys (1997) 24(10):1557–98. doi: 10.1118/1.597966
18. Optimization and Decision-Making in Radiological Protection. A Report of a Task Group of Committee 4 of the International Commission on Radiological Protection. Ann ICRP (1989) 20(1):1–60.
19. Orlandi E, Bonomo P, Ferella L, D’Angelo E, Maddalo M, Alterio D, et al. Long-Term Outcome of Re-Irradiation for Recurrent or Second Primary Head and Neck Cancer: A Multi-Institutional Study of AIRO-Head and Neck Working Group. Head Neck (2019) 41(10):3684–92. doi: 10.1002/hed.25890
20. Bots WTC, van den Bosch S, Zwijnenburg EM, Dijkema T, van den Broek GB, Weijs WLJ, et al. Reirradiation of Head and Neck Cancer: Long-Term Disease Control and Toxicity. Head Neck (2017) 39(6):1122–30. doi: 10.1002/hed.24733
21. Choi SH, Chang JS, Choi J, Park SH, Keum KC, Park KR, et al. Re-Irradiation Using Intensity-Modulated Radiotherapy for Recurrent and Second Primary Head and Neck Cancer. Anticancer Res (2018) 38(5):3165–73. doi: 10.21873/anticanres.12580
22. Spencer SA, Harris J, Wheeler RH, Machtay M, Schultz C, Spanos W, et al. Final Report of RTOG 9610, a Multi-Institutional Trial of Reirradiation and Chemotherapy for Unresectable Recurrent Squamous Cell Carcinoma of the Head and Neck. Head Neck (2008) 30(3):281–8. doi: 10.1002/hed.20697
23. Tortochaux J, Tao Y, Tournay E, Lapeyre M, Lesaunier F, Bardet E, et al. Randomized Phase III Trial (GORTEC 98-03) Comparing Re-Irradiation Plus Chemotherapy Versus Methotrexate in Patients With Recurrent or a Second Primary Head and Neck Squamous Cell Carcinoma, Treated With a Palliative Intent. Radiother Oncol (2011) 100(1):70–5. doi: 10.1016/j.radonc.2011.06.025
Keywords: recurrent head and neck cancer, brachytherapy, radioactive seed implantation, squamous carcinoma, radiotherapy
Citation: Jiang Y, Zhen P, Dai J, Li Y, Liu S, Xu J, Wang Y, Tian S, Cui Y, Ji Z, Guo F, Qiu B, Sun H, Fan J and Wang J (2021) Long-Term Safety and Efficacy of CT-Guided I125 Radioactive Seed Implantation as a Salvage Therapy for Recurrent Head and Neck Squamous Carcinoma: A Multicenter Retrospective Study. Front. Oncol. 11:645077. doi: 10.3389/fonc.2021.645077
Received: 04 January 2021; Accepted: 18 May 2021;
Published: 01 July 2021.
Edited by:
Christian Simon, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandReviewed by:
Mark Prince, University of Michigan, United StatesCopyright © 2021 Jiang, Zhen, Dai, Li, Liu, Xu, Wang, Tian, Cui, Ji, Guo, Qiu, Sun, Fan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junjie Wang, anVuamlld2FuZ0Bwa3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.