- School of Medicine, Texas Christian University/University of North Texas Health Sciences Center, Fort Worth, TX, United States
Background: The liver is the second most common site of breast cancer metastasis. Liver directed therapies including hepatic resection, radiofrequency ablation (RFA), transarterial chemo- and radioembolization (TACE/TARE), and hepatic arterial infusion (HAI) have been scarcely researched for breast cancer liver metastasis (BCLM). The purpose of this review is to present the known body of literature on these therapies for BCLM.
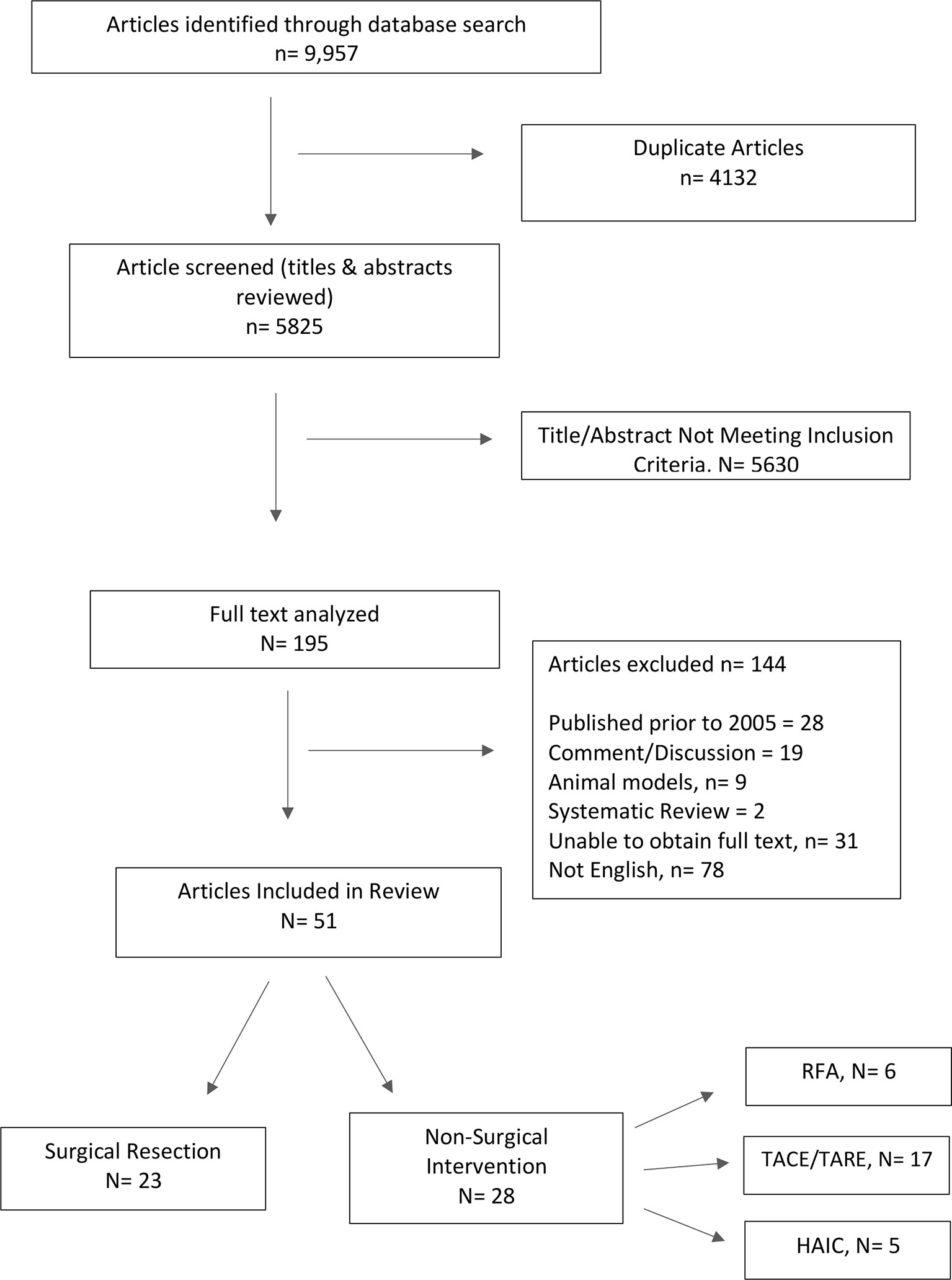
Methods: A systematic review was performed with pre-specified search terms using PubMed, MEDLINE, EMBASE, and Cochrane Review resulting in 9,957 results. After review of abstracts and application of exclusion criteria, 51 studies were included in this review.
Results: Hepatic resection afforded the longest median overall survival (mOS) and 5-year survival (45 mo, 41%) across 23 studies. RFA was presented in six studies with pooled mOS and 5-year survival of 38 mo and 11–33%. Disease burden and tumor size was lower amongst hepatic resection and RFA patients. TACE was presented in eight studies with pooled mOS and 1-year survival of 19.6 mo and 32–88.8%. TARE was presented in 10 studies with pooled mOS and 1-year survival of 11.5 mo and 34.5–86%. TACE and TARE populations were selected for chemo-resistant, unresectable disease. Hepatic arterial infusion was presented in five studies with pooled mOS of 11.3 months.
Conclusion: Although further studies are necessary to delineate appropriate usage of liver directed therapies in BCLM, small studies suggest hepatic resection and RFA, in well selected patients, can result in prolonged survival. Longitudinal studies with larger cohorts are warranted to further investigate the effectiveness of each modality.
Introduction
Among women worldwide, breast cancer is the most frequently diagnosed malignancy and second most common cause of cancer death (1). The liver is the primary site of spread in 15% of patients with metastatic breast cancer (2). Outcomes for breast cancer liver metastasis (BCLM) are grim with a median overall survival of 4–8 months, if untreated (2); 22 to 26 months following systemic chemotherapy alone with no reported 5-year survivors, and 37% 5-year survival after introduction of anti-HER2 therapy, although there is considerable variation of outcomes (3). Five-year survival rates for patients with BCLM with combination chemotherapy, immunotherapy, and hormone therapy are only 3.8–12% (median overall survival [mOS], 4–21 months) (4). Despite this, survival for patients with metastatic breast cancer has steadily improved over the last two decades (5) as evidenced by a single report of increased mOS from 17 months (1999) to 23.4 months (2008) (6).
BCLM management is an area of ongoing research. Advancement in liver directed therapies for the treatment of colorectal liver metastasis (CRLM) has open doors to the potential for improvement in survival for BCLM. The most direct intervention is hepatic resection, although the current oncologic dogma is that patients with BCLM are usually unresectable at diagnosis. Through successes with percutaneous and laparoscopic ablative technologies (radiofrequency and microwave) in CRLM, studies have emerged on its efficacy in treatment of BCLM. The introduction of trans-arterial chemoembolization (TACE) and radioembolization (TARE), the latter using yttrium-90 (Y-90), in the treatment unresectable liver metastasis has provided an option which focal therapies do not afford. Lastly, hepatic arterial infusion (HAI) has gained ground in CRLM. Although very few in number, this technique has been evaluated in management of BCLM.
