- 1Otorhinolaryngology, Head and Neck Surgery, University Hospitals Leuven, Leuven, Belgium
- 2Department of Neurosciences, Experimental Otorhinolaryngology, KU Leuven, Leuven, Belgium
- 3Department of Microbiology, Immunology and transplantation, Allergy and Clinical Immunology Research Unit, KU Leuven, Leuven, Belgium
- 4Department of Oncology, Section Head and Neck Oncology, KU Leuven, Leuven, Belgium
- 5Neurosurgery, University Hospitals Leuven, Leuven, Belgium
- 6Neurosciences, Research Group Experimental Neurosurgery and Neuroanatomy and Leuven Brain Institute, Leuven, Belgium
- 7Radiology, University Hospitals Leuven, Leuven, Belgium
- 8Endocrinology, University Hospitals Leuven, Leuven, Belgium
Background: The endoscopic endonasal transsphenoidal approach (EETA) is an established technique for the resection of a large variety of benign sellar and suprasellar lesions, mostly pituitary adenomas. It has clear advantages over the microscopic approach, like a superior close-up view of the relevant anatomy and the tumor-gland interface, an enlarged working angle, as well as an increased panoramic vision inside the surgical area. We have been performing the EETA for over a decade, and this study will focus on perioperative and postoperative outcomes and complications and their association with the learning curve.
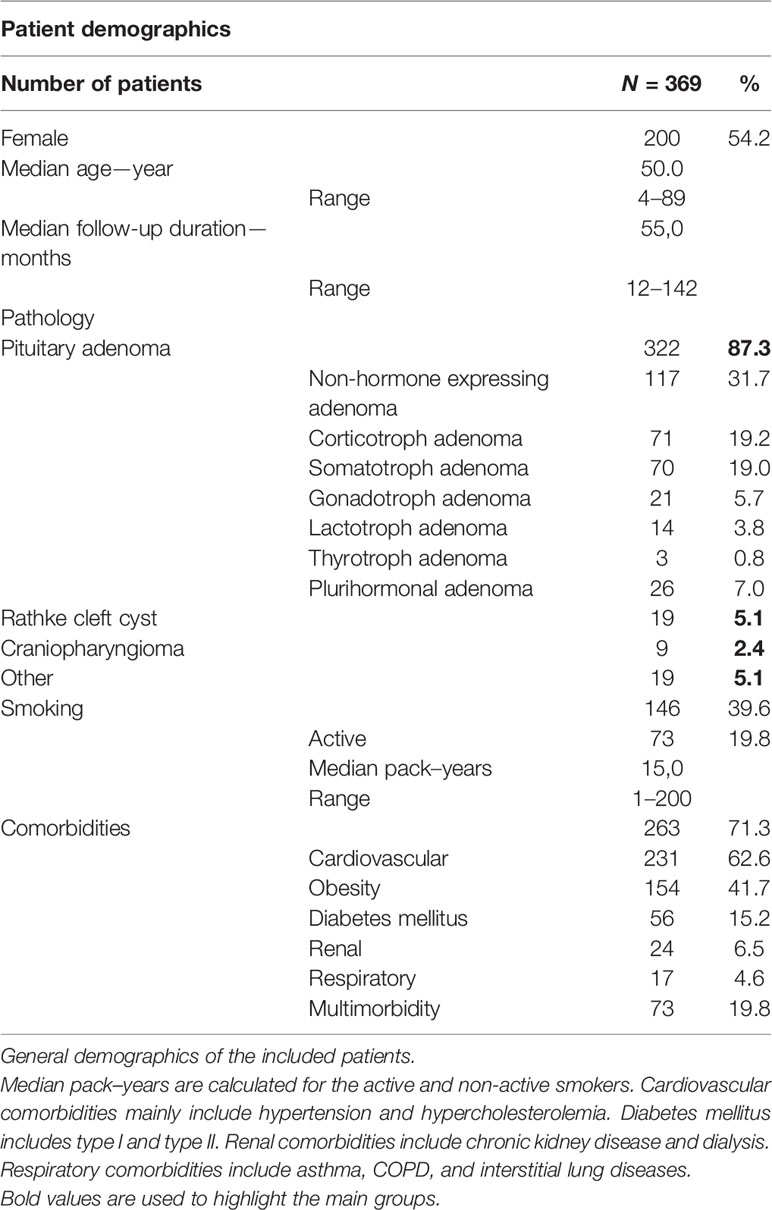
Material and Methods: All patients in our tertiary referral center (n = 369) undergoing an EETA for a lesion of the sellar and suprasellar region between January 1st 2008 and December 31st 2018 were included, and data were retrospectively retrieved from the electronic patient records.
Results: Median follow-up after surgery was 55 months. Pituitary adenomas (n = 322) were the most frequent pathology. Headache (43.4%) and loss of vision (29.3%) were the most common presenting symptoms. Median procedure duration was significantly longer during the initial 5 years (106 versus 79 minutes; p <0.0001), but incidence of peri- and postoperative CSF leaks in the early years was not significantly higher. Knosp grade >2 was associated with perioperative CSF leak (p =0.002), and perioperative CSF leak was associated with postoperative CSF leak (p <0.001). Almost all cases of meningitis were preceded by a postoperative CSF leak. In 22.4% of patients, tumor recurrence required additional therapy. Perioperative (iatrogenic) mortality was 0.8%. The overall hospital stay decreased over time from an average of 7 to 5 days, and the case load increased yearly (p =0.015).
Conclusion: The EETA is an excellent technique with complication rates comparable to or even lower than those in large microsurgical series in the literature. EETA has a significant learning curve affecting the procedure duration. Throughout the first 10 years following the transition from the microscopic approach to the EETA in our cohort, the caseload increased and hospital stay was reduced, while no increase in peri- and postoperative complications was observed.
Introduction
Tumors with the highest incidence located in the sellar and suprasellar region are benign pituitary adenomas (1). They are derived from differentiated hormone-expressing cells located in the anterior part of the pituitary gland and are classified based on their size in microadenomas (<10 mm), macroadenomas (10–40 mm) or giant adenomas (>40 mm) or on their hormone-producing capacity (functional versus non-functional adenomas).
Functional adenomas (e.g. corticotropinomas, somatotropinomas, thyrotropinomas, and prolactinomas) generally arise from only one type of hormone-expressing cells and typically present as hypersecretory syndromes (e.g. Cushing’s disease, acromegaly, hyperthyroidism, and hyperprolactinemia). Prolactinomas only require surgery when medical treatment is insufficient or not tolerated (2). Usually, gonadotropinomas do not lead to hypersecretory syndromes and are diagnosed similarly to the non-functional adenomas (3). Non-functional adenomas can originate from any differentiated hormone-expressing cell, but are generally diagnosed when symptoms occur due to the size of the tumor (4). This so called mass-effect can lead to headache, hypopituitarism and/or visual field deficits. These visual symptoms arise through compression following increasing size of the longitudinal axis and typically cause hemi-anopsia. Rarely, palsy of the 3rd, 4th, and/or 6th cranial nerves develops as a consequence of cavernous sinus invasion (5, 6). In very rare cases, pituitary apoplexy can occur which is characterized by sudden onset of severe headache and rapidly worsening visual field deficits or double vision caused by compression of nerves surrounding the gland. This is often followed by acute symptoms caused by lack of secretion of essential hormones. Additionally, incidentalomas in the pituitary region have become more frequent as the use of ever improving medical imaging techniques increased (7).
Less frequent benign pathologies in the sellar and suprasellar region are Rathke’s cleft cysts and craniopharyngiomas. The former are embryological remnants of the Rathke pouch and only require surgical removal in case of mass-effect or progressive growth (8, 9). The latter are congenital tumors of the central nervous system, believed to arise from residual ectoblastic cells of the craniopharyngeal duct. Craniopharyngiomas are most often located above the pituitary gland and can be resected through the endoscopic endonasal transsphenoidal approach (EETA), but often require additional radiotherapy for optimal treatment (10, 11).
Other lesions of the sellar and suprasellar region that may need to be approached for biopsy or resection are meningiomas, gliomas, and germ cell tumors, although the EETA for these lesions is less widely applied.
