- 1Department of Endocrine and Breast Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Chongqing Key Laboratory of Molecular Oncology and Epigenetics, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 3Department of Ophthalmology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 4College of Foreign Languages, Chongqing Medical University, Chongqing, China
Background and Objectives: Currently, the location of primary tumor was an independent prognostic factor of breast cancer. Tumors in the central and nipple portion (TCNP) had poor prognosis compared to other peripheral quadrants. The breast-conserving therapy (BCT) is becoming increasingly common worldwide in breast cancer operations. However, whether the availability of BCT was performed for TCNP remained a matter of debate. We sought to investigate whether BCT was suitable for TCNP with respect to survival outcomes, compared with mastectomy therapy.
Methods: Utilizing the Surveillance, Epidemiology, and End Results (SEER) database, we obtained TCNP breast cancer patients diagnosed during the period of 2010–2015. One-to-one (1:1) propensity score matching (PSM) was applied to construct a matched sample consisting of pairs of BCT and mastectomy groups. Univariate and multivariate Cox proportional hazard models were applied to estimate the factors associated with breast cancer-specific survival (BCSS) and overall survival (OS). Survival analysis was performed with the Kaplan–Meier method.
Results: In the overall cohort, a total of 9,900 patients were enrolled. We found that patients with BCT showed significantly better BCSS (log-rank, p < 0.001) and OS (log-rank, p < 0.001) than the mastectomy group before PSM. The same finding was also shown in 5,820 patients after PSM. Additionally, none of the subgroups, including age, sex, race, histological grade, AJCC stage, and molecular subtype undergoing mastectomy therapy, had better BCSS than BCT.
Conclusions: Our study was the first research to show that BCT exhibited superior prognosis in the cohort of TCNP from SEER databases than mastectomy therapy. This finding could provide a cue for treatment strategies for suitable TCNP patients, especially those with a strong willingness to conserve their breasts.
Introduction
As the most common cancer in American women, breast cancer remains second to lung cancer in mortality rate, accounting for about 15% of all cancers this year (1). Although the death rate of female breast cancer has dropped from its peak by 40% since 1989, the decline rate slowed down in recent years (1). Additionally, some early-stage patients still have worse survival in clinical studies (2, 3). A series of studies revealed that age, primary tumor size, tumor location, positive axillary lymph nodes, histological grade, molecular subtype, and BRCA mutation were closely correlated with the prognosis of breast cancer (4–10).
Of note, the location of primary tumor, which has been reported by a wide variety of investigations, is an independent prognostic factor (9, 11–13). A similar finding was demonstrated in subsets of patients with colon, gastric, and lung cancer (14–16). Importantly, tumors in the central and nipple portion (TCNP) had poorer prognosis and more aggressive clinicopathological characteristics than peripheral quadrants (9). With consideration of recurrence rate and survival time, previous surgeons mostly made non-conservative operations for tumor in the central location (17, 18). Recently, with widespread popularization of oncologic concepts and increasingly mature plastic technology, the breast-conserving therapy (BCT), breast-conserving surgery with adjuvant whole breast radiotherapy (RT), is becoming gradually common worldwide (19, 20). Indeed, BCT showed more benefits for patients relative to mastectomy therapy, including advantages such as reducing operative time, diminishing psychological burden, improving cosmetic outcomes, and limiting side effects (21). However, the availability and safety of BCT for TCNP remained a matter of debate (22). Additionally, large-sample studies concerning the comparison of prognosis between BCT and mastectomy therapy for TCNP of breast cancer are scarce.
Therefore, based on the Surveillance, Epidemiology, and End Results (SEER) database, we aimed to explore whether BCT is more suitable for TCNP of breast cancer compared with mastectomy therapy.
Materials and Methods
Study Design and Data Sources
Using SEER 18 Regs Custom Data (with additional treatment fields), we abstracted Breast Cancer cases from the SEER data released in November 2018. A total of 11,872 patients were originally identified according to the following criteria: tumor in the nipple (C50.0) and central portion (C50.1), the first and only malignant primary tumor, age at diagnosis 20 years, year of diagnosis (2010–2015), race (white, black, Asian/Pacific Islander, and American Indian/Alaska Native), site recode (breast), histological grade (I to IV), AJCC stages (I–IV), breast molecular subtype (Luminal A, Luminal B, HER2 enriched, and Triple Negative), and record of radiation therapy. Criteria for exclusion of the patients were as follows: unknown unilateral tumor (n = 2), unknown primary surgery type (n = 860), unknown T stage (n = 49), unknown N stage (n = 50), and treatment of breast-conserving surgery without radiation therapy (n = 1,011) (Figure 1). A total of 9,900 breast cancer patients were included in this retrospective cohort study.
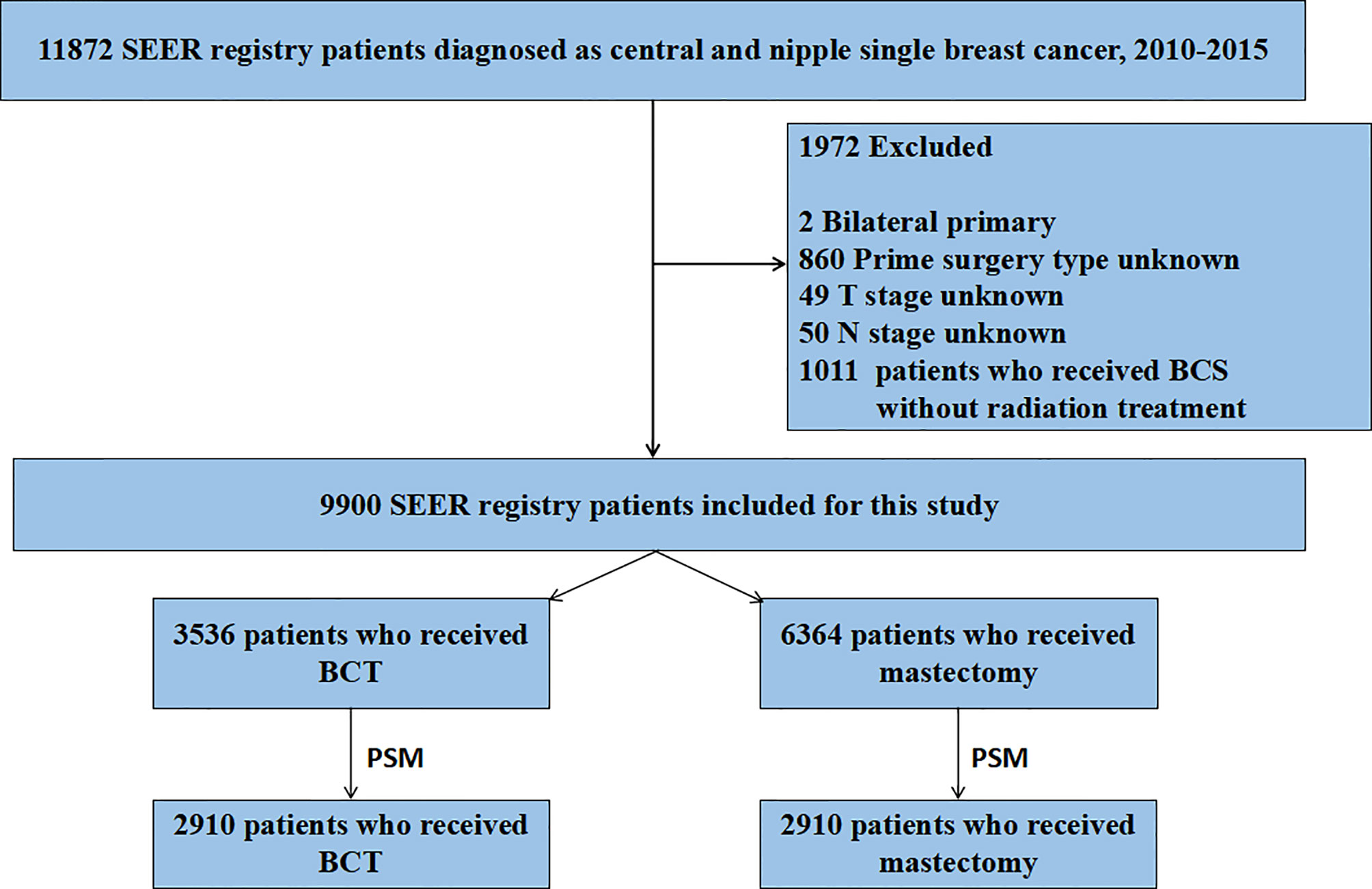
Figure 1 Flow chart for screening patients. SEER, Surveillance Epidemiology, and End Results; PSM, propensity score matching. BCT, breast-conserving therapy.
The primary end points of our study were breast cancer-specific survival (BCSS) and overall survival (OS). BCSS was calculated from the date of diagnosis to the date of death from breast cancer or the last follow-up, and OS was calculated from the date of diagnosis to the date of death for any cause or the last follow-up.