The current body of literature on this topic is fragmented, with various small retrospective studies proving proof of concept for management of BCLM. There is no single published review examining the various methods of liver specific treatment options for BCLM. The purpose of this manuscript is to present the known body of literature on the topic of hepatic resection, RFA, TACE/TARE, and HAI for the treatment of BCLM. Using the available data, the authors will present an argument for use of multimodal therapies in highly selected cases of BCLM.
Materials and Methods
Registration, Search Strategy, and Study Selection
PROSPERO registration was obtained (CRD42020184009) for the systematic review after review of published works confirming lack of similar reviews in the literature.
Literature searches were performed by two independent reviewers (KW and KR) utilizing four electronic databases: PubMed, MEDLINE, EMBASE, and Cochrane Review from inception to May 2020. The following search terms were used: “liver metastasis,” “liver metastasis breast cancer,” “breast cancer liver metastases,” “breast cancer hepatic metastasis.” The outcome was a total of 9,957 results from the initial search.
Simple title review for the following key terms was performed: “hepatectomy,” “hepatic resection,” “Radiofrequency Ablation,” “Microwave Ablation,” “Transarterial chemoembolization,” “Transarterial radioembolization,” and “Hepatic Arterial Infusion.” All duplicates were removed resulting in a total of 195 studies.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) contains study of interventions in the treatment of BCLM; (2) publication as a full research article in English language, (3) related surgery/procedure detail and outcome indicators were reported.
One hundred thirty-nine studies were excluded (Figure 1). After exclusions, 51 studies were chosen for inclusion.
Data Extraction and Management
Fifty-one studies were reviewed in full text format. Data was extracted directly from the text of the article and collated into the following categories: study design, type of intervention, total patients, mean age, histology of primary tumor, hormone receptor status of primary tumor, adjuvant therapy, extent of hepatic resection (major vs minor), resection margin status, time from primary to liver metastasis, RECIST response, post-procedure complications, 30-day mortality, median overall survival, disease free survival, 1-, 3-, and 5-year survival. All relevant text, tables, and figures were reviewed for data extraction.
For safety and effectiveness outcomes, overall rates of each complication were calculated using the unweighted median figures given in each study. In studies where a mean was presented when a median value is most commonly used, the data will be provided for table completion, but notation is made to identify the difference in reporting method. Consensus discussion resolved all discrepancies between reviewers.
Quality and Methodological Assessment
There were no randomized studies found in the literature search. The non-randomized studies comparing two interventions were evaluated using the Risk of Bias in Non-randomized Studies–of Interventions (ROBINS-I) Assessment Tool (7). This tool was utilized to examine and measure seven specific bias domains: confounding, selection of participants, classification of interventions, deviation from intended interventions, missing data, measurement of outcomes, and selection of reported result. There were a total of five studies that met criteria: hepatic resection versus systemic chemotherapy (8, 9), laparoscopic RFA versus systemic chemotherapy (10), and TACE versus TARE (11), TACE versus systemic chemotherapy (12) (Table 1). All other included studies were identified as retrospective or, rarely, prospective and observational, and therefore offered no comparison of interventions. These studies are subject to the biases inherent to their study design.
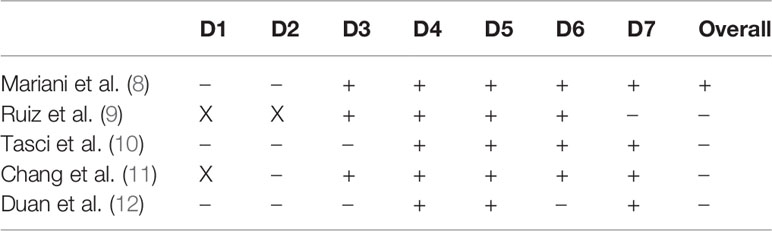
Table 1 ROBINS-I Assessment of Non-randomized studies: D1, Bias due to confounding; D2, Bias in selection of participants into the study; D3, Bias in classification of interventions; D4, Bias due to deviations from intended interventions; D5, Bias due to missing data; D6, Dias in measurement of outcomes; D7, Bias in selection of the reported result; X, high; –, some concern; +, low.
