
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 17 June 2021
Sec. Gastrointestinal Cancers
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.644670
This article is part of the Research Topic Advanced Molecular Targets in the Diagnosis and Treatment of Gastrointestinal Cancers View all 55 articles
 Guo Wu1,2†
Guo Wu1,2† Jungang Liu1,2,3†
Jungang Liu1,2,3† Haizhou Liu4†
Haizhou Liu4† Lan Jin3
Lan Jin3 Xiaoliang Huang1,2
Xiaoliang Huang1,2 Xianwei Mo1,2
Xianwei Mo1,2 Huage Zhong1,2
Huage Zhong1,2 Yanhua Li3,5
Yanhua Li3,5 Yawei Zhang6*
Yawei Zhang6* Weizhong Tang1,2*
Weizhong Tang1,2*Purpose: This study aimed to elucidate the prognostic significance of a novel inflammation-joined and nutrition-related clinicopathological marker for colorectal cancer (CRC).
Methods: Various factors from preoperative fasting blood samples from 2471 patients with CRC were retrospectively analyzed. Factors related to prognosis were evaluated using univariate and multivariate analyses. The Kaplan–Meier method was used to generate survival curves, while the log-rank test was used to measure survival differences between groups.
Results: Univariate analysis revealed that C-reactive protein (CRP)/mean corpuscular volume (MCV) ratio, TNM stage, differentiation, right-sided tumor, age, carcinoembryonic antigen (CEA) level, and CRP level were significantly associated with poor prognosis in CRC. In contrast, adjuvant chemotherapy is regarded as a protective factor. Elevation of CRP/MCV ratio (odds ratio [OR]: 1.535, 95% confidence interval [CI]: 1.121–2.104, P = 0.008), TNM stage (OR: 2.747, 95% CI: 2.175–3.469, P < 0.001), and differentiation (OR, 1.384; 95% CI, 1.150–1.666; P = 0.001) were prognostic risk factors in the multivariate analyses. Subgroup analysis showed that CRP/MCV, TNM staging system, and differentiation also independently affected survival in patients with lymph node-positive CRC. The nomogram based on these three indicators showed that CRP/MCV had a greater prognostic value and clinical significance for lymph node-positive patients with poorly differentiated tumors at the late stage.
Conclusion: A novel nomogram using the clinicopathologic index of inflammation and nutrition was constructed to predict the prognosis of CRC. Early interventions should be emphasized for advanced-stage patients with severe inflammation and poor nutritional status.
Colorectal cancer (CRC) is a major health problem worldwide and the third most common cancer and the second leading cause of cancer-related deaths (1). CRC is estimated to be the second most common cancer in China and ranks as the fifth leading cause of cancer-related deaths regardless of age and sex (1). The incidence and mortality rates of CRC vary significantly worldwide. Even for patients of the same stage, the biological behavior of the tumor and the patient’s prognosis are quite different. Colorectal tumors are heterogeneous, and individualized risk stratification helps guide clinical treatment. It is well documented that inflammatory and nutritional status are both important factors affecting tumor development and clinical outcomes (2, 3). The systemic inflammatory response in cancer patients can lead to malnutrition, which can alter immune responses, increasing the risks of postoperative infection and poor wound healing in surgical patients (4). Early identification of patients who are in danger of a hyperinflammatory state and malnourishment is vital to reduce the risk of surgical complications and mortality, improve clinical outcomes, and relieve the financial burden (2).
Tumors that occur in the digestive tract are more closely related to inflammation and nutrition. On the one hand, they are directly stimulated by digestive juice; on the other hand, intestinal microbes participate in the inflammatory response, and chronic inflammation eventually leads to the occurrence of tumors. For example, Helicobacter pylori infection has been shown to be an important risk factor for gastric cancer, and a persistent chronic inflammatory environment resulting from this infection leads to a series of damages in the gastrointestinal tract (5, 6). Accordingly, Bacteroides fragilis and Enterococcus faecalis settled in the intestine produce enterotoxins and reactive oxygen species that cause oxidative DNA damage, induce inflammation, and damage the epithelial barrier (7). In addition, the main functions of the digestive tract are digestion and absorption. Tumors that occur in the digestive tract are more likely to develop malnutrition, causing symptoms and signs related to malnutrition. Nutrition, inflammation, immunity, and cancer constitute a triangular relationship, and the imbalance of the qualitative and quantitative nutritional intake is directly related to inflammation and immunity of the body, leading to time-dependent functional degradation and indirectly leading to the occurrence and development of cancer (8). However, few studies have combined the two organically to guide the treatment and prognosis of CRC.
Chronic inflammatory conditions associated with carcinogenesis are characterized by various clinicopathological markers. Many previous studies have investigated the role of preoperative systemic inflammatory markers in CRC prognosis, including the Glasgow prognostic score (GPS), neutrophil-to-lymphocyte ratio (NLR), C-reactive protein to albumin (CAR), lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR), and systemic organ score (SIS) (9–13). However, patients with gastrointestinal tumors tended to show a different degree of nutritional deficiency, and some patients began to see a doctor because of unknown anemia or even cachexia. In general, right-sided colon cancer (RC) has a high prevalence of microcytic anemia due to luminal blood loss (14). Unlike other nutritional indicators, such as albumin and lymphocytes, MCV is more stable with fewer affected factors (15). MCV, a parameter measuring the variation in red blood cell volume and distinguishing the type of anemia, has been used as a host nutrition index to predict the long-term outcomes in patients with different cancers, including esophageal squamous cell carcinoma, non-small-cell lung cancer, gastroesophageal adenocarcinoma, liver cancer, and CRC (16–21). A retrospective analysis from Japan suggested that preoperative anemia, especially microcytic anemia (MCV<80fl), may serve as an easily available predictor of outcome in CRC.
CRP is an acute phase protein (APR) produced by the liver that has long been employed for clinical purposes, and it can influence multiple phases of inflammation by acting proinflammatory and anti-inflammatory roles (22). Its rapidly rising levels have been linked to a variety of diseases, including the prognosis of various tumors (23, 24). Numerous experimental studies have suggested that CRP upregulation is associated with the development of CRC (25, 26). However, few studies have comprehensively evaluated the prognostic value of CRP/MCV in CRC (27). By combining these two accessible factors, CRP and MCV, we propose an applicable inflammation-joined and nutrition-related clinicopathologic marker for overall survival (OS) prediction in colorectal cancer.
Overall, 2471 consecutive CRC patients who underwent surgery at the Department of Gastrointestinal Surgery of Guangxi Clinical Research Center for Colorectal Cancer between 2004 and 2019 were enrolled. The inclusion criteria were as follows: (a) patients with histologically confirmed CRC; (b) patients who underwent primary tumor resection; and (c) patients who had no treatment prior to the blood test. The exclusion criteria were as follows: (a) familial adenomatous polyposis or hereditary colon cancer; (b) there were no signs of clinical infection such as fever on the day of blood collection (28), and (c) patients with other neoplastic diseases during the same period. Baseline clinicopathologic parameters, including general basic information; past, personal, and family history; preoperative and postoperative blood routine examination; serological markers and inflammation-related indicators; enhanced computed tomography (CT) and MRI; degree of histological differentiation and pathological TNM staging; KRAS and BRAFV600E mutation status and microsatellite instability (MSI) testing; and preoperative and postoperative adjuvant chemoradiotherapy, were derived from the medical records. We used the Cox regression model to identify mean corpuscular volume (MCV) as a promising nutritional predictor of prognosis, while C-reactive protein (CRP) was used as an inflammatory index. To explore the value of the CRP/MCV ratio in specific subgroups, we performed a subgroup analysis based on different clinicopathologic parameters.
The CRP/MCV was obtained by dividing the absolute number of CRP by the absolute number of erythrocyte MCV. MSI testing is characterized by defects in mismatch repair genes (MLH1, MSH2, MSH6, and PMS2). Left-sided colon cancer (LC) was defined as a tumor diagnosed from the splenic flexure of the colon, descending colon, sigmoid colon, and rectum, while right-sided colon cancer (RC) included the ileocecum, ascending colon, hepatic flexure of the colon, and transverse colon, which were consistent with other promulgated articles (29, 30). Routine blood tests were performed using a Mindray BC-6900, and CRP levels were determined using an automatic immunoturbidimetric assay. Tumor markers, including carcinoembryonic antigen (CEA), were measured using an ARCHITECTi2000SR automatic electrochemical luminescence instrument and supporting reagents (Abbott Laboratories, Chicago). TNM staging was evaluated based on the 8th edition of the Union for International Cancer Control classification. The normal ranges CRP were 0–5.0 mg/L for indicates and 80.0–100.0 fl for MCV. The cutoff CEA level was 5 ng/mL.