Historically, the gold standard for surgical removal or biopsy of all of the above pathologies has been the microscopic transsphenoidal approach. Since the year 2000, skull base tumors have increasingly successfully been approached in an endoscopic way, and our team has been among the pioneers (12–15). The EETA has clear advantages, like the increased panoramic vision inside the surgical area, resulting in better orientation for the surgeons and better close-up view of the tumor–gland interface and the relevant anatomical landmarks (16–20). Typically, neurosurgeons and otorhinolaryngologists collaborate in these skull base approaches, where they combine their knowledge and expertise during the “two nostrils–four hands” surgery. However, EETA has its limitations as well, and there are some major drawbacks coming from a microscopic approach, mainly the loss of three-dimensional vision and the longer learning curve when the surgeon is unfamiliar with endoscopic procedures.
In our tertiary referral center we have been performing the EETA for lesions in the sellar region since April 2008, after a long period of using the microscopic approach. In this retrospective, monocentric cohort study we describe our 10 year experience with the EETA and evaluate the perioperative and postoperative outcomes, with emphasis on extent of tumor resection, cerebrospinal fluid (CSF) leakage, cranial nerve damage, recurrence, and the effects of the learning curve.
Patients and Methods
Study Design and Data Collection
The study was approved by the Medical Ethical Committee of the University Hospitals Leuven (S63665).
Figure 1 depicts the flow diagram of the selection of potential patients in our electronic heath record system using two search queries between 2008 and 2018 (included). We did not include patients after 2018 to ensure a follow-up period of at least 1 year. Firstly, all patients who were billed for ‘‘Transsphenoidal pituitary surgery’’ (N = 426) were identified. Secondly all patients who had the word ‘‘Transsphenoidal’’ (N = 2191) mentioned anywhere in their electronic health record system were also identified. After removing the duplicates, 529 unique patients were found. We excluded patients that were operated via the microscopic approach (before April 2008), patients operated in other hospitals but in follow-up at our hospital, other types of surgery in the sellar/suprasellar region like closure of idiopathic/traumatic CSF leaks via a transsphenoidal approach and some other exceptions (see Figure 1). Subsequent removal of the patients who did not meet the inclusion criteria resulted in 369 unique patients. There was no age restriction. Patients who presented with a recurrence after surgery elsewhere or with a recurrence after previous microsurgical resection were also included.
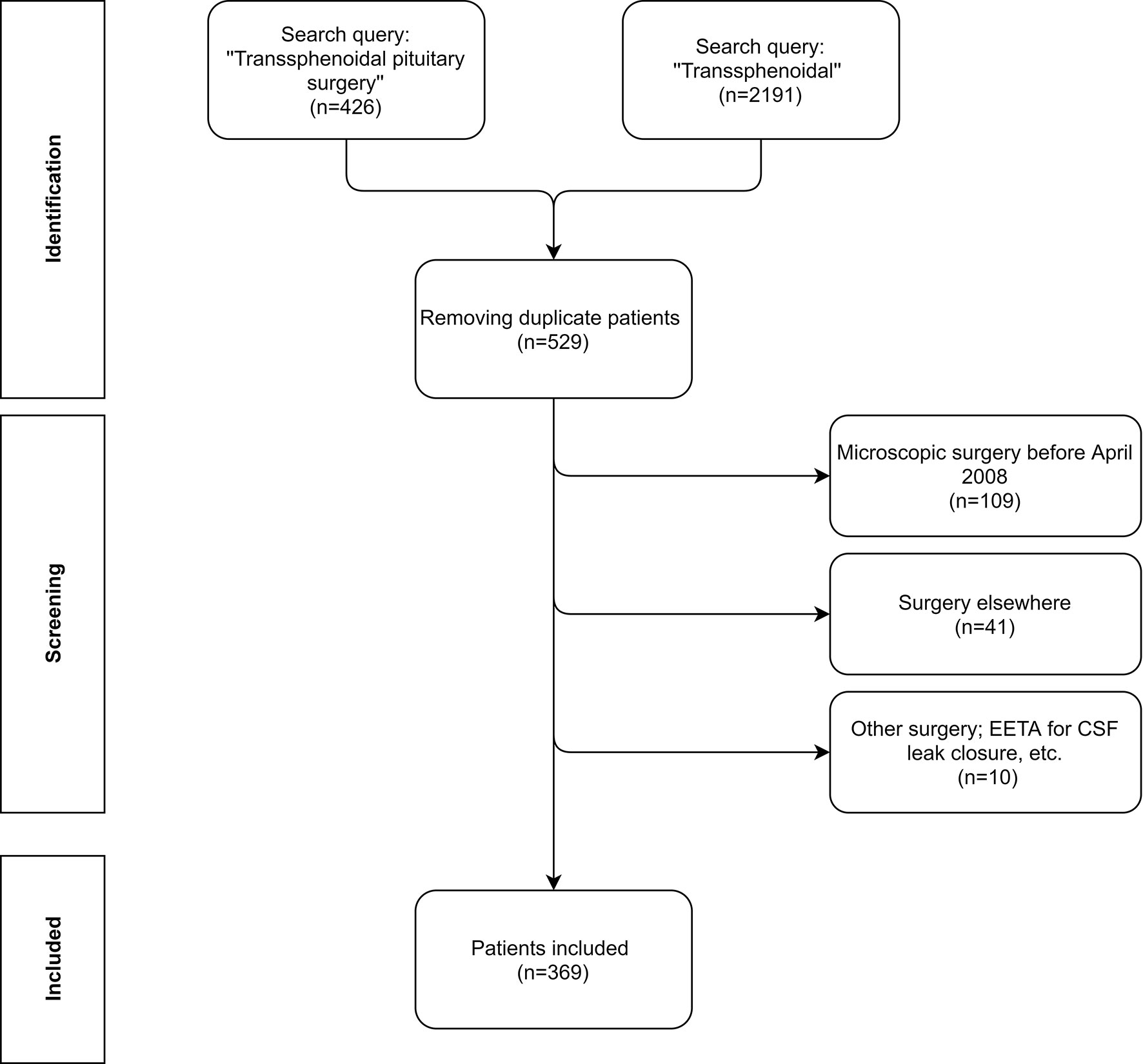
Figure 1 Flow diagram of the patient inclusion process. The patients were initially identified using two search queries: ‘‘Transsphenoidal pituitary surgery’’ ‘‘Transsphenoidal’’ in 2 databases; 1 was the billing file, the other the medical records. Afterwards, all of the unique patients were screened for the inclusion criteria. Note that at our hospital we do not have a separate code for CSF leak closure. Finally 369 unique patients were included in this study.
The electronic health medical records were reviewed and analyzed for clinical, biochemical, and radiological data, procedure characteristics, perioperative complications, pathological examination of the tumor, postoperative outcomes, morbidities, and mortalities.
Patient Work-Up and Surgical Procedure
All patients were operated under general anesthesia by a team consisting of an experienced neurosurgeon and an otorhinolaryngologist. Our standard preoperative workup included a clinical and biochemical evaluation, an MRI of the sella, and a CT scan for neuronavigation (Brainlab ®, Munich, Germany). If visual impairment was suspected, an ophthalmological examination was performed before surgery. All patients received perioperative antibiotic prophylaxis and a corticosteroid stress-dose. In all patients, a bilateral approach was used in three phases: the nasal, sphenoidal, and sellar phase.
After careful out-fracture of the inferior and middle turbinate with the Cottle, the natural ostium of the sphenoidal sinuses was reached via the paraseptal corridor (nasal phase). To enlarge the natural ostium of the sphenoidal sinuses, the inferior 3rd of the superior turbinate was removed by a monopolar cutting. The access was then enlarged by a mushroom punch and Kerrison rongeurs with caution not to damage the septal branch of the sphenopalatine artery (posterior septal artery) in patients where the use of a nasoseptal flap was anticipated. After finishing the bilateral sphenoidotomy, a posterior septectomy with resection of the rostrum and intersinus septum allowed a wide access to the face of the sella with optimal identification of the optico-carotic recess (OCR), carotic and optic protuberance on both sides, and the clival indentation (sphenoidal phase). At this point we start the two-nostril, four-handed technique to remove the sellar bone with a Kerrison punch or microdrill using a diamond burr, depending on the erosion of the bone, open the inner periosteum, and perform a meticulous endoscopy-guided resection of the tumor (sellar phase). For macro-adenoma, the inferior and lateral components of the tumor were resected before approaching the superior aspect to avoid limited vision after descent of the redundant diaphragm into the operative field. For micro-adenoma, the most challenging step was always identification of the right tumor-gland plane. In case of unclear identification, pathologic tissue which differs in color and consistency from normal pituitary tissue was removed until normal gland-tissue could be recognized.