Statistical Analysis
Statistical analysis was performed by virtue of the SPSS version 21.0 software package (IBM SPSS Statistics, Chicago, IL, USA). The following variables were analyzed: age, sex, race, laterality, histological grade, AJCC stage, tumor size, LN status, distant metastasis, breast molecular subtype, chemotherapy, radiotherapy, and treatment (BCT vs. mastectomy). The differences in clinicopathological characteristics between the two groups (BCT and mastectomy therapy) were examined using the Chi-square test. To reduce the obvious differences in baseline covariates and inherent selection bias, we conducted a one-to-one propensity score matching (PSM) analysis (23) between the two treatment groups, including these following variables: age, sex, race, laterality, histological grade, AJCC stage, breast molecular subtype, and chemotherapy. In this PSM, we set the match tolerance as 0.02. The BCSS and OS survival curves were estimated with the Kaplan–Meier method, and survival differences were assessed by the log-rank test. The Cox proportional hazards regression model was conducted on univariate and multivariate analyses of BCSS and OS. Univariate Cox regression analysis was performed for each prognostic variable, and those variables with p < 0.05 were included in the multivariate Cox model analysis. All reported p values are two-sided, and differences at p < 0.05 were considered statistically significant.
Results
Demographics and Clinicopathological Findings
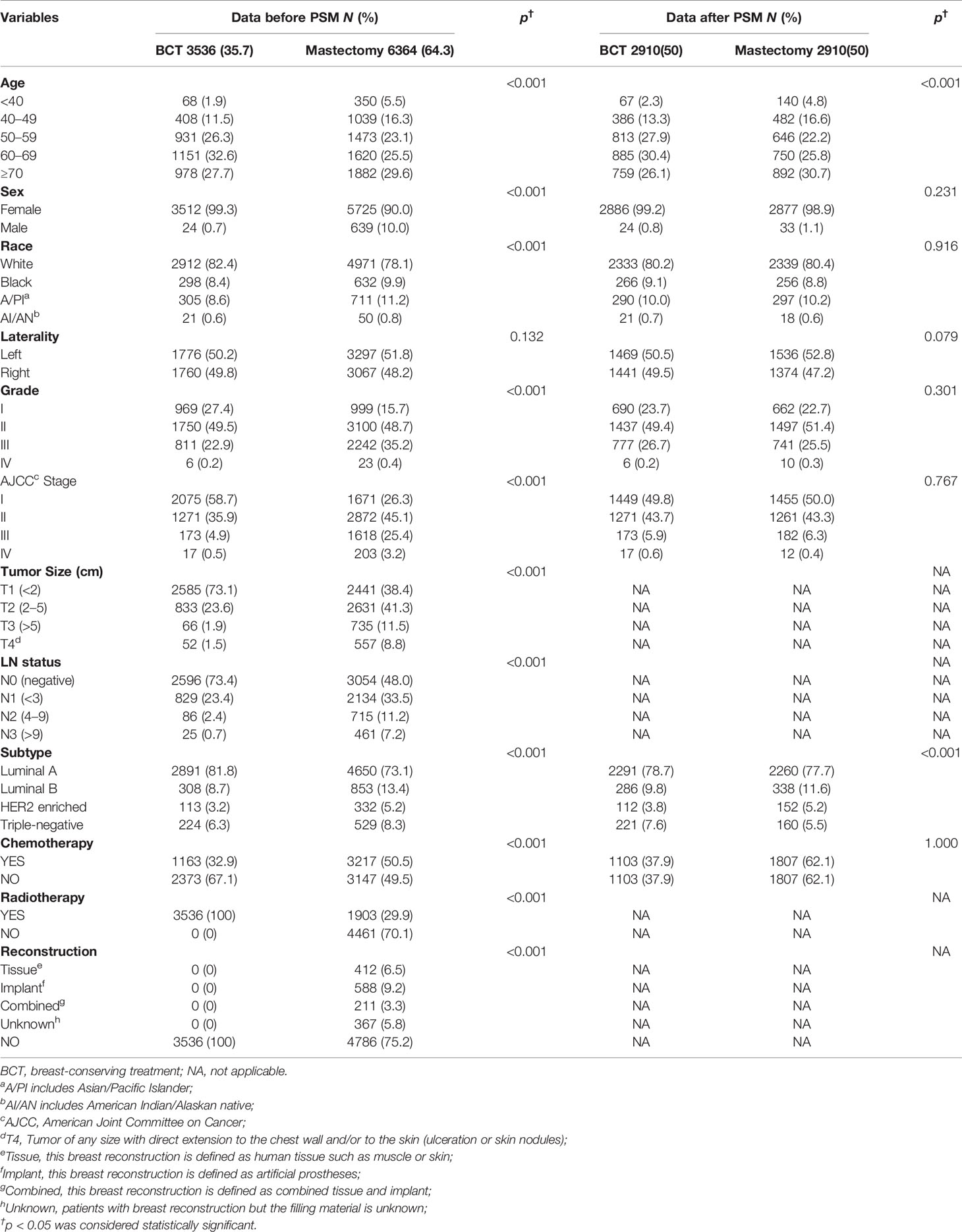
This retrospective cohort study included 9,900 patients with breast cancer (9,237 women and 663 men). According to the type of surgery and receipt of RT, all of these patients were divided into two groups: the BCT group (n = 3,536, 35.7%) and the mastectomy therapy group (n = 6,364, 64.3%). The demographic and clinicopathological characteristics of patients before or after PSM are listed in Table 1. Except for laterality, notable differences were detected in relevant variables between the two groups before PSM. The majority of patients in both the BCT group and the mastectomy group were aged older than 50 years (86.6% and 78.2%). We observed that the BCT group, compared with the mastectomy group, exhibited higher rates of female patients (99.3% vs. 90.0%, p < 0.001), white race (82.4% vs. 78.1%, p < 0.001), histological grade I and II (27.4% vs. 15.7% and 49.5% vs. 48.7%, respectively, p < 0.001), AJCC stage I (58.7% vs. 26.3%), and Luminal A (81.8% vs. 73.1%, p < 0.001). Besides, the proportion of patients with tumors smaller than 2 cm and negative lymph node (LN) metastasis was also higher in the BCT group than in the mastectomy group (73.1% vs. 38.4% and 73.4% vs. 48.0%, respectively, p < 0.001). Moreover, the BCT group presented a lower percentage of chemotherapy than the mastectomy group (32.9% vs. 50.5%, p < 0.001). Last but not least, a total of 1,578 breast reconstruction cases of 9,900 patients were in the mastectomy group (Table 1).
After 1:1 matching, 2,910 patients in the BCT group were matched as compared with 2,910 patients in the mastectomy group. Except for age and molecular subtype, there were no notable differences between the two treatment groups after PSM.
Survival Analysis for OS and BCSS
Subsequently, we investigated the outcome of BCSS and OS in patients with TCNP between the BCT group and the mastectomy group, in the use of the Kaplan–Meier method. Before PSM, patients who received BCT showed significantly better BCSS and OS (p < 0.001) than those undergoing mastectomy therapy (Figures 2A, B). Similarly, we still found the notable difference between the two groups after PSM (p < 0.001; Figures 2C, D). We next assessed the prognostic values of the BCT group compared with the mastectomy group in various subgroups, including age, sex, race, histological grade, AJCC stage, and molecular subtype (Figures 3–8, Supplementary Figures 1–6). Interestingly, these results showed that the BCT had poorer survival in none of all these clinicopathological parameters subgroups. Compared with the patients who are undergoing mastectomy therapy, BCT was a better prognostic factor for BCSS in patients aged between 50 and 59 or those aged 70 and above (p = 0.016 and p < 0.001, respectively; Figures 3C, E), female patients (p < 0.001; Figure 4A), those of white race (p < 0.001; Figure 5A), and those with histological grade II (moderate differentiation) and III (poor differentiation) (p = 0.001 and p < 0.001, respectively; Figures 6B, C). Except for Stage IV, patients with Stage I–III showed better survival with BCT (p = 0.011, p = 0.001, and p < 0.001, respectively; Figures 7A–C). Similar outcomes were present in the molecular subtype of patients including Luminal A, Luminal B, and Triple Negative (p < 0.001, p = 0.002, and p = 0.018, respectively; Figures 8A, B, D). Besides, as for OS, BCT group still had better prognosis than the mastectomy group in most of the above subgroups (Supplementary Figures 1–6).