All statistical analyses were performed using R software (R version 3.6.2; www.r-project.org). Categorical variables were analyzed using the chi-square test or Fisher’s exact test, and continuous variables were analyzed using Student’s t-test. The cutoff of CRP/MCV was 0.06 × 10–15 mg/L2 based on the exhaustive method (EXM) to optimize selection. Survival curves were constructed using the Kaplan–Meier method and compared using the log-rank test. Both univariate and multivariate Cox regression models were performed using the Kaplan-Meier survival (Version: 3.1-8) package to evaluate hazard ratios (HRs) and confidence intervals (CIs) for survival based on CRP/MCV and other selected clinicopathological factors. Statistical significance was set at P < 0.05.
A total of 2471 patients consisting of 1500 (60.7%) men and 971 (39.3%) women were enrolled in this study, and approximately half had rectal cancer. The optimum cutoff value was obtained by considering the correlation of the CRP/MCV ratio with OS in patients. A total of 1468 (59.4%) patients had low risk (CRP/MCV ≤ 0.06) and 1003 (40.6%) patients with high risk (CRP/MCV > 0.06). The male to female ratio was 1.37:1 in the low-risk group compared to 1.86:1 in the high-risk group (P < 0.001; Table 1). Moreover, the mean age in the low-risk cohort was 57.20 ± 12.67 years, which was lower than that in the high-risk group (59.02 ± 13.16 years; P = 0.001) (Table 2). The low-risk category tended to have more rectal cancer, and most of them were located on the left side, with 67.6% of cases diagnosed as moderately differentiated. Poorly differentiated, advanced T stage, high probability of metastasis, and microsatellite instability appeared more frequently in the high-risk group. The relationship between the CRP/MCV ratio and baseline clinicopathological characteristics is shown in Figure 1. Except for the N stage and KRAS status, the increased CRP-MCV was associated with males; older age (older than 60 years); presenting advanced T stage, M stage, and later TNM stage; accompanied by microsatellite instability; and right-sided and poorly differentiated colon cancer.
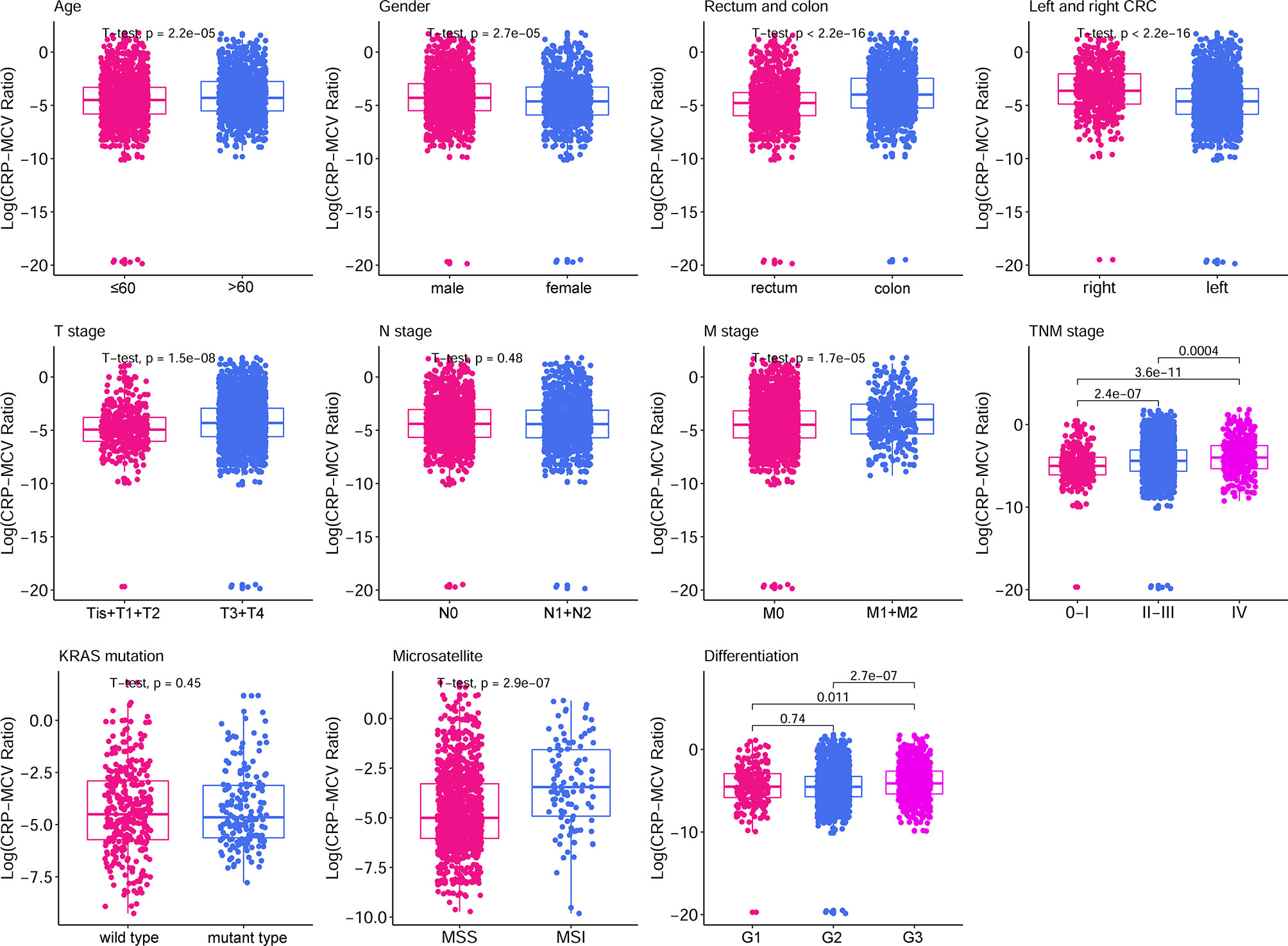
Figure 1 Box plot of clinicopathological features based on CRP/MCV classification. The boxplots show the 5% and 95% confidence intervals. The box plot lower extreme is the first quartile, and the box plot upper extreme is the third quartile. Box plots show the median and whiskers are the minimum and maximum, respectively. Elevated median levels indicated a higher CRP/MCV ratio. The statistical method used for each group was the Student’s t-test. CRP, C-reactive protein; MCV, mean corpuscular volume.
According to univariate analysis (Figure 2), significant differences in cumulative survival were observed for the CRP/MCV ratio together with the TNM stage, differentiation, right side, age, CEA, and CRP levels. Adjuvant chemotherapy was considered to be a protective factor. Sex, MCV level, neoadjuvant chemotherapy, KRAS, and microsatellite status did not show significant differences.
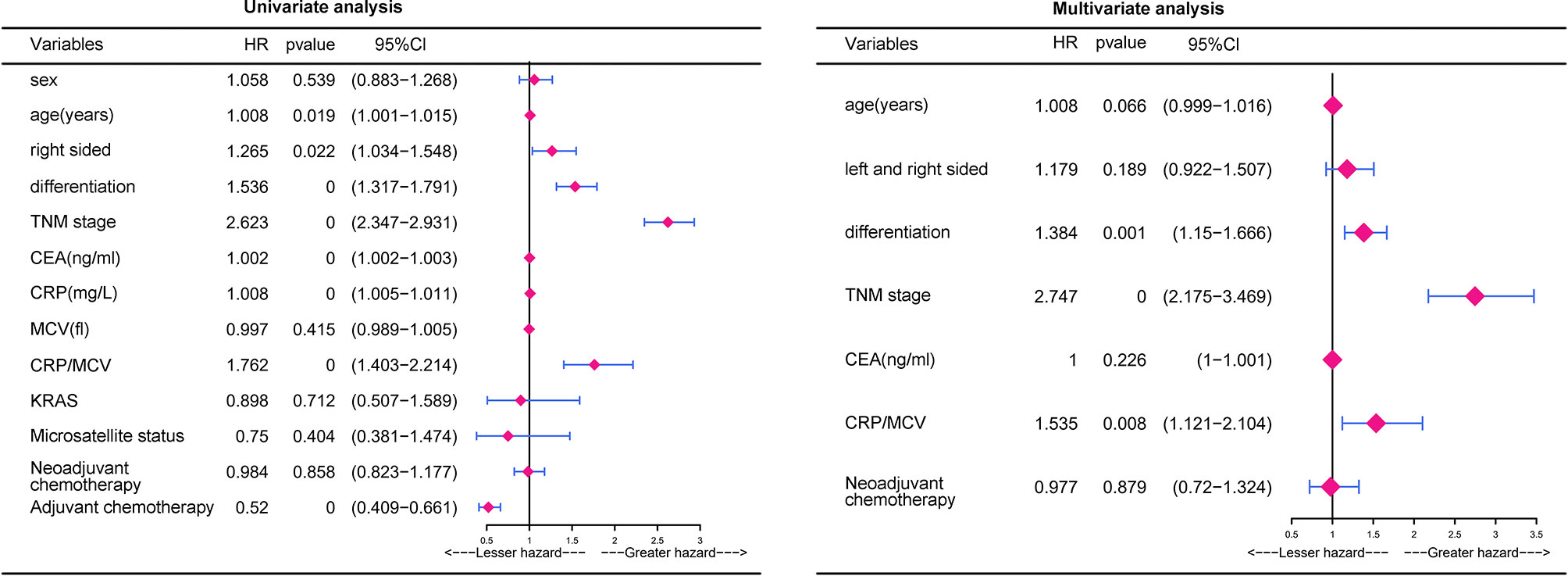
Figure 2 Univariate analysis and multivariate analysis of risk factors for the prognosis in CRC. P value was based on Student’s t-test for continuous factors and Chi-square test (case number ≥ 5) or Fisher’s exact test (case number < 5) for categorical factors.
