
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol. , 09 March 2021
Sec. Pediatric Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.641450
This article is part of the Research Topic Adverse and Toxic Effects of Childhood Cancer Treatments View all 13 articles
 Serena Brancati1†
Serena Brancati1† Lucia Gozzo1,2†
Lucia Gozzo1,2† Laura Longo1
Laura Longo1 Daniela Cristina Vitale1
Daniela Cristina Vitale1 Giovanna Russo3
Giovanna Russo3 Filippo Drago1,2,4*
Filippo Drago1,2,4*Fertility preservation represents one important goal of cancer patients’ management due to the high impact on health and quality of life of survivors. The available preventive measures cannot be performed in all patients and are not feasible in all health-care facilities. Therefore, the pharmacological treatment with GnRHa has become a valuable non-invasive and well-tolerated alternative, especially in those who cannot access to cryopreservation options due to clinical and/or logistic issues. Supporting data demonstrate a significant advantage for the survivors who received GnRHa in the long-term maintenance of ovarian function and preservation of fertility. The prevention of the risk of ovarian failure with GnRHa is a typical off-label use, defined as the administration of a medicinal product not in accordance with the authorized product information. Italy has officially recognized the off-label use of GnRHa in adult women at risk of premature and permanent menopause following chemotherapy. However, fertility preservation still represents an unmet medical need in adolescents who cannot access to other treatment options.
In Europe, nearly 80% of children and adolescents with cancer treated on current protocols survive at least 5 years on average (1). Improved survival rates have increased the number of childhood cancer survivors (CCSs) entering adulthood after treatment for malignancy, which account for 0.1–0.15% of the general population (2). The high survival rate in children and adolescents is accompanied by a substantial risk of late adverse events (LAEs). Above all, treatment may interfere with physiological growth and development and have an important impact on health status later in life, whilst some late toxicities may cause premature death. Fertility is one of the most important concerns of CCSs (3, 4). The occurrence of fertility impairment in female—reduced pregnancy rates and increased risk of early menopause—after pelvic, abdominal, or spinal radiotherapy, total body irradiation, or chemotherapy regimens containing alkylant agents during childhood and adolescence has been widely documented (5–8).
Treatments may deplete or accelerate the decline of the non-renewable pool of primordial follicles in the ovary leading to POF and infertility (9, 10). Gonadal toxicity is affected by type, doses and length of therapy (11, 12), and by age at treatment (females treated at a younger age are less likely to develop POF, probably because of a higher number of primordial follicles at the time of treatment). POF results in a reduced fertile lifespan and associated risk for involuntary childlessness, which can negatively impact the quality of life (13–15), but also accelerates the risk of developing menopause-associated conditions, such as osteoporosis and cardiovascular disease (16). Therefore, fertility preservation (FP) represents an important issue for oncologists, fertility specialists and patients. Most recent guidelines (12, 17, 18) state FP should be discussed with the parents (or guardians) of adolescents soon after diagnosis and before starting anticancer treatments.
In pubertal and postpubertal adolescents, oocyte cryopreservation is one of the available option for FP during gonadotoxic chemotherapy (18) (Table 1). Oocyte cryopreservation (21) requires ovarian stimulation with gonadotropin hormone, ultrasound-assisted oocyte collection, and selection and freezing of oocytes (22). There are many barriers to the adoption of this option into standard of care. First, oocyte cryopreservation should be carried out before starting chemotherapy and requires time (17) for ovarian stimulation and follicular growing, with consequent delay in the initiation of oncological treatment. This can be a problem especially in pediatric cancers, which often require urgency to start treatment. The random-start controlled ovarian stimulation protocol, providing for a stimulation at any time of the menstrual cycle, can reduce the time needed for cryopreservation, but for patients with very aggressive diseases no delay is allowed (23). Moreover, this technique is associated with additional challenges as it requires to acquire oocytes through transvaginal approach (24), a painful procedure to be performed with sedation and with specialized equipment, which is not often available in pediatric hospitals. Not less important is its emotional and psychological impact for most adolescents, despite their sexual maturity.
Ovarian tissue cryopreservation (OTC), although still considered as experimental, is commonly proposed as an alternative to oocyte cryopreservation (18). Cryopreservation of the ovarian tissue requires a laparoscopic procedure under general anesthesia and the subsequent freezing of the tissue that contains most primordial follicles (25). Differently from oocyte cryopreservation, OTC can be performed at any time with less delay in starting cancer therapy. Once the patient is disease-free, an autotransplantation can be carried out. Major issues of OTC include ischemic damage to the tissue and the theoretical risk of reintroducing malignant cells, especially. Indeed, transplantation is not in patients with diseases associated with a high risk of ovarian metastases (26, 27). Another limitation is represented by the availability of a center with adequate cryoconservation competences and able to perform the most sensitive and updated histological analysis before transplantation to avoid relapses (28).
An alternative, that could represent a more accessible FP option burdened by less discomfort for postpubertal patients, could be the concomitant use of gonadotropin-releasing hormone (GnRH) analogs (triptorelin, goserelin, leuprolide) during the course of chemotherapy (18, 29, 30). This non-invasive and less expensive approach could allow preventing POF in cancer survivors and has been recently integrated in clinical guidelines (17, 18, 31–34). In particular, the updated European Society for Medical Oncology (ESMO) and American Society of Clinical Oncology (ASCO) recommendations state that GnRHa should be used in addition to the other options (17, 35).
It is noteworthy that in adolescents and young women with malignancies GnRHa are widely used as an alternative to estro-progestinic combinations for menstrual suppression (36–41), in order to reduce the risk of heavy menstrual bleeding associated with hematologic malignancies or myelosuppression induced by chemotherapy. In this setting, despite significant side-effects simulating the physiology of menopause and the risk of loss of bone mineral density with prolonged use (usually > 6 months) (42), GnRHa are well tolerated and effective option for menses suppression (43, 44) and are generally preferred to oral contraceptives for several reasons. Oral contraceptives have some disadvantages such as the daily regimen, in contrast to the monthly administration of GnRHa. Furthermore, the efficacy of oral contraceptives can be reduced by an erratic absorption due to mucositis, diarrhea, and emesis. Moreover, the use of estrogen-based oral contraceptives for menstrual suppression is associated with an increased risk of venous thromboembolism (41, 45). However, one of the most important reason for choosing GnRHa is represented by their possible gonadal protective effect (41).
