
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 21 May 2021
Sec. Gastrointestinal Cancers
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.641487
 Peng Liu1†
Peng Liu1† Jinhong Wei2†
Jinhong Wei2† Feiyu Mao1
Feiyu Mao1 Zechang Xin3
Zechang Xin3 Heng Duan3
Heng Duan3 Yan Du3
Yan Du3 Xiaodong Wang4
Xiaodong Wang4 Zhennan Li4
Zhennan Li4 Jianjun Qian4
Jianjun Qian4 Jie Yao1,4*
Jie Yao1,4*Hepatocellular carcinoma (HCC) is one of the most common types of cancer worldwide and its incidence continues to increase year by year. Endoplasmic reticulum stress (ERS) caused by protein misfolding within the secretory pathway in cells and has an extensive and deep impact on cancer cell progression and survival. Growing evidence suggests that the genes related to ERS are closely associated with the occurrence and progression of HCC. This study aimed to identify an ERS-related signature for the prospective evaluation of prognosis in HCC patients. RNA sequencing data and clinical data of patients from HCC patients were obtained from The Cancer Genome Atlas (TCGA) and The International Cancer Genome Consortium (ICGC). Using data from TCGA as a training cohort (n=424) and data from ICGC as an independent external testing cohort (n=243), ERS-related genes were extracted to identify three common pathways IRE1, PEKR, and ATF6 using the GSEA database. Through univariate and multivariate Cox regression analysis, 5 gene signals in the training cohort were found to be related to ERS and closely correlated with the prognosis in patients of HCC. A novel 5-gene signature (including HDGF, EIF2S1, SRPRB, PPP2R5B and DDX11) was created and had power as a prognostic biomarker. The prognosis of patients with high-risk HCC was worse than that of patients with low-risk HCC. Multivariate Cox regression analysis confirmed that the signature was an independent prognostic biomarker for HCC. The results were further validated in an independent external testing cohort (ICGC). Also, GSEA indicated a series of significantly enriched oncological signatures and different metabolic processes that may enable a better understanding of the potential molecular mechanism mediating the progression of HCC. The 5-gene biomarker has a high potential for clinical applications in the risk stratification and overall survival prediction of HCC patients. In addition, the abnormal expression of these genes may be affected by copy number variation, methylation variation, and post-transcriptional regulation. Together, this study indicated that the genes may have potential as prognostic biomarkers in HCC and may provide new evidence supporting targeted therapies in HCC.
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer-related mortality worldwide (1). It has been reported that most liver cancers are closely related to hepatitis B virus (HBV) infection and liver cirrhosis (2). Studies have shown that the morbidity and mortality associated with HCC continuing to increasing year on year and has become a major global public health problem (3). Clinical outcomes for HCC patients are difficult to predict based on classical histological classifications due to tumor heterogeneity (4). There is an urgent need to establish efficient and accurate risk assessment models to assess the prognosis in HCC patients to improve diagnosis and treatment.
Specific tumor biomarkers including α-fetoprotein, carcinoembryonic antigen, cytokines, nucleic acids and microRNAs have been used for early diagnosis and prognosis of HCC (5, 6). However, the prognosis remains unsatisfactory. Studies have revealed that ERS is positively associated with the occurrence and development of various human diseases including cancer (7), Studies have revealed that Pekinenin E can inhibit the growth of HCC by promoting ERS-mediated cell death and cell cycle arrest (8), and Endoplasmic reticulum stress can also promote HCC immune escape by transferring specific miRNAs to macrophages infiltrating the tumor microenvironment (9) yet the role of ERS-related prediction model in HCC remains to be determined.
In this study, we identified 374 cases of HCC that involved ERS-related genes from the TCGA database. A Cox regression analysis was applied to identify an ERS-related 5 gene signature that was used to construct a prognostic model. The model was validated using data from the ICGC database. We performed Kaplan-Meier survival and ROC (receiver operating characteristic) analyses to evaluate the performance of the model. The nomogram performed well in predicting the overall survival rate of HCC patients and can be used to predict prognosis in HCC. Based on comprehensive genomic data analysis, we aimed to demonstrate the value of an ERS-related signature to improve prognosis in HCC.
Harmonized RNA sequencing data (HTSeq-FPKM) and associated clinical information for Liver Hepatocellular Carcinoma (TCGA-LIHC) were obtained from TCGA database (https://portal.gdc.cancer.gov/). RNA-seq data included 424 cases, 374 of which were tumor samples and 50 adjacent normal tissues. The samples with survival time less than 90 days were deleted, and finally 329 samples with complete clinical information were used for analysis. The test dataset of gene expression and clinical trait data (the Liver Cancer-RIKEN JP) were downloaded from the ICGC database (https://dcc.icgc.org/). The ERS-related genes were obtained from the Molecular signatures Database (https://www.gsea-msigdb.org/gsea/msigdb/index.jsp), and involved the IRE1(M10426), PERK(M14369) and ATF6(M23457) pathways. Studies have shown that these three pathways play a pivotal role in the process of endoplasmic reticulum stress response (10). Then we obtained the expression levels of ERS-related genes in the training and testing cohort for subsequent analysis. Multi-omics used GSCAlite web tool (http://bioinfo.life.hust.edu.cn/web/GSCALite/) for subsequent analysis (11).
Univariate and multivariate regression analyses were conducted using the “survival together with forestplot” software package in R to screen ERS-related genes with significant prognostic value (p<0.01). Multivariate Cox regression analysis showed the minimum value of Akaike’s information criterion was 1032.78. This was the best cutoff point. The prognostic gene signature was constructed based on the linear combination of Cox regression coefficient multiplied by gene expression.
The risk score was calculated as = (coefficientGene 1 × expression level of Gene 1) + (coefficientGene 2×expression level of Gene 2) +… + (coefficient Gene x × expression level of Gene x). According to the median risk score, the same parameters were used to classify patients in the training and validation cohorts into high and low-risk groups. We then used the “survival” package in R to draw a Kaplan–Meier survival curve to assess the survival rate between the high and low risk groups. The Receiver Operating Characteristic (ROC) curve and the area under the curve (AUC) over time were used to assess the predictive performance of the genetic and clinical factors in predicting survival at 1, 3 and 5 years. Also, the “pheatmap” package in R was used to illustrate the risk map. In the survival analysis, P-values < 0.05 were considered statistically significant and used for subsequent analysis
We downloaded the corresponding liver cancer transcriptome data and clinical information from the ICGC database and used the same risk score formula to calculate the risk score for each patient. We adopted the median risk score as the best parameter to divide the patients into high and low risk groups. We then used the ROC and Kaplan-Meier curves to test the predictive performance of the prognostic gene model.
Since the average survival time of HCC patients in clinical practice is about 6 months, in order to exclude the influence of premature death caused by postoperative infection, weakness, and other external factors, we filtered out samples with a survival period of less than 90 days. A total of 329 HCC patients were retained. The samples were divided into two groups based on each gene expression level, including the low and high expression groups. p value were calculated by the logrank test and the overall survival of patients was assessed by Kaplan-Meier survival analysis.
To determine the independent prognostic parameters and verify the independent prognostic value of gene signatures, we performed univariate and multivariate regression analysis including the 5-gene risk score and common clinic-pathological parameters (such as age, gender, stage, histological grade, vascular invasion, hepatitis virus infection and alcohol consumption). P-values of <0.05 were statistically significant.
Nomograms are widely applied to prognostic devices in oncology and medicine (12). The 5-gene signals determined by multivariate regression analysis were used as independent prognostic factors and included in the construction of the prognostic nomogram. The time-dependent receiver operating characteristic (ROC) curves were used to compare OS at 1, 3 and 5 years. We used the calibration chart and the consistency index (C index) to investigate the calibration and identification of the Nomogram (through the bootstrap method of 1000 resampling).
To clarify the underlying molecular mechanisms of the prognostic genetic models, Genomic Enrichment Analysis (GSEA) was used to explore the enrichment terminology in the pathway of the gene signature Kyoto Encyclopedia of Genes and Genomes (KEGG) related to ERS (13, 14). A p-value of < 0.05 was considered statistically significant.
To determine whether our ERS-related 5-gene signature is superior to other HCC prognostic models, we used receiver operating curve (ROC) to compare 7-mRNA signature (15) and 9-mRNA signature (16). We obtained relevant genes in these models from the literature, and constructed 1 years ROC curves for the entire TCGA cohort. Then compared these gene prognostic models obtained from the literature with the ERS-related 5-gene signature of this study to evaluate the advantages and disadvantages of each.
Multiple omics data are involved in this study regarding the selected target genes. We retrieved the whole exome sequencing results, copy number variants, methylation profile and miRNA expression profile from the TCGA GDC portal. The maf format file including the genomic variants was downloaded by maftools R package. The other omics data were retrieved by TCGAbiolinks R package. All the transcripts, mutations, probes and miRNAs are restricted to the target genes.
The R software package (4.0.2) was used for statistical analysis. A Pearson χ2 test and Fisher’s exact test were used to analyze the qualitative variables.
First, we identified three gene sets associated with ERS-related by searching the keyword “IRE1 PERK ATF6” in the MSigDB database. To investigate the expression level of these genes in HCC, we downloaded RNA-seq data from TCGA. In 329 liver cancer specimens, 88 genes of ERS-related were identified, The expression profiles of the 88 ERS- related genes in the training cohort were used to establish a prognostic model. The clinical-pathological characteristics of liver cancer patients from the two databases were shown in Table 1.
The univariate Cox regression model was used to identify a total of 20 ERS-related genes related to overall survival (OS) (Figure 1). Further multivariate Cox regression analysis was performed and finally five ERS-related genes were defined and used to create a prognostic model. Multivariate Cox regression analysis of the five ERS-related genes in the training cohort is shown in Table 2.