Statistical Analysis
All studies were case series comprised of retrospective studies (n = 45) and prospective studies (n = 7). The outcomes measures varied widely, precluding meta-analysis. Therefore, the results of this review are presented in descriptive terms only.
Results
Hepatic Resection
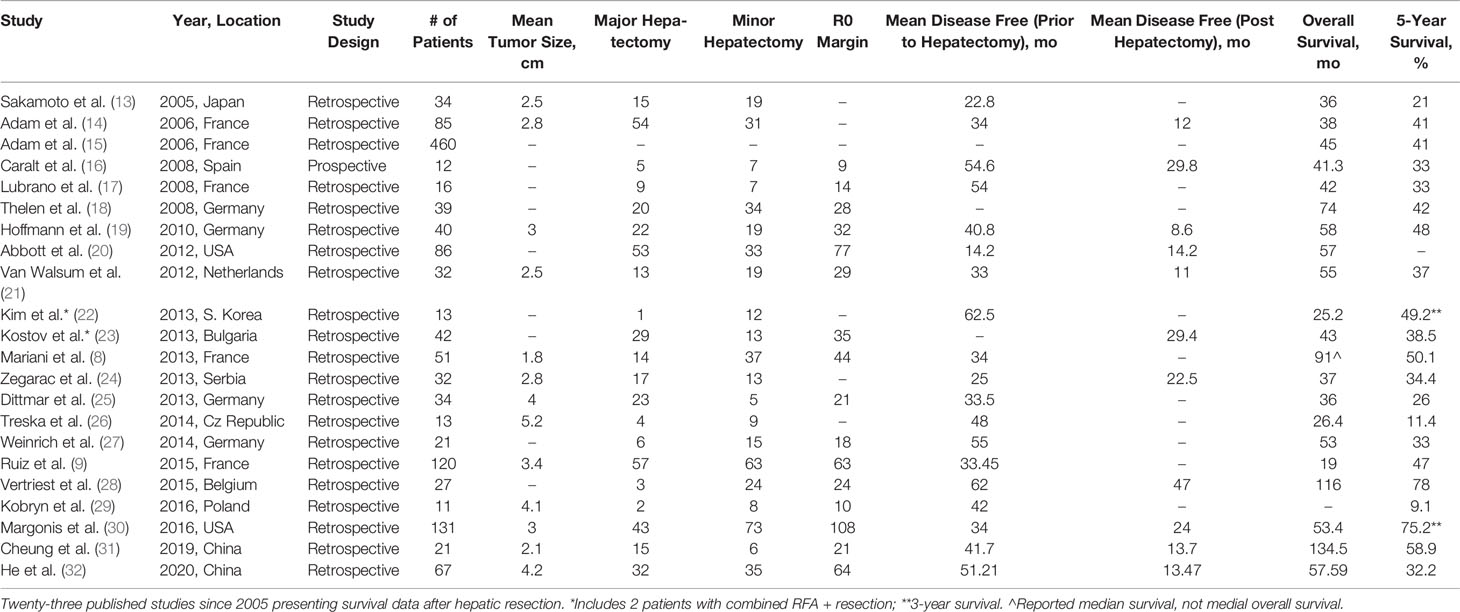
Hepatic resection is the most published intervention for management of BCLM (Table 2). Of 23 studies, 13 provided mean diameter of liver metastasis (8, 9, 14, 19, 21, 24–26, 29–32). The median tumor size was 3 cm (1.8–5.2 cm). Twenty-one studies provided detail of surgical resection (8, 9, 13, 16–32)—there were a total of 437 major hepatectomies (47.1%). Approximately 597 (79.6%) obtained R0 resection (8, 9, 16–21, 23, 25, 27–30, 32). Mortality rates were available for 19 studies (8, 9, 13–21, 23, 25, 27–32) which was consistently low across with a median of 0% (0–5.5%). It is worth noting, however, that these values are difficult to compare since mortality rates were not standardized. Some reported “perioperative” mortality, while others reported “in-hospital” mortality, or “30-day” mortality. Tumor histology was provided in 12 studies, totaling 424 patients with ductal carcinoma, 77 with lobular carcinoma, and 10 mixed or other histology (8, 15–17, 19–25, 28, 30, 32). No studies stratified outcomes based on primary tumor histology. Thirteen studies provided detail on hormone status of the primary tumor (8, 9, 14, 17, 20–25, 28, 30–32). Hormone receptor positive tumors totaled 431 in the included studies. Her2 positive tumors totaled 117 patients. Nineteen studies reported mean time between diagnosis of primary of liver metastasis (8, 9, 13, 15–17, 19–32). Six studies provided details of receptor status at time of hepatectomy (16, 17, 20, 25, 28, 30). One hundred eighty tumors were estrogen receptor positive, 125 tumors progesterone receptor positive, and 54 tumors Her2 positive. The median time to diagnosis of liver metastasis was 41.7 months. Twenty-two studies provided a mOS after hepatectomy (8, 9, 13–28, 30–32). The mOS was 45 months (range 19–134.5 months). Five-year survival data was provided for 22 studies (8, 9, 13–19, 21–32). Median 5-year survival was 41% (21–78%). Twenty studies reported univariate and multivariate analysis in an attempt to ascertain factors that affect overall survival of their cohorts (17, 18, 20, 21, 23–28, 30–32). Factors identified on univariate analysis that improved overall survival include: partial response to neoadjuvant chemotherapy, hormone receptor positivity, R0 resection, disease-free interval >2 years, HER2 positive primary tumor treated with trastuzumab, age >49.1 years at diagnosis of liver metastasis, age >45.2 years at mastectomy, Pringle maneuver during hepatectomy, size of liver metastasis <4 cm, negative portal lymph nodes, solitary metastasis, and uni-lobar metastasis distribution.
Radiofrequency Ablation (RFA)
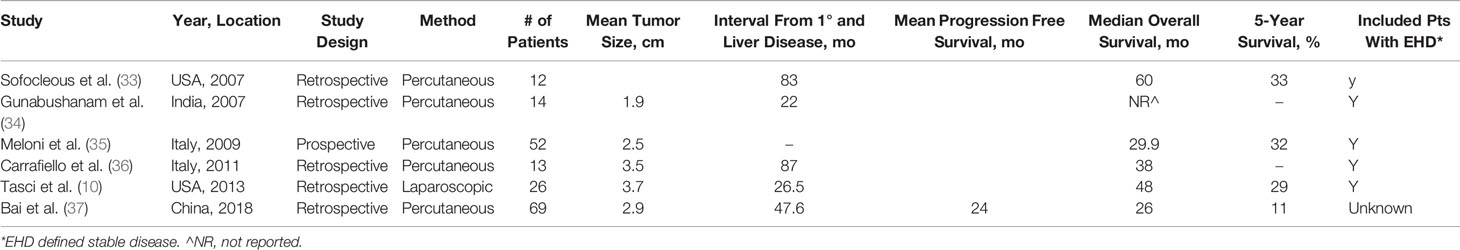
Percutaneous and laparoscopic RFA (perc-RFA and lap-RFA) for the treatment of BCLM was presented in six studies (Table 3). All studies included patients who received primary treatment with systemic chemotherapy, targeted therapies, or immunotherapy. Five studies presented results of perc-RFA (33–37) and one study of solely lap-RFA (10). There were a total of 186 patients in the pooled cohort. Three studies provided histology data on the primary tumor (10, 35, 37). There were 92 ductal carcinoma, 7 lobular carcinoma, and 27 mixed or other histology. Fifty-one tumors were hormone receptor positive and 32 tumors were Her2 positive. The mean tumor size was 2.9 cm (1.9–3.7 cm) as published in five studies (10, 34–37). Five studies provided median interval between diagnosis of primary and liver metastasis (10, 33, 34, 36, 37). The median interval was 47.6 months (22–87 months). Five studies provided mOS (10, 33, 35–37), which was 38 months (26–60 months). Four studies provided 5-year survival ranging from 11 to 33% (10, 33, 35, 37).