The rational for the use of GnRH analogs (GnRHa) for the reduction of ovarian toxicity is based on the observation that chemotherapy mostly affects tissues with rapid cellular turn-over, as gonadal one (46). Moreover, the gonadotoxicity is lower in prepubertal girls than in adult women (47, 48), probably as a consequence of their higher ovarian reserve, in addition to the hypogonadotropic prepubertal milieu. This led to the speculation that ovarian suppression in postpubertal female patients might mitigate the adverse effects of treatment on ovarian function (49). Preclinical data have confirmed the efficacy of GnRHa in reducing cyclophosphamide-induced gonadotoxicity (50–53). A prospective randomized study in primates demonstrated that co-treatment with GnRHa significantly decreased the rate of follicular decline and the total number of primordial follicles lost compared with cyclophosphamide alone (53).
GnRHa may protect the ovaries against chemotherapy-induced damage through the inhibition of the hypothalamic-pituitary-ovarian axis with the induction of a prepubertal state (54). The increased rate of non-resting follicles loss leads to a decrease in the secretion of sex steroids and inhibins produced by these follicles at different stages of maturation and differentiation. The resultant low systemic concentrations of these endogen molecules induce a feedback on the hypothalamus and pituitary gland, increasing the gonadotropins secretion, mainly follicle-stimulating hormone (FSH) (54), which enhance the follicle recruitment and maturation. These growing follicles are more exposed to the gonadotoxic effects, ending in an accelerated rate of follicular apoptosis and degeneration. This vicious cycle may be interrupted by preventing the increase in FSH through the GnRHa administration (55). Moreover, GnRHa may exert beneficial effects through the decrease in utero-ovarian perfusion resulting from the hypoestrogenic state (56, 57), with lower exposure of the ovaries to chemotherapy. In addition, human gonads express GnRH receptors (58–63), which activation may decrease apoptosis (61). Other hypothetical gonadoprotective mechanisms include an increased formation of intragonadal antiapoptotic molecules (64, 65) and protection of the undifferentiated germline stem cells (54), with the latter mechanism not yet tested under an experimental model.
Several randomized clinical trials (RCTs) have shown a clear benefit and a good safety profile of GnRHa in the prevention of chemotherapy damage in pre-menopausal women aged 18–45 years with breast cancer (66–76) (Supplementary 1). In these RCTs, patients with normal ovarian function were randomized to receive chemotherapy plus GnRHa or alone. The study population was heterogeneous, with different age, chemotherapy regimens, selection criteria and follow-up duration. The markers for FP were mainly represented by the return of ovarian function, and the assessment of ovarian reserve by measurement of hormone levels. Only few studies evaluated the long-term pregnancy rate in survivors, the most appropriate marker of fertility that requires a prolonged follow-up, especially in a young population. The three larger phase III RCTs [US POEMS–SWOG/S0230 (73, 74), Italian PROMISE–GIM6 (68) and Anglo Celtic Group OPTION trial (75)], demonstrated a statistically significant reduction in POF and a significant increase in pregnancy rate in the GnRHa arms. A large meta-analysis of 12 RCTs including patients with breast cancer (77) confirmed these positive findings showing a significant reduction of the risk of POF at 12 months from the end of chemotherapy, and a greater number of pregnancies. The efficacy and safety of GnRHa as a clinical option to reduce POF and improve fertility was further confirmed by a recent meta-analysis and systematic review (78), that showed a statistically greater number of pregnancies in the GnRHa group and no differences in progression-free survival.
The similar or even improved survival outcomes of premenopausal breast cancer patients who received GnRHa, reported by the main RCTs and meta-analysis (79) dispelled the safety concern on the potential antagonism between concurrent GnRHa and chemotherapy.
Moreover, no significant increase in the occurrence of GnRHa-associated toxicities (e.g. hot flashes, sweating, headache, vaginal dryness, and thromboembolic events) has been reported (68, 73).
In the light of these results, the most updated guidelines consider temporary ovarian suppression with GnRHa during chemotherapy as an option to be discussed with breast cancer patients interested in preserving ovarian function (17, 80, 81).
Currently, limited evidence exists on the role of this strategy in women diagnosed with tumors other than breast cancer. One randomized trial has assessed the temporary ovarian suppression with GnRHa in 30 young patients with ovarian cancer (82). The study showed a significant reduction in the risk of chemotherapy-induced POF, although no information on post-treatment pregnancies was reported.
Randomized trials performed in women with hematological malignancies showed no protective effect of GnRHa or suggested a partial protective effect with only a delaying in the appearance of POF (83–86). It is noteworthy that all these studies had a small sample size and were not powered to find a possible advantage of GnRHa. Indeed, when the gonadotoxicity is either very low or very high (>90%) the needed power to detect a difference between the study arms requires hundreds of patients. However, other large retrospective or prospective studies and case series have shown a potential protective effect of GnRHa during chemotherapy also in women with hematological malignancies (87–97).
Nevertheless, at present, only very limited evidences from RCTs regarding the use of GnRHa in adolescents with cancer are available (Table 2, Supplementary 2). This could be due, at least in part, to the difficulty of carrying out, in such a young population, clinical trials with a follow-up long enough to allow the evaluation of reliable fertility preservation indicators, such as pregnancy rate. Indeed, menses resumption is an indirect marker of fertility, but patients resuming menses may have a subclinical and irreversible depletion of ovarian reserve and may experience early menopause (102, 103). The only prospective phase III RCT including postmenarchal adolescent patients, affected by ovarian malignancy, demonstrated the gonadoprotective effect of GnRHa even in the younger population (82). Six months after chemotherapy, all the patients in the GnRHa group had normal menstrual bleeding and normal titer of FSH/LH, whereas 33% in the control group had amenorrhea and POF. A phase II trial evaluated the gonadoprotective effect of leuprolide in adolescent and young women affected by hematologic malignancies who underwent to hematopoietic stem cell transplantation (HSCT) (98). In this case only seven patients (16%) regained ovarian function and leuprolide failed to significantly preserve fertility.
However, such poor outcome could be explained by the fact that almost all patients received at least one prior chemotherapy regimen (median number before HSCT = 2), and 12 patients also received prior local radiation. Therefore, ovarian reserve was probably affected by previous gonadotoxic exposure. Another limitation was the use of a very high dosage of GnRHa. Whereas previous studies used monthly 3.75 mg triptorelin or monthly 3.6 mg goserelin or 11.25 mg leuprolide every 3 months, in this study 22.5 mg leuprolide were administered in 3-month depot injection within 2 months of HSCT. The high doses used in this trial led to intolerable side effects and treatment discontinuation in some patients (104).
Several prospective non-randomized studies have shown the ability of GnRHa to provide a powerful instrument for protection of the ovarian function even in adolescents with hematological malignancies (88, 96, 99, 101, 105).