Multivariate analysis demonstrated that elevated CRP/MCV ratio [odds ratio (OR): 1.535, 95% CI: 1.121–2.104, P = 0.008], TNM stage (OR: 2.747, 95% CI: 2.175–3.469, P < 0.001), and differentiation (OR, 1.384; 95% CI, 1.150–1.666; P = 0.001) were significant predictors of overall survival in patients with CRC (Figure 2). Kaplan–Meier curves showed significantly worse survival for patients with high risk than low risk according to the CRP/MCV ratio (Figure 3).
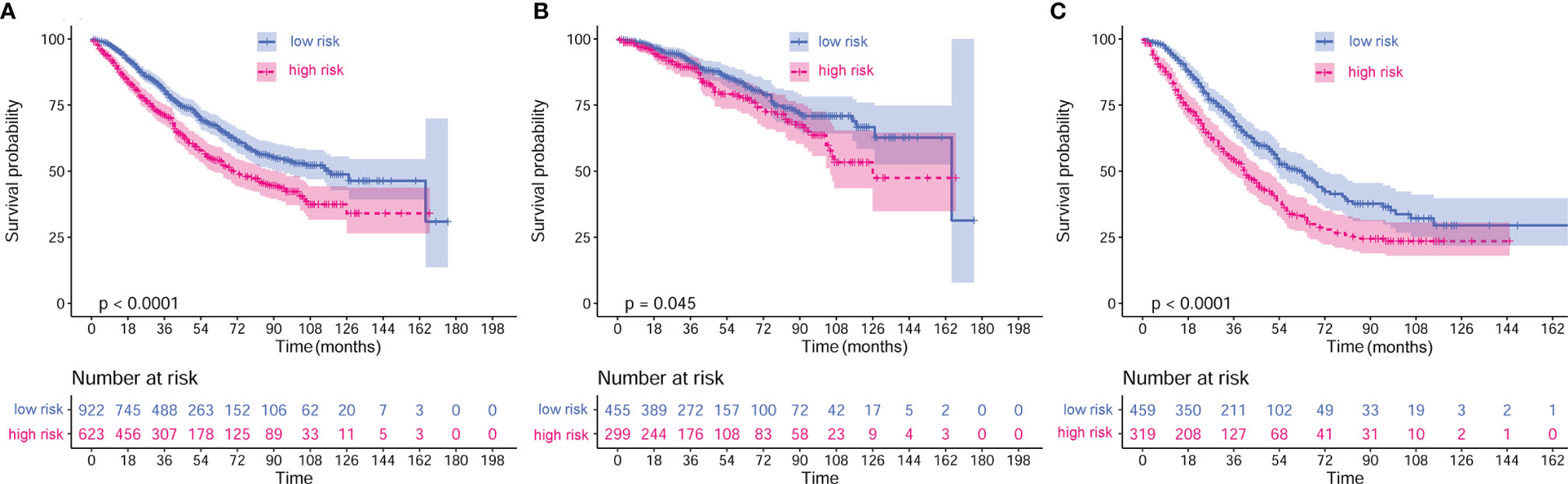
Figure 3 Kaplan–Meier curves of different stages based on CRP/MCV risk stratification. (A) Kaplan–Meier curves of all stages based on CRP/MCV risk stratification. (B) Kaplan–Meier curves of stage I and II based on CRP/MCV risk stratification. (C) Kaplan–Meier curves of stage III and IV based on CRP/MCV risk stratification. A CRP/MCV value above 0.06 indicates high risk and vice versa. The abscissa represents time in months. CRC, colorectal cancer; CRP, C-reactive protein; MCV, mean corpuscular volume.
The adverse association of CRP/MCV with overall survival seems to be stronger among women, poor differentiation, early T stage, advanced N stage, advanced TNM stage, and right-side tumor patients (Figure 4). As shown in Figure 5, the Kaplan–Meier curves in the subgroup analysis expounded the impact of CRP/MCV combined with other clinical features on prognosis. In the sex group, both women and men with CRC in the high CRP/MCV group had a poor prognosis. In the age group, patients with CRC aged > 60 years had a higher CRP/MCV value and worse prognosis. As for the location group, both in the RC and LC cancers had higher CRP/MCV values, which were associated with a worse prognosis. Furthermore, CRC patients with a higher CRP/MCV value diagnosed as microsatellite stable had a worse prognosis than those diagnosed with MSI, which was also found in some favorable randomized control trials (31). The log-rank test suggested that confounding factors affected the prognosis of the KRAS group based on the CRP/MCV ratio (P = 0.34, Figure 5).
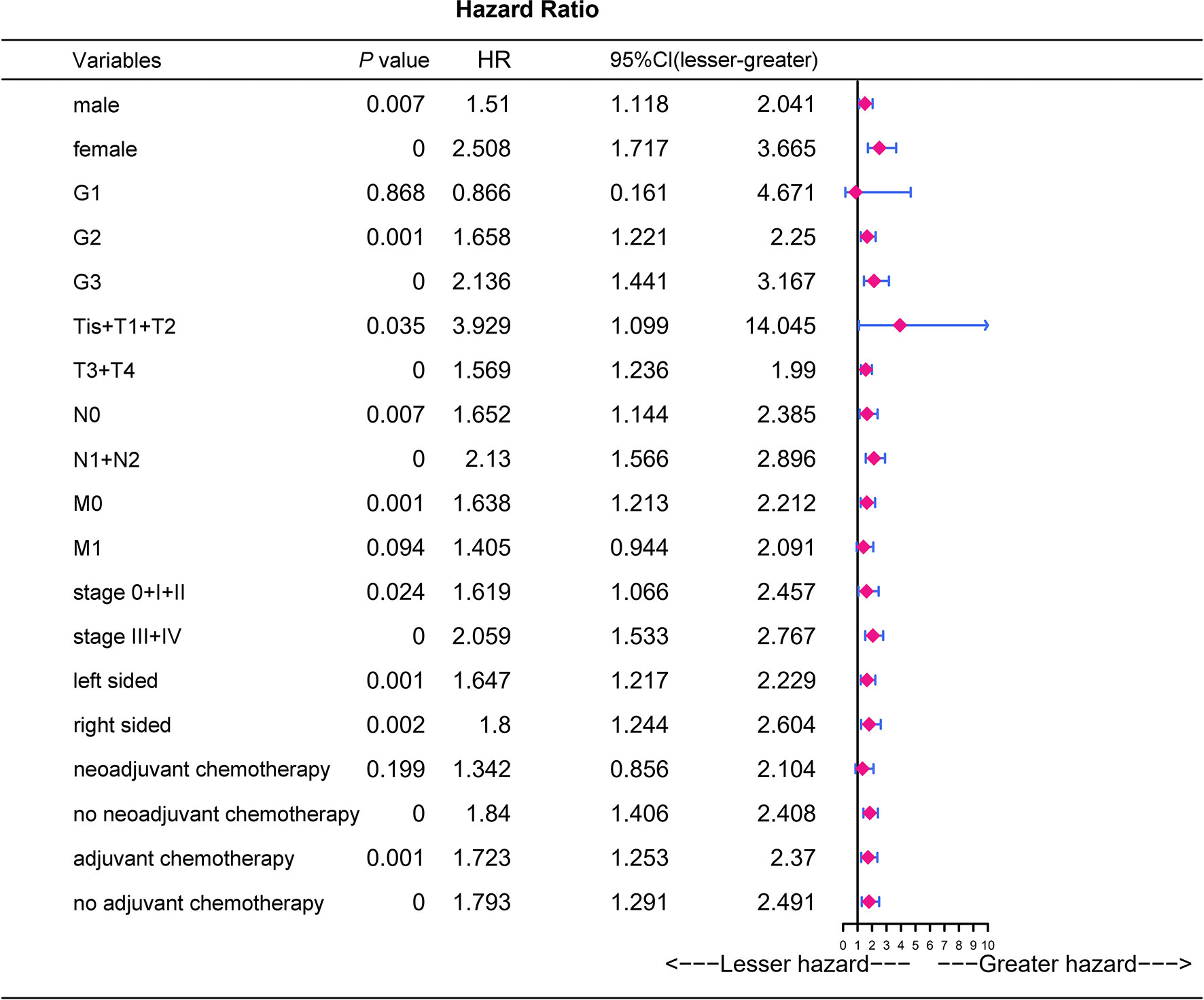
Figure 4 Prognostic analysis of CRP/MCV values in different subgroups. Univariate logistic regression analysis was performed in different subgroups to determine the relationship between CRP/MCV values and prognosis. P value was based on Student’s t-test for continuous factors, and on chi-square test (case number ≥ 5) or Fisher’s exact test (case number < 5) for categorical factors. CRC, colorectal cancer; CRP, C-reactive protein; MCV, mean corpuscular volume.
