- Department of Radiation Oncology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China
Background: Elevated pretreatment lactate dehydrogenase (LDH) has been associated with poor prognosis in various malignancies; however, its prognostic role in hypopharyngeal cancer remains elusive. In this study, we aimed to assess the association between pretreatment LDH and clinical outcome of hypopharyngeal cancer.
Methods: We retrospectively collected 198 hypopharyngeal cancer patients treated with surgery in our institution between 2004 and 2018. The prognostic role of pretreatment LDH was explored by using univariate and multivariate analyses. Besides, subgroup analysis was performed based on T stage.
Results: Three-year and Five-year of disease-free survival (DFS, 67.0 vs. 57.4%, 65.8 vs. 39.8%, p = 0.007) and overall survival (OS, 74.8 vs. 68.9%, 66.8 vs. 50.8%, p = 0.006) exhibited significant differences between low LDH level and high LDH level groups. Univariate analysis showed that pretreatment elevated serum LDH served as an unfavorable determinant with regard to DFS and OS. Further multivariate analysis also confirmed that LDH was an independent predictor for DFS and OS. Additionally, N status and age were also found to be significantly associated with both DFS and OS.
Conclusion: Pretreatment elevated serum LDH is an inferior prognostic factor for patients with hypopharyngeal cancer. These results should be validated by more multicenter and prospective studies.
Introduction
Among head and neck squamous cell carcinomas, hypopharyngeal cancer is comparatively uncommon and aggressive (1). Hypopharyngeal cancer occurs predominantly in males (2) with heavy alcohol consumption and tobacco use (3). It is mainly arising from pyriform sinus, and early symptoms of this malignancy are often neglected, which contributes to the fact that hypopharyngeal cancer patients usually present with an advanced stage at their first diagnosis (4, 5). Therefore, despite advancements in diagnosis and treatment modalities, the 5-year disease-free survival (DFS) rate and overall survival (OS) rate of hypopharyngeal cancer remain unsatisfied, which were in the range of 35.8–65.5% and 26.9–58.5%, respectively, according to different studies (6–14).
Surgery is one of the main initial treatment option. The surgery modality for locally advanced hypopharyngeal cancer is pharyngectomy with total or partial laryngectomy, in conjunction with selective or radical neck dissection, with reasonable adjuvant treatment based on the presence of clinical adverse pathologic features (15). Given the poor prognosis of advanced hypopharyngeal cancer, a novel prognostic determinant that can accurately predict clinical outcomes for these patients is urgently needed.
Lactate dehydrogenase(LDH) is a glycolytic enzyme (16), comprised of four polypeptide subunits, in charge of the transformation of pyruvate into lactate in aerobic conditions (17). Distinguish from non-proliferating cells, which are dominated by mitochondrial tricarboxylic acid (TCA) cycle to generate ATP needed for cellular activities, a majority of cancer cells are alternatively characterized by aerobic glycolysis, a phenomenon called “the Warburg effect.” To better understand Warburg effect, we should note that proliferating cells have more crucial metabolic requirements beyond ATP, such as carbon and NADPH, which are needed for macromolecular synthesis like nucleotides, amino acids, and lipids. Despite the inefficient ATP production of aerobic glycolysis, the superiority it conferred to cancer cells is the robust generation of NADPH and excretion of excess carbon. The enzyme LDH is the main player in the conversion of both glucose and glutamine to lactate, and one by-product of the conversion is the generation of NADPH (18). The inhibition of LDH resulted in reduced cell proliferation (19). Former research studies showed that LDH not merely associates with the activation of some proto-oncogenes, such as Myc and HIF-α (20), but also plays a crucial role in the maintenance of tumor invasiveness (21, 22), metastatic potential (23, 24), chemoresistance (25), and radioresistance (26). Moreover, it also showed that LDH takes part in immunosuppression (27), and LDH can be regarded as an immune surveillance prognostic marker (28) and its abnormal rise was forerunner of unfavorable outcomes in cancer patients (29).
The prognostic significance of pretreatment serum LDH in multiple malignancies is well established, such as biliary tract cancer (30), lung cancer (31, 32), breast cancer (33), gastric cancer (34), colorectal cancer (35), and so on (36). However, the clinical significance of LDH in hypopharyngeal cancer is quite unclear, and it is worthwhile to explore the prognostic role of LDH. Serum LDH is promising to become a cost effective, non-invasive, and easily reproducible marker, which can predict survival outcomes in hypopharyngeal cancer. Therefore, it is imperative to evaluate whether LDH can serve as a prognostic factor in hypopharyngeal cancer patients.
Materials and Methods
Patients
We retrospectively reviewed patients diagnosed with hypopharyngeal cancer between March 2004 and December 2018 in our institution (Sun Yat-Sen Memorial hospital). Exclusion criteria included: initial definitive chemoradiotherapy, no available pretreatment serum LDH, inadequate follow-up, data and renunciation of medical treatment. We enrolled the patients treated with surgery and with eligible pretreatment serum LDH. Overall, we collected 198 histologically confirmed hypopharyngeal cancer patients in this study. Patients’ baseline clinical and pathological characteristics, including age, gender, tumor differentiation, surgical margin, vascular invasion, adjuvant therapy, type of surgery, adjuvant radiotherapy, chemotherapy regimen and cycles, T stage and N status were collected and analyzed. This study was performed under the review and approval of the Institutional Review Board at Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University.
Laboratory Testing of LDH
Peripheral blood samples were collected and analyzed for laboratory tests within 2 weeks before the beginning of treatment. Serum LDH levels were measured employing a Hitachi Automatic Analyzer 7600-020 (Hitachi High-Technologies, Tokyo, Japan). Normal serum LDH enzyme levels were defined to 108–252 U/L. Based on pretreatment serum LDH levels, patients were classified into high LDH group (LDH ≥178 U/L) and low LDH group (LDH <178 U/L) (37).
Clinical Staging
All patients underwent pretreatment evaluations, including: physical examinations, hematologic and biochemical tests, chest radiography, electronic laryngoscope, esophageal barium meal examination. Contrast-enhanced magnetic resonance imaging (MRI) or computed tomography (CT) of the head and neck was performed on 64 and three patients, respectively. MRI and CT scan were aimed to evaluate the size and surrounding invasion of the tumor, as well as the status of lymph node metastasis. Abdominal ultrasonography and whole body bone scan using single-photon emission computed tomography (SPECT), which was used to detect distant metastasis, were carried out in 143 and 85 patients, respectively.
The TNM classification of all patients were staged according to the 7th edition of the American Joint Committee on Cancer (AJCC) for hypopharyngeal cancer.
Treatment of Patients
All of the patients received surgery with or without adjuvant therapy. Treatment option was decided upon thorough evaluation and made by a multidisciplinary team, regarding treatment efficacy, function maintenance and complication. Surgery modality including excision of hypopharyngeal cancer in the absent of neck dissection, pharyngectomy with total or partial laryngectomy, in conjunction with radical, modified radical, selective or extended neck dissection. According to the guideline proposed by the American Head and Neck Society (38) for patients with hypopharyngeal cancer, (1) radical neck dissection referred to ipsilateral cervical lymphadenectomy of levels I–V, (2) modified radical neck dissection spared one or more non-lymphatic structures, (3) lymph nodes in levels II–IV were removed in selective neck dissection, (4) removal of structures not contained in radical neck dissection termed extended neck dissection. Alterations could be made depending on the sophisticated clinical circumstances.