In a prospective case series with control (88), postpubertal adolescents with normal ovarian function who received monthly leuprolide before and during polychemotherapy for lymphoma, resumed their menstrual cycles and ovulation. After a follow-up of five years, three normal pregnancies were reported. In contrast, patients in the control group had permanent hypergonadotropic amenorrhea.
A long-term follow-up analysis (up to 15 years) of adolescent and young adult with Hodgkin lymphoma co-treated with triptorelin, confirmed the gonadoprotective effect of GnRHa (92). Indeed 96.9% in the GnRHa group resumed ovulation and regular menses, throughout a median follow-up of 8 years (range 2–15), compared with 63% in the control group.
Interestingly, a case report (91, 106) documented four spontaneous pregnancies and two successful deliveries in a patient previously undergoing repeated SCTs and monthly GnRHa co-treatment. SCT almost invariably induces POF owing to higher chemotherapy doses and possible total-body irradiation (107, 108). The estimated odds for spontaneous conception after two SCTs became negligible. These results are highly suggestive that the administration of GnRHa before and during chemotherapy might have minimized the gonadotoxic effects and increased the chance of spontaneous ovulation and successful conception and delivery.
More recently, a prospective, non-randomized study compared the rate of POF after SCT in adolescent and young women receiving GnRHa with gonadotoxic chemotherapy vs chemotherapy alone (94). The study found that GnRHa co-treatment may significantly decrease the POF rate from 82–33% in patients with lymphomas. Moreover, a recent single-center retrospective study on postmenarchal adolescent patients (median age 14, range 11 to 18) showed that GnRHa preserved ovarian function and fertility in adolescents treated for acute lymphoblastic leukemia, acute myeloid leukemia, Hodgkin lymphoma, or other cancers (100). On the last clinical visit, 29 patients (81%) had a regular menstrual cycle, three (8%) oligomenorrhea, and four (11%) amenorrhea. All the four patients with amenorrhea received HSCT. No differences were observed among patients’ disease.
Even these trials confirmed the acceptable safety profile of GnRHa in this setting, with only frequent estrogen deprivation symptoms, reversible upon discontinuation, and bone metabolism alterations not significant for therapies <6 months.
Globally, these clinical data suggest that GnRHa may represents a very useful tool even in post-pubertal adolescents not only for reducing the risk of hypermenorrhea associated with hematologic malignancies or myelosuppressive chemotherapy, but also for preserving ovarian function and fertility, especially when the other established methods of FP (e.g. oocyte cryopreservation) cannot be performed, but also in combination with them in order to increase the odds of success for a specific patient.
At the moment, a phase II/III (NCT02856048), and two phase II (NCT04536467 and NCT03475758) randomized open-label trials including adolescents and pediatric patients are ongoing (Table 3).
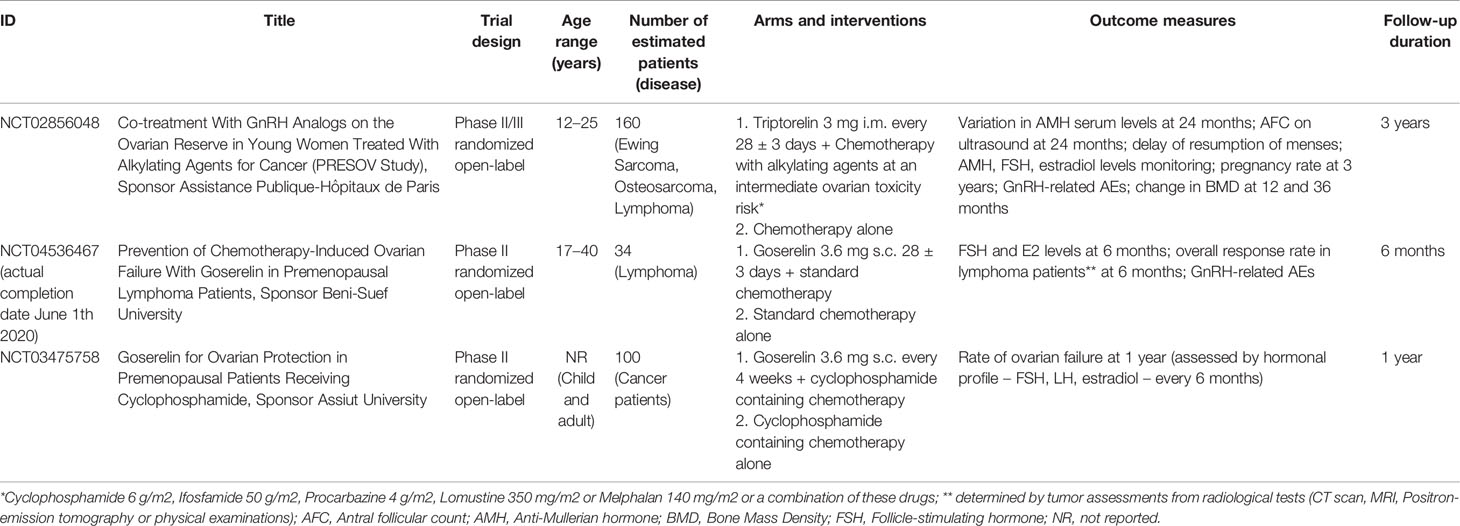
Table 3 Ongoing clinical trials (www.clinicaltrial.gov; update November 2020).
To date the three analogues (triptorelin, goserelin, leuprolide) are authorized for various therapeutic indications, such as cancer, endometriosis, uterine fibroids, and precocious puberty. Therefore, the prescription for preventing the risk of POF is typically an off-label use, defined as the use of a medicinal product “for a medical purpose not in accordance with the authorized product information” (109). European Union. Study on off-label use of medicinal products in the European Union. Available at: https://ec.europa.eu/health/sites/health/files. Off-label is not regulated at the European level, but specific national measures have been adopted (109, 110). In general, this use is not reimbursable excluding selected cases defined by law. For example, the Recommandations Temporaires d’Utilisation (RTU) provide coverage of recognized off-label treatment by France Health Insurance (111).
Moreover, the Italian national health system (NHS) reimburses an off-label use according to Law 648/1996 based on results from at least phase II trials (112). The inclusion into the 648/1996 list of reimbursable drugs ensures a nationwide access according to criteria for appropriate use and monitoring defined by the AIFA Scientific Committee in the light of clinical evidence.