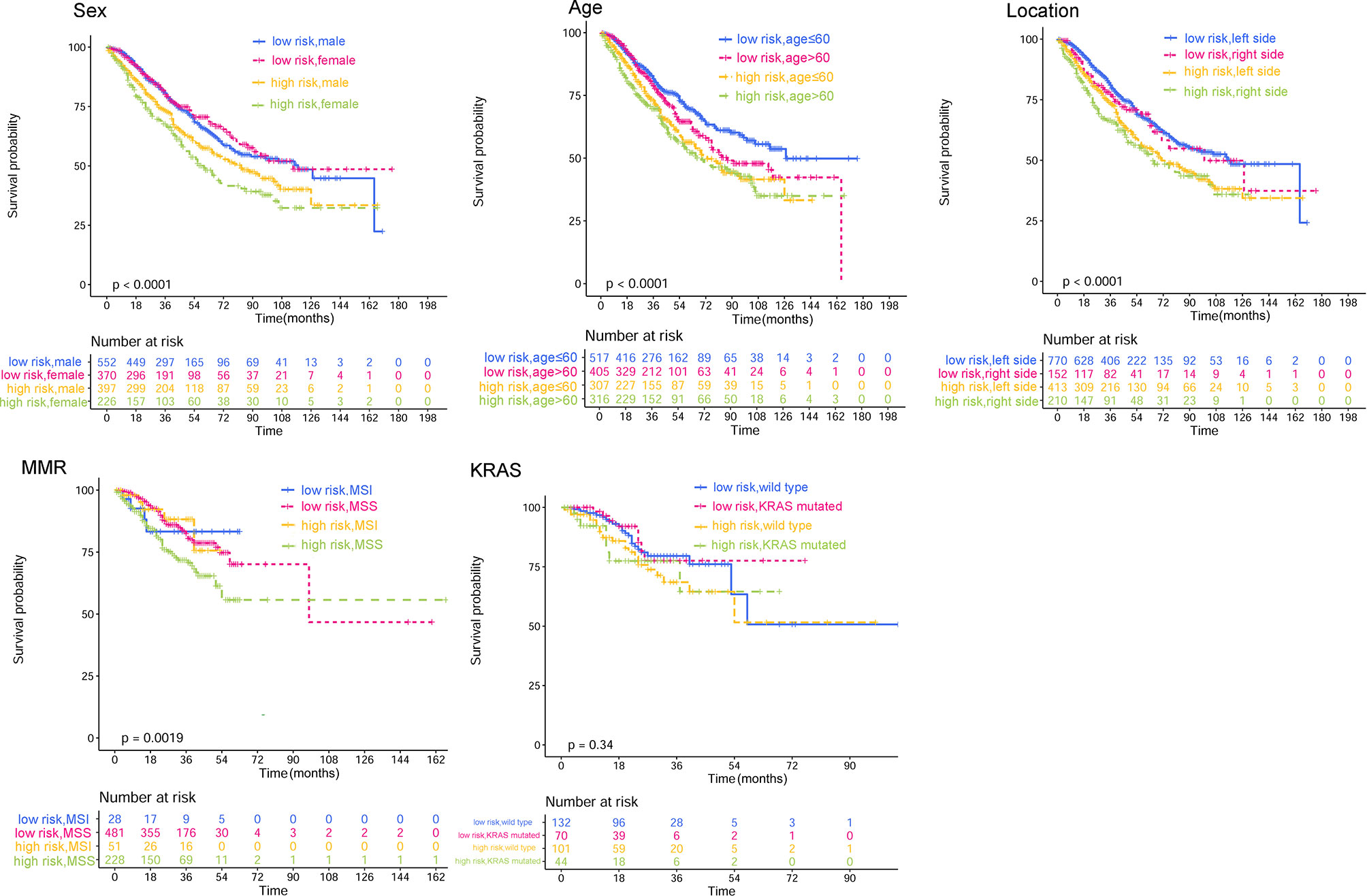
Figure 5 Kaplan–Meier curves of subgroups based on CRP/MCV stratification. The subgroup analysis types are displayed in the upper left corner of the graph, followed by sex, age location, microsatellite status, and KRAS gene type. A CRP/MCV value above 0.06 indicates high risk and vice versa. The abscissa represents time in months. CRP, C-reactive protein; MCV, mean corpuscular volume.
To identify the subgroups, the relationship between CRP/MCV value and overall survival is stronger, we performed further studies based on the above results. Patients with lymph node-positive CRC were found to have comparable results. Patients (n = 1109) were assigned to a training set (n = 776) and validation set (n = 333). The prognostic nomogram was constructed using a training set and internal verification using the receiver operating characteristic (ROC) curve and the area under the curve (AUC). The goodness of fit between the observed event rates and predicted values was assessed using calibration curves, and Kaplan–Meier curves were used for risk stratification. In multivariate analysis, lymph node-positive CRC patients also demonstrated that elevation of the TNM stage (OR, 3.157; 95% CI, 2.445–4.077; P < 0.001), CRP/MCV ratio (OR: 1.512, 95% CI: 1.091–2.094, P = 0.013), and differentiation (OR: 1.452, 95% CI: 1.184–1.779, P < 0.001) were significant risk factors (Figure 6). Adjuvant chemotherapy was considered to be a protective factor. The three independent features listed above were used to construct a prognostic nomogram (Figure 7). The predictive nomogram showed that the CRP/MCV ratio was one of the major factors in addition to TNM stage and differentiation. The calibration plot demonstrated a favorable agreement between the predicted and observed values in the primary and validation datasets (Figure 8). The ROC curve analysis showed that our nomogram had superior AUC values (0.694) than the TNM staging system alone (0.642) (Figure 9), and the model had better and stable predictive values at all times (Figure 9). Furthermore, CRP/MCV values significantly distinguished lymph node-positive patient outcomes in both the validation and training sets (Figure 10).
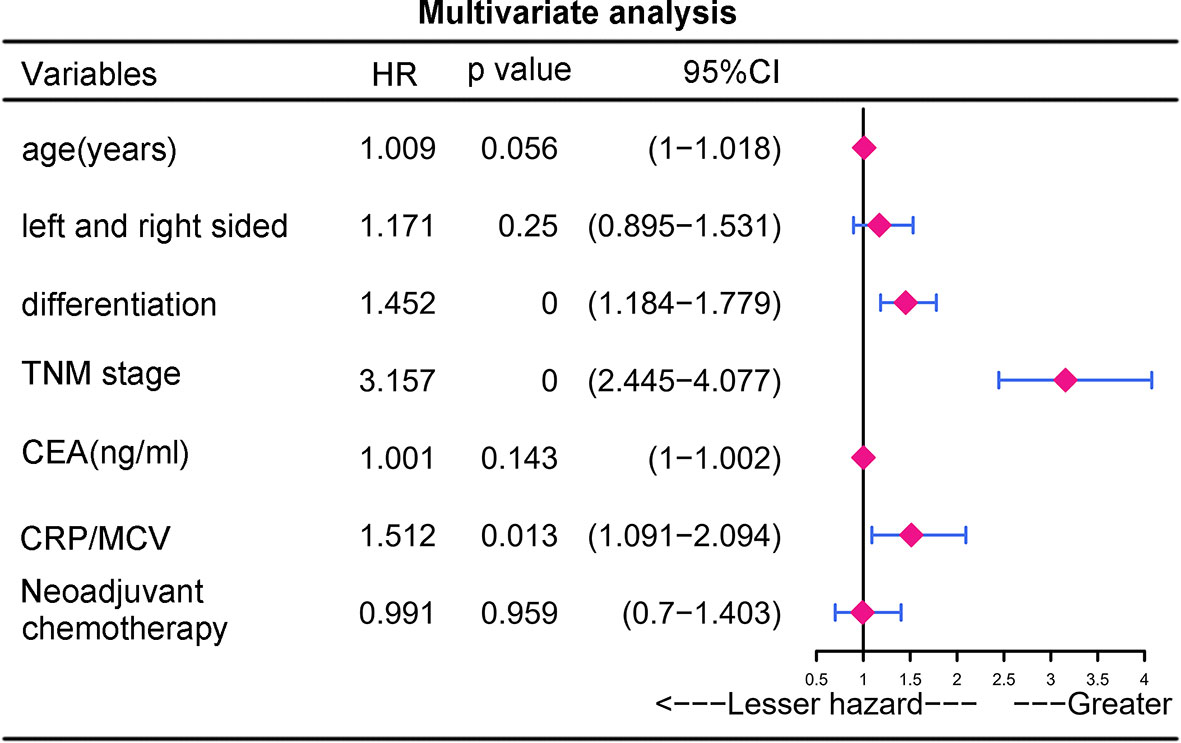
Figure 6 Multivariate analysis of prognostic risk factors for lymph node positive CRC. Multivariate analysis is a multivariate regression analysis of the variables found by univariate analysis.