Adjuvant treatment was carried out based on the presence of clinical adverse pathologic features such as extranodal extension, positive margin, pT3 or pT4 primary, multiple positive lymph nodes, perineural invasion and lymphatic invasion. 119 (60.1%) patients were prescribed with adjuvant chemotherapy; regimen of TP (taxol and platinum) (73.1%) was the most commonly used regimen in this population. Other chemotherapeutic schemes included TPF (taxol, platinum and 5-fluorouracil) and PF (platinum and 5-fluorouracil). For advanced hypopharyngeal cancer patients with adverse pathologic features, postoperative radiotherapy, either 3D conformal RT or IMRT was performed. The range of total prescribed radiation doses was 60–66 Gy for tumor bed, and 50–56 Gy for lymphatic drainage area (2.0 Gy/fraction; daily Monday–Friday; in 6–7 weeks).
Follow-Up Evaluation
After the completion of surgery and adjuvant therapy, patients were followed up every 3 months for the first 2 years, semiannually for the subsequent 3 years, and annually thereafter. The observation of follow-up included physical examination, esophagography, chest X-ray, MRI of hypopharynx and neck. Overall survival (OS) was defined as the time interval from the beginning of treatment to death. Disease-free survival (DFS) was the time from the initiation of therapy to recurrence or death of any cause. Locoregional recurrence-free survival (LRFS) was the duration between the beginning of treatment and the first local recurrence or regional lymph node metastasis. Distant metastasis-free survival (DMFS) was defined as the duration from the date treatment began to the time of distant metastasis.
Statistical Analysis
Data statistical analysis was carried out by employing SPSS 20.0 software. We chose the median level of LDH to separate all the patients into two groups. The correlation between categorical variables and clinical outcomes (DFS and OS) was analyzed by chi-square test. Kaplan–Meier method was used to evaluate survival distribution (DFS and OS), and the log-rank test was performed to calculate the significant differences of survival between groups. Univariate and multivariate Cox proportional hazard regression model was employed to further find out the risk factors for DFS and OS. Patients’ clinical characteristics including age, gender, T stage, N status, tumor differentiation, surgical margin, vascular invasion, and adjuvant chemotherapy were included as covariates in multivariate analysis. Multivariate analysis was carried out by forward stepwise regression procedure. A two-sided P-value ≤0.05 was deemed statistically significant.
Results
Baseline Characteristics
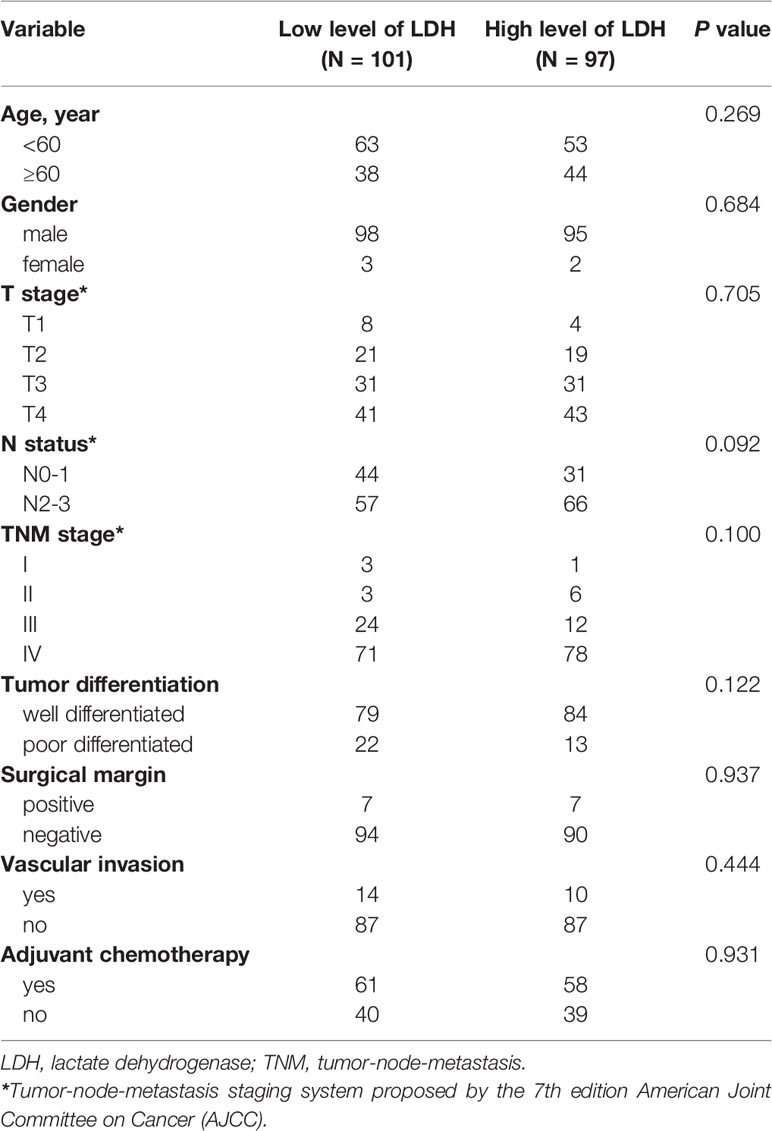
The clinical characteristics of 198 eligible patients diagnosed with hypopharyngeal cancer and underwent surgery were retrospectively collected and evaluated. The median follow-up time was 4.63 years (range: 0.58–13.17 years). All of the patients were divided into two groups: low LDH group (≤178 U/L) and high LDH group (>178 U/L). Among these 198 patients, 193 were males and five were females, with a median age of 58 years (range: 36–79 years). Squamous cell carcinoma was the most common histology, and the rest were carcinosarcoma and sarcoma. With regard to histologic grade, the number of well differentiated and poorly differentiated was 163 and 35, respectively. 167 (84.3%) patients had a history of heavy cigarette smoking, and 115 (58.1%) patients were heavy drinkers. According to the 7th edition TNM cancer staging system of AJCC, 185 (93.4%) patients were presented at stages III–IV, 52 (26.3%) patients were diagnosed with T1–2 stage, and 146 (73.7%) patients were diagnosed with T3–4 stage. With regard to lymph node metastasis status, the patient numbers of N0–1 and N2–3 were 75 (37.9%) and 123 (62.1%) respectively. Patients’ clinical and pathological characteristics are presented in Table 1.