Transarterial Chemoembolization (TACE) and Transarterial Radioembolization (TARE)
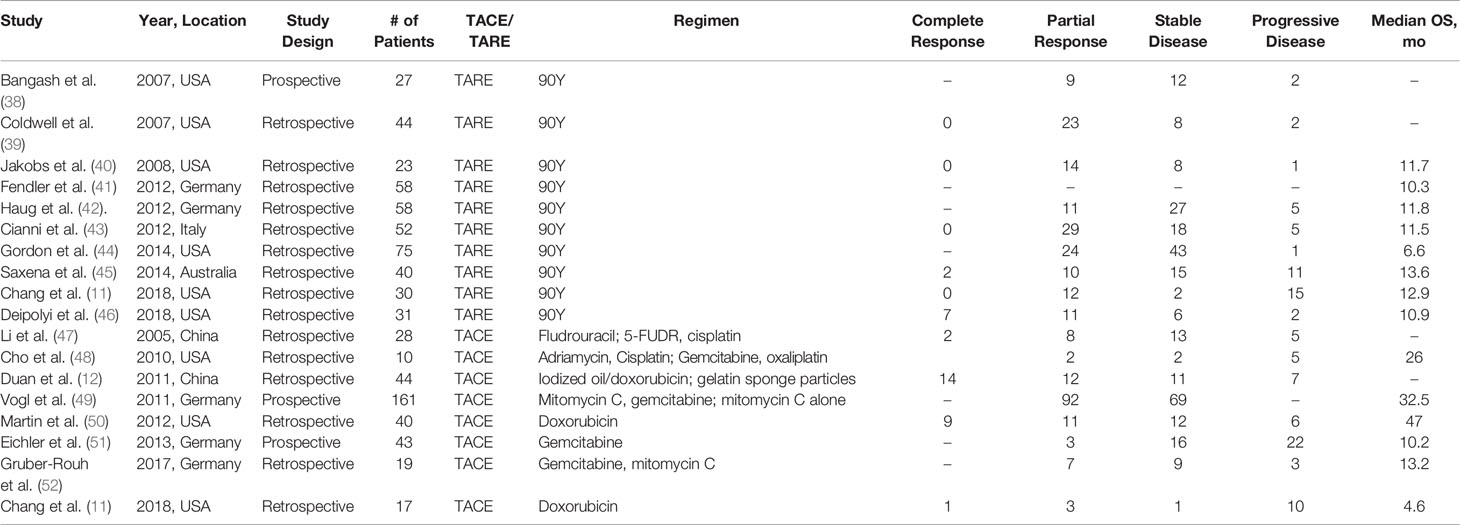
TACE has been described in eight studies, including one comparing TACE to TARE (Table 4). All studies provided RECIST response data (362 patients). There were an astonishing 26 complete responders (7.2%); 132 partial responders (38.1%); 133 patients (36.7%) with stable disease; and 58 patients (16%) had disease progression. mOS data from six studies (11, 48–52) showed a median of 19.6 months (4.6–32 months). One-year survival data was provided in four studies (12, 47, 49, 51) ranging from 32 to 88.8%. Cox regression analysis was performed in two studies (12, 47) revealing the following characteristics suggestive of improved outcomes on univariate analysis: N0 status, stage I or II disease, and Child-Pugh A at diagnosis of liver metastasis.
TARE was presented 10 published studies (Table 4). Y-90 is the most commonly used radioembolizing product. A total of nine studies provided RECIST response data (11, 38–40, 42–46). There were nine complete responders (2.3%) out of the 380 total patients. One hundred forty-three patients (37.6%) had partial response; 139 patients (36.5%) had stable disease; and 44 patients (11.5%) had disease progression. Eight studies provided a mOS (11, 40–46). The mOS of the pooled data was 11.5 months (6.6–13.6 months). Three studies provided 1-year survival data (39, 44, 45) which ranged from 34.5 to 86%. COX regression analysis was reported in four studies (11, 42, 45, 46). Through univariate analysis, SUVmax response, lower volume of hepatic parenchyma involvement, chemotherapy after TARE, and radiologic response to treatment were identified as factors improving overall survival. A single study identified presence of PI3K mutation as a factor increasing the likelihood of radiologic response after treatment.
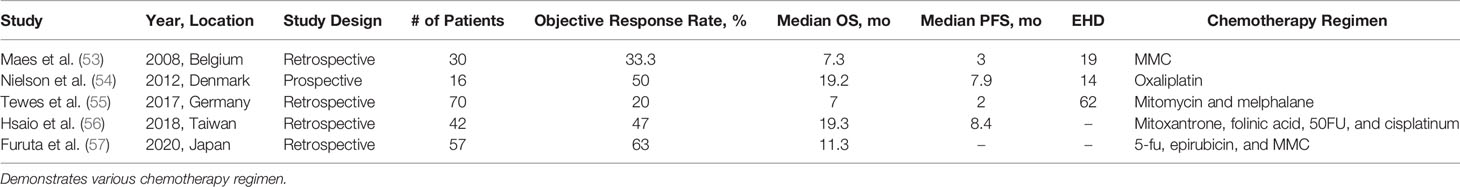
Hepatic Arterial Infusion (HAI)
Five studies were identified in which HAI was used for the treatment of BCLM (Table 5). Four studies reported RECIST response rates. There were two patients (1.2%) in the pooled cohort (N = 158) with complete response; 48 patients (30.4%) with partial response; 49 patients (31%) with stable disease; and 60 patients (38%) with progressive disease. Two studies presented univariate and multivariate analysis data (56, 57). The findings on univariate analysis suggest the following factors contribute to overall survival: ECOG status, hormone receptor status, maximum size of liver metastasis, and response to systemic chemotherapy. The median PFS of the pooled data was 5.45 months (2–8.4 months). mOS data was presented in all studies (53–57). The mOS for all pooled data was 11.3 months (7–19.3 months).
Discussion
Early Detection, Predictive Models, and Treatment of BCLM
Hepatic resection is a rare option for BCLM as the vast majority of patients have bilobar, unresectable disease at diagnosis. This difference in discovery of liver metastasis at a resectable state between CRLM and BCLM could stem from the lack of surveillance recommendations for BCLM. National Comprehensive Cancer Network (NCCN) guidelines recommend follow-up computed tomography (CT) of the abdomen and pelvis every 6–12 months for 5 years in stage II and higher colon cancer. Ninety-three percent of all CRLM were diagnosed within 3 years of the primary (58). Breast cancer, on the other hand, carries no recommendation for imaging surveillance for liver metastasis. Median time to liver metastasis from all included studies in this review is 41.35 months (22–83 months), comparable to that of CRLM.
As there are many patients with metastatic breast cancer without liver disease, there must be tumor specific factors that promote liver metastasis. Kimbung, et al. identified 17 liver metastasis-selective genes of prognostic relevance in early breast cancer which independently identify patients at higher risk within both luminal A and luminal B molecular subtype (59). This group also identified the novel role for claudin-2 as a prognostic biomarker for the likelihood of breast cancer recurrence, specifically liver recurrence (60). Lin et al. developed a nomogram for prediction of liver metastasis in breast cancer patients based on eight characteristics at diagnosis of primary disease: sex, histology, nodal involvement, histologic grade, ER/PR/Her2 status, and age at diagnosis (4). There has been no prospective external validation of this nomogram found within the literature.
This presents a major area of further research, as developing a reliable predictive model for development of liver metastasis based on characteristics at diagnosis of primary disease could pave the way for selective surveillance for liver metastatic disease. This would, in theory, result in earlier diagnosis of BCLM, which could, in turn, broader treatment options and potential survival advantage.
Hepatic Resection
Hepatic resection is the mainstay in definitive treatment for primary liver malignancies and metastatic disease to the liver. Although few metastatic diseases afford an aggressive approach, hepatic resection is gaining more traction, most notably in CRLM.