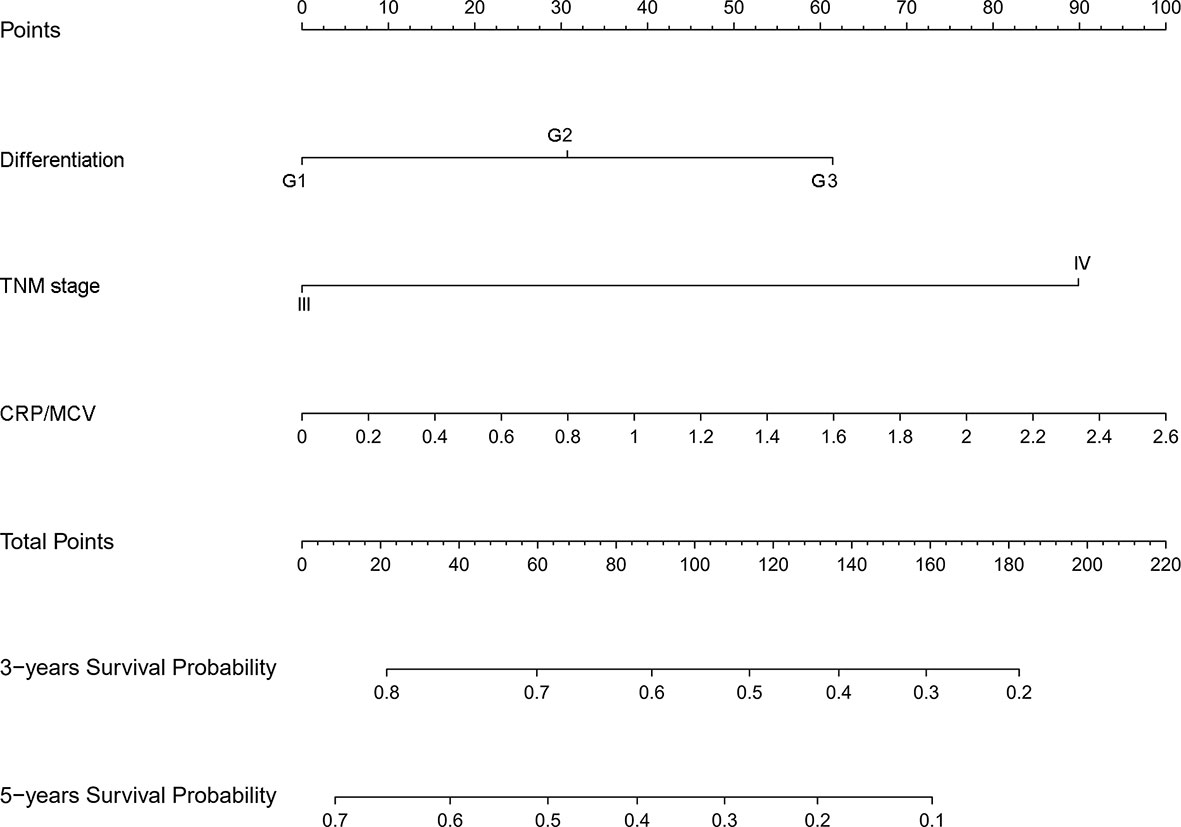
Figure 7 Nomogram for predicting prognosis in lymph node positive CRC. The nomogram was developed in the primary cohort, with the TNM stage, CRP/MCV ratio, and differentiation incorporated.
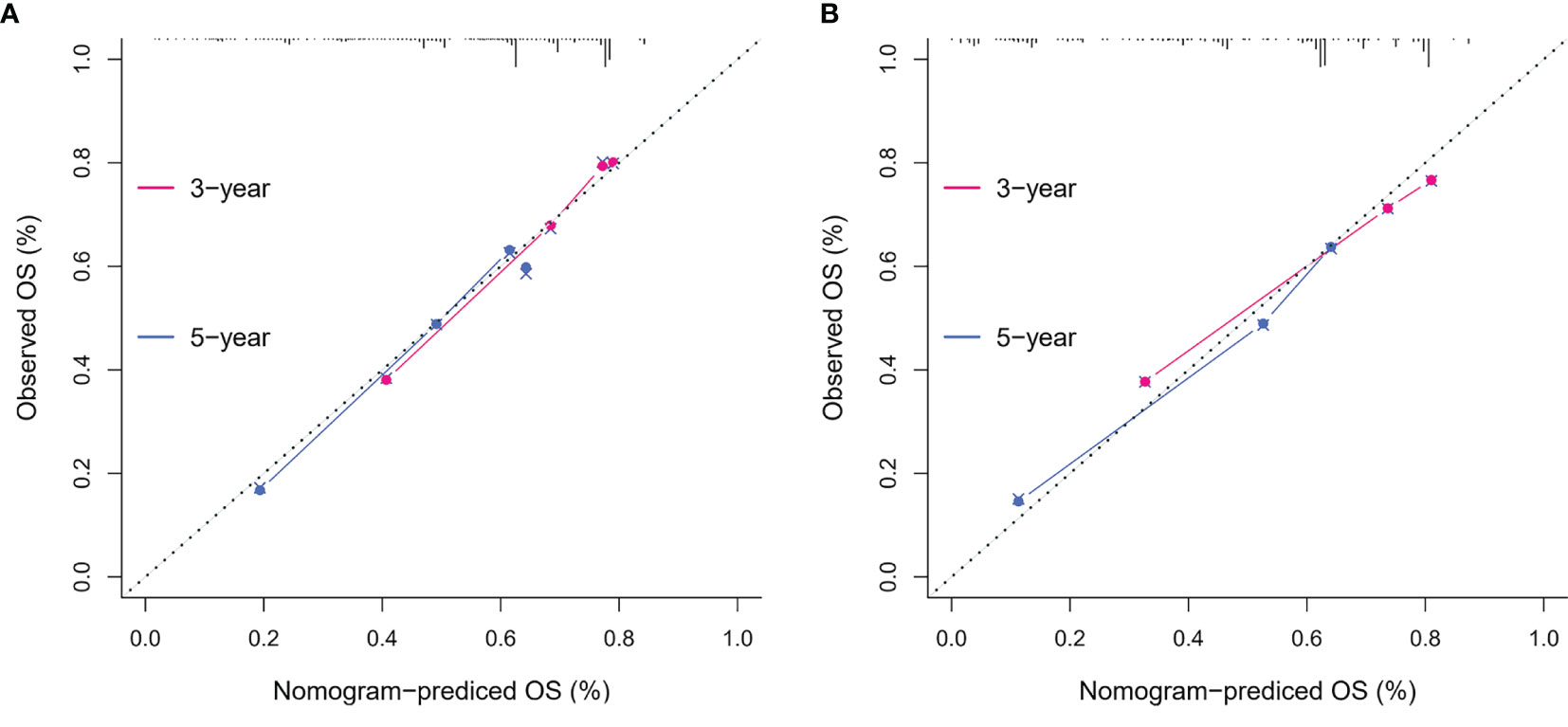
Figure 8 The calibration curves on 3-year and 5-year OS of the nomogram. The x-axis represents the nomogram-predicted probability of overall survival, and the y-axis represents the observed overall survival. The reference line is 45°, which indicates perfect calibration. (A) Calibration curves of training set. (B) Calibration curves of the validation set.