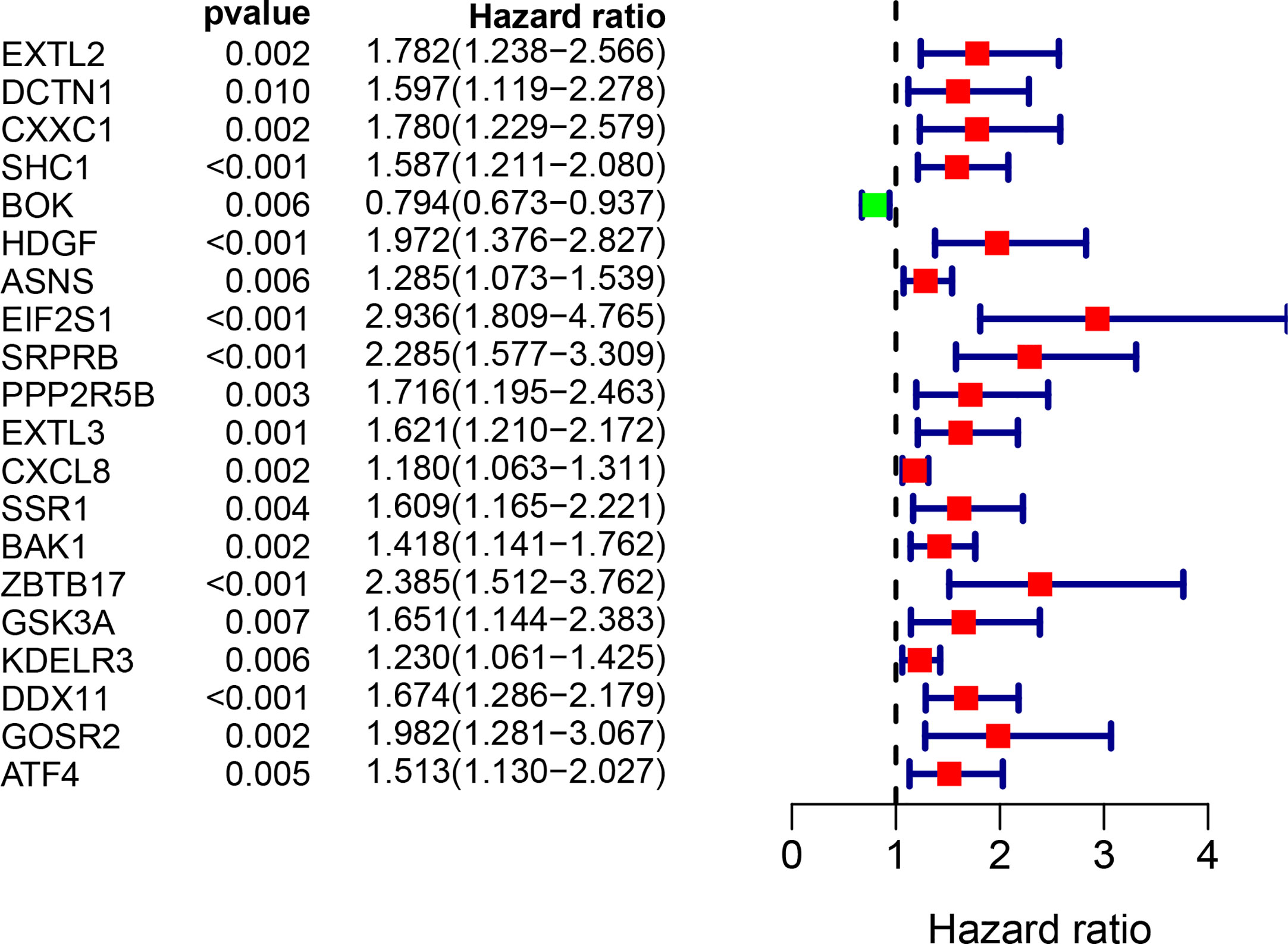
Figure 1 Univariate Cox regression analysis of 20 genes related to ERS in the training set of TCGA cohort.
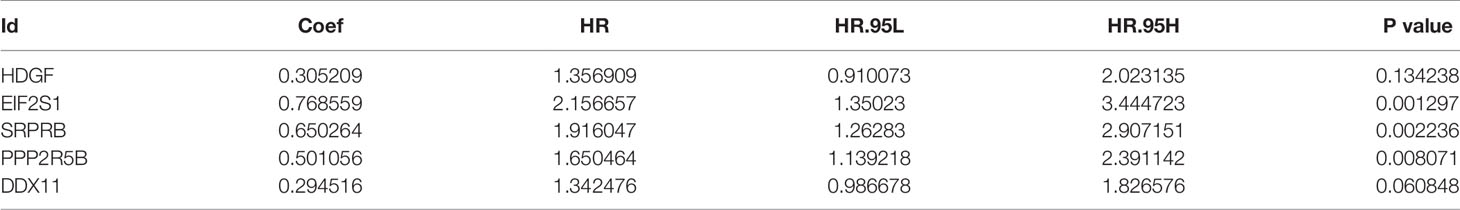
Table 2 Multivariate Cox regression analysis of the 5 genes associated with ERS in the training set of TCGA cohort.
The five genes related to ERS include HDGF, EIF2S1, SRPRB, PPP2R5B and DDX11. The model risk score =0.305* HDGF+0.769* EIF2S1+0.650* SRPRB+0.501* PPP2R5B+0.295* DDX11. Taking the median risk score (0.929 for the training cohort and 2.671 for the test cohort) as the threshold, the patients were separated into high and low-risk groups. Time-dependent ROC (AUC value) was applied to evaluate the prognostic ability of the 5-gene signals. In the training cohort, the AUC values of 1, 3 and 5 years OS predictions for the risk score were 0.815, 0.727 and 0.688 respectively (Figure 2A). However, in the test cohort, the AUC values of 1, 3 and 5 years were 0.631, 0.719 and 0.714 (Figure 2C). With increasing risk scores, the mortality rate of patients in the training and test cohorts gradually increased (Figures 2B, D). Subsequently, we also performed ROC analysis to evaluate whether 5-gene risk score can predict a better prognosis compared with common clinicopathological characteristics. As shown in the Figure 2E, the 5-gene risk score (AUC=0.804) has a better predictive effect on the prognosis of HCC than age, gender, stage, grade and TNM stage. These results indicate that the prognostic model has good predictive power and specificity.
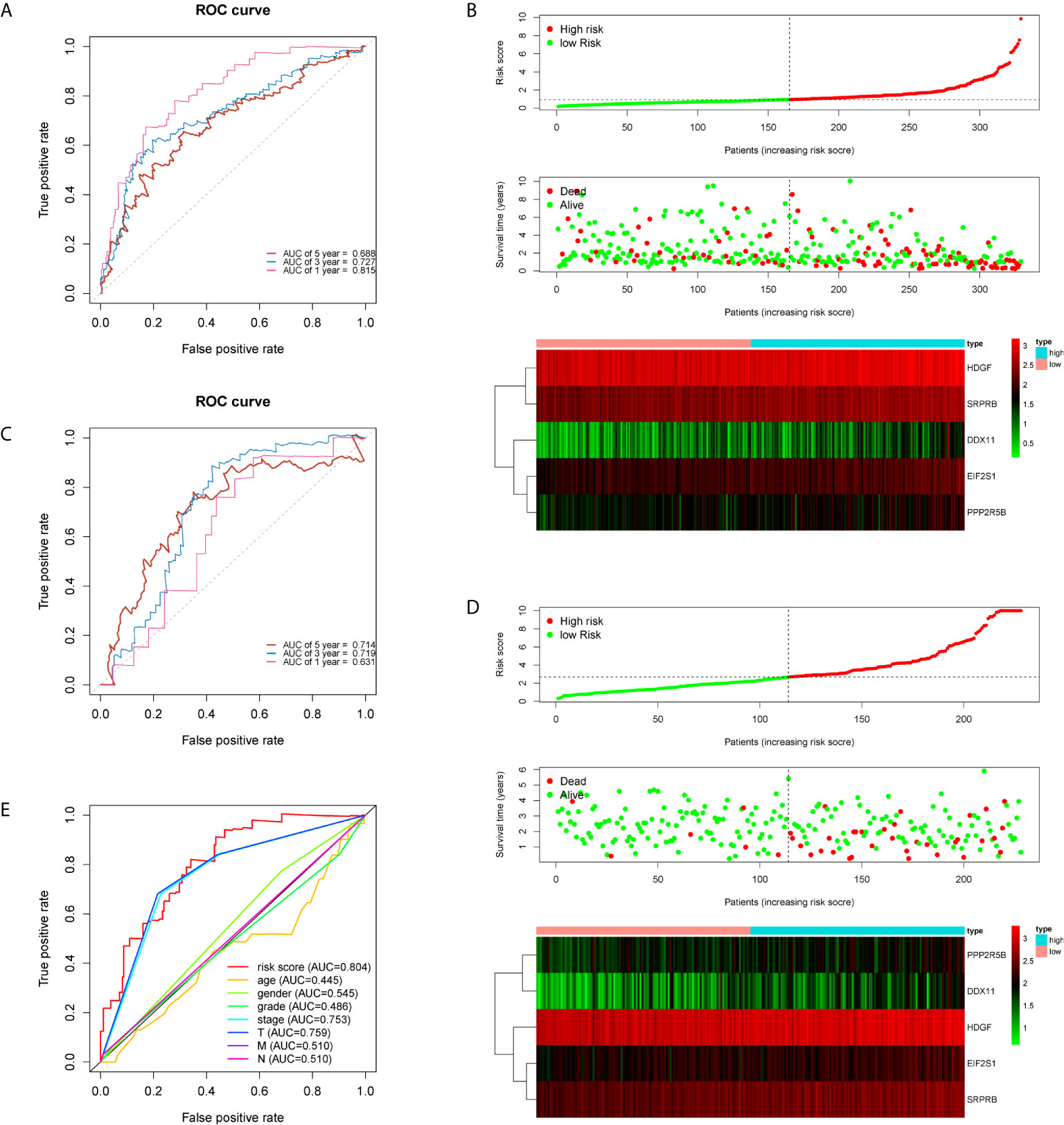
Figure 2 Time-dependent ROC analysis and risk scoring analysis were used for the 5-gene signature in HCC. Time-related ROC analysis (A), and risk score (B) in the training set of TCGA cohort. Time-related ROC analysis (C), and risk score (D) in the testing set of ICGC cohort. multi-index ROC analysis in the TCGA cohort (E).
To verify the prognostic value of the 5 genes, we divided the samples into high expression group and low expression group according to the expression value of each gene. The Kaplan-Meier survival analysis showed that patients with high expression of the five target genes tend to have a poor prognosis, while the low expression samples have a relatively good prognosis (Figure 3).