The longest mOS after hepatic resection is reported as 134.5 months as reported by Cheung et al. (31). Although a small study (21 patients), all underwent R0 resection. The cause for prolonged survival in this cohort compared to others is likely due to patient selection. The patient population was younger, with median age of 45 at diagnosis and selected for only those with oligometastatic liver disease. On univariate analysis, estrogen receptor positivity was found to be a protective factor for overall survival (OR = 0.159; 95% CI: 0.030–0.848; P = 0.067). Alternatively, triple negative status had a negative impact on overall survival (OR = 5.580; 95% CI: 1.210–25.731; P = 0.027). These findings are similar to those reported by Vertriest et al. (28), including a high R0 resection rate (88.9%), long disease-free interval prior to liver metastasis (62 months), long mOS after hepatectomy (116 months), and high 5-year survival (78%). Similar to that employed in the Cheung study, only those without macroscopic EHD, with disease-free interval >12 months, no more than three liver lesions, and response to systemic chemo- or hormone therapy were offered resection.
Of note, Sadot et al. published their experience with a combination of hepatic resection and ablation for management of BCLM (61). The retrospectively reviewed 167 patients with isolated BCLM comparing outcomes with those who received standard chemotherapy alone. There were 69 patients who underwent resection [42], percutaneous ablation [29], combination resection and ablation [2], and 98 patients who received standard chemotherapy. The hepatic tumor burden was less and the time from primary diagnosis to resection was longer in the surgical intervention group. The surgical group had a median recurrence free period 28.5 months and 10 patients were recurrence free at 5 years. They found no difference in mOS or 5-year survival between the medical and surgical cohorts. The obvious limitation of this study is its retrospective nature. Additionally, it is worth mentioning that the groups were significantly dissimilar in the most important ways. As mentioned by the authors, the tumor burden was lower in hepatic resection patients, but more importantly, the percutaneous ablation group were, by nature, not candidates for resection, whether due to location of metastasis or comorbidities. As this published study is an outlier, it begs further research to evaluate the role of hepatic resection and RFA in the treatment of this disease. Further studies are needed to determine if patients who are deemed resectable but go on to systemic chemotherapy only have similar survival to those who are resected.
Based on the evidence presented, it appears that younger patients with resectable tumors less than 3 cm in size, good functional status, long interval between diagnosis of the primary and liver metastasis, and no extra-hepatic disease (or stable bone-only EHD) have significantly improved survival after hepatic resection. Further studies are necessary to clearly delineate which subset of patients gain the greatest survival benefit from hepatic resection.
Radiofrequency Ablation (RFA)
RFA was used in combination with systemic chemotherapy, metastasectomy, hormonal therapy, or radioembolization. The largest study to date was published by Bai et al. study (37), which involved 69 patients over a 15-year period. The mOS from diagnosis was 36 months. Approximately half the cohort had up to four liver metastases. Additionally, approximately half the cohort had extrahepatic disease at time of RFA. Due to these factors, the mOS was expected to be lower than other studies retrieved. Nevertheless, the 5-year survival from time of diagnosis was 20.7%, which gives cause for further research.
Tasci et al. is the sole study reviewing their experience with lap-RFA (10). There were no reported complications. Furthermore, mOS and 5-year actuarial survival compared with standard chemotherapy alone were 48 months vs 9 months and 29 vs 0%, respectively. The mOS, 5-year actuarial survival, and clinical benefits of this treatment modality over percutaneous RFA seem to favor lap-RFA. Yet, there is a need for a study that directly compares lap-RFA and perc-RFA to determine if there is a statistically significant difference in outcomes.
As RFA is often used in the management of other malignant liver diseases when hepatic resection is not feasible and the burden of disease is small, the utility of this modality may follow similar guidelines in BCLM. Further longitudinal studies among larger patient populations are warranted to characterize the role of RFA as an adjunct treatment for a subset of patients with BCLM.
TACE/TARE
Within the past 15 years, several studies have confirmed the efficacy of TACE and TARE in the treatment of BCLM. Although few in number, these studies demonstrate similar or improved survival compared to systemic chemotherapy alone.
In published BCLM studies, TACE rarely resulted in complete radiographic response. Most notably, a study by Duan et al. (12). showed their center’s experience with 44 patients receiving TACE with doxorubicin. TACE resulted in 14 patients with complete radiologic response. This is an astonishing number of complete responders. However, there was no mOS or progression free survival data, which could be of value for those in the complete response group. The overall 1- and 3-year survival was 76.2 and 47.6% respectively, significantly higher than their control group (48.1 and 7.4%). From this body of data in the setting of unresectable BCLM, it is clear that the role of TACE in the treatment algorithm cannot be understated, however, more research is necessary to delineate which patients would most benefit.
Similar to TACE, TARE rarely produces complete radiographic response. Usage of Y-90 resin or glass microspheres allows for high dose radiation targeted to a specific area without radiation effects to the surrounding organs. Saxena et al. reported their experience with TARE in unresectable, chemo-resistant BCLM (45). The cohort consisted of 40 patients, of which six underwent hepatic resection prior to TARE. The median time to progression was 6.8 months, mOS was 13.6 months, and 1- and 2-year survival of 61 and 39%. This cohort underscores the impact TARE could make on overall survival in patients with chemo-resistant disease. Nevertheless, further studies are necessary to determine true survival data for patients with chemo-resistant disease.
The most obvious difficulty with interpreting this data is that for TACE, no two chemoembolization regimen were the same, precluding our ability to combine the data in a reproducible fashion. It appears, however, that there is some benefit of these therapies in patients with good ECOG status and unresectable, chemo-resistant disease. This is an area where further studies are necessary, in light of the fact that there are a considerable number of patients with complete and/or partial response.
Hepatic Arterial Infusion
Similar to the previously discussed methods, HAI for the treatment of BCLM remains in its infancy. The earliest study reviewing HAI for BCLM by Arai et al. was published in 1994 with 56 patients (62). The chemotherapy regimen consisted of 5-FU, Adriamycin, and mitomycin C. Complete response was observed in 11 patients (19%), partial response in 33 (58.9%), stable disease in 6 (10.7%), and progressive disease in 4 (7.1%).
HAI and TACE share similar challenges, most notably determining appropriate chemotherapy regimen. The most intensive therapy regimen was reported by Furuta et al., which included 5-FU, epirubicin, and mitomycin C (57). This study also had the most robust objective response rate. Further research is required to determine the optimal regimen for treatment of BCLM, however it appears HAI could be of value in the management of unresectable BCLM as either an adjunct therapy or to convert to resectability.
Limitations
As stated previously, there were no randomized controlled trials found in the literature on the topic of hepatic resection, RFA, TACE/TARE, or HAI for the treatment of BCLM. Therefore, all provided studies are retrospective in nature, placing considerable risk of bias in the presented studies. Selection bias is of great concern across the published studies as patients undergoing hepatic resection, for example, were highly specific: often those without extrahepatic disease (an uncommon phenomenon) or those with stable extrahepatic bone-only disease. Additionally, there is publication bias, as there was only one study published which suggested non-superiority of hepatic resection and RFA compared with chemotherapy alone (61). This may be partially related to the current dogma in treatment of BCLM, and therefore, treatment is largely systemic chemotherapy alone. Also, it cannot be ignored that this may be because others have attempted hepatic resection, RFA, TACE/TARE, and HAI with poor results which were not published. Lastly, within the literature provided, there can be lead time bias based on the surveillance methods employed by various oncology departments. This could result in the semblance of prolonged survival after intervention, when none exists if a standardized surveillance method was used. In light of the various limitations of these studies, further controlled trials and well-designed studies are necessary to answer this very important question. Despite the biases presented, however, some broad conclusions can be made in regards to which patients and under which circumstances each of these treatment methods could be studied further.