Prognostic Role of Pretreatment Serum LDH for the Whole Group
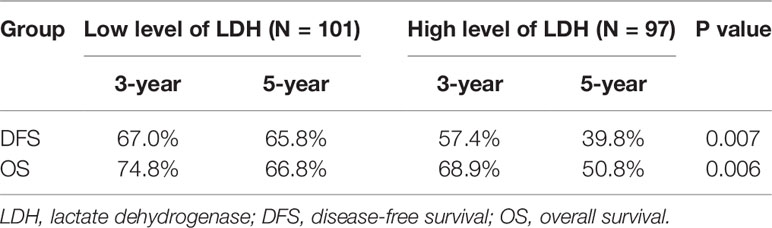
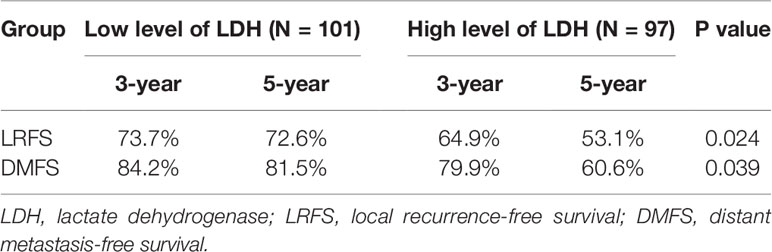
Survival analysis showed that three- and five-year of DFS (67.0 vs. 57.4%, 65.8 vs. 39.8%, p = 0.007) and OS (74.8 vs. 68.9%, 66.8 vs. 50.8%, p = 0.006) exhibited significant difference between low LDH and high LDH groups (Table 2). Additionally, three- and five-year of LRFS (low vs. high, 73.7 vs. 64.9%, 72.6 vs. 53.1%, p = 0.024) and DMFS (low vs. high, 84.2 vs. 79.9%, 81.5 vs. 60.6%, p = 0.039) also proved a significant difference between groups (Table 3). The Kaplan–Meier curves for OS and DFS for patients with LDH ≤178 U/L and LDH >178 U/L are presented in Figures 1, 2.
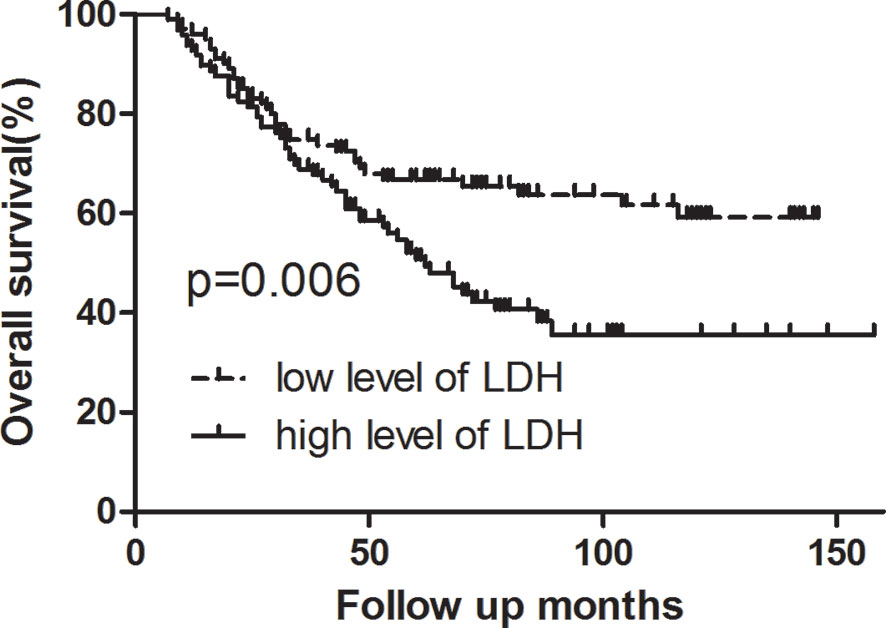
Figure 1 Kaplan–Meier curve for overall survival, stratified by pretreatment serum LDH ≤178 U/L and >178U/L. Log-rank test, P < 0.05.
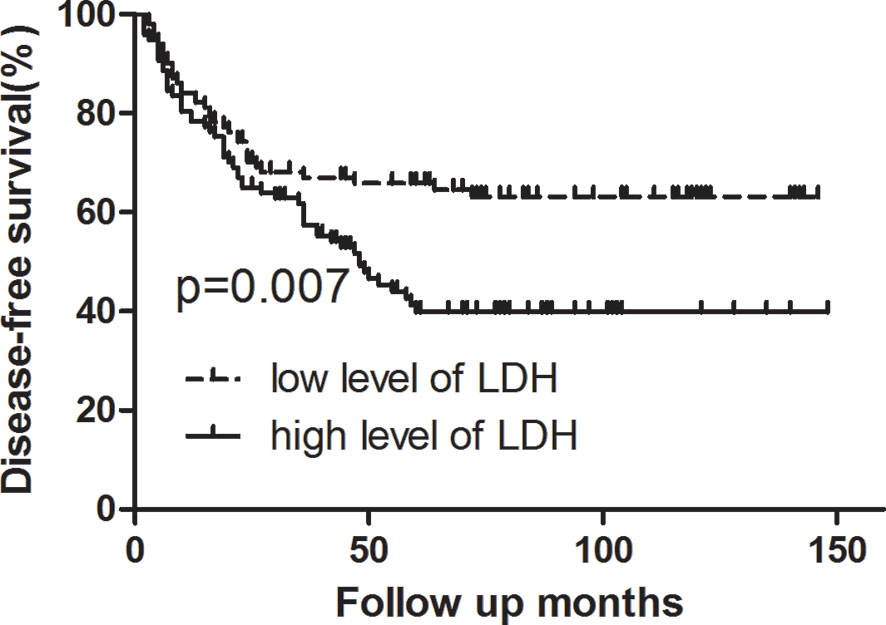
Figure 2 Kaplan–Meier curve for disease-free survival, stratified by pretreatment serum LDH ≤178 U/L and >178U/L. Log-rank test, P < 0.05.