In case of craniopharyngiomas, a resection of the solid part of the tumor and of the wall of the cystic component was attempted. In case of Rathke’s cleft cysts, a broad opening of the cyst was performed to drain the contents, and a biopsy of the wall was taken.
In the absence of perioperative complications, Spongostan® (Ethicon, Edinburgh, Scotland) and Tisseel®(Baxter, Deerfield, Illinois, U.S.) were used to close the sellar defect. In case of a small (punctiform) intra-operative CSF-leak, a multilayer reconstruction using fascia and/or fat and a free mucosal graft in overlay were used. In case of large intra-operative CSF-leaks (macro-adenoma, malignancies), in cases in which the arachnoid was thinned out and herniated into the sella, and in case of postoperative CSF-leak, a more extensive, multilayer closure was warranted using a mucoperiosteal flap (nasoseptal/Hadad flap) in overlay instead of a free flap. Placement of lumbo-external drainage and postoperative nasal packing was not included in our standard of care but was only performed in indicated cases.
Statistical Analysis
All statistical analysis was performed using IBM SPSS Statistics 27® software or Microsoft Excel 2016. Categorical variables were expressed in frequencies and proportions. Normally distributed continuous variables were presented as means and their standard deviations, skewed continuous data as median and range. Normality was tested using Shapiro–Wilk test. Means were compared using Independent Samples T-test; medians were compared by non-parametric tests. Significance was set at p <0.05. One-way ANOVA was performed to investigate the association between categorical and continuous variables when appropriate, otherwise a Kruskal–Wallis test was performed. Pearson Chi-Square test was used for the association between categorical variables. Kaplan–Meier curves were calculated, and log-rank tests were subsequently performed. Recurrence-free interval was defined as time in months from date of operation until moment of either hormone suppression therapy, additional surgery, radiotherapy, or last follow-up depending on which event takes place first. Overall survival interval was defined as time in months from date of operation until date of death or last follow-up.
Results
Patient Characteristics
A total of 369 patients were analyzed (Table 1). More than half of the cohort (54.2%) was female, and the median age at surgery was 50 y (range 4–89). The median follow-up duration was 55.0 months.
Obesity was a common comorbidity (in 67%) with 154 patients classified as overweight (25 ≤ BMI < 30), 66 as class I obesity (30 ≤ BMI < 35), 23 as class II obesity (35 ≤ BMI < 40), and four as class III obesity (BMI ≥ 40). Five patients were diagnosed with multiple endocrine neoplasia syndrome.
Forty-five patients (12%) in this cohort study presented with a recurrence after previous microsurgical resection.
Clinical and Biochemical Manifestations
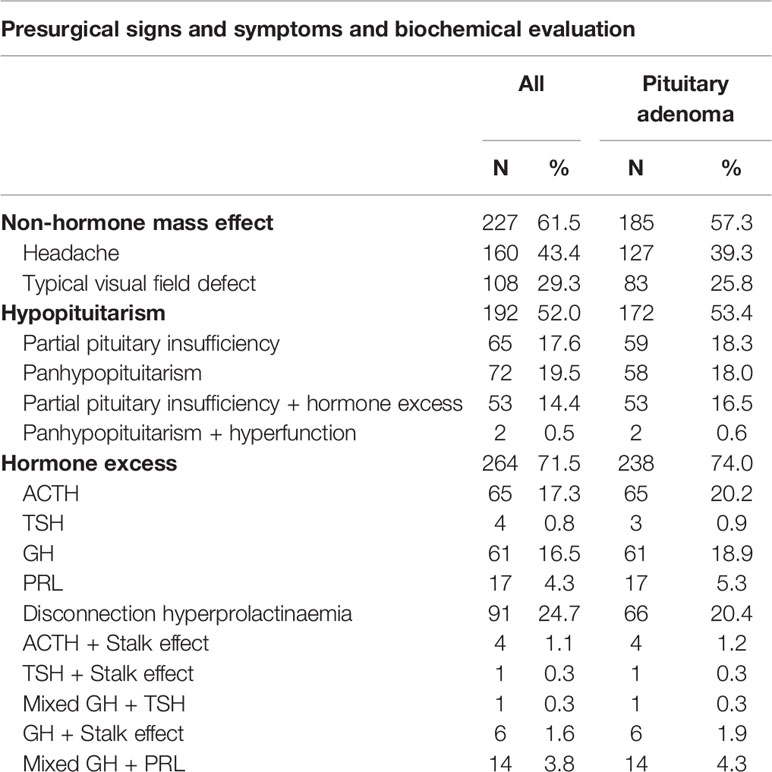
Over half of the patient population presented with a non-hormonal mass effect (61.5%) (Table 2). Typical visual symptoms were diagnosed in 108 patients during ophthalmologic screening and included 90 patients with bitemporal hemianopsia, 15 patients with diplopia, and three with both symptoms. Eleven patients presented with pituitary apoplexy. Seventeen percent (35/201) of female patients presented with amenorrhea, and 30 patients reported sexual dysfunction. Fatigue was also a very common symptom in our cohort (151 patients).
Biochemical evaluation revealed that 52% of patients had a central deficiency in at least one hormonal axis (Table 2). Hormone excess in this surgical series involved mostly the somatotropic axis (82 patients), followed by the corticotropic axis (69 patients).
Tumor Characteristics and Histopathology
The most frequently encountered tumors were pituitary adenomas (87.3%), followed by Rathke ‘s cleft cyts (5.1%) and craniopharyngiomas (2.4%). Most of the pituitary adenomas were macroadenomas (231/322), followed by microadenomas (75/322) and giant adenomas (14/322) (Figure 2A). Tumor size was not known in two. Cavernous sinus invasion by pituitary adenomas was radiologically classified using the Knosp staging system (Figure 2B). Non-hormone expressing adenoma was the most frequent pathological diagnosis (31.7%), followed by corticotroph adenoma (19.2%) and somatotroph adenoma (19%) (Table 1). The plurihormonal adenomas were further classified according to their main hormone expression pattern, and the results are visualized in Figure 2C. Furthermore, the EETA was used in 19 less frequent pathologies: six meningiomas, two chordomas, two oncocytomas, two plasmacytomas, two cholesterol granulomas, one germinoma, one chondrosarcoma, one neurinoma, one post-radiation sarcoma, and one leiomyosarcoma.
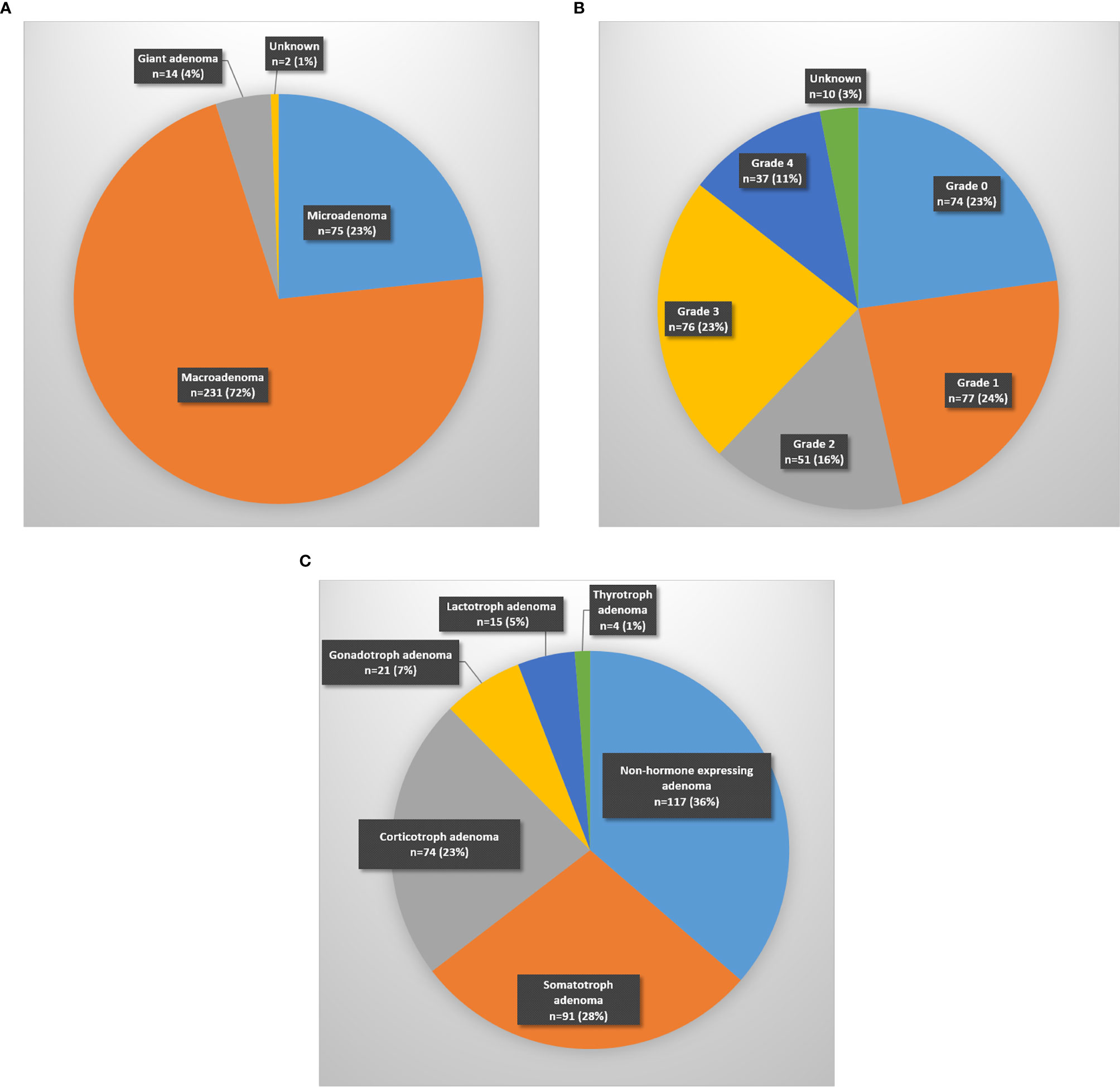
Figure 2 Distribution of pituitary adenomas according to (A) size (micro < 10 mm; macro 10–40 mm; giant > 40 mm), (B) Knosp classification-grade, (C) main hormone expression pattern.