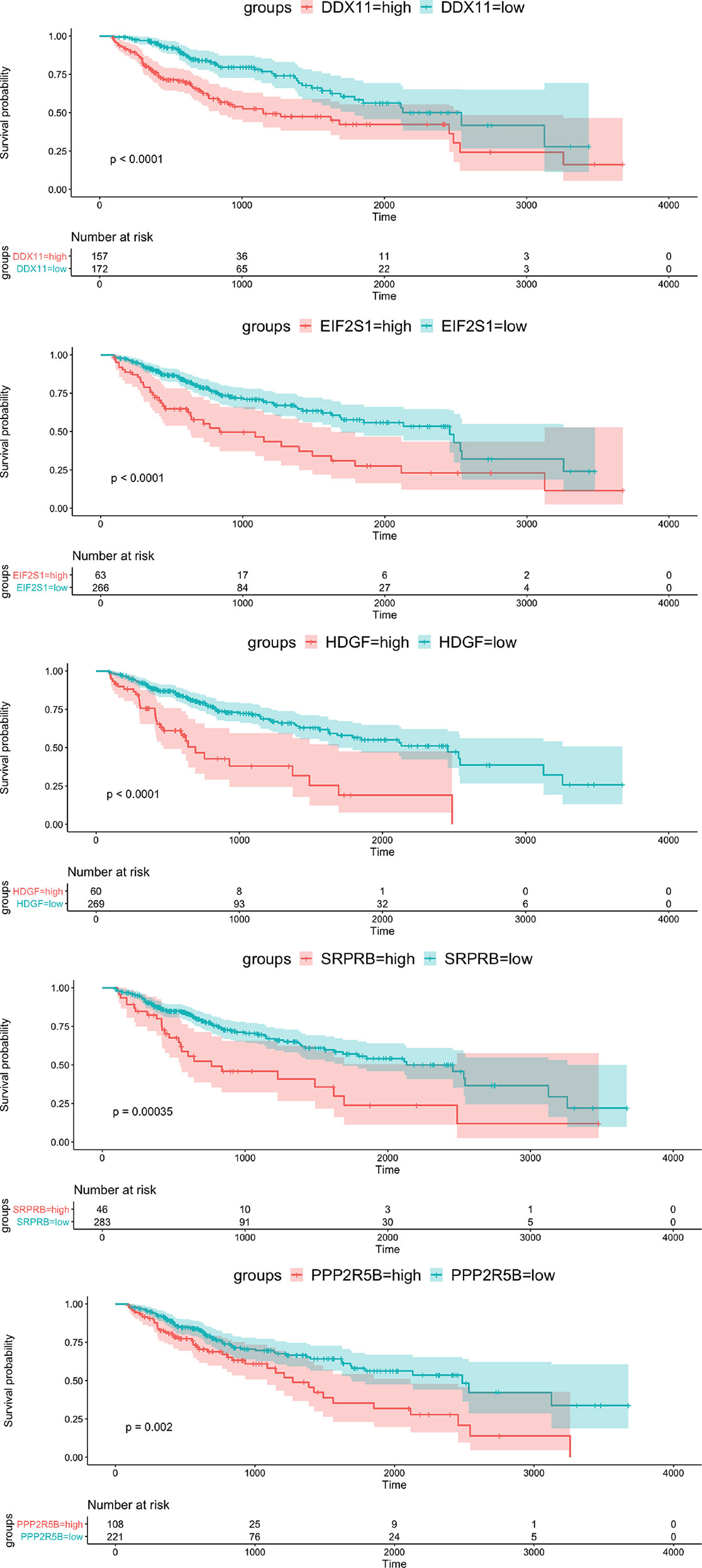
Figure 3 Kaplan-Meier survival curve of OS for 5 hub genes with survival significance in the TCGA cohort. Red and green colors represent high and low expression, respectively.
In order to further integrate clinical information to achieve multivariate survival analysis, we collected all clinical information records of liver cancer patients in the TCGA database, and filtered out indicators with missing values of more than 50%, and finally retained 35 clinical indicators, including age, gender, staging, Classification. We used the multivariate cox regression algorithm to identify clinical features that significantly affect the prognosis. For the results of the multivariate cox regression analysis, see the Supplementary Table S1. To better visualize the influence pattern of each clinical indicator on the prognosis, we used the significant clinical features to construct a forest map model, as shown in Figure 4A. It can be seen that whether to receive chemotherapy and radiotherapy after surgery were protective factors for survival and prognosis. Conversely, factors such as tumor status, tumor residual, creatinine value, total bilirubin value, histologic grade and pathological stage are risk factors. Next, to determine whether the prognostic model is independent of the common clinical features that predict the prognosis of HCC, we used two independent TCGA and ICGC data sets to perform univariate and multivariate Cox regression analysis. The results obtained consistently indicate that the prognostic risk model constructed based on endoplasmic reticulum stress-related genes is a significantly independent factor (Table 3). After integrating all 5 target genes and clinical information, the predictive performance of the risk model for TCGA samples and the predictive performance on independent verification data were shown in (Figures 4B, C). Results analysis revealed that the multivariate risk model can significantly distinguish two patients with different survival prognosis on both TCGA samples and independent validation data, and the logrank test p value is less than 0.0001. The nomogram of 1, 3 and 5 years OS in the cohort were shown in Figure 4D. The calibration chart shows the accuracy of assessing the prediction nomogram. The nomogram performed well when predicting the probability of survival at 1, 3 and 5 years (Figure 4E).
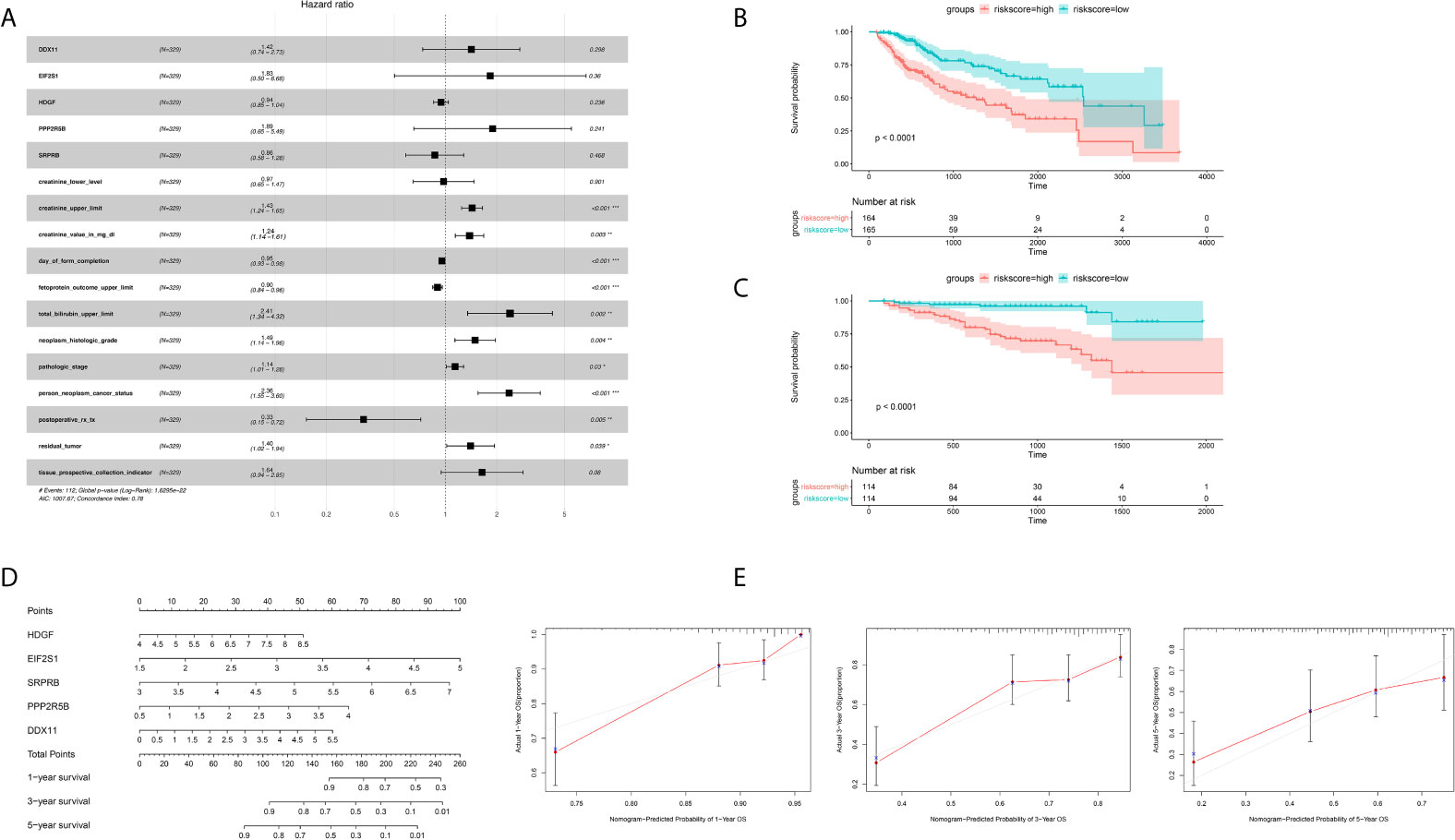
Figure 4 Identification of independent prognostic factors and establish forecast nomogram. (A) Forrest chart of HCC multivariate cox analysis in the TCGA cohort. (B) Kaplan-Meier curve of 5 gene markers in the TCGA population for the training set. (C) Kaplan-Meier curve of 5 gene markers in the ICGC population for the testing cohort. (D) Establishment of nomogram combining 5 gene markers in the TCGA cohort. (E) The Calibration curves, used for internal verification nomogram in the TCGA cohort. *p < 0.05, **p < 0.01, ***p < 0.001.