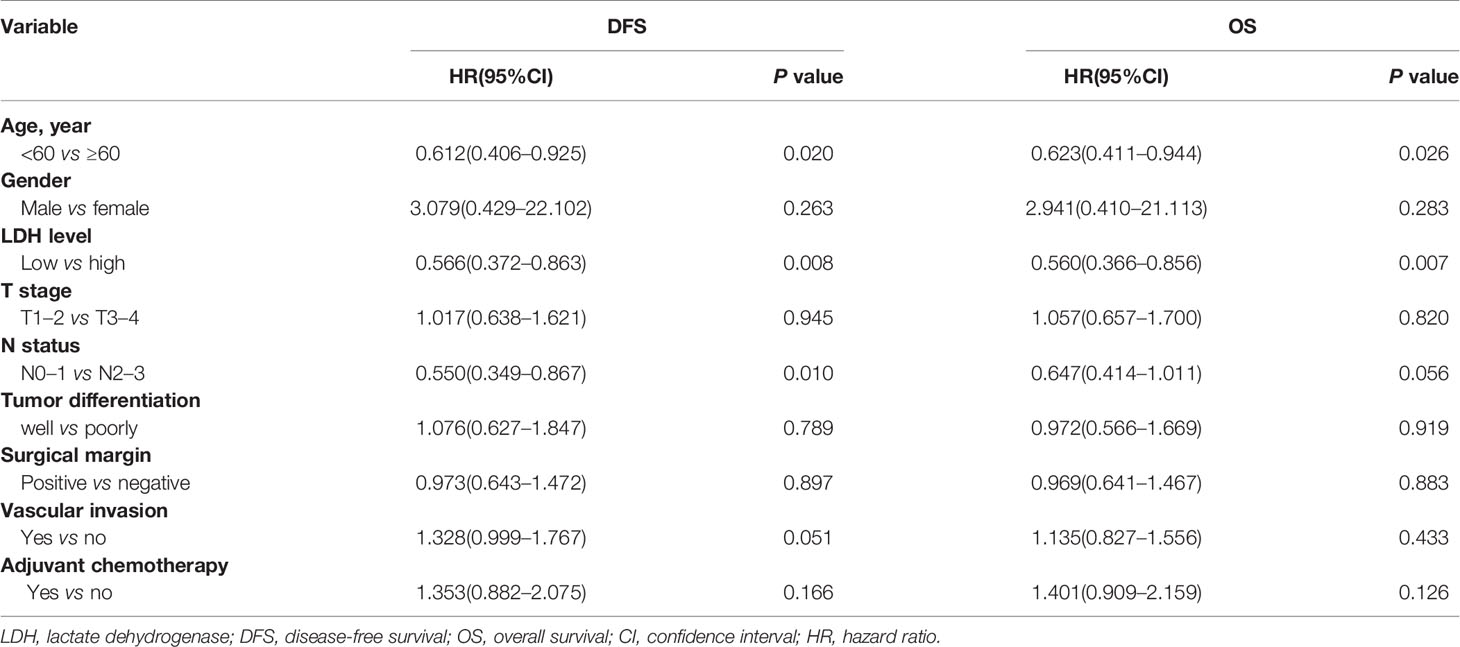
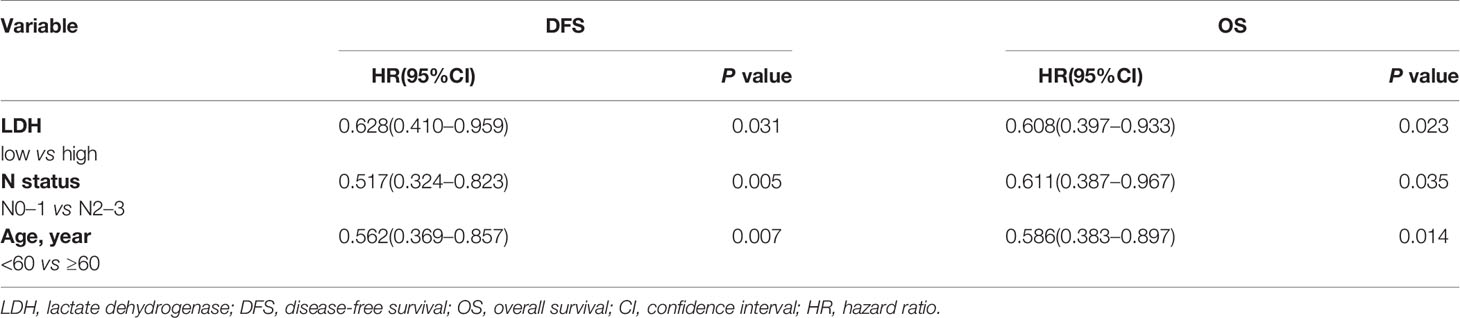
Univariate and multivariate analyses were performed to evaluate the relationship between clinicopathologic variables and survival outcomes. According to univariate analysis, high pretreatment serum LDH served as an unfavorable determinant with regard to DFS (low vs. high, p = 0.008; HR = 0.566, 95% CI 0.372–0.863) and OS (low vs. high, p = 0.007; HR = 0.560, 95% CI 0.366–0.856). Additionally, age was also proven to be correlated significantly with both DFS and OS, and N status was correlated with DFS. However, gender, T stage, tumor differentiation, surgical margin, and so on were not associated with survival prognosis (Table 4). Moreover, multivariate analysis further revealed that LDH (low vs. high, DFS, p = 0.031; HR = 0.628, 95% CI 0.410–0.959; OS, p = 0.023; HR = 0.608, 95% CI 0.397–0.933), N status (N0–1 vs. N2–3, DFS, p = 0.005; HR = 0.517, 95% CI 0.324 -0.823; OS, p = 0.035; HR = 0.611, 95% CI 0.387–0.967) and age (<60 vs. ≥60, DFS, p = 0.007; HR = 0.562, 95% CI 0.369–0.857; OS, p = 0.014; HR = 0.586, 95% CI 0.383–0.897) were independent prognostic factors for both DFS and OS (Table 5).
Prognostic Role of Pretreatment Serum LDH for T3–4 Patients
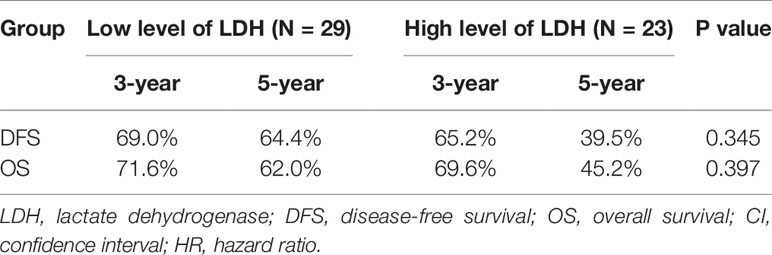
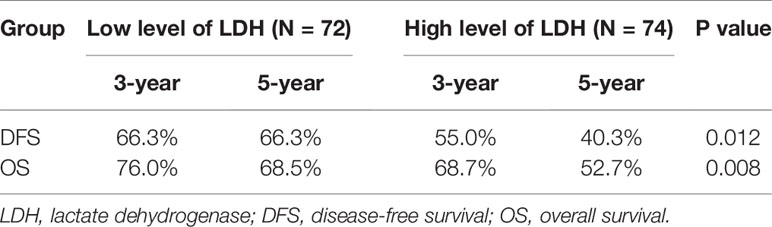
Further subgroup analysis exhibited that no significant tendency was observed between LDH and survival outcomes in the T1–2 group (Table 6). However, in the T3–4 group, high level of LDH was correlated significantly with worse survival outcomes (low vs. high; 3-year DFS: 66.3 vs. 55.0%, 5-year DFS: 66.3 vs. 40.3%, p = 0.012; 3-year OS: 76.0 vs. 68.7%, 5-year OS: 68.5 vs. 52.7%, p = 0.008) (Table 7).