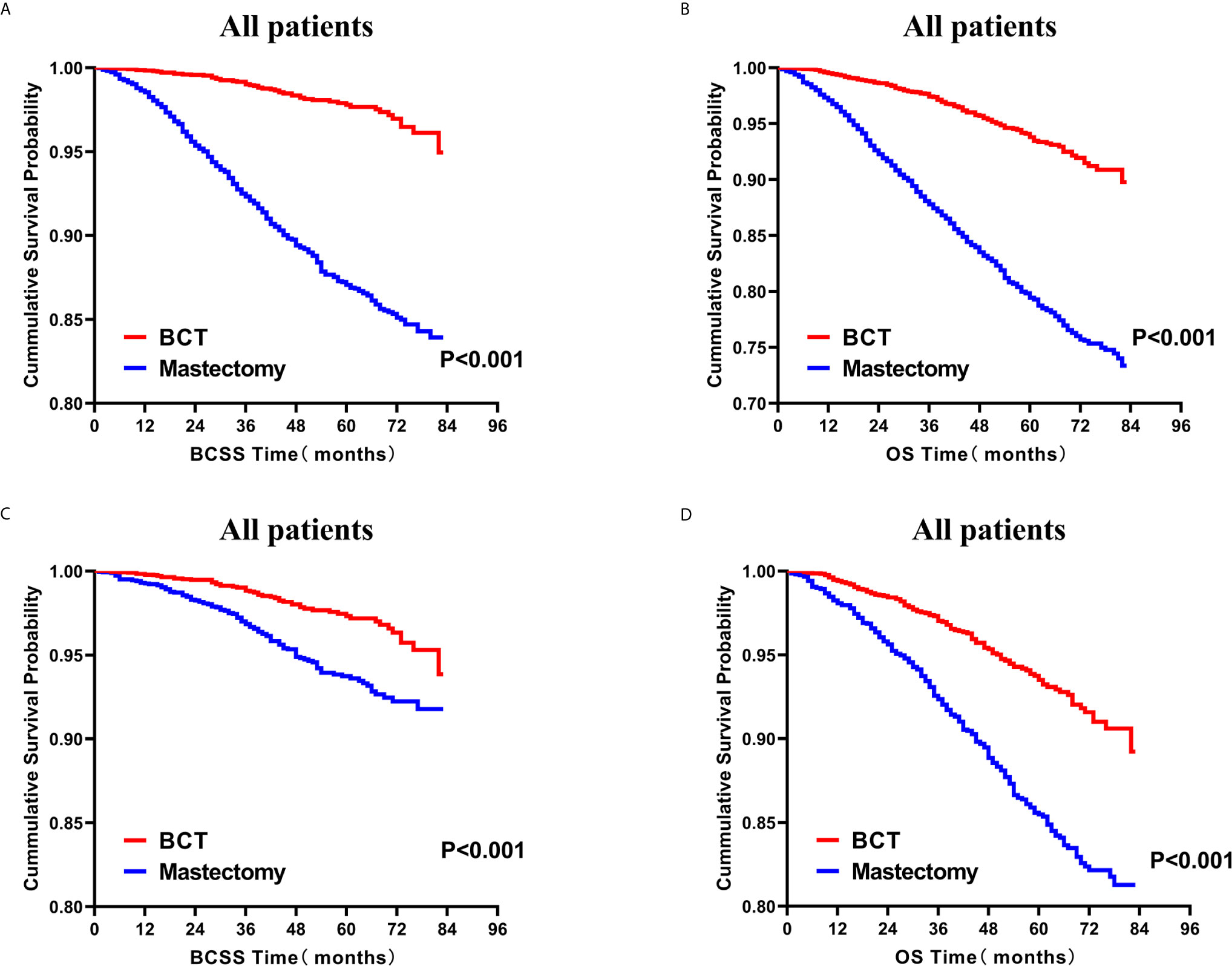
Figure 2 Kaplan–Meier curves for BCSS (A) and OS (B) by treatment type for all patients before PSM and BCSS (C) and OS (D) by treatment type for all patients after PSM; BCT versus mastectomy. BCSS, breast cancer specific survival; OS, overall survival; PSM, propensity score matching; BCT, breast-conserving therapy.

Figure 3 Kaplan–Meier curves for BCSS by treatment type for all patients, stratified by age at diagnosis: (A) Age < 40 years. (B) Age of 40–49 years. (C) Age of 50–59 years. (D) Age of 60–69 years. (E) Age ≥ 70 years. BCSS, breast cancer specific survival; BCT, breast-conserving therapy.
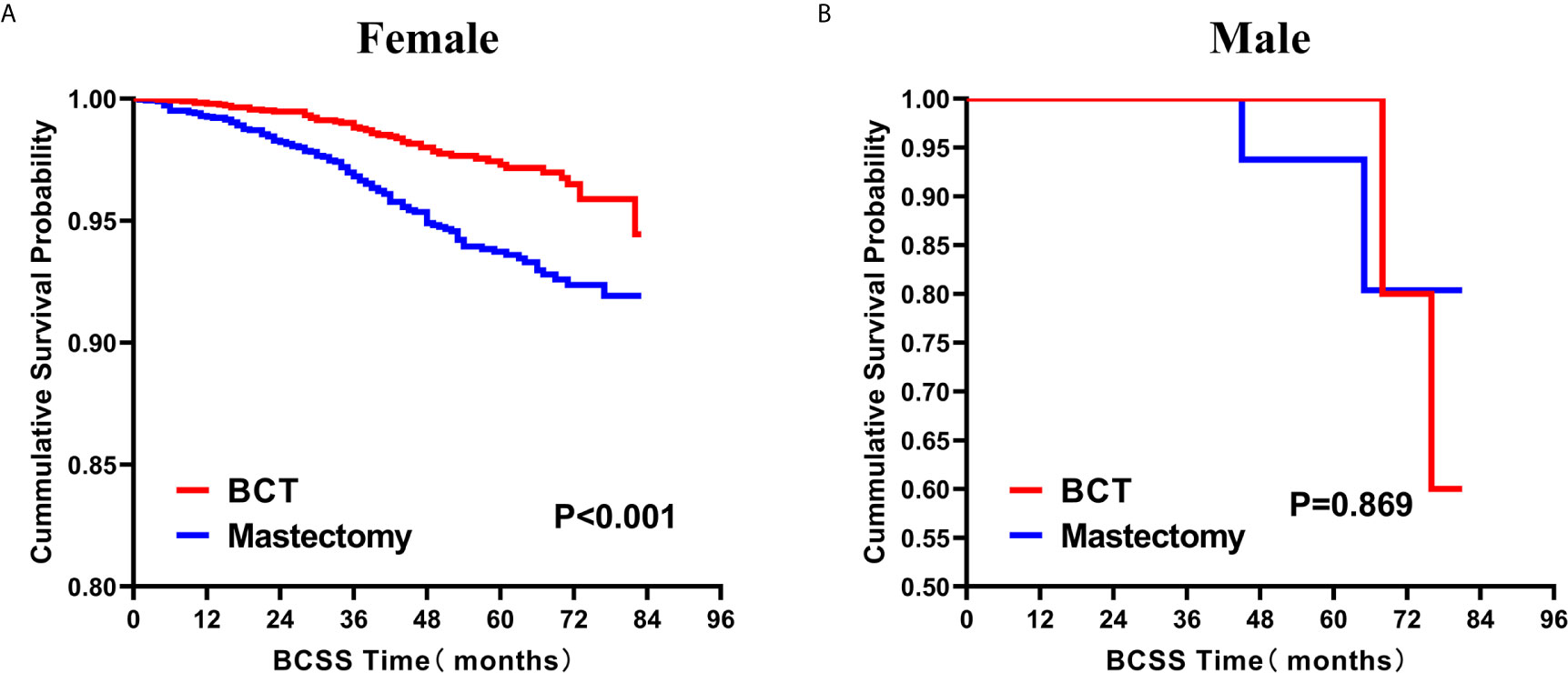
Figure 4 Kaplan–Meier Curves for BCSS by treatment type for all patients, stratified by sex: (A) Female. (B) Male. BCSS, breast cancer specific survival; BCT, breast-conserving therapy.
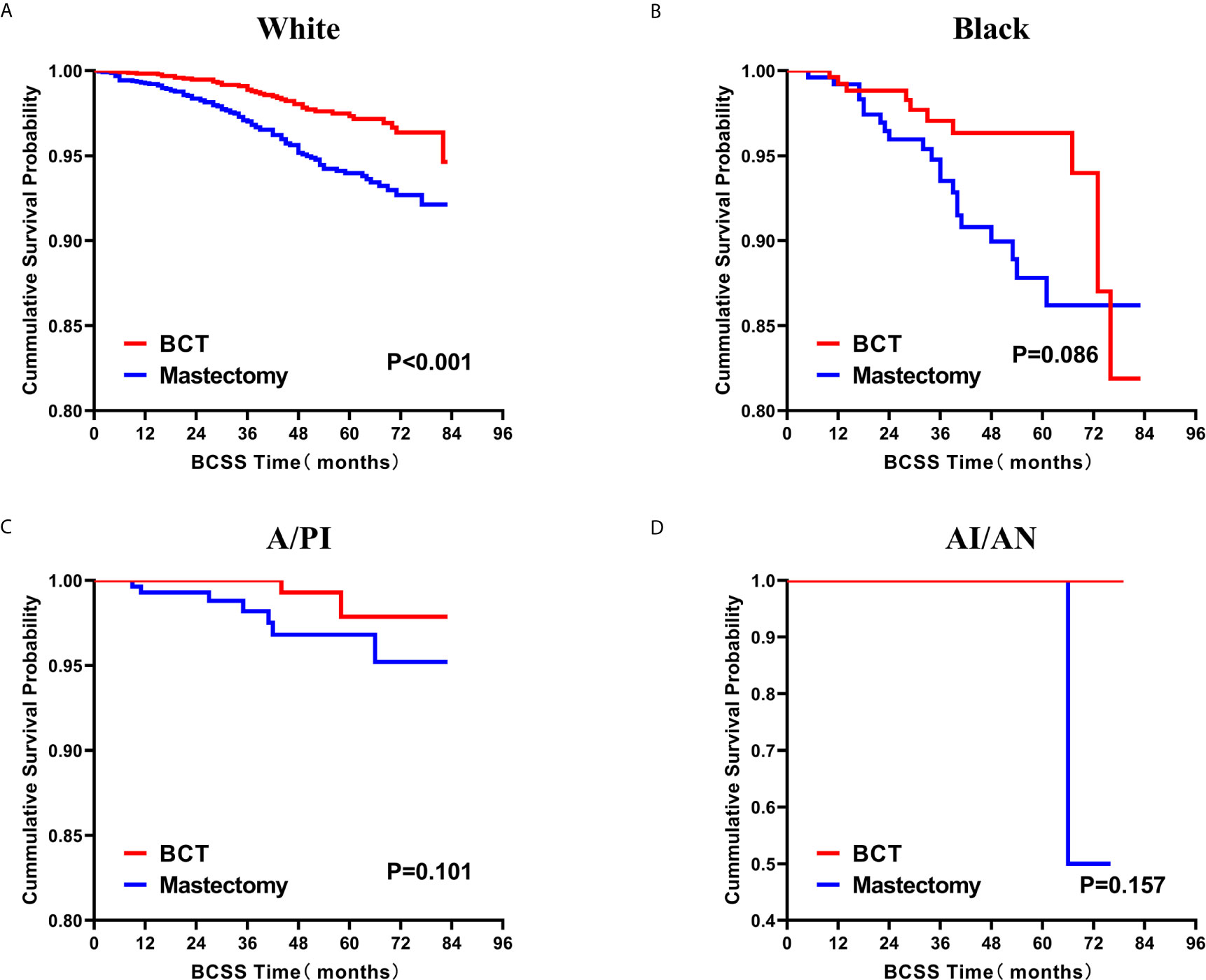
Figure 5 Kaplan–Meier curves for BCSS by treatment type for all patients, stratified by race: (A) White. (B) Black. (C)A/PI. (D) AI/AN. BCSS, breast cancer specific survival; BCT, breast-conserving therapy; A/PI, Asian/Pacific Islander; AI/AN, American Indian/Alaskan native.