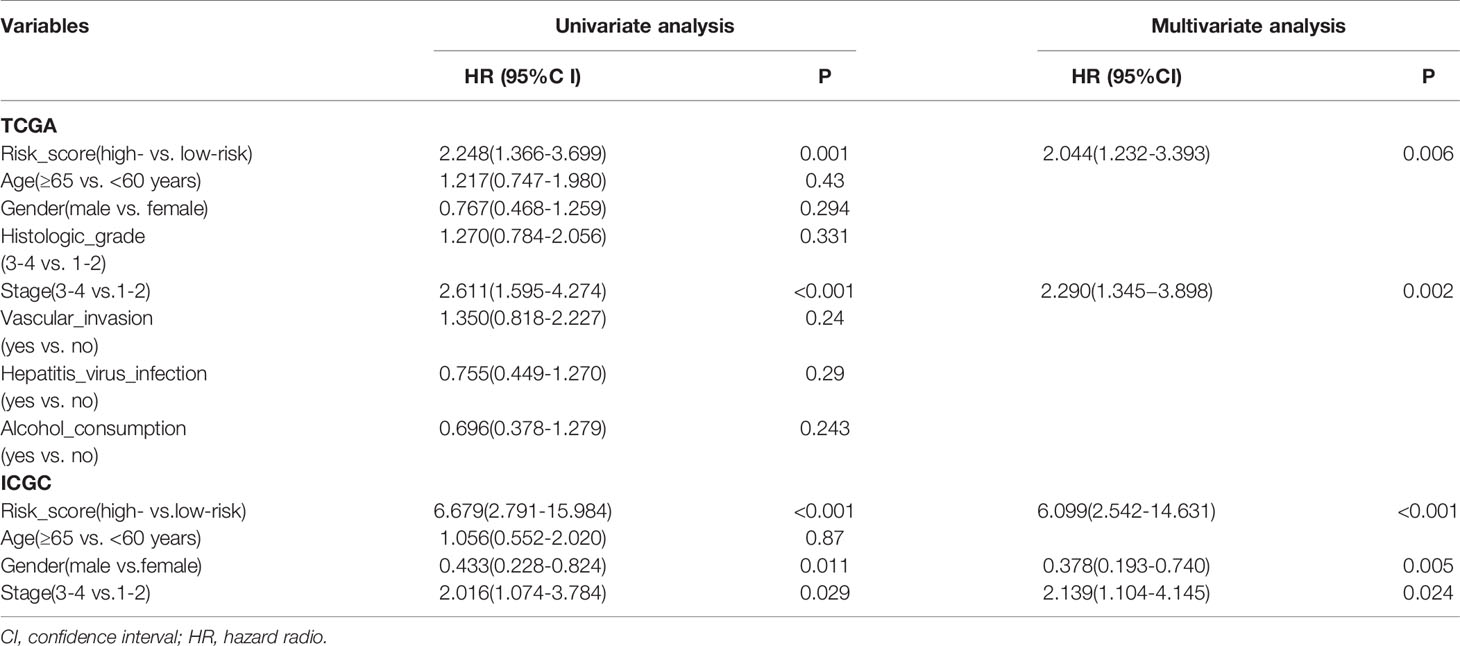
Table 3 Univariate and multivariate Cox regression analysis of prognostic factors of hepatocellular carcinoma patients in TCGA and ICGC cohorts.
PCA is often used to visualize the distribution of risk amongst different populations in line with the risk gene sets, ERS gene sets and the whole genome expression sets. We performed PCA analysis on the entire gene expression profiles and showed unclear separation in risk statuses (Figure 5A). According to the all genes related to ERS, the low and high-risk groups tended to be divided into two aspects. Patients in the low and the high-risk groups had different ERS status (Figure 5B). However, when based on the risk model genome there were significant differences between the high and low risk groups, shown a clearly separated state (Figure 5C).
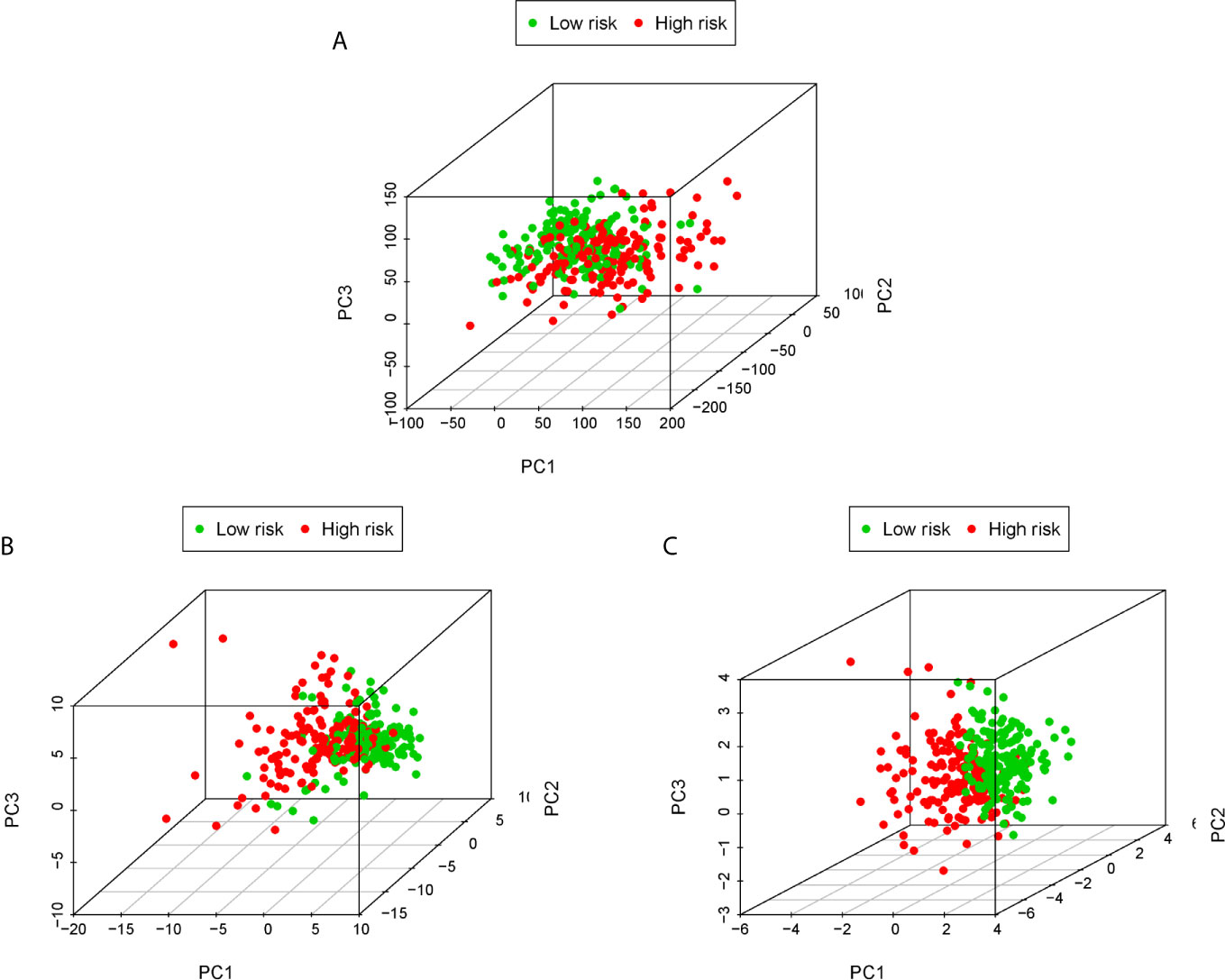
Figure 5 PCA analysis of each genome in the TCGA cohort. PCA analysis based on the entire genome expression profile (A), the all genes related to ERS (B) and risk model genome (C).
GSEA showed that the ERS-related gene signature was commonly concentrated in the basal transcription factors, cell cycle neurotrophin signaling pathway, oocyte meiosis, progesterone mediated oocyte maturation, purine metabolism, pyrimidine metabolism, RNA degradation, spliceosome and ubiquitin mediated proteolysis in high-risk patients (Figure 6A). In low-risk patients the main pathways involved were butanoate metabolism, complement and coagulation cascades, drug metabolism cytochrome p450, acid metabolism, glycine serine and threonine metabolism, primary bile acid biosynthesis, retinol metabolism, tryptophan metabolism, valine leucine and isoleucine degradation (Figure 6B).
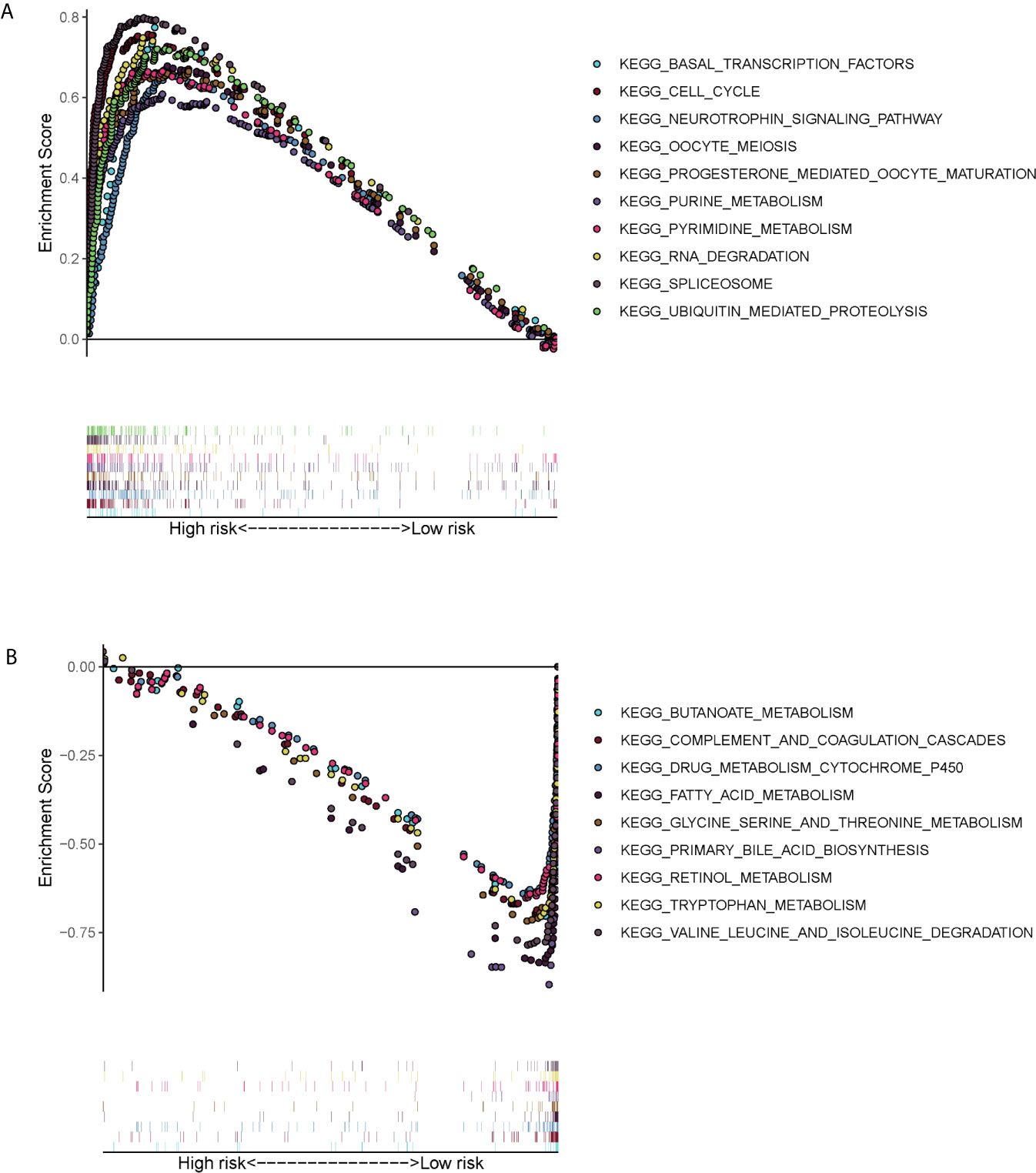
Figure 6 Gene set enrichment analysis (KEGG pathways) in the TCGA cohort. (A) Enrichment pathways for high-risk patients. (B) Enrichment pathways for low-risk patients.
