- 1Department of Neurosurgery, University of Miami Miller School of Medicine, Miami, FL, United States
- 2University of Miami Brain Tumor Initiative, Department of Neurosurgery, University of Miami Miller School of Medicine, Miami, FL, United States
- 3Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, Miami, FL, United States
Glioblastoma (GBM) remains one of the most lethal primary brain tumors in both adult and pediatric patients. Targeting tumor metabolism has emerged as a promising-targeted therapeutic strategy for GBM and characteristically resistant GBM stem-like cells (GSCs). Neoplastic cells, especially those with high proliferative potential such as GSCs, have been shown to upregulate UCP2 as a cytoprotective mechanism in response to chronic increased reactive oxygen species (ROS) exposure. This upregulation plays a central role in the induction of the highly glycolytic phenotype associated with many tumors. In addition to shifting metabolism away from oxidative phosphorylation, UCP2 has also been implicated in increased mitochondrial Ca2+ sequestration, apoptotic evasion, dampened immune response, and chemotherapeutic resistance. A query of the CGGA RNA-seq and the TCGA GBMLGG database demonstrated that UCP2 expression increases with increased WHO tumor-grade and is associated with much poorer prognosis across a cohort of brain tumors. UCP2 expression could potentially serve as a biomarker to stratify patients for adjunctive anti-tumor metabolic therapies, such as glycolytic inhibition alongside current standard of care, particularly in adult and pediatric gliomas. Additionally, because UCP2 correlates with tumor grade, monitoring serum protein levels in the future may allow clinicians a relatively minimally invasive marker to correlate with disease progression. Further investigation of UCP2’s role in metabolic reprogramming is warranted to fully appreciate its clinical translatability and utility.
Introduction
In recent years, oncologic treatment plans have continued to emphasize the importance of highly specific, personalized, and targeted therapeutic modalities to combat malignancies in the clinic. Whereas some cancers have seen drastic improvements in prognostic outcomes, certain primary brain tumors have proven to be particularly refractory to most drugs. Very little improvement has been made in the management of patients with gliomas and Glioblastomas, specifically, with 5-year survival in adults and children remaining dismally low (at 7.2% and 17.7%, respectively) (1–3). With very few efficacious drugs available to these patients, there exists an obvious and desperate need for novel approaches and new potential interventions to improve patients’ quality of life. Challenges that have historically hindered glioblastoma drug discovery and utility are impermeability of the blood brain barrier, drastic intra-tumoral heterogeneity, being extremely invasive in nature, and the presence of neurocritical structures commonly surrounding the tumor. Isolating and targeting populations of common progenitor cells, or glioma stem-like cells (GSCs), within the tumor has recently emerged as a potentially effective strategy to eliminate the cells responsible for much of the rapid proliferation commonly observed in these pathologies (4, 5).
Neoplastic cells exhibit a multitude of mitotic, biochemical, and cellular aberrancies. Many solid tumors have been shown to exhibit an increased dependency on glycolytic metabolism and may even forgo oxidative phosphorylation in the presence of oxygen. In fact, it is this often-overlooked increased glycolytic flux on the part of cancer cells that enables clinicians to effectively localize tumors and metastatic outgrowths via fluorodeoxyglucose positron emission tomography (PET scan) on a regular basis. This phenomenon, originally described by Otto Warburg nearly a century ago, has been highlighted as one of the central tenants of tumorigenesis and an “emerging hallmark of cancer” (6–8). While this switch in metabolism has been well documented in the literature for some time, the underlying mechanism by which malignant cells undergo this transition is still in question.
Existing hypotheses concerning the Warburg effect posit that forgoing oxidative phosphorylation and generating energy exclusively via glycolysis may be a more efficient way to generate metabolic intermediates, nucleotides for further proliferation, and drive angiogenesis to the hypoxic, acidic microenvironment. Another theory is that increased competition for shared metabolic resources in the tumor’s vicinity cause the neoplastic cells to seek the path of less resistance and opt for the “evolutionarily less efficient pathway” to generate energy (9). Additionally, this shift in metabolism has been shown to increase cell proliferation as long as the supply of glucose is not limiting. Recent literature suggests that cells may be shutting off mitochondrial cellular respiration by increasing the expression of mitochondrial uncoupling protein 2 (UCP2), in an effort to mitigate the effect of cytotoxic reactive oxygen species (ROS), thereby inducing the Warburg effect via UCP2 upregulation (10, 11). In non-cancerous cells, UCP2 upregulation may facilitate, rather than inhibit, continued fatty acid metabolism by mitigating increased ROS generation and actually result in decreased glycolytic flux (12, 13). However, in neurons where basal ROS levels are consistently elevated, as is the case in many cancerous cells, UCP2 upregulation has shown to decrease the cell’s ability to sense glucose appropriately and results in disruption of carbohydrate homeostasis (14, 15).
Rapidly dividing stem cells with high proliferative and anabolic capabilities have been shown to overexpress UCP2, whereas induction of neuronal differentiation causes a loss of UCP2 expression (16). Similarly, human pluripotent stem cells have been shown to overexpress UCP2 and metabolize primarily via glycolysis until differentiation, wherein they repress UCP2 expression and switch to primarily oxidative phosphorylation (17). These data suggest that UCP2 may be upregulated in situations where proliferative resources are limited, in situations where cells need to generate metabolites quickly to aid in division, and in states of developmental regression into a more anaplastic phenotype as seen in oncogenesis. The central role of UCP2 in driving the metabolic switch in aggressive neoplasms, and its potential therapeutic implications have yet to be thoroughly investigated. Here we sought to investigate the potential role UCP2 could serve as a biomarker to stratify glioma patients for adjunctive metabolic therapies as well as review the implications of prolonged ROS elevation leading to UCP2 overexpression in malignancy.