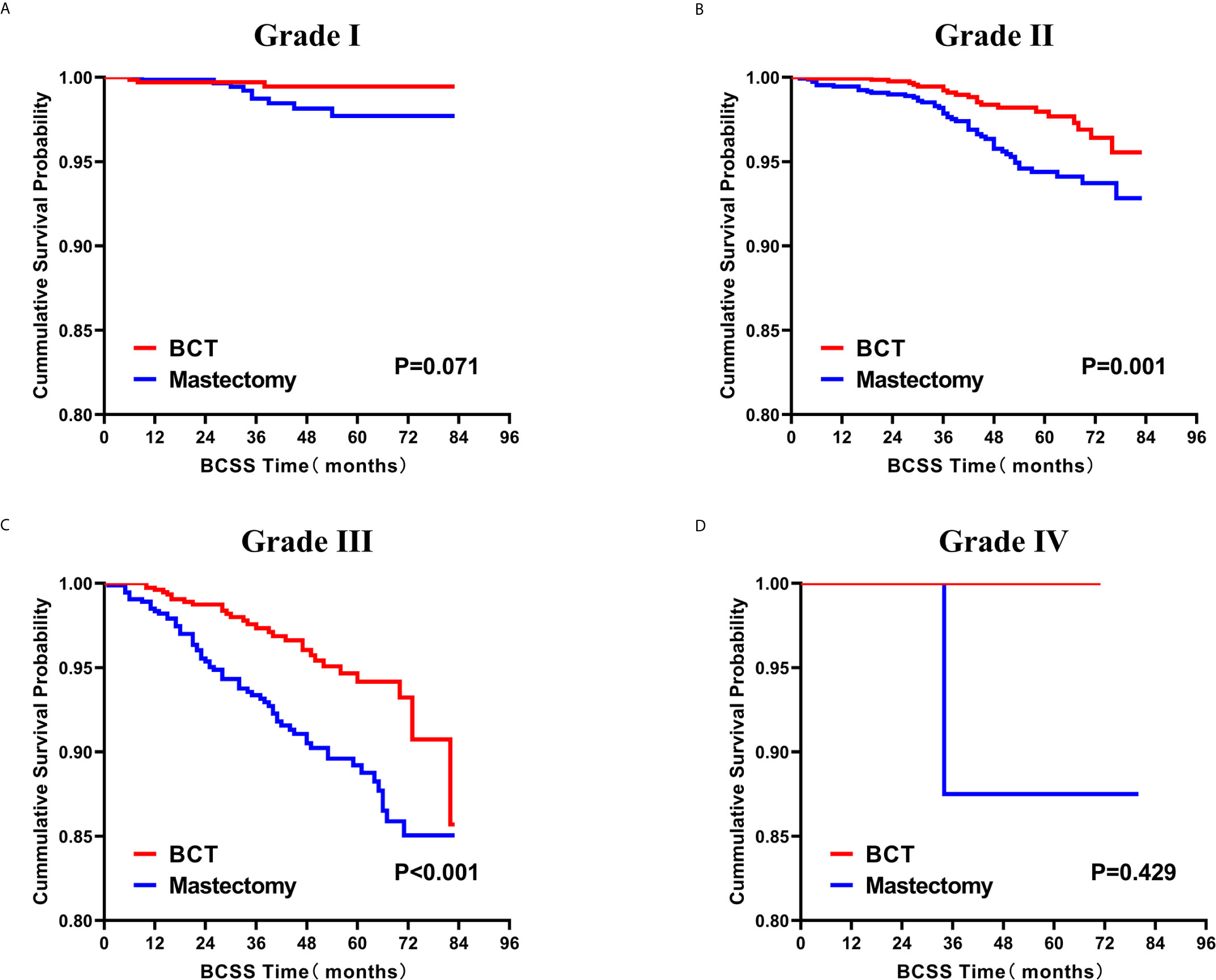
Figure 6 Kaplan–Meier curves for BCSS by treatment type for all patients, stratified by histological grade: (A) Grade I. (B) Grade II. (C) Grade III. (D) Grade IV. BCSS, breast cancer specific survival; BCT, breast-conserving therapy.
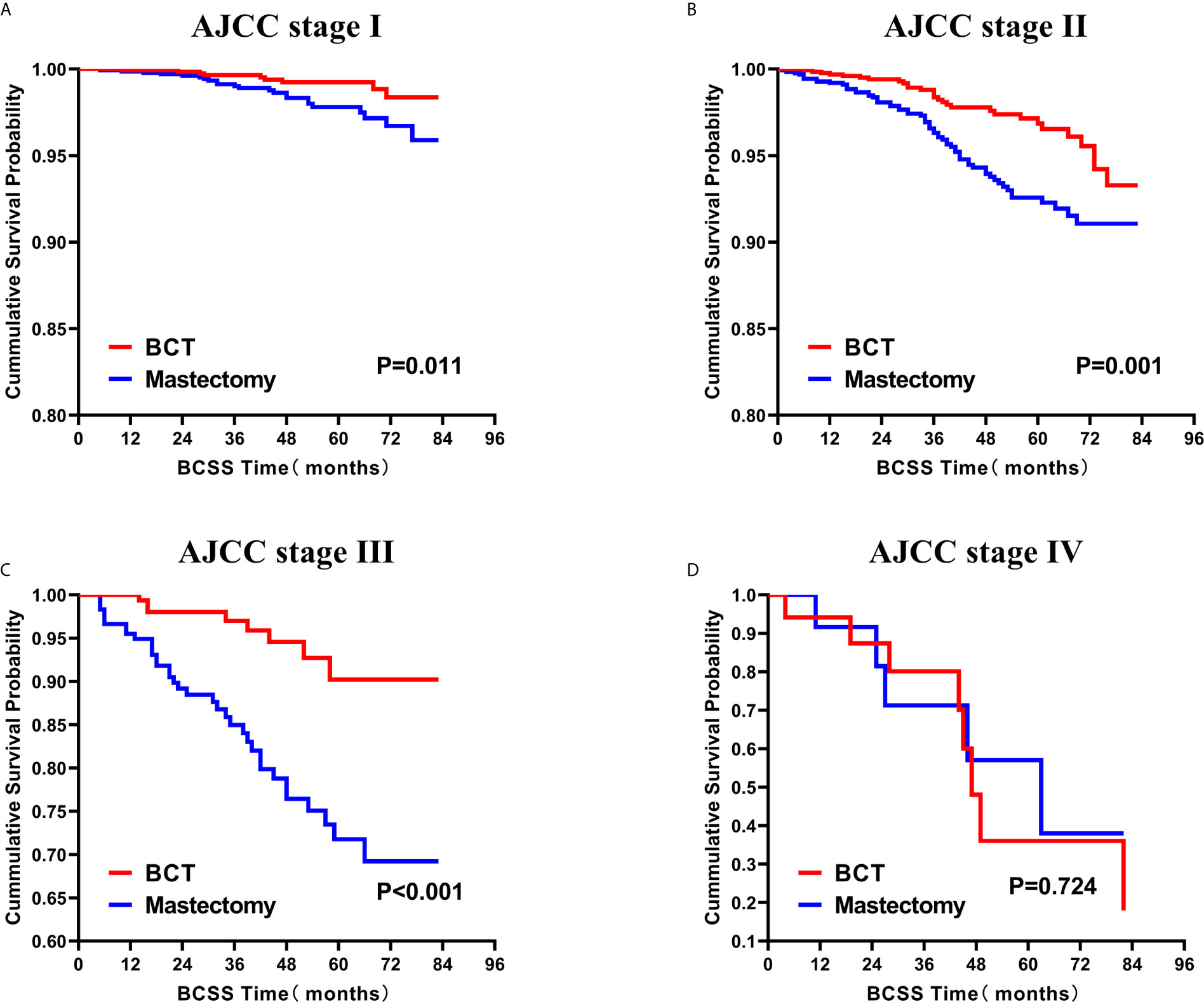
Figure 7 Kaplan–Meier Curves for BCSS by treatment type for all patients, stratified by AJCC stage: (A) AJCC stage I. (B) AJCC stage II. (C) AJCC stage III. (D) AJCC stage IV. BCSS, breast cancer specific survival; BCT, breast-conserving therapy.