From 2016 this Italian law allows to reimburse GnRHa for the preservation of ovarian function in pre-menopausal women at risk of premature and permanent menopause following chemotherapy treatment (29). The Italian regulatory authority defined the eligibility criteria, including age (>18 and <43) and lack of adequate alternative options. Thus, currently the use in the post-pubertal age is not approved and falls within the Italian Law 94/1998 (113), by which physicians can perform off-label prescriptions (not covered by the NHS) but in individual and exceptional cases.
This represents to date a limit for the treatment of this population that could be overcome if the eligibility criteria of Law 648/96 will be modified in order to include even pediatric patients.
Currently, to the best of our knowledge no other countries gave a nation-wide approval for this systematic off-label use.
Ovarian failure following chemotherapy represents an adverse event with an important impact on health and quality of life of survivors. Oocyte and tissue cryopreservation are the main options for fertility preservation. However, these techniques cannot be performed in all patients in all health-care facilities. Pharmacological treatment with GnRHa is a non-invasive and well-tolerated alternative, which can be offered to all subjects if cryopreservation is not feasible due to clinical or logistic issues. Moreover, combining several methods may increase the odds of success of fertility preservation in eligible patients. The available evidence demonstrates a significant advantage for the survivors who received the GnRHa in the long-term maintenance of ovarian function and preservation of fertility. Italy has officially recognized the off-label use of GnRHa in women at risk of premature and permanent menopause following chemotherapy. However, fertility preservation still represents an unmet medical need in adolescents, especially in those who cannot access to other therapeutic options.
SB and LG wrote the first draft of the manuscript. FD checked and revised the draft manuscript. All authors contributed to the article and approved the submitted version.
The open access publication fees will be funded within the AIFA pharmacovigilance grant, project ADR-648 “Monitoring of safety and efficacy of drugs prescribed under Law 648/96”.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.641450/full#supplementary-material
1. Gatta G, Botta L, Rossi S, Aareleid T, Bielska-Lasota M, Clavel J, et al. Childhood cancer survival in Europe 1999-2007: results of EUROCARE-5–a population-based study. Lancet Oncol (2014) 15(1):35–47. doi: 10.1016/S1470-2045(13)70548-5
2. Olsen JH, Moller T, Anderson H, Langmark F, Sankila R, Tryggvadottir L, et al. Lifelong cancer incidence in 47,697 patients treated for childhood cancer in the Nordic countries. J Natl Cancer Inst (2009) 101(11):806–13. doi: 10.1093/jnci/djp104
3. Hudson MM. Reproductive outcomes for survivors of childhood cancer. Obstet Gynecol (2010) 116(5):1171–83. doi: 10.1097/AOG.0b013e3181f87c4b
4. Nieman CL, Kinahan KE, Yount SE, Rosenbloom SK, Yost KJ, Hahn EA, et al. Fertility preservation and adolescent cancer patients: lessons from adult survivors of childhood cancer and their parents. Cancer Treat Res (2007) 138:201–17. doi: 10.1007/978-0-387-72293-1_15
5. Byrne J, Mulvihill JJ, Myers MH, Connelly RR, Naughton MD, Krauss MR, et al. Effects of treatment on fertility in long-term survivors of childhood or adolescent cancer. N Engl J Med (1987) 317(21):1315–21. doi: 10.1056/NEJM198711193172104
6. Chow EJ, Stratton KL, Leisenring WM, Oeffinger KC, Sklar CA, Donaldson SS, et al. Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol (2016) 17(5):567–76. doi: 10.1016/S1470-2045(16)00086-3
7. Reulen RC, Zeegers MP, Wallace WH, Frobisher C, Taylor AJ, Lancashire ER, et al. Pregnancy outcomes among adult survivors of childhood cancer in the British Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev (2009) 18(8):2239–47. doi: 10.1158/1055-9965.EPI-09-0287
8. Sklar CA, Mertens AC, Mitby P, Whitton J, Stovall M, Kasper C, et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst (2006) 98(13):890–6. doi: 10.1093/jnci/djj243
9. Larsen EC, Muller J, Schmiegelow K, Rechnitzer C, Andersen AN. Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab (2003) 88(11):5307–14. doi: 10.1210/jc.2003-030352
10. van den Berg MH, Overbeek A, Lambalk CB, Kaspers GJL, Bresters D, van den Heuvel-Eibrink MM, et al. Long-term effects of childhood cancer treatment on hormonal and ultrasound markers of ovarian reserve. Hum Reprod (2018) 33(8):1474–88. doi: 10.1093/humrep/dey229
11. Barton SE, Najita JS, Ginsburg ES, Leisenring WM, Stovall M, Weathers RE, et al. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol (2013) 14(9):873–81. doi: 10.1016/S1470-2045(13)70251-1
12. Peccatori FA, Azim HA Jr, Orecchia R, Hoekstra HJ, Pavlidis N, Kesic V, et al. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2013) 24 Suppl 6:vi160–70. doi: 10.1093/annonc/mdt199
13. Benedict C, Shuk E, Ford JS. Fertility Issues in Adolescent and Young Adult Cancer Survivors. J Adolesc Young Adult Oncol (2016) 5(1):48–57. doi: 10.1089/jayao.2015.0024
14. Gorman JR, Su HI, Roberts SC, Dominick SA, Malcarne VL. Experiencing Reproductive Concerns as a Female Cancer Survivor Is Associated With Depression. Cancer-Am Cancer Soc (2015) 121(6):935–42. doi: 10.1002/cncr.29133
15. Maclaran K, Horner E, Panay N. Premature ovarian failure: long-term sequelae. Menopause Int (2010) 16(1):38–41. doi: 10.1258/mi.2010.010014
16. Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas (2010) 65(2):161–6. doi: 10.1016/j.maturitas.2009.08.003
17. Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, et al. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol (2018) 36(19):1994–2001. doi: 10.1200/JCO.2018.78.1914
18. AIOM. Linee guida per la preservazione della fertilità nei pazienti oncologici. Italian Association of Medical Oncology (AIOM) (2020).