Normal Function of Uncoupling Protein 2
Three homologous mitochondrial uncoupling protein domains exist at locus 11q13.4. These three isoforms, UCP1, UCP2, and UCP3 all pertain to a family of mitochondrial anion carrier proteins. Although they are differentially expressed in different tissue types, they all function to diminish the proton gradient across the inner mitochondrial membrane in mammalian cells by releasing energy in the form of heat rather than by ATP anabolism. Whereas UCP1 is predominantly expressed in brown adipose tissue and facilitates thermogenesis, UCP2 and UCP3 expression is greatest in skeletal muscle and is thought to be more involved in protecting against the cytotoxic effects of ROS (18, 19). UCP2 is located on the inner mitochondrial membrane (IMM) and acts to uncouple the proton gradient across this membrane, of which the primary function is to drive ATP synthesis via ATP synthase. Under normal circumstances, protons accumulate in the inner membrane space and ATP synthase facilitates them to readily flow into the matrix, generating ATP in the process. When UCP2 is upregulated, the opposite occurs, in that the exit of anions and protons from the matrix is facilitated. Although the mechanism by which UCP2 facilitates this proton transport is not fully understood, by allowing protons to leak across the IMM, the driving force behind ATP production via the electron transport chain is decreased, resulting in heat-energy release.
As is seen in many cancers, this metabolic shift away from mitochondrial cellular respiration causes cells to increasingly depend on glycolysis to meet their metabolic demands. Additionally, recent studies have suggested that UCP2 may have a role in global homeostatic glucose regulation due to its expression in the arcuate nucleus and pro-opiomelanocortin neurons which project into the hypothalamus (14, 20, 21). Consistent with its potential homeostatic role in metabolic function, UCP2 over-expression has been linked to both α and ß-cell dysfunction and increased mRNA transcripts for UCP2 have been detected in the pancreatic islets of several animal models with type 2 diabetes (22–24). A common UCP2 promoter polymorphism -866G/A has been shown to increase transcriptional activity by allowing for easier binding of the pancreatic transcription factor PAX6, increasing the risk of glucose dysregulation and type II diabetes in several human populations (25–29).
Understood Role of Uncoupling Protein 2 in Gliomas and Other Malignancies
Several cancers, including gliomas, have been observed to upregulate UCP2 expression when compared with their non-neoplastic cells of origin. Upregulation of this protein has been shown to directly increase AKT pathway signaling and enhance glycolysis by activating phosphofructokinase 2, a key regulatory protein in the glycolytic pathway (30). Recent studies suggest that UCP2 plays a critical role in protecting the cell from metabolically generated reactive oxygen species (ROS), which are known to become increasingly present as cells develop more malignant phenotypes. Neoplastic cells are therefore engaged in a cytotoxic positive feedback loop in which they increase carbohydrate metabolism, dramatically increasing intracellular ROS, leading to the upregulation of UCP2, which further dysregulates glycolytic function allowing cells to continue taking in glucose even in states of “satiety” (31, 32).
This phenomenon in which cells shunt metabolic away from oxidative phosphorylation to protect themselves from free radical damage induced by ROS illustrates one possible reason why cancers exhibit high glycolytic dependence even when oxygen is available. UCP2 has also been shown to facilitate mitochondrial Ca2+ sequestration from the endoplasmic reticulum specifically (33). While the link between high intracellular Ca2+ and apoptosis has long been understood, recent work posits that multiple potentially apoptogenic Ca2+ influx pathways exist, due to entering from the extracellular matrix or release from the endoplasmic reticulum (34–37). Therefore, the dramatic increase in UCP2 expression seen across multiple malignancies may also be, in part, due to aiding in sequestering rising intracellular Ca2+ in the mitochondria to protect the cell against apoptosis.
By facilitating apoptotic evasion, overexpression of UCP2 may similarly aide in chemotherapeutic resistance. Overexpression of UCP2 in multiple human cancer cell lines has consistently shown to favor a highly glycolytic phenotype, inhibits ROS accumulation, and prevents apoptosis after exposure to chemotherapeutic agents (38–40). Temozolomide is the chemotherapeutic drug of choice in glioma management and has been shown to trigger dramatic bursts of ROS leading to potential autophagy secondary to ERK activation (41). Previously, our lab has shown that GSCs are resistant to Temozolomide doses well above the peak therapeutic doses reportedly achieved in patient brain tissue and cerebrospinal fluid (42, 43). This resistance may be, in part, due to an increase of UCP2 to protect against ROS accumulation. Taken together, these findings suggest that the upregulation of UCP2 as a cytoprotective mechanism may be largely responsible for inducing this metabolic switch towards aerobic glycolysis, rather than being a consequence of an upstream metabolic alteration (Figure 1A).