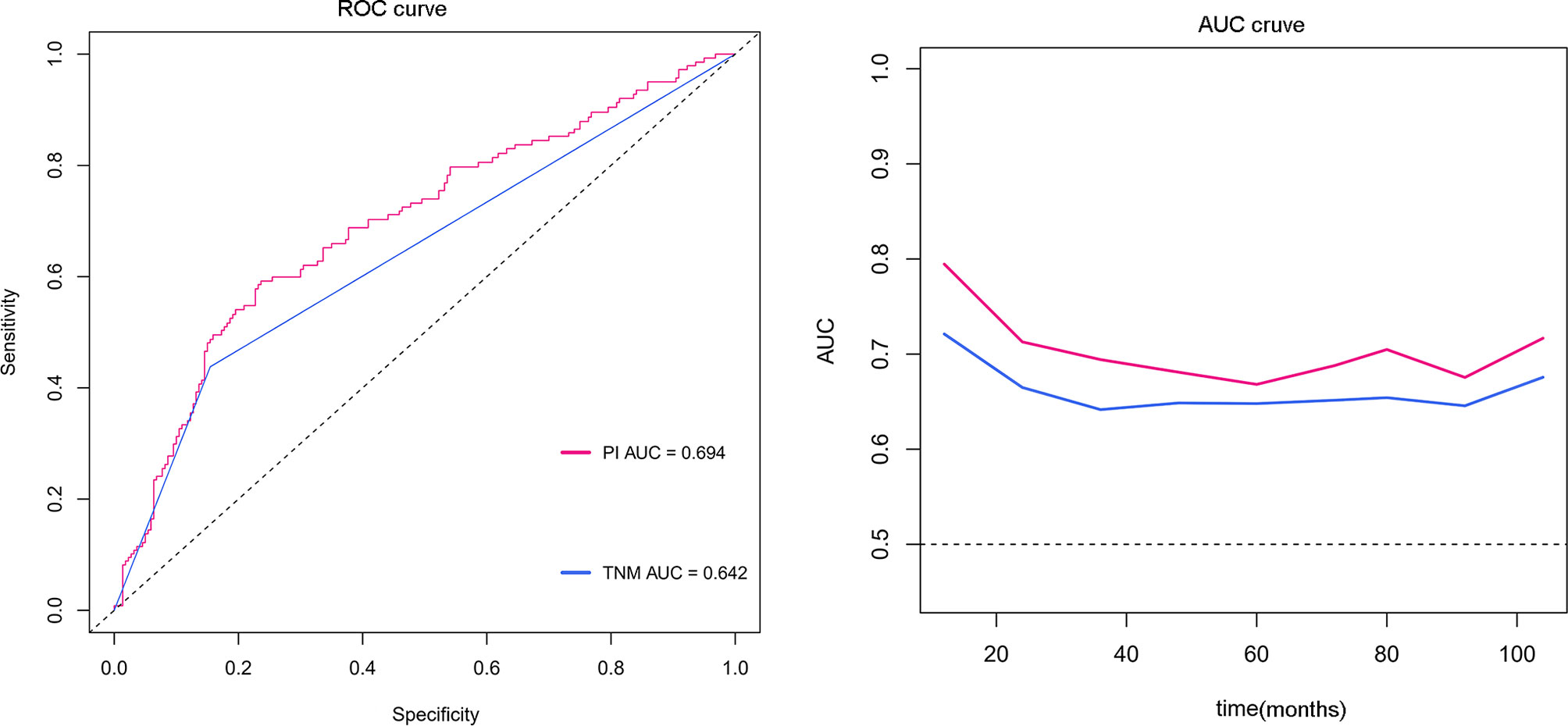
Figure 9 ROC and time-dependent AUC curves for the nomogram. The red line represents the prognostic nomogram, and the blue line represents the TNM staging system alone.
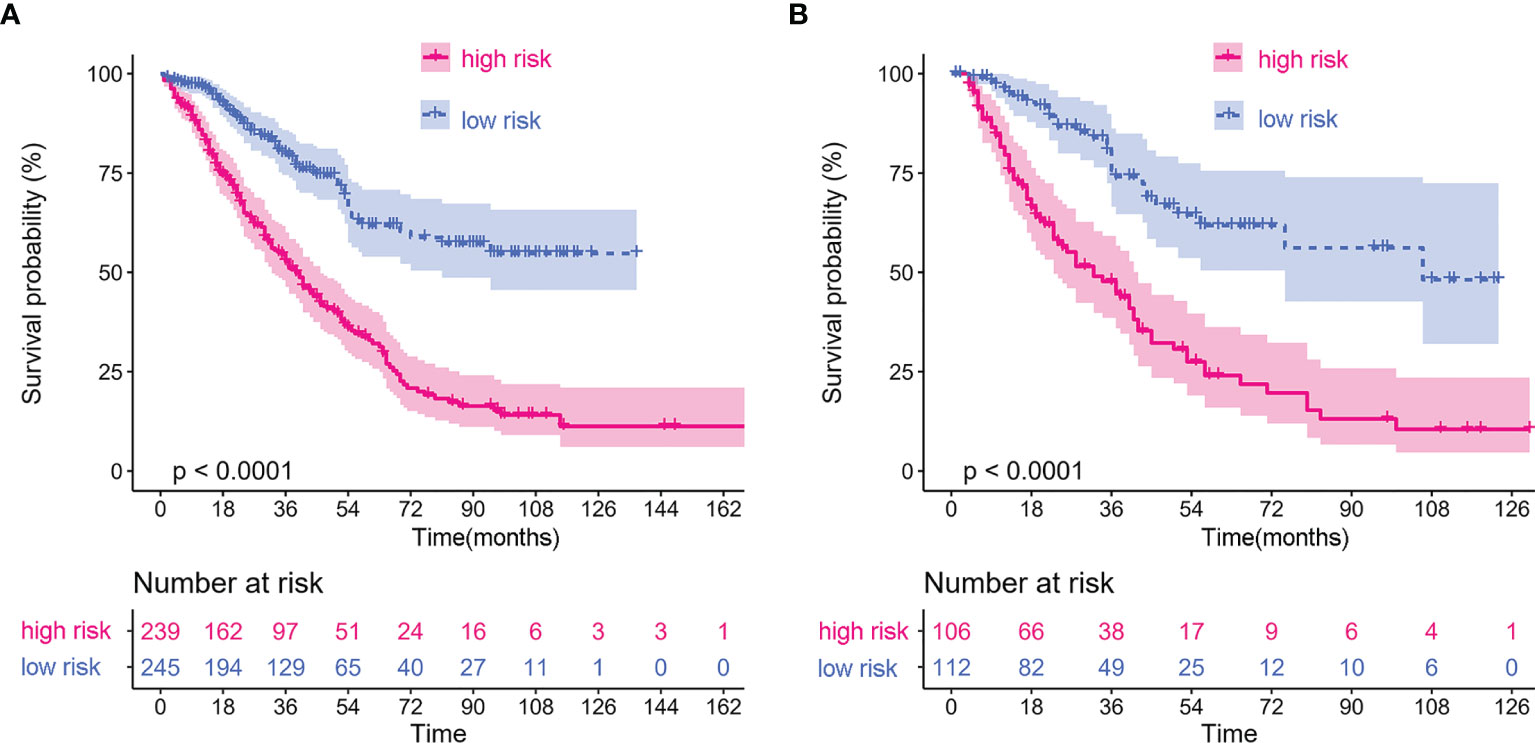
Figure 10 Kaplan–Meier curves of lymph node positive patients based on CRP/MCV stratification. (A) The Kaplan–Meier curve of lymph node positive patients in the training dataset. (B) The Kaplan–Meier curve of lymph node positive patients in the validation dataset. The abscissa represents time in months.
This is a retrospective study of clinical big data on laboratory markers for guiding clinical practice in a single center. Among various laboratory indicators, we screened CRP as an inflammation indicator and MCV as a nutritional indicator to form a pioneering prognostic indicator. If the tumor is regarded as “a battle,” CRP can reflect the intensity of the battlefield and the lethality of weapons, while MCV is a fortress against damage. The combination of the two can vividly describe the outcome of a battle.