21. Practice Committees of the American Society for Reproductive M, the Society for Assisted Reproductive T. Mature oocyte cryopreservation: a guideline. Fertil Steril (2013) 99(1):37–43. doi: 10.1016/j.fertnstert.2012.09.028
22. Burns KC, Hoefgen H, Strine A, Dasgupta R. Fertility Preservation Options in Pediatric and Adolescent Patients With Cancer. Cancer (2018) 124(9):1867–76. doi: 10.1002/cncr.31255
23. Vadaparampil S, Quinn G, King L, Wilson C, Nieder M. Barriers to fertility preservation among pediatric oncologists. Patient Educ Counseling (2008) 72(3):402–10. doi: 10.1016/j.pec.2008.05.013
24. Dudzinski DM. Ethical issues in fertility preservation for adolescent cancer survivors: oocyte and ovarian tissue cryopreservation. J Pediatr Adolesc Gynecol (2004) 17(2):97–102. doi: 10.1016/j.jpag.2004.01.004
25. Donnez J, Dolmans MM. Cryopreservation and transplantation of ovarian tissue. Clin Obstet Gynecol (2010) 53(4):787–96. doi: 10.1097/GRF.0b013e3181f97a55
26. Dolmans MM, Masciangelo R. Risk of transplanting malignant cells in cryopreserved ovarian tissue. Minerva Ginecol (2018) 70(4):436–43. doi: 10.23736/S0026-4784.18.04233-8
27. Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra’anani H, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod (2008) 23(5):1007–13. doi: 10.1093/humrep/den055
28. Lau GA, Schaeffer AJ. Current standing and future directions in pediatric oncofertility: a narrative review. Transl Androl Urol (2018) 7(Suppl 3):S276–S82. doi: 10.21037/tau.2018.05.04
29. AIFADetermina n. 1005 del 22 luglio 2016 - Inserimento degli analoghi dell’ormone di rilascio delle gonadotropine (triptorelina, goserelina, leuprolide) nell’elenco dei medicinali erogabili a totale carico del Servizio sanitario nazionale, ai sensi della legge 23 dicembre 1996, n. 648, per la preservazione della funzionalita’ ovarica nelle donne in pre-menopausa affette da patologie neoplastiche che debbano sottoporsi a trattamento chemioterapico in grado di causare menopausa precoce e permanente e per le quali opzioni maggiormente consolidate di preservazione della fertilita’ (crioconservazione di ovociti) non siano considerate adeguate. (GU Serie Generale n.183 del 06-08-2016).
30. Blumenfeld Z. Fertility Preservation Using GnRH Agonists: Rationale, Possible Mechanisms, and Explanation of Controversy. Clin Med Insights Reprod Health (2019) 13:1179558119870163. doi: 10.1177/1179558119870163
31. Lambertini M, Peccatori FA, Demeestere I, Amant F, Wyns C, Stukenborg JB, et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines(dagger). Ann Oncol (2020) 31(12):1664–78. doi: 10.1016/j.annonc.2020.09.006
32. Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2019) 30(10):1674. doi: 10.1093/annonc/mdz189
33. National Comprehensive Cancer Network (NCCN) Guidelines. Available at: https://www.nccn.org/professionals/physician_gls/pdf/aya.pdf (Accessed January 2021).
34. National Comprehensive Cancer Network (NCCN) Guidelines. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (Accessed January 2021).
35. Dolmans MM, Taylor HS, Rodriguez-Wallberg KA, Blumenfeld Z, Lambertini M, von Wolff M, et al. Utility of gonadotropin-releasing hormone agonists for fertility preservation in women receiving chemotherapy: pros and cons. Fertil Steril (2020) 114(4):725–38. doi: 10.1016/j.fertnstert.2020.08.011
36. Purisch SE, Shanis D, Zerbe C, Merideth M, Cuellar-Rodriguez J, Stratton P. Management of uterine bleeding during hematopoietic stem cell transplantation. Obstet Gynecol (2013) 1211):424–7. doi: 10.1097/aog.0b013e318270ecd3
37. Bates JS, Buie LW, Woodis CB. Management of menorrhagia associated with chemotherapy-induced thrombocytopenia in women with hematologic malignancy. Pharmacotherapy (2011) 31(11):1092–110. doi: 10.1592/phco.31.11.1092
38. Chiusolo P, Salutari P, Sica S, Scirpa P, Laurenti L, Piccirillo N, et al. Luteinizing hormone-releasing hormone analogue: leuprorelin acetate for the prevention of menstrual bleeding in premenopausal women undergoing stem cell transplantation. Bone Marrow Transplant (1998) 21(8):821–3. doi: 10.1038/sj.bmt.1701187
39. Kirkham YA, Ornstein MP, Aggarwal A, McQuillan S, Canpago C. Menstrual suppression in special circumstances. J Obstet Gynaecol Can (2014) 36(10):915–24. doi: 10.1016/S1701-2163(15)30442-4
40. Quinn SM, Louis-Jacques J. Menstrual management and reproductive concerns in adolescent and young adult women with underlying hematologic or oncologic disease. Curr Opin Pediatr (2016) 28(4):421–7. doi: 10.1097/MOP.0000000000000359
41. Close AG, Jones KA, Landowski A, Switzer GE, Kazmerski TM, Miller E, et al. Current practices in menstrual management in adolescents with cancer: A national survey of pediatric oncology providers. Pediatr Blood Cancer (2019) 66(12):e27961. doi: 10.1002/pbc.27961
42. Divasta AD, Laufer MR, Gordon CM. Bone density in adolescents treated with a GnRH agonist and add-back therapy for endometriosis. J Pediatr Adolesc Gynecol (2007) 20(5):293–7. doi: 10.1016/j.jpag.2007.04.008
43. Quaas AM, Ginsburg ES. Prevention and treatment of uterine bleeding in hematologic malignancy. Eur J Obstet Gynecol Reprod Biol (2007) 134(1):3–8. doi: 10.1016/j.ejogrb.2007.03.012
44. Meirow D, Rabinovici J, Katz D, Or R, Shufaro Y, Ben-Yehuda D. Prevention of severe menorrhagia in oncology patients with treatment-induced thrombocytopenia by luteinizing hormone-releasing hormone agonist and depo-medroxyprogesterone acetate. Cancer-Am Cancer Soc (2006) 107(7):1634–41. doi: 10.1002/cncr.22199
45. Adegite EA, Goyal RK, Murray PJ, Marshal M, Sucato GS. The management of menstrual suppression and uterine bleeding: a survey of current practices in the Pediatric Blood and Marrow Transplant Consortium. Pediatr Blood Cancer (2012) 59(3):553–7. doi: 10.1002/pbc.23360
46. Rivkees SA, Crawford JD. The relationship of gonadal activity and chemotherapy-induced gonadal damage. JAMA (1988) 259(14):2123–5. doi: 10.1001/jama.259.14.2123
47. Fidler MM, Reulen RC, Winter DL, Kelly J, Jenkinson HC, Skinner R, et al. Long term cause specific mortality among 34 489 five year survivors of childhood cancer in Great Britain: population based cohort study. BMJ (2016) 354:i4351. doi: 10.1136/bmj.i4351
48. Ortin TT, Shostak CA, Donaldson SS. Gonadal status and reproductive function following treatment for Hodgkin’s disease in childhood: the Stanford experience. Int J Radiat Oncol Biol Phys (1990) 19(4):873–80. doi: 10.1016/0360-3016(90)90007-7
49. Blumenfeld Z, Katz G, Evron A. ‘An ounce of prevention is worth a pound of cure’: the case for and against GnRH-agonist for fertility preservation. Ann Oncol (2014) 25(9):1719–28. doi: 10.1093/annonc/mdu036
50. Glode LM, Robinson J, Gould SF. Protection from cyclophosphamide-induced testicular damage with an analogue of gonadotropin-releasing hormone. Lancet (1981) 1(8230):1132–4. doi: 10.1016/S0140-6736(81)92301-1
51. Bokser L, Szende B, Schally AV. Protective effects of D-Trp6-luteinising hormone-releasing hormone microcapsules against cyclophosphamide-induced gonadotoxicity in female rats. Br J Cancer (1990) 61(6):861–5. doi: 10.1038/bjc.1990.192
52. Ataya KM, McKanna JA, Weintraub AM, Clark MR, LeMaire WJ. A luteinizing hormone-releasing hormone agonist for the prevention of chemotherapy-induced ovarian follicular loss in rats. Cancer Res (1985) 45(8):3651–6.