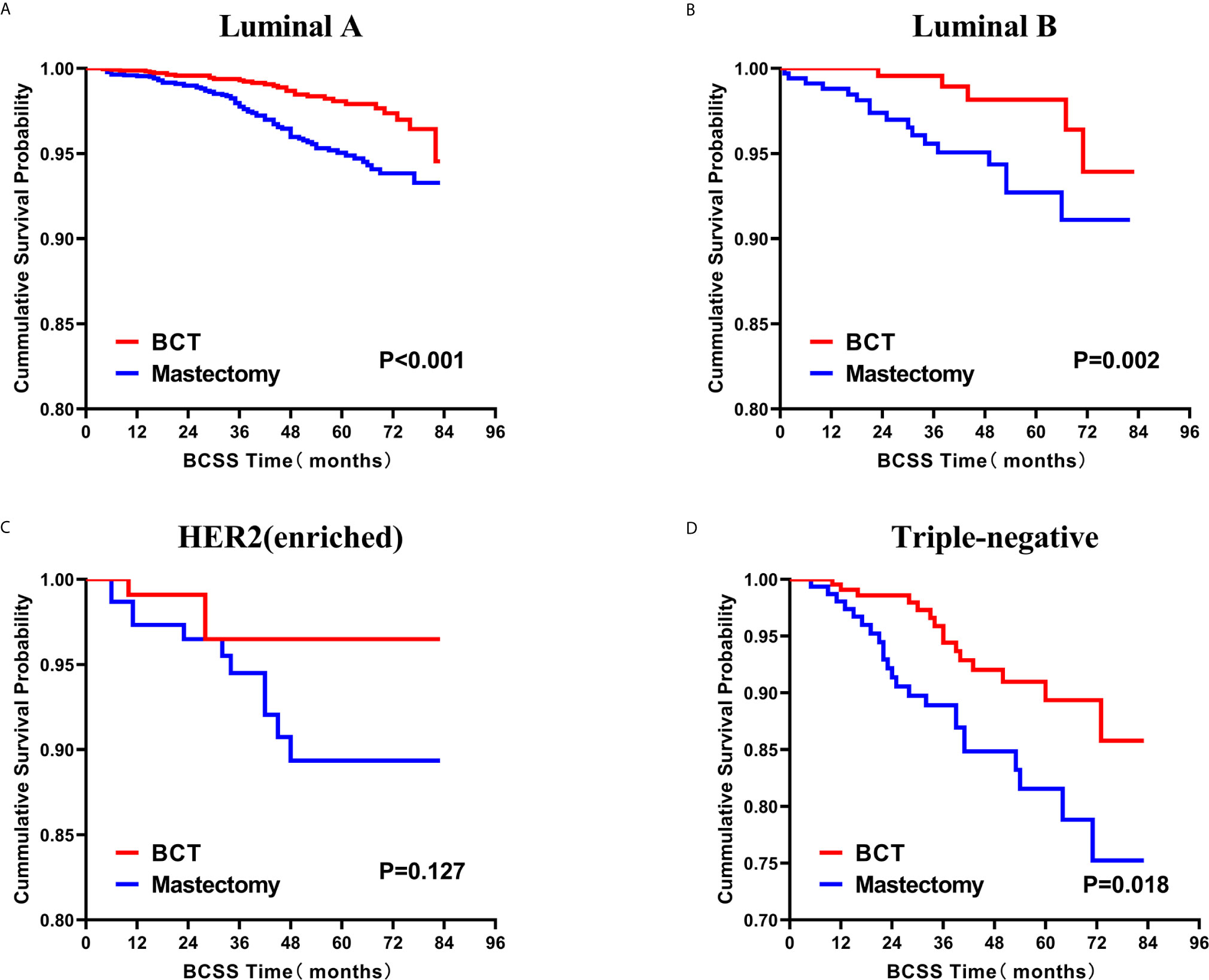
Figure 8 Kaplan–Meier curves for BCSS by treatment type for all patients, stratified by molecular subtype: (A) Luminal A. (B) Luminal B. (C) HER2 enriched. (D) Triple-negative. BCSS, breast cancer specific survival; BCT, breast-conserving therapy.
Prognostic Factors for TCNP Patients
Furthermore, we next explored the prognostic factors associated with BCSS and OS in the cohort of patients. Univariable Cox regression analysis revealed that age, race, histological grade, AJCC stage, molecular subtype, receipt of chemotherapy, and treatment type were important factors associated with BCSS, while age, sex, race, histological grade, AJCC stage, molecular subtype, and treatment type were visibly associated with OS (Table 2). After we included the covariates that were clinically worth exploring or had p < 0.05 in the univariate analysis into the multivariate analysis, the parameters age, sex, race, histological grade, AJCC stage, molecular subtype, and treatment type were independent prognostic factors of BCSS in TCNP patients. While the factors age, race, histological grade, AJCC stage, molecular subtype, receipt of chemotherapy, and treatment type were significantly associated with OS (Table 3).
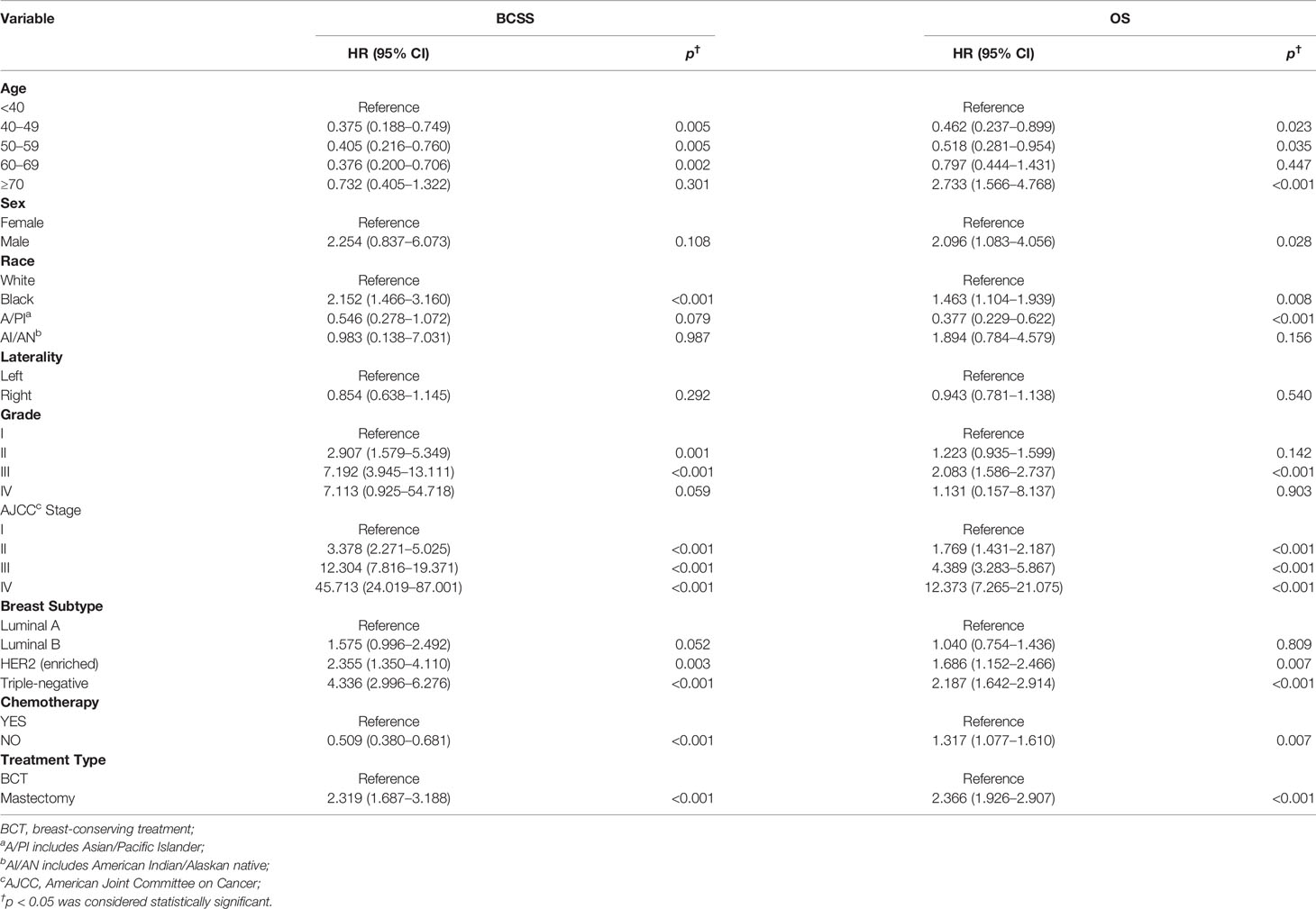
Table 2 Univariable Cox proportional hazard regression model of breast cancer-specific survival (BCSS) and overall survival (OS).
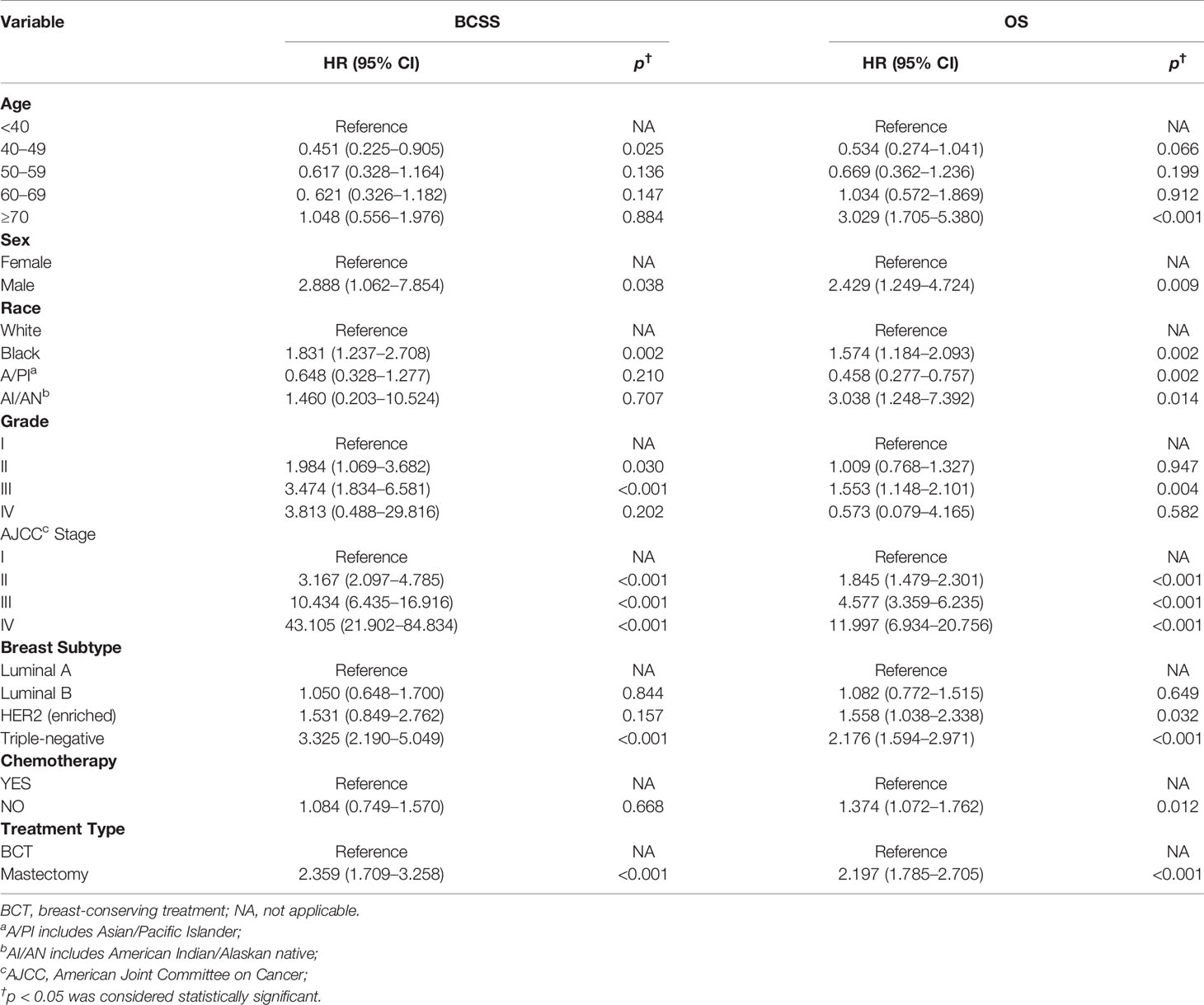
Table 3 Multivariable Cox proportional hazard regression model of breast cancer-specific survival (BCSS) and overall survival (OS).
Discussion
To our knowledge, this is the first research to explore the role of different treatments on survival of TCNP breast cancer patients. In the retrospective analysis of 9,900 cases, we uncovered that the BCT group exhibited better BCSS and OS than mastectomy group. Furthermore, we also conducted a subgroup analysis of all the independent influencing parameters for BCSS, including age, sex, race, histological grade, AJCC stage, and molecular subtype. We observed that none of subgroups undergoing the mastectomy therapy had better BCSS than BCT.
National Comprehensive Cancer Network (NCCN) guidelines and relevant studies demonstrated that the long-term survival rate of the BCT is similar to that of the mastectomy therapy. Therefore, BCT should be recommended for patients with early breast cancer (24–27). A Dutch study showed that BCT, compared with mastectomy therapy, was significantly correlated with improved 10-year overall survival in the whole cohort of 37,207 T1-2N0-1M0 stage patients (28). Additionally, another population-based study on 10-year follow-up for 3,071 T1-2N2 stage breast cancer showed that patients who underwent BCT had superior survival time and lower rate of distant metastasis and regional recurrence (29). Our same findings further confirmed the availability and security of BCT and the range of application, such as AJCC stage I–III. These findings also agreed with the NCCN guidelines and Chinese breast cancer guidelines that patients in stage III (except for inflammatory breast cancer) who have been downgraded by neoadjuvant chemotherapy to meet the criteria for breast preservation can also be carefully considered (22, 24). Therefore, BCT has been gradually accepted by more surgeons and popularized by more patients worldwide in recent years. However, which type of patients undergoing BCT would have longer survival time and better quality of life is still an important unsolved issue.
Indeed, tumor location has been proven to be a strong predictive factor for survival (9). However, the opinion of whether BCT is suitable for TCNP breast cancer patients remains controversial (30–32). A study of 105,037 patients showed that TCNP was significantly correlated with older years, larger tumor sizes, higher AJCC stage, and worse survival time relative to other peripheral quadrants (9). Additionally, Chinese breast cancer guidelines indicated that tumor in the nipple was one of the relative contraindications for BCT (22). Intriguingly, our research of 9,900 AJCC stage I–IV TCNP patients showed that BCT exhibited better prognosis in most clinicopathological subgroups, supporting another recent investigation on early-stage T1 or T2 centrally located breast cancer (32). Our results constituting a wide range and a variety of influential factors from the SEER database provided more convincing evidence that BCT may not be a relative contraindication in clinical decision-making. Additionally, we consider that the superior BCSS outcome of BCT, to some extent, may due to the role of RT. Previous studies had demonstrated that timely adjuvant RT after BCT could decrease locoregional recurrence and distant metastasis (33–35). Another pre-clinical study also demonstrated that RT could potentially induce an anti-tumoral immune response in breast cancer (36).
Currently, breast cancers are classified into four distinct subtypes: Luminal A, Luminal B, HER2-enriched, and TNBC, according to the presence or absence of molecular markers for estrogen or progesterone receptors and HER2 (8). These subtypes have distinct risk profiles and treatment strategies (37). In particular, TNBC tends to exhibit a more aggressive clinical biological characteristics and worse prognosis than other subtypes (8, 38, 39). However, several professionals indicated that BCT might not be considered a contraindication for TNBC (40, 41). From our analysis of the TNBC subgroup, although the BCT group had a higher proportion of TNBC, it showed a superior BCSS survival than mastectomy therapy.
Of note, the application of BCT for TCNP patients should allow for an adequate safety tumor-free margin of at least 2 mm (22). This undoubtedly increases technical difficulties in operations and makes surgeons hesitate to conduct BCT in consideration of locoregional recurrence (42). However, oncoplastic breast surgery (OBS) could broaden the general indication of BCT by achieving wider excision margins, which could ensure better local control of the disease (43). Eggemann, Holm et al. had provided excellent applicability of the B technique for breast cancer localized in the central portion of the breast (20). An inverted T method invented by McCulley et al. also made a similar satisfactory outcome for TCNP (44). Additionally, when BCT was implemented with the OBS technique, the rate of cosmetic failure could drop to <7% within 2 years and <10% within 5 years (45). Kijima et al. produced good cosmetic results for TCNP by multifarious flaps, including Latissimus dorsi mini flap, Grisotti flap, hole-shaped skin glandular flap, and Free dermal fat graft (19). Therefore, surgeons could consider BCT for more TCNP patients, with the help of the OBS technique.
This present study has several potential limitations. Firstly, we could not obtain information about disease recurrence from the SEER database. Therefore, we could not compare the different rates of locoregional recurrence between BCT and mastectomy therapy. Secondly, SEER could not provide information about neoadjuvant therapy. Some patients in the BCT group should undergo neoadjuvant chemotherapy before operation to meet the indication of breast conserving. Therefore, we could not assess the potential role of neoadjuvant therapy on our clinical outcomes. Thirdly, the SEER database collected large numbers of patients’ information from 18 population-based cancer registries. Some clinicopathologic information may be miscoded or missed during the registration process. For instance, patients’ BRCA gene mutations, Ki-67 in immunohistochemical analysis, were not provided in the current dataset.
Collectively, our study was the first investigation to show that BCT exhibited better prognosis than mastectomy therapy in the cohort of TCNP from SEER databases. This finding could provide a cue for treatment strategies for suitable TCNP patients, especially those with a strong willingness to conserve their breasts. However, some other factors, such as the potential locoregional recurrence rate, and whether physical condition could withstand the adverse effects of radiotherapy, should also be taken into account. Therefore, whole-process therapeutic strategies for TCNP patients still warrant further in-depth research.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
Conception and design: JW, HL, and GR. Data acquisition, analysis, and interpretation: JW, ZZ, and XL. Material support: XL, XW, YL, and HL. Study supervision: GR. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (#31420103915 and #81472475).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank all medical personnel of the Department of Endocrine and Breast Surgery and Chongqing Key Laboratory of Molecular Oncology and Epigenetics, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China, for their technical assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.642571/full#supplementary-material
Supplementary Figure 1 | Kaplan-Meier Curves for OS by Treatment Type for All Patients, Stratified by Age at Diagnosis: (A) Age <40 years. (B) Age of 40-49 years. (C) Age of 50-59 years. (D) Age of 60-69 years. (E) Age ≥ 70 years. BCSS, breast cancer specific survival; BCT, breast-conserving therapy.
Supplementary Figure 2 | Kaplan-Meier Curves for OS by Treatment Type for All Patients, Stratified by Sex: (A) Female. (B) Male. BCSS, breast cancer specific survival; BCT, breast-conserving therapy.
Supplementary Figure 3 | Kaplan-Meier Curves for OS by Treatment Type for All Patients, Stratified by Race: (A) White. (B) Black. (C) A/PI. (D) AI/AN. BCSS, breast cancer specific survival; BCT, breast-conserving therapy; A/PI, Asian/Pacific Islander; AI/AN, American Indian/Alaskan native.
Supplementary Figure 4 | Kaplan-Meier Curves for OS by Treatment Type for All Patients, Stratified by Histological Grade: (A) Grade I. (B) Grade II. (C) Grade III. (D) Grade IV. BCSS, breast cancer specific survival; BCT, breast-conserving therapy.
Supplementary Figure 5 | Kaplan-Meier Curves for OS by Treatment Type for All Patients, Stratified by AJCC stage: (A) AJCC stage I. (B) AJCC stage II. (C) AJCC stage III. (D) AJCC stage IV. BCSS, breast cancer specific survival; BCT, breast-conserving therapy.
Supplementary Figure 6 | Kaplan-Meier Curves for OS by Treatment Type for All Patients, Stratified by Molecular Subtype: (A) Luminal A. (B) Luminal B. (C) HER2 enriched. (D) Triple-negative. BCSS, breast cancer specific survival; BCT, breast-conserving therapy.
References
1. Siegel R, Miller K, Jemal A. Cancer Statistics, 2020. CA: Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
2. Prat A, Guarneri V, Paré L, Griguolo G, Pascual T, Dieci M, et al. A Multivariable Prognostic Score to Guide Systemic Therapy in Early-Stage HER2-Positive Breast Cancer: A Retrospective Study With an External Evaluation. Lancet Oncol (2020) 21(11):1455–64. doi: 10.1016/S1470-2045(20)30450-2
3. Li H, Chen Y, Wang X, Tang L, Guan X. T1-2n0m0 Triple-Negative Breast Cancer Treated With Breast-Conserving Therapy Has Better Survival Compared to Mastectomy: A SEER Population-Based Retrospective Analysis. Clin Breast Cancer (2019) 19(6):e669–82. doi: 10.1016/j.clbc.2019.05.011
4. Cai S, Zuo W, Lu X, Gou Z, Zhou Y, Liu P, et al. The Prognostic Impact of Age at Diagnosis Upon Breast Cancer of Different Immunohistochemical Subtypes: A Surveillance, Epidemiology, and End Results (SEER) Population-Based Analysis. Front Oncol (2020) 10:1729. doi: 10.3389/fonc.2020.01729
5. Cianfrocca M, Goldstein LJ. Prognostic and Predictive Factors in Early-Stage Breast Cancer. Oncologist (2004) 9(6):606–16. doi: 10.1634/theoncologist.9-6-606
6. Andersson Y, Bergkvist L, Frisell J, de Boniface J. Long-Term Breast Cancer Survival in Relation to the Metastatic Tumor Burden in Axillary Lymph Nodes. Breast Cancer Res Treat (2018) 171(2):359–69. doi: 10.1007/s10549-018-4820-0
7. Ehinger A, Malmström P, Bendahl P, Elston C, Falck A, Forsare C, et al. Histological Grade Provides Significant Prognostic Information in Addition to Breast Cancer Subtypes Defined According to St Gallen 2013. Acta Oncol (Stockholm Sweden) (2017) 56(1):68–74. doi: 10.1080/0284186X.2016.1237778
8. Boyle P. Triple-Negative Breast Cancer: Epidemiological Considerations and Recommendations. Ann Oncol (2012) 23(Suppl 6):vi7–12. doi: 10.1093/annonc/mds187
9. Ji F, Xiao W, Yang C, Yang M, Zhang L, Gao H, et al. Tumor Location of the Central and Nipple Portion Is Associated With Impaired Survival for Women With Breast Cancer. Cancer Manage Res (2019) 11:2915–25. doi: 10.2147/CMAR.S186205
10. Wunderle M, Gass P, Häberle L, Flesch V, Rauh C, Bani M, et al. BRCA Mutations and Their Influence on Pathological Complete Response and Prognosis in a Clinical Cohort of Neoadjuvantly Treated Breast Cancer Patients. Breast Cancer Res Treat (2018) 171(1):85–94. doi: 10.1007/s10549-018-4797-8
11. Wu S, Zhou J, Ren Y, Sun J, Li F, Lin Q, et al. Tumor Location Is a Prognostic Factor for Survival of Chinese Women With T1-2N0M0 Breast Cancer. Int J Surg (2014) 12(5):394–8. doi: 10.1016/j.ijsu.2014.03.011
12. Hwang KT, Kim J, Kim EK, Jung H, Sohn G, Kim SI, et al Poor Prognosis of Lower Inner Quadrant in Lymph Node–negative Breast Cancer Patients Who Received No Chemotherapy: A Study Based on Nationwide Korean Breast Cancer Registry Database. Clin Breast Cancer (2017) 17(4):e169–84. doi: 10.1016/j.clbc.2016.12.011
13. Farag SS, Archer KJ, Mrózek K, Ruppert AS, Carroll AJ, Vardiman JW, et al. Pretreatment Cytogenetics Add to Other Prognostic Factors Predicting Complete Remission and Long-Term Outcome in Patients 60 Years of Age or Older With Acute Myeloid Leukemia: Results From Cancer and Leukemia Group B 8461. Blood (2006) 108(1):63–73. doi: 10.1182/blood-2005-11-4354
14. Ishizuka M, Shimizu T, Shibuya N, Takagi K, Hachiya H, Nishi Y, et al. Impact of Primary Tumor Location on Survival After Curative Resection in Patients With Colon Cancer: A Meta-Analysis of Propensity Score-Matching Studies. Oncologist (2021) 26(3):196–207. doi: 10.1002/onco.13555
15. Talamonti M, Kim S, Yao K, Wayne J, Feinglass J, Bennett C, et al. Surgical Outcomes of Patients With Gastric Carcinoma: The Importance of Primary Tumor Location and Microvessel Invasion. Surgery (2003) 134(4):720–7discussion 727–729. doi: 10.1016/S0039-6060(03)00337-4
16. Wang Z, Li M, Teng F, Kong L, Yu J. Primary Tumor Location Is an Important Predictor of Survival in Pulmonary Adenocarcinoma. Cancer Manage Res (2019) 11:2269–80. doi: 10.2147/CMAR.S192828
17. Hill D, White V, Giles G, Collins J, Kitchen P. Changes in the Investigation and Management of Primary Operable Breast Cancer in Victoria. Med J Aust (1994) 161(2):110–1:114, 118 passim. doi: 10.5694/j.1326-5377.1994.tb127341.x
18. Deemarski L, Seleznev I. Extended Radical Operations on Breast Cancer of Medial or Central Location. Surgery (1984) 96(1):73–7. doi: 10.3109/14017438409109909
19. Kijima Y, Yoshinaka H, Shinden Y, Hirata M, Nakajo A, Arima H, et al. Oncoplastic Breast Surgery for Centrally Located Breast Cancer: A Case Series. Gland Surg (2014) 3(1):62–73. doi: 10.3978/j.issn.2227-684X.2013.11.01
20. Eggemann H, Ignatov A, Elling D, Lampe D, Lantzsch T, Weise M, et al. Efficacy and Patient Satisfaction of Breast Conserving Therapy for Central Breast Cancer by the B Technique. Ann Surg Oncol (2013) 20(11):3438–45. doi: 10.1245/s10434-013-3030-6
21. Nijenhuis MV, Rutgers EJ. Who Should Not Undergo Breast Conservation? Breast (2013) 22(Suppl 2):S110–4. doi: 10.1016/j.breast.2013.07.021
22. Zhongwei C, Depei C, Kai C, Qiang Z, Yinhua L, Erwei S, et al. [A Consensus Statement on the Breast-Conserving Surgery of Early-Stage Breast Cancer (2019)]. Zhonghua Wai Ke Za Zhi (2019) 57(2):81–4. doi: 10.3760/cma.j.issn.0529-5815.2019.02.001
23. Mccaffrey DF, Ridgeway G, Morral AR. Propensity Score Estimation With Boosted Regression for Evaluating Causal Effects in Observational Studies. Psychol Methods (2005) 9(4):403–25. doi: 10.1037/1082-989X.9.4.403
24. Gradishar WJ, Anderson BO, Abraham J, Aft R, Kumar R. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Network: JNCCN (2020) 18(4):452–78. doi: 10.3760/cma.j.issn.0529-5815.2019.02.001
25. Litière S, Werutsky G, Fentiman IS, Rutgers E, Christiaens MR, Van Limbergen E, et al. Breast Conserving Therapy Versus Mastectomy for Stage I-II Breast Cancer: 20 Year Follow-Up of the EORTC 10801 Phase 3 Randomised Trial. Lancet Oncol (2012) 13(4):412–9. doi: 10.1016/S1470-2045(12)70042-6
26. Zhimin S, Junjie L. Interpretation of “Guideline and Standard for the Diagnosis and Treatment of Breast Cancer by Chinese Anti-Cancer Association(2015 Edition)”: Surgical Section. Chin J Breast Disease(Electronic Edition) (2016) 10(01):1–5. doi: CNKI:SUN:ZHRD.0.2016-01-001
27. Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol: Off J Eur Soc Med Oncol (2015) 26(Suppl 5):v8–30. doi: 10.1093/annonc/mdv298
28. van Maaren M, de Munck L, de Bock G, Jobsen J, van Dalen T, Linn S, et al. 10 Year Survival After Breast-Conserving Surgery Plus Radiotherapy Compared With Mastectomy in Early Breast Cancer in the Netherlands: A Population-Based Study. Lancet Oncol (2016) 17(8):1158–70. doi: 10.1016/S1470-2045(16)30067-5
29. van Maaren MC, de Munck L, Jobsen JJ, Poortmans P, de Bock GH, Siesling S, et al. Breast-Conserving Therapy Versus Mastectomy in T1-2N2 Stage Breast Cancer: A Population-Based Study on 10-Year Overall, Relative, and Distant Metastasis-Free Survival in 3071 Patients. Breast Cancer Res Treat (2016) 160(3):511–21. doi: 10.1007/s10549-016-4012-8
30. Fowble B, Solin LJ, Schultz DJ, Weiss MC. Breast Recurrence and Survival Related to Primary Tumor Location in Patients Undergoing Conservative Surgery and Radiation for Early-Stage Breast Cancer. Int J Radiat Oncol Biol Phys (1992) 23(5):933–9. doi: 10.1016/0360-3016(92)90897-Q
31. McCready DR. Mastectomy or Lumpectomy? The Choice of Operation for Clinical Stages I and II Breast Cancer. The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Canadian Association of Radiation Oncologists. CMAJ: Can Med Assoc J = J l’Assoc Med Can (1998) 158 Suppl 3(5):S9. doi: 10.9774/GLEAF.978-1-909493-38-4_2
32. Zhang M, Wu K, Zhang P, Wang M, Bai F, Chen H. Breast-Conserving Surgery Is Oncologically Safe for Well-Selected, Centrally Located Breast Cancer. Ann Surg Oncol (2021) 28(1):330–9. doi: 10.1245/s10434-020-08793-z
33. Gajdos C, Tartter PI, Bleiweiss IJ. Subareolar Breast Cancers. Am J Surg (2000) 180(3):167–70. doi: 10.1016/S0002-9610(00)00477-3
34. Goodman CR, Seagle BL, Friedl TWP, Rack B, Lato K, Fink V, et al. Association of Circulating Tumor Cell Status With Benefit of Radiotherapy and Survival in Early-Stage Breast Cancer. JAMA Oncol (2018) 4(8):e180163. doi: 10.1001/jamaoncol.2018.0163
35. Knauerhase H, Strietzel M, Gerber B, Reimer T, Fietkau R. Tumor Location, Interval Between Surgery and Radiotherapy, and Boost Technique Influence Local Control After Breast-Conserving Surgery and Radiation: Retrospective Analysis of Monoinstitutional Long-Term Results. Int J Radiat Oncol Biol Phys (2008) 72(4):1048–55. doi: 10.1016/j.ijrobp.2008.02.007
36. Jatoi I, Benson J, Kunkler I. Hypothesis: Can the Abscopal Effect Explain the Impact of Adjuvant Radiotherapy on Breast Cancer Mortality? NPJ Breast Cancer (2018) 4:8. doi: 10.1038/s41523-018-0061-y
37. Waks A, Winer E. Breast Cancer Treatment: A Review. JAMA (2019) 321(3):288–300. doi: 10.1001/jama.2018.19323
38. Seong M, Kim K, Chung I, Kang E, Lee J, Park S, et al. A Multi-Institutional Study on the Association Between BRCA1/BRCA2 Mutational Status and Triple-Negative Breast Cancer in Familial Breast Cancer Patients. Breast Cancer Res Treat (2014) 146(1):63–9. doi: 10.1007/s10549-014-3006-7
39. Chen Q, Wang X, Lin P, Zhang J, Li J, Song C, et al. The Different Outcomes Between Breast-Conserving Surgery and Mastectomy in Triple-Negative Breast Cancer: A Population-Based Study From the SEER 18 Database. Oncotarget (2017) 8(3):4773–80. doi: 10.18632/oncotarget.13976
40. Abdulkarim B, Cuartero J, Hanson J, Deschênes J, Lesniak D, Sabri S. Increased Risk of Locoregional Recurrence for Women With T1-2N0 Triple-Negative Breast Cancer Treated With Modified Radical Mastectomy Without Adjuvant Radiation Therapy Compared With Breast-Conserving Therapy. J Clin Oncol: Off J Am Soc Clin Oncol (2011) 29(21):2852–8. doi: 10.1200/JCO.2010.33.4714
41. Adkins F, Gonzalez-Angulo A, Lei X, Hernandez-Aya L, Mittendorf E, Litton J, et al. Triple-Negative Breast Cancer Is Not a Contraindication for Breast Conservation. Ann Surg Oncol (2011) 18(11):3164–73. doi: 10.1245/s10434-011-1920-z
42. Multon O, Bourgeois D, Validire P, Vilcoq J, Durand J, Clough K. [Breast Cancers With Central Localization: Conservative Treatment by Tumorectomy With Ablation of the Areolar Plaque]. Presse Med (Paris France: 1983) (1997) 26(21):988–94.
43. Bertozzi N, Pesce M, Santi P, Raposio E. Oncoplastic Breast Surgery: Comprehensive Review. Eur Rev Med Pharmacol Sci (2017) 21(11):2572–85. doi: 10.1016/j.amsu.2017.07.048
44. McCulley S, Durani P, Macmillan R. Therapeutic Mammaplasty for Centrally Located Breast Tumors. Plast Reconstruct Surg (2006) 117(2):366–73. doi: 10.1097/01.prs.0000200874.31320.c2
Keywords: tumors in the central and nipple portion, breast-conserving therapy, mastectomy, prognosis, SEER
Citation: Wang J, Wang X, Zhong Z, Li X, Sun J, Li J, Huang J, Li Y, Ren G and Li H (2021) Breast-Conserving Therapy Has Better Prognosis for Tumors in the Central and Nipple Portion of Breast Cancer Compared with Mastectomy: A SEER Data-Based Study. Front. Oncol. 11:642571. doi: 10.3389/fonc.2021.642571
Received: 16 December 2020; Accepted: 22 July 2021;
Published: 12 August 2021.
Edited by:
Emanuel Petricoin, George Mason University, United StatesReviewed by:
Jianjun Liu, University of Science and Technology of China, ChinaZeming Liu, Zhongnan Hospital of Wuhan University, China
Copyright © 2021 Wang, Wang, Zhong, Li, Sun, Li, Huang, Li, Ren and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guosheng Ren, cmVuZ3M3MjZAMTI2LmNvbQ==; Hongzhong Li, MjAzODUxQGNxbXUuZWR1LmNu
 Jing Wang
Jing Wang Xiaoyu Wang
Xiaoyu Wang Zhenyu Zhong
Zhenyu Zhong Xue Li
Xue Li Jiazheng Sun1,2
Jiazheng Sun1,2 Guosheng Ren
Guosheng Ren Hongzhong Li
Hongzhong Li