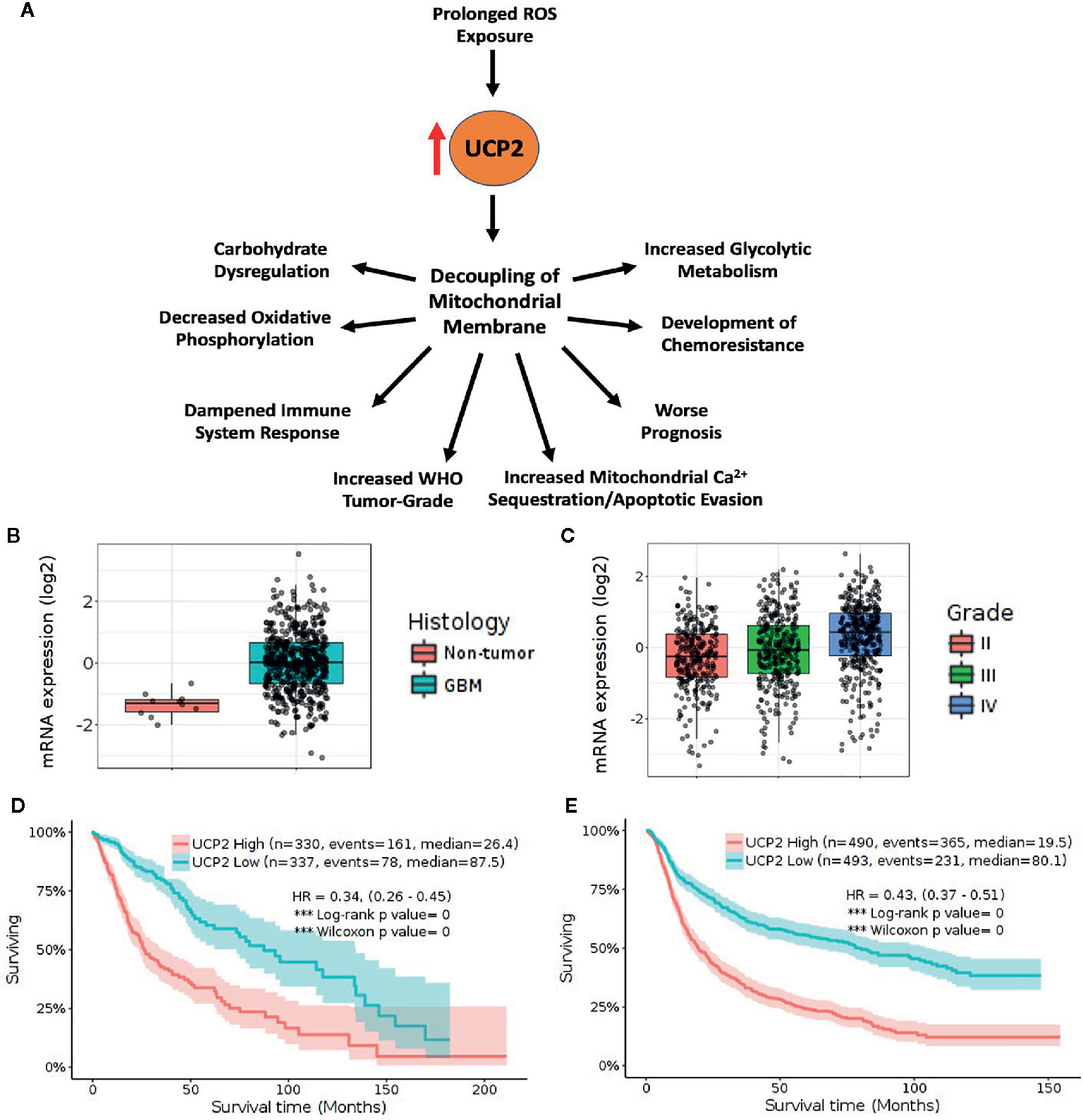
Figure 1 High UCP2 expression is indicative of advanced tumor-grade and is associated with decreased survival. (A) UCP2 upregulation has many downstream consequences including worse prognosis. (B) TCGA_GBM database platform HG-U133A containing 10 non-tumor and 528 GBM tumor samples shows UCP2 is upregulated in tumor vs. non-tumor (p= 1.3E-05). (C) UCP2 expression correlates with tumor grade as seen in query of CGGA database including 625 low-grade and 388 high-grade gliomas (Grade II vs. Grade III p= 4.2E-02, Grade II vs. Grade IV. p= 2.4E-13, Grade III vs. Grade IV p= 6.3E-07). (D) Elevated UCP2 expression is indicative of worse prognosis in survival data from the TCGA GBMLGG dataset containing 515 low-grade and 152 high-grade tumors. (E) Elevated UCP2 expression is indicative of worse prognosis in survival data from the CGGA dataset. (Expression data were analyzed via pairwise group comparison using p-value with Bonferroni correction. Kaplan–Meyer data were analyzed via computation of Log-rank p-values).
Uncoupling Protein 2 as a Potential Biomarker in Glioma
Rising levels of cytotoxic ROS have been shown to directly correlate both with increased glioma grade and with UCP2 expression. We sought to analyze if UCP2 expression alone would correlate with tumor grade. Genomic data on GBM patients from TCGA were analyzed using an open-access brain tumor database, GlioVis (gliovis. bioinfo.cnio.es) (44). Consistent with previous findings, UCP2 expression was shown to be significantly greater in GBM tissue than non-tumor tissue in an analysis of the TCGA GBM database containing 10 non-tumor samples and 528 Glioblastoma samples (p<0.05, Figure 1B). UCP2 expression was shown to positively correlate with tumor grade in both the CGGA RNA-seq database containing 625 low-grade glioma samples and 388 high-grade glioma samples and the TCGA GBMLGG database containing 515 low grade and 152 high-grade glioma samples (p<0.05, Figure 1C).