Conclusion
In conclusion, interventional management of BCLM is in the earliest stages of development, particularly in determining the indications for hepatic resection, RFA, TACE/TARE, and HAI. Several studies confirm the safety and efficacy of each intervention, however there are many questions that require further investigation to determine the appropriate usage of each intervention as well as optimal chemotherapeutics for TACE and HAI. Hepatic resection may improve survival in highly selected patients with BCLM who are younger at diagnosis, have smaller tumor size, and no extra-hepatic disease. RFA requires further study, both laparoscopically and percutaneously for this disease, as it is unclear the benefit of this intervention either in combination with hepatic resection or alone. TACE may improve survival in patients with unresectable, chemo-resistant BCLM, however, there is no consistent chemoembolization regimen. TARE, on the other hand, does not show improved survival consistently across published studies. Lastly, HAI requires further research to determine appropriate selection for usage and chemotherapy regimen which is varied in the literature. This is a vast area of further research and the authors hope this review will encourage further study on these topics.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. National Comprehensive Cancer Network. Breast Cancer (Version 5.2020) . Available at: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdft (Accessed August 25, 2020).
2. Chen X, Zheng Z, Chen L, Zheng H. MAPK, NFKB, and VEGF signaling pathways regulate breast cancer liver metastasis. Oncotarget (2017) 8:101452–60. doi: 10.18632/oncotarget.20843
3. Golse N, Adam R. Liver Metastases From Breast Cancer: What Role for Surgery? Indications Results Clin Breast Cancer (2017) 17:256–65. doi: 10.1016/j.clbc.2016.12.012
4. Lin Z, Yan S, Zhang J, Pan Q. A Nomogram for Distinction and Potential Prediction of Liver Metastasis in Breast Cancer Patients. J Cancer (2018) 9(12):2098–106. doi: 10.7150/jca.24445
5. Arciero C, Guo Y, Jiang R, Behera M, O’Regan R, Peng L. ER+/HER2+ Breast Cancer Has Different Metastatic Patterns and Better Survival Than ER-/HER2- Breast Cancer. Clin Breast Cancer (2019) 19(4):236–45. doi: 10.1016/j.clbc.2019.02.001
6. Ruiterkamp J, Ernst M, Munck L, Loo M, Bastiaannet E, Poll-Franse L. Improved survival of patients with primary distant metastatic breast cancer in the period of 1995-2008. A nationwide population-based study in the Netherlands. Breast Cancer Res Treat (2011) 128:495–503. doi: 10.1007/s10549-011-1349-x
7. Sterne JAC, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (2016) 355. doi: 10.1136/bmj.i4919
8. Mariani P, Servois V, Rycke Y, Bennett S, Feron J, Almubarak M. Liver metastases from breast cancer: Surgical resection or not? A case-matched control study in highly selected patients.” EJSO (2013) 39:1377–83. doi: 10.1016/j.ejso.2013.09.021
9. Ruiz A, van Hillegersberg R, Siesling S, Castro-Benitez C, Sebagh M, Wicherts D. Surgical resection versus systemic therapy for breast cacner liver metastases: Results of a European case matched comparison. Euro J Cancer (2018) 95:1–10. doi: 10.1016/j.ejca.2018.02.024
10. Tasci Y, Aksoy E, Taskin H, Aliyev S, Moore H, Agcaoglu O. A comparison of laparoscopic radiofrequency ablation versus systemic therapy alone in the treatment of breast cancer metastasis to the liver. HPB (2013) 15:789–93. doi: 10.1111/hpb.12133
11. Chang J, Charalel R, Noda C, Ramaswamy R, Kim S, Darcy M. Liver-dominant Breast Cancer Metastasis: A Comparative Outcomes Study of Chemoembolization versus Radioembolization. Anticancer Res (2018) 38:3063–68. doi: 10.21873/anticanres.12563
12. Duan XF, Dong NN, Zhang T, Li Q. Treatment outcome of patients with liver-only metastases from breast cancer after mastectomy: a retrospective analysis. J Cancer Res Clin Oncol (2011) 137:1363–70. doi: 10.1007/s00432-011-1008-y
13. Sakamoto Y, Yamamoto J, Yoshimoto M, Kasumi F, Kosuge T, Kokudo N. Hepatic Resection for Metastatic Breast Center: Prognostic Analysis of 34 Patients. World J Surg (2005) 29:524–7. doi: 10.1007/s00268-004-7688-6
14. Adam R, Aloia T, Krissat J, Bralet M, Paule B, Giacchetti S. Is Liver Resection Justified for Patients with Hepatic Metastases From Breast Cancer. Ann Surg (2006) 244:897–908. doi: 10.1097/01.sla.0000246847.02058.1b
15. Adam R, Chiche L, Aloia T, Elias D, Salmon R, Rivoire M. Hepatic Resection for Noncolorectal Nonendocrine Liver Metastases: Analysis of 1452 Patients and Development of a Prognostic Model. Ann Surg (2006) 244:524–35 doi: 10.1097/01.sla.0000239036.46827.5f
16. Caralt M, Bilbao I, Cortes J, Escartin A, Lazaro J, Dopazo C. Hepatic Resection for Liver Metastases as Part of the “Oncosurgical” Treatment of Metastatic Breast Cancer. Ann Surg Oncol (2008) 15:2804–10. doi: 10.1245/s10434-008-0072-2
17. Lubrano J, Roman H, Tarrab S, Resch B, Marpeau L, Scotte M. Liver Resection for Breast Cancer Metastasis: Does it Improve Survival? Surg Today (2008) 38:293–99. doi: 10.1007/s00595-007-3617-2
18. Thelen A, Benckert C, Jonas S, Lopez-Hannihen E, Sehouli J, Neumann U. Liver Resection for Metastases from Breast Cancer. J Surg Onc (2008) 97:25–9. doi: 10.1002/jso.20911
19. Hoffmann K, Franz C, Hinz U, Schirmacher P, Herfarth C, Eichbaum M. Liver Resection for Multimodal Treatment of Breast Cancer Metastases: Identification of Prognostic Factors. Ann Surg Oncol (2010) 17:1546–54. doi: 10.1245/s10434-010-0931-5
20. Abbott D, Brouquet A, Mittendorf EA, Andreou A, Meric-Bernstam F, Valero V. Resection of liver metastases from breast cancer: estrogen receptor status and response to chemotherapy prior to surgery define outcome. Surgery (2012) 151:710–16. doi: 10.1016/j.surg.2011.12.017
21. Van Walsum G, de Ridder J, Verhoef C, Bosscha K, Van Gulik T, Hesselink E. Resection of liver metastases in patients with breast cancer: Survival and prognostic factors. EJSO (2012) 38:910–7. doi: 10.1016/j.ejso.2012.04.015
22. Kim J, Park J, Lee S, Kim J, Jeong J, Yoon D. Does Liver Resection Provide Long-Term Survival Benefits for Breast Cancer Patients with Liver Metastasis? A Single Hospital Experience. Yonsei Med J (2014) 55:558–62. doi: 10.3349/ymj.2014.55.3.558
23. Kostov D, Kobakov G, Yankov D. Prognostic Factors Related to Surgical Outcome of Liver Metastases of Breast Cancer. J Breast Cancer (2013) 16:184–92. doi: 10.4048/jbc.2013.16.2.184
24. Zegarac M, Nikolic S, Gavrilovic D, Jevric M, Kolarevic D, Nikolic-Tomasevic Z. Prognostic factors for longer disease free survival and overall survival after surgical resection of isolated liver metastasis from breast cancer. JBUON (2013) 18:859–65.