Surgical Procedure and Perioperative Complications
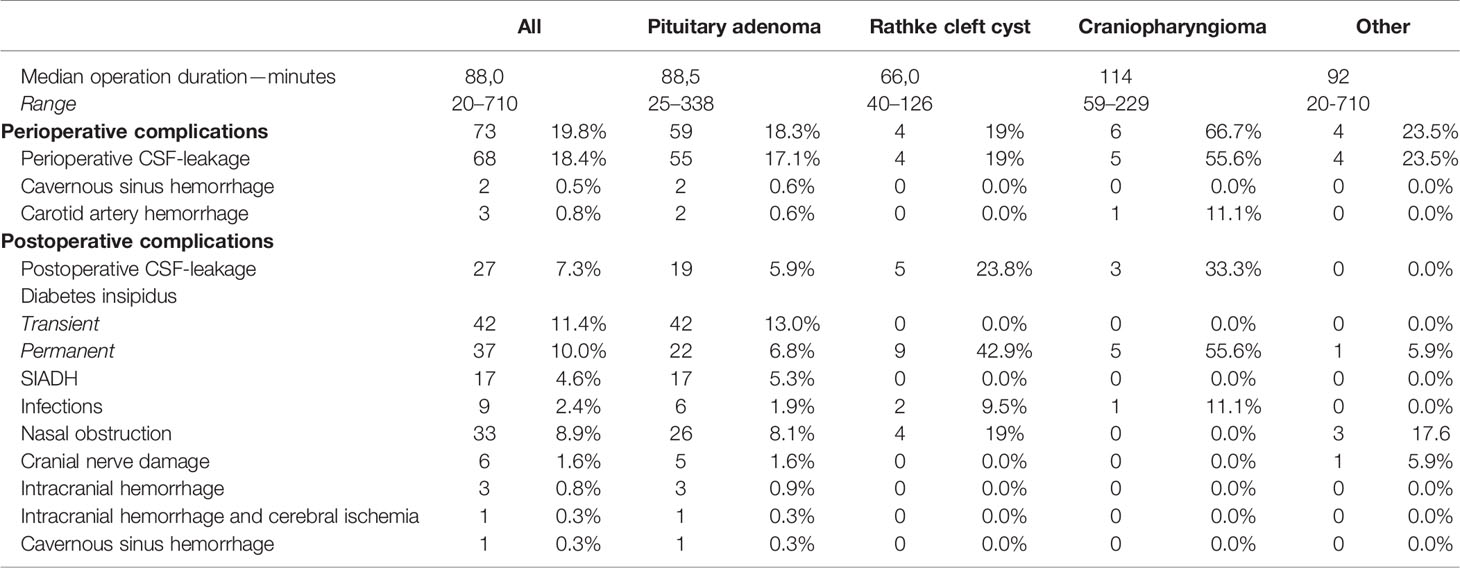
Overall, we observed a progressive increase of EETA-procedures over the last decade (R2 of 0.499; p =0.015) with a median yearly operated number of patients of 33 (range 15–43). Moreover, a significant reduction in operation time between the first 2 years of EETA [2008–2010: median 110.5 min, range (50; 710)] and the last three operation periods [2013–2014: median 79 min, range (40; 229); 2015–2016: median 95 min, range (20; 338); 2017–2018: median 80 min, range (20; 196)] (p < 0.001) could be observed (31.5; 15.5; 30.5 min respectively).
The most common perioperative complication was a CSF leak, with a significantly higher rate in the craniopharyngioma group than in the pituitary adenoma group (p = 0.014) (Table 3). There was no significant decrease in perioperative CSF leak rate over the years (p = 0.999). Knosp grade >2 was significantly associated with a higher perioperative CSF leak incidence (p <0.001) in the pituitary adenoma group.
In two patients, profuse bleeding from the cavernous sinus impaired visualization resulting in an incomplete tumor resection.
Three patients suffered from a perioperative carotid artery hemorrhage. In only one patient, the bleeding could be controlled during the operation using Flo-Seal® (Baxter, Deerfield, Illinois, U.S). The other two patients required interventional radiological therapy, which was successful in one patient.
In general, a macroscopically complete resection was achieved in 78% of the patients.
Postoperative Complications
Median hospitalization duration was 6 days (range 1–62 days). Pairwise comparisons after Bonferroni correction showed that for operation period 2008–2010 (7 days, range 2–62), 2011–2012 (7 days, range 3–20) and 2013–2014 (6 days, range 2–59) the median hospitalization duration was significantly longer than for operation period 2017–2018 (5 days, range 1–17) (p = 0.001; p < 0.001; p = 0.004 respectively).
Transient and permanent diabetes insipidus were the most common postoperative complications (Table 3), followed by postoperative CSF leak and syndrome of inappropriate antidiuretic hormone secretion (SIADH).
Zooming in on postoperative CSF leak, 15 cases (4.0%) were diagnosed during the hospitalization period and 12 after hospital discharge (3.3%). Looking at factors that were potentially associated with postoperative CSF leak, surgical experience with EETA did not seem to play a role as no significant decrease was seen over the years (p = 0.0725). Far lateral extension (Knosp grade > 2) was also not associated with a higher incidence of postoperative CSF leak (p =0.875). We did see that the occurrence of perioperative CSF leak was associated with higher postoperative CSF leak incidence (p < 0.001). For the management of this complication, placement of a lumbo-external drainage (LED) alone was sufficient in seven patients; LED combined with surgical closure was needed in 16 patients. One patient required ventriculo-external drainage (VED) with surgical closure and another required ventriculo-peritoneal drainage (VPD) with surgical closure. One patient received only surgical closure without LED, and one patient refused a re-intervention and received only antibiotics. The average duration of temporary CSF drainage was 7 days (range 5–30 days). Surgical closure was always done by a multilayer reconstruction using a graft with or without a free/pedicled mucoperiosteal flap in overlay. A free muscle graft was used in 11 cases, four patients received a nasoseptal flap (overlay) combined with a muscle graft (inlay). In two patients a fat graft was used and in one patient a fascia graft.
Meningitis (eight cases) was the most frequent postoperative infection; in all but one patient this was preceded by a CSF leak in the postoperative phase. One sellar abscess developed after resection of a non-hormone expressing adenoma.
A postoperative intracranial hemorrhage was observed in four patients. In two patients symptoms of (permanent) third cranial nerve damage (diplopia and ipsilateral mydriasis) lead to the diagnosis of intracranial hemorrhage resulting in localized brain stem or cerebral ischemia. One of these patients presented with a giant adenoma with extensive cavernous sinus invasion (Knosp grade 4). The other case was a recurrence with extensive suprasellar invasion. In the other two patients the intracranial hemorrhage was found following decreased consciousness, headache, and decreased visual acuity. In total, six patients had ophthalmological confirmed cranial nerve damage after surgery. In three patients the right third cranial nerve was permanently damaged. The other three patients had transient visual problems. A last complication was severe epistaxis requiring surgical intervention in two patients. No septal perforations were observed.