53. Ataya K, Rao LV, Lawrence E, Kimmel R. Luteinizing hormone-releasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol Reprod (1995) 52(2):365–72. doi: 10.1095/biolreprod52.2.365
54. Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist (2007) 12(9):1044–54. doi: 10.1634/theoncologist.12-9-1044
55. Lobo RA. Potential options for preservation of fertility in women. N Engl J Med (2005) 353(1):64–73. doi: 10.1056/NEJMra043475
56. Saitta A, Altavilla D, Cucinotta D, Morabito N, Frisina N, Corrado F, et al. Randomized, double-blind, placebo-controlled study on effects of raloxifene and hormone replacement therapy on plasma no concentrations, endothelin-1 levels, and endothelium-dependent vasodilation in postmenopausal women. Arterioscler Thromb Vasc Biol (2001) 21(9):1512–9. doi: 10.1161/hq0901.095565
57. Kitajima Y, Endo T, Nagasawa K, Manase K, Honnma H, Baba T, et al. Hyperstimulation and a gonadotropin-releasing hormone agonist modulate ovarian vascular permeability by altering expression of the tight junction protein claudin-5. Endocrinology (2006) 147(2):694–9. doi: 10.1210/en.2005-0700
58. Blumenfeld Z, von Wolff M. GnRH-analogues and oral contraceptives for fertility preservation in women during chemotherapy. Hum Reprod Update (2008) 14(6):543–52. doi: 10.1093/humupd/dmn022
59. Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev (2009) 30(6):624–712. doi: 10.1210/er.2009-0012
60. Webb R, Garnsworthy PC, Gong JG, Armstrong DG. Control of follicular growth: local interactions and nutritional influences. J Anim Sci (2004) 82 E-Suppl:E63–74. doi: 10.2527/2004.8213_supplE63x
61. Grundker C, Emons G. Role of gonadotropin-releasing hormone (GnRH) in ovarian cancer. Reprod Biol Endocrinol (2003) 1:65. doi: 10.1186/1477-7827-1-65
62. Harrison GS, Wierman ME, Nett TM, Glode LM. Gonadotropin-releasing hormone and its receptor in normal and malignant cells. Endocr Relat Cancer (2004) 11(4):725–48. doi: 10.1677/erc.1.00777
63. Leung PC, Cheng CK, Zhu XM. Multi-factorial role of GnRH-I and GnRH-II in the human ovary. Mol Cell Endocrinol (2003) 202(1-2):145–53. doi: 10.1016/S0303-7207(03)00076-5
64. Paris F, Perez GI, Fuks Z, Haimovitz-Friedman A, Nguyen H, Bose M, et al. Sphingosine 1-phosphate preserves fertility in irradiated female mice without propagating genomic damage in offspring. Nat Med (2002) 8(9):901–2. doi: 10.1038/nm0902-901
65. Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol (2003) 4(5):397–407. doi: 10.1038/nrm1103
66. Badawy A, Elnashar A, El-Ashry M, Shahat M. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: prospective randomized study. Fertil Steril (2009) 91(3):694–7. doi: 10.1016/j.fertnstert.2007.12.044
67. Sverrisdottir A, Nystedt M, Johansson H, Fornander T. Adjuvant goserelin and ovarian preservation in chemotherapy treated patients with early breast cancer: results from a randomized trial. Breast Cancer Res Treat (2009) 117(3):561–7. doi: 10.1007/s10549-009-0313-5
68. Del Mastro L, Boni L, Michelotti A, Gamucci T, Olmeo N, Gori S, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA (2011) 306(3):269–76. doi: 10.1001/jama.2011.991
69. Lambertini M, Boni L, Michelotti A, Gamucci T, Scotto T, Gori S, et al. Ovarian Suppression With Triptorelin During Adjuvant Breast Cancer Chemotherapy and Long-term Ovarian Function, Pregnancies, and Disease-Free Survival: A Randomized Clinical Trial. JAMA (2015) 314(24):2632–40. doi: 10.1001/jama.2015.17291
70. Song G, Gao H, Yuan Z. Effect of leuprolide acetate on ovarian function after cyclophosphamide-doxorubicin-based chemotherapy in premenopausal patients with breast cancer: results from a phase II randomized trial. Med Oncol (2013) 30(3):667. doi: 10.1007/s12032-013-0667-8
71. Jiang FY ZQ, Zeng J. Protective effect of GnRHa on chemo-therapy induced ovarian damage in breast cancer patients. Shandong Med J (2013) 53(8):16–8.
72. Karimi-Zarchi M, Forat-Yazdi M, Vafaeenasab MR, Nakhaie-Moghadam M, Miratashi-Yazdi A, Teimoori S, et al. Evaluation of the effect of GnRH agonist on menstrual reverse in breast cancer cases treated with cyclophosphamide. Eur J Gynaecol Oncol (2014) 35(1):59–61.