25. Dittmar Y, Altendorf-Hofmann A, Schule S, Ardelt M, Dirsch O, Runnebaum I. Liver resection in selected patients with metastatic breast cancer: a single-centre analysis and review of literature. J Cancer Res Clin Oncol (2013) 139:1317–25. doi: 10.1007/s00432-013-1440-2
26. Treska V, Cerna M, Liska V, Treskova I, Narsanska A, Bruha J. Surgery for Breast Cancer Liver Metastases – Factors Determining Results. Anticancer Res (2014) 34:1281–86.
27. Weinrich M, Weib C, Schuld J, Rau B. Liver Resections of Isolated Liver Metastasis in Breast Cancer: Results and Possible Prognostic Factors. HPB Surg (2014) 2014:893829. doi: 10.1155/2014/893829
28. Vertriest C, Berardi G, Tomassini F, Broucke R, Depypere H, Cocquyt V. Resection of Single Metachronous Liver Metastases from Breast Cancer Stage I-II Yield Excellent Overall and Disease-Free Survival. Single Center Experience and Review of the Literature. Dig Surg (2015) 32:52–9. doi: 10.1159/000375132
29. Kobryn E, Kobryn K, Wroblewski T, Kobryn K, Pietrzak R, Rykowski P. Is there a rationale for aggressive breast cacner liver metastases resection in Polish female patients? Analysis of overall survival following hepatic resection at a single centre in Poland. Ann Agr Environ Med (2016) 23:683–87. doi: 10.5604/12321966.1226866
30. Margonis GA, Buettner S, Sasaki K, Kim Y, Ratti F, Russolillo N. The role of liver-directed surgery in patients with hepatic metastasis from primary breast cancer: a multi-institutional analysis. HPB (2016) 18:700–05. doi: 10.1016/j.hpb.2016.05.014
31. Cheung T, Chok K, Chan A, Tsang S, Dai W, Yau T. Survival analysis of breast cancer liver metastasis treated by hepatectomy: A propensity score analysis for Chinese women in Hong Kong. Hepatobiliary Pancreatic Dis Int (2019) 18:452–57. doi: 10.1016/j.hbpd.2019.08.001
32. He X, Zhang Q, Feng Y, Li Z, Pan Q, Zhao Y. Resection of liver metastases from breast cancer: a multicentre analysis. Clin Trans Oncol (2020) 22:512–21. doi: 10.1007/s12094-019-02155-2
33. Sofocleous C, Nascimento R, Gonen M, Theodoulou M, Covey A, Brody L. Radiofrequency Ablation in the Management of Liver Metastases from Breast Cancer. AJR (2007) 189:883–9. doi: 10.2214/AJR.07.2198
34. Gunabushanam G, Sharma S, Thulkar S, Srivastava D, Rath G, Julka P. Radiofrequency Ablation of Liver Metastases from Breast Cancer: Results in 14 Patients. J Vasc Interv Radiol (2007) 18:67–72. doi: 10.1016/j.jvir.2006.10.014
35. Meloni M, Andreano A, Laeseke P, Livraghi T, Sironi S, Lee F. Breast Cancer Liver Metastases: US-guided Percutaneous Radiofrequency Ablation-Intermediate and Long-term Survival Rates. Radiology (2009) 253:861–9. doi: 10.1148/radiol.2533081968
36. Carrafiello G, Fonatana F, Cotta E, Petulla M, Brunese L, Mangini M. Ultrasound-guided thermal radiofrequency ablation (RFA) as an adjunct to systemic chemotherapy for breast cancer liver metastases. Radiol Med (2011) 116:1059–66. doi: 10.1007/s11547-011-0697-2
37. Bai X, Yang W, Zhang Z, Jiang A, Wu W, Lee J. Long-term outcomes and prognostic analysis of percutraneous radiofrequency ablation in liver metastasis from breast cancer. Int J Hyperthermia (2019) 35(1):183–93. doi: 10.1080/02656736.2018.1488279
38. Bangash A, Atassi B, Kaklamani V, Rhee T, Yu M, Lewandowski R. 90Y Radioembolization of Metastatic Breast Cancer to the Liver: Toxicity, Imaging Response, Survival. J Vasc Interv Radiol (2007) 18:621–28. doi: 10.1016/j.jvir.2007.02.019
39. Coldwell D, Kennedy A, Nutting C. Use of Yttrium-90 Microspheres in the Treatment of unresectable Hepatic Metastases from Breast Cancer. Int J Radiat Oncol Biol Phys (2007) 69:800–4. doi: 10.1016/j.ijrobp.2007.03.056
40. Jakobs TF, Hoffmann RT, Fischer T, Stemmler H, Tatsch K, Fougere C. Radioembolization in Patients with Hepatic Metastases from Breast Cancer. J Vasc Interv Radiol (2008) 19:683–90. doi: 10.1016/j.jvir.2008.01.009
41. Fendler WP, Lechner H, Todica A, Paprottka K, Paprottka P, Jakobs T. Safety, Efficiacy, and Prognostic Factors After Radioembolization of Hepatic Metastases from Breast Cancer: A Large Single Center Experience in 81 Patients. J Nucl Med (2016) 57:517–23. doi: 10.2967/jnumed.115.165050
42. Haug A, Donfack B, Trumm C, Zech C, Michl M, Laubender R. 18F-FDG PET/CT Predicts Survival After Radioembolization of Hepatic Metastases from Breast Cancer. J Nucl Med (2012) 53:371–77. doi: 10.2967/jnumed.111.096230
43. Cianni R, Pelle G, Notarianni E, Saltarelli A, Rabuffi P, Bagni O. Radioembolisation with 90Y-labelled resin microspheres in the treatment of liver metastasis from breast cancer. Eur Radiol (2013) 23:182–9. doi: 10.1007/s00330-012-2556-5
44. Gordon AC, Gradishar WJ, Kaklamani VG, Thuluvath A, Ryu R, Sato K. Yttrium-90 radioembolization stops progression of targeted breast cancer liver metastasis after failed chemotherapy: 90Y Radioembolization for BCLM. J Vasc Interv Radiol (2014) 25:1523–32. doi: 10.1016/j.jvir.2014.07.007
45. Saxena A, Kapoor J, Meteling B, Morris D, Bester L. Yttrium-90 Radioembolization for Unresectable, Chemoresistant Breast Cancer Liver Metastases: A Large Single-Center Experience of 40 Patients. Ann Surg Oncol (2014) 21:1296–303. doi: 10.1245/s10434-013-3436-1