Recurrence and Overall Survival
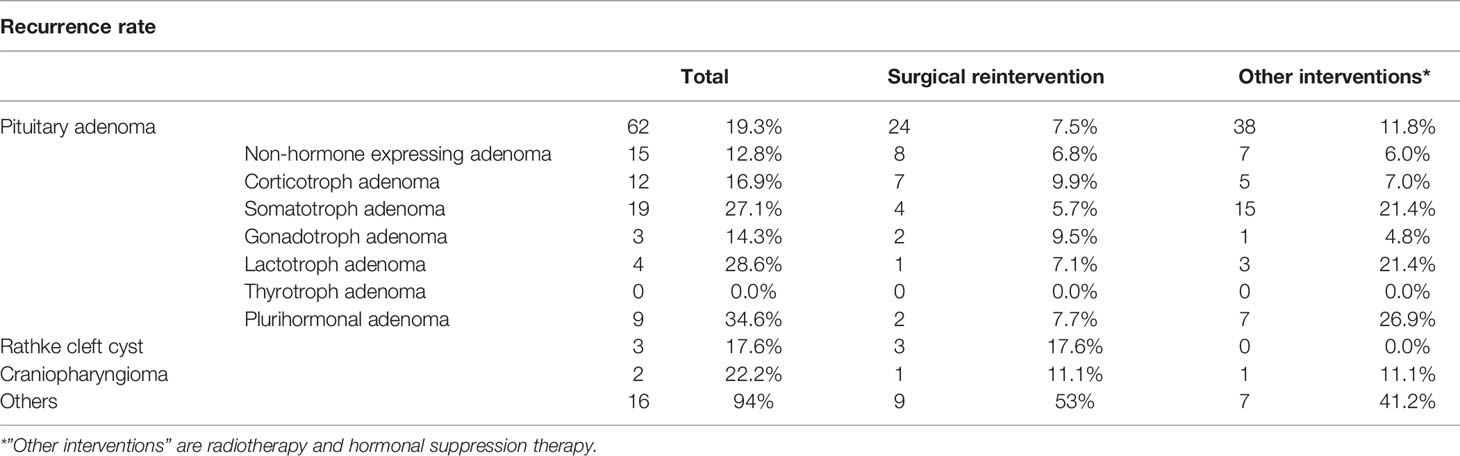
At last follow-up, local control after EETA was 83.2% (Table 4). Focusing on the factors determining local control, previous surgical therapy and tumor size did not decrease the chance of local control (p =0.576, p = 0.462). Tumor regrowth requiring surgical reintervention was seen in 19.3% of patients with pituitary adenomas. The median time to additional surgical intervention was 15.3 months (range 1–96.5 months). Ultimately, 93.0% of patients had their tumor controlled either through additional surgery, radiotherapy, medication or a combination. Table 4 displays recurrence rates and reintervention rates per disease category. In the pituitary adenoma group, most often additional therapy was needed for plurihormonal (34.6%), lactotroph (28.6%) and somatotroph (27.1%) adenomas. Corticotroph and gonadotroph adenomas had the lowest recurrence rates (16.9% and 14.3%), but with a high surgical reintervention rate (9.9% and 9.5%) as there is often no hormonal suppression therapy for these tumors. Figure 3A shows the Kaplan–Meier recurrence-free interval curves for the three most frequent types of pathology. There was no significant difference between the groups in recurrence-free interval (p > 0.76). Figure 3B shows the Kaplan–Meier recurrence-free interval curves for the pituitary adenomas operated during the first 5 years in comparison to the last 5 years. There was no significant difference between the first 5 years and last 5 years (p = 0.886).
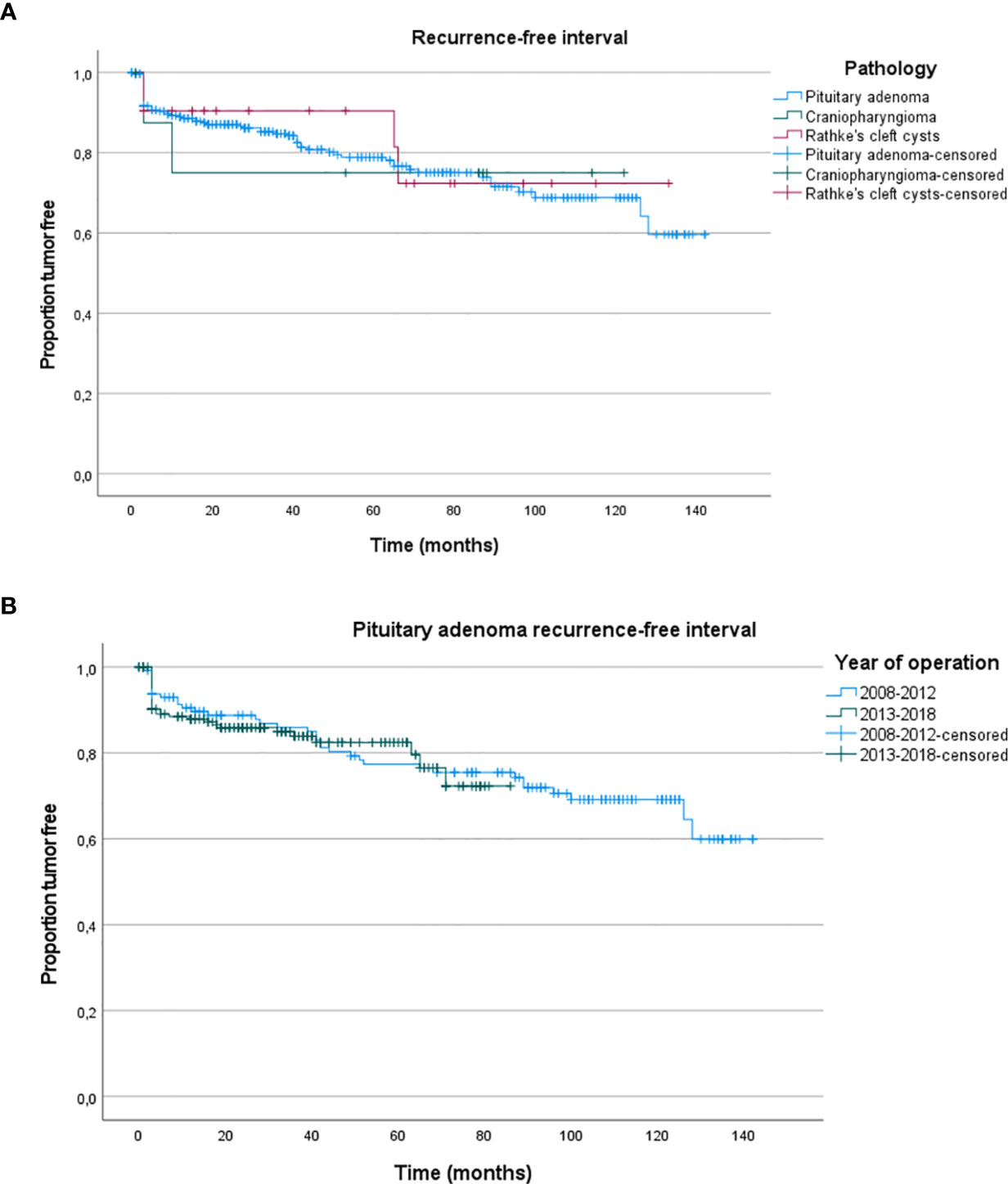
Figure 3 (A) Kaplan–Meier recurrence-free interval curves for the three most frequent histological types. (B) Kaplan–Meier recurrence-free interval curves for the pituitary adenomas operated during the first 5 years in comparison to the last 5 years.
Overall, three patients died due to iatrogenic complications: one after a carotid artery hemorrhage, one due to a tonsillar herniation with a Chiari I malformation as predisposing factor, and one after an intracranial hemorrhage and unsuccessful rehabilitation.
Discussion
In our large cohort of 369 patients that were operated by an EETA for a (para)sellar lesion between 2008 and 2018, demographics were very comparable to those of other large retrospective analyses of pituitary pathologies (1, 21). The incidence of pituitary adenomas is generally higher in females mainly due to a higher frequency and earlier signs of hyperprolactinemia in females (e.g. amenorrhea) (22). Not surprisingly, the majority of sellar and suprasellar lesions were pituitary adenomas (21).