73. Moore HC, Unger JM, Phillips KA, Boyle F, Hitre E, Porter D, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med (2015) 372(10):923–32. doi: 10.1056/NEJMoa1413204
74. Moore HCF, Unger JM, Phillips KA, Boyle F, Hitre E, Moseley A, et al. Final Analysis of the Prevention of Early Menopause Study (POEMS)/SWOG Intergroup S0230. J Natl Cancer Inst (2019) 111(2):210–3. doi: 10.1093/jnci/djy185
75. Leonard RCF, Adamson DJA, Bertelli G, Mansi J, Yellowlees A, Dunlop J, et al. GnRH agonist for protection against ovarian toxicity during chemotherapy for early breast cancer: the Anglo Celtic Group OPTION trial. Ann Oncol (2017) 28(8):1811–6. doi: 10.1093/annonc/mdx184
76. Zhang Y, Ji YJ, Li JW, Lei L, Wu SY, Zuo WJ, et al. Sequential versus simultaneous use of chemotherapy and gonadotropin-releasing hormone agonist (GnRHa) among estrogen receptor (ER)-positive premenopausal breast cancer patients: effects on ovarian function, disease-free survival, and overall survival. Breast Cancer Res Tr (2018) 168(3):679–86. doi: 10.1007/s10549-018-4660-y
77. Lambertini M, Ceppi M, Poggio F, Peccatori FA, Azim HA, Ugolini D, et al. Ovarian suppression using luteinizing hormone-releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: a meta-analysis of randomized studies. Ann Oncol (2015) 26(12):2408–19. doi: 10.1093/annonc/mdv374
78. Lambertini M, Moore HCF, Leonard RCF, Loibl S, Munster P, Bruzzone M, et al. Gonadotropin-Releasing Hormone Agonists During Chemotherapy for Preservation of Ovarian Function and Fertility in Premenopausal Patients With Early Breast Cancer: A Systematic Review and Meta-Analysis of Individual Patient-Level Data. J Clin Oncol (2018) 36(19):1981–90. doi: 10.1200/JCO.2018.78.0858
79. Cuzick J, Ambroisine L, Davidson N, Jakesz R, Kaufmann M, Regan M, et al. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet (2007) 369(9574):1711–23. doi: 10.1016/S0140-6736(07)60778-8
80. Paluch-Shimon S, Cardoso F, Partridge AH, Abulkhair O, Azim HA Jr, Bianchi-Micheli G, et al. ESO-ESMO 4th International Consensus Guidelines for Breast Cancer in Young Women (BCY4). Ann Oncol (2020) 31(6):674–96. doi: 10.1016/j.annonc.2020.03.284
81. Lambertini M, Cinquini M, Moschetti I, Peccatori FA, Anserini P, Valenzano Menada M, et al. Temporary ovarian suppression during chemotherapy to preserve ovarian function and fertility in breast cancer patients: A GRADE approach for evidence evaluation and recommendations by the Italian Association of Medical Oncology. Eur J Cancer (2017) 71:25–33. doi: 10.1016/j.ejca.2016.10.034
82. Gilani MM HM, Ghaemmaghami F, Ramazanzadeh F. Ovarian preservation with gonadotropin-releasing hormone analog during chemotherapy. Asia Pac. J Clin Oncol (2007) 3:79–83. doi: 10.1111/j.1743-7563.2007.00089.x
83. Waxman JH, Ahmed R, Smith D, Wrigley PF, Gregory W, Shalet S, et al. Failure to preserve fertility in patients with Hodgkin’s disease. Cancer Chemother Pharmacol (1987) 19(2):159–62. doi: 10.1007/BF00254570
84. Giuseppe L, Attilio G, Edoardo DN, Loredana G, Cristina L, Vincenzo L. Ovarian function after cancer treatment in young women affected by Hodgkin disease (HD). Hematology (2007) 12(2):141–7. doi: 10.1080/10245330600954072
85. Behringer K, Wildt L, Mueller H, Mattle V, Ganitis P, van den Hoonaard B, et al. No protection of the ovarian follicle pool with the use of GnRH-analogues or oral contraceptives in young women treated with escalated BEACOPP for advanced-stage Hodgkin lymphoma. Final results of a phase II trial from the German Hodgkin Study Group. Ann Oncol (2010) 21(10):2052–60. doi: 10.1093/annonc/mdq066
86. Demeestere I, Brice P, Peccatori FA, Kentos A, Dupuis J, Zachee P, et al. No Evidence for the Benefit of Gonadotropin-Releasing Hormone Agonist in Preserving Ovarian Function and Fertility in Lymphoma Survivors Treated With Chemotherapy: Final Long-Term Report of a Prospective Randomized Trial. J Clin Oncol (2016) 34(22):2568–U35. doi: 10.1200/JCO.2015.65.8864
87. Blumenfeld Z, Eckman A. Preservation of fertility and ovarian function and minimization of chemotherapy-induced gonadotoxicity in young women by GnRH-a. J Natl Cancer Inst Monogr (2005) 34):40–3. doi: 10.1093/jncimonographs/lgi015
88. Pacheco BP, Ribas JMM, Milone G, Fernandez I, Kvicala R, Mila T, et al. Use of GnRH analogs for functional protection of the ovary and preservation of fertility during cancer treatment in adolescents: A preliminary report. Gynecol Oncol (2001) 81(3):391–7. doi: 10.1006/gyno.2001.6181
89. Castelo-Branco C, Nomdedeu B, Camus A, Mercadal S, Martinez de Osaba MJ, Balasch J. Use of gonadotropin-releasing hormone agonists in patients with Hodgkin’s disease for preservation of ovarian function and reduction of gonadotoxicity related to chemotherapy. Fertil Steril (2007) 87(3):702–5. doi: 10.1016/j.fertnstert.2006.10.004
90. Blumenfeld Z, Dann E, Avivi I, Epelbaum R, Rowe JM. Fertility after treatment for Hodgkin’s disease. Ann Oncol (2002) 13 Suppl 1:138–47. doi: 10.1093/annonc/13.S1.138
91. Blumenfeld Z, Zuckerman T. Repeated spontaneous pregnancies and successful deliveries after repeated autologous stem cell transplantation and GnRH-agonist treatment. Oncologist (2010) 15(1):59–60. doi: 10.1634/theoncologist.2009-0269
92. Blumenfeld Z, Avivi I, Eckman A, Epelbaum R, Rowe JM, Dann EJ. Gonadotropin-releasing hormone agonist decreases chemotherapy-induced gonadotoxicity and premature ovarian failure in young female patients with Hodgkin lymphoma. Fertil Steril (2008) 89(1):166–73. doi: 10.1016/j.fertnstert.2007.02.010
93. Behringer K, Thielen I, Mueller H, Goergen H, Eibl AD, Rosenbrock J, et al. Fertility and gonadal function in female survivors after treatment of early unfavorable Hodgkin lymphoma (HL) within the German Hodgkin Study Group HD14 trial. Ann Oncol (2012) 23(7):1818–25. doi: 10.1093/annonc/mdr575