46. Deipolyi AR, Riedl C, Bromberg J, Chandarlapaty S, Klebanoff C, Sofocleous C. Association of PI3K Pathway Mutations with Early PET/CT Imaging Response after Radiormbolization for Breast Cancer Liver Metastases: Results of a Single Center Retrospective Pilot Study. J Vasc Interv Radiol (2018) 29:1226–35. doi: 10.1016/j.jvir.2018.04.018
47. Li XP, Meng ZQ, Guo WJ, Li J. Treatment for liver metastases from breast cancer: Results and prognostic factors. World J Gastroenterol (2005) 11:3782–87. doi: 10.3748/wjg.v11.i24.3782
48. Cho SW, Kitisin K, Buck D, Steel J, Brufsky A, Gillespie R. Transcatheter Arterial Chemoembolization Is a Feasible Palliative Locoregional Therapy for Breast Cancer Liver Metastases. Int J Surg Onc (2010) 2010:251621. doi: 10.1155/2010/251621
49. Vogl T, Naguib N, Nour-Eldin N, Mack M, Zangos S, Abskharon J. Repeated Chemoembolization Followed by Laser-Induced Thermotherapy for Liver Metastasis of Breast Cancer. AJR (2011) 196(1):W66–72. doi: 10.2214/AJR.09.3836
50. Martin RCG, Robbins K, Fages JF, Romero F, Rustein L, Tomalty D. Optimal outcomes for liver-dominant metastatic breast cancer with transarterial chemoembolization with drug-eluting beads loaded with doxorubicin. Breast Cancer Res Treat (2012) 132:753–63. doi: 10.1007/s10549-011-1926-z
51. Eichler K, Jakobi S, Gruber-Rough T, Hammerstingl R, Vogl T, Zangos S. Transarterial chemoembolization (TACE) with gemcitabine: Phase II study in patients with liver metastases of breast cancer. Euro J Rad (2013) 82:e816–22. doi: 10.1016/j.ejrad.2013.08.046
52. Gruber-Rouh T, Lagenback M, Naguib NN, Nour-Eldin N, Vogl T, Zangos S. Transarterial chemoperfusion for the treatment of liver metastases of breast cancer and colorectal cancer: Clinical results in palliative care patients. World J Clin Oncol (2017) 8:343–50. doi: 10.5306/wjco.v8.i4.343
53. Maes T, Wildiers H, Heye S, Demey W, Maleux G, Neven P. Intra-hepatic Mitomycin C bolus infusion in the treatment of extensive liver metastases of breast cancer. Breast Cancer Res Treat (2008) 110:135–42. doi: 10.1007/s10549-007-9707-4
54. Nielsen DL, Norgaard H, Vestermark L, Pfeiffer P, Jensen B, Nelausen K. Intrahepatic and systemic therapy with oxaliplatin combined with capecitabine in patients with hepatic metastases from breast cancer. Breast (2012) 21:556–61. doi: 10.1016/j.breast.2012.05.003
55. Tewes M, Peis MW, Bogner S, Theysohn J, Reinboldt M, Schuler M. Hepatic arterial infusion chemotherapy for extensive liver metastases of breast cancer: efficacy, safety, and prognostic parameters. J Cancer Res Clin Oncol (2017) 143:2131–41. doi: 10.1007/s00432-017-2462-y
56. Hsiao J, Chang H, Tseng Y, Chiang C, Chen I, Chen Y. Hepatic Arterial Infusion Chemotherapy Is a Feasible Treatment Option for Breast Cancer with Liver-predominant Metastatic Disease. In Vivo (2018) 32:1635–41. doi: 10.21873/invivo.11425
57. Furuta M, Watanabe J, Aramaki T, Notsu A, Yasui H. Hepatic Arterial Infusion Chemotherapy for Metastatic Breast Cancer Patients with Resistance to Standard Systemic Chemotherapies. In Vivo (2020) 34:275–82. doi: 10.21873/invivo.11771
58. Engstrand J, Nilsson H, Stromberg C, Jonas E, Freedman J. Colorectal cancer liver metastases – a population-based study on incidence, management and survival. BMC Cancer (2018) 18:78. doi: 10.1186/s12885-017-3925-x
59. Kimbung S, Johansson I, Danielsson A, Veerla S, Brage S, Stolt M. Transcriptional Profiling of Breast Cancer Metastases Identified Liver Metastasis-Selective Genes Assocated with Adverse Outcome in Luminal A Primary Breast Cancer. Clin Cancer Res (2016) 22:146–57. doi: 10.1158/1078-0432.CCR-15-0487
60. Kimbung S, Kovacs A, Bendahl P, Malmstrom P, Ferno M, Hatschek T. Claudin-2 is an independent negative prognostic factor in breast cancer and specifically predicts early liver recurrences. Mol Oncol (2014) 8:119–28. doi: 10.1016/j.molonc.2013.10.002
61. Sadot E, Lee SY, Sofocleous CT, Solomon S, Gonen M, Kingham T. Hepatic resection or ablation for isolated breast cancer liver metastasis: A case-control study with comparison to medically treated patients. Ann Surg (2016) 264:147–54. doi: 10.1097/SLA.0000000000001371
Keywords: breast cancer liver metastasis, hepatic resection, radiofrequency ablation, transarterial chemoembolization, transarterial radioembolization, liver directed therapies
Citation: Rivera K, Jeyarajah DR and Washington K (2021) Hepatectomy, RFA, and Other Liver Directed Therapies for Treatment of Breast Cancer Liver Metastasis: A Systematic Review. Front. Oncol. 11:643383. doi: 10.3389/fonc.2021.643383
Received: 18 December 2020; Accepted: 25 January 2021;
Published: 26 March 2021.
Edited by:
Damiano Caputo, Campus Bio-Medico University, ItalyReviewed by:
Matteo De Pastena, University of Verona, ItalyGiuseppe Bianco, Catholic University of the Sacred Heart, Italy
Copyright © 2021 Rivera, Jeyarajah and Washington. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kimberly Washington, ay5sLndhc2hpbmd0b25AdGN1LmVkdQ==
 Kevin Rivera
Kevin Rivera Kimberly Washington
Kimberly Washington