Since many previous studies have investigated the relationship between inflammation, nutrition, and tumors (8, 32, 33), it is urgent to develop an exact practical clinical index to calculate their prognostic value. CRP plays a role in tumor inflammation, while MCV indicates the level of host nutrition among miscellaneous preoperative indicators. Elevated CRP levels are thought to reflect host reactions to the biological behavior of a tumor (34). The mechanism of CRP upregulation is controlled by proinflammatory cytokines from tumor cells or the immune system, which leads to repeated stimulation and chronic inflammation, forming a carcinogenic microenvironment that favors the development of cancer (35). MCV could serve as an anemia marker that is closely linked with host nutrition, as anemia is caused by tumor bleeding, poor nutrition, and chronic inflammation due to cancer progression (27). A previous report suggested that an insufficient blood supply caused by chronic blood loss and malnutrition induced hypoxia in the tumor microenvironment, leading not only to HIF-1α upregulation but also to T-cell apoptosis, which decreases total lymphocyte levels and contributes to tumor revascularization and proliferation (36). In addition, Nagai et al. showed that in patients with an elevated MCV level, the benefits of 5-FU-based chemotherapy could be predicted by blocking thymidylate synthase (TS), which is involved in DNA synthesis (18). Based on the above facts, we hypothesized that CRP divided by MCV was used as a germane indicator to assess the therapeutic effect and predict long-term outcomes in CRC patients.
In the present study, the CRP/MCV ratio, TNM stage, and differentiation were identified as three independent prognostic indicators in the multivariate analysis. Although the pathological TNM stage was the most important prognostic indicator in patients with CRC, it was not as preoperatively available and dynamically changed to CRP/MCV or CEA. Instead, CRP/MCV can be considered a clinically friendly indicator. Conventional tumor markers such as CEA are well known to be significant indicators of disease burden, posttreatment surveillance, and prognostic value, as they are thought to be secreted from the tumor itself (37, 38). Interestingly, CRP as a prognostic indicator in univariate analysis no longer made sense in the multivariate analysis. When combined with MCV, multivariate analysis revealed that the CRP/MCV ratio was superior to CEA in this respect. Further work is required to evaluate whether a combination of CRP divided by MCV is more valuable for early diagnosis and recurrence compared with CEA.
According to the CRP/MCV classification, the high-risk population was closely associated with men over 60 years of age who presented with advanced T, M, and TNM stages, accompanied by MSI, right-sided colon cancer, and poor differentiation. In other words, preoperative CRP/MCV could predict aggressive tumor biology (39), which is conducive to risk stratification and guiding clinical work. Indeed, estimation of changes in CRP reflects the presence and intensity of an inflammatory process and differentiates inflammatory from non-inflammatory conditions, which are useful in managing the patient’s disease and predicting the prognostic value in certain diseases (40). It has been reported that anemia in CRC frequently shows a microcytic phenotype (39, 41), especially in high-grade T stage, proximal colon tumor location, lymph node metastasis, and elevated serum CRP with or without hypoalbuminemia (14, 27). Based on the above factors, we can speculate that a high level of CRP indicates that the inflammatory response of the tumor is obvious, while low levels of MCV appear in the manner of chronic anemia and poor nutritional status of the host, which leads to increased CRP/MCV values and poor prognosis in patients with CRC. The Kaplan–Meier curves of subgroup analysis showed that the CRP/MCV ratio could also separate the stand or fall of prognosis according to sex, age, location, and microsatellite status, which means that our indicator can easily distinguish a poor prognosis group from a good prognosis group.
Finally, CRP/MCV was also a significant prognostic indicator in patients with positive lymph nodes in the subgroup analysis. Multivariate analysis showed that CRP/MCV was one of the main prognostic factors in addition to the TNM staging system. The nomogram showed that CRP/MCV had more prognostic and clinical significance in lymph node-positive patients with poorly differentiated tumors at the late stage. Within the range of 30–80% of the 3-year survival probability, the corresponding line intervals decreased. For example, when the CRP/MCV value increased by the same amount, patients at stage IV had a lower 3-year survival rate than those with the same differentiation at stage III. Thus, in more advanced patients, it is more difficult to survive in the early 3 years if the inflammatory response is obvious and the nutritional status is poor, which guides clinical interventions. Improving nutrition and reducing inflammation may help advanced cancer patients reach early stages.
This study has several limitations. First, it had a retrospective design and was conducted in a single institution, which could have resulted in selection bias. Second, several diseases, such as iron deficiency anemia, alpha or beta-thalassemia minor, and liver disease, which can affect the value of MCV, were not screened; this may have led to a selection bias. Third, the use of a single parameter to assess nutritional status has been questioned since many other nutritional factors affect outcomes (40). Lastly, this study was conducted over a long period between 2004 and 2019, which can be associated with historical biases in treatment strategy and perioperative management.
In conclusion, a new applicable inflammation-joined and nutrition-related clinicopathologic measurement was constructed to predict the prognosis of patients with CRC. Elevated CRP/MCV can predict the biological behavior of CRC. Severe inflammation and malnutrition suggest poor early prognosis and guide early clinical intervention.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
GW, JL, HL, and WT contributed to study designs. GW, XM, and HZ contributed to material support and data acquisition. XH, JL, and HL performed the statistical analysis, and GW drafted the manuscript. LJ, LY, and YZ have revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the 2019 Guangxi University High-level Innovation Team, the Project of Outstanding Scholars Program, Guangxi Science and Technology Project (2019AC03004), Guangxi Clinical Research Center for Colorectal Cancer (Guike: AD19245197), Research Basic Ability Improvement Project for Guangxi Young College Teachers (2021KY0087), Innovation Project of Guangxi Graduate Education (YCSW2021133), and Future Academic Star of Guangxi Medical University (WLXSZX21125).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Wei-shun Xie, Yong-sheng Meng, Jian-hong Chen, Chao Tian, and Ling-xu Huang for their efforts in establishing the database. We would like to thank Editage (www.editage.cn) for English language editing.
1. Global Cancer Observatory (2020). Available at: http://gco.iarc.fr/ (Accessed May, 2021).
2. Almasaudi AS, McSorley ST, Dolan RD, Edwards CA, McMillan DC. The Relation Between Malnutrition Universal Screening Tool (MUST), Computed Tomography–Derived Body Composition, Systemic Inflammation, and Clinical Outcomes in Patients Undergoing Surgery for Colorectal Cancer. Am J Clin Nutr (2019) 110(6):1327–34. doi: 10.1093/ajcn/nqz230
3. Tsuchihashi K, Ito M, Moriwaki T, Fukuoka S, Taniguchi H, Takashima A, et al. Role of Predictive Value of the Modified Glasgow Prognostic Score for Later-Line Chemotherapy in Patients With Metastatic Colorectal Cancer. Clin Colorectal Cancer (2018) 17(4):e687–e97. doi: 10.1016/j.clcc.2018.07.004
4. Mainous MR, Deitch EA. Nutrition and Infection. Surg Clinics North America (1994) 74(3):659–76. doi: 10.1016/s0039-6109(16)46335-8
5. Malfertheiner P, Chan FKL, McColl KEL. Peptic Ulcer Disease. Lancet (2009) 374(9699):1449–61. doi: 10.1016/s0140-6736(09)60938-7