To determine whether our ERS-related 5-gene signature is superior to other HCC prognostic models, we compared 7-mRNA signature (15) and 9-mRNA signature (16) for the entire TCGA cohort. Among the two prognostic models, 7-mRNA signature show that its 1 year AUC values was 0.773, the 9-mRNA signature show that its 1 year AUC values was 0.733. The above results indicate that two models have certain predictive power. However, compared with our 5-gene signature of this study, the predictive power of the ERS-related 5-gene signature is significantly higher than the three prognostic models (Figure 7).
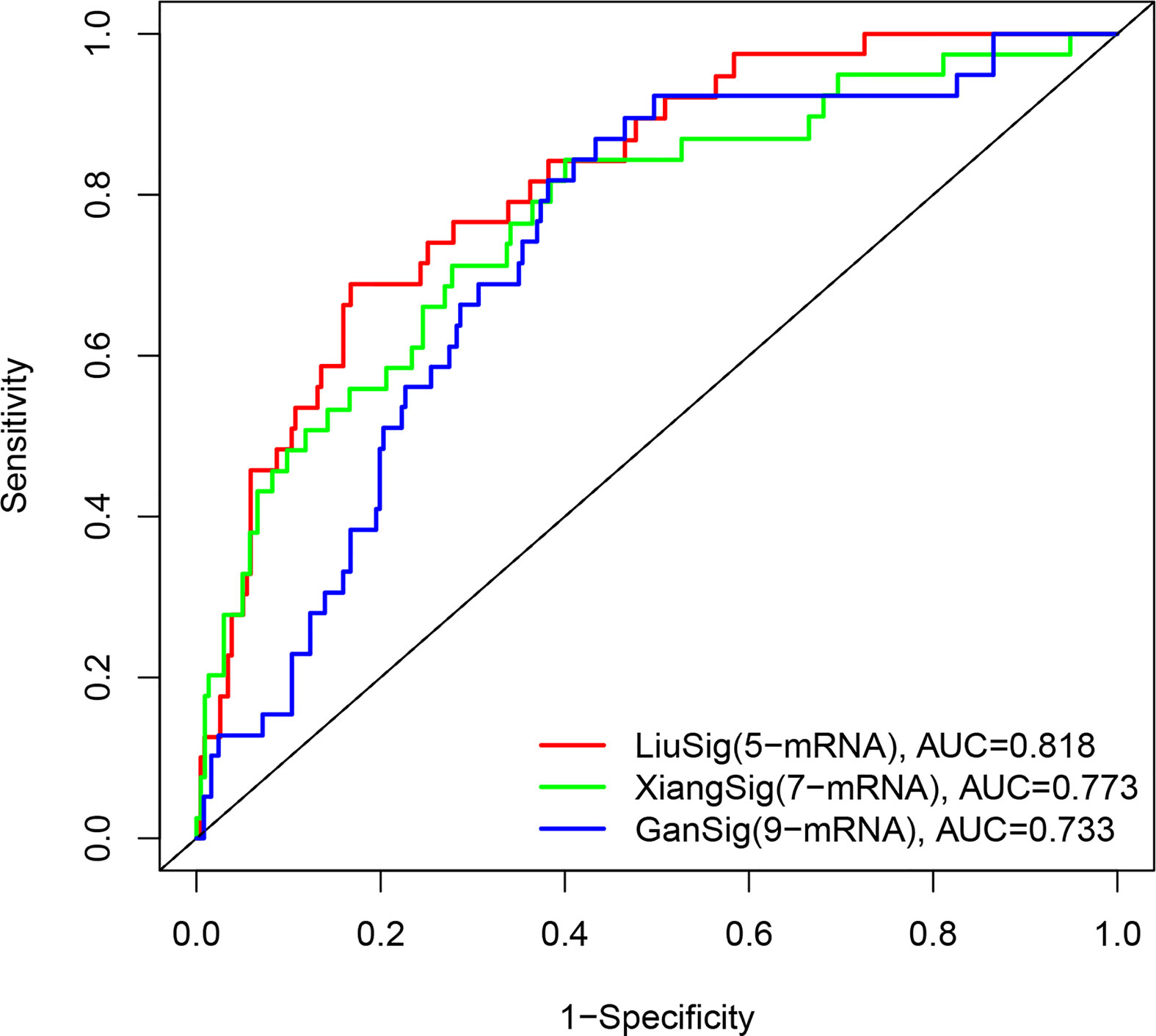
Figure 7 The ROC analysis at 1 years of overall survival for our ERS-5 gene, Xiang-7 gene and Gan-9 gene signatures in the entire TCGA cohort.
Through above systematic statistical analysis, we found that the expression levels of 5 target genes can significantly interfere with the survival and prognosis of liver cancer patients. In order to further investigate the biological mechanism of the abnormal expression of the five target genes, we analyzed the five genes from different omics dimensions, including the genome level, copy number level, methylation level and post-transcriptional regulation level.
We used the R package maftools to obtain all exon variation data of liver cancer samples in maf format. The mutation data contains all liver cancer somatic mutations identified by the MuTect2 algorithm. Two of the five target genes have high frequency mutations in liver cancer patients, and they were mainly missense mutations. As shown in Figure 8A, the gene DDX11 has mutations in 5 liver cancer samples, while PPP2R5B has mutations in 3 samples. Since the five target genes showed differential expression in most liver cancer samples, this result suggests that the abnormal expression of the five target genes may not be related to mutations.
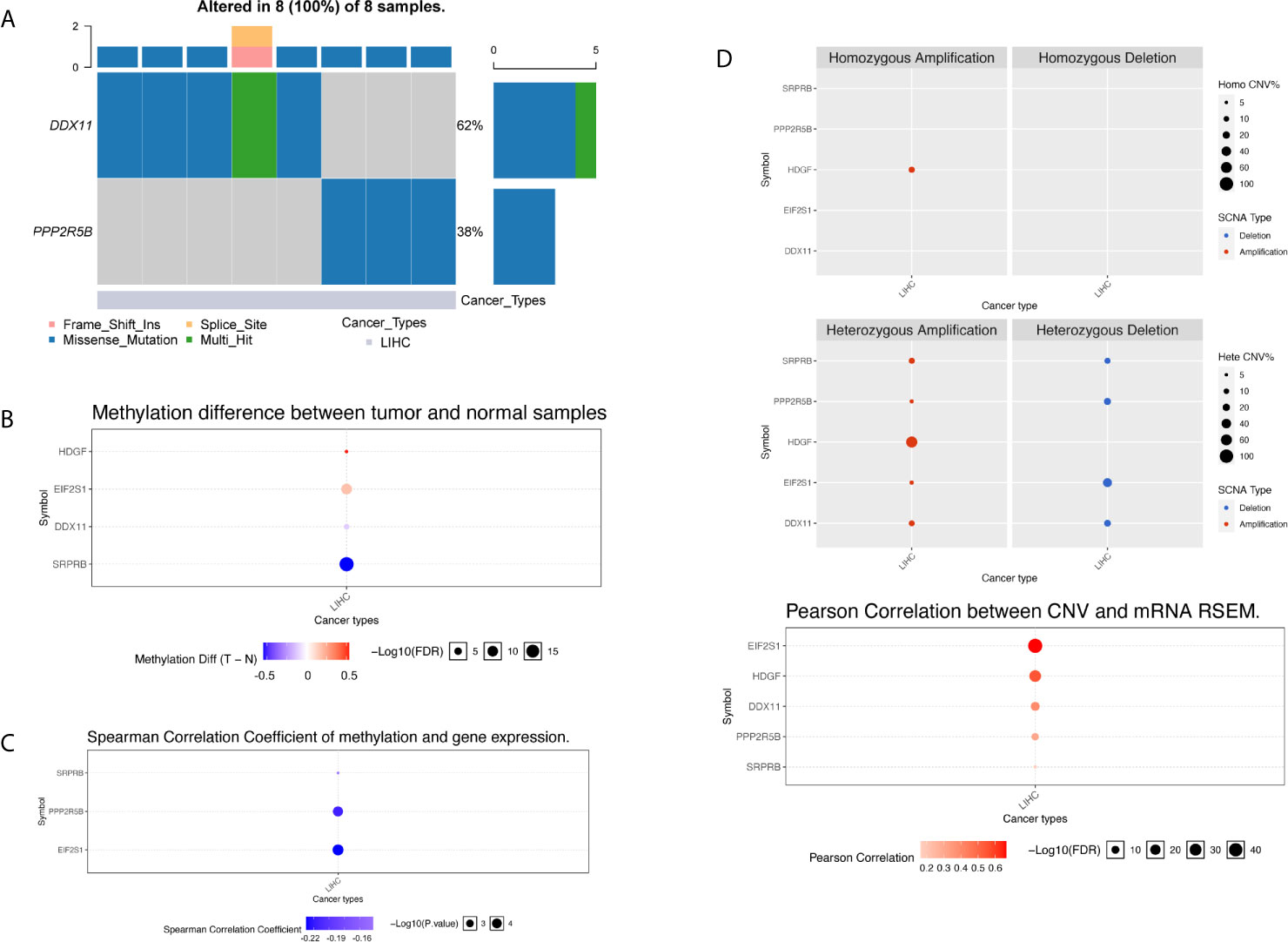
Figure 8 Multi-omics analysis of five gene signatures in TCGA cohort by GSCALite. (A) In the oncoplot graph, different color represents different types of variation. (B) Methylation difference between tumor and normal samples. (C) Spearman correlation coefficient of methylation and gene expression. (D) Copy number variation pattern of 5 gene signatures.