More notably, in an analysis of Kaplan-Meyer curves based on gene expression the TCGA GBMLGG shows that, across low- and high-grade gliomas, higher UCP2 expression is associated with significantly shorter median survival when compared to low UCP2 expression (High=26.4, Low=87.5 months, Figure 1D). Additionally, in a combined analysis of samples from oligodendrogliomas, oligoastrocytomas, astrocytomas, anaplastic oligodendrogliomas, anaplastic oligoastrocytomas, anaplastic astrocytomas, and GBMs the CGGA shows a significant and dramatically poorer median prognosis in tumors which have high UCP2 expression (High=19.5, Low=80.1 months, Figure 1E). In short, UCP2 expression increases with increased WHO tumor-grade and is associated with much poorer prognosis across a cohort of brain tumors.
Additionally, high UCP2 expression is known to favor a highly glycolytic metabolic profile, therefore suggesting that more aggressive gliomas may have an increased dependency on glycolysis and may be more susceptible to anti-glycolytic treatments. This finding has large implications for metabolic management of high-grade gliomas. Liquid biopsy has recently been proposed as a method of monitoring or diagnosing tumors via non-invasive, low-cost methodology by detecting circulating neoplastic cells, DNA, RNA, or proteins secreted by tumor cells (45). The feasibility of measuring UCP2 levels in patient serum has been previously demonstrated (46–48). Theoretically, patients may be able to establish baseline UCP2 measurements after surgical resection and monitor UCP2 trending upward, indicating disease progression, or trending downward, indicating efficacy of treatment due to either cell death or induction of differentiation. Similarly, following UCP2 levels may aide in the surveillance of low-grade gliomas progressing into more aggressive phenotypes. While UCP2 protein levels have been shown to decrease via western blot in response to cellular differentiation, the level of mRNA transcripts were shown to remain relatively stable (16). The consistent presence of mRNA is due to an upstream open reading frame in exon 2 of the UCP2 gene coding for ORF1 which has been shown to strongly inhibit the protein’s expression (49). By regulating this factor, cells are able to quickly increase UCP2 expression in response to metabolic stress without having to generate entirely new transcripts (50, 51). Because of this, UCP2 should be investigated at the protein level should clinicians wish to follow it as a tumor marker in the future. Importantly, determining an individual’s intratumoral UCP2 expression level can lead to targeted metabolic therapeutic interventions. Multi-modality treatment plans incorporating metabolic therapies such as glycolytic inhibition or exogenous ketone body supplementation may be more seriously considered in application to more advanced disease with more dramatic glycolytic demands.
Therapeutic Implications of Uncoupling Protein 2 Expression Level in Gliomas
The role inflammation plays in glioma progression has yet to be fully understood. While the link between glucose and inflammation has been well documented in the scientific literature, the effect on the tumor’s microenvironment of the metabolic shift accompanying UCP2 upregulation also warrants further investigation. Several studies have demonstrated that, in UCP2 knockout mice, macrophages mount a higher immune response to pathogens when compared with the UCP2 wild-type macrophage response, potentially due to the increased peroxide and superoxide generation inside mitochondria (52–54).
This finding suggests that UCP2 upregulation may be due to not only increased need for ROS mitigation, but also as a way to dampen the immune response to the tumor, allowing evasion of immune-system recognition. This potential effect of UCP2 on the microenvironment is consistent with previous studies showing that gliomas and GBMs are “immuno-cold”, and are oftentimes not engaged or targeted by patients’ immune systems in immunotherapy clinical trials. Conversely, by inducting this metabolic shift which increases glucose availability in the tumor microenvironment, UCP2 may also have a relationship to pro-inflammatory cascades which favor tumorigenesis and progression. Inflammatory tumor associated macrophages (TAMs) in the microenvironment have been associated with more aggressive malignancies with decreased patient survival (55, 56).
Two strategies exist in the metabolic targeting of UCP2 over-expression (Figure 2). One strategy is to target tumors with high levels of expression, exhibiting uncoupled oxidative phosphorylation with extremely high levels of glycolytic metabolism, with glycolytic inhibitors or glucose starvation as is suggestive with ketogenic therapy (57). An in vitro study on five different GBM cell lines by our lab found that UCP2 was directly upregulated in response to exogenous acetoacetate supplementation, and concurrent glycolytic inhibition produced a dramatic synergistic loss of cell viability (58). Treating UCP2-overexpressing HCT116 cells with 2-deoxy-D-glucose was also shown to halt cell growth, further suggesting the efficacy of glycolytic inhibitor therapy where levels of UCP2 are increased (59). By increasing the glycolytic flux to the tumor, clinicians can aim to lower the supply of glucose available to the tumors while also utilizing anti-glycolytic drugs with minimal toxicity to peripheral tissues.

Figure 2 Therapeutic implications of tumor UCP2 expression. UCP2 can be directly measured in tumor tissue and compared to baseline UCP2 expression levels. Tumors with high expression likely to exhibit carbohydrate dysregulation may benefit from adjunctive metabolic treatments such as glycolytic inhibition. Basal levels of UCP2 can be used to monitor tumor progression with potential serum measurements at follow-up. In tumors where UCP2 is inhibited or tumoral expression of UCP2 is very low, mitochondrial metabolism may remain intact suggesting an oxidative phosphorylation inhibitor may be warranted.