6. Fock KM, Graham DY, Malfertheiner P. Helicobacter Pylori Research: Historical Insights and Future Directions. Nat Rev Gastroenterol Hepatol (2013) 10(8):495–500. doi: 10.1038/nrgastro.2013.96
7. Saus E, Iraola-Guzman S, Willis JR, Brunet-Vega A, Gabaldon T. Microbiome and Colorectal Cancer: Roles in Carcinogenesis and Clinical Potential. Mol Aspects Med (2019) 69:93–106. doi: 10.1016/j.mam.2019.05.001
8. Zitvogel L, Pietrocola F, Kroemer G. Nutrition, Inflammation and Cancer. Nat Immunol (2017) 18(8):843–50. doi: 10.1038/ni.3754
9. Ishizuka M, Nagata H, Takagi K, Horie T, Kubota K. Inflammation-Based Prognostic Score is a Novel Predictor of Postoperative Outcome in Patients With Colorectal Cancer. Ann Surg (2007) 246(6):1047–51. doi: 10.1097/SLA.0b013e3181454171
10. Chan JC, Chan DL, Diakos CI, Engel A, Pavlakis N, Gill A, et al. The Lymphocyte-to-Monocyte Ratio is a Superior Predictor of Overall Survival in Comparison to Established Biomarkers of Resectable Colorectal Cancer. Ann Surg (2017) 265(3):539–46. doi: 10.1097/SLA.0000000000001743
11. Mallappa S, Sinha A, Gupta S, Chadwick SJ. Preoperative Neutrophil to Lymphocyte Ratio >5 is a Prognostic Factor for Recurrent Colorectal Cancer. Colorectal Dis (2013) 15(3):323–8. doi: 10.1111/codi.12008
12. Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K. Clinical Significance of the C-Reactive Protein to Albumin Ratio for Survival After Surgery for Colorectal Cancer. Ann Surg Oncol (2016) 23(3):900–7. doi: 10.1245/s10434-015-4948-7
13. Suzuki Y, Okabayashi K, Hasegawa H, Tsuruta M, Shigeta K, Kondo T, et al. Comparison of Preoperative Inflammation-Based Prognostic Scores in Patients With Colorectal Cancer. Ann Surg (2018) 267(3):527–31. doi: 10.1097/SLA.0000000000002115
14. Väyrynen JP, Tuomisto A, Väyrynen SA, Klintrup K, Karhu T, Mäkelä J, et al. Preoperative Anemia in Colorectal Cancer: Relationships With Tumor Characteristics, Systemic Inflammation, and Survival. Sci Rep (2018) 8(1):1126. doi: 10.1038/s41598-018-19572-y
15. Hoffmann JJ, Nabbe KC, van den Broek NM. Effect of Age and Gender on Reference Intervals of Red Blood Cell Distribution Width (RDW) and Mean Red Cell Volume (MCV). Clin Chem Lab Med (2015) 53(12):2015–9. doi: 10.1515/cclm-2015-0155
16. Zheng YZ, Dai SQ, Li W, Cao X, Li Y, Zhang LJ, et al. Prognostic Value of Preoperative Mean Corpuscular Volume in Esophageal Squamous Cell Carcinoma. World J Gastroenterol (2013) 19(18):2811–7. doi: 10.3748/wjg.v19.i18.2811
17. Yoon HJ, Kim K, Nam YS, Yun JM, Park M. Mean Corpuscular Volume Levels and All-Cause and Liver Cancer Mortality. Clin Chem Lab Med (2016) 54(7):1247–57. doi: 10.1515/cclm-2015-0786
18. Nagai H, Yuasa N, Takeuchi E, Miyake H, Yoshioka Y, Miyata K. The Mean Corpuscular Volume as a Prognostic Factor for Colorectal Cancer. Surg Today (2018) 48(2):186–94. doi: 10.1007/s00595-017-1575-x
19. Qu X, Zhang T, Ma H, Sui P, Du J. Lower Mean Corpuscular Hemoglobin Concentration is Associated With Unfavorable Prognosis of Resected Lung Cancer. Future Oncol (2014) 10(14):2149–59. doi: 10.2217/fon.14.121
20. Jomrich G, Hollenstein M, John M, Ristl R, Paireder M, Kristo I, et al. High Mean Corpuscular Volume Predicts Poor Outcome for Patients With Gastroesophageal Adenocarcinoma. Ann Surg Oncol (2019) 26(4):976–85. doi: 10.1245/s10434-019-07186-1
21. Yoshida N, Kosumi K, Tokunaga R, Baba Y, Nagai Y, Miyamoto Y, et al. Clinical Importance of Mean Corpuscular Volume as a Prognostic Marker After Esophagectomy for Esophageal Cancer: A Retrospective Study. Ann Surg (2020) 271(3):494–501. doi: 10.1097/SLA.0000000000002971
22. Marnell L, Mold C, Du Clos TW. C-Reactive Protein: Ligands, Receptors and Role in Inflammation. Clin Immunol (2005) 117(2):104–11. doi: 10.1016/j.clim.2005.08.004
23. Allin KH, Nordestgaard BG. Elevated C-Reactive Protein in the Diagnosis, Prognosis, and Cause of Cancer. Crit Rev Clin Lab Sci (2011) 48(4):155–70. doi: 10.3109/10408363.2011.599831
24. Shrotriya S, Walsh D, Bennani-Baiti N, Thomas S, Lorton C. C-Reactive Protein is an Important Biomarker for Prognosis Tumor Recurrence and Treatment Response in Adult Solid Tumors: A Systematic Review. PloS One (2015) 10(12):e0143080. doi: 10.1371/journal.pone.0143080
25. Moshage HJ, Janssen JA, Franssen JH, Hafkenscheid JC, Yap SH. Study of the Molecular Mechanism of Decreased Liver Synthesis of Albumin in Inflammation. J Clin Invest (1987) 79(6):1635–41. doi: 10.1172/JCI113000
26. Miki C, Konishi N, Ojima E. C-Reactive Protein as a Prognostic Variable That Reflects Uncontrolled Up-Regulation of the IL-1-IL-6 Network System in Colorectal Carcinoma. Digestive Dis Sci (2004) 49(6):970–6. doi: 10.1023/B:DDAS.0000034556.48527.6e
27. Tokunaga R, Nakagawa S, Miyamoto Y, Ohuchi M, Izumi D, Kosumi K, et al. The Impact of Preoperative Anaemia and Anaemic Subtype on Patient Outcome in Colorectal Cancer. Colorectal Dis (2019) 21(1):100–9. doi: 10.1111/codi.14425
28. Ding N, Huang J, Li N, Yuan J, Wang S, Xiao Z. Roles of Neutrophil/Lymphocyte Ratio in Prognosis and in Differentiation of Potential Beneficiaries in HER2-Positive Breast Cancer With Trastuzumab Therapy. BMC Cancer (2020) 20(1):235. doi: 10.1186/s12885-020-06750-3
29. Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, et al. Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol (2017) 3(2):194–201. doi: 10.1001/jamaoncol.2016.3797
30. Lee MS, Menter DG SK. Right Versus Left Colon Cancer Biology: Integrating the Consensus Molecular Subtypes. J Natl Compr Canc Netw (2017) 15(3):411–9. doi: 10.6004/jnccn.2017.0038
31. Phipps AI, Alwers E, Harrison T, Banbury B, Brenner H, Campbell PT, et al. Association Between Molecular Subtypes of Colorectal Tumors and Patient Survival, Based on Pooled Analysis of 7 International Studies. Gastroenterology (2020) 158(8):2158–68 e4. doi: 10.1053/j.gastro.2020.02.029
32. Mattox TW. Cancer Cachexia: Cause, Diagnosis, and Treatment. Nutr Clin Pract (2017) 32(5):599–606. doi: 10.1177/0884533617722986
33. Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J Clin Oncol (2016) 34(35):4270–6. doi: 10.1200/JCO.2016.67.4283
34. Ikeda M, Natsugoe S, Ueno S, Baba M, Aikou T. Significant Host- and Tumor-Related Factors for Predicting Prognosis in Patients With Esophageal Carcinoma. Ann Surg (2003) 238(2):197–202. doi: 10.1097/01.sla.0000080822.22415.cb
35. Balkwill F, Mantovani A. Inflammation and Cancer: Back to Virchow? Lancet (2001) 357(9255):539–45. doi: 10.1016/s0140-6736(00)04046-0
36. Kumar V, Gabrilovich DI. Hypoxia-Inducible Factors in Regulation of Immune Responses in Tumour Microenvironment. Immunology (2014) 143(4):512–9. doi: 10.1111/imm.12380
37. Goldstein M, Mitchell EP. Carcinoembryonic Antigen in the Staging and Follow-Up of Patients With Colorectal Cancer. Cancer Invest (2005) 23(4):338–51. doi: 10.1081/cnv-200058878
38. Clinical Practice Guidelines for the Use of Tumor Markers in Breast and Colorectal Cancer. Adopted on may 17, 1996 by the American Society of Clinical Oncology. J Clin Oncol (1996) 14(10):2843–77. doi: 10.1200/JCO.1996.14.10.2843
39. Sadahiro S, Suzuki T, Tokunaga N, Mukai M, Tajima T, Makuuchi H, et al. Anemia in Patients With Colorectal Cancer. J Gastroenterol (1998) 33(4):488–94. doi: 10.1007/s005350050120
40. Schneider C, Bodmer M, Jick SS, Meier CR. Colorectal Cancer and Markers of Anemia. Eur J Cancer Prev (2018) 27(6):530–8. doi: 10.1097/CEJ.0000000000000397
Keywords: colorectal cancer, C-reactive protein, mean corpuscular volume, clinical intervention, risk stratification
Citation: Wu G, Liu J, Liu H, Jin L, Huang X, Mo X, Zhong H, Li Y, Zhang Y and Tang W (2021) An Applicable Inflammation-Joined and Nutrition-Related Prognostic Indicator in Patients With Colorectal Cancer. Front. Oncol. 11:644670. doi: 10.3389/fonc.2021.644670
Received: 21 December 2020; Accepted: 31 May 2021;
Published: 17 June 2021.
Edited by:
Raluca Ioana Stefan-van Staden, National Institute of Research and Development for Electrochemistry and Condensed Matter (INCEMC), RomaniaReviewed by:
Engin Altintas, Mersin University, TurkeyCopyright © 2021 Wu, Liu, Liu, Jin, Huang, Mo, Zhong, Li, Zhang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weizhong Tang, dGFuZ3dlaXpob25nQGd4bXUuZWR1LmNu; Yawei Zhang, emhhbmd5YTY5QGZveG1haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.