We further investigated the abnormality of methylation level. There were four genes at the methylation level that were significantly different in tumor patients and normal tissues, as shown in Figure 8B. The gene HDGF and EIF2S1 were mainly hypermethylation, while the DDX11 and SRPRB were hypomethylation, the most significant of which were SRPRB and EIF2S1. As we all know, abnormal methylation plays an important role in the regulation of expression level, so we further compared the correlation between methylation level and expression level. It was found that there was a correlation between the differences in methylation and expression levels of three genes, and the results are shown in Figure 8C. Hypermethylation tends to inhibit gene expression, so the significant negative correlation suggests that the cause of expression differences may be caused by abnormal regulation of the apparent level. Although abnormal expression levels may be affected by multiple dimensions of omics, the correlation between methylation and expression levels suggests that the expression levels of SPPRB, PPP2R5B and EIF2S1 were regulated by abnormal methylation.
Next, we compared the copy number variation from two perspectives of heterozygosity and homozygosity, and found that the main manifestations of liver cancer patients were loss of heterozygosity and amplification. The HDGF gene was all amplified variants, as shown in Figure 8D. At the same time, we found that there was a significant correlation between the copy number variation and the expression level variation of the 5 genes, suggested that the genome copy number variation affects the quantification of gene expression in RNAseq. Therefore, abnormal gene expression may be due to the combined effect of copy number variation and methylation variation.
We know that in addition to the effects of mutation, methylation, and copy number on expression levels, post-transcriptional regulation also plays an important role. The most important noncoding rna is mirna. Through the analysis of the mirna target database, we obtained mirna for targeted regulation of 5 target genes. for the specific results, see the Supplementary Table S2. The regulatory relationship between mirna and target genes was shown in Figure 9. The yellow and blue circles in the figure represent the target gene and mirna, respectively. The arrows represent regulatory relationships. We found that some mirna can simultaneously regulate multiple target genes. Since target genes are significantly related to the prognosis of liver cancer, if the mirna involved in post-transcriptional regulation is also significantly related to the prognosis, it suggests that the regulatory relationship of mirna-gene plays an important role in the progression of liver cancer.
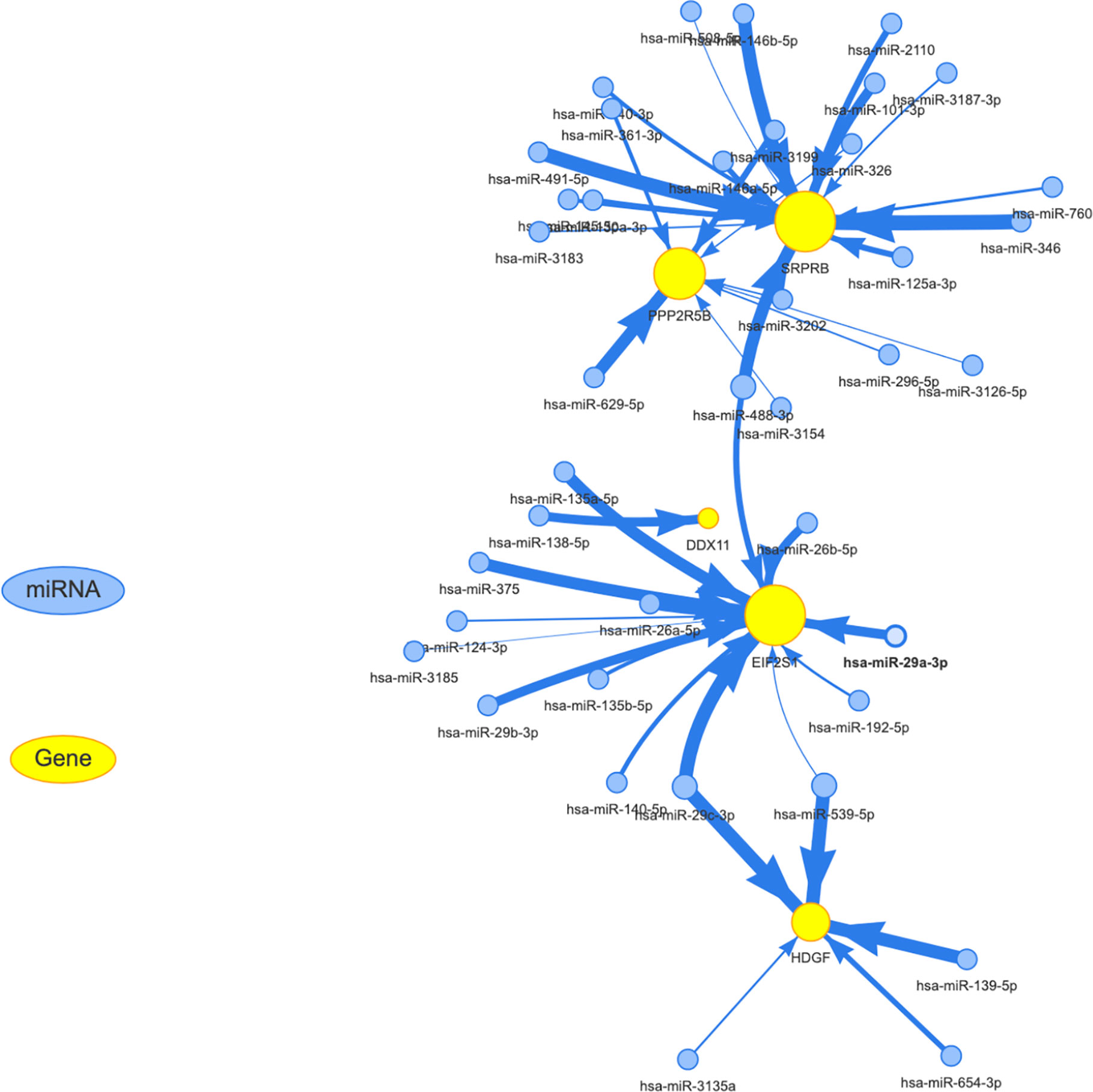
Figure 9 Establishment of miRNA-mRNA regulatory network. The yellow and blue circles represent the target gene and miRNA, respectively. The arrows represent regulatory relationships.
Firstly, we used the mirna expression data of liver cancer samples downloaded from the TCGA database. Then, all miRNAs were screened by Univariate Cox regression, and the most significant top 6 miRNAs were screened according to P value less than 0.01, and the results were shown in the Supplementary Table S3. Subsequently, boxplot was used to visualize the expression distribution of 6 miRNAs in patients with different risk of HCC, as shown in Figure 10A. The expression level of mirna is significantly different in different risk groups. We further used the KM curve to analyze the relationship between the expression of 6 mirna and the survival prognosis of patients, as shown in Figure 10B. All the 6 miRNAs were significantly correlated with the prognosis of HCC patients. The main target genes involved in the regulation of these six miRNAs were EIF2S1 and HDGF. Therefore, the post-transcriptional regulation of EIF2S1 and HDGF plays a key role in the progression of liver cancer patients.
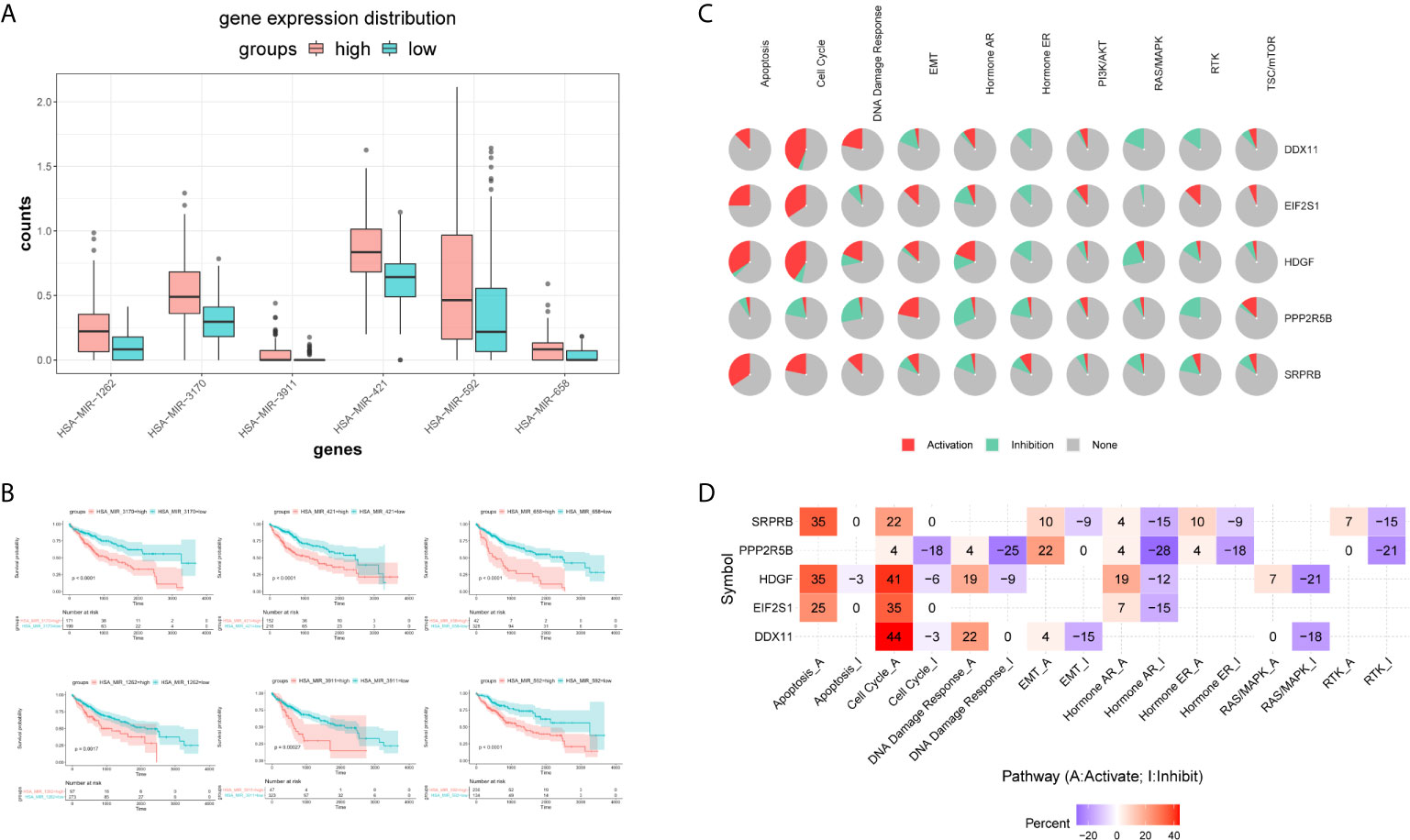
Figure 10 Expression distribution and survival analysis of miRNA in the TCGA dataset. (A) miRNA expression distribution in different risk groups. (B) KM survival analysis of 6 miRNAs. (C) Functional analysis of 5 target genes. Red and green in the pie chart represent the direction and proportion of expression. (D) Inferred activity of the identified 5 target genes in biological pathways. A and I to mark the active and inhibited pathways, respectively.