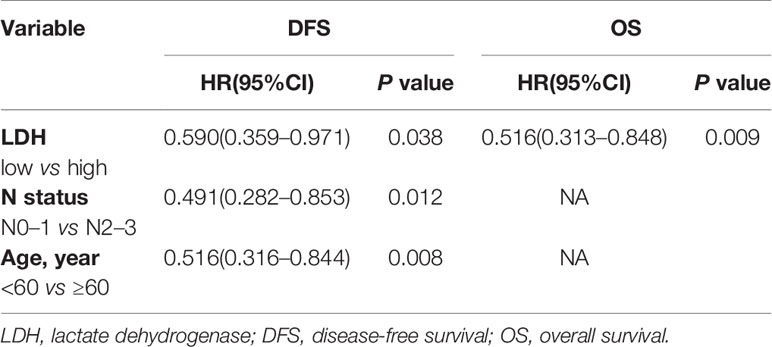
Furthermore, multivariate analysis of DFS and OS for patients with T3–4 showed that LDH was an independent prognostic factor for both DFS (low vs. high, p = 0.038; HR = 0.590, 95% CI 0.359–0.971) and OS (low vs. high, p = 0.009; HR = 0.516, 95% CI 0.313–0.848), whereas N status (N0–1 vs. N2–3, p = 0.012; HR = 0.491, 95% CI 0.282–0.853) and age (<60 vs. ≥60, p = 0.008; HR = 0.516, 95% CI 0.316–0.844) were independent prognostic factors for only DFS (Table 8).
Discussion
Among LDH family members, LDH-5, consisting LDHA subunit only, is the one that has the most vigorous capacity to catalyze pyruvate into lactic acid. Despite the availability of oxygen and the much less ATP produced, cancer cells prevailingly convert into aerobic glycolysis, which contributes to the synthesis of the substrates needed for cancer cell proliferation. Liang S et al. reported that Phosphoenolpyruvate carboxykinase 1 (PCK1) inhibited LDHA expression and thus impeded tumor cells’ growth and metastasis (39). In nasopharyngeal carcinoma, JMJD2A was predicted to boost the Warburg effect through LDHA activation, and fuel tumorigenesis and progression ultimately (40). Jiujie C et al. discovered that FOXM1 and LDHA were overexpressed simultaneously in pancreatic cancer. FOXM1 upregulated LDHA expression by promoting its transcription and was further attributed to pancreatic cancer cell proliferation and metastasis (41). The previous findings of LDH demonstrated the important impact of LDH on tumor development, and therefore, the clinical significance of LDH in cancer merits further investigation.
Elevated serum LDH has long been regarded as an adverse prognostic factor in various malignancies (36). Faloppi L et al. suggested that high pretreatment serum LDH levels significantly associated with poor clinical outcomes in biliary tract cancer patients receiving first-line chemotherapy (30). Pelizzari G et al. reported that during first-line treatment in metastatic breast cancer patients, elevated serum LDH levels served as independent ominous prognostic factor for PFS (33). Additionally, several meta-analysis studies revealed that elevated pretreatment serum LDH was an inferior factor in metastatic prostate cancer (42), osteosarcoma (43), urothelial carcinoma (44), breast cancer (45) and nasopharyngeal carcinoma (46). Moreover, in addition to serving as a prognostic factor, serum LDH also emerges as an indicator of treatment option. Previous studies found that serum LDH was able to distinguish renal cell carcinoma patients who were more likely to benefit from TORC1 inhibition temsirolimus (47), and identify locally advanced cervical cancer patients who could take advantage of neoadjuvant chemotherapy (37). Nevertheless, the reports about LDH’ value in hypopharyngeal cancer is scarcely any.
Hypopharyngeal cancer is a head and neck squamous cell carcinoma, predominantly debuted with advanced stage and accompanied with poor oncologic outcomes. To our best information, our study, for the first time, evaluated the prognostic impact of pretreatment serum LDH in hypopharyngeal cancer patients who underwent primary surgery, and the results confirmed that high level of LDH was an inferior factor in terms of DFS (low vs. high, p = 0.008; HR = 0.566, 95% CI 0.372–0.863) and OS (low vs. high, p = 0.007; HR = 0.560, 95% CI 0.366–0.856).
More importantly, our results showed that the significant correlation between LDH and survival outcomes was present specifically in T3–4 patients, rather than in T1–2 patients. Additionally, multivariate analysis of DFS and OS for patients with T3–4 showed that LDH was an independent prognostic factor for both DFS (low vs. high, p = 0.038; HR = 0.590, 95% CI 0.359–0.971) and OS (low vs. high, p = 0.009; HR = 0.516, 95% CI 0.313–0.848). Besides, multivariate analysis also disclosed that N2–3 and age ≥60 were correlated with worse prognosis, and these results were consistent with previous study (15, 48, 49). Based on the findings above, patients with high LDH, N2–3 and age ≥60 tend to experience worse survival outcomes, and this group of patients may need to adopt more intensive follow-up care.
One former study showed that prognostic nutritional index (PNI) played an important role in predicting survival outcomes in hypopharyngeal cancer patients who received surgery; however, the population enrolled in this study was relatively small (48). Recent studies also looked into inflammation index such as NLR and CPR (50, 51). The superior role of NLR, CPR, and LDH in predicting survival was controversial; nevertheless, it may be of great value to incorporate the parameters above in evaluating hypopharyngeal cancer patients prognosis (52, 53).
Nasopharyngeal cancer (NPC) is another head and neck cancer that affects the throat, and the role of LDH in nasopharyngeal cancer had been exhaustively evaluated. Jun M et al. reported that high baseline LDH levels were related with advanced stage and served as an inferior predictor for OS, DFS, and DMFS for NPC patients. Patients with elevated LDH levels may benefit from more aggressive treatment modalities (54). However, Ming-huang H et al. found that LDH was too, a poor predictor for local relapse-free in survival NPC patients (55). For metastatic NPC patients, elevated LDH also was an adverse prognostic factor (56). Moreover, longitudinal variation of serum LDH predicted chemotherapy response in metastatic NPC (57). In addition, prognostic nomogram incorporating LDH and other variables showed satisfactory efficacy in predicting OS of NPC patients (58, 59).
Meanwhile, our study still has certain limitations. First, the present study was performed retrospectively, and therefore, more prospective studies are urgently desired to validate our analysis. Second, the cut-off LDH value was to define the higher LDH level; however, different institutions may have various parameters, which limit the utility of this LDH cut-off value. For patients with advanced disease, concurrent cisplatin-based chemotherapy and radiotherapy is recommended (60), for having equivalent treatment efficacy and maintaining larynx function intact compared to surgery (10–12, 61, 62). However, with radical surgery as the major treatment modality in our institution, the present study failed to investigate LDH prognostic value in patients who received definitive chemoradiotherapy as their initial treatment option. Moreover, our study did not evaluate the expression level of LDHA in tumor samples. Further investigation of the expression levels of LDHA in tumor tissues, its relation with serum LDH, and the clinical significance is warranted. Besides, we noticed significantly low incidence rate (2.5%) of female hypopharyngeal cancer patients during the data collecting process. In contrast, the incidences of female hypopharyngeal cancer in America (16.3–20%) were reported to be higher than that in our study (1, 63). However, our data regarding the low incidence of female patients was supported by other studies carried out in China, Japan, and Korea (1.6–6.2%) (48, 61, 64). Therefore, our results and conclusions may have limited utility for the female population. Above all, our study shed some light on LDH clinical utility in predicting hypopharyngeal cancer patients’ oncological outcomes.