Another strategy is to inhibit UCP2 directly or preventing its transcription in an effort to slow rampant carbohydrate uptake and restore a more normal metabolic phenotype. In one study, lactate accumulation was diminished after siRNA knockout of UCP2, albeit not to a statistically significantly extent. However, the same study found that a 33% reduction of UCP2 expression resulted in a 22% protection from the proglycolytic-loss of mitochondrial membrane potential, suggesting that UCP2 knockout can restore normal metabolic phenotype via enabling oxidative phosphorylation (60). Also, although purine nucleotides are known to inhibit UCP2 expression under normal physiological conditions, the compound genipin successfully inhibited UCP2-mediated proton leak and reversed high-glucose induced ß cell dysfunction in both isolated kidney mitochondria and pancreatic islets (61, 62). Lending to the idea that UCP2 is expressed in highly proliferative, embryonic, stem-like states, UCP2 knockout was shown to suppress murine skin carcinogenesis of both benign papilloma and malignant squamous cell carcinoma (63). Recently, a series of in-vitro experiments demonstrated that UCP2 knockdown inhibited migration, invasiveness, clonogenicity, proliferation, and promoted via ROS-mediated cell apoptosis, in addition to reducing tumorgenicity in nude mice by inhibiting the p38 MAPK pathway (64). As previously described, stem cells with high proliferative potential have increased UCP2 expression which is only downregulated upon differentiation. Therefore, knocking out UCP2 may be a promising strategy to induce differentiation and halt cell-division or conversely facilitate apoptosis via ROS accumulation.
In regard to intratumoral heterogeneity, some subpopulations of glioblastoma cells may actually exhibit decreased glycolysis, underscoring the need for multi-modal treatment approaches and regular monitoring of the tumor’s metabolic profile to adjust therapy accordingly. One proposed escape mechanism by which cancer cells may evade glycolytic therapy is via the p53-mediated induction of SCO2, pushing for oxidative phosphorylation, and TIGAR, which lowers the levels of glycolytic substrate fructose-2,6-bisphosphate (65–67). The mitochondrial permeability transition pore (mPTP) mitigates ROS accumulation via transient opening, stabilizing the mitochondrial membrane potential (68). Shi et al. recently demonstrated that glioblastoma and other cancers lack properly functioning mPTP, and that treating these cells with a metabolically stable analogue of Gboxin, an inhibitor of oxidative phosphorylation, inhibits glioblastoma allograft and patient-derived xenografts (69). Therefore, monitoring the expression of UCP2 can help clinicians understand the metabolic profile associated with each unique tumor and give insight as to whether certain metabolic therapies may be effective. Highly glycolytic tumors expressing high levels of UCP2 may benefit from glycolytic inhibition synergizing with glucose deprivation, whereas tumors with low UCP2 expression or with UCP2 knockout metabolizing primarily via oxidative phosphorylation may benefit from differentiation and OXPHOS inhibition with Gboxin-like compounds. Patients are in desperate need of novel approaches to combat these malignancies, glioblastoma in particular. UCP2’s implications on tumor metabolism warrants more investigation. Just as PET scans are commonplace in oncologic medicine, further understanding of glioma metabolism may allow full clinical exploitation of these aberrant pathways by implementing targeted therapies into future multi-modal treatment plans.
Conclusion
Although it has been common scientific knowledge that cancers and gliomas specifically are highly glycolytic, the clinical utility of this tendency has yet to be fully exploited. As ROS increases and gliomas advance in grade, so too does UCP2 expression rise and the tumor become more dependent on glycolytic metabolism. Additionally, high UCP2 expression has shown to correlate with poorer survival outcomes. This finding suggests that more aggressive tumors with high levels of UCP2 expression, which are highly dependent on glycolysis, may benefit from multi-modal treatment approaches which aim to shut down glycolysis. Conversely, UCP2 knockout may aide in restoring normal metabolic phenotype and pushing stem-like cancer cells towards differentiation. UCP2 expression could potentially serve as a biomarker to stratify patients for adjunctive anti-tumor metabolic therapies, particularly in adult and pediatric gliomas.
Future Perspective
Clinicians in the future may be able to harness UCP2 expression profiles to better direct targeted treatments against aberrant tumor metabolism. Further investigation of UCP2’s role in metabolic reprogramming, the ability to monitor it’s expression as a serum tumor marker, and in vivo experiments exhibiting a survival benefit with appropriate stratification for additional therapies is warranted to fully appreciate it’s clinical translatability and utility.
Author Contributions
FV wrote the manuscript, developed the ideas, conducted the review of literature, and made the figures. SV reviewed the manuscript and helped with the editing process, and helped in guiding FV. RG wrote the manuscript, developed the ideas, and guided FV in creating the figures and focusing on the topic idea. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the Mystic Force Foundation for their continued support of our research.
References
1. Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol (2017) 19:v1–v88. doi: 10.1093/neuonc/nox158
2. Lukas RV, Wainwright DA, Ladomersky E, Sachdev S, Sonabend AM, Stupp R. Newly Diagnosed Glioblastoma: A Review on Clinical Management. Oncology (2019) 33:91–100.
3. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013-2017. Neuro Oncol (2020) 22:iv1–iv96. doi: 10.1093/neuonc/noaa200
4. Garnier D, Renoult O, Alves-Guerra M-C, Paris F, Pecqueur C. Glioblastoma Stem-Like Cells, Metabolic Strategy to Kill a Challenging Target. Front Oncol (2019) 9:118. doi: 10.3389/fonc.2019.00118
5. Kalkan R. Glioblastoma Stem Cells as a New Therapeutic Target for Glioblastoma. Clin Med Insights Oncol (2015) 9:95–103. doi: 10.4137/CMO.S30271
6. Warburg O, Wind F, Negelein E. The Metabolism Of Tumors In The Body. J Gen Physiol (1927) 8:519–30. doi: 10.1085/jgp.8.6.519
7. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
8. Schwartz L, Supuran CT, Alfarouk KO. The Warburg Effect and the Hallmarks of Cancer. Anticancer Agents Med Chem (2017) 17:164–70. doi: 10.2174/1871520616666161031143301
9. Epstein T, Gatenby RA, Brown JS. The Warburg effect as an adaptation of cancer cells to rapid fluctuations in energy demand. PloS One (2017) 12:e0185085. doi: 10.1371/journal.pone.0185085
10. Baffy G. Uncoupling protein-2 and cancer. Mitochondrion (2010) 10:243–52. doi: 10.1016/j.mito.2009.12.143
11. Ji R, Chen W, Wang Y, Gong F, Huang S, Zhong M, et al. The Warburg Effect Promotes Mitochondrial Injury Regulated by Uncoupling Protein-2 in Septic Acute Kidney Injury. Shock (2020) 1–24. doi: 10.1097/SHK.0000000000001576
12. Pecqueur C, Bui T, Gelly C, Hauchard J, Barbot C, Bouillaud F, et al. Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysis-derived pyruvate utilization. FASEB J (2008) 22:9–18. doi: 10.1096/fj.07-8945com
13. Kukat A, Dogan SA, Edgar D, Mourier A, Jacoby C, Maiti P, et al. Loss of UCP2 attenuates mitochondrial dysfunction without altering ROS production and uncoupling activity. PloS Genet (2014) 10:e1004385. doi: 10.1371/journal.pgen.1004385
14. Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature (2007) 449:228–32. doi: 10.1038/nature06098
15. Kong D, Vong L, Parton LE, Ye C, Tong Q, Hu X, et al. Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab (2010) 12:545–52. doi: 10.1016/j.cmet.2010.09.013
16. Rupprecht A, Sittner D, Smorodchenko A, Hilse KE, Goyn J, Moldzio R, et al. Uncoupling protein 2 and 4 expression pattern during stem cell differentiation provides new insight into their putative function. PloS One (2014) 9:e88474. doi: 10.1371/journal.pone.0088474
17. Zhang J, Khvorostov I, Hong JS, Oktay Y, Vergnes L, Nuebel E, et al. UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J (2011) 30:4860–73. doi: 10.1038/emboj.2011.401
18. Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, et al. Superoxide activates mitochondrial uncoupling proteins. Nature (2002) 415:96–9. doi: 10.1038/415096a
19. Hass DT, Barnstable CJ. Uncoupling proteins in the mitochondrial defense against oxidative stress. Prog Retin Eye Res (2021) 1–25. doi: 10.1016/j.preteyeres.2021.100941
20. Coppola A, Liu ZW, Andrews ZB, Paradis E, Roy MC, Friedman JM, et al. A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab (2007) 5:21–33. doi: 10.1016/j.cmet.2006.12.002
21. Haigh JL, New LE, Filippi BM. Mitochondrial Dynamics in the Brain Are Associated With Feeding, Glucose Homeostasis, and Whole-Body Metabolism. Front Endocrinol (Lausanne) (2020) 11:580879. doi: 10.3389/fendo.2020.580879
22. Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell (2001) 105:745–55. doi: 10.1016/S0092-8674(01)00378-6
23. Kassis N, Bernard C, Pusterla A, Casteilla L, Penicaud L, Richard D, et al. Correlation between pancreatic islet uncoupling protein-2 (UCP2) mRNA concentration and insulin status in rats. Int J Exp Diabetes Res (2000) 1:185–93. doi: 10.1155/EDR.2000.185
24. Allister EM, Robson-Doucette CA, Prentice KJ, Hardy AB, Sultan S, Gaisano HY, et al. UCP2 regulates the glucagon response to fasting and starvation. Diabetes (2013) 62:1623–33. doi: 10.2337/db12-0981
25. Krempler F, Esterbauer H, Weitgasser R, Ebenbichler C, Patsch JR, Miller K, et al. A functional polymorphism in the promoter of UCP2 enhances obesity risk but reduces type 2 diabetes risk in obese middle-aged humans. Diabetes (2002) 51:3331–5. doi: 10.2337/diabetes.51.11.3331
26. Sesti G, Cardellini M, Marini MA, Frontoni S, D’Adamo M, Del Guerra S, et al. A common polymorphism in the promoter of UCP2 contributes to the variation in insulin secretion in glucose-tolerant subjects. Diabetes (2003) 52:1280–3. doi: 10.2337/diabetes.52.5.1280
27. Sasahara M, Nishi M, Kawashima H, Ueda K, Sakagashira S, Furuta H, et al. Uncoupling protein 2 promoter polymorphism -866G/A affects its expression in beta-cells and modulates clinical profiles of Japanese type 2 diabetic patients. Diabetes (2004) 53:482–5. doi: 10.2337/diabetes.53.2.482