Pathway analysis showed that the five target genes were mainly involved in regulating apoptosis, cell cycle, hormone receptors and other cancer-related pathways (Figure 10C). In order to further study whether each pathway was activated or inhibited, we used A and I to mark the active pathway and the inhibited pathway, as shown in Figure 10D. It was obvious that the apoptosis pathway was activated in liver cancer patients under the regulation of five genes. The RAS/MAPK and RTK pathways were mainly inhibited. Cell cycle, DNA damage response, hormone receptors, and EMT pathways were both activated and/or inhibited.
The complexity of tumor heterogeneity in HCC remains a major challenge to accurate patient prognosis. It is particularly important to search for new prognostic biomarkers and to establish more accurate prognostic models to predict the survival of liver cancer patients. Many previous studies have focused on mRNA gene signatures and achieved promising results in predicting the prognosis of HCC (16–19). However, the predictive abilities of these biomarkers are limited and there is a need to develop more accurate signatures. Experimental data suggest that ER stress and unfolded protein response are involved in HCC development, aggressiveness and response to treatment, However, the exact role of ERS stress in HCC needs further study (20). In this study, we aimed to discover a novel prognostic marker related to ERS to provide new approaches in the treatment of liver cancer.
In this study, we created a new 5-gene signature based on ERS (including HDGF, EIF2S1, SRPRB, PPP2R5B and DDX11) for prognosis in HCC. Our 5-gene signature was demonstrated as an independent prognostic factor in HCC and the overall survival of the high-risk group was significantly lower than the low-risk group. The nomogram combining the 5-gene markers showed that it performed best in predicting survival indicating that the risk model and had utility in evaluating the prognosis of HCC. GSEA analysis revealed a series of significantly enriched oncology features and some metabolic processes in the two groups that may help to understand the underlying molecular mechanism of the disease.
HDGF is a critical regulator of cancer cell activities and plays central roles in transformation, apoptosis, angiogenesis and metastasis in several cancer types (21–24). In HCC, HDGF is considered a liver cancer-derived growth factor and it has been shown that targeted inhibition of HDGF may have efficacy as a new type of HCC treatment (25). EIF2S1 is a phosphorylated form of eIF2α that inhibits eIF2α-mediated translation Studies have shown that it is related to oxidative stress and ER protein folding and acts to maintain the differentiation state of β-cells (26). However, the role and mechanism of EIF2S1 in HCC remain unclear.
SRPRB is a novel human gene whose transcript is upregulated in apoptotic MCF-7 cells (27). It is commonly expressed in the liver, pancreas, thyroid, testis and 25 other tissues in the human body. Studies have shown that SRPRB regulates HHCC cell proliferation and apoptosis by down-regulating TIMP3 and up-regulating CDK inhibitor p21. Also, it may interact with SERP1 to affect the prognosis of pancreatic cancer (28, 29).
The mechanism of SRPRB in the prognosis of liver cancer merits further investigation. PPP2R5B is the regulatory subunit of protein phosphatase 2 (PP2A). As PP2A is generally recognized as a tumor suppressor, studies have shown that highly expressed mitotic regulators and low-expressed PP2A class I subunits (such as PPP2R5B) can improve survival rate (30). Previous studies have also shown that SRPRB is responsible for the dephosphorylation and inactivation of Akt protein and can lead to insulin resistance in adipocytes induced by chronic hyperinsulinemia (31).
The role of PPP2R5B in liver cancer remains unclear. DDX11 is a DNA helicase, involved in the cohesion of sister chromatids and DNA repair pathways (32). The expression of DDX11 is dysregulated and has a significant effect on the progression of many tumors such as lung adenocarcinoma (33), melanoma (34) and Warsaw breakage Syndrome (35). Some evidence has shown that DDX11 regulates the proliferation and invasion of HCC cells by activating the PI3K/AKT/mTOR signaling pathway (36).
Hepatocellular carcinoma is a multistep process of genetic and epigenetic alterations involving many genes. Recent studies have shown that multi-omics data integration strategies spanning different cell function levels across the genome, epigenome, transcriptome, proteome, metabolome, and microbiome provide a new perspective for the typing, diagnosis and prognosis of complex diseases (such as cancer) (37). With the advancement of high-throughput technology, the mutation, methylation, copy number and gene expression patterns of various cancer types have been identified and characterized. Previous reports in the literature usually indicate that mutations in driving oncogenes are associated with poor prognosis. For example, the PIK3CA mutation in breast cancer (38), the NBN mutation in prostate cancer (39), and the TP53 mutation in gastric cancer (40). However, the specificity and sensitivity of this statement needs further confirmation. As we all know, copy number variation (CNV) is generally considered the source of genetic variation, and its importance has been confirmed in recent studies (41). In addition to gene mutations, CNV also plays a very important role in the occurrence and development of many cancers, such as ovarian cancer (42), bladder cancer (43), gastric cancer (44), and so on. A recent study analyzed the genome profiles of 17,879 tumors in patients with known outcomes, and found that almost all mutations in cancer driver genes contained very little information about the patient’s prognosis. However, the copy number changes in these same driver genes have obvious prognostic power (45). It indicates that the identification of copy number may be an important target for future tumor treatment. As an important epigenetic modification, DNA methylation participates in the regulation of gene transcription and maintains the stability of the genome (46). Methylation changes, usually including hypomethylation of proto-oncogenes and methylation of tumor suppressor genes, play a key role in regulating the expression of many tumor genes. In this study, we found that the abnormal expression of 5 genes may be due to the combined effect of copy number variation and methylation variation, and its abnormal expression may not be related to mutation. Multi-omics analysis of these genes will deepen the understanding of the occurrence, development and potential mechanism of liver cancer.
A 5-gene model and the nomograms related to stresses are yet to be rarely reported. Our biomarker may be a useful tool in the prognosis and treatment of HCC. However, our study had several shortcomings including the need for a lack of external validation. In addition, due to the limitations of the database, the clinical data of some important parameters were incomplete, such as BCLC staging, preoperative AFP, and CEA were excluded from the scoring system analysis. It may affect statistical power. Therefore, our study cannot rule out that the survival of patients may be affected by other key clinical factors. Although the 5-gene model had implications for the diagnosis and treatment of liver cancer, more complex mechanisms associated with the prognosis of liver cancer require further exploration.
Our study indicated that a 5-gene signature based on ERS-related independent prognostic significance could potentially serve as a prognostic indicator for clinical decision-making in the treatment of HCC patients. Multi-omics analysis of the role of these genes in promoting tumors in liver cancer will provide a new perspective for elucidating the potential mechanisms of liver cancer prognosis.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
PL conceived and designed this study. The software and the data analyses were carried out by PL and JW. Other authors including FM, ZX, HD and YD also participated in the data analyses of this study. The manuscript was written by PL and supervised by JW. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work was appropriately investigated and resolved. PL and JW have contributed equally to this work. All authors contributed to the article and approved the submitted version.