Headache is a very common preoperative complaint of the patient, and our results are comparable to the literature, but unfortunately headache is also very common in the general population and therefore unspecific (23–25). Visual signs and more specifically, bitemporal hemianopsia are much more specific and also more common (28–100%) when there is pathology located at the (para)sellar region, according to the literature (26). Interestingly, the prevalence of visual signs in our cohort (29.3%) is located at the lower end of the reported incidence in the literature. This is unlikely to be due to smaller tumor size, as the incidence of macroadenomas in our cohort is roughly the same as in literature (27). A more plausible explanation is the fact that we only reported visual symptoms confirmed by an ophthalmologic examination.
In our experience, there was a clear learning curve reflected in duration of the surgical procedure. The EETA-procedures were initially longer, and surgical time dropped significantly going from the first two years to years 3 and 4 and then further during years 5 and 6, to then stabilize. Other authors did not observe this reduction in operative procedure duration, attributing this phenomenon to an increase in familiarity with EETA paralleling a higher acceptance of more complicated cases (28, 29). Nonetheless, we also noted a clear increase in case load over time.
Overall, the EETA is a less traumatic route to the sella as previous studies have reported (19, 30). In our cohort we did not observe any iatrogenic septal perforation, and the prevalence of epistaxis was slightly lower than reported in other series (1.25–11%) (31–33).
Looking at the complications, our observed rate of perioperative CSF leak (18.4%) compares favorably to what other authors reported, with incidences ranging from 15 to 25% with even reports up to 60% (29, 34–37). Younus et al. reported a decrease in perioperative CSF leak (from 60 to 33%) when the surgeon gained more experience (38). We could not observe this trend in our cohort even after including our earliest cases. This can be explained by a meticulous surgical technique ahead from the very beginning. Knosp grade is used to determine the cavernous sinus invasion in order to see preoperatively if macroscopic total resection is feasible or not (39). We found that a Knosp grade >2 is associated with higher perioperative CSF leak. A higher Knosp grade is associated with a higher invasiveness and in order to achieve a macroscopic total resection, the surgeon is required to do more extended manipulations, resulting in an increased chance of damaging the arachnoid (40, 41). Patel et al. reported that cavernous sinus invasion was not associated with perioperative CSF-leak, but did not specify their definition of cavernous sinus invasion (42).
Postoperative CSF leakage is a frequent, serious, and costly complication resulting in a higher risk of meningitis and a longer hospital stay (43, 44). The cause of this complication is either lack of recognition of a perioperative CSF leak or an incomplete closure of the leak, but small perioperative CSF leaks are not always noticeable without enhanced visualization (45).
Our rate of postoperative CSF leakage is 7.3%. Other authors report rates ranging from 1.4 to 16.9% (46, 47). Not surprisingly, there are a vast number of studies investigating how to prevent this complication (36, 48, 49). Our study shows, not surprisingly, that a perioperative CSF leak is predictive for a postoperative leak, which has been suggested in the past (50, 51). More recent literature has shown that a more intensive therapy including a perioperative lumbar drain and nasoseptal flaps in high risk patients, like those undergoing revision surgery, could be beneficial (29, 36, 37, 52, 53).
The hospitalization duration has also significantly decreased over the years. However, our hospitalization is still slightly longer (median of 5 days) than in some recently published reports, describing short-hospital-stay protocols of 3 days or less. This is mainly due to the organization of patient care in our hospital, not to a higher frequency of postoperative morbidities (54, 55).
Recurrence in pituitary adenoma occurred in around 20% of cases, which is lower than previously reported in the literature, although strongly dependent on the tumor-characteristics (24, 49, 50).
According to a recent meta-analysis, the pooled surgical remission for acromegaly is 54.8%, which is lower than the 72.9% observed in our cohort (56).
For corticotroph adenoma, Braun et al. reported that the recurrence rate ranged from 1 to 41% depending on the study, but with an average rate of 14% which is in line with the recurrence rate of our corticotroph adenoma subgroup of 16.9% (Table 4) (57).
Both of these types of adenomas can recur either as a macroscopically visible adenoma or as a microscopic adenoma, even undetectable on imaging, but merely based on evolution of hormonal levels. In the former case, surgery can be repeated, but in the latter, medical therapy or radiation therapy is to be considered. Lactotroph adenomas are generally not treated by surgical intervention. Only after failed medical therapy or intolerance, surgery is considered. However, surgery is often insufficient to reach complete remission. Our results in lactotroph adenomas (71.4%) are comparable to those previously reported (58–60).
Conclusion
In this large historical cohort with long-term follow-up, EETA has proven to be a safe and efficient technique. Surgical teams that want to switch from a microscopic to an endoscopic approach should take into account the initial slightly longer operation time. However, in our series, already in the initial years, the caseload increased and hospital stay was reduced, while no increase in peri- and postoperative complications was observed. This series further adds to the body of evidence that EETA is the new gold standard for treating patients with (para) sellar lesions.
Data Availability Statement
The data in this study are available upon reasonable request to the corresponding author. Requests to access these datasets should be directed to dmluY2VudC52YW5kZXJwb29ydGVuQHV6bGV1dmVuLmJl.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Ethical Committee of the University Hospitals Leuven (S63665). Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
LG and ZQ: conception, data collection, drafting the article, and revising the article for important intellectual content. AS: initial data collection and revising the article for important intellectual content. MJ, JM, JLo, SV, JLa, and MB: revising the article for important intellectual content. VV: conception, data collection, drafting the article, and revising the article for important intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Asemota AO, Ishii M, Brem H, Gallia GL. Comparison of Complications, Trends, and Costs in Endoscopic vs Microscopic Pituitary Surgery: Analysis From a US Health Claims Database. Clin Neurosurg (2017) 81(3):458–71. doi: 10.1093/neuros/nyx350
2. Molitch ME. Diagnosis and Treatment of Pituitary Adenomas: A Review. JAMA - J Am Med Assoc (2017) 317:516–24. doi: 10.1001/jama.2016.19699
3. Melmed S. Mechanisms for Pituitary Tumorigenesis: The Plastic Pituitary. J Clin Invest (2003) 112:1603–18. doi: 10.1172/JCI20401
4. Melmed S. Pituitary-Tumor Endocrinopathies. Longo DL, Editor. N Engl J Med (2020) 382(10):937–50. doi: 10.1056/NEJMra1810772
5. Suri H, Dougherty C. Clinical Presentation and Management of Headache in Pituitary Tumors. Curr Pain Headache Rep (2018) 22:1–6. doi: 10.1007/s11916-018-0710-8
6. Kim SH, Lee KC, Kim SH. Cranial Nerve Palsies Accompanying Pituitary Tumour. J Clin Neurosci (2007) 14(12):1158–62. doi: 10.1016/j.jocn.2006.07.016
7. Vernooij MW, Ikram MA, Tanghe HL, Vincent AJPE, Hofman A, Krestin GP, et al. Incidental Findings on Brain MRI in the General Population. N Engl J Med (2007) 357(18):1821–8. doi: 10.1056/NEJMoa070972
8. Barkhoudarian G, Palejwala SK, Ansari S, Eisenberg AA, Huang X, Griffiths CF, et al. Rathke’s Cleft Cysts: A 6-Year Experience of Surgery vs. Observation With Comparative Volumetric Analysis. Pituitary (2019) 22(4):362–71. doi: 10.1007/s11102-019-00962-y
9. Larkin S, Karavitaki N, Ansorge O. Rathke’s Cleft Cyst. In: Handbook of Clinical Neurology. Amsterdam: Elsevier B.V (2014). p. 255–69. doi: 10.1016/B978-0-444-59602-4.00017-4
11. O’Steen L, Indelicato DJ. Advances in the Management of Craniopharyngioma [Version 1; Peer Review: 3 Approved]. F1000 Res (2018) 7. doi: 10.12688/f1000research.15834.1
12. Goffart Y, Jorissen M, Daele J, Vander Poorten V, Born J, Deneufbourg JM, et al. Minimally Invasive Endoscopic Management of Malignant Sinonasal Tumours. Acta Otorhinolaryngol Belg (2000) 54(2):221–32.