28. Andersen G, Dalgaard LT, Justesen JM, Anthonsen S, Nielsen T, Thorner LW, et al. The frequent UCP2 -866G>A polymorphism protects against insulin resistance and is associated with obesity: a study of obesity and related metabolic traits among 17 636 Danes. Int J Obes (Lond) (2013) 37:175–81. doi: 10.1038/ijo.2012.22
29. Gomathi P, Samarth AP, Raj N, Sasikumar S, Murugan PS, Nallaperumal S, et al. The -866G/A polymorphism in the promoter of the UCP2 gene is associated with risk for type 2 diabetes and with decreased insulin levels. Gene (2019) 701:125–30. doi: 10.1016/j.gene.2019.03.041
30. Sreedhar A, Petruska P, Miriyala S, Panchatcharam M, Zhao Y. UCP2 overexpression enhanced glycolysis via activation of PFKFB2 during skin cell transformation. Oncotarget (2017) 8:95504–15. doi: 10.18632/oncotarget.20762
31. Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature (2008) 454:846–51. doi: 10.1038/nature07181
32. Robinson AJ, Hopkins GL, Rastogi N, Hodges M, Doyle M, Davies S, et al. Reactive Oxygen Species Drive Proliferation in Acute Myeloid Leukemia via the Glycolytic Regulator PFKFB3. Cancer Res (2020) 80:937–49. doi: 10.1158/0008-5472.CAN-19-1920
33. Waldeck-Weiermair M, Malli R, Naghdi S, Trenker M, Kahn MJ, Graier WF. The contribution of UCP2 and UCP3 to mitochondrial Ca(2+) uptake is differentially determined by the source of supplied Ca(2+). Cell Calcium (2010) 47:433–40. doi: 10.1016/j.ceca.2010.03.004
34. Giorgi C, Romagnoli A, Pinton P, Rizzuto R. Ca2+ signaling, mitochondria and cell death. Curr Mol Med (2008) 8:119–30. doi: 10.2174/156652408783769571
35. Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene (2008) 27:6407–18. doi: 10.1038/onc.2008.308
36. Koshenov Z, Oflaz FE, Hirtl M, Bachkoenig OA, Rost R, Osibow K, et al. The contribution of uncoupling protein 2 to mitochondrial Ca(2+) homeostasis in health and disease - A short revisit. Mitochondrion (2020) 55:164–73. doi: 10.1016/j.mito.2020.10.003
37. Motloch LJ, Larbig R, Gebing T, Reda S, Schwaiger A, Leitner J, et al. By Regulating Mitochondrial Ca2+-Uptake UCP2 Modulates Intracellular Ca2+. PloS One (2016) 11:e0148359. doi: 10.1371/journal.pone.0148359
38. Dalla Pozza E, Fiorini C, Dando I, Menegazzi M, Sgarbossa A, Costanzo C, et al. Role of mitochondrial uncoupling protein 2 in cancer cell resistance to gemcitabine. Biochim Biophys Acta (2012) 1823:1856–63. doi: 10.1016/j.bbamcr.2012.06.007
39. Yu G, Liu J, Xu K, Dong J. Uncoupling protein 2 mediates resistance to gemcitabine-induced apoptosis in hepatocellular carcinoma cell lines. Biosci Rep (2015) 35:1–7. doi: 10.1042/BSR20150116
40. Yu J, Shi L, Lin W, Lu B, Zhao Y. UCP2 promotes proliferation and chemoresistance through regulating the NF-kappaB/beta-catenin axis and mitochondrial ROS in gallbladder cancer. Biochem Pharmacol (2020) 172:113745. doi: 10.1016/j.bcp.2019.113745
41. Lin CJ, Lee CC, Shih YL, Lin TY, Wang SH, Lin YF, et al. Resveratrol enhances the therapeutic effect of temozolomide against malignant glioma in vitro and in vivo by inhibiting autophagy. Free Radic Biol Med (2012) 52:377–91. doi: 10.1016/j.freeradbiomed.2011.10.487
42. Gersey ZC, Rodriguez GA, Barbarite E, Sanchez A, Walters WM, Ohaeto KC, et al. Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer (2017) 17:99. doi: 10.1186/s12885-017-3058-2
43. Portnow J, Badie B, Chen M, Liu A, Blanchard S, Synold TW. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res (2009) 15:7092–8. doi: 10.1158/1078-0432.CCR-09-1349
44. Bowman RL, Wang Q, Carro A, Verhaak RG, Squatrito M. GlioVis data portal for visualization and analysis of brain tumor expression datasets. Neuro Oncol (2017) 19:139–41. doi: 10.1093/neuonc/now247
45. Karachaliou N, Mayo-de-Las-Casas C, Molina-Vila MA, Rosell R. Real-time liquid biopsies become a reality in cancer treatment. Ann Transl Med (2015) 3:36. doi: 10.3978/j.issn.2305-5839.2015.01.16
46. Pan HC, Lee CC, Chou KM, Lu SC, Sun CY. Serum levels of uncoupling proteins in patients with differential insulin resistance: A community-based cohort study. Med (Baltimore) (2017) 96:e8053. doi: 10.1097/MD.0000000000008053
47. Kakarla M, Puppala VK, Tyagi S, Anger A, Repp K, Wang J, et al. Circulating levels of mitochondrial uncoupling protein 2, but not prohibitin, are lower in humans with type 2 diabetes and correlate with brachial artery flow-mediated dilation. Cardiovasc Diabetol (2019) 18:148. doi: 10.1186/s12933-019-0956-4
48. Huang W, Wang X, Zhang H, Wang C, Liu D. The Value of Serum Uncoupling Protein-2 Level for the Patients With Sepsis. Shock (2020) 54:301–7. doi: 10.1097/SHK.0000000000001523
49. Pecqueur C, Alves-Guerra MC, Gelly C, Levi-Meyrueis C, Couplan E, Collins S, et al. Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J Biol Chem (2001) 276:8705–12. doi: 10.1074/jbc.M006938200