This work was supported by a grant from the National Natural Science Foundation of China (No. 81772516).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.641487/full#supplementary-material
1. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A Global View of Hepatocellular Carcinoma: Trends, Risk, Prevention and Management. Nat Rev Gastroenterol Hepatol (2019) 16(10):589–604. doi: 10.1038/s41575-019-0186-y
2. Villanueva A. Hepatocellular Carcinoma. New Engl J Med (2019) 380(15):1450–62. doi: 10.1056/NEJMra1713263
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
4. Losic B, Craig AJ, Villacorta-Martin C, Martins-Filho SN, Akers N, Chen X, et al. Intratumoral Heterogeneity and Clonal Evolution in Liver Cancer. Nat Commun (2020) 11(1):291. doi: 10.1038/s41467-019-14050-z
5. Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the Early Diagnosis of Hepatocellular Carcinoma. World J Gastroenterol (2015) 21(37):10573–83. doi: 10.3748/wjg.v21.i37.10573
6. Zhao YJ, Ju Q, Li GC. Tumor Markers for Hepatocellular Carcinoma. Mol Clin Oncol (2013) 1(4):593–8. doi: 10.3892/mco.2013.119
7. da Silva DC, Valentão P, Andrade PB, Pereira DM. Endoplasmic Reticulum Stress Signaling in Cancer and Neurodegenerative Disorders: Tools and Strategies to Understand its Complexity. Pharmacol Res (2020) 155:104702. doi: 10.1016/j.phrs.2020.104702
8. Fan L, Xiao Q, Chen Y, Chen G, Duan J, Tao W. Pekinenin E Inhibits the Growth of Hepatocellular Carcinoma by Promoting Endoplasmic Reticulum Stress Mediated Cell Death. Front Pharmacol (2017) 8:424. doi: 10.3389/fphar.2017.00424
9. Liu J, Fan L, Yu H, Zhang J, He Y, Feng D, et al. Endoplasmic Reticulum Stress Causes Liver Cancer Cells to Release Exosomal miR-23a-3p and Up-regulate Programmed Death Ligand 1 Expression in Macrophages. Hepatol (Baltimore Md) (2019) 70(1):241–58. doi: 10.1002/hep.30607
10. Di Conza G, Ho PC. Er Stress Responses: An Emerging Modulator for Innate Immunity. Cells (2020) 9(3):695. doi: 10.3390/cells9030695
11. Liu CJ, Hu FF, Xia MX, Han L, Zhang Q, Guo AY. Gscalite: A Web Server for Gene Set Cancer Analysis. Bioinf (Oxford England) (2018) 34(21):3771–2. doi: 10.1093/bioinformatics/bty411
12. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in Oncology: More Than Meets the Eye. Lancet Oncol (2015) 16(4):e173–80. doi: 10.1016/s1470-2045(14)71116-7
13. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc Natl Acad Sci United States America (2005) 102(43):15545–50. doi: 10.1073/pnas.0506580102
14. Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a Reference Resource for Gene and Protein Annotation. Nucleic Acids Res (2016) 44(D1):D457–62. doi: 10.1093/nar/gkv1070
15. Xiang XH, Yang L, Zhang X, Ma XH, Miao RC, Gu JX, et al. Seven-Senescence-Associated Gene Signature Predicts Overall Survival for Asian Patients With Hepatocellular Carcinoma. World J Gastroenterol (2019) 25(14):1715–28. doi: 10.3748/wjg.v25.i14.1715
16. Gan X, Luo Y, Dai G, Lin J, Liu X, Zhang X, et al. Identification of Gene Signatures for Diagnosis and Prognosis of Hepatocellular Carcinomas Patients At Early Stage. Front Genet (2020) 11:857. doi: 10.3389/fgene.2020.00857
17. Long J, Zhang L, Wan X, Lin J, Bai Y, Xu W, et al. A Four-Gene-Based Prognostic Model Predicts Overall Survival in Patients With Hepatocellular Carcinoma. J Cell Mol Med (2018) 22(12):5928–38. doi: 10.1111/jcmm.13863
18. Zhang Y, Zhang L, Xu Y, Wu X, Zhou Y, Mo J. Immune-Related Long Noncoding RNA Signature for Predicting Survival and Immune Checkpoint Blockade in Hepatocellular Carcinoma. J Cell Physiol (2020) 235(12):9304–16. doi: 10.1002/jcp.29730
19. Ouyang G, Yi B, Pan G, Chen X. A Robust Twelve-Gene Signature for Prognosis Prediction of Hepatocellular Carcinoma. Cancer Cell Int (2020) 20:207. doi: 10.1186/s12935-020-01294-9
20. Wei J, Fang D. Endoplasmic Reticulum Stress Signaling and the Pathogenesis of Hepatocarcinoma. Int J Mol Sci (2021) 22:(4):1799. doi: 10.3390/ijms22041799
21. Bao C, Wang J, Ma W, Wang X, Cheng Y. HDGF: A Novel Jack-of-All-Trades in Cancer. Future Oncol (London England) (2014) 10(16):2675–85. doi: 10.2217/fon.14.194
22. Zhang C, Chang X, Chen D, Yang F, Li Z, Li D, et al. Downregulation of HDGF Inhibits the Tumorigenesis of Bladder Cancer Cells by Inactivating the PI3K-AKT Signaling Pathway. Cancer Manage Res (2019) 11:7909–23. doi: 10.2147/cmar.S215341
23. Wang Q, Chen C, Ding Q, Zhao Y, Wang Z, Chen J, et al. METTL3-Mediated M(6)a Modification of HDGF mRNA Promotes Gastric Cancer Progression and has Prognostic Significance. Gut (2020) 69(7):1193–205. doi: 10.1136/gutjnl-2019-319639
24. Jiang H, Fu Q, Song X, Ge C, Li R, Li Z, et al. HDGF and PRKCA Upregulation is Associated With a Poor Prognosis in Patients With Lung Adenocarcinoma. Oncol Lett (2019) 18(5):4936–46. doi: 10.3892/ol.2019.10812
25. Enomoto H, Nakamura H, Liu W, Iwata Y, Nishikawa H, Takata R, et al. Down-Regulation of HDGF Inhibits the Growth of Hepatocellular Carcinoma Cells in Vitro and In Vivo. Anticancer Res (2015) 35(12):6475–9.
26. Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, et al. Translation Attenuation Through eIF2alpha Phosphorylation Prevents Oxidative Stress and Maintains the Differentiated State in Beta Cells. Cell Metab (2009) 10(1):13–26. doi: 10.1016/j.cmet.2009.06.002
27. Yan W, Wang WL, Zhu F, Chen SQ, Li QL, Wang L. Isolation of a Novel Member of Small G Protein Superfamily and its Expression in Colon Cancer. World J Gastroenterol (2003) 9(8):1719–24. doi: 10.3748/wjg.v9.i8.1719
28. Li Q, Yan W, Cheng S, Guo S, Wang W, Zhang Z, et al. Introduction of G1 Phase Arrest in Human Hepatocellular Carcinoma Cells (HHCC) by APMCF1 Gene Transfection Through the Down-Regulation of TIMP3 and Up-Regulation of the CDK Inhibitors P21. Mol Biol Rep (2006) 33(4):257–63. doi: 10.1007/s11033-006-9007-9
29. Ma Q, Wu X, Wu J, Liang Z, Liu T. SERP1 is a Novel Marker of Poor Prognosis in Pancreatic Ductal Adenocarcinoma Patients Via Anti-Apoptosis and Regulating SRPRB/NF-κb Axis. Int J Oncol (2017) 51(4):1104–14. doi: 10.3892/ijo.2017.4111
30. Cunningham CE, Li S, Vizeacoumar FS, Bhanumathy KK, Lee JS, Parameswaran S, et al. Therapeutic Relevance of the Protein Phosphatase 2A in Cancer. Oncotarget (2016) 7(38):61544–61. doi: 10.18632/oncotarget.11399
31. Beg M, Srivastava A, Shankar K, Varshney S, Rajan S, Gupta A, et al. PPP2R5B, a Regulatory Subunit of PP2A, Contributes to Adipocyte Insulin Resistance. Mol Cell Endocrinol (2016) 437:97–107. doi: 10.1016/j.mce.2016.08.016
32. Pisani FM, Napolitano E, Napolitano LMR, Onesti S. Molecular and Cellular Functions of the Warsaw Breakage Syndrome Dna Helicase Ddx11. Genes (2018) 9(11):564. doi: 10.3390/genes9110564
33. Li J, Liu L, Liu X, Xu P, Hu Q, Yu Y. The Role of Upregulated DDX11 as A Potential Prognostic and Diagnostic Biomarker in Lung Adenocarcinoma. J Cancer (2019) 10(18):4208–16. doi: 10.7150/jca.33457
34. Bhattacharya C, Wang X, Becker D. The DEAD/DEAH Box Helicase, DDX11, is Essential for the Survival of Advanced Melanomas. Mol Cancer (2012) 11:82. doi: 10.1186/1476-4598-11-82
35. Bharti SK, Khan I, Banerjee T, Sommers JA, Wu Y, Brosh RM Jr. Molecular Functions and Cellular Roles of the ChlR1 (DDX11) Helicase Defective in the Rare Cohesinopathy Warsaw Breakage Syndrome. Cell Mol Life Sci CMLS (2014) 71(14):2625–39. doi: 10.1007/s00018-014-1569-4
36. Yu Y, Zhao D, Li K, Cai Y, Xu P, Li R, et al. E2F1 Mediated DDX11 Transcriptional Activation Promotes Hepatocellular Carcinoma Progression Through PI3K/AKT/mTOR Pathway. Cell Death Dis (2020) 11(4):273. doi: 10.1038/s41419-020-2478-0
37. Menyhárt O, Győrffy B. Multi-Omics Approaches in Cancer Research With Applications in Tumor Subtyping, Prognosis, and Diagnosis. Comput Struct Biotechnol J (2021) 19:949–60. doi: 10.1016/j.csbj.2021.01.009
38. Oshiro C, Kagara N, Naoi Y, Shimoda M, Shimomura A, Maruyama N, et al. PIK3CA Mutations in Serum DNA are Predictive of Recurrence in Primary Breast Cancer Patients. Breast Cancer Res Treat (2015) 150(2):299–307. doi: 10.1007/s10549-015-3322-6
39. Cybulski C, Wokołorczyk D, Kluźniak W, Jakubowska A, Górski B, Gronwald J, et al. An Inherited NBN Mutation is Associated With Poor Prognosis Prostate Cancer. Br J Cancer (2013) 108(2):461–8. doi: 10.1038/bjc.2012.486
40. Tahara T, Shibata T, Okamoto Y, Yamazaki J, Kawamura T, Horiguchi N, et al. Mutation Spectrum of TP53 Gene Predicts Clinicopathological Features and Survival of Gastric Cancer. Oncotarget (2016) 7(27):42252–60. doi: 10.18632/oncotarget.9770
41. Sebat J, Lakshmi B, Troge J, Alexander J, Young J, Lundin P, et al. Large-Scale Copy Number Polymorphism in the Human Genome. Sci (New York NY) (2004) 305(5683):525–8. doi: 10.1126/science.1098918
42. Despierre E, Moisse M, Yesilyurt B, Sehouli J, Braicu I, Mahner S, et al. Somatic Copy Number Alterations Predict Response to Platinum Therapy in Epithelial Ovarian Cancer. Gynecol Oncol (2014) 135(3):415–22. doi: 10.1016/j.ygyno.2014.09.014
43. Bonberg N, Pesch B, Behrens T, Johnen G, Taeger D, Gawrych K, et al. Chromosomal Alterations in Exfoliated Urothelial Cells From Bladder Cancer Cases and Healthy Men: A Prospective Screening Study. BMC Cancer (2014) 14:854. doi: 10.1186/1471-2407-14-854
44. Liang L, Fang JY, Xu J. Gastric Cancer and Gene Copy Number Variation: Emerging Cancer Drivers for Targeted Therapy. Oncogene (2016) 35(12):1475–82. doi: 10.1038/onc.2015.209
45. Smith JC, Sheltzer JM. Systematic Identification of Mutations and Copy Number Alterations Associated With Cancer Patient Prognosis. eLife (2018) 7. doi: 10.7554/eLife.39217
Keywords: hepatocellular carcinoma, The Cancer Genome Atlas, endoplasmic reticulum stress, signature, prognosis, multi-omics
Citation: Liu P, Wei J, Mao F, Xin Z, Duan H, Du Y, Wang X, Li Z, Qian J and Yao J (2021) Establishment of a Prognostic Model for Hepatocellular Carcinoma Based on Endoplasmic Reticulum Stress-Related Gene Analysis. Front. Oncol. 11:641487. doi: 10.3389/fonc.2021.641487
Received: 19 December 2020; Accepted: 09 April 2021;
Published: 21 May 2021.
Edited by:
Shuyun Rao, George Washington University, United StatesReviewed by:
Jihane N Benhammou, UCLA David Geffen School of Medicine, United StatesCopyright © 2021 Liu, Wei, Mao, Xin, Duan, Du, Wang, Li, Qian and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Yao, ZG9jeWFvQGhvdG1haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.