13. Bogaerts S, Vander Poorten V, Nuyts S, Van Den Bogaert W, Jorisser M. Results of Endoscopic Resection Followed by Radiotherapy for Primarily Diagnosed Adenocarcinomas of the Paranasal Sinuses. Head Neck (2008) 30(6):728–36. doi: 10.1002/hed.20771
14. Van Gerven L, Jorissen M, Nuyts S, Hermans R, Vander Poorten V. Long-Term Follow-Up of 44 Patients With Adenocarcinoma of the Nasal Cavity and Sinuses Primarily Treated With Endoscopic Resection Followed by Radiotherapy. Head Neck (2011) 33(6):898–904. doi: 10.1002/hed.21556
15. Camp S, Van Gerven L, Vander Poorten V, Nuyts S, Hermans R, Hauben E, et al. Long-Term Follow-Up of 123 Patients With Adenocarcinoma of the Sinonasal Tract Treated With Endoscopic Resection and Postoperative Radiation Therapy. Head Neck (2016) 38(2):294–300. doi: 10.1002/hed.23900
16. Rolston JD, Han SJ, Aghi MK. Nationwide Shift From Microscopic to Endoscopic Transsphenoidal Pituitary Surgery. Pituitary (2016) 19:248–50. doi: 10.1007/s11102-015-0685-y
17. Rotenberg B, Tam S, Ryu WHA, Duggal N. Microscopic Versus Endoscopic Pituitary Surgery: A Systematic Review. Laryngoscope (2010) 120:1292–7. doi: 10.1002/lary.20949
18. Agam MS, Wedemeyer MA, Wrobel B, Weiss MH, Carmichael JD, Zada G. Complications Associated With Microscopic and Endoscopic Transsphenoidal Pituitary Surgery: Experience of 1153 Consecutive Cases Treated At a Single Tertiary Care Pituitary Center. J Neurosurg (2019) 130(5):1576–83. doi: 10.3171/2017.12.JNS172318
19. Li A, Liu W, Cao P, Zheng Y, Bu Z, Zhou T. Endoscopic Versus Microscopic Transsphenoidal Surgery in the Treatment of Pituitary Adenoma: A Systematic Review and Meta-Analysis. World Neurosurg (2017) 101:236–46. doi: 10.1016/j.wneu.2017.01.022
20. Little AS, Kelly DF, White WL, Gardner PA, Fernandez-Miranda JC, Chicoine MR, et al. Results of a Prospective Multicenter Controlled Study Comparing Surgical Outcomes of Microscopic Versus Fully Endoscopic Transsphenoidal Surgery for Nonfunctioning Pituitary Adenomas: The Transsphenoidal Extent of Resection (Transspher) Study. J Neurosurg (2020) 132(4):1043–53. doi: 10.3171/2018.11.JNS181238
21. Lüdecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S, Saeger W. Pathohistological Classification of Pituitary Tumors: 10 Years of Experience With the German Pituitary Tumor Registry. Eur J Endocrinol (2007) 156(2):203–16. doi: 10.1530/eje.1.02326
22. Franks S, Nabarro JDN, Jacobs HS. Prevalence and Presentation of Hyperprolactinaemia in Patients With “Functionless” Pituitary Tumours. Lancet (1977) 309(8015):778–80. doi: 10.1016/S0140-6736(77)92959-2
23. Losa M, Donofrio CA, Barzaghi R, Mortini P. Presentation and Surgical Results of Incidentally Discovered Nonfunctioning Pituitary Adenomas: Evidence for a Better Outcome Independently of Other Patients’ Characteristics. Eur J Endocrinol (2013) 169(6):735–42. doi: 10.1530/EJE-13-0515
24. Gravdahl GB, Tronvik EA, Fougner SL, Solheim O. Pituitary Adenoma and Non-acute Headache: Is There an Association, and Does Treatment Help? World Neurosurg (2016) 92:284–91. doi: 10.1016/j.wneu.2016.04.071
25. Almutairi RD, Muskens IS, Cote DJ, Dijkman MD, Kavouridis VK, Crocker E, et al. Gross Total Resection of Pituitary Adenomas After Endoscopic vs. Microscopic Transsphenoidal Surgery: A Meta-Analysis. Acta Neurochirurg (2018) 160:1005–21. doi: 10.1007/s00701-017-3438-z
26. Muskens IS, Zamanipoor Najafabadi AH, Briceno V, Lamba N, Senders JT, van Furth WR, et al. Visual Outcomes After Endoscopic Endonasal Pituitary Adenoma Resection: A Systematic Review and Meta-Analysis. Pituitary (2017) 20(5):539–52. doi: 10.1007/s11102-017-0815-9
27. Ntali G, Wass JA. Epidemiology, Clinical Presentation and Diagnosis of non-Functioning Pituitary Adenomas. Pituitary (2018) 21:111–8. doi: 10.1007/s11102-018-0869-3
28. Younus I, Gerges MM, Uribe-Cardenas R, Morgenstern PF, Eljalby M, Tabaee A, et al. How Long is the Tail End of the Learning Curve? Results From 1000 Consecutive Endoscopic Endonasal Skull Base Cases Following the Initial 200 Cases. J Neurosurg (2020) 7:1–11. doi: 10.3171/2019.12.JNS192600
29. Bora SK, Suri A, Khadgawat R, Tandon N, Suri V, Chand Sharma M, et al. Management of Cushing’s Disease: Changing Trend From Microscopic to Endoscopic Surgery. World Neurosurg (2020) 134:e46–54. doi: 10.1016/j.wneu.2019.08.165
30. Gao Y, Zhong C, Wang Y, Xu S, Guo Y, Dai C, et al. Endoscopic Versus Microscopic Transsphenoidal Pituitary Adenoma Surgery: A Meta-Analysis. World J Surg Oncol (2014) 12:94. doi: 10.1186/1477-7819-12-94
31. Magro E, Graillon T, Lassave J, Castinetti F, Boissonneau S, Tabouret E, et al. Complications Related to the Endoscopic Endonasal Transsphenoidal Approach for Nonfunctioning Pituitary Macroadenomas in 300 Consecutive Patients. World Neurosurg (2016) 89:442–53. doi: 10.1016/j.wneu.2016.02.059
32. Thompson CF, Wang MB, Kim BJ, Bergsneider M, Suh JD. Incidence and Management of Epistaxis After Endoscopic Skull Base Surgery. ORL (2012) 74(6):315–9. doi: 10.1159/000345500
33. Younus I, Gerges MM, Dobri GA, Ramakrishna R, Schwartz TH. Readmission After Endoscopic Transsphenoidal Pituitary Surgery: Analysis of 584 Consecutive Cases. J Neurosurg (2020) 133(4):1242–7. doi: 10.3171/2019.7.JNS191558
34. Han ZL, He DS, Mao ZG, Wang HJ. Cerebrospinal Fluid Rhinorrhea Following Trans-Sphenoidal Pituitary Macroadenoma Surgery: Experience From 592 Patients. Clin Neurol Neurosurg (2008) 110(6):570–9. doi: 10.1016/j.clineuro.2008.02.017
35. Pereira MP, Oh T, Joshi RS, Haddad AF, Pereira KM, Osorio RC, et al. Clinical Characteristics and Outcomes in Elderly Patients Undergoing Transsphenoidal Surgery for Nonfunctioning Pituitary Adenoma. Neurosurg Focus (2020) 49(4):E19. doi: 10.3171/2020.7.FOCUS20524
36. Villalonga JF, Solari D, Cavallo LM, Cappabianca P, Prevedello DM, Carrau R, et al. The Sellar Barrier on Preoperative Imaging Predicts Intraoperative Cerebrospinal Fluid Leak: A Prospective Multicenter Cohort Study. Pituitary (2021) 24(1):27–37. doi: 10.1007/s11102-020-01082-8
37. Thakur JD, Corlin A, Mallari RJ, Huang W, Eisenberg A, Sivakumar W, et al. Pituitary Adenomas in Older Adults (≥ 65 Years): 90-Day Outcomes and Readmissions: A 10-Year Endoscopic Endonasal Surgical Experience. Pituitary (2020) 1:3. doi: 10.1007/s11102-020-01081-9
38. Younus I, Gerges MM, Uribe-Cardenas R, Morgenstern P, Kacker A, Tabaee A, et al. The Slope of the Learning Curve in 600 Consecutive Endoscopic Transsphenoidal Pituitary Surgeries. Acta Neurochir (Wien) (2020) 162(10):2361–70. doi: 10.1007/s00701-020-04471-x