94. Blumenfeld Z, Patel B, Leiba R, Zuckerman T. Gonadotropin-releasing hormone agonist may minimize premature ovarian failure in young women undergoing autologous stem cell transplantation. Fertil Steril (2012) 98(5):1266–70.e1. doi: 10.1016/j.fertnstert.2012.07.1144
95. Huser M, Smardova L, Janku P, Crha I, Zakova J, Stourac P, et al. Fertility status of Hodgkin lymphoma patients treated with chemotherapy and adjuvant gonadotropin-releasing hormone analogues. J Assist Reprod Genet (2015) 32(8):1187–93. doi: 10.1007/s10815-015-0452-z
96. Blumenfeld Z, Zur H, Dann EJ. Gonadotropin-Releasing Hormone Agonist Cotreatment During Chemotherapy May Increase Pregnancy Rate in Survivors. Oncologist (2015) 20(11):1283–9. doi: 10.1634/theoncologist.2015-0223
97. Phelan R, Mann E, Napurski C, Defor TE, Petryk A, Miller WP, et al. Ovarian function after hematopoietic cell transplantation: a descriptive study following the use of GnRH agonists for myeloablative conditioning and observation only for reduced-intensity conditioning. Bone Marrow Transpl (2016) 51(10):1369–75. doi: 10.1038/bmt.2016.150
98. Cheng YC, Takagi M, Milbourne A, Champlin RE, Ueno NT. Phase II Study of Gonadotropin-Releasing Hormone Analog for Ovarian Function Preservation in Hematopoietic Stem Cell Transplantation Patients. Oncologist (2012) 17(2):233–8. doi: 10.1634/theoncologist.2011-0205
99. Blumenfeld Z, Avivi I, Linn S, Epelbaum R, Ben-Shahar M, Haim N. Prevention of irreversible chemotherapy-induced ovarian damage in young women with lymphoma by a gonadotrophin-releasing hormone agonist in parallel to chemotherapy. Hum Reprod (1996) 11(8):1620–6. doi: 10.1093/oxfordjournals.humrep.a019457
100. Meli M, Caruso-Nicoletti M, La Spina M, Nigro LL, Samperi P, D’Amico S, et al. Triptorelin for Fertility Preservation in Adolescents Treated With Chemotherapy for Cancer. J Pediatr Hematol Oncol (2018) 40(4):269–76. doi: 10.1097/MPH.0000000000001144
101. Gini G, Annibali O, Lupasco D, Bocci C, Tomarchio V, Sampaolo M, et al. Gonadal Function Recovery and Fertility in Women Treated with Chemo- and/or Radiotherapy for Hodgkin’s and Non-Hodgkin Lymphoma. Chemotherapy (2019) 64(1):36–41. doi: 10.1159/000499535
102. Partridge A, Gelber S, Gelber RD, Castiglione-Gertsch M, Goldhirsch A, Winer E. Age of menopause among women who remain premenopausal following treatment for early breast cancer: Long-term results from International Breast Cancer Study Group Trials V and VI. Eur J Cancer (2007) 43(11):1646–53. doi: 10.1016/j.ejca.2007.04.006
103. Lutchman Singh K, Muttukrishna S, Stein RC, McGarrigle HH, Patel A, Parikh B, et al. Predictors of ovarian reserve in young women with breast cancer. Br J Cancer (2007) 96(12):1808–16. doi: 10.1038/sj.bjc.6603814
104. Blumenfeld Z. Gonadotropin-releasing hormone analog cotreatment for preservation of ovarian function. Oncologist (2012) 17(2):162–3. doi: 10.1634/theoncologist.2011-0351
105. Castelo-Branco C, Nomdedeu B, Camus A, Mercadal S, de Osaba MJM, Balasch J. Use of gonadotropin-releasing hormone agonists in patients with Hodgkin’s disease for preservation of ovarian function and reduction of gonadotoxicity related to chemotherapy. Fertil Steril (2007) 87(3):703–6. doi: 10.1016/j.fertnstert.2006.10.004
106. Blumenfeld Z, Benaroush M, Zuckerman T. Spontaneous pregnancy and normal delivery after repeated autologous bone marrow transplantation and GnRH agonist treatment. Hum Reprod (2007) 22(8):2346. doi: 10.1093/humrep/dem066
107. Salooja N, Szydlo RM, Socie G, Rio B, Chatterjee R, Ljungman P, et al. Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective survey. Lancet (2001) 358(9278):271–6. doi: 10.1016/S0140-6736(01)05482-4
108. Carter A, Robison LL, Francisco L, Smith D, Grant M, Baker KS, et al. Prevalence of conception and pregnancy outcomes after hematopoietic cell transplantation: report from the Bone Marrow Transplant Survivor Study. Bone Marrow Transplant (2006) 37(11):1023–9. doi: 10.1038/sj.bmt.1705364
109. EU European Union. Study on off-label use of medicinal products in the European Union. European Union (EU) (2017). Available at: https://ec.europa.eu/health/sites/health/files.
110. Gozzo L, Longo L, Vitale DC, Drago F. The Regulatory Challenges for Drug Repurposing During the Covid-19 Pandemic: The Italian Experience. Front Pharmacol (2020) 11:588132. doi: 10.3389/fphar.2020.588132
111. https://www.ansm.sante.fr/Activites/Recommandations-Temporaires-d-Utilisation-RTU/Les-Recommandations-Temporaires-d-Utilisation-Principes-generaux/(offset)/0.
112. Law 648. Conversione in legge del decreto-legge 21 ottobre 1996, n. 536, recante misure per il contenimento della spesa farmaceutica e la rideterminazione del tetto di spesa per l"anno (1996). Available at: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=1996-12-23&atto.codiceRedazionale=096G0680&elenco30giorni=false (Accessed October 21, 1996).
Keywords: GnRHa, chemotherapy, adverse event, off-label, regulatory issue
Citation: Brancati S, Gozzo L, Longo L, Vitale DC, Russo G and Drago F (2021) Fertility Preservation in Female Pediatric Patients With Cancer: A Clinical and Regulatory Issue. Front. Oncol. 11:641450. doi: 10.3389/fonc.2021.641450
Received: 14 December 2020; Accepted: 29 January 2021;
Published: 09 March 2021.
Edited by:
Antonio Ruggiero, Catholic University of the Sacred Heart, ItalyReviewed by:
Zeev Blumenfeld, Technion Israel Institute of Technology, IsraelCopyright © 2021 Brancati, Gozzo, Longo, Vitale, Russo and Drago. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Filippo Drago, Zi5kcmFnb0B1bmljdC5pdA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.