Conclusions
Pretreatment serum LDH is an independent prognostic factor in hypopharyngeal cancer patients who underwent primary surgery. High pretreatment serum LDH predicts poor prognosis. Patients with high LDH, N2–3 and age ≥60 are inclined to experience worse survival outcomes. Therefore, more intensive follow-up care should be given to these patients.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: institutional data constrained by IRB. Requests to access these datasets should be directed to bGl1eWltaW5jbkAxMzkuY29t.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board at Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JW was involved in the design, data collection, and writing. YL and XQ were taking part in conception and revision. KY and CC were involved in conception, data analysis, writing, and revision. All remaining authors took part in data collection and writing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
3. Hashim D, Genden E, Posner M, Hashibe M, Boffetta P. Head and neck cancer prevention: from primary prevention to impact of clinicians on reducing burden. Ann Oncol: Off J Eur Soc Med Oncol (2019) 30(5):744–56. doi: 10.1093/annonc/mdz084
4. Ho AS, Kim S, Tighiouart M, Gudino C, Mita A, Scher KS, et al. Association of Quantitative Metastatic Lymph Node Burden With Survival in Hypopharyngeal and Laryngeal Cancer. JAMA Oncol (2018) 4(7):985–9. doi: 10.1001/jamaoncol.2017.3852
5. Deng XY, Su Y, Zheng L, Xie CM, Gu MF, Zeng RF, et al. Analysis of cervical and retropharyngeal lymph node metastases in the patients with hypopharyngeal carcinoma with computed tomography and magnetic resonance imaging. Chin J Cancer (2010) 29(2):189–93. doi: 10.5732/cjc.009.10298
6. Krishnatreya M, Kataki AC, Sharma JD, Baishya N, Rahman T, Bhattcharyya M, et al. A Survival Analysis of Hypopharyngeal Cancer Patients: A Hospital-Cancer registry Based Study. Indian J Otolaryngol Head Neck Surgery: Off Publ Assoc Otolaryngologists India (2019) 71(Suppl 1):798–804. doi: 10.1007/s12070-018-1556-4
7. Jakobsen KK, Wingstrand VL, Jensen JS, Grønhøj C, Jensen DH, Karnov K, et al. Incidence and survival of hypopharyngeal cancer: a Danish Nation-Wide Study from 1980 to 2014. Acta Oncol (Stockholm Sweden) (2019) 58(11):1570–6. doi: 10.1080/0284186x.2019.1657585
8. Hochfelder CG, McGinn AP, Mehta V, Castellucci E, Kabarriti R, Ow TJ. Treatment sequence and survival in locoregionally advanced hypopharyngeal cancer: A surveillance, epidemiology, and end results-based study. Laryngoscope (2019) 130(11):2611–21. doi: 10.1002/lary.28452
9. Petersen JF, Timmermans AJ, van Dijk BAC, Overbeek LIH, Smit LA, Hilgers FJM, et al. Trends in treatment, incidence and survival of hypopharynx cancer: a 20-year population-based study in the Netherlands. Eur Arch Oto Rhino Laryngol: Off J Eur Fed Oto Rhino Laryngol Societies (EUFOS): Affiliated German Soc Oto Rhino Laryngol - Head Neck Surg (2018) 275(1):181–9. doi: 10.1007/s00405-017-4766-6
10. Chung EJ, Jeong WJ, Jung YH, Kwon SK, Kwon TK, Ahn SH, et al. Long-term oncological and functional outcomes of induction chemotherapy followed by (chemo)radiotherapy vs definitive chemoradiotherapy vs surgery-based therapy in locally advanced stage III/IV hypopharyngeal cancer: Multicenter review of 266 cases. Oral Oncol (2019) 89:84–94. doi: 10.1016/j.oraloncology.2018.12.015
11. Su X, He HC, Ye ZL, Zhou DL, Liu Q, Yang XH, et al. A 10-Year Study on Larynx Preservation Compared With Surgical Resection in Patients With Locally Advanced Laryngeal and Hypopharyngeal Cancers. Front Oncol (2020) 10:535893. doi: 10.3389/fonc.2020.535893
12. Iwae S, Fujii M, Hayashi R, Hasegawa Y, Fujii T, Okami K, et al. Matched-pair analysis of patients with advanced hypopharyngeal cancer: surgery versus concomitant chemoradiotherapy. Int J Clin Oncol (2017) 22(6):1001–8. doi: 10.1007/s10147-017-1151-9
13. Rodrigues J, Breda E, Monteiro E. Surgically-Treated Locoregionally Advanced Hypopharyngeal Cancer: Outcomes. Int Arch Oto Rhino Laryngol (2018) 22(4):443–8. doi: 10.1055/s-0038-1641562
14. Lo WC, Wu CT, Wang CP, Yang TL, Lou PJ, Ko JY, et al. Lymph Node Ratio Predicts Recurrence and Survival for Patients with Resectable Stage 4 Hypopharyngeal Cancer. Ann Surg Oncol (2017) 24(6):1707–13. doi: 10.1245/s10434-017-5770-1
15. Heng Y, Zhu X, Zhou L, Zhang M, Zhou H, Tao L. The presence of risk factors and corresponding treatment strategies post-surgical resection in stage IV hypopharyngeal squamous cell carcinoma patients: a retrospective cohort study. Ann Trans Med (2020) 8(5):189. doi: 10.21037/atm.2020.01.102
16. Augoff K, Hryniewicz-Jankowska A, Tabola R. Lactate dehydrogenase 5: an old friend and a new hope in the war on cancer. Cancer Lett (2015) 358(1):1–7. doi: 10.1016/j.canlet.2014.12.035
17. Świderek K, Tuñón I, Martí S, Moliner V. Protein Conformational Landscapes and Catalysis. Influence of Active Site Conformations in the Reaction Catalyzed by L-Lactate Dehydrogenase. ACS Catalysis (2015) 5(4):1172–85. doi: 10.1021/cs501704f
18. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Sci (New York NY) (5930) 2009) 324:1029–33. doi: 10.1126/science.1160809
19. Yeung C, Gibson AE, Issaq SH, Oshima N, Baumgart JT, Edessa LD, et al. Targeting Glycolysis through Inhibition of Lactate Dehydrogenase Impairs Tumor Growth in Preclinical Models of Ewing Sarcoma. Cancer Res (2019) 79(19):5060–73. doi: 10.1158/0008-5472.Can-19-0217
20. Serganova I, Cohen IJ, Vemuri K, Shindo M, Maeda M, Mane M, et al. LDH-A regulates the tumor microenvironment via HIF-signaling and modulates the immune response. PloS One (2018) 13(9):e0203965. doi: 10.1371/journal.pone.0203965
21. Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL. Lactate dehydrogenase 5 expression in operable colorectal cancer: strong association with survival and activated vascular endothelial growth factor pathway–a report of the Tumour Angiogenesis Research Group. J Clin Oncol: Off J Am Soc Clin Oncol (2006) 24(26):4301–8. doi: 10.1200/jco.2006.05.9501
22. Azuma M, Shi M, Danenberg KD, Gardner H, Barrett C, Jacques CJ, et al. Serum lactate dehydrogenase levels and glycolysis significantly correlate with tumor VEGFA and VEGFR expression in metastatic CRC patients. Pharmacogenomics (2007) 8(12):1705–13. doi: 10.2217/14622416.8.12.1705
23. Jin L, Chun J, Pan C, Alesi GN, Li D, Magliocca KR, et al. Phosphorylation-mediated activation of LDHA promotes cancer cell invasion and tumour metastasis. Oncogene (2017) 36(27):3797–806. doi: 10.1038/onc.2017.6
24. Rizwan A, Serganova I, Khanin R, Karabeber H, Ni X, Thakur S, et al. lactate, and metastases in 4T1 breast tumors. Clin Cancer Research: an Off J Am Assoc Cancer Res (2013) 19(18):5158–69. doi: 10.1158/1078-0432.Ccr-12-3300
25. Jin HF, Wang JF, Shao M, Zhou K, Ma X, Lv XP. Down-Regulation of miR-7 in Gastric Cancer Is Associated With Elevated LDH-A Expression and Chemoresistance to Cisplatin. Front Cell Dev Biol (2020) 8:555937. doi: 10.3389/fcell.2020.555937
26. Koukourakis MI, Kakouratos C, Kalamida D, Bampali Z, Mavropoulou S, Sivridis E, et al. Hypoxia-inducible proteins HIF1α and lactate dehydrogenase LDH5, key markers of anaerobic metabolism, relate with stem cell markers and poor post-radiotherapy outcome in bladder cancer. Int J Radiat Biol (2016) 92(7):353–63. doi: 10.3109/09553002.2016.1162921
27. Seth P, Csizmadia E, Hedblom A, Vuerich M, Xie H, Li M, et al. Deletion of Lactate Dehydrogenase-A in Myeloid Cells Triggers Antitumor Immunity. Cancer Res (2017) 77(13):3632–43. doi: 10.1158/0008-5472.Can-16-2938
28. Van Wilpe S, Koornstra R, Den Brok M, De Groot JW, Blank C, De Vries J, et al. Lactate dehydrogenase: a marker of diminished antitumor immunity. Oncoimmunology (2020) 9(1):1731942. doi: 10.1080/2162402x.2020.1731942
29. Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomarkers: Section A Dis Markers (2017) 19(4):353–63. doi: 10.3233/cbm-160336
30. Faloppi L, Del Prete M, Casadei Gardini A, Santini D, Silvestris N, Bianconi M, et al. The correlation between LDH serum levels and clinical outcome in advanced biliary tract cancer patients treated with first line chemotherapy. Sci Rep (2016) 6:24136. doi: 10.1038/srep24136
31. Deng T, Zhang J, Meng Y, Zhou Y, Li W. Higher pretreatment lactate dehydrogenase concentration predicts worse overall survival in patients with lung cancer. Medicine (2018) 97(38):e12524. doi: 10.1097/md.0000000000012524
32. Sagman U, Feld R, Evans WK, Warr D, Shepherd FA, Payne D, et al. The prognostic significance of pretreatment serum lactate dehydrogenase in patients with small-cell lung cancer. J Clin Oncol: Off J Am Soc Clin Oncol (1991) 9(6):954–61. doi: 10.1200/jco.1991.9.6.954
33. Pelizzari G, Basile D, Zago S, Lisanti C, Bartoletti M, Bortot L, et al. Lactate Dehydrogenase (LDH) Response to First-Line Treatment Predicts Survival in Metastatic Breast Cancer: First Clues for A Cost-Effective and Dynamic Biomarker. Cancers (2019) 11(9):1243. doi: 10.3390/cancers11091243
34. Namikawa T, Ishida N, Tsuda S, Fujisawa K, Munekage E, Iwabu J, et al. Prognostic significance of serum alkaline phosphatase and lactate dehydrogenase levels in patients with unresectable advanced gastric cancer. Gastric cancer: Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2019) 22(4):684–91. doi: 10.1007/s10120-018-0897-8
35. Kostakis ID, Vaiopoulos AG, Philippou A, Papavassiliou AG, Koutsilieris M, Kouraklis G. Preoperative serum lactate dehydrogenase levels in colorectal and gastric cancer: a hospital-based case-control study. Biomarkers Med (2013) 7(1):131–7. doi: 10.2217/bmm.12.86
36. Petrelli F, Cabiddu M, Coinu A, Borgonovo K, Ghilardi M, Lonati V, et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol (Stockholm Sweden) (2015) 54(7):961–70. doi: 10.3109/0284186x.2015.1043026
37. Li J, Wu MF, Lu HW, Chen Q, Lin ZQ, Wang LJ. Pretreatment serum lactate dehydrogenase is an independent prognostic factor for patients receiving neoadjuvant chemotherapy for locally advanced cervical cancer. Cancer Med (2016) 5(8):1863–72. doi: 10.1002/cam4.779
38. Robbins KT, Clayman G, Levine PA, Medina J, Sessions R, Shaha A, et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Oto Laryngology–head Neck Surg (2002) 128(7):751–8. doi: 10.1001/archotol.128.7.751
39. Shi L, An S, Liu Y, Liu J, Wang F. PCK1 Regulates Glycolysis and Tumor Progression in Clear Cell Renal Cell Carcinoma Through LDHA. OncoTargets Ther (2020) 13:2613–27. doi: 10.2147/ott.S241717
40. Su Y, Yu QH, Wang XY, Yu LP, Wang ZF, Cao YC, et al. JMJD2A promotes the Warburg effect and nasopharyngeal carcinoma progression by transactivating LDHA expression. BMC Cancer (2017) 17(1):477. doi: 10.1186/s12885-017-3473-4
41. Cui J, Shi M, Xie D, Wei D, Jia Z, Zheng S, et al. FOXM1 promotes the warburg effect and pancreatic cancer progression via transactivation of LDHA expression. Clin Cancer Research: an Off J Am Assoc Cancer Res (2014) 20(10):2595–606. doi: 10.1158/1078-0432.Ccr-13-2407
42. Li F, Xiang H, Pang Z, Chen Z, Dai J, Chen S, et al. Association between lactate dehydrogenase levels and oncologic outcomes in metastatic prostate cancer: A meta-analysis. Cancer Med (2020) 9(19):7341–51. doi: 10.1002/cam4.3108
43. Fu Y, Lan T, Cai H, Lu A, Yu W. Meta-analysis of serum lactate dehydrogenase and prognosis for osteosarcoma. Medicine (2018) 97(19):e0741. doi: 10.1097/md.0000000000010741