39. Knosp E, Steiner E, Kitz K, Matula C. Pituitary Adenomas With Invasion of the Cavernous Sinus Space: A Magnetic Resonance Imaging Classification Compared With Surgical Findings. Neurosurgery (1993) 33(4):610–8. doi: 10.1227/00006123-199310000-00008
40. Esquenazi Y, Essayed WI, Singh H, Mauer E, Ahmed M, Christos PJ, et al. Endoscopic Endonasal Versus Microscopic Transsphenoidal Surgery for Recurrent and/or Residual Pituitary Adenomas. World Neurosurg (2017) 101:186–95. doi: 10.1016/j.wneu.2017.01.110
41. Broersen LHA, Biermasz NR, van Furth WR, de Vries F, Verstegen MJT, Dekkers OM, et al. Endoscopic vs. Microscopic Transsphenoidal Surgery for Cushing’s Disease: A Systematic Review and Meta-Analysis. Pituitary (2018) 21(5):524–34. doi: 10.1007/s11102-018-0893-3
42. Patel PN, Stafford AM, Patrinely JR, Smith DK, Turner JH, Russell PT, et al. Risk Factors for Intraoperative and Postoperative Cerebrospinal Fluid Leaks in Endoscopic Transsphenoidal Sellar Surgery. Otolaryngol - Head Neck Surg (2018) 158(5):952–60. doi: 10.1177/0194599818756272
43. Tang R, Mao S, Li D, Ye H, Zhang W. Treatment and Outcomes of Iatrogenic Cerebrospinal Fluid Leak Caused by Different Surgical Procedures. World Neurosurg (2020) 143:e667–75. doi: 10.1016/j.wneu.2020.08.069
44. Parasher AK, Lerner DK, Glicksman JT, Miranda SP, Dimentberg R, Ebesutani D, et al. Drivers of In-Hospital Costs Following Endoscopic Transphenoidal Pituitary Surgery. Laryngoscope (2021) 131:760–4. doi: 10.1002/lary.29041
45. Jakimovski D, Bonci G, Attia M, Shao H, Hofstetter C, Tsiouris AJ, et al. Incidence and Significance of Intraoperative Cerebrospinal Fluid Leak in Endoscopic Pituitary Surgery Using Intrathecal Fluorescein. World Neurosurg (2014) 82:E513–23. doi: 10.1016/j.wneu.2013.06.005
46. Lobatto DJ, de Vries F, Zamanipoor Najafabadi AH, Pereira AM, Peul WC, Vliet Vlieland TPM, et al. Preoperative Risk Factors for Postoperative Complications in Endoscopic Pituitary Surgery: A Systematic Review. Pituitary (2018) 21(1):84–97. doi: 10.1007/s11102-017-0839-1
47. Lee JA, Cooper RL, Nguyen SA, Schlosser RJ, Gudis DA. Endonasal Endoscopic Surgery for Pediatric Sellar and Suprasellar Lesions: A Systematic Review and Meta-Analysis. Otolaryngol - Head Neck Surg (United States) (2020) 163:284–92. doi: 10.1177/0194599820913637
48. Cavallo LM, Solari D, Somma T, Cappabianca P. The 3f (Fat, Flap, and Flash) Technique For Skull Base Reconstruction After Endoscopic Endonasal Suprasellar Approach. World Neurosurg (2019) 126:439–46. doi: 10.1016/j.wneu.2019.03.125
49. Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, et al. A Novel Reconstructive Technique After Endoscopic Expanded Endonasal Approaches: Vascular Pedicle Nasoseptal Flap. Laryngoscope (2006) 116(10):1882–6. doi: 10.1097/01.mlg.0000234933.37779.e4
50. Strickland BA, Lucas J, Harris B, Kulubya E, Bakhsheshian J, Liu C, et al. Identification and Repair of Intraoperative Cerebrospinal Fluid Leaks in Endonasal Transsphenoidal Pituitary Surgery: Surgical Experience in a Series of 1002 Patients. J Neurosurg (2018) 129(2):425–9. doi: 10.3171/2017.4.JNS162451
51. Mehta GU, Oldfield EH. Prevention of Intraoperative Cerebrospinal Fluid Leaks by Lumbar Cerebrospinal Fluid Drainage During Surgery for Pituitary Macroadenomas: Clinical Article. J Neurosurg (2012) 116(6):1299–303. doi: 10.3171/2012.3.JNS112160
52. Xiaoming X, Zhu Y, Hong Y. Efficacy and Safety of Intraoperative Lumbar Drain in Endoscopic Skull Base Tumor Resection: A Meta-Analysis. Front Oncol (2020) 10:606. doi: 10.3389/fonc.2020.00606
53. Tan J, Song R, Huan R, Huang N, Chen J. Intraoperative Lumbar Drainage can Prevent Cerebrospinal Fluid Leakage During Transsphenoidal Surgery for Pituitary Adenomas: A Systematic Review and Meta-Analysis. BMC Neurol (2020) 20(1):303. doi: 10.1186/s12883-020-01877-z
54. Thomas JG, Gadgil N, Samson SL, Takashima M, Yoshor D. Prospective Trial of a Short Hospital Stay Protocol After Endoscopic Endonasal Pituitary Adenoma Surgery. World Neurosurg (2014) 81:576–83. doi: 10.1016/j.wneu.2013.11.014
55. Lobatto DJ, Vliet Vlieland TPM, van den Hout WB, de Vries F, de Vries AF, Schutte PJ, et al. Feasibility, Safety, and Outcomes of a Stratified Fast-Track Care Trajectory in Pituitary Surgery. Endocrine (2020) 69(1):175–87. doi: 10.1007/s12020-020-02308-2
56. Starnoni D, Daniel RT, Marino L, Pitteloud N, Levivier M, Messerer M. Surgical Treatment of Acromegaly According to the 2010 Remission Criteria: Systematic Review and Meta-Analysis. Acta Neurochir (Wien) (2016) 158(11):2109–21. doi: 10.1007/s00701-016-2903-4
57. Braun LT, Rubinstein G, Zopp S, Vogel F, Schmid-Tannwald C, Escudero MP, et al. Recurrence After Pituitary Surgery in Adult Cushing’s Disease: A Systematic Review on Diagnosis and Treatment. Endocrine (2020) 70:218–31. doi: 10.1007/s12020-020-02432-z
58. Hamilton DK, Vance ML, Boulos PT, Laws ER. Surgical Outcomes in Hyporesponsive Prolactinomas: Analysis of Patients With Resistance or Intolerance to Dopamine Agonists. Pituitary (2005) 8(1):53–60. doi: 10.1007/s11102-005-5086-1
59. Donoho DA, Laws ER. The Role of Surgery in the Management of Prolactinomas. Neurosurg Clinics North America (2019) 30:509–14. doi: 10.1016/j.nec.2019.05.010
Keywords: endoscopic endonasal surgery (EES), transsphenoidal approaches, pituitary tumor, cerebrospinal fluid (CSF) leak, pituitary adenoma
Citation: Van Gerven L, Qian Z, Starovoyt A, Jorissen M, Meulemans J, van Loon J, De Vleeschouwer S, Lambert J, Bex M and Vander Poorten V (2021) Endoscopic, Endonasal Transsphenoidal Surgery for Tumors of the Sellar and Suprasellar Region: A Monocentric Historical Cohort Study of 369 Patients. Front. Oncol. 11:643550. doi: 10.3389/fonc.2021.643550
Received: 18 December 2020; Accepted: 06 April 2021;
Published: 07 May 2021.
Edited by:
Aviram Mizrachi, Rabin Medical Center, IsraelReviewed by:
J. Manuel Revuelta Barbero, Emory University, United StatesMaxim Kutin, N.N. Burdenko National Scientific and Practical Center for Neurosurgery, Russia
Copyright © 2021 Van Gerven, Qian, Starovoyt, Jorissen, Meulemans, van Loon, De Vleeschouwer, Lambert, Bex and Vander Poorten. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vincent Vander Poorten, dmluY2VudC52YW5kZXJwb29ydGVuQHV6bGV1dmVuLmJl
†These authors have contributed equally to this work and share first authorship
 Laura Van Gerven
Laura Van Gerven Zhen Qian
Zhen Qian Anastasiya Starovoyt
Anastasiya Starovoyt Mark Jorissen1
Mark Jorissen1 Jeroen Meulemans
Jeroen Meulemans Steven De Vleeschouwer
Steven De Vleeschouwer Julie Lambert
Julie Lambert Marie Bex
Marie Bex Vincent Vander Poorten
Vincent Vander Poorten