50. Rupprecht A, Moldzio R, Modl B, Pohl EE. Glutamine regulates mitochondrial uncoupling protein 2 to promote glutaminolysis in neuroblastoma cells. Biochim Biophys Acta Bioenerg (2019) 1860:391–401. doi: 10.1016/j.bbabio.2019.03.006
51. Yu G, Wang J, Xu K, Dong J. Dynamic regulation of uncoupling protein 2 expression by microRNA-214 in hepatocellular carcinoma. Biosci Rep (2016) 36:1–6. doi: 10.1042/BSR20160062
52. Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet (2000) 26:435–9. doi: 10.1038/82565
53. Carrion J, Abengozar MA, Fernandez-Reyes M, Sanchez-Martin C, Rial E, Dominguez-Bernal G, et al. UCP2 deficiency helps to restrict the pathogenesis of experimental cutaneous and visceral leishmaniosis in mice. PloS Negl Trop Dis (2013) 7:e2077. doi: 10.1371/journal.pntd.0002077
54. Bai Y, Onuma H, Bai X, Medvedev AV, Misukonis M, Weinberg JB, et al. Persistent nuclear factor-kappa B activation in Ucp2-/- mice leads to enhanced nitric oxide and inflammatory cytokine production. J Biol Chem (2005) 280:19062–9. doi: 10.1074/jbc.M500566200
55. Lin JY, Li XY, Tadashi N, Dong P. Clinical significance of tumor-associated macrophage infiltration in supraglottic laryngeal carcinoma. Chin J Cancer (2011) 30:280–6. doi: 10.5732/cjc.010.10336
56. Yagi T, Baba Y, Okadome K, Kiyozumi Y, Hiyoshi Y, Ishimoto T, et al. Tumour-associated macrophages are associated with poor prognosis and programmed death ligand 1 expression in oesophageal cancer. Eur J Cancer (2019) 111:38–49. doi: 10.1016/j.ejca.2019.01.018
57. Schwartz L, Seyfried T, Alfarouk KO, Da Veiga Moreira J, Fais S. Out of Warburg effect: An effective cancer treatment targeting the tumor specific metabolism and dysregulated pH. Semin Cancer Biol (2017) 43:134–8. doi: 10.1016/j.semcancer.2017.01.005
58. Vallejo FA, Shah SS, de Cordoba N, Walters WM, Prince J, Khatib Z, et al. The contribution of ketone bodies to glycolytic inhibition for the treatment of adult and pediatric glioblastoma. J Neurooncol (2020) 147:317–26. doi: 10.1007/s11060-020-03431-w
59. Derdak Z, Mark NM, Beldi G, Robson SC, Wands JR, Baffy G. The mitochondrial uncoupling protein-2 promotes chemoresistance in cancer cells. Cancer Res (2008) 68:2813–9. doi: 10.1158/0008-5472.CAN-08-0053
60. Samudio I, Fiegl M, McQueen T, Clise-Dwyer K, Andreeff M. The warburg effect in leukemia-stroma cocultures is mediated by mitochondrial uncoupling associated with uncoupling protein 2 activation. Cancer Res (2008) 68:5198–205. doi: 10.1158/0008-5472.CAN-08-0555
61. Zackova M, Skobisova E, Urbankova E, Jezek P. Activating omega-6 polyunsaturated fatty acids and inhibitory purine nucleotides are high affinity ligands for novel mitochondrial uncoupling proteins UCP2 and UCP3. J Biol Chem (2003) 278:20761–9. doi: 10.1074/jbc.M212850200
62. Qiu W, Zhou Y, Jiang L, Fang L, Chen L, Su W, et al. Genipin inhibits mitochondrial uncoupling protein 2 expression and ameliorates podocyte injury in diabetic mice. PloS One (2012) 7:e41391. doi: 10.1371/journal.pone.0041391
63. Li W, Zhang C, Jackson K, Shen X, Jin R, Li G, et al. UCP2 knockout suppresses mouse skin carcinogenesis. Cancer Prev Res (Phila) (2015) 8:487–91. doi: 10.1158/1940-6207.CAPR-14-0297-T
64. Wu S, Luo C, Hameed NUF, Wang Y, Zhuang D. UCP2 silencing in glioblastoma reduces cell proliferation and invasiveness by inhibiting p38 MAPK pathway. Exp Cell Res (2020) 394:112110. doi: 10.1016/j.yexcr.2020.112110
65. Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, et al. p53 regulates mitochondrial respiration. Science (2006) 312:1650–3. doi: 10.1126/science.1126863
66. Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell (2006) 126:107–20. doi: 10.1016/j.cell.2006.05.036
67. Han CY, Patten DA, Richardson RB, Harper ME, Tsang BK. Tumor metabolism regulating chemosensitivity in ovarian cancer. Genes Cancer (2018) 9:155–75. doi: 10.18632/genesandcancer.176
68. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol Rev (2014) 94:909–50. doi: 10.1152/physrev.00026.2013
Keywords: uncoupling protein 2 (UCP2), cancer, glioma, biomarker, metabolism, Warburg effect, Glioblastoma, precision-medicine
Citation: Vallejo FA, Vanni S and Graham RM (2021) UCP2 as a Potential Biomarker for Adjunctive Metabolic Therapies in Tumor Management. Front. Oncol. 11:640720. doi: 10.3389/fonc.2021.640720
Received: 11 December 2020; Accepted: 01 February 2021;
Published: 08 March 2021.
Edited by:
Federica Sotgia, University of Salford, United KingdomReviewed by:
Khalid Omer Alfarouk, Alfarouk Biomedical Research LLC, United StatesHsueh-Wei Chang, Kaohsiung Medical University, Taiwan
Copyright © 2021 Vallejo, Vanni and Graham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Regina M. Graham, UkdyYWhhbUBtZWQubWlhbWkuZWR1
 Frederic A. Vallejo
Frederic A. Vallejo Steven Vanni1
Steven Vanni1 Regina M. Graham
Regina M. Graham