44. Wu M, Lin P, Xu L, Yu Z, Chen Q, Gu H, et al. Prognostic Role of Serum Lactate Dehydrogenase in Patients With Urothelial Carcinoma: A Systematic Review and Meta-Analysis. Front Oncol (2020) 10:677. doi: 10.3389/fonc.2020.00677
45. Liu D, Wang D, Wu C, Zhang L, Mei Q, Hu G, et al. Prognostic significance of serum lactate dehydrogenase in patients with breast cancer: a meta-analysis. Cancer Manage Res (2019) 11:3611–9. doi: 10.2147/cmar.S199260
46. Zhang M, Wei S, Su L, Lv W, Hong J. Prognostic significance of pretreated serum lactate dehydrogenase level in nasopharyngeal carcinoma among Chinese population: A meta-analysis. Medicine (2016) 95(35):e4494. doi: 10.1097/md.0000000000004494
47. Armstrong AJ, George DJ, Halabi S. Serum lactate dehydrogenase predicts for overall survival benefit in patients with metastatic renal cell carcinoma treated with inhibition of mammalian target of rapamycin. J Clin Oncol: Off J Am Soc Clin Oncol (2012) 30(27):3402–7. doi: 10.1200/jco.2011.40.9631
48. Ye LL, Oei RW, Kong FF, Du CR, Zhai RP, Ji QH, et al. The prognostic value of preoperative prognostic nutritional index in patients with hypopharyngeal squamous cell carcinoma: a retrospective study. J Trans Med (2018) 16(1):12. doi: 10.1186/s12967-018-1391-0
49. Visini M, Giger R, Shelan M, Elicin O, Anschuetz L. Predicting Factors for Oncological and Functional Outcome in Hypopharyngeal Cancer. Laryngoscope (2020). doi: 10.1002/lary.29186
50. Lo WC, Wu CT, Wang CP, Yang TL, Lou PJ, Ko JY, et al. The Pretreatment Neutrophil-to-Lymphocyte Ratio is a Prognostic Determinant of T3-4 Hypopharyngeal Squamous Cell Carcinoma. Ann Surg Oncol (2017) 24(7):1980–8. doi: 10.1245/s10434-017-5865-8
51. Katano A, Takahashi W, Yamashita H, Yamamoto K, Ando M, Yoshida M, et al. The impact of elevated C-reactive protein level on the prognosis for oro-hypopharynx cancer patients treated with radiotherapy. Sci Rep (2017) 7(1):17805. doi: 10.1038/s41598-017-18233-w
52. Inomata M, Hirai T, Seto Z, Tokui K, Taka C, Okazawa S, et al. Clinical Parameters for Predicting the Survival in Patients with Squamous and Non-squamous-cell NSCLC Receiving PD-1 Inhibitor Therapy. Pathol Oncol Research: POR (2020) 26(1):327–33. doi: 10.1007/s12253-018-0473-x
53. Galvano A, Peri M, Guarini AA, Castiglia M, Grassadonia A, De Tursi M, et al. Analysis of systemic inflammatory biomarkers in neuroendocrine carcinomas of the lung: prognostic and predictive significance of NLR, LDH, ALI, and LIPI score. Ther Adv Med Oncol (2020) 12:1758835920942378. doi: 10.1177/1758835920942378
54. Zhou GQ, Tang LL, Mao YP, Chen L, Li WF, Sun Y, et al. Baseline serum lactate dehydrogenase levels for patients treated with intensity-modulated radiotherapy for nasopharyngeal carcinoma: a predictor of poor prognosis and subsequent liver metastasis. Int J Radiat Oncol Biology Phys (2012) 82(3):e359–65. doi: 10.1016/j.ijrobp.2011.06.1967
55. Wan XB, Wei L, Li H, Dong M, Lin Q, Ma XK, et al. High pretreatment serum lactate dehydrogenase level correlates with disease relapse and predicts an inferior outcome in locally advanced nasopharyngeal carcinoma. Eur J Cancer (Oxford England: 1990) (2013) 49(10):2356–64. doi: 10.1016/j.ejca.2013.03.008
56. Jin Y, Ye X, Shao L, Lin BC, He CX, Zhang BB, et al. Serum lactic dehydrogenase strongly predicts survival in metastatic nasopharyngeal carcinoma treated with palliative chemotherapy. Eur J Cancer (Oxford England: 1990) (2013) 49(7):1619–26. doi: 10.1016/j.ejca.2012.11.032
57. Huang L, Sim AYL, Wu Y, Liang Z, Li K, Du Y, et al. Lactate dehydrogenase kinetics predict chemotherapy response in recurrent metastatic nasopharyngeal carcinoma. Ther Adv Med Oncol (2020) 12:1758835920970050. doi: 10.1177/1758835920970050
58. Zhang LL, Xu F, Song D, Huang MY, Huang YS, Deng QL, et al. Development of a Nomogram Model for Treatment of Nonmetastatic Nasopharyngeal Carcinoma. JAMA Netw Open (2020) 3(12):e2029882. doi: 10.1001/jamanetworkopen.2020.29882
59. Jiang Y, Qu S, Pan X, Huang S, Zhu X. Prognostic Nomogram For Locoregionally Advanced Nasopharyngeal Carcinoma. Sci Rep (2020) 10(1):861. doi: 10.1038/s41598-020-57968-x
61. Kim JW, Kim MS, Kim SH, Kim JH, Lee CG, Kim GE, et al. Definitive Chemoradiotherapy Versus Surgery Followed by Adjuvant Radiotherapy in Resectable Stage III/IV Hypopharyngeal Cancer. Cancer Res Treatment: Off J Korean Cancer Assoc (2016) 48(1):45–53. doi: 10.4143/crt.2014.340
62. Cui J, Wang L, Piao J, Huang H, Chen W, Chen Z, et al. Initial surgical versus non-surgical treatments for advanced hypopharyngeal cancer: A meta-analysis with trial sequential analysis. Int J Surg (London England) (2020) 82:249–59. doi: 10.1016/j.ijsu.2020.04.059
63. Kılıç S, Kılıç SS, Hsueh WD, Eloy JA, Baredes S, Woo Park RC, et al. Radiotherapy modality as a predictor of survival in hypopharyngeal cancer. Head Neck (2018) 40(11):2441–8. doi: 10.1002/hed.25360
Keywords: lactate dehydrogenase (LDH), hypopharyngeal cancer, prognosis, retrospective, T3–4
Citation: Wu J, You K, Chen C, Zhong H, Jiang Y, Mo H, Song J, Qiu X and Liu Y (2021) High Pretreatment LDH Predicts Poor Prognosis in Hypopharyngeal Cancer. Front. Oncol. 11:641682. doi: 10.3389/fonc.2021.641682
Received: 14 December 2020; Accepted: 02 February 2021;
Published: 11 March 2021.
Edited by:
Yury O. Nunez Lopez, AdventHealth, United StatesReviewed by:
Hong-Quan Duong, Hanoi University of Public Health, VietnamCarlotta Granchi, University of Pisa, Italy
Copyright © 2021 Wu, You, Chen, Zhong, Jiang, Mo, Song, Qiu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yimin Liu, bGl1eWltaW5jbkAxMzkuY29t; Xingsheng Qiu, cWl1eGluZ3NoZW5nQHNpbmEuY24=
†These authors have contributed equally to this work and share first authorship
 Jialing Wu
Jialing Wu Kaiyun You
Kaiyun You Changlong Chen†
Changlong Chen† Yimin Liu
